Abstract
Purpose
3-hydroxyisobutryl-CoA hydrolase (HIBCH) deficiency is a rare disorder of valine metabolism. We present a family with the oldest reported subjects with HIBCH deficiency and provide support that HIBCH deficiency should be included in the differential for elevated hydroxy-C4-carnitine in newborn screening (NBS).
Methods
Whole exome sequencing (WES) was performed on one affected sibling. HIBCH enzymatic activity was measured in patient fibroblasts. Acylcarnitines were measured by electrospray ionization tandem mass spectrometry (ESI-MS/MS). Disease incidence was estimated using a cohort of 61,434 individuals.
Results
Two siblings presented with infantile-onset, progressive neurodegenerative disease. WES identified a novel homozygous variant in HIBCH c.196C>T; p.Arg66Trp. HIBCH enzymatic activity was significantly reduced in patients’ fibroblasts. Acylcarnitine analysis showed elevated hydroxy-C4-carnitine in blood spots of both affected siblings, including in their NBS cards, while plasma acylcarnitines were normal. Estimates show HIBCH deficiency incidence as high as 1 in ~130,000 individuals.
Conclusion
We describe a novel family with HIBCH deficiency at the biochemical, enzymatic and molecular level. Disease incidence estimates indicate HIBCH deficiency may be under-diagnosed. This together with the elevated hydroxy-C4-carnitine found in the retrospective analysis of our patient’s NBS cards suggests that this disorder could be screened by NBS programs and should be added to the differential diagnosis for elevated hydroxy-C4-carnitine which is already measured in most NBS programs using MS/MS.
Keywords: HIBCH deficiency, hydroxy-C4-carnitine, valine metabolism, newborn screening, Leigh syndrome
INTRODUCTION
HIBCH deficiency (OMIM #250620) is a rare inborn error of metabolism caused by a defect in the HIBCH enzyme resulting in deficiency in the conversion of 3-hydroxy-isobutryl-CoA to 3-hydroxy-isobutyric acid, a critical step in valine catabolism [1]. Only 9 patients from 7 unrelated families, 3 of which are consanguineous, have been reported [1–7]. This autosomal recessive condition is characterized by developmental delay of motor milestones in early infancy and neurological regression within the first year of life. MRI abnormalities are striking for bilateral involvement of the basal ganglia with varying degrees of white matter atrophy [1,2,4]. Strikingly, in all HIBCH patients reported to date, the clinical presentation and MRI findings led to a general diagnosis of Leigh syndrome (OMIM 256000). Leigh’s is a relatively common neurometabolic condition associated with several different gene disorders which features infantile onset progressive encephalopathy and characteristic brain MRI findings including bilateral basal ganglia and white matter changes. Leigh syndrome is caused mainly, but not exclusively, by defects in oxidative phosphorylation and may be accompanied with elevated lactic acid [8]. . Due to this, mitochondrial diseases are naturally considered in the top differential diagnoses for children presenting with Leigh’s disease while HIBCH, which is considered to be exceedingly rare, is not typically investigated.
A definitive diagnosis of HIBCH deficiency can be achieved through measurement of HIBCH enzymatic activity in patient tissues. Affected individuals have significantly decreased enzymatic activity as compared to healthy controls [1,4]. However, this assay is not readily performed in clinical laboratories. A significant clinical diagnostic finding for HIBCH deficiency is elevation of 3-hydroxy-isobutyryl-carnitine, which occurs secondary to the accumulation of 3-hydroxy-isobutyryl-CoA and has been reported in almost all subjects with HIBCH deficiency in whom this metabolite was measured [1,4,6]. In addition, urinary excretion of 2-methyl-2,3-dihydroxybutyric acid can be seen in small quantities by urine organic acid analysis and methacrylyl-CoA metabolites, S-(2-carboxypropyl)cysteine and S-(2-carboxypropyl)cysteamine, can be detected through urine MS/MS analysis [7,9]. However, these metabolites, which are not routinely measured in most laboratories, are also present in individuals with a deficiency of short-chain enoyl-CoA hydratase (SCEH), a mitochondrial enzyme immediately upstream of HIBCH in the valine catabolic pathway encoded by ECHS1 [10]. Further obfuscation of the diagnosis of HIBCH deficiency occurs when diagnostic measurement of acylcarnitine levels does not include hydroxy-C4-carnitine in the report, and/or when hydroxy-C4-carnitine falls within normal limits [6,7]. All of these factors may lead to the under-diagnosis of HIBCH deficiency.
Here, we report a novel family with two siblings affected with HIBCH deficiency confirmed by enzymatic, biochemical and molecular studies. We present detailed biochemical profiling of these cases including retrospective evaluation of newborn screening cards showing elevated hydroxy-C4-carnitine. We also estimate incidence for this disorder and find compelling results that imply HIBCH deficiency is under-diagnosed and more common than currently held. Our report highlights the clinical and biochemical presentation of this disease and provides evidence that HIBCH deficiency should be included in the differential diagnosis for elevated hydroxy-C4-carnitine in NBS programs using MS/MS.
METHODS
Human Subjects
Patients 1 and 2 are similarly affected siblings with parents of Lebanese origin. The family self-reports consanguinity as parents being first cousins. Informed consent was obtained for all subjects under approved Institutional Review Boards #130990 at CHOC Children’s. Genomic DNA was extracted from peripheral-blood lymphocytes or cultured fibroblasts according to standard protocols.
