Abstract
Schizophrenia is a devastating psychiatric illness with high heritability. Brain structure and function differ, on average, between schizophrenia cases and healthy individuals. As common genetic associations are emerging for both schizophrenia and brain imaging phenotypes, we can now use genome-wide data to investigate genetic overlap. Here we integrated results from common variant studies of schizophrenia (33,636 cases, 43,008 controls) and volumes of several (mainly subcortical) brain structures (11,840 subjects). We did not find evidence of genetic overlap between schizophrenia risk and subcortical volume measures either at the level of common variant genetic architecture or for single genetic markers. The current study provides proof-of-concept (albeit based on a limited set of structural brain measures), and defines a roadmap for future studies investigating the genetic covariance between structural/functional brain phenotypes and risk for psychiatric disorders.
Keywords: schizophrenia, MRI, brain imaging, genetics, GWAS, meta-analysis, endophenotype
Introduction
Schizophrenia is a devastating, highly heritable psychiatric disorder that affects approximately 1% of the population. 1 Despite marked recent successes in identifying genetic risk factors and pathways involved in schizophrenia, 1-4 the neurobiology of schizophrenia remains poorly understood.
Many differences in brain function and structure have been reported in cases with schizophrenia compared with controls, although there is considerable inter-individual heterogeneity. Of specific relevance to this study, a recent meta-analysis found that schizophrenia cases had smaller hippocampus, amygdala, thalamus, nucleus accumbens, and intracranial volumes along with larger pallidum and lateral ventricle volumes.5,6 Hippocampal and lateral ventricle volumes were influenced by antipsychotic medication use.5 In addition, mean hippocampal volume is smaller in high-risk individuals and in unaffected first-degree relatives of schizophrenia cases. 7,8
Structural brain measurements, such as those from magnetic resonance imaging (MRI), typically have high reproducibility and low measurement error and can be highly heritable. 9,10 Increasingly large studies of brain morphometry are being performed, and are being used to evaluate the effects of common and rare genetic contributions on brain structure. 9,11
With genome-wide association results available from large samples for schizophrenia and for MRI-based brain phenotypes, we can now use genomic approaches to evaluate the genetic link between disease risk and such brain measures. Findings of covariation would help us develop new hypotheses about the structures involved in the primary disease process of schizophrenia. In this proof-of-concept study, we created a roadmap for the analysis of genetic covariation using a battery of complementary methods. We evaluated the overlap of common genetic variation at the high level of genetic architecture as well as of individual genetic variants. We also evaluated common genetic variant effect sizes on neuroimaging phenotypes and schizophrenia. The data we analyzed are from large mega-analyses by the PGC (Psychiatric Genomics Consortium) for schizophrenia 3 and meta-analyses from the ENIGMA consortium (Enhancing NeuroImaging Genetics through Meta-Analysis) for eight MRI volumetric measures (amygdala, caudate nucleus, hippocampus, nucleus accumbens, pallidum, putamen, thalamus, and intracranial volume (ICV)). 9 Our results suggest that common genetic variation predisposing to schizophrenia does not show evidence of overlap with common genetic variation influencing these eight brain structure volumes. Genetic effect sizes did not differ significantly for neuroimaging and schizophrenia phenotypes.
Results
We analyzed genome-wide association data for schizophrenia (33,636 cases and 43,008 controls) and eight structural MRI brain measures (11,840 individuals). Sample characteristics are presented in Supplementary Table 1. These data were used for a comprehensive set of comparisons of common variant genetic sharing between schizophrenia and brain volumetric measures.
Comparisons of common variant genetic architectures
Linkage disequilibrium score regression (LDSR)
Using GWA summary statistics (excluding the extended MHC region), we used LDSR 12 to estimate the heritability of schizophrenia due to common SNPs at 25.5% (SE=1.1%) along with eight brain volumetric measures (Table 1). The SNP-based heritability estimates for the MRI measures ranged from 11% (nucleus accumbens) to 30% (putamen). The heritability for amygdala volume was non-significant in this sample. The genetic correlations of MRI volumetric measures with schizophrenia were all non-significant (Table 1). These negative findings stand in contrast to the relatively high common-variant correlations of schizophrenia with bipolar disorder and major depressive disorder. 13,14
Table 1.
SNP-heritability analyses for MRI brain volume and genetic correlations with schizophrenia*.
| Brain region* | N | Heritability | SE | Genetic correlation with SCZ | SE | Z | P |
|---|---|---|---|---|---|---|---|
| Intracranial volume | 9,826 | 0.157 | 0.050 | −0.010 | 0.072 | −0.137 | 0.891 |
| Caudate nucleus | 11,624 | 0.260 | 0.043 | −0.095 | 0.057 | −1.674 | 0.094 |
| Hippocampus | 11,621 | 0.135 | 0.041 | −0.147 | 0.081 | −1.826 | 0.068 |
| Nucleus accumbens | 11,603 | 0.105 | 0.045 | −0.094 | 0.090 | −1.051 | 0.293 |
| Pallidum | 11,595 | 0.137 | 0.047 | −0.038 | 0.069 | −0.546 | 0.585 |
| Putamen | 11,598 | 0.303 | 0.052 | 0.013 | 0.052 | 0.256 | 0.798 |
| Thalamus | 11,646 | 0.118 | 0.041 | −0.113 | 0.087 | −1.298 | 0.194 |
amygdala heritability was too low to allow a valid analysis
Genetic predisposition scores
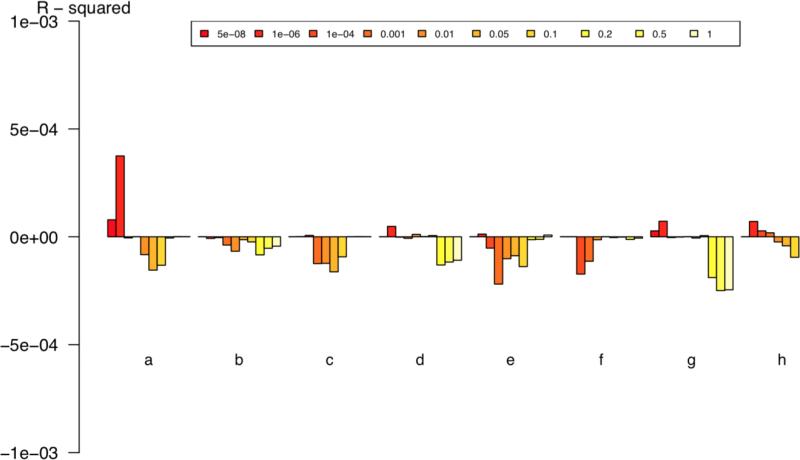
In the genetic “risk” score approach, 15 we considered the ENIGMA GWA results as “training” sets in order to compute common variant genetic predisposition to (for instance) greater ICV for each schizophrenia case and control. We then compared the mean polygenic predisposition score in cases to that in controls. None of the correlations was significant after correction for eight comparisons (Figure 1 and Table 2). The strongest effect (for hippocampal volume) was almost entirely driven by one SNP (rs2268894), 9 but only three SNPs met the p-value threshold of 1×10−6 for inclusion in this analysis. These null results are in contrast to the robust evidence for common variant genetic correlations between schizophrenia and other psychiatric disorders. 16
Figure 1.
Genetic predisposition score analyses examining the predictive capacity of ENIGMA brain volumetric results on schizophrenia case-control status using different P-value thresholds. X-axis: (a) hippocampus, (b) ICV, (c) nucleus accumbens, (d) amygdala, (e) caudate nucleus, (f) pallidum, (g) putamen, (h) thalamus. Y-axis shows Nagelkerke's R2. Positive values indicate SNP effects for increasing brain structure volume and increased risk for schizophrenia. Negative values indicate SNP effects for decreasing brain structure volume in and increased risk for schizophrenia. Significance values are given in Table 2.
Table 2.
Two outcome variables derived from genetic predisposition analysis.
| Phenotype | P- | R2 | AUC | OR (95% CI) |
|---|---|---|---|---|
| Intracranial volume | 0.247 | −2.46×10−5 | 0.512 | 0.944 ( 0.877,1.016) |
| Caudate nucleus | 0.033 | −8.35×10−5 | 0.502 | 0.928 (0.864,0.997) |
| Hippocampus | 0.010 | −1.23×10−4 | 0.506 | 0.917 (0.853,0.986) |
| Nucleus accumbens | 0.002 | −1.74 ×10−4 | 0.500 | 0.928 (0.862,0.9996) |
| Pallidum | 0.985 | 6.21 ×10−9 | 0.513 | 1.034 (0.963,1.111) |
| Putamen | 0.607 | −4.87×10−6 | 0.515 | 0.971 (0.891,1.059) |
| Thalamus | 0.221 | −2.75×10−5 | 0.510 | 0.959 (0.888,1.036) |
| Amygdala | 0.806 | 1.11×10−6 | 0.509 | 1.021 (0.951,1.096) |
P=significance uncorrected for multiple testing. R2=correlation (Nagelkerke) on the observed scale corrected for principal components. AUC=area under receiver operating characteristic curve. OR=odds ratio. CI=confidence interval
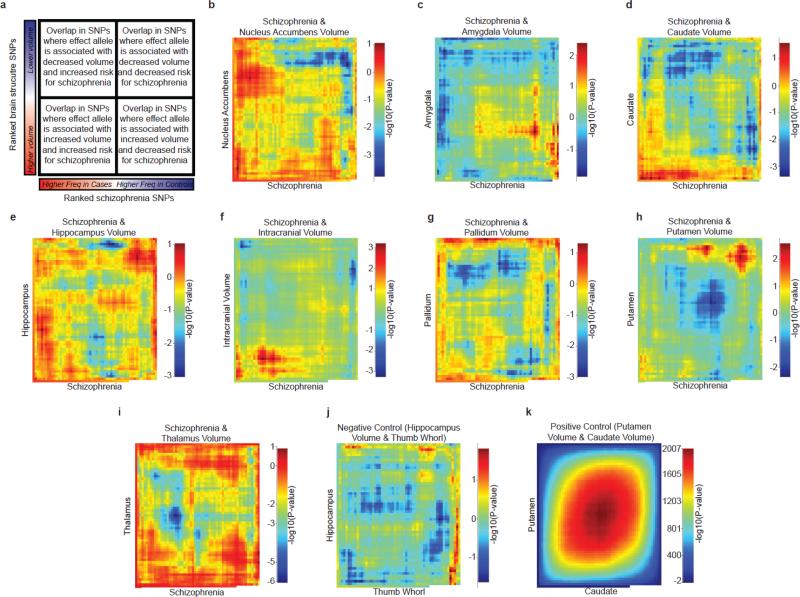
Rank-rank hypergeometric overlap test (RRHO) 17
We quantified overlap between pairs of GWA results ranked by their association statistics using RRHO based on 172,652 SNPs. The overlap of rank-ordered lists of genetic variants influencing any of the brain MRI volumes and those conferring risk for schizophrenia was not statistically significant (Figure 2). The overlap between genetic contributions to putamen and caudate nucleus volumes was used as a positive control; the overlap between genetic contributions to hippocampal volume and the presumably unrelated trait of thumb whorl structure 18 was used as a negative control. The latter comparison showed similar overlap to that of brain structure and schizophrenia.
Figure 2.
Evaluating the genome-wide overlap between genetic influences on schizophrenia and subcortical volumes. (a) A cartoon describing the output map. (b-i) independent SNPs present in both ENIGMA and PGC schizophrenia results were selected independent of association to any phenotype (see on-line methods). Association results were ordered based on the significance of their association to the phenotype (–log10(P-value) multiplied by the sign of the effect), and statistical significance was evaluated using RRHO test. The same test for overlap was conducted with a (j) finger whorl phenotype, expected to have no overlap with brain structure genetics, and (k) the overlap between caudate and putamen volume, expected to have very strong overlap. Overlap in the rank-ordered lists between genetic variants influencing any of the eight brain phenotypes and those creating risk for schizophrenia was not statistically significant. The overlap between hippocampal volume and presence of a whorl on the left thumb was used as a negative control and showed similar levels of overlap to brain structure and schizophrenia.
Sign tests
We compared the pattern of GWA results by checking whether the signs of the regression coefficients 3 were consistently in the same direction between the top associations for schizophrenia and those for the MRI volumetric measures. None of the sign tests showed consistent directions of effect (Table 3).
Table 3.
Sign tests of directional effects among 94 genome-wide significant associations with schizophrenia (P<5×10−8) and the top 231 associations (P<1×10−6).
| Brain region | P threshold | N same direction | Proportion | P |
|---|---|---|---|---|
| Intracranial volume | <5×10−8 | 49 | 0.52 | 0.379 |
| Caudate nucleus | <5×10−8 | 47 | 0.50 | 0.541 |
| Hippocampus | <5×10−8 | 46 | 0.49 | 0.621 |
| Nucleus accumbens | <5×10−8 | 48 | 0.51 | 0.459 |
| Pallidum | <5×10−8 | 51 | 0.54 | 0.235 |
| Putamen | <5×10−8 | 52 | 0.55 | 0.177 |
| Thalamus | <5×10−8 | 49 | 0.52 | 0.379 |
| Amygdala | <5×10−8 | 49 | 0.52 | 0.379 |
| Intracranial volume | <1×10−6 | 121 | 0.52 | 0.255 |
| Caudate nucleus | <1×10−6 | 113 | 0.49 | 0.653 |
| Hippocampus | <1×10−6 | 105 | 0.45 | 0.926 |
| Nucleus accumbens | <1×10−6 | 109 | 0.47 | 0.821 |
| Pallidum | <1×10−6 | 117 | 0.51 | 0.448 |
| Putamen | <1×10−6 | 115 | 0.50 | 0.552 |
| Thalamus | <1×10−6 | 115 | 0.50 | 0.552 |
| Amygdala | <1×10−6 | 109 | 0.47 | 0.821 |
The expected proportion under the null is 0.5.
