Abstract
Background
Hürthle cell neoplasms (HCNs) are rare tumors of the thyroid gland. The definitive treatment for Hürthle cell carcinoma (HCC) is total thyroidectomy, while thyroid lobectomy is adequate for Hürthle cell adenoma (HCA). However, differentiating HCC from HCA either before or during surgery is a challenge. The purpose of this study was to identify factors that predict malignancy in patients with HCN.
Methods
Between May 1994 and January 2007, 1,199 patients underwent thyroid surgery at an academic medical center. Medical records of 55 consecutive patients who underwent thyroid resections for the preoperative diagnosis of HCN were reviewed.
Results
Of the 55 patients with HCN, 46 (84%) had adenomas and 9 (16%) had carcinomas. Patients with HCC were significantly older than those with HCA (66 ± 6 years versus 53 ± 2 years, P = 0.01). Patients with carcinoma also had significantly larger thyroid nodules (4.5 ± 0.7 cm versus 2.5 ± 0.2 cm, P < 0.001). All HCNs less than 2 cm in diameter were benign. The malignancy rate increased with nodule size: 18% of nodules measuring 2–4 cm, and 44% of those larger than 4 cm were HCC. One patient with HCC had recurrence of the disease, but there were no disease-related deaths.
Conclusion
Advanced patient age and larger nodule size are two important factors that predict malignancy in patients with HCN. In patients with these and other known risk factors for HCC, total thyroidectomy should be considered.
Keywords: Hürthle cell neoplasms, Hürthle cell carcinoma, Hürthle cell adenoma, Malignant thyroid neoplasms, Thyroid surgery, Fine-needle aspiration biopsy
Hürthle cells, also known as oncocytic or oxyphilic cells, are large polygonal cells containing granular cytoplasm and pleomorphic nuclei that occur in the thyroid gland in association with Hashimoto’s thyroiditis, nodular hyperplasia, Graves’ disease, and other thyroid conditions.1 Hürthle cell neoplasms (HCNs) are defined as encapsulated thyroid lesions consisting of at least 50% Hürthle cells with minimal or absent colloid.1,2
HCNs are subdivided into benign Hürthle cell adenomas (HCAs) and malignant Hürthle cell carcinomas (HCCs). Many experienced surgeons recommend total thyroidectomy for the treatment of HCC, while thyroid lobectomy is considered adequate for HCA.3,4 However, discriminating between HCC and HCA either before or during surgery is a challenge. The diagnosis of HCC is made upon histopathological examination of the surgical specimen, when tumor cells are seen to invade the thyroid capsule, blood vessels, or adjacent tissues, or when metastases are found in lymph nodes or distant organs. Fine-needle aspiration biopsy (FNAB) can establish the presence of a HCN.5 However, neither FNAB nor frozen-section analysis is a reliable technique for the differentiation of HCC and HCA.2,4–8 Therefore, most patients with HCNs are treated with diagnostic lobectomy as the initial operation.
The purpose of this study was to determine if any pre- or intraoperative factors are predictive of malignancy in patients with HCNs. Identification of such factors might help guide the selection of the initial surgical procedure in the management of patients with this disease.
METHODS
From May 1994 to January 2007, 1,198 patients underwent thyroid surgery at the University of Wisconsin, and 55 patients received the final histopathological diagnosis of HCC or HCA. For these 55 patients with HCN, a retrospective medical record review was performed to collect data on patient demographics, clinical features, management including diagnostic and therapeutic procedures, results of FNA and frozen-section biopsies, and tumor characteristics.
All FNAB and other cytopathology reports were reviewed. A HCN was defined as a lesion with scant colloid consisting predominantly (>50%) of Hürthle cells. Cytopathology and final pathology results were compared to calculate the true-positive and false-negative rates of FNAB for HCN.
Pathology reports were reviewed to confirm the diagnosis of HCC or HCA. Any lesion with evidence of capsular or vascular invasion was considered malignant, unless the focus of invasion was deemed by the pathologist to be an artifact from prior FNAB. Tumor size was obtained from the gross pathology report. Secondary pathologic diagnoses, including incidental papillary thyroid carcinomas, were recorded but not included in calculations of the HCN malignancy rate.