Molecular Analysis
Whole exome sequencing was performed at the Human Genome Sequencing Center (HGSC) at Baylor College of Medicine through the Baylor-Hopkins Center for Mendelian Genomics initiative. Using 1 μg of DNA an Illumina paired-end pre-capture library was constructed according to the manufacturer’s protocol. Four pre-captured libraries were pooled and then hybridized in solution to the HGSC CORE design (52Mb, NimbleGen) according to the manufacturer’s protocol NimbleGen SeqCap EZ Exome Library SR User’s Guide (Version 2.2) with minor revisions. The sequencing run was performed with a sequencing yield of 9.4 Gb, the sample achieved 91% of the targeted exome bases covered to a depth of 20X or greater. Sequence data were aligned, single nucleotide variants (SNVs) and small insertions and deletions (InDels) were called by GATK [11,12]. Quality control filtering of variants was based on coverage, strand bias, mapping quality, and base quality custom Perl scripts were used to annotate variants as previously described [13]. Multiple metrics for prediction of potential functional consequences of variants were applied: CADD [14], SIFT [15] and PolyPhen2 [16], Genomic Evolutionary Rate Profiling (GERP) [17,18], and PhyloP [19]. Filtering of variants included five criteria: 1. Allele frequency must be less than 2% in any reference population. 2. Scaled CADD of ≥ 15 or two or more of PhyloP must be greater than 2 OR GERP_RS greater than 5 or maximum damaging scores for SIFT or PolyPhen2_HDIV. 3. Variants should be homozygous or compound heterozygous. 4. Variants must not be present in a segmental duplication. 5. Variant must not be present in a SNP cluster defined as the presence of 5 or more SNVs within 1 Kb of each other. The Exome Aggregation Consortium (ExAC) Cohort was used as reference population data for variant filtering of exome data (Exome Aggregation Consortium (ExAC) Cohort, Cambridge, MA, http://exac.broadinstitute.org, accessed December, 2014).
The HIBCH variant identified through exome sequencing in Patient 2 was orthogonally validated and recessive segregation through the subject’s pedigree was confirmed using PCR-based Sanger sequencing.
All genetic alleles studied were submitted to ClinVar http://www.ncbi.nlm.nih.gov/clinvar/ and were annotated in reference to HIBCH NM_014362.3 for cDNA and NP_055177.2 for protein.
Enzyme Analysis
HIBCH enzymatic activity was measured in primary fibroblasts at the Laboratory Genetic Metabolic Diseases at the Academic Medical Center according to published methods [1].
Acylcarnitine Analysis
Acylcarnitines were measured at CHOC Children’s Metabolic Laboratory from dried blood spots (Schleicher & Schuell 903, Keene, NH, U.S.A), and retrospectively from the patient’s newborn screening cards (stored 12 – 13 yrs). Ten birth-matched control samples (5 for each patient) obtained at the same time and stored in the same manner were also analyzed. The laboratory was blind regarding the sample identity (patient or control). Acylcarnitine butyl-esters were analyzed by ESI-MS/MS (Waters Alliance 2795 Quattro Micro) . Precursor ion scans of the peak at m/z 85 were monitored in the range m/z 250-500.
Disease Incidence Estimate
Disease incidence was estimated based on the Hardy-Weinberg Equation, 1 = p2 + 2pq + q2, using carrier allele frequencies. Any variant meeting the above criteria for being considered ‘damaging’ were considered pathogenic and the frequency of these ‘damaging’ alleles were determined in the ExAC cohort.
RESULTS
Clinical Report
Patient 1
The family history was positive for six first trimester miscarriages, thought to be related to a uterine septum which was later on surgically corrected but left scar tissue. A female term baby ,was born after the surgical procedure, but died of hypoplastic left heart at six days of age (Fig. 1A). Patient 1 (IV-2), a girl, the product of in vitro fertilization (IVF), was the second child born at 38 weeks gestational age by cesarean section after an uneventful pregnancyEarly developmental milestones were normal. She was able to sit unsupported but was never able to bear weight and never developed language. From 4-5 months of age, our patient began to lose previously acquired milestones. Initial neurological examination at 11 months of age noted normal head circumference, no dysmorphic features, dystonia, spastic quadriplegia and absent reflexes in the lower extremities. Visual inattention prompted an ophthalmological examination which revealed optic atrophy. A gastrostomy tube was placed at 1.6 years of age due to poor feeding and failure to thrive. At 2 years of age brain MRI demonstrated high signal lesions in the globus pallidus and head of the caudate nucleus with patchy high signal in the periventricular white matter and a focal lesion in the left cerebral peduncle (Fig. 2A, a-c). Repeat MRI at 6.3 years of age showed improved high signal abnormality in the basal ganglia, however new associated atrophy was seen (Fig. 2A, d-f). The most recent brain MRI at 12 years of age showed residual cystic changes in the globus pallidus, basal ganglia atrophy, and persistent generalized brain atrophy and white matter changes (Fig. 2A, g-i). Patient is currently 13 years of age. Physical examination is remarkable for microcephaly and severely impaired vision. She is unable to fix or follow, has strabismus, intermittent roving eye movements and optic nerve atrophy. She is unable to talk but is aware of her surroundings and communicates by making sounds, smiling or grimacing. Patient has poor head control, is unable to sit-up or ambulate (wheel chair bound). At baseline she has truncal hypotonia with hypertonia in extremities. Intermittent dystonia in the trunk, extremities and face are evident with positional changes or distress. Patient has a gastric tube for feedings, severe scoliosis and mild joint contractures, which developed overtime secondary to decreased movements, and a dislocated hip. She is in Tanner Stage IV, with a history of precocious puberty. Baclofen and Clonazepam have been used for treatment of her dystonia, with partial response.