Analysis of single genetic variants
Genome-wide significant associations
We evaluated the 128 genome-wide significant schizophrenia index SNPs 3 for association with brain volumes. 9 One association survived correction for 876 comparisons: rs2909457*A (chr2:162,845,855, intergenic between SLC4A10 and DPP4) was associated with decreased hippocampal volume (P=1.2×10−6, effect size=−23 mm3 per allele) and decreased risk for schizophrenia (odds ratio=0.94, P=4.6×10−8). However, this finding was in the opposite direction of expectations given previous observations of smaller hippocampal volumes in cases relative to controls (Supplementary Table 2). 6 Starting with the eight SNPs previously found associated with the brain volumes, 9 no significant associations with schizophrenia were observed (Supplementary Table 2).
SNP meta-analyses
We also performed GWA meta-analyses of the schizophrenia and brain structure results. The Manhattan plots for these analyses are shown in Supplementary Figures 1-8. In Supplementary Table 3, the genome-wide significant findings are given. In most instances, the results were entirely driven by the association with schizophrenia.
Conjunction analysis
To identify individual SNPs that influence risk for both schizophrenia and brain structure, we implemented a conjunction test. 19 No SNP showed genome-wide significant association with both schizophrenia and brain structure, although several loci were detected at sub-threshold levels (Supplementary Figure 9).
Comparison of genetic effect sizes for clinical and brain volume measures
Some investigators have suggested that common genetic variants underlying continuous brain imaging endophenotypes may have larger effect sizes than those for neuropsychiatric disorders (e.g., schizophrenia). 20-22 To test this hypothesis, we compared the maximum effect sizes from replicated genetic associations for each trait. For comparability across quantitative or binary traits, effect sizes were assessed as percent of variance explained (for MRI volumes) or percent of variance explained on the liability scale (for schizophrenia). 23 As shown in Supplementary Figure 10, individual common variants had only a small influence on either brain structure or schizophrenia. Effect sizes for individual SNPs were similar for both brain structure and schizophrenia, and of the same order as those observed for anthropometric traits such as height. 24
Discussion
In this proof-of-concept study, we evaluated the relationship between common genetic variants implicated in schizophrenia and those associated with subcortical brain volumes and ICV. The sample sizes were the largest yet applied to these questions. With a comprehensive set of analyses, we did not find evidence for notable genetic correlations, either at a high level (i.e., common variant genetic architecture) or for single genetic markers. Our findings do not support the hypothesis that these subcortical brain volume measures and ICV are causally associated with schizophrenia risk. Similarly, we did not find evidence that common SNPs have pleiotropic effects on these MRI volumes and schizophrenia. Our results suggest alternative hypotheses that require consideration and refutation – that the volumetric differences observed in schizophrenia cases may be epiphenomena unrelated to its primary genetic causes, a result of prenatal environment, or result from reverse causation. 25 Finally, the effect sizes of SNPs implicated in schizophrenia and those associated with brain volumes were broadly similar.
We studied a limited set of brain MRI measures. Our study should be considered a proof-of-concept for evaluating genetic covariation rather than decisively addressing the full range of hypotheses pertaining to the genetic overlap of brain imaging measures with neuropsychiatric disease risk. We provide a rigorous roadmap for more definitive and larger future studies. Full elucidation of the brain correlates of schizophrenia will require a fuller set of structural and functional imaging measures (perhaps at the voxel level) along with evaluation of common and rare genetic variation.
The null findings of this study should be interpreted in light of several qualifiers. First, several brain regions that are not expected a priori to overlap with schizophrenia were included for completeness (e.g., caudate and putamen volumes are uncorrelated with schizophrenia, 5,6 and amygdala volume did not have SNP-heritability different from zero in our study). Second, other neuroimaging phenotypes could be more informative for schizophrenia (e.g., cortical thickness, ventricular volume, diffusion tensor imaging, or functional activity). 26,27 Indeed, genetic variants associated with disease may influence distinct cell types within circumscribed neural circuits that may not be captured by MRI. Third, the ENIGMA MRI protocol served to harmonize images obtained from different scanners and protocols. While we have shown this performs well, genetic signal might have been lessened. Fourth, in this study of adults, we may not have observed the brain regions at the most appropriate time for identifying genetic overlap with schizophrenia, given that the volumes of most subcortical brain structures plateau in late adolescence to early adulthood. While schizophrenia is widely believed to be a neurodevelopmental disorder, 28 its onset generally follows the period of greatest growth for these structures. Fifth, relatively small genetic correlations between schizophrenia and these brain volumes may have been masked by combining datasets in a meta-analytic framework (e.g., heterogeneous sample characteristics such as age, sex, and technical noise resulting from different MRI scanners or acquisition sequences may remain). It is conceivable that this resulted in the lower than expected SNP-heritability for some of these measures. Mega-analysis could be an important way to improve control for heterogeneity. Sixth, we evaluated only common genetic variation. Although common genetic variation explains far more of the risk for schizophrenia than rare copy number variation or rare deleterious exonic variation, 2 rare genetic effects on brain structure could be salient for some cases of schizophrenia. Finally, the sample sizes and statistical power of the schizophrenia and neuroimaging data sets differed. The PGC has attained a sample size sufficient to detect many common loci of small effect, whereas ENIGMA is earlier in the discovery arc. 29
Brain volume heritability estimates from genome-wide data obtained using LDSR 14 were lower than observed in previous studies. 30 This was expected for the subcortical regions, as those were corrected for ICV. For ICV, a likely source of difference with previous studies is the removal of the extended MHC region from our analysis.
Although we found no evidence for genetic correlation between subcortical volumes and schizophrenia, we also investigated whether effect sizes of genetic variants are larger for brain measures than for schizophrenia. This point has been debated with respect to “endophenotype” studies, which attempt to identify quantifiable brain measures or other biomarkers thought to be intermediate between genotype and the liability to a disorder. 31-33 An endophenotype that lies on a causal pathway to a clinical disorder could increase power for genetic studies. Prior studies addressed this hypothesis in far smaller samples. We compared SNP effect sizes for the top findings for schizophrenia with those for subcortical volumes (hippocampus, putamen, caudate) and ICV. The results of this analysis showed similar effect sizes. Importantly, the endophenotype concept is unlikely to be sufficiently addressed in these analyses given the reasons noted above.
In conclusion, this paper presents a roadmap for comprehensive evaluation of genetic covariation between neuropsychiatric disease liability and brain imaging measures. The current analysis was limited to a small number of brain volume phenotypes, and no evidence of genetic overlap was identified. More extensive brain-wide and genome-wide analyses may help in the mechanistic dissection of genetic risk for disease.
Online Methods
A supplementary methods checklist is available. The data used for the analyses described here are available to researchers. The ENIGMA data can be obtained from http://enigma.ini.usc.edu/enigma-vis. The PGC data can be downloaded from http://www.med.unc.edu/pgc/downloads.
PGC schizophrenia
We mega-analyzed individual genotype data from 46 European-ancestry schizophrenia GWAS datasets (full details in reference 3). Briefly, quality control and imputation were performed by the PGC Statistical Analysis Group for each dataset separately. Genotype imputation was with the pre-phasing/imputation stepwise approach implemented in IMPUTE2/SHAPEIT (chunk size of 3 Mb and default parameters) using the 1000 Genomes Project dataset (phase 1, August 2012, URLs). After imputation, we identified autosomal SNPs with high imputation accuracy across all samples. For robust relatedness testing and population structure analysis, we evaluated a subset of SNPs following LD-pruning (r2 > 0.02) and frequency filtering (MAF > 0.05). For association testing, we evaluated the 46 datasets separately using an additive logistic regression model including ancestry principal components as covariates, and then conducted a meta-analysis of the 52 sets of results using an inverse-weighted fixed effects model. After excluding subjects who were also in ENIGMA (N=458, see below), 33,636 cases and 43,008 controls were used for calculations (Supplementary Table 1).
ENIGMA, sample with brain volume measures and assessment of endophenotype
The data analyzed here are from the ENIGMA analysis of eight MRI volumetric measures (full details in reference 9). MRI brain scans and genome-wide genotype data were available for 11,840 subjects from 22 cohorts (Supplementary Table 1). Only cohorts without schizophrenia cases and controls overlapping with the PGC schizophrenia samples were included. Participants clustered with subjects of known European ancestry as verified by multidimensional scaling (MDS) analysis. Genomic data were imputed to a reference panel (1000 Genomes, v3 phase1) comprising only European samples and with monomorphic SNPs removed. Imputation was performed at each site using MaCH for phasing and minimac for imputation. 34 Only SNPs with an imputation score of RSQ > 0.5 and minor allele counts > 10 within each site were included. Tests of association were conducted separately for eight MRI volumetric phenotypes (nucleus accumbens, amygdala, caudate nucleus, hippocampus, pallidum, putamen, thalamus, and ICV) with the following covariates in a multiple linear regression framework: age, age2, sex, 4 MDS components (to account for population structure), ICV (for subcortical brain phenotypes), and diagnosis (when applicable). The GWA statistics from each of the 22 sites were combined using a fixed-effect inverse variance-weighted meta-analysis as implemented in METAL. 35
Removal of duplicated individuals
Subject overlap between all PGC and ENIGMA cohorts was evaluated using a checksum algorithm in order to ensure the robustness of our results given that some analyses were sensitive to the presence of duplicate individuals. For each individual, ten checksum numbers were created based on ten batches of 50 SNP genotypes and compared between individuals from both consortia. Based on these comparisons and a general exclusion of cohorts containing schizophrenia cases, 1,517 individuals were removed from ENIGMA and 458 subjects were removed from the PGC.
Linkage disequilibrium score regression (LDSR)
For LDSR, each dataset underwent additional filtering. Only markers overlapping with HapMap Project Phase 3 SNPs and passing the following filters were included: INFO score > 0.9 (where available), study missingness of 0, and MAF >1%. Indels and strand-ambiguous SNPs were removed. To remove a potential source of bias, all SNPs in the extended MHC region (chr6:25-35 Mb) were removed from all datasets. The schizophrenia analysis included only results from European studies were used (LDSR requires LD data from a comparable sample). For the ENIGMA amygdala results, the mean Χ2 was too low (1.0051) to reliably estimate heritability using LDSR.
The analysis was conducted using a two-step procedure with the LD-scoring analysis package. 12,14 An unconstrained regression was run to estimate the regression intercepts for each phenotype, followed by an analysis with regression intercepts constrained to those estimated in the first step and the covariance intercept defined as zero (note that we took steps to exclude overlapping samples). Standard errors were estimated using a block jackknife procedure and used to calculate P-values.
Genetic predisposition analyses
To investigate the combined impact of ENIGMA association results on case-control status in the PGC schizophrenia data, we performed a series or genetic predisposition score analyses. For each ENIGMA volumetric phenotypes, we excluded SNPs with MAF <2%, indels, and SNPs in the extended MHC region (chr6:25-34 Mb). We then “clumped” the data, discarding variants within 500 kb of and in r2 ≥ 0.1 with another more significant marker. We performed genetic predisposition score prediction of target subgroups as originally described 15 for several P-value thresholds (5×10−8, 1×10−6, 1×10−4, 0.001, 0.01, 0.05, 0.1, 0.2, 0.5, 1.0), multiplying the effect size of the ENIGMA phenotype of each variant by the imputation probability for the risk allele in each individual. The resulting values were summed so that each individual had a genetic predisposition score for further analyses. Two outcome variables are reported in Table 2: the significance of the case-control score difference analyzed by logistic regression (including ancestry-based principal components and a study indicator as covariates) and the proportion of variance explained (Nagelkerke's R2) computed by comparison of a full model (covariates + polygenic risk scores) score to a reduced model (covariates only). Note that these R2 estimates are biased due to recruitment of the case-control studies and as the numbers of cases and controls do not reflect the underlying risk of disease in the population.
Rank-rank-hypergeometric overlap test (RRHO)
RRHO 17 tests the hypothesis that ordering of two lists (LD-pruned GWAS results for schizophrenia versus a brain structure phenotype) by the strength of their association is arbitrary. The number of independent SNPs in common between the two ordered lists is evaluated at specified step sizes. Two lists that show similar ordering of SNPs demonstrate a global pattern of similarity of associations. Independent SNPs were selected based on the 1000 Genomes European dataset for 200 SNP windows shifted at five SNP intervals using an r2 threshold of 0.25. SNPs found in both PGC and ENIGMA data with MAF ≥ 0.01 were retained (172,652 SNPs). The SNPs were then ordered by the –log10(p-value) of association multiplied by the effect size. A two-sided RRHO test that allowed testing for either over- or under-enrichment was used with a step-size of 3000 SNPs.
Finger whorl data used as control in conjunction analysis
A GWAS of a dermatoglyphic trait (presence of a whorl on the left thumb), collected as part of an ongoing study at the Queensland Institute of Medical Research, 18 was used to provide a negative control for the RRHO test. Briefly, rolled ink prints were collected on archival quality paper, and fingerprint patterns were manually coded. Complete data from 3,314 participants (twins and their family members) were available. Genotypes were imputed to the 1000 Genomes Project reference (phase 1 version 3). GWAS was conducted using Merlin-offline to account for relatedness and zygosity.