Statistical analysis was performed using SPSS (SPSS, Inc., Chicago, Illinois) and Stata (StataCorp, College Station, Texas) statistical software to identify variables associated with the diagnosis of HCC. The independent-samples Student’s t-test was used to analyze continuous data and the chi-squared test was used to analyze categorical data. Statistical significance was defined as a P-value of <0.05. We also calculated the Pearson product moment correlation coefficient for thyroid nodule sizes measured by preoperative ultrasound and surgical pathology examination.
RESULTS
Patient Diagnoses and Demographics
After undergoing thyroid resection, 46 patients received the final diagnosis of HCA, and nine the diagnosis of HCC. Therefore, 16% of the patients in this sample had HCC. Patients with HCC were significantly older than those with HCA (66 ± 6 versus 53 ± 2 years, P = 0.014). Seventy-four percent of the patients with HCA were female, compared with 56% of the HCC patients. This difference in gender between the two groups was not statistically significant (Table 1). No patient in this series had a history of radiation exposure.
TABLE 1.
Patient demographics
| Adenomas | Carcinomas | P-value | |
|---|---|---|---|
| n | 46 | 9 | |
| Age (mean ± SEM) | 53 ± 2 | 66 ± 6 | 0.014 |
| Sex | |||
| Male | 12 (26%) | 4 (44%) | 0.234 |
| Female | 34 (74%) | 5 (55%) |
Diagnostic Studies: FNAB and Frozen-Section Analysis
Previous studies have demonstrated that FNAB is a reliable technique for the identification of HCNs.5 We reviewed the medical records of the 55 patients with HCNs to determine the utilization and accuracy of FNAB in this cohort. FNAB was performed in 47 (85%) of the 55 patients with HCNs. One FNAB was deemed to be nondiagnostic while all others were considered adequate for analysis. Of the 46 adequate FNAB specimens, 41 (89%) were reported as HCNs. The FNAB test results for these 41 patients with HCNs were considered to be true positives. The FNAB results were classified as false negatives in the remaining five (11%) patients. In the five patients with false-negative FNABs, four of the FNAB were categorized as benign lesions without Hürthle cells and the other one was reported as suspicious for follicular carcinoma. After resection the final pathologic diagnosis for these five patients was HCA.
Of the 55 patients with HCNs on final pathology, 30 (55%) underwent intraoperative frozen-section analysis. The frozen-section report mentioned the presence of Hürthle cells in 17 patients (57% of those who had frozen sections). In no case did the results of the frozen-section analysis alter the planned extent of thyroid resection (e.g., from lobectomy to total thyroidectomy), and in no case was the diagnosis of Hürthle cell cancer made on the basis of frozen section.
Patient Management and Clinical Outcomes
The surgical management of the patients with HCNs is shown in Table 2. Of the 46 patients with HCA, 42 (92%) underwent thyroid lobectomy and 4 (8%) had total thyroidectomy. There were no perioperative complications. As one would expect for a benign diagnosis, no patient with an adenoma was treated with radioactive iodine therapy, and there were no recurrences of HCA.
TABLE 2.
Patient management
| Adenomas | Carcinomas | |
|---|---|---|
| n | 46 | 9 |
| Operation | ||
| Thyroid lobectomy | 42 (91%) | 1 (11%) |
| Total thyroidectomy | 4 (9%) | 8 (89%) |
| Initial | 4 | 2 |
| Completion | 0 | 6 |
| Radioactive iodine treatment | 0 (0%) | 6 (67%) |
| Tumor recurrence | 0/46 | 1/9 (11%) |
| Disease-related mortality | 0/46 | 0/9 |
Of the nine patients with HCC, only one had a thyroid lobectomy. This patient received thyroid lobe resection for the preoperative diagnosis of HCN, and HCC measuring 3.3 cm in diameter with “minimal vascular invasion” was discovered upon pathologic examination of the resected lobe. After a discussion of the options, the patient elected to pursue a strategy of watchful waiting rather than undergo completion thyroidectomy. The remaining eight patients underwent total thyroidectomy, with two undergoing initial total thyroid resection and the other six having a completion thyroidectomy after initial lobectomy. There were no perioperative complications in the patients with HCC. Five patients with HCC underwent postoperative I-131 scanning, and there was positive uptake in three cases. The serum thyroglobulin levels in these patients with positive I-131 scans were 150.9 and 32.5 ng/mL; in one patient the thyroglobulin level was not recorded. Six of the nine patients with HCC (67%) underwent remnant ablation with radioactive iodine following surgery.