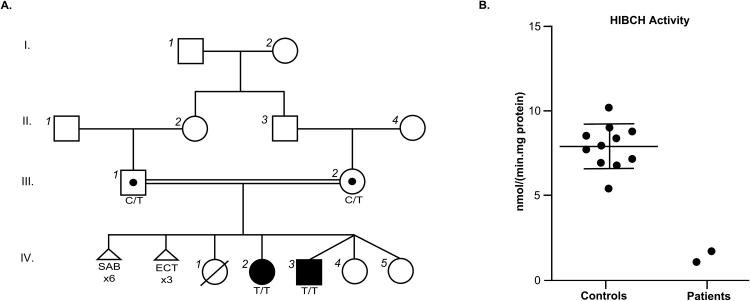
Figure 1. Novel homozygous HIBCH variant results in decreased enzymatic activity measurements in cultured skin fibroblasts of affected siblings.
(A) A four-generation familial pedigree highlighting consanguinity is positive for a pathogenic homozygous missense variant in HIBCH NM_014362.3 c.196C>T in both affected siblings (filled black symbol), with carrier parents (unfilled circle with a black dot). SAB, spontaneous abortion. ECT, ectopic pregnancy. (B) HIBCH activity measurements in control fibroblasts compared to affected patients. Reference range for the control population is 5.3 – 10.5 nmol/(min.mg). Whisker plot denotes the mean (large horizontal line) and standard deviation of the mean (small horizontal lines; n=11).
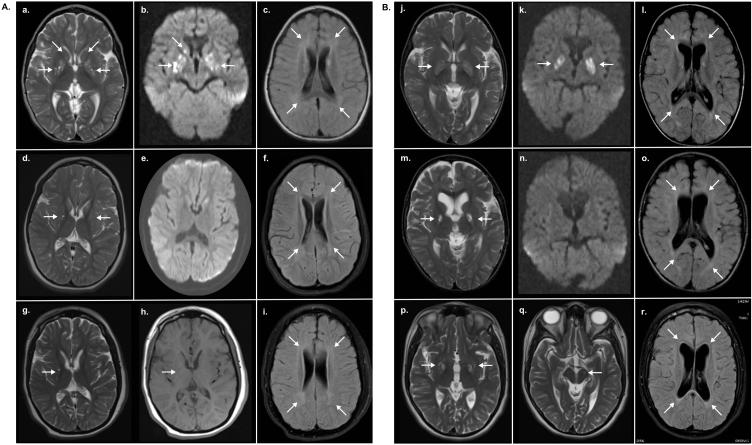
Figure 2. MRI over a ten year time period reveals classic findings of HIBCH deficiency. (A) Patient 1: Initial brain MRI obtained at 2 years of age.
(a) Axial T2 weighted images show high signal lesions in the globus pallidus and head of the caudate. (b) B1000 DWI shows corresponding restricted diffusion, and (d) flair images show patchy high signal in the periventricular white matter.
(d) Repeat MRI obtained at 6.3 years of age shows improved high signal abnormality in the globus pallidus with associated atrophy on T2 weighted images. (e) The abnormal signals in B1000 DWI have now normalized and (f) the patchy high signal in flair throughout the white matter persists; there is now generalized atrophy. (g-h) Most recent MRI obtained at 12 years of age shows residual cystic changes in the globus pallidus on T2 and T1, with overall basal ganglia atrophy and resolution of the caudate signal abnormalities. (i) Flair images demonstrate persistent white matter signal changes and increased atrophy.
(B) Patient 2: (j) Initial brain MRI performed at 1.6 years of age demonstrates swelling of the globus pallidus bilaterally with diffuse abnormal high signal in T2. (k) B1000 DWI shows corresponding restricted diffusion and (l) flair images show generalized atrophy with bilateral patchy high signal throughout the deep white matter. (m) At 3.6 years of age T2 images show interval development of cystic changes in the globus pallidus with atrophy. (n) The abnormal signals in B1000 DWI have now normalized and (o) flair sequence again shows generalized atrophy and deep white matter hyperintensities.
(p) At 11 years of age, T2 images show stable cystic changes in the globus pallidus and (q) a small high signal intensity lesion in the left cerebral peduncle. (r) Flair images show improved patchy white matter hyperintensities.