Lookup of top GWAS SNP findings
Evidence for an effect of the reported 128 independent schizophrenia-associated SNPs on subcortical brain volumes and ICV was studied through a look-up of results. rs115329265 was not available in the ENIGMA data and was replaced by a SNP in moderate LD (chr6:28305863R; r2=0.64); rs77149735 was not available in ENIGMA and could not be replaced by a SNP in LD. Three chrX SNPs (rs1378559, rs5937157, and rs12845396) were excluded, because chrX data were not available from ENIGMA. Effects of the eight independent SNPs associated with brain volumes reported by ENIGMA on schizophrenia risk were studied through a look-up of results in the PGC data.
Multiple comparison correction was performed by estimating the effective number of independent tests (Meff). This method considers the correlation structure (Supplementary Table 4) between brain measures and calculates the Meff based on the observed eigenvalue variance of the different brain volume measures using matSpD (see URLs). The p-value for significance was 0.05 divided by the sum of (a) Meff times the number of SNPs included in the lookup from PGC to ENIGMA (n=124), and (b) the number of SNPs included in the lookup from ENIGMA to PGC (n=8). Eight brain volumes resulted in seven independent tests, and only SNPs with a P < 5.7×10−5 were considered significant.
SNP sign test in the top GWAS findings
To investigate a potential accumulation of same or opposite direction effects of SNPs between PGC schizophrenia and ENIGMA, we counted the number of same direction effects for the top-findings from the schizophrenia dataset (94 LD-independent genome-wide significant SNPs, 231 with P < 1×10−6) in the different brain structure datasets and tested the significance of the result in a binomial test (n=14 tests for 7 effective ENIGMA phenotypes and 2 P-value thresholds).
Conjunction analysis
To determine whether a particular SNP is linked to both brain structure and risk for schizophrenia, a conjunction analysis was used. 19 This analysis makes inference on the alternative hypothesis that both null hypotheses are false. This is in distinction to a traditional meta-analysis method which infers on an alternative hypothesis that one or more null hypotheses are false. A conjunction analysis is calculated as: Pconj = max(Pbrain, Pcase-control), where Pbrain is the significance of the SNP associated to brain structure and Pcase-control is the significance of the SNP association to schizophrenia. As conjunction tests can be very conservative, an adjustment to this test 36 based on the estimated fraction of false nulls was used here with modifications (P'conj). Over 7.5 million SNPs found in both the ENIGMA and PGC datasets with MAF ≥ 0.01 were evaluated.
A conjunction null hypothesis is the union of the individual null hypotheses, producing a ‘composite null hypothesis’. In standard testing situations a “point null hypothesis” is used, meaning that there is exactly one configuration of the unknown parameters of interest that corresponds to the null. For example, “no gene-brain association, no case-control association” is a point null hypothesis. A composite null has multiple configurations. For example, both of these configurations fall into the conjunction null hypothesis: “true gene-brain association, no case-control association”; “no gene-brain association; true case-control association”. A valid conjunction test has to control false positive risk over all possible configurations in the conjunction null. Put another way, a conjunction test has to be calibrated for the worst possible configuration of true signals, and as a result can be quite conservative when the true state of the model is not one of the extreme cases.
The method of Deng et al. 36 attempts to reduce the conservativeness of the conjunction procedure in the multiple testing setting. The authors propose a method that estimates prevalence of null hypotheses in each of the individual tests being combined. With this information, a “relaxed” test can be constructed that is less conservative. However, a crucial equation in that paper is in error. The equation below provides the estimator for the proportion of false null hypothesis for each of the two tests to be combined. The expression is based on the method of Storey 37, who posed it as an estimate of the proportion of true null hypotheses. Deng et al. 36 apparently inverted the result incorrectly; the correct expression is:
In our analyses, the λ parameter in the equation above was set to 0.25.
SNP meta-analysis
We combined the association P-values of SNPs associated with schizophrenia with SNPs associated with the seven subcortical brain volumes and ICV from ENIGMA. Using METAL, 35 we conducted a sample size-weighted meta-analysis for schizophrenia (effective sample size 71,715) and ENIGMA (variable sample sizes per SNP ranging from 8,000-11,000). SNPs were excluded if they were not present in both datasets and for MAF < 1% (per analysis). The total number of SNPs present in the eight meta-analyses ranged from 7,847,762 to 7,945,194.
SNP effect size comparisons
SNP effect sizes were extracted from studies of brain structure (ENIGMA), 9 schizophrenia (PGC), 3 height (GIANT), 24 and educational attainment (EduYears). 38 The five highest effect size SNPs were selected for schizophrenia and height, all genome-wide significant SNPs were displayed for brain structure volumes and EduYears. Percent variance was calculated on the liability scale for schizophrenia for comparison with quantitative traits. 23 For brain structures, height, and EduYears, percent variance explained was calculated as R2g|c/(1-R2c) = (t2/((n-k-1)+t2))*100, where the t-statistic is calculated as the ß-coefficient for a given SNP from the regression model (controlling for covariates) divided by the standard error of the ß-estimate, n is the total number of subjects, and k is the total number of covariates. 95% confidence intervals were calculated by transforming percent variance explained to a Z-statistic using Fisher's Z transformation, finding the 95% confidence intervals of the Z-statistic, and transforming this interval back into percent variance explained.
Supplementary Material
Summary.
The authors defined a roadmap for the investigation of the genetic covariance between structural/functional brain phenotypes and risk for psychiatric disorders. Their proof-of-concept study using the largest available common variant datasets for schizophrenia and volumes of several (mainly subcortical) brain structures did not find evidence of genetic overlap.
Acknowledgements
Psychiatric Genomics Consortium (PGC). The authors are grateful to the many family members who participated in the studies that recruited these samples, to the many clinicians who assisted in their recruitment, and to our team members without whom this study would have been impossible. Core funding for the PGC is from the US National Institute of Mental Health (U01 MH094421). Statistical analyses were carried out on the Genetic Cluster Computer (http://www.geneticcluster.org) hosted by SURFsara and financially supported by the Netherlands Scientific Organization (NWO 480-05-003) along with a supplement from the Dutch Brain Foundation and the VU University Amsterdam. The GRAS data collection was supported by the Max Planck Society, the Max-Planck-Förderstiftung, and the DFG Center for Nanoscale Microscopy & Molecular Physiology of the Brain (CNMPB), Göttingen, Germany. The Boston CIDAR project was supported by the NIMH (P50 MH080272, RWM; U01 MH081928, LJS; R01 MH092380, TLP) and the Massachusetts General Hospital Executive Committee on Research (TLP). P.H.L. is supported by NIMH K99 MH101367. ISC Portugal: CNP and MTP have been supported by NIMH grants MH085548, MH085542, MH071681, MH061884, MH58693, and MH52618, and the NCRR RR026075. CNP, MTP, and AHF have been supported by grants from the Department of Veterans Affairs Merit Review Program. The Danish Aarhus study was supported by grants from Lundbeck Foundation, Danish Strategic Research Council, Aarhus University, and Stanley Research Foundation. Work in Cardiff was supported by MRC Centre (G0800509) and MRC Programme (G0801418) Grants, the European Community's Seventh Framework Programme (HEALTH-F2-2010-241909, Project EU-GEI)) the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement n°279227, a fellowship to JW from the MRC/Welsh Assembly Government and the Margaret Temple Award from the British Medical Association. We thank Novartis for their input in obtaining CLOZUK samples, and staff at The Doctor's Laboratory (Lisa Levett/Andrew Levett) for help with sample acquisition and data linkage, and in Cardiff (Kiran Mantripragada/Lucinda Hopkins) for sample management. CLOZUK and some other samples were genotyped at the Broad Institute or by the WTCCC and WTCCC2 (WT 083948/Z/07/Z). We acknowledge use of the British 1958 Birth Cohort DNA (MRC: G0000934) and the Wellcome Trust (068545/Z/0/ and 076113/C/04/Z), the UK Blood Services Common Controls (UKBS-CC collection), funded by the WT (076113/C/04/Z) and by NIHR programme grant to NHSBT (RP-PG-0310-1002). VCU investigators were supported by NIMH grants R01 MH083094, R01 MH041953, and R01 MH068881, and WTCCC2 grant WTCCC-084710. Recruitment of families in Bulgaria was funded by the Janssen Research Foundation, Beerse, Belgium. We thank the staff in the Neuroscience Biomarkers Genomic Lab led by Reyna Favis at Janssen for sample processing and the staff at Illumina for genotyping Janssen DNA samples. We also thank Anthony Santos, Nicole Bottrel, Monique-Andree Franc, William Cafferty of Janssen Research & Development) for operational support. Funding from the Netherlands Organization for Health Research and Development (ZonMw), within the Mental Health program (GROUP consortium), and NIMH R01 MH078075. The Danish Council for Strategic Research (Journ.nr. 09-067048); The Danish National Advanced Technology Foundation (Journ.nr. 001-2009-2); The Lundbeck Foundation (Journ.nr. R24-A3243); EU 7th Framework Programme (PsychGene; Grant agreement nr. 218251); EU 7th Framework Programme (PsychDPC; Grant agreement nr. 286213). The Wellcome Trust supported this study as part of the WTCCC2 project. E. Bramon holds a MRC New Investigator Award and a MRC Centenary Award. The TOP Study was supported by the Research Council of Norway (#213837, # 217776, # 223273), South-East Norway Health Authority (#2013-123) and K.G. Jebsen Foundation. This work was supported by the Donald and Barbara Zucker Foundation, the North Shore – Long Island Jewish Health System Foundation, and grants from Stanley Foundation (AKM), NARSAD (AKM), NIMH (MH065580 to TL; MH001760 to AKM), and NIMH RC2 MH089964 and R01 MH084098. SynSys, EU FP7-242167, Sigrid Juselius Foundation, The Academy of Finland (grant number: 251704), Sohlberg Foundation. The Swedish Research Council (grants 2006-4472, 2009-5269, 2009-3413) and the County Councils of Västerbotten and Norrbotten, Sweden supported the collection of the Umeå samples. The Betula Study, from which the Umeå controls were recruited, is supported by grants from the Swedish Research Council (grants 345-2003-3883, 315-2004-6977) and the Bank of Sweden Tercentenary Foundation, the Swedish Council for Planning and Coordination of Research, the Swedish Council for Research in the Humanities and Social Sciences and the Swedish Council for Social Research. We acknowledge support from NIMH K01 MH085812 (PI Keller) and NIMH R01 MH100141 (PI Keller). EGCUT work was supported by the Targeted Financing from the Estonian Ministry of Science and Education (SF0180142s08); NIH R01 DK075787; the Development Fund of the University of Tartu (grant SP1GVARENG); the European Regional Development Fund to the Centre of Excellence in Genomics (EXCEGEN; grant 3.2.0304.11-0312); and FP7 grant 313010. MM was supported by CZ.2.16/3.1.00/24022OPPK, NT/13770–4and 00064203 FN Motol. Funding from the National Medical Research Council (NMRC/TCR/003/2008) and the Biomedical Research Council (A*STAR) is acknowledged. Genotyping of the Swedish Hubin sample was performed by the SNP&SEQ Technology Platform in Uppsala, which is supported by Uppsala University, Uppsala University Hospital, Science for Life Laboratory, and the Swedish Research Council (Contracts 80576801 and 70374401). The Swedish Hubin sample was supported by Swedish Research Council (IA, EGJ) and Stockholm County Council and the Karolinska Insititutet (EGJ). B.J.M., V.J.C., R.J.S., S.V.C., F.A.H., A.V.J., C.M.L., P.T.M., C.P., and U.S. were supported by the Australian Schizophrenia Research Bank, which is supported by an Enabling Grant from the National Health and Medical Research Council (No. 386500), the Pratt Foundation, Ramsay Health Care, the Viertel Charitable Foundation and the Schizophrenia Research Institute and the NSW Department of Health. C.P. is supported by a Senior Principal Research Fellowship from the National Health and Medical Research Council (Australia). The Perth sample collection was funded by Australian National Health and Medical Research Council project grants and the Australian Schizophrenia Research Bank. The Bonn/Mannheim sample was genotyped within a study that was supported by the German Federal Ministry of Education and Research (BMBF) through the Integrated Genome Research Network (IG) MooDS (Systematic Investigation of the Molecular Causes of Major Mood Disorders and Schizophrenia; grant 01GS08144 to M.M.N. and S.C., grant 01GS08147 to M.R.), under the National Genome Research Network plus (NGFNplus), and the Integrated Network IntegraMent (Integrated Understanding of Causes and Mechanisms in Mental Disorders), under e:Med Programme (GSK control sample; Müller-Myhsok). This work has been funded by the Bavarian Ministry of Commerce and by the Federal Ministry of Education and Research in the framework of the National Genome Research Network, Förderkennzeichen 01GS0481 and the Bavarian Ministry of Commerce. M.M.N. is a member of the DFG-funded Excellence-Cluster ImmunoSensation. M.M.N. also received support from the Alfried Krupp von Bohlen und Halbach-Stiftung. M.R. was supported by the 7th Framework Programme of the European Union (ADAMS project, HEALTH-F4-2009-242257; CRESTAR project, HEALTH-2011-1.1-2) grant 279227. J.K. holds the Joanne Murphy Professor in Behavioural Science. The Stanley Center for Psychiatric Research at the Broad Institute acknowledges funding from the Stanley Medical Research Institute. Support for the Sweden Schizophrenia Study (PIs Sullivan, Hultman, and Sklar) was provided by the NIMH (R01 MH077139 and R01 MH095034), the Stanley Center for Psychiatric Research, the Sylvan Herman Foundation, the Friedman Brain Institute at the Mount Sinai School of Medicine, the Karolinska Institutet, Karolinska University Hospital, the Swedish Research Council, the Swedish County Council, the Söderström Königska Foundation. We acknowledge use of DNA from The UK Blood Services collection of Common Controls (UKBS collection), funded by the Wellcome Trust grant 076113/CI04/Z, by the Juvenile Diabetes Research Foundation grant WT0618S8, and by the National Institute of Health Research of England. The Multicenter Genetics Studies of Schizophrenia and Molecular Genetics of Schizophrenia studies study were supported by NIMH grant R01 MH062276 (to DF Levinson, C Laurent, M Owen and D Wildenauer), grant R01 MH068922 (to PV Gejman), grant R01 MH068921 (to AE Pulver) and grant R01 MH068881 (to B Riley). D.F.L. was supported by the Walter E. Nichols, M.D., Professorship in the School of Medicine, the Eleanor Nichols Endowment, the Walter F. & Rachael L. Nichols Endowment and the William and Mary McIvor Endowment, Stanford University. This study was supported by NIH R01 grants (MH67257 to N.G.B., MH59588 to B.J.M., MH59571 to P.V.G., MH59565 to R.F., MH59587 to F.A., MH60870 to W.F.B., MH59566 to D.W.B., MH59586 to J.M.S., MH61675 to D.F.L., MH60879 to C.R.C., and MH81800 to P.V.G.), NIH U01 grants (MH46276 to C.R.C., MH46289 to C. Kaufmann, MH46318 to M.T. Tsuang, MH79469 to P.V.G., and MH79470 to D.F.L.), the Genetic Association Information Network (GAIN), and by The Paul Michael Donovan Charitable Foundation. Genotyping was carried out by the Center for Genotyping and Analysis at the Broad Institute of Harvard and MIT (S. Gabriel and D. B. Mirel), supported by grant U54 RR020278 from the National Center for Research Resources. We thank S. We (DRW, RS) thank the staff of the Lieber Institute and the Clinical Brain Disorders Branch of the IRP, NIMH for their assistance in data collection and management. We acknowledge the Irish contribution to the International Schizophrenia Consortium (ISC) study, the WTCCC2 SCZ study & WTCCC2 controls from the 1958BC and UKNBS, the Science Foundation Ireland (08/IN.1/B1916). We acknowledge use of the Trinity Biobank sample from the Irish Blood Transfusion Service & the Trinity Centre for High Performance Computing. Funding for this study was provided by the Wellcome Trust Case Control Consortium 2 project (085475/B/08/Z and 085475/Z/08/Z), the Wellcome Trust (072894/Z/03/Z, 090532/Z/09/Z and 075491/Z/04/B), NIMH grants (MH 41953 and MH083094) and British 1958 Birth Cohort DNA collection funded by the Medical Research Council (grant G0000934) and the Wellcome Trust (grant 068545/Z/02) and of the UK National Blood Service controls funded by the Wellcome Trust. We acknowledge Hong Kong Research Grants Council project grants GRF 774707M, 777511M, 776412M and 776513M.