After a median follow-up time of 10 months (range 2–143 months), there was only one case of recurrent HCC. This patient was diagnosed with multifocal Hürthle cell carcinoma in the left thyroid lobe, and underwent total thyroidectomy. Three years later, a nodule was found between the trachea and the left carotid artery, and this recurrent HCC lesion was resected. The cancer-specific mortality in this series was zero.
Tumor Characteristics
Tumor size was not recorded for five of the 55 patients with HCNs. In the remaining 50 patients, HCCs were significantly larger than HCAs (mean tumor diameter 4.50 ± 0.71 cm versus 2.46 ± 0.20 cm; P < 0.001). The relationship between tumor size and the rate of malignancy is displayed in Fig. 1. Interestingly, no tumors measuring 2 cm or less in diameter (n = 19) were malignant. Eighteen percent of tumors that were between 2 cm and 4 cm in diameter (n = 22) were found to be HCC. The rate of malignancy increased to 44% for tumors larger than 4 cm (n = 9).
FIG. 1.
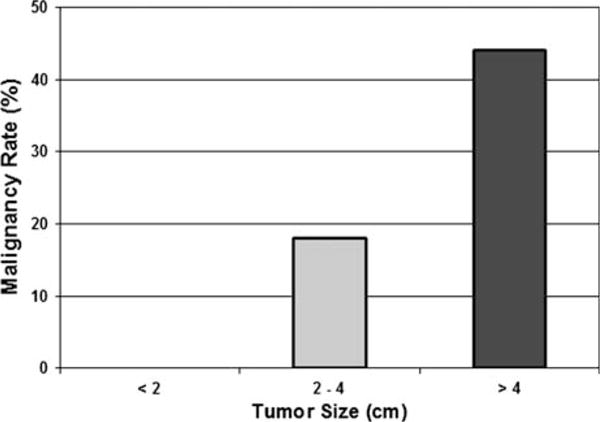
Tumor size is predictive of malignancy in Hürthle cell neoplasms. Larger tumor diameter was associated with an increased rate of malignancy.
Twelve patients had nodule sizes recorded by preoperative thyroid ultrasound. The Pearson correlation coefficient for nodule size measured by preoperative ultrasound and postoperative surgical pathology examination was 0.76, indicating a strong positive correlation.
Multiple thyroid nodules were noted in 32% of patients with HCA and 55% with HCC. This difference in the prevalence of multiple nodules was not significant. Two patients in the HCA group had papillary thyroid cancer (PTC), while none in the HCC group had a concomitant PTC. This difference was also not statistically significant.
DISCUSSION
Hürthle cells are large polygonal cells with hyperchromatic nuclei and abundant mitochondria resulting in fine, granular, eosinophilic cytoplasm. When Hürthle cells comprise more than 50% of an encapsulated nodule, the lesion is termed a Hürthle cell neoplasm. Hürthle cell neoplasms are either benign Hürthle cell adenomas or malignant Hürthle cell carcinomas. In this study we describe a series of 55 patients with HCN, including nine patients with HCC, yielding a malignancy rate of 16%. The prevalence of HCC in patients with HCN in reports from other institutions has ranged from 4% to 69%.2–4,9–13 This broad range likely reflects differences in patient referral patterns.
Compared to papillary and follicular thyroid carcinoma, HCC is thought to be characterized by higher rates of recurrence, metastasis, and cancer-related mortality.1,12,14 In the current series, there was only one case of tumor recurrence, and no patient with HCC has died. These favorable outcomes in surgically treated HCC patients are consistent with the experience of other endocrine surgery centers.2
The majority of the patients with HCC in this series received total thyroidectomy followed by radioactive iodine (RAI) therapy. Compared with thyroid lobectomy, total thyroidectomy offers several advantages for the treatment of HCC. These include a decreased risk of local recurrence, and the facilitation of RAI therapy. HCC generally has a lower affinity for RAI than other types of well-differentiated thyroid cancer.14–16 In the absence of contraindications, however, total thyroidectomy followed by RAI is a reasonable approach to the initial management of HCC, with the goal of eliminating all gross and microscopic disease. After total thyroidectomy and RAI remnant ablation, serum thyroglobulin may be measured to detect recurrent disease.