Patient 2
Patient 2 (IV-3), the brother of patient 1, was the second child of a triplet pregnancy conceived by IVF, with two healthy sisters. He was delivered via cesarean section and born with good Apgar scores with no perinatal complications. The patient’s clinical course is similar to that of his older sister. Symptoms first became apparent at 6 months of age with failure to thrive and developmental delay. Gastrostomy tube was placed due to poor feedings at 2.10 years of age. Ophthalmological evaluation was also consistent with optic nerve atrophy. At 1.6 years of age, brain MRI demonstrated swelling of the globus pallidus bilaterally with diffuse abnormal high signal and generalized atrophy throughout the deep white matter (Fig. 2B, j-l). A new MRI at 3.6 years of age showed interval development of cystic changes in the basal ganglia with atrophy and a questionable lesion in the left cerebral peduncle in addition to persistent generalized atrophy and deep white matter hyperintensities (Fig. 2B, m-o). The latest brain MRI at 11 years of age showed stable cystic changes in the basal ganglia, a more pronounced lesion in the left cerebral peduncle and improvement of patchy white matter high signal (Fig. 2B, p-r). Patient is currently 12 years old. Physical examination and overall clinical course are remarkably similar to his sister’s. He underwent spinal surgery for scoliosis at the age of 11 years and also has a history of femur fractures secondary to osteopenia.
Laboratory Testing
Investigations for suspected mitochondrial disease included lactate in plasma and CSF, organic acids, acylcarnitines, and plasma and CSF amino acids, all of which were reported as normal. A muscle biopsy was done in patient 1 which showed normal histology, including cytochrome c oxidase (COX) stain, and normal respiratory chain activity. Further testing included pyruvate dehydrogenase complex activity in fibroblasts, which was normal.
Molecular Testing Identifies Pathogenic HIBCH Variant
Chromosomes, microarray and testing for common mitochondrial DNA mutations in blood and muscle were normal, as well as Southern blot testing for deletions in muscle. Sanger sequencing of SURF1, SCO1, SCO2, COX10, NDUFV1, NDUFS7, and PANK2 revealed no pathogenic mutation.
Whole exome sequencing of Patient 2 identified a homozygous variant in HIBCH NM_014362.3 c.196C>T; NP_055177.2 p.Arg66Trp that is predicted damaging to protein function with maximally damaging scores from both SIFT and PolyPhen2. This SNV received a scaled CADD score of 16.4. Metrics of constraint and conservation were not impressive with GERP = 3.49 and PhyloP = 0.6. This variant was not observed in NHLBI ESP 6,500 cohort or the 1000 Genomes Project and was observed in 2 out of 122,868 chromosomes in the ExAC cohort. Sanger sequencing showed that both affected siblings were homozygous for this variant and each parent was heterozygous, thus confirming recessive inheritance through the pedigree.
HIBCH Enzymatic Activity in Patient Cells Confirms Diagnosis
Definitive diagnosis of HIBCH deficiency was achieved through testing of HIBCH enzymatic activity in fibroblasts from the affected children. HIBCH activity in both patient’s fibroblasts was markedly reduced compared to the reference range at 1.1 and 1.7 nmol/(min.mg protein) for patients 1 and 2 respectively (reference range 5.3–10.5 nmol/(min.mg protein), Fig. 1B), thus confirming the diagnosis of HIBCH deficiency at both a molecular and enzymatic level.
Acylcarnitine Analysis Demonstrates Potential for Newborn Screening
Acylcarnitine levels tested in our patients prior to their referral to our center were reported as normal, however, the profile did not include assessment of hydroxy-C4-carnitine. After results of exome sequencing pointed to HIBCH as a potential cause of pathogenicity, acylcarnitine analysis in blood spots showed elevated hydroxy-C4-carnitine in both patients at 0.88 μM and 1.23 μM for patient 1 and 2 respectively (control < 0.3 μM). Acylcarnitine analysis by flow injection MS/MS as it is typically performed in clinical laboratories cannot discriminate between the isomers 3-hydroxy-butyryl and 3-hydroxy-isobutyryl-carnitine, but based on the enzymatic, molecular data and published information [1]; we assume that the isomer elevated in our patients was 3-hydroxy-isobutyryl-carnitine. Retrospective analysis on the original NBS cards stored for over 10 years, also showed elevated hydroxy-C4-carnitine in both siblings at 0.78 μM and 0.43 μM for patient 1 and 2 respectively compared with very low hydroxy-C4-carnitine in all control samples (patient 1 controls = .074 μM ± .028; patient 2 controls = .074 μM ± .057; Fig. 3 A-C). This demonstrates the potential to successfully screen for HIBCH deficiency in NBS. To determine if hydroxy-C4-carnitine had been persistently elevated in our patients, the plasma acylcarnitines which were reported as normal but did not include C4OH levels, were reviewed by the performing labs which confirmed that levels of hydroxy-C4-carnitine were within normal limits .
