Summary
Background
It has previously been shown that higher serum TSH is associated with increased thyroid cancer incidence and advanced-stage disease. In the healthy adult population, mean TSH increases with age. As age over 45 years is a known prognostic indicator for thyroid cancer, it is important to know whether higher TSH in patients with thyroid cancer occurs independent of age.
Objective
To determine the relationship between higher TSH, cancer and age.
Design
A retrospective cohort study.
Patients and methods
A total of 1361 patients underwent thyroid surgery between May 1994 and December 2007 at a single institution. Of these patients, 954 had pathological data, pre-operative TSH and complete surgical history available. Data were analysed in relation to age and TSH.
Results
Mean TSH was significantly higher in cancer patients regardless of age < 45 years or ≥ 45 years (P = 0·046 and P = 0·027, respectively). When examining age groups < 20, 20–44, 45–59 and ≥ 60 years, there was a trend of rising mean TSH with age. Despite the rise in the benign subgroups, mean TSH was consistently higher in those with cancer vs. those without. On multivariate analysis, higher TSH was independently associated with cancer (P = 0·039) and pathological features of Hashimoto’s thyroiditis (P=0·001) but not with age (P = 0·557). On multivariate analysis of high-risk features associated with poor prognosis, there was a significant association between higher TSH and extrathyroidal extension (P = 0·002), whereas there was no clear relationship with age, tumour size > 4 cm, and distant metastases.
Conclusion
Independent of age, thyroid cancer incidence correlates with higher TSH. Higher TSH is associated with extrathyroidal extension of disease.
Introduction
An association between higher serum TSH and both thyroid cancer incidence and advanced-stage disease was recently revealed.1,2 Although the relationship between higher TSH and thyroid cancer seems plausible given the role of TSH as a growth factor, the potential that cancer is a confounder in an alternative relationship with high TSH has not been ruled out. In addition, an explanation for the association between higher TSH and aggressive disease has not been provided.
Unique among malignancies, age is an important prognostic indicator in staging systems for differentiated thyroid cancer (DTC).3,4 Survival has a weak dependence on age when the cancer is localized.5,6 By contrast, patients with regional or distant metastases have a survival rate that correlates strongly with age.5–9 The association between age and survival is not dependent on different stage at diagnosis, tumour differentiation, treatment or socioeconomic variables.5–9
Our previously published data suggest that higher TSH is associated with advanced-stage thyroid cancer.1 Staging of thyroid cancer is heavily dependent on age as patients aged less than 45 years can have distant metastases and a maximum TNM stage of II. By contrast, tumour size, extrathyroidal extension, local lymph-node metastases and distant metastases play a role in determining stage I–IV disease in those patients aged over 45 years.4 Our previous analysis did not clarify whether TSH correlates with cancer stage independent of age. Furthermore, if age is not the reason for higher TSH in advanced-stage patients, it is unclear which high-risk cancer features (extrathyroidal extension, tumour size > 4 cm, or distant metastases) correlate with higher TSH.
In addition, the previous finding of higher TSH and increased thyroid cancer incidence1,2 could also be explained by age. Given that there is a rise in TSH with age in the healthy adult population10,11 and an increased incidence of microcarcinomas in patients over age 45,12 it is not certain whether the correlation between higher TSH and thyroid cancer is secondary to advanced age or TSH itself. In this study, we examined the relationship between TSH, age and high-risk cancer features with the aim of clarifying the relationship between higher TSH and thyroid cancer.
Subjects and methods
Between May 1994 and December 2007, 1361 patients underwent thyroid surgery at our institution. Institutional Review Board (IRB) approval was obtained and demographic and pathological data from 980 patients with preoperative TSH were collected. Eight patients who did not have records from their first surgery available and 18 patients who had a thyroid malignancy other than DTC were excluded from the analysis. This left a total of 954 patients, of whom 249 were patients with DTC and 705 were patients with benign thyroid disease. The 705 patients with benign thyroid disease included patients with symptomatic goitres, follicular adenomas, large benign nodules, and rarely Graves’ disease. The cohort included 772 women and 182 men.
All analysis was completed with SPSS statistical software version 10·0 (SPSS Inc., Chicago, IL, USA). The variables age and TSH were compared for thyroid cancer vs. benign disease. TSH was evaluated as a continuous variable and as a mean. Age was evaluated as a continuous variable and categorically within age ranges < 45 vs. ≥ 45 years and then < 20, 20–44, 45–59 and ≥ 60 years. The age 45 was used as a cut-off because the same extent of disease results in worse prognosis in patients ≥ 45 years vs. < 45 years.4 The categories of age ranges < 20 and ≥ 60 years were used because the likelihood of cancer recurrence is higher in patients less than age 20 or ≥ 60 years.13 Variables such as gender, age, nodule size, nodule number, cancer and pathological features of Hashimoto’s disease were examined in relation to TSH.