ENIGMA. ENIGMA was supported in part by a Consortium grant (U54 EB020403 to PMT) from the NIH Institutes contributing to the Big Data to Knowledge (BD2K) Initiative, including the NIBIB and NCI. ADNI and ADNI2GO: Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer's Association; Alzheimer's Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of Southern California. BETULA: this sample collection was supported by a Wallenberg Scholar grant from the Knut and Alice Wallenberg (KAW) foundation and a grant from Torsten and Ragnar Söderbergs Foundation to LN, a grant from HelseVest RHF (Grant 911554) to SLH. Bipolar Family Study (BFS): The Bipolar Family Study wishes to thank the Scottish Mental Health Research Network for research assistant support, the Brain Research Imaging Centre Edinburgh, a center in the Scottish Funding Council Scottish Imaging Network–A Platform for Scientific Excellence (SINAPSE) Collaboration, for image acquisition and the Wellcome Trust Clinical Research Facility for genotyping. Genotyping was supported by the National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award (to A.M.M.), and data collection was supported by the Health Foundation Clinician Scientist Fellowship. BIG: This work makes use of the BIG (Brain Imaging Genetics) database, first established in Nijmegen, The Netherlands, in 2007. This resource is now part of Cognomics (www.cognomics.nl), a joint initiative by researchers of the Donders Centre for Cognitive Neuroimaging, the Human Genetics and Cognitive Neuroscience departments of the Radboud university medical centre and the Max Planck Institute for Psycholinguistics in Nijmegen. The Cognomics Initiative is supported by the participating departments and centres and by external grants, i.e. the Biobanking and Biomolecular Resources Research Infrastructure (Netherlands) (BBMRI-NL), the Hersenstichting Nederland, and the Netherlands Organisation for Scientific Research (NWO). We wish to thank all persons who kindly participated in the BIG research. The research leading to these results also receives funding from the European Community's Seventh Framework Programme (FP7/2007– 2013) under grant agreements #602450 (IMAGEMEND) and #602805 (Aggressotype), and from ERC-2010-AdG 268800-NEUROSCHEMA. B. Franke is supported by a Vici grant from the Netherlands Organisation for Scientific Research (NWO; grant # 016.130.669). Brain Genomics Superstruct Project (GSP): Data were provided [in part] by the Brain Genomics Superstruct Project of Harvard University and the Massachusetts General Hospital, with support from the Center for Brain Science Neuroinformatics Research Group, the Athinoula A. Martinos Center for Biomedical Imaging, and the Center for Human Genetic Research. 20 individual investigators at Harvard and MGH generously contributed data to GSP. GIG: The GIG (Genomic Imaging Göttingen) sample was established at the Center for Translational Research in Systems Neuroscience and Psychiatry at Göttingen University. We thank Maria Keil, Esther Diekhof, Tobias Melcher and Ilona Henseler for assistance in MRI data acquisition, and Elisabeth Binder and Holger Mohr for their valuable help with genotyping. We are grateful to all persons who kindly participated in the GIG study. IMAGEN: IMAGEN was supported by the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement- related behaviour in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the FP7 projects IMAGEMEND (602450) and MATRICS (603016), and the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Programme Grant “Developmental pathways into adolescent substance abuse” (93558), as well as the NIHR-biomedical Research Center “Mental Health”. Further support was provided by the Swedish Research Council FORMAS and the German Federal Ministry for Education and Research BMBF (eMED SysAlc 01ZX1311A; Forschungsnetz AERIAL; 1EV0711). MooDS: The establishment of the MooDS sample was funded by the German Federal Ministry of Education and Research (BMBF) through the Integrated Genome Research Network (IG) MooDS (Systematic Investigation of the Molecular Causes of Major Mood Disorders and Schizophrenia; grant 01GS08144 to Markus M. Nöthen and Sven Cichon, grant 01GS08147 to Marcella Rietschel and Andreas Meyer-Lindenberg and grant 01GS08148 to Andreas Heinz), under the auspices of the National Genome Research Network plus (NGFNplus), and through the Integrated Network IntegraMent (Integrated Understanding of Causes and Mechanisms in Mental Disorders), under the auspices of the e:Med Programme (grant 01ZX1314A to Markus M. Nöthen, grant 01ZX1314C to Hendrik Walter, grant 01ZX1314G to Marcella Rietschel). MPIP: The MPIP Munich Morphometry Sample comprises images acquired as part of the Munich Antidepressant Response Signature Study and the Recurrent Unipolar Depression (RUD) Case-Control study performed at the MPIP, and control subjects acquired at the Ludwig-Maximilians-University, Munich, Department of Psychiatry. We wish to acknowledge Anna Olynyik and radiographers Rosa Schirmer, Elke Schreiter, Reinhold Borschke and Ines Eidner for image acquisition and data preparation. We thank Dorothee P. Auer for local study management in the initial phase of the RUD study. We are grateful to GlaxoSmithKline for providing the genotypes of the Recurrent Unipolar Depression Case-Control Sample. We thank the staff of the Center of Applied Genotyping (CAGT) for generating the genotypes of the MARS cohort. The study is supported by a grant of the Exzellenz-Stiftung of the Max Planck Society. This work has also been funded by the Federal Ministry of Education and Research (BMBF) in the framework of the National Genome Research Network (NGFN), FKZ 01GS0481. NCNG: this sample collection was supported by grants from the Bergen Research Foundation and the University of Bergen, the Dr Einar Martens Fund, the K.G. Jebsen Foundation, the Research Council of Norway, to SLH, VMS and TE. NESDA: Funding was obtained from the Netherlands Organization for Scientific Research (Geestkracht program grant 10-000-1002); the Center for Medical Systems Biology (CSMB, NWO Genomics), Biobanking and Biomolecular Resources Research Infrastructure (BBMRI-NL), VU University's Institutes for Health and Care Research (EMGO+) and Neuroscience Campus Amsterdam, University Medical Center Groningen, Leiden University Medical Center, National Institutes of Health (NIH, R01D0042157-01A, MH081802, Grand Opportunity grants 1RC2 MH089951 and 1RC2 MH089995). Part of the genotyping and analyses were funded by the Genetic Association Information Network (GAIN) of the Foundation for the National Institutes of Health. Computing was supported by BiG Grid, the Dutch e-Science Grid, which is financially supported by NWO. NeuroIMAGE: The NeuroIMAGE was supported by NIH Grant R01MH62873 (to Stephen V. Faraone), NWO Large Investment Grant 1750102007010 (to Jan Buitelaar) and grants from Radboud University Nijmegen Medical Center, University Medical Center Groningen and Accare, and VU University Amsterdam. The research leading to these results also receives funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreements n° 602450 (IMAGEMEND), n° 278948 (TACTICS) and n° 602805 (Aggressotype). NTR-Adults and Brainscale: We would like to thank all twin participants from the Netherlands Twin Register. The NTR-adult and Brainscale studies were supported by the Netherlands Organization for Scientific Research NWO [MW904-61-193 (E.d.G & D.B), MaGW-nr: 400-07-080 (D. v`t E.), MagW 480-04-004 (D.B), (51.02.060 (H.H.), 668.772 (D.B. & H.H.); NWO/SPI 56-464-14192 (D.B.), the European Research Council (ERC-230374) (D.B.), High Potential Grant Utrecht University (H.H.), NWO Brain and Cognition 433-09-220 (H.H.) and the Neuroscience Campus Amsterdam (NCA). Older Australian Twins Study (OATS): We would like to acknowledge and thank the OATS participants, their supporters and respective Research Teams. This work was supported by a number of sources. OATS is supported by the NHMRC/Australian Research Council Strategic Award 401162 and NHMRC Project Grant 1045325 to P. Sachdev and colleagues. OATS was facilitated through access to the Australian Twin Registry, a national research resource supported by the NHMRC Enabling Grant 310667, administered by the University of Melbourne. DNA was extracted by Genetic Repositories Australia, an Enabling Facility supported by the NHMRC Grant 401184. OATS genotyping was partly funded by a Commonwealth Scientific and Industrial Research Organisation Flagship Collaboration Fund Grant. Henry Brodaty is supported by the Australian Government funded Dementia Collaborative Research Centre (DCRC), UNSW. Nicola Armstrong was supported by the NHMRC Project Grant 525453 and Karen Mather is supported by an Alzheimer's Australia Dementia Research Foundation Postdoctoral Fellowship and the NHMRC Capacity Building Grant 568940. QTIM: DPH, NJ, CRKC, and PMT are supported, in part, by NIH grants R01 NS080655, R01AG040060, R01 EB008432, R01 MH097268, U01 AG024904, R01 MH085667, R01 MH089722, P41 EB015922, and R01 MH094343. RKW is supported by National Science Foundation (BCS-1229450). JLS was supported by the NIMH (K99MH102357) and Autism Speaks. SEM and GZ are supported by Future Fellowships (FT110100548, FT0991634) from the Australian Research Council, and GWM is supported by a National Health and Medical Research Council (NHMRC), Australia, Fellowship (619667). The QTIM study is supported by grants from NIH (R01 HD050735) and the NHMRC (389875, 486682, 1009064). We thank the twins and siblings for their participation, Marlene Grace and Ann Eldridge for twin recruitment, Aiman Al Najjar and other radiographers for scanning, Kerrie McAloney and Daniel Park for research support, and Anjali Henders and staff for DNA sample processing and preparation. SHIP: The Study of Health in Pomerania (SHIP) is supported by the German Federal Ministry of Education and Research (grants 01ZZ9603, 01ZZ0103 and 01ZZ0403) and the German Research Foundation (DFG; GR 1912/5-1). Genome-wide data and MRI scans were supported by a joint grant from Siemens Healthcare, Erlangen, Germany, and the Federal State of Mecklenburg–West Pomerania. SHIP-TREND-0: This cohort is part of the Community Medicine Research net (CMR) of the University of Greifswald, which is funded by the German Federal Ministry of Education and Research and the German Ministry of Cultural Affairs, as well as by the Social Ministry of the Federal State of Mecklenburg–West Pomerania. CMR encompasses several research projects that share data from the population-based Study of Health in Pomerania (SHIP; see URLs). MRI scans were supported by a joint grant from Siemens Healthcare, Erlangen, Germany, and the Federal State of Mecklenburg–West Pomerania. The SHIP authors are grateful to Mario Stanke for the opportunity to use his server cluster for SNP imputation as well as to Holger Prokisch and Thomas Meitinger (HelmholtzZentrum München) for genotyping the SHIP-TREND cohort which was supported by the Federal Ministry of Education and Research (grant 03ZIK012). We thank all staff members and participants of the SHIP studies, as well as all of the genotyping staff for generating the SHIP SNP data set. D. J. is supported by a scholarship from the Gerhard-Domagk programme of the University Medicine Greifswald. Sydney Memory and Ageing Study (Sydney MAS): We would like to thank the Sydney MAS participants, their supporters and respective Research Teams. Sydney MAS was supported by the Australian National Health and Medical Research Council (NHMRC) Program Grants 350833 and 568969 to P Sachdev, H Brodaty and G Andrews. DNA was extracted by Genetic Repositories Australia, an Enabling Facility supported by the NHMRC Grant 401184. Henry Brodaty is supported by the Australian Government funded Dementia Collaborative Research Centre (DCRC), UNSW. Nicola Armstrong was supported by the NHMRC Project Grant 525453 and Karen Mather is supported by an Alzheimer's Australia Dementia Research Foundation Postdoctoral Fellowship. Both Simone Reppermund and Karen Mather are supported by the NHMRC Capacity Building Grant 568940.