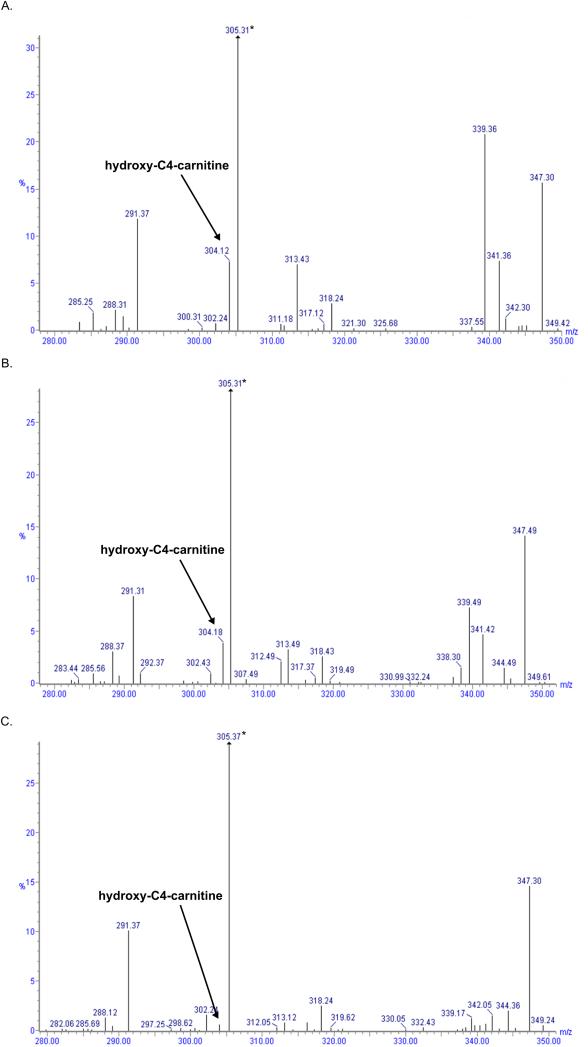
Figure 3. Retrospective acylcarnitine analysis of patient’s NBS cards reveals elevated hydroxy-C4-carnitine.
Acylcarnitine mass spectra from NBS cards of (A) patient 1 and (B) patient 2 with elevated mass 304 (arrow), hydroxy-C4-carnitine. Deuterated-C5-carnitine was used as quantitative internal standard (asterisk). (C) Mass spectrum from a normal control with very low levels of mass 304 detected.
Incidence Estimates show HIBCH Deficiency is Under-Diagnosed
Disease incidence was estimated based on Hardy Weinberg principles and using carrier frequencies in the ExAC cohort. To avoid ascertainment bias and since few HIBCH deficiency patients have been reported, any variant meeting criteria for being considered ‘damaging’ was considered pathogenic and the frequency of these ‘damaging’ alleles were determined in the ExAC cohort. At time of reporting the ExAC cohort was comprised of 122,868 chromosomes representing multiple worldwide populations: European, Finnish, South Asian, East Asian, African, Latino, and other. Incidence ranges from the lowest population incidence in Europeans at 1 in 551,545 to highest incidence of 1 in 127,939 South Asian individuals (Table 1).
Table 1.
Estimates of HIBCH deficiency incidence
| Population | Num. Chroms | Num. Carriers | Incidence (1 in N) |
|---|---|---|---|
| European | 67 710 | 87 | 551 545 |
| Finnish | 6 748 | 14 | 200 787 |
| African | 10 584 | 20 | 241 744 |
| American | 11 608 | 20 | 331 922 |
| East Asian | 8 766 | 22 | 148 019 |
| South Asian | 16 628 | 46 | 127 939 |
Discussion
We present a newly diagnosed family with HIBCH deficiency confirmed at the biochemical, enzymatic and molecular level. The children in this family are the oldest individuals reported with HIBCH deficiency which enables this report to extend the understanding of the natural history of this disorder. In addition, we demonstrate that HIBCH deficiency can be detected through NBS cards and present evidence that this disorder may be under-diagnosed and more prevalent than currently appreciated
The clinical presentation of HIBCH deficiency in our family is consistent with that of previously described patients. The disease is first observed as loss of milestones and neurodegeneration within the first year of life. Our subjects showed dystonia, spastic quadriplegia and absent reflexes in the lower extremities before the age of one. Another hallmark feature of HIBCH deficiency is early MRI abnormalities showing bilateral, symmetrical T2 hyperintensity in the basal ganglia, specifically the globus pallidus, with corresponding restricted diffusion in the acute/sub-acute phase. Our patients displayed this abnormality as well as asymmetric cerebral peduncle involvement and transient T2 hyperintensity in the caudate nuclei which have previously been reported in patients with HIBCH deficiency [5].
Over time the disease in our patients evolved in to a slowly progressive encephalopathy. In the second decade of life our subjects’ truncal hypotonia remains along with more pronounced hypertonia in extremities, and intermittent dystonia in the trunk, extremities and face. Patients are still able to interact and partially communicate with sounds and facial expression. No other organ system complications are apparent. The initial edema, bilateral T2 in the MRI transiently improved after a 2-4 year interval. However, in later stages of disease, cystic encephalomalacia and atrophy developed in the basal ganglia. To our knowledge, the progression of MRI findings in HIBCH deficiency over a period of several years has not been previously illustrated.
There is significant overlap in the clinical presentation of HIBCH deficiency and Leigh syndrome. The infantile-onset progressive neurodegeneration and bilateral, symmetrical abnormalities in the basal ganglia found in HIBCH deficiency are typical of Leigh syndrome, which is a genetically and clinically heterogeneous entity [4,20]. In addition to HIBCH, other inborn errors of metabolism, including pyruvate dehydrogenase complex (PDHC) deficiency [8], biotin-thiamine basal ganglia disease [21], pyruvate carboxylase [22,23] and SCEH deficiencies [10], mirror the radiological findings associated with Leigh syndrome. These clinical similarities caused by multiple independent genetic etiologies highlight the importance of comprehensive diagnostic testing, including acylcarnitine analysis, in the clinical work-up of patients presenting with Leigh’s disease.When biochemical investigations are negative or inconclusive, NGS gene panels that include HIBCH or WES may be reasonable approaches.