High-risk cancer features such as tumour size > 4 cm, distant metastases and extrathyroidal extension were also analysed in relation to TSH. Lymph-node involvement was not included as a high-risk feature for the following reasons. First, there is no agreement about the prognostic value of lymph-node metastases.3 Second, central neck dissection is not routine at our institution and thus analysis would probably exclude patients with undetected lymph-node metastases. All analyses was repeated excluding the 118 patients on preoperative levothyroxine (LT4) because treatment with LT4 leads to an artificially determined TSH value. Values were reported as mean ± SEM. Univariate and multivariate analyses were used to determine the relationship between age and cancer with respect to higher TSH and then to analyse the relationship between high-risk cancer features and TSH. P<0·05was considered significant.
This analysis is a follow-up study to our previous work that evaluated TSH as a cancer predictor.1 The current analysis includes an updated number of patients and evaluates TSH as the dependent variable on multivariate analysis instead of cancer as the dependent variable. This is a separate analysis as there are several variables that are known cancer predictors but are not predictors of high TSH. The current analysis also looks more extensively at stage in order to determine which high-risk feature accounts for the association between higher TSH and advanced-stage disease. Evaluating specific high-risk features has implications for the pathogenesis of the association between higher TSH and thyroid cancer.
Results
Patient and tumour characteristics based on age
Patients less than age 45 differed significantly from those aged 45 and older in multiple categories. The younger patients were more likely to have DTC, a smaller nodule size and a solitary nodule. Surgical patients under age 45 had a 31% (118/383) incidence of DTC vs. those ≥ 45 who had a 23% (131/571) likelihood of DTC (P=0·004). The primary nodule resulting in surgery was 2·37 ± 0·01 cm in the younger patients vs. 2·74 ± 0·01 cm in the older subset (P = 0·007). On final pathology, multiple nodules were detected in 173/383 (46%) of the patients aged < 45 vs. 302/571 (53%) of those ≥ 45 (P = 0·012). Mean TSH was also substantially higher in older patients vs. younger. In patients aged ≥ 45, mean TSH was 2·24 ± 0·24 mIU/l and in patients aged < 45, mean TSH was 1·52 ± 0·11 mIU/l (P=0·018). Of note, even the male to female ratio differed in those patients over age 45 vs. less than 45. There were 53/383 (14%) male patients in the younger age group vs. 129/571 (22%) male patients in the older age group.
The only demographic or pathological variable that did not differ significantly between groups was Hashimoto’s thyroiditis on final pathology. There was a 21% (83/383) incidence of Hashimoto’s in the patients aged < 45 years and a 19% (107/571) incidence in patients aged ≥ 45 years (Table 1). The prevalence of Hashimoto’s thyroiditis was the same in patients with cancer and with benign thyroid disease. Two of the cancer patients did not have detailed pathology reports, but of those 247 who did have a comprehensive pathology report, 51 had Hashimoto’s thyroiditis (20·6%). Four of the patients with benign thyroid disease did not have detailed pathology reports, but of the remaining 701 cases, 139 had Hashimoto’s thyroiditis (19·8%), P-value=0·424.
Table 1.