Data used in preparing this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu). Many investigators within ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
PGC-SCZ consortium collaborators include
Stephan Ripke 1,7,8,9, Benjamin M Neale 7,8,11,38,41, Aiden Corvin 45, James TR Walters 39, Kai-How Farh 7, Peter A Holmans 39,40, Phil Lee 8,11,12, Brendan Bulik-Sullivan 7,8, David A Collier 46,47, Hailiang Huang 7,41, Tune H Pers 41, Ingrid Agartz 48,49,50, Esben Agerbo 20, Margot Albus 51, Madeline Alexander 52, Farooq Amin 53,54, Silviu A Bacanu 55, Martin Begemann 56, Richard A Belliveau Jr 8, Judit Bene 57,58, Sarah E Bergen 8,42, Elizabeth Bevilacqua 8, Tim B Bigdeli 55, Donald W Black 59, Richard Bruggeman 60, Nancy G Buccola 61, Randy L Buckner 62,63,64, William Byerley 65, Wiepke Cahn 66, Guiqing Cai 67,68, Murray J Cairns 69,70,71, Dominique Campion 72, Rita M Cantor 73, Vaughan J Carr 69,74, Noa Carrera 39, Stanley V Catts 69,75, Kimberley D Chambert 8, Raymond CK Chan 76, Eric YH Chen 77,78, Ronald YL Chen 78, Wei Cheng 79, Eric FC Cheung 80, Siow Ann Chong 81, C Robert Cloninger 82, David Cohen 83, Nadine Cohen 84, Paul Cormican 45, Nick Craddock 39,40, Benedicto Crespo-Facorro 85,86, James J Crowley 43, David Curtis 87,88, Michael Davidson 89, Kenneth L Davis 67, Franziska Degenhardt 90,91, Jurgen Del Favero 92, Lynn E DeLisi 12, Ditte Demontis 20, Dimitris Dikeos 93, Timothy Dinan 94, Srdjan Djurovic 48,95, Gary Donohoe 45,96, Elodie Drapeau 67, Jubao Duan 97,98, Frank Dudbridge 99, Peter Eichhammer 100, Johan Eriksson 101,102,103, Valentina Escott-Price 39, Laurent Essioux 104, Ayman H Fanous 105,106,107,108, Martilias S Farrell 43, Josef Frank 109, Lude Franke 110, Robert Freedman 111, Nelson B Freimer 112, Joseph I Friedman 67, Menachem Fromer 7,8,11, Giulio Genovese 8, Lyudmila Georgieva 39, Elliot S Gershon 113, Ina Giegling 114,115, Paola Giusti-Rodríguez 43, Stephanie Godard 116, Jacqueline I Goldstein 7,41, Srihari Gopal 117, Jacob Gratten 118, Lieuwe de Haan 119, Christian Hammer 56, Marian L Hamshere 39, Mark Hansen 120, Thomas Hansen 20, Vahram Haroutunian 67,121,122, Annette M Hartmann 114, Frans A Henskens 69,123,124, Stefan Herms 90,91,125, Joel N Hirschhorn 41, Per Hoffmann 90,91,125, Andrea Hofman 90,91, Mads V Hollegaard 126, David M Hougaard 126, Masashi Ikeda 127, Inge Joa 128, Antonio Julià 129, Anna K Kähler 42, René S Kahn 66, Luba Kalaydjieva 130,131, Sena Karachanak-Yankova 132, Juha Karjalainen 110, David Kavanagh 39, Matthew C Keller 133, Brian J Kelly 70, James L Kennedy 134,135,136, Andrey Khrunin 137, Yunjung Kim 43, Janis Klovins 138, James A Knowles 139, Bettina Konte 114, Vaidutis Kucinskas 140, Zita Ausrele Kucinskiene 140, Hana Kuzelova-Ptackova 141, Claudine Laurent 52,142, S Hong Lee 118, Jimmy Lee Chee Keong 81,143, Sophie E Legge 39, Bernard Lerer 144, Miaoxin Li 77,78,145, Tao Li 146, Kung-Yee Liang 147, Jeffrey Lieberman 148, Svetlana Limborska 137, Jouko Lönnqvist 149, Carmel M Loughland 69,70, Jan Lubinski 150, Milan Macek Jr 151, Patrik KE Magnusson 42, Brion S Maher 152, Wolfgang Maier 153, Jacques Mallet 154, Sara Marsal 129, Manuel Mattheisen 19,20,21, Morten Mattingsdal 48,155, Robert W McCarley 12, Colm McDonald 156, Andrew M McIntosh 16, Sandra Meier 157, Carin J Meijer 119, Bela Melegh 57,58, Ingrid Melle 23, Raquelle I Mesholam-Gately 12, Andres Metspalu 158, Patricia T Michie 69,159, Lili Milani 158, Vihra Milanova 160, Younes Mokrab 161, Derek W Morris 45,96, Ole Mors 20, Bertram Müller-Myhsok 162,163,164, Kieran C Murphy 165, Robin M Murray 166, Inez Myin-Germeys 167, Mari Nelis 158, Igor Nenadic 168, Deborah A Nertney 169, Gerald Nestadt 170, Kristin K Nicodemus 171, Liene Nikitina-Zake 138, Laura Nisenbaum 172, Annelie Nordin 173, Eadbhard O'Callaghan 174, Colm O'Dushlaine 8, F Anthony O'Neill 175, Sang-Yun Oh 176, Ann Olincy 111, Line Olsen 20, Jim Van Os 167,177, Christos Pantelis 69,178, George N Papadimitriou 93, Sergi Papiol 56, Elena Parkhomenko 67, Michele T Pato 139, Tiina Paunio 179,180, Psychosis Endophenotypes International Consortium 181, Diana O Perkins 44, Olli Pietiläinen 179,182, Jonathan Pimm 88, Andrew J Pocklington 39, John Powell 166, Alkes Price 41, Ann E Pulver 170, Shaun M Purcell 183, Digby Quested 184, Henrik B Rasmussen 20, Abraham Reichenberg 67, Mark A Reimers 55, Alexander L Richards 39,40, Joshua L Roffman 63,64, Panos Roussos 183,185, Douglas M Ruderfer 39, Veikko Salomaa 102, Alan R Sanders 97,186, Ulrich Schall 69,70, Christian R Schubert 187, Thomas G Schulze 109,188, Sibylle G Schwab 189, Edward M Scolnick 8, Rodney J Scott 69,71,190, Larry J Seidman 12, Jianxin Shi 191, Jeremy M Silverman 67,192, Kang Sim 81, Petr Slominsky 137, Jordan W Smoller 8,11,12, Hon-Cheong So 78, Erik Söderman 50, Chris C A Spencer 193, Eli A Stahl 41, Elisabeth Stogmann 194, Richard E Straub 195, Eric Strengman 66,196, Jana Strohmaier 157, T Scott Stroup 148, Mythily Subramaniam 81, Jaana Suvisaari 149, Dragan M Svrakic 82, Jin P Szatkiewicz 43, Srinivas Thirumalai 197, Draga Toncheva 198, Paul A Tooney 69,71,199, Juha Veijola 200,201, John Waddington 202, Dermot Walsh 203, Dai Wang 117, Qiang Wang 204, Bradley T Webb 55, Mark Weiser 89, Dieter B Wildenauer 205, Nigel M Williams 39, Stephanie Williams 43, Stephanie H Witt 109, Aaron R Wolen 55, Emily HM Wong 78, Brandon K Wormley 55, Jing Qin Wu 69,71, Hualin Simon Xi 206, Clement C Zai 134,135, Xuebin Zheng 207, Fritz Zimprich 194, Naomi R Wray 118, Peter M Visscher 118, Wellcome Trust Case-Control Consortium 2 208, Rolf Adolfsson 173, Ole A Andreassen 22,23, Douglas HR Blackwood 209, Anders D Børglum 20, Elvira Bramon 210, Joseph D Buxbaum 67,68,122,211, Sven Cichon 90,91,125,212, Ariel Darvasi 213, Enrico Domenici 214, Hannelore Ehrenreich 56, Tõnu Esko 41, Pablo V Gejman 97,186, Michael Gill 45, Hugh Gurling 88, Christina M Hultman 42, Nakao Iwata 127, Assen V Jablensky 69,215,216,217, Erik G Jönsson 48,50, Kenneth S Kendler 55, George Kirov 39, Jo Knight 134,135,136, Todd Lencz 218,219,220, Douglas F Levinson 52, Qingqin S Li 117, Jianjun Liu 207,221, Anil K Malhotra 218,219,220, Steven A McCarroll 8, Andrew McQuillin 88, Jennifer L Moran 8, Preben B Mortensen 20, Bryan J Mowry 169,222, Markus M Nöthen 90,91, Roel A Ophoff 66,73,112, Michael J Owen 39,40, Aarno Palotie 8,11, Carlos N Pato 139, Tracey L Petryshen 8,12, Danielle Posthuma 223,224,225, Marcella Rietschel 109, Brien P Riley 55, Dan Rujescu 114,115, Pak C Sham 77,78,145, Pamela Sklar 122,183,185, David St Clair 226, Daniel R Weinberger 195,227, Jens R Wendland 187, Thomas Werge 20, Mark J Daly 7,8,41, Patrick F Sullivan 38,42,43,44, Michael C O'Donovan 38,39,40
45 Neuropsychiatric Genetics Research Group, Department of Psychiatry, Trinity College Dublin, Dublin 8, Ireland
46 Eli Lilly and Company Limited, Erl Wood Manor, Sunninghill Road, Windlesham, Surrey, GU20 6PH, UK
47 Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, King's College London, London, SE5 8AF, UK
48 NORMENT, KG Jebsen Centre for Psychosis Research, Institute of Clinical Medicine, University of Oslo, 0424 Oslo, Norway
49 Department of Psychiatry, Diakonhjemmet Hospital, 0319 Oslo, Norway
50 Department of Clinical Neuroscience, Psychiatry Section, Karolinska Institutet, SE-17176 Stockholm, Sweden
51 State Mental Hospital, 85540 Haar, Germany
52 Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, California 94305, USA
53 Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, Georgia 30322, USA
54 Department of Psychiatry and Behavioral Sciences, Atlanta Veterans Affairs Medical Center, Atlanta, Georgia 30033, USA
55 Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, VA, USA
56 Clinical Neuroscience, Max Planck Institute of Experimental Medicine, Göttingen 37075, Germany
57 Department of Medical Genetics, University of Pécs, Pécs H-7624, Hungary
58 Szentagothai Research Center, University of Pécs, Pécs H-7624, Hungary
59 Department of Psychiatry, University of Iowa Carver College of Medicine, Iowa City, Iowa 52242, USA
60 University Medical Center Groningen, Department of Psychiatry, University of Groningen, NL-9700 RB, The Netherlands
61 School of Nursing, Louisiana State University Health Sciences Center, New Orleans, Louisiana 70112, USA
62 Center for Brain Science, Harvard University, Cambridge, Massachusetts 02138, USA
63 Department of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts 02114, USA
64 Athinoula A Martinos Center, Massachusetts General Hospital, Boston, Massachusetts 02129, USA
65 Department of Psychiatry, University of California at San Francisco, San Francisco, California, 94143 USA
66 University Medical Center Utrecht, Department of Psychiatry, Rudolf Magnus Institute of Neuroscience, 3584 Utrecht, The Netherlands
67 Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York 10029, USA
68 Department of Human Genetics, Icahn School of Medicine at Mount Sinai, New York, New York 10029, USA
69 Schizophrenia Research Institute, Sydney NSW 2010, Australia
70 Priority Centre for Translational Neuroscience and Mental Health, University of Newcastle, Newcastle NSW 2300, Australia
71 School of Biomedical Sciences and Pharmacy, University of Newcastle, Callaghan NSW 2308, Australia
72 Centre Hospitalier du Rouvray and INSERM U1079 Faculty of Medicine, 76301 Rouen, France
73 Department of Human Genetics, David Geffen School of Medicine, University of California, Los Angeles, California 90095, USA
74 School of Psychiatry, University of New South Wales, Sydney NSW 2031, Australia
75 Royal Brisbane and Women's Hospital, University of Queensland, Brisbane QLD 4072, Australia
76 Institute of Psychology, Chinese Academy of Science, Beijing 100101, China
77 State Key Laboratory for Brain and Cognitive Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China
78 Department of Psychiatry, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China
79 Department of Computer Science, University of North Carolina, Chapel Hill, North Carolina 27514, USA
80 Castle Peak Hospital, Hong Kong, China
81 Institute of Mental Health, Singapore 539747, Singapore
82 Department of Psychiatry, Washington University, St Louis, Missouri 63110, USA
83 Department of Child and Adolescent Psychiatry, Assistance Publique Hospitaux de Paris, Pierre and Marie Curie Faculty of Medicine and Institute for Intelligent Systems and Robotics, Paris, 75013, France
84 Blue Note Biosciences, Princeton, New Jersey 08540, USA
85 University Hospital Marques de Valdecilla, Instituto de Formacion e Investigacion Marques de Valdecilla, University of Cantabria, E-39008 Santander, Spain
86 Centro Investigacion Biomedica en Red Salud Mental, Madrid, Spain
87 Department of Psychological Medicine, Queen Mary University of London, London E1 1BB, UK
88 Molecular Psychiatry Laboratory, Division of Psychiatry, University College London, London WC1E 6JJ, UK
89 Sheba Medical Center, Tel Hashomer 52621, Israel
90 Institute of Human Genetics, University of Bonn, D-53127 Bonn, Germany
91 Department of Genomics, Life and Brain Center, D-53127 Bonn, Germany
92 Applied Molecular Genomics Unit, VIB Department of Molecular Genetics, University of Antwerp, B-2610 Antwerp, Belgium
93 First Department of Psychiatry, University of Athens Medical School, Athens 11528, Greece
94 Department of Psychiatry, University College Cork, Co Cork, Ireland
95 Department of Medical Genetics, Oslo University Hospital, 0424 Oslo, Norway
96 Cognitive Genetics and Therapy Group, School of Psychology and Discipline of Biochemistry, National University of Ireland Galway, Co Galway, Ireland
97 Department of Psychiatry and Behavioral Sciences, NorthShore University HealthSystem, Evanston, Illinois 60201, USA
98 Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, Illinois 60637,, USA
99 Department of Non-Communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK
100 Department of Psychiatry, University of Regensburg, 93053 Regensburg, Germany
101 Folkhälsan Research Center, Helsinki, Finland, Biomedicum Helsinki 1, Haartmaninkatu 8, FI-00290, Helsinki, Finland
102 National Institute for Health and Welfare, PO BOX 30, FI-00271 Helsinki, Finland
103 Department of General Practice, Helsinki University Central Hospital, University of Helsinki PO BOX 20, Tukholmankatu 8 B, FI-00014, Helsinki, Finland