The enzymatic block in HIBCH deficiency causes an accumulation of 3-hydroxy-isobutyryl-CoA. As a direct result hydroxy-C4-carnitine has been reported to be elevated in almost all confirmed patients in whom this has been measured [3]. However, it is proposed that disease pathogenesis and neurotoxicity is not due to the build-up of 3-hydroxy-isobutyryl-CoA, but rather of highly reactive methacrylyl-CoA metabolites. 3-Hydroxy-isobutyryl-CoA fluxes upward through the reversible crotonase enzyme resulting in the accumulation of methacrylyl-CoA, a proximal metabolite in the valine catabolic pathway which is hypothesized to react with mitochondrial enzymes containing essential cysteine residues including PDHC and respiratory chain enzymes [1,3,10,24]. This could be a reason for the elevated lactate and abnormal electron transport chain activity seen in some of the previously reported patients [1,4]. A similar pathophysiology has been proposed for SCEH deficiency, another recently described defect in valine metabolism [10].
The estimated incidence of HIBCH deficiency in the general population ranges from as high as 1 in 127,939 in East Asians to 1 in 551,545 in Europeans. This incidence is comparable to or higher than other metabolic disorders that are currently screened for by either selective or NBS methods. For example, glutaric aciduria type 1 has a birth prevalence of ~1 in 100,000, propionic aciduria ~1 in 100,000 – 150,000 and beta-ketothiolase deficiency has an estimated birth prevalence that ranges from 1 in 232,000 – <1 in a million [25–27]. Our results suggest that HIBCH may not be as rare as previously thought supporting the need for greater awareness of and appropriate diagnostic work-up for HIBCH deficiency.
Many NBS programs using MS/MS technology to screen for multiple disorders of amino acid metabolism, including organic acidurias caused by defects in the valine catabolic pathway, and already measure hydroxy-C4-carnitine [28]. This metabolite could be increased due to the build-up of one of 2 isomers, 3-hydroxy-butyryl-carnitine or 3-hydroxy-isobutyryl-carnitine, the former of which is used for the diagnosis of medium-/short-chain 3-hydroxy-acyl-CoA dehydrogenase (M/SCHAD) deficiency, a rare disorder of fatty acid oxidation [29].
Retrospective analysis of state NBS cards showed elevated hydroxy-C4-carnitine in both siblings, indicating that this disorder could be screened for by NBS programs. The actual values of 0.78 μM and 0.43 μM, in blood spots in filter paper measured after >10 years of storage, likely would have been higher had they been measured in the neonatal period, as some degradation for acylcarnitines occurs over time, even for samples preserved at −20ºC [30]. In comparison with the controls, the values in our patients were >6 SD above the mean. C4OH levels in the NBS card have only been reported for one other patient with HIBCH deficiency whose levels were 1.2 uMol, just below the established cut-off of the NBS program. This result led the NBS program to lower their cut-off from 1.3 to 1.0 uMol, a level that is still higher than the levels used by most NBS laboratories (cite). Prospective studies will have to determine if all HIBCH patients can be detected by NBS programs, with adequate cutoffs established depending on each program’s population, [7,31]., and potentially employing ratios and / or second tier tests.
There is no treatment currently available for HIBCH deficiency and as such this disorder may not be a priority for NBS [32–34]. However, HIBCH deficiency could be added to the differential diagnosis for elevations of hydroxy-C4-carnitine as this analyte is already measured by NBS programs using MS/MS as the primary marker for M/SCHAD deficiency, a secondary NBS condition [33]. Current American College of Medical Genetics and Genomics diagnostic algorithms prompts confirmatory testing for M/SCHAD in newborns with elevated hydroxy-C4-carnitine [23,34]. Therefore we propose a simple modification to the existing algorithm that includes HIBCH deficiency in the differential diagnosis for elevated hydroxy-C4-carnitine (Fig. 4). Of importance, for NBS programs using underivatized methods, hydroxy-C4-carnitine is isobaric to malonyl-carnitine (C3DC) and subsequently indistinguishable from this metabolite. Therefore, when underivatized methodology is used, it is necessary that follow-up for samples that screen positive for C3DC include confirmatory testing for both elevated malonyl-carnitine and hydroxy-C4-carnitine [35].
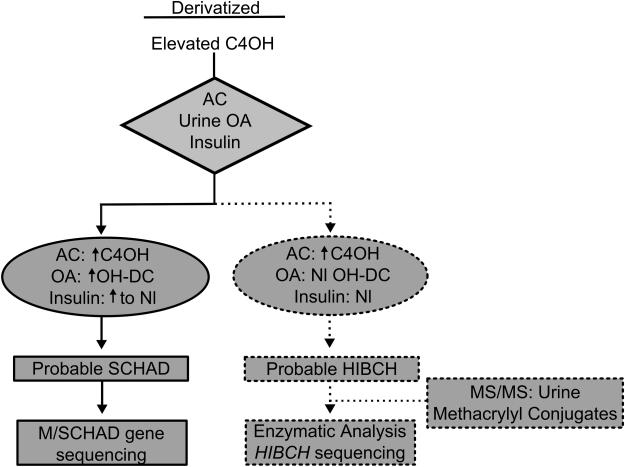
Figure 4. Proposed algorithm for laboratory follow-up of neonates with isolated elevations of hydroxy-C4-carnitine in NBS.