Many thyroid surgeons utilize FNAB for preoperative diagnosis. In this series of patients with final pathology proven HCNs, the true-positive and false-negative rates of FNAB were 89% and 11%, respectively. While FNAB is incapable of distinguishing HCC from HCA, the results show that FNAB is valuable in identifying Hürthle cell neoplasms. We recommend the routine use of FNABs for preoperative diagnosis in all patients with thyroid nodules. In our experience, the routine use of FNAB allows the planning of definitive initial surgery and the avoidance of unnecessary procedures.5
The majority of the patients in this series also had frozen-section analysis performed intraoperatively. Due to the retrospective nature of the study and incomplete documentation, it was not possible to determine the motive for frozen section in each case. Importantly, in no instance did the result of the frozen section alter the planned operation. We conclude that it is unnecessary to perform routine frozen-section analysis during thyroid resection for HCN. This is consistent with the findings of previous studies which found that routine frozen-section analysis in patients with follicular thyroid lesions had low sensitivity and poor cost effectiveness.6,17
While FNAB and frozen-section analysis are inadequate for the purpose of differentiating HCC from HCA, we hypothesized that other patient and tumor-related factors might be more informative. Some investigators have noted an association between older age and HCC,11,12,14 while others have not.2,4,10 In the current study, patients with HCC were more than a decade older than those with HCA, and this difference was statistically significant. Authors have also reported an association between a history of prior head and neck irradiation and HCC.18 We were unable to examine this relationship because none of the patients in our series had a history of radiation exposure or treatment.
Whether or not tumor size is predictive of malignancy in HCN is a subject of controversy. Several authors have observed an association between tumor size and the rate of cancer,2,4,11,19–26 while others have not found this association to be significant.27–31 In our series, on average, HCCs were significantly larger than HCAs (4.50 cm versus 2.46 cm). Strikingly, in HCNs measuring 2 cm or less in diameter, the malignancy rate was zero. In contrast, in tumors larger than 4 cm, the cancer rate was 44%.
We found a strong positive correlation between nodule size as measured by preoperative ultrasound and the actual tumor size in the resected specimen. Therefore, because of the strong association between tumor size and malignancy, and the accuracy of measuring nodule size by ultrasound, we recommend that sonographic thyroid examination should be done routinely in patients with suspected HCN.
An understanding of the molecular biology of HCNs may lead to the development of new methods for reliably diagnosing HCC in the preoperative setting. Research has shown that Ki67, a marker of cell proliferation, and cyclin D1, a cell cycle promoter, are expressed at higher levels in HCC relative to HCA. Conversely, Bcl-2, an antiapoptotic protein, is downregulated in HCC.32–34 Other investigators have used gene microarray technology to look for differences in gene expression between HCA and HCC.35 In the future, it may be possible for physicians to diagnose HCC based on molecular profiling of FNAB cytology samples.
In the absence of a reliable preoperative diagnostic test to differentiate HCC from HCA, surgeons and patients should consider the presence or absence of risk factors for cancer when deciding upon the initial surgical treatment for HCN. In the current study, advanced patient age and large tumor size were strongly associated with malignancy. An important finding is that no HCN measuring 2 cm or less in diameter was malignant. For patients with tumors less than 2 cm, we recommend thyroid lobectomy. Almost half of HCNs larger than 4 cm were malignant. Advanced patient age was also predictive of cancer. Therefore we recommend total thyroidectomy as the initial surgical treatment for patients with HCNs larger than 4 cm, as well as for patients over the age of 65 years with tumors larger than 2 cm.