104 Translational Technologies and Bioinformatics, Pharma Research and Early Development, FHoffman-La Roche, CH-4070 Basel, Switzerland
105 Mental Health Service Line, Washington VA Medical Center, Washington DC 20422, USA
106 Department of Psychiatry, Georgetown University School of Medicine, Washington DC 20057, USA
107 Department of Psychiatry, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, USA
108 Department of Psychiatry, Keck School of Medicine of the University of Southern California, Los Angeles, California 90033, USA
109 Department of Genetic Epidemiology in Psychiatry, Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, Heidelberg, D-68159 Mannheim, Germany
110 Department of Genetics, University of Groningen, University Medical Centre Groningen, 9700 RB Groningen, The Netherlands
111 Department of Psychiatry, University of Colorado Denver, Aurora, Colorado 80045, USA
112 Center for Neurobehavioral Genetics, Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles, California 90095, USA
113 Departments of Psychiatry and Human Genetics, University of Chicago, Chicago, Illinois 60637 USA
114 Department of Psychiatry, University of Halle, 06112 Halle, Germany
115 Department of Psychiatry, University of Munich, 80336, Munich, Germany
116 Departments of Psychiatry and Human and Molecular Genetics, INSERM, Institut de Myologie, Hôpital de la Pitiè-Salpêtrière, Paris, 75013, France
117 Neuroscience Therapeutic Area, Janssen Research and Development, Raritan, New Jersey 08869, USA
118 Queensland Brain Institute, The University of Queensland, Brisbane, QLD 4072, Australia
119 Academic Medical Centre University of Amsterdam, Department of Psychiatry, 1105 AZ Amsterdam, The Netherlands
120 Illumina, La Jolla, California, California 92122, USA
121 JJ Peters VA Medical Center, Bronx, New York, New York 10468, USA
122 Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, New York 10029, USA
123 School of Electrical Engineering and Computer Science, University of Newcastle, Newcastle NSW 2308, Australia
124 Priority Research Centre for Health Behaviour, University of Newcastle, Newcastle NSW 2308, Australia
125 Division of Medical Genetics, Department of Biomedicine, University of Basel, Basel, CH-4058, Switzerland
126 Section of Neonatal Screening and Hormones, Department of Clinical Biochemistry, Immunology and Genetics, Statens Serum Institut, Copenhagen, DK-2300, Denmark
127 Department of Psychiatry, Fujita Health University School of Medicine, Toyoake, Aichi, 470-1192, Japan
128 Regional Centre for Clinical Research in Psychosis, Department of Psychiatry, Stavanger University Hospital, 4011 Stavanger, Norway
129 Rheumatology Research Group, Vall d'Hebron Research Institute, Barcelona, 08035, Spain
130 Centre for Medical Research, The University of Western Australia, Perth, WA 6009, Australia
131 The Perkins Institute for Medical Research, The University of Western Australia, Perth, WA 6009, Australia
132 Department of Medical Genetics, Medical University, Sofia 1431, Bulgaria
133 Department of Psychology, University of Colorado Boulder, Boulder, Colorado 80309, USA
134 Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, Ontario, M5T 1R8, Canada
135 Department of Psychiatry, University of Toronto, Toronto, Ontario, M5T 1R8, Canada
136 Institute of Medical Science, University of Toronto, Toronto, Ontario, M5S 1A8, Canada
137 Institute of Molecular Genetics, Russian Academy of Sciences, Moscow 123182, Russia
138 Latvian Biomedical Research and Study Centre, Riga, LV-1067, Latvia
139 Department of Psychiatry and Zilkha Neurogenetics Institute, Keck School of Medicine at University of Southern California, Los Angeles, California 90089, USA
140 Faculty of Medicine, Vilnius University, LT-01513 Vilnius, Lithuania
141 Department of Biology and Medical Genetics, 2nd Faculty of Medicine and University Hospital Motol, 150 06 Prague, Czech Republic
142 Department of Child and Adolescent Psychiatry, Pierre and Marie Curie Faculty of Medicine, Paris 75013, France
143 Duke-NUS Graduate Medical School, Singapore 169857, Singapore
144 Department of Psychiatry, Hadassah-Hebrew University Medical Center, Jerusalem 91120, Israel
145 Centre for Genomic Sciences, The University of Hong Kong, Hong Kong, China
146 Mental Health Centre and Psychiatric Laboratory, West China Hospital, Sichuan University, Chengdu, 610041, Sichuan, China
147 Department of Biostatistics, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland 21205, USA
148 Department of Psychiatry, Columbia University, New York, New York 10032, USA
149 Department of Mental Health and Substance Abuse Services; National Institute for Health and Welfare, PO BOX 30, FI-00271 Helsinki, Finland
150 Department of Genetics and Pathology, International Hereditary Cancer Center, Pomeranian Medical University in Szczecin, 70-453 Szczecin, Poland
151 Department of Biology and Medical Genetics, 2nd Faculty of Medicine and University Hospital Motol, 150 06, Prague, Czech Republic
152 Department of Mental Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland 21205, USA
153 Department of Psychiatry, University of Bonn, D-53127 Bonn, Germany
154 Centre National de la Recherche Scientifique, Laboratoire de Génétique Moléculaire de la Neurotransmission et des Processus Neurodégénératifs, Hôpital de la Pitié Salpêtrière, 75013, Paris, France
155 Research Unit, Sørlandet Hospital, 4604 Kristiansand, Norway
156 Department of Psychiatry, National University of Ireland Galway, Co Galway, Ireland
157 Department of Genetic Epidemiology in Psychiatry, Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, Heidelberg, D-68159 Mannheim, Germany
158 Estonian Genome Center, University of Tartu, Tartu 50090, Estonia
159 School of Psychology, University of Newcastle, Newcastle NSW 2308, Australia
160 First Psychiatric Clinic, Medical University, Sofia 1431, Bulgaria
161 Eli Lilly and Company Limited, Erl Wood Manor, Sunninghill Road, Windlesham, Surrey, GU20 6PH UK
162 Max Planck Institute of Psychiatry, 80336 Munich, Germany
163 Institute of Translational Medicine, University of Liverpool, Liverpool L69 3BX, UK
164 Munich Cluster for Systems Neurology (SyNergy), 80336 Munich, Germany
165 Department of Psychiatry, Royal College of Surgeons in Ireland, Dublin 2, Ireland
166 King's College London, London SE5 8AF, UK
167 Maastricht University Medical Centre, South Limburg Mental Health Research and Teaching Network, EURON, 6229 HX Maastricht, The Netherlands
168 Department of Psychiatry and Psychotherapy, Jena University Hospital, 07743 Jena, Germany
169 Queensland Centre for Mental Health Research, University of Queensland, Brisbane QLD 4076, Australia
170 Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, USA
171 Department of Psychiatry, Trinity College Dublin, Dublin 2, Ireland
172 Eli Lilly and Company, Lilly Corporate Center, Indianapolis, 46285 Indiana, USA
173 Department of Clinical Sciences, Psychiatry, Umeå University, SE-901 87 Umeå, Sweden
174 DETECT Early Intervention Service for Psychosis, Blackrock, Co Dublin, Ireland
175 Centre for Public Health, Institute of Clinical Sciences, Queen's University Belfast, Belfast BT12 6AB, UK
176 Lawrence Berkeley National Laboratory, University of California at Berkeley, Berkeley, California 94720, USA
177 Institute of Psychiatry, King's College London, London SE5 8AF, UK
178 Melbourne Neuropsychiatry Centre, University of Melbourne & Melbourne Health, Melbourne VIC 3053, Australia
179 Public Health Genomics Unit, National Institute for Health and Welfare, PO BOX 30, FI-00271 Helsinki, Finland
180 Department of Psychiatry, University of Helsinki, PO BOX 590, FI-00029 HUS, Helsinki, Finland
181 PEIC
182 Institute for Molecular Medicine Finland, FIMM, University of Helsinki, PO BOX 20 FI-00014, Helsinki, Finland
183 Division of Psychiatric Genomics, Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York 10029, USA
184 Department of Psychiatry, University of Oxford, Oxford, OX3 7JX, UK
185 Institute for Multiscale Biology, Icahn School of Medicine at Mount Sinai, New York, New York 10029, USA
186 Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, Illinois 60637, USA
187 PharmaTherapeutics Clinical Research, Pfizer Worldwide Research and Development, Cambridge, Massachusetts 02139, USA
188 Department of Psychiatry and Psychotherapy, University of Gottingen, 37073 Gottingen, Germany
189 Psychiatry and Psychotherapy Clinic, University of Erlangen, 91054 Erlangen, Germany
190 Hunter New England Health Service, Newcastle NSW 2308, Australia
191 Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland 20892, USA
192 Research and Development, Bronx Veterans Affairs Medical Center, New York, New York 10468, USA
193 Wellcome Trust Centre for Human Genetics, Oxford, OX3 7BN, UK
194 Department of Clinical Neurology, Medical University of Vienna, 1090 Wien, Austria
195 Lieber Institute for Brain Development, Baltimore, Maryland 21205, USA
196 Department of Medical Genetics, University Medical Centre Utrecht, Universiteitsweg 100, 3584 CG, Utrecht, The Netherlands
197 Berkshire Healthcare NHS Foundation Trust, Bracknell RG12 1BQ, UK
198 Department of Medical Genetics, Medical University, Sofia1431, Bulgaria
199 Priority Research Centre for Translational Neuroscience and Mental Health, University of Newcastle, Newcastle NSW 2300, Australia
200 Department of Psychiatry, University of Oulu, PO BOX 5000, 90014, Finland
201 University Hospital of Oulu, PO BOX 20, 90029 OYS, Finland
202 Molecular and Cellular Therapeutics, Royal College of Surgeons in Ireland, Dublin 2, Ireland
203 Health Research Board, Dublin 2, Ireland
204 Mental Health Centre and Psychiatric Laboratory, West China Hospital, Sichuan University, Chendu, 610041, Sichuan, China
205 School of Psychiatry and Clinical Neurosciences, The University of Western Australia, Perth WA 6009, Australia
206 Computational Sciences CoE, Pfizer Worldwide Research and Development, Cambridge, Massachusetts 02139, USA
207 Human Genetics, Genome Institute of Singapore, A*STAR, Singapore 138672, Singapore
208 WTCCC2
209 Division of Psychiatry, University of Edinburgh, Edinburgh EH16 4SB, UK
210 University College London, London WC1E 6BT, UK 211 Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, New York 10029, USA
212 Institute of Neuroscience and Medicine (INM-1), Research Center Juelich, 52428 Juelich, Germany
213 Department of Genetics, The Hebrew University of Jerusalem, 91905 Jerusalem, Israel
214 Neuroscience Discovery and Translational Area, Pharma Research and Early Development, FHoffman-La Roche, CH-4070 Basel, Switzerland
215 School of Psychiatry and Clinical Neurosciences, The University of Western Australia, Perth, WA 6009, Australia
216 Centre for Clinical Research in Neuropsychiatry, School of Psychiatry and Clinical Neurosciences, The University of Western Australia, Medical Research Foundation Building, Perth WA 6000, Australia
217 The Perkins Institute for Medical Research, The University of Western Australia, Perth, WA 6009, Australia
218 The Zucker Hillside Hospital, Glen Oaks, New York 11004, USA
219 The Feinstein Institute for Medical Research, Manhasset, New York 11030, USA
220 The Hofstra NS-LIJ School of Medicine, Hempstead, New York 11549, USA
221 Saw Swee Hock School of Public Health, National University of Singapore, Singapore 117597, Singapore
222 Queensland Brain Institute, The University of Queensland, Brisbane QLD 4072, Australia
223 Department of Functional Genomics, Center for Neurogenomics and Cognitive Research, Neuroscience Campus Amsterdam, VU University, Amsterdam 1081, The Netherlands
224 Department of Complex Trait Genetics, Neuroscience Campus Amsterdam, VU University Medical Center Amsterdam, Amsterdam 1081, The Netherlands
225 Department of Child and Adolescent Psychiatry, Erasmus University Medical Centre, Rotterdam 3000, The Netherlands
226 University of Aberdeen, Institute of Medical Sciences, Aberdeen, AB25 2ZD, UK
227 Departments of Psychiatry, Neurology, Neuroscience and Institute of Genetic Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland 21205, USA
ENIGMA2 consortium collaborators include