Elevated hydroxy-C4-carnitine prompts confirmatory testing to rule out M/SCHAD deficiency (solid bars) and could include HIBCH deficiency in the differential diagnosis (dashed bars). AC, acylcarnitines; OA, organic acids; Nl, normal; OH-DC, hydroxy-dicarboxylic; C4OH, hydroxy-C4-carnitine; HIBCH, 3-hydroxy-isobutyryl-CoA hydrolase; M/SCHAD, medium-/short-chain hydroxy-acyl-CoA hydratase deficiency.
In summary, we report the two oldest known living patients with HIBCH deficiency with extensive neuroradiological examinations highlighting the progression of this rare disorder of valine catabolism. Our report stresses the importance of a thorough metabolic evaluation, beyond mitochondrial diseases, in patients presenting with Leigh’s disease which includes acylcarnitine analysis by a laboratory that measures and reports hydroxy-C4-carnitine. As the elevation of this metabolite may be intermittent, a repeat testing may be indicated if the initial test results are normal. We show that hydroxy-C4-carnitine can be elevated on NBS cards of HIBCH deficient patients, suggesting that this disorder could be screened for by NBS programs. In light of these findings, we propose a modified algorithm that includes HIBCH deficiency in the differential diagnosis for newborns that screen positive with elevated hydroxy-C4-carnitine.
ACKNOWLEDGEMENTS
We thank the family for their steadfast commitment to participation in this study; the generous support of the Fry Family Foundation to CHOC Children’s Metabolic Program; and Jos Ruiter (Laboratory Genetic Metabolic Diseases at the Academic Medical Center, Emma Children’s Hospital, University of Amsterdam) for technical assistance in performing the enzymatic assay. The exome sequencing was performed at Baylor-Hopkins Center for Mendelian Genomics funded by the NIH National Human Genome Research Institute (U54HG006542). Research reported in this publication was supported by National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R01NS08372.
References
- 1.Ferdinandusse S, Waterham H, Heales SJR, Brown GK, Hargreaves IP, Taanman J-W, et al. HIBCH mutations can cause Leigh-like disease with combined deficiency of multiple mitochondrial respiratory chain enzymes and pyruvate dehydrogenase. Orphanet J Rare Dis. 2013;8:1–11. doi: 10.1186/1750-1172-8-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loupatty FJ, Clayton PT, Ruiter J, Ofman R, Ijlst L, Brown GK, et al. Mutations in the gene encoding 3-hydroxyisobutyryl-CoA hydrolase results in progressive infantile neurodegeneration. Am J Hum Genet. 2007;80:195–9. doi: 10.1086/510725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown GK, Hunt SM, Scholem R, Fowler K, Grimes A, Mercer JFB, et al. Beta-Hydroxyisobutyryl Coenzyme A Deacylase Deficiency: A Defect in Valine Metabolism Associated with Physical Malformations. Pediatrics. 1982;70:532–8. [PubMed] [Google Scholar]
- 4.Reuter MS, Sass JO, Leis T, Köhler J, Mayr JA, Feichtinger RG, et al. HIBCH deficiency in a patient with phenotypic characteristics of mitochondrial disorders. Am J Med Genet A. 2014;164:3162–9. doi: 10.1002/ajmg.a.36766. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Naiki M, Hoshino S, Kitaura Y, Kondo Y, Nomura N, et al. Clinical and biochemical characterization of 3-hydroxyisobutyryl-CoA hydrolase (HIBCH) deficiency that causes Leigh-like disease and ketoacidosis. Mol Genet Metab Rep. 2014;1:455–60. doi: 10.1016/j.ymgmr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soler-Alfonso C, Enns GM, Koenig MK, Saavedra H, Bonfante-Mejia E, Northrup H. Identification of HIBCH Gene Mutations Causing Autosomal Recessive Leigh Syndrome: A Gene Involved in Valine Metabolism. Pediatr Neurol. 2014 doi: 10.1016/j.pediatrneurol.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Peters H, Wanders R, Ruiter J, Ferdinandusse S, Boneh A, Pitt JJ. Novel biochemical findings in 3-hydroxyisobutyryl-CoA hydrolase deficiency: implications for screening. Journal Inherit Metab Dis. 2013;36:S104. [Google Scholar]
- 8.Ruhoy IS, Saneto RP. The genetics of Leigh syndrome and its implications for clinical practice and risk management. Appl Clin Genet. 2014;7:221–34. doi: 10.2147/TACG.S46176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitt J, Eggington M, Kahler SG. Comprehensive screening of urine samples for IEM by ESI MSMS. Clin Chem. 2002;48:1970–80. [PubMed] [Google Scholar]
- 10.Peters H, Buck N, Wanders R, Ruiter J, Waterham H, Koster J, et al. ECHS1 mutations in Leigh disease: a new inborn error of metabolism affecting valine metabolism. Brain. 2014;137:2903–8. doi: 10.1093/brain/awu216. [DOI] [PubMed] [Google Scholar]