References
- 1.Chen H, Udelsman R. Hurthle cell adenoma, carcinoma. In: Clark OH, Duh QY, editors. Textbook of endocrine surgery. 2nd. W. B. Saunders; Philadelphia PA: 2005. pp. 123–8. [Google Scholar]
- 2.Chen H, Nicol TL, Zeiger MA, et al. Hurthle cell neoplasms of the thyroid: are there factors predictive of malignancy? Ann Surg. 1998;227:542–6. doi: 10.1097/00000658-199804000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gosain AK, Clark OH. Hurthle cell neoplasms. Arch Surg. 1984;119:515–9. doi: 10.1001/archsurg.1984.01390170015004. [DOI] [PubMed] [Google Scholar]
- 4.Azadian A, Rosen IB, Walfish PG, et al. Management considerations in Hurthle cell carcinoma. Surgery. 1995;118:711–4. doi: 10.1016/s0039-6060(05)80039-x. discussion 714–5. [DOI] [PubMed] [Google Scholar]
- 5.Greenblatt DY, Woltman T, Harter J, et al. Fine-needle aspiration optimizes surgical management in patients with thyroid cancer. Ann Surg Oncol. 2006;13:859–63. doi: 10.1245/ASO.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Nicol TL, Udelsman R. Follicular lesions of the thyroid. does frozen section evaluation alter operative management? Ann Surg. 1995;222:101–6. doi: 10.1097/00000658-199507000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McHenry CR, Sandoval BA. Management of follicular and hurthle cell neoplasms of the thyroid gland. Surg Oncol Clin North Am. 1998;7:893–910. [PubMed] [Google Scholar]
- 8.Elliott DD, Pitman MB, Bloom L, et al. Fine-needle aspiration biopsy of hurthle cell lesions of the thyroid gland: a cytomorphologic study of 139 cases with statistical analysis. Cancer. 2006;108:102–9. doi: 10.1002/cncr.21716. [DOI] [PubMed] [Google Scholar]
- 9.Rosen IB, Luk S, Katz I. Hurthle cell tumor behavior: dilemma and resolution. Surgery. 1985;98:777–83. [PubMed] [Google Scholar]
- 10.Arganini M, Behar R, Wu TC, et al. Hurthle cell tumors: a twenty-five-year experience. Surgery. 1986;100:1108–15. [PubMed] [Google Scholar]
- 11.Carcangiu ML, Bianchi S, Savino D, et al. Follicular Hurthle cell tumors of the thyroid gland. Cancer. 1991;68:1944–53. doi: 10.1002/1097-0142(19911101)68:9<1944::aid-cncr2820680917>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.McDonald MP, Sanders LE, Silverman ML, et al. Hurthle cell carcinoma of the thyroid gland: prognostic factors and results of surgical treatment. Surgery. 1996;120:1000–4. doi: 10.1016/s0039-6060(96)80046-8. discussion 1004–5. [DOI] [PubMed] [Google Scholar]
- 13.Besic N, Hocevar M, Zgajnar J, et al. Aggressiveness of therapy and prognosis of patients with Hurthle cell papillary thyroid carcinoma. Thyroid. 2006;16:67–72. doi: 10.1089/thy.2006.16.67. [DOI] [PubMed] [Google Scholar]
- 14.Sanders LE, Silverman M. Follicular and Hurthle cell carcinoma: predicting outcome and directing therapy. Surgery. 1998;124:967–74. [PubMed] [Google Scholar]
- 15.Watson RG, Brennan MD, Goellner JR, et al. Invasive Hurthle cell carcinoma of the thyroid: natural history and management. Mayo Clin Proc. 1984;59:851–5. doi: 10.1016/s0025-6196(12)65621-3. [DOI] [PubMed] [Google Scholar]
- 16.Besic N, Vidergar-Kralj B, Frkovic-Grazio S, et al. The role of radioactive iodine in the treatment of Hurthle cell carcinoma of the thyroid. Thyroid. 2003;13:577–84. doi: 10.1089/105072503322238845. [DOI] [PubMed] [Google Scholar]
- 17.Callcut RA, Selvaggi SM, Mack E, et al. The utility of frozen section evaluation for follicular thyroid lesions. Ann Surg Oncol. 2004;11:94–8. doi: 10.1007/BF02524352. [DOI] [PubMed] [Google Scholar]
- 18.Ron E, Modan B, Preston D, et al. Thyroid neoplasia following low-dose radiation in childhood. Radiat Res. 1989;120:516–31. [PubMed] [Google Scholar]
- 19.Thompson NW, Dunn EL, Batsakis JG, et al. Hurthle cell lesions of the thyroid gland. Surg Gynecol Obstet. 1974;139:555–60. [PubMed] [Google Scholar]