Derrek P Hibar 5, Jason L Stein 1,5,6, Miguel E Renteria 35, Alejandro Arias-Vasquez 2,3,4,10, Sylvane Desrivières 228, Neda Jahanshad 229, Roberto Toro 230, Katharina Wittfeld 231,232, Lucija Abramovic 233, Micael Andersson 234, Benjamin S Aribisala 235,236,237, Nicola J Armstrong 25, Manon Bernard 238, Marc M Bohlken 233, Marco P Boks 233, Janita Bralten 2,4,10, Andrew A Brown 22, M Mallar Chakravarty 239,240, Qiang Chen 195, Christopher R K Ching 229, Gabriel Cuellar-Partida 35, Anouk den Braber 241, Sudheer Giddaluru 242,243, Aaron L Goldman 195, Oliver Grimm 18, Tulio Guadalupe 244,245, Johanna Hass 32, Girma Woldehawariat 246, Avram J Holmes 63, Martine Hoogman 2,4, Deborah Janowitz 232, Tianye Jia 228, Sungeun Kim 29,30, Marieke Klein 2,4, Bernd Kraemer 24, Phil Lee 8,11,12, Loes M Olde Loohuis 247, Michelle Luciano 248, Christine Macare 228, Karen A Mather 25, Manuel Mattheisen 19,20,21, Yuri Milaneschi 249, Kwangsik Nho 29,30, Martina Papmeyer 16, Adaikalavan Ramasamy 250,251, Shannon L Risacher 29,30, Roberto Roiz-Santiañez 27,28, Emma J Rose 45, Alireza Salami 234, Philipp G Sämann 252, Lianne Schmaal 249, Andrew J Schork 253,254, Jean Shin 238, Lachlan T Strike 35,36, Alexander Teumer 255, Marjolein M J van Donkelaar 2,4, Kristel R van Eijk 233, Raymond K Walters 7,41, Lars T Westlye 256,257, Christopher D Whelan 258, Anderson M Winkler 259, Marcel P Zwiers 4, Saud Alhusaini 258,260, Lavinia Athanasiu 22, Stefan Ehrlich 32, Marina M H Hakobjan 2,4, Cecilie B Hartberg 22, Unn Haukvik 22, Angelien J G A M Heister 2,4, David Höhn 252, Dalia Kasperaviciute 261,262, David C M Liewald 248, Lorna M Lopez 248, Remco R R Makkinje 2,4, Mar Matarin 261, Marlies A M Naber 2,4, David R McKay 263,264, Margaret Needham 45, Allison C Nugent 246, Benno Pütz 252, Natalie A Royle 235,237,248, Li Shen 29,30, Emma Sprooten 16, Daniah Trabzuni 251,265, Saskia S L van der Marel 2,4, Kimm J E van Hulzen 2,4, Esther Walton 32, Christiane Wolf 252, Laura Almasy 266, David Ames 267,268, Sampath Arepalli 269, Amelia A Assareh 25, Mark E Bastin 235,237,248,270, Henry Brodaty 25, Kazima B Bulayeva 271, Melanie A Carless 266, Sven Cichon 90,91,125,212, Aiden Corvin 45, Joanne E Curran 266, Michael Czisch 252, Greig I de Zubicaray 36, Allissa Dillman 269, Ravi Duggirala 266, Thomas D Dyer 266, Susanne Erk 9, Iryna O Fedko 241, Luigi Ferrucci 272, Tatiana M Foroud 30,31, Peter T Fox 273, Masaki Fukunaga 274, Raphael Gibbs 251,269, Harald H H Göring 266, Robert C Green 275,276, Sebastian Guelfi 251, Narelle K Hansell 35, Catharina A Hartman 277, Katrin Hegenscheid 278, Andreas Heinz 9, Dena G Hernandez 251,269, Dirk J Heslenfeld 279, Pieter J Hoekstra 277, Florian Holsboer 252, Georg Homuth 280, Jouke-Jan Hottenga 241, Masashi Ikeda 127, Clifford R Jack Jr 281, Mark Jenkinson 282, Robert Johnson 283, Ryota Kanai 284,285, Maria Keil 24, Jack W Kent Jr 266, Peter Kochunov 286, John B Kwok 287,288, Stephen M Lawrie 16, Xinmin Liu 246,289, Dan L Longo 290, Katie L McMahon 291, Eva Meisenzahl 292, Ingrid Melle 23, Sebastian Mohnke 9, Grant W Montgomery 35, Jeanette C Mostert 2,4, Thomas W Mühleisen 212, Michael A Nalls 269, Thomas E Nichols 13,14, Lars G Nilsson 234, Markus M Nöthen 90,91, Kazutaka Ohi 293, Rene L Olvera 273, Rocio Perez-Iglesias 28,177, G Bruce Pike 294,295, Steven G Potkin 296, Ivar Reinvang 257, Simone Reppermund 25, Marcella Rietschel 109, Nina Romanczuk-Seiferth 9, Glenn D Rosen 297,298, Dan Rujescu 114,115, Knut Schnell 188, Peter R Schofield 287,288, Colin Smith 299, Vidar M Steen 242,243, Jessika E Sussmann 16, Anbupalam Thalamuthu 25, Arthur W Toga 300, Bryan Traynor 269, Juan Troncoso 301, Jessica A Turner 33,34, Maria C Valdés Hernández 270, Dennis van 't Ent 241, Marcel van der Brug 302, Nic J A van der Wee 303, Marie-Jose van Tol 304, Dick J Veltman 249, Thomas H Wassink 305, Eric Westman 306, Ronald H Zielke 283, Alan Zonderman 307, David G Ashbrook 308, Reinmar Hager 308, Lu Lu 309,310, Francis J McMahon 17, Derek W Morris 45,96, Robert W Williams 309,310, Han G Brunner 2,4, Randy L Buckner 62,63,64, Jan K Buitelaar 4,10, Wiepke Cahn 66, Vince D Calhoun 311,312, Gianpiero L Cavalleri 258, Benedicto Crespo-Facorro 85,86, Anders M Dale 313,314, Gareth E Davies 315, Norman Delanty 258,316, Chantal Depondt 317, Srdjan Djurovic 48,95, Wayne C Drevets 246,318, Thomas Espeseth 256,257, Randy L Gollub 63, Beng-Choon Ho 319, Wolfgang Hoffmann 231,255, Norbert Hosten 278, René S Kahn 66, Stephanie Le Hellard 242,243, Andreas Meyer-Lindenberg 18, Bertram Müller-Myhsok 162,163,164, Matthias Nauck 320, Lars Nyberg 234, Massimo Pandolfo 317, Brenda W J H Penninx 249, Joshua L Roffman 63,64, Sanjay M Sisodiya 261, Jordan W Smoller 8,11,12, Hans van Bokhoven 2,4, Neeltje E M van Haren 233, Henry Völzke 255, Henrik Walter 9, Michael W Weiner 321, Wei Wen 25, Tonya White 322,323, Ingrid Agartz 48,49,50, Ole A Andreassen 22,23, John Blangero 266, Dorret I Boomsma 241, Rachel M Brouwer 233, Dara M Cannon 246,324, Mark R Cookson 269, Eco J C de Geus 241, Ian J Deary 248, Gary Donohoe 45,96, Guillén Fernández 4,10, Simon E Fisher 4, Clyde Francks 4, David C Glahn 263,264, Hans J Grabe 232,325, Oliver Gruber 24, John Hardy 251, Ryota Hashimoto 326, Hilleke E Hulshoff Pol 233, Erik G Jönsson 48,50, Iwona Kloszewska 327, Simon Lovestone 184, Venkata S Mattay 195, Patrizia Mecocci 328, Colm McDonald 156, Andrew M McIntosh 16, Roel A Ophoff 66,73,112, Tomas Paus 329,330, Zdenka Pausova 238,331, Mina Ryten 250,251, Perminder S Sachdev 25,26, Andrew J Saykin 29,30,31, Andy Simmons 332,333,334, Andrew Singleton 269, Hilkka Soininen 335,336, Joanna M Wardlaw 235,237,248,270, Michael E Weale 250, Daniel R Weinberger 195,227, Hieab H H Adams 323,337, Lenore J Launer 338, Stephan Seiler 339, Reinhold Schmidt 339, Ganesh Chauhan 340, Claudia L Satizabal 341,342, James T Becker 343,344,345, Lisa Yanek 346, Sven J van der Lee 337, Maritza Ebling 347,348, Bruce Fischl 347,348, W T Longstreth 349, Douglas Greve 347,348, Helena Schmidt 350, Paul Nyquist 351, Louis N Vinke 347,348, Cornelia M van Duijn 337, Xue Luting 352, Bernard Mazoyer 353, Joshua C Bis 354, Vilmundur Gudnason 355, Sudha Seshadri 341,342, M Arfan Ikram 323,337, Nicholas G Martin 35, Margaret J Wright 35,36, Gunter Schumann 228, Barbara Franke 1,2,3,4, Paul M Thompson 5,38, Sarah E Medland 35,38
228 MRC-SGDP Centre, Institute of Psychiatry, King's College London, London, SE5 8AF, UK
229 Imaging Genetics Center, Institute for Neuroimaging & Informatics, Keck School of Medicine of the University of Southern California, Los Angeles, 90292, USA
230 Institut Pasteur, Paris, 75015, France
231 German Center for Neurodegenerative Diseases (DZNE) Rostock/Greifswald, Greifswald, 17487, Germany
232 Department of Psychiatry, University Medicine Greifswald, Greifswald, 17489, Germany
233 Brain Center Rudolf Magnus, Department of Psychiatry, UMC Utrecht, Utrecht, 3584, The Netherlands
234 Umeå Centre for Functional Brain Imaging (UFBI), Umeå University, Umeå, 901 87, Sweden
235 Brain Research Imaging Centre, University of Edinburgh, Edinburgh, EH4 2XU, UK
236 Department of Computer Science, Lagos State University, Lagos, Nigeria
237 Scottish Imaging Network, A Platform for Scientific Excellence (SINAPSE) Collaboration, Department of Neuroimaging Sciences, University of Edinburgh, Edinburgh, EH4 2XU, UK
238 Hospital for Sick Children, University of Toronto, Toronto, M5G 1X8, Canada
239 Cerebral Imaging Centre, Douglas Mental Health University Institute, Montreal, H4H 1R3, Canada
240 Department of Psychiatry and Biomedical Engineering, McGill University, Montreal, H3A 2B4, Canada
241 Biological Psychology, Neuroscience Campus Amsterdam, VU University & VU Medical Center, Amsterdam, 1081 BT, The Netherlands
242 NORMENT - KG Jebsen Centre for Psychosis Research, Department of Clinical Science, University of Bergen, 5021, Norway
243 Dr Einar Martens Research Group for Biological Psychiatry, Center for Medical Genetics and Molecular Medicine, Haukeland University Hospital, Bergen, 5021, Norway
244 Language and Genetics Department, Max Planck Institute for Psycholinguistics, Nijmegen, 6525 XD, The Netherlands
245 International Max Planck Research School for Language Sciences, Nijmegen, 6525 XD, The Netherlands
246 National Institute of Mental Health Intramural Research Program, Bethesda, 20892, USA
247 Center for Neurobehavioral Genetics, University of California, Los Angeles, California, 90095, USA
248 Centre for Cognitive Ageing and Cognitive Epidemiology, Psychology, University of Edinburgh, Edinburgh, EH8 9JZ, UK
249 Department of Psychiatry, Neuroscience Campus Amsterdam, VU University Medical Center, Amsterdam, 1007 MB, The Netherlands
250 Department of Medical and Molecular Genetics, King's College London, London, SE1 9RT, UK
251 Reta Lila Weston Institute and Department of Molecular Neuroscience, UCL Institute of Neurology, London, WC1N 3BG, UK
252 Max Planck Institute of Psychiatry, Munich, 80804, Germany
253 Multimodal Imaging Laboratory, Department of Neurosciences, University of California, San Diego, 92093, USA
254 Department of Cognitive Sciences, University of California, San Diego, 92161, USA
255 Institute for Community Medicine, University Medicine Greifswald, Greifswald, 17489, Germany
256 NORMENT - KG Jebsen Centre, Division of Mental Health and Addiction, Oslo University Hospital, Oslo, 0315, Norway
257 NORMENT - KG Jebsen Centre, Department of Psychology, University of Oslo, Oslo, 0373, Norway
258 Molecular and Cellular Therapeutics, The Royal College of Surgeons, Dublin, 2, Ireland
259 The Oxford Center for Functional MRI of the Brain, Nuffield Department of Clinical Neurosciences, Oxford University, Oxford, OX3 9DU, UK
260 Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montreal, H3A 2B4, Canada
261 UCL Institute of Neurology, London, United Kingdom and Epilepsy Society, WC1N 3BG, UK
262 Department of Medicine, Imperial College London, London, SW7 2AZ, UK
263 Department of Psychiatry, Yale University, New Haven, Connecticut, 06511, USA
264 Olin Neuropsychiatric Research Center, Hartford, Connecticut, 06114, USA
265 Department of Genetics, King Faisal Specialist Hospital and Research Centre, Riyadh, 12713, Saudi Arabia
266 Texas Biomedical Research Institute, San Antonio, Texas, 78227, USA
267 National Ageing Research Institute, Royal Melbourne Hospital, Melbourne, 3052, Australia
268 Academic Unit for Psychiatry of Old Age, University of Melbourne, 3101, Australia
269 Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, Maryland, 20892, USA
270 Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, EH4 2XU, UK
271 NI Vavilov Institute of General Genetics, Russian Academy of Sciences, Moscow, 119333, Russia
272 Clinical Research Branch, National Institute on Aging, Baltimore, Maryland, 20892, USA
273 University of Texas Health Science Center, San Antonio, 78229, USA
274 Biofunctional Imaging, Immunology Frontier Research Center, Osaka University, Osaka, 565-0871, Japan
275 Harvard Medical School, Cambridge, Massachusetts, 02115, USA
276 Division of Genetics, Department of Medicine, Brigham and Women's Hospital, Boston, Massachusetts, 02115, USA
277 Department of Psychiatry, University of Groningen, University Medical Center Groningen, Groningen, 9700, The Netherlands
278 Institute of Diagnostic Radiology and Neuroradiology, University Medicine Greifswald, Greifswald, 17489, Germany
279 Department of Psychology, VU University Amsterdam, Amsterdam, 1081 BT, The Netherlands
280 Interfaculty Institute for Genetics and Functional Genomics, University Medicine Greifswald, Greifswald, 17489, Germany
281 Radiology, Mayo Clinic, Rochester, Minesota?, 55905, USA
282 FMRIB Centre, University of Oxford, Oxford, OX3 9DU, UK
283 NICHD Brain and Tissue Bank for Developmental Disorders, University of Maryland Medical School, Baltimore, Maryland, 21201, USA
284 School of Psychology, University of Sussex, Brighton, BN1 9QH, UK
285 Institute of Cognitive Neuroscience, University College London, London, WC1N 3AR, UK
286 Department of Psychiatry, University of Maryland, Catonsville, Maryland, 21201, USA
287 Neuroscience Research Australia, Sydney, 2031, Australia
288 School of Medical Sciences, UNSW, Sydney, 2052, Australia
289 Columbia University Medical Center, New York, 10032, USA
290 Lymphocyte Cell Biology Unit, Laboratory of Immunology, National Institute on Aging, National Institutes of Health, Baltimore, Maryland, 21224, USA
291 Centre for Advanced Imaging, University of Queensland, Brisbane, 4072, Australia