- 11.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–8. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnen PE, Yarham JW, Besse A, Wu P, Faqeih EA, Al-Asmari AM, et al. Mutations in FBXL4 cause mitochondrial encephalopathy and a disorder of mitochondrial DNA maintenance. Am J Hum Genet. 2013;93:471–81. doi: 10.1016/j.ajhg.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–5. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–74. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper GM, Stone EA, Asimenos G, NISC Comparative Sequencing Program. Green ED, Batzoglou S, et al. Distribution and intensity of constraint in mammalian genomic sequence. Genome Res. 2005;15:901–13. doi: 10.1101/gr.3577405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S. Identifying a high fraction of the human genome to be under selective constraint using GERP++ PLoS Comput Biol. 2010;6:e1001025. doi: 10.1371/journal.pcbi.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siepel A, Pollard KS, Haussler D. New methods for detecting lineage-specific selection. BMC Bioinformatics. 2006:190–205. [Google Scholar]
- 20.Krishna SH, McKinney AM, Lucato LT. Congenital genetic inborn errors of metabolism presenting as an adult or persisting into adulthood: neuroimaging in the more common or recognizable disorders. Semin Ultrasound CT MR. 2014;35:160–91. doi: 10.1053/j.sult.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Sremba LJ, Chang RC, Elbalalesy NM, Cambray-Forker EJ, Abdenur JE. Whole exome sequencing reveals compound heterozygous mutations in SLC19A3 causing biotin-thiamine responsive basal ganglia disease. Mol Genet Metab Rep. 2014;1:368–72. doi: 10.1016/j.ymgmr.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pronicka E, Kulczycka H, Chmielik J, Krajewska G, Szeffer J. Suspected pyruvate carboxylase deficiency in 4 children with Leigh disease. Neurol Neurochir Pol. 1986;20:89–94. [PubMed] [Google Scholar]
- 23.Ito H, Mori K, Ito M, Naito E, Yokota I, Kuroda Y. Case of methylmalonic acidemia presenting clinically Leigh encephalopathy. No To Hattatsu. 2004;36:324–9. [PubMed] [Google Scholar]
- 24.Wanders RJA, Duran M, Loupatty FJ. Enzymology of the branched-chain amino acid oxidation disorders: the valine pathway. Journal Inherit Metab Dis. 2012;35:5–12. doi: 10.1007/s10545-010-9236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumgartner HF, Dionisi-Vici C, Haliloglu G, Karall D, Chapman KA, Huemer M, et al. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J Rare Dis. 2014;9:1–36. doi: 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarafoglou K, Matern D, Redlinger-Grosse K, Bentler K, Gaviglio A, Harding CO, et al. Siblings with mitochondrial acetoacetyl-CoA thiolase deficiency not identified by newborn screening. Pediatrics. 2011;128:e246–50. doi: 10.1542/peds.2010-3918. [DOI] [PubMed] [Google Scholar]
- 27.Zaki OK, Elabd HS, Ragheb SG, Ghoraba DA, Elghawaby AE. Demographic and clinical features of glutaric acidemia type 1; a high frequency among isolates in Upper Egypt. Egyptian J Med Hum Gen. 2014;15:187–92. [Google Scholar]
- 28.Chace DH, Kalas TA. A biochemical perspective on the use of tandem mass spectrometry for newborn screening and clinical testing. Clin Biochem. 2005;38:296–309. doi: 10.1016/j.clinbiochem.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Chace DH, Kalas TA, Naylor EW. Use of Tandem Mass Spectrometry for Multianalyte Screening of Dried Blood Specimens from Newborns. Clinical Chemistry. 2003;49:1797–817. doi: 10.1373/clinchem.2003.022178. [DOI] [PubMed] [Google Scholar]
- 30.Strnadová KA, Holub M, Mühl A, Heinze G, Ratschmann R, Mascher H, et al. Long-term stability of amino acids and acylcarnitines in dried blood spots. Clin Chem. 2007;53:717–22. doi: 10.1373/clinchem.2006.076679. [DOI] [PubMed] [Google Scholar]
- 31.McHugh DMS, Cameron CA, Abdenur JE, Abdulrahman M, Adair O, Al Nuaimi SA, et al. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: a worldwide collaborative project. Genet Med. 2011;13:230–54. doi: 10.1097/GIM.0b013e31820d5e67. [DOI] [PubMed] [Google Scholar]
- 32.Wilson JM, Jungner G. Principles and practice of screening for disease: Public health papers. 34th World Health Organization; Geneva: 1968. pp. 1–163. [Google Scholar]
- 33.Watson MS, Mann MY, Lloyd-Puryear MA, Rinaldo P, Howell RR. Newborn screening: toward a uniform screening panel and system. Genet Med. 2006;8(Suppl 1):s1–s11. doi: 10.1097/01.gim.0000223891.82390.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindner M, Hoffmann GF, Matern D. Newborn screening for disorders of fatty-acid oxidation: experience and recommendations from an expert meeting. Journal Inherit Metab Dis. 2010;33:521–6. doi: 10.1007/s10545-010-9076-8. [DOI] [PubMed] [Google Scholar]
- 35.Popa FI, Perlini S, Teofoli F, Degani D, Funghini S, La Marca G, et al. 3-hydroxyacyl-coenzyme a dehydrogenase deficiency: identification of a new mutation causing hyperinsulinemic hypoketotic hypoglycemia, altered organic acids and acylcarnitines concentrations. JIMD Rep. 2011;2:71–7. doi: 10.1007/8904_2011_50. [DOI] [PMC free article] [PubMed] [Google Scholar]