- 20.Johnson TL, Lloyd RV, Burney RE, et al. Hurthle cell thyroid tumors an immunohistochemical study. Cancer. 1987;59:107–12. doi: 10.1002/1097-0142(19870101)59:1<107::aid-cncr2820590123>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Taneri F, Tekin E, Salman B, et al. Huerthle cell neoplasms of the thyroid: predicting malignant potential. Endocr Regul. 2000;34:19–21. [PubMed] [Google Scholar]
- 22.Lopez-Penabad L, Chiu AC, Hoff AO, et al. Prognostic factors in patients with Hurthle cell neoplasms of the thyroid. Cancer. 2003;97:1186–94. doi: 10.1002/cncr.11176. [DOI] [PubMed] [Google Scholar]
- 23.Giorgadze T, Rossi ED, Fadda G, et al. Does the fine-needle aspiration diagnosis of “Hurthle-cell neoplasm/follicular neoplasm with oncocytic features” denote increased risk of malignancy? Diagn Cytopathol. 2004;31:307–12. doi: 10.1002/dc.20132. [DOI] [PubMed] [Google Scholar]
- 24.Pisanu A, Sias L, Uccheddu A. Factors predicting malignancy of Hurthle cell tumors of the thyroid: influence on surgical treatment. World J Surg. 2004;28:761–5. doi: 10.1007/s00268-004-7312-9. [DOI] [PubMed] [Google Scholar]
- 25.Melck A, Bugis S, Baliski C, et al. Hemithyroidectomy: the preferred initial surgical approach for management of Hurthle cell neoplasm. Am J Surg. 2006;191:593–7. doi: 10.1016/j.amjsurg.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Paunovic I, Krgovic K, Tatic S, et al. Surgery for thyroid Hurthle cell tumours–a single institution experience. Eur J Surg Oncol. 2006;32:458–61. doi: 10.1016/j.ejso.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Gauger PG, Reeve TS, Delbridge LW. Intraoperative decision making in follicular lesions of the thyroid: is tumor size important? J Am Coll Surg. 1999;189:253–8. doi: 10.1016/s1072-7515(99)00134-9. [DOI] [PubMed] [Google Scholar]
- 28.McHenry CR, Thomas SR, Slusarczyk SJ, et al. Follicular or Hurthle cell neoplasm of the thyroid: can clinical factors be used to predict carcinoma and determine extent of thyroidectomy? Surgery. 1999;126:798–802. discussion 802–4. [PubMed] [Google Scholar]
- 29.Sugino K, Ito K, Mimura T, et al. Hurthle cell tumor of the thyroid: analysis of 188 cases. World J Surg. 2001;25:1160–3. doi: 10.1007/BF03215865. [DOI] [PubMed] [Google Scholar]
- 30.Chao TC, Lin JD, Chen MF. Surgical treatment of Hurthle cell tumors of the thyroid. World J Surg. 2005;29:164–8. doi: 10.1007/s00268-004-7669-9. [DOI] [PubMed] [Google Scholar]
- 31.Alaedeen DI, Khiyami A, McHenry CR. Fine-needle aspiration biopsy specimen with a predominance of Hurthle cells: a dilemma in the management of nodular thyroid disease. Surgery. 2005;138:650–6. doi: 10.1016/j.surg.2005.06.047. discussion 656–7. [DOI] [PubMed] [Google Scholar]
- 32.Papotti M, Torchio B, Grassi L, et al. Poorly differentiated oxyphilic (Hurthle cell) carcinomas of the thyroid. Am J Surg Pathol. 1996;20:686–94. doi: 10.1097/00000478-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Erickson LA, Jin L, Goellner JR, et al. Pathologic features, proliferative activity, and cyclin D1 expression in Hurthle cell neoplasms of the thyroid. Mod Pathol. 2000;13:186–92. doi: 10.1038/modpathol.3880034. [DOI] [PubMed] [Google Scholar]
- 34.Hoos A, Stojadinovic A, Singh B, et al. Clinical significance of molecular expression profiles of Hurthle cell tumors of the thyroid gland analyzed via tissue microarrays. Am J Pathol. 2002;160:175–83. doi: 10.1016/S0002-9440(10)64361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finley DJ, Zhu B, Fahey TJ., 3rd Molecular analysis of Hurthle cell neoplasms by gene profiling. Surgery. 2004;136:1160–8. doi: 10.1016/j.surg.2004.05.061. [DOI] [PubMed] [Google Scholar]