292 Ludwig-Maximilians-Universität, Munich, 80539, Germany
293 Department of Psychiatry, Osaka University Graduate School of Medicine, Osaka, 565-0871, Japan
294 Department of Neurology, University of Calgary, Calgary, T2N 2T9, Canada
295 Department of Clinical Neuroscience, University of Calgary, Calgary, T2N 2T9, Canada
296 Psychiatry and Human Behavior, University of California, Irvine, California, 92697, USA
297 Beth Israel Deaconess Medical Center, Boston, Massachusetts, 02215, USA
298 Department of Neurology, Harvard Medical School, Boston, Massachusetts, 02115, USA
299 Department of Neuropathology, MRC Sudden Death Brain Bank Project, University of Edinburgh, Edinburgh, EH8 9AG, UK
300 Laboratory of Neuro Imaging, Institute for Neuroimaging and Informatics, Keck School of Medicine of the University of Southern California, Los Angeles, California, 90033, USA
301 Brain Resource Center, Johns Hopkins University, Baltimore, Maryland, 21287, USA
302 The Scripps Research Institute, Jupiter, Florida, 33458, USA
303 Leiden University Medical Center, Leiden, 2333 ZA, The Netherlands
304 Neuroimaging Centre, University of Groningen, University Medical Center Groningen, Groningen, 9713 AW, The Netherlands
305 Department of Psychiatry, Carver College of Medicine, University of Iowa, Iowa City, 52242, USA
306 Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, SE-141 83, Sweden
307 Research Resources Branch, National Institute on Aging, National Institutes of Health, Bethesda, Maryland, 20892, USA
308 Faculty of Life Sciences, University of Manchester, Manchester, M13 9PL, UK
309 Center for Integrative and Translational Genomics, University of Tennessee Health Science Center, Memphis, Tennessee, 38163, USA
310 Department of Anatomy and Neurobiology, University of Tennessee Health Science Center, Memphis, Tennessee, 38163, USA
311 The Mind Research Network & LBERI, Albuquerque, New Mexico, 87106, USA
312 Department of ECE, University of New Mexico, Albuquerque, New Mexico, 87131, USA
313 Center for Translational Imaging and Personalized Medicine, University of California, San Diego, 92093, California, USA
314 Departments of Neurosciences, Radiology, Psychiatry, and Cognitive Science, University of California, San Diego, 92093, California, USA
315 Avera Institute for Human Genetics, Sioux Falls, 57108, USA
316 Neurology Division, Beaumont Hospital, Dublin, 9, Ireland
317 Department of Neurology, Hopital Erasme, Universite Libre de Bruxelles, Brussels, 1070, Belgium
318 Janssen Research & Development, Johnson & Johnson, New Jersey, 08560, USA
319 Department of Psychiatry, University of Iowa, Iowa City, 52242, USA
320 Institute of Clinical Chemistry and Laboratory Medicine, University Medicine Greifswald, Greifswald, 17489, Germany
321 Center for Imaging of Neurodegenerative Disease, San Francisco VA Medical Center, University of California, San Francisco, 94121, USA
322 Department of Child Psychiatry, Erasmus University Medical Centre, Rotterdam, 3015 CE, The Netherlands
323 Department of Radiology, Erasmus University Medical Centre, Rotterdam, 3015 CE, The Netherlands
324 Clinical Neuroimaging Laboratory, College of Medicine, Nursing and Health Sciences, National University of Ireland Galway, Galway, SW4 794, Ireland
325 Department of Psychiatry and Psychotherapy, HELIOS Hospital Stralsund, 18435, Germany
326 Molecular Research Center for Children's Mental Development, United Graduate School of Child Development, Osaka University, Osaka, 565-0871, Japan
327 Medical University of Lodz, Lodz, 90-419, Poland
328 Section of Gerontology and Geriatrics, Department of Medicine, University of Perugia, Perugia, 06123, Italy
329 Rotman Research Institute, University of Toronto, Toronto, M6A 2E1, Canada
330 Departments of Psychology and Psychiatry, University of Toronto, M5T 1R8, Canada
331 Departments of Physiology and Nutritional Sciences, University of Toronto, M5S 3E2, Canada
332 Department of Neuroimaging, Institute of Psychiatry, King's College London, London, SE5 8AF, UK
333 Biomedical Research Centre for Mental Health, King's College London, London, SE5 8AF, UK
334 Biomedical Research Unit for Dementia, King's College London, London, SE5 8AF, UK
335 Institute of Clinical Medicine, Neurology, University of Eastern Finland, Kuopio, FI-70211, Finland
336 Neurocentre Neurology, Kuopio University Hospital, FI-70211, Finland
337 Department of Epidemiology, Erasmus University Medical Centre, Rotterdam, 3015 GE, The Netherlands
338 Laboratory of Epidemiology and Population Sciences, Intramural Research Program, National Institute on Aging, Bethesda, Maryland, 20892, USA
339 Department of Neurology, Clinical Division of Neurogeriatrics, Medical University Graz, Graz, 8036, Austria
340 INSERM U897, University of Bordeaux, 33076, France
341 Department of Neurology, Boston University School of Medicine, Boston, Massachusetts, 02118, USA
342 Framingham Heart Study, Framingham, 01702, USA
343 Department of Neurology, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, 15213, USA
344 Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, 15213, USA
345 Department of Psychology, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, 15213, USA
346 General Internal Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, 21205, USA
347 Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, 02129, USA
348 Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, 02114, USA
349 Department of Neurology University of Washington, Seattle, 98104, USA
350 Institute of Molecular Biology and Biochemistry, Medical University Graz, 8036, Austria
351 Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland, 21205, USA
352 Department of Biostatistics, Boston University School of Public Health, Boston, Massachuestts, 02118, USA
353 UMR5296 CNRS, CEA and University of Bordeaux, Bordeaux, 33076, France
354 Cardiovascular Health Research Unit, Department of Medicine, University of Washington, Seattle, 98101, USA
355 Icelandic Heart Association, Kopavogur, University of Iceland, Faculty of Medicine, Reykjavik, 101, Iceland
Footnotes
URLs
Psychiatric Genomics Consortium (http://pgc.unc.edu); ENIGMA (http://enigma.ini.usc.edu); 1000 Genomes Project imputation panel (http://mathgen.stats.ox.ac.uk/impute); matSpD interface (http://genepi.qimr.edu.au/general/daleN/matSpD).
Conflicts of Interest
Several of the authors/contributors are employees of companies: Johnson and Johnson, Pfizer (C.R.S., J.R.W., H.S.X), F. Hoffman-La Roche (E.D., L.E), Eli Lilly (D.C., Y.M., L.N), Janssen (S.G., D.W., Q.S.L.), and deCODE genetics (S.G, K.S., H.S.). P.F.S is a scientific advisor to Pfizer. None of these companies influenced the design of the study, the interpretation of the data, the amount of data reported, or financially profit by publication of these pre-competitive results. The other authors do not report conflicts of interest.
References
- 1.Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nature Reviews Genetics. 2012;13:537–51. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purcell SM, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–90. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Network & Pathway Analysis Subgroup of Psychiatric Genomics, C. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18:199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Erp TG, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haijma SV, et al. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–38. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 8.Thermenos HW, et al. A review of neuroimaging studies of young relatives of individuals with schizophrenia: a developmental perspective from schizotaxia to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:604–35. doi: 10.1002/ajmg.b.32170. [DOI] [PubMed] [Google Scholar]
- 9.Hibar DP, et al. Common genetic variants influence human subcortical brain structures. Nature. 2015 doi: 10.1038/nature14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blokland GA, de Zubicaray GI, McMahon KL, Wright MJ. Genetic and environmental influences on neuroimaging phenotypes: a meta-analytical perspective on twin imaging studies. Twin Res Hum Genet. 2012;15:351–71. doi: 10.1017/thg.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefansson H, et al. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2014;505:361–6. doi: 10.1038/nature12818. [DOI] [PubMed] [Google Scholar]
- 12.Bulik-Sullivan BK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nature Genetics. 2015;47:291–5. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross-Disorder Group of the Psychiatric Genomics Consortium Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature genetics. 2013;45:984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulik-Sullivan BK, et al. An atlas of genetic correlations across human diseases and traits. Nature Genetics. 2015 doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Schizophrenia Consortium Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cross-Disorder Group of the Psychiatric Genomics Consortium Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–9. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plaisier SB, Taschereau R, Wong JA, Graeber TG. Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 2010;38:e169. doi: 10.1093/nar/gkq636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho YYW. Variants within ADAMTS9-AS2 influence whorls in fingerprint patterns. Submitted. [Google Scholar]
- 19.Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Rose EJ, Donohoe G. Brain vs behavior: an effect size comparison of neuroimaging and cognitive studies of genetic risk for schizophrenia. Schizophr Bull. 2013;39:518–26. doi: 10.1093/schbul/sbs056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. 2010;15:918–27. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- 22.Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–70. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- 23.Witte JS, Visscher PM, Wray NR. The contribution of genetic variants to disease depends on the ruler. Nat Rev Genet. 2014;15:765–76. doi: 10.1038/nrg3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood AR, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46:1173–86. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toulopoulou T, et al. Reciprocal causation models of cognitive vs volumetric cerebral intermediate phenotypes for schizophrenia in a pan-European twin cohort. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.152. [DOI] [PubMed] [Google Scholar]
- 26.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–22. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narr KL, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–19. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- 28.Weinberger DR. On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology. 1996;14:1S–11S. doi: 10.1016/0893-133X(95)00199-N. [DOI] [PubMed] [Google Scholar]
- 29.Visscher PM, Brown MA, McCarthy MI, Yang J. Five Years of GWAS Discovery. American journal of human genetics. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge T, et al. Massively expedited genome-wide heritability analysis (MEGHA). Proc Natl Acad Sci U S A. 2015;112:2479–84. doi: 10.1073/pnas.1415603112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 32.Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Mol Psychiatry. 2010;15:789–97. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annual Review of Clinical Psychology. 2006;2:267–90. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- 34.Fuchsberger C, Abecasis GR, Hinds DA. minimac2: faster genotype imputation. Bioinformatics. 2015;31:782–4. doi: 10.1093/bioinformatics/btu704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng X, Xu J, Wang C. Improving the power for detecting overlapping genes from multiple DNA microarray-derived gene lists. BMC Bioinformatics. 2008;9(Suppl 6):S14. doi: 10.1186/1471-2105-9-S6-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storey JD. A direct approach to false discovery rates. Journal of the Royal Statistical Society: Seried B (Statistical Methodology) 2002;63:479–98. [Google Scholar]
- 38.Rietveld CA, et al. GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science. 2013;340:1467–71. doi: 10.1126/science.1235488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.