Patient and tumour characteristics
| Age (years)
|
P-value | ||
|---|---|---|---|
| < 45 | ≥ 45 | ||
| N | 383 | 571 | |
| DTC, n (%) | 118 (31) | 131 (23) | 0·004 |
| Mean TSH (mIU/l) | 1·52 ± 0·11 | 2·24 ± 0·24 | 0·018 |
| Gender | |||
| Male, n (%) | 53 (14) | 129 (22) | 0·001 |
| Female, n (%) | 330 (86) | 442 (77) | |
| Mean nodule size (cm) | 2·37 ± 0·01 | 2·74 ± 0·01 | 0·007 |
| > 1 Nodule, n (%) | 173 (46) | 302 (53) | 0·012 |
| Hashimoto’s thyroiditis on pathology, n (%) | 83 (21) | 107 (19) | 0·155 |
Age and TSH
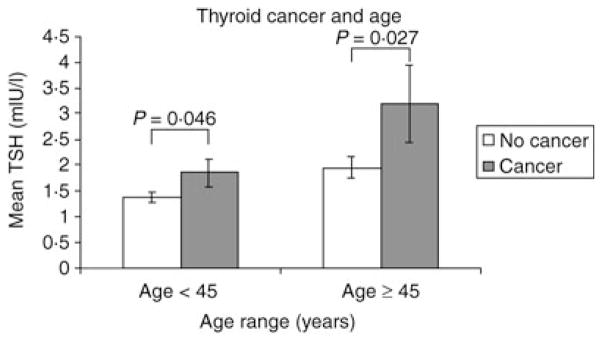
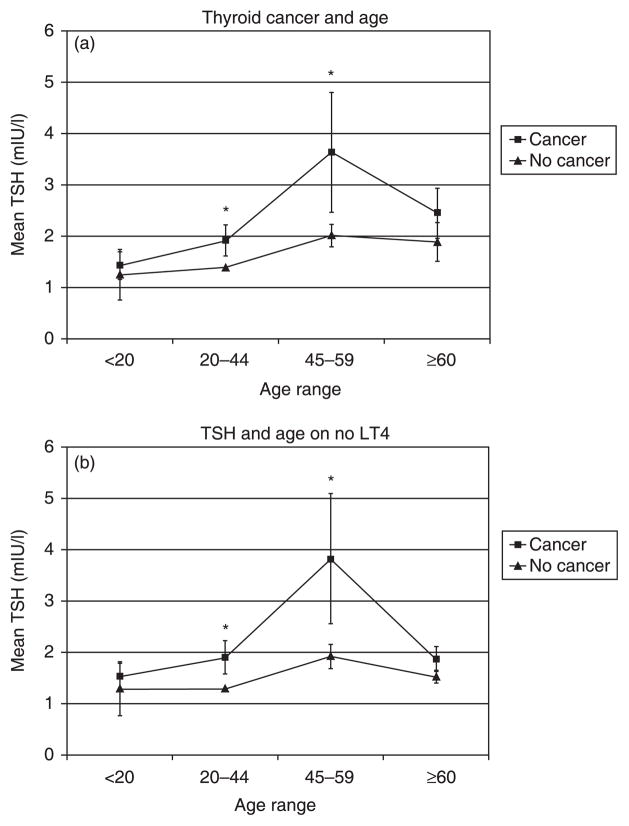
In both age groups, < 45 years and ≥ 45 years, mean TSH was significantly higher in patients with cancer compared to those without (P = 0·046 and 0·027, respectively) (Fig. 1). When the patients were further subdivided by age < 20, 20–44, 45–59 and ≥ 60 years, there was a nonsignificant trend towards a rise in TSH with age in the benign subgroups and a parallel but more rapid increase in TSH in the groups with cancer (Fig. 2a). The difference between cancer vs. no cancer within each subgroup was statistically significant in those aged 20–44 and 45–59 years (P = 0·039 and 0·035, respectively). Of note, significance was established in the two groups with the largest number of patients. There were 48 patients, 14 of these with malignancy, in the age group < 20 years. There were 335 patients, 104 of these with malignancy, in the 20–44-year age group. There were 334 patients, 82 of these with malignancy, in the 45–59-year age group. In the age group ≥ 60 years, there were 237 patients, ofwhom49 had cancer (Fig. 2a).
Fig. 1.
In the age categories < 45 years and ≥ 45 years, patients with thyroid cancer have a significantly higher mean TSH than those without cancer.
Fig. 2.
(a) Across all age categories, patients with thyroid cancer have a higher mean TSH. Significance is established in the age categories 20–44 years and 45–59 years. *P < 0·05. (b) When the 118 patients on preoperative LT4 are excluded from analysis, the same pattern of higher mean TSH in cancer patients versus those without cancer persists. Significance is established in age categories 20–44 and 45–59 years. *P < 0·05.
As treatment with LT4 leads to an artificially set TSH, all 118 patients receiving preoperative LT4 were excluded and the analysis was repeated. Again, the same trend of higher mean TSH in cancer patients was seen, with a significant difference in the age groups 20–44 and 45–59 years (P = 0·019 and 0·027, respectively) (Fig. 2b). Of note, although the mean TSH was higher in the cancer subgroups, it was consistently within the normal range.
On multivariate analysis, higher TSH had a significant association with thyroid cancer incidence. There was a trend for an association between higher TSH and advanced age when only age and cancer were compared (cancer P = 0·01, age P = 0·124). However, once patients on preoperative LT4 were excluded and the analysis expanded to include gender, nodule size, nodule number and Hashimoto’s thyroiditis, only Hashimoto’s (P = 0·001) and thyroid cancer (P = 0·039) were independently associated with higher TSH (Table 2).
Table 2.
Multivariate analysis of variables associated with higher TSH (N = 836; excludes 118 patients on preoperative LT4)
| Variable | P-value |
|---|---|
| Age | 0·557 |
| Thyroid cancer | 0·039* |
| Hashimoto’s | 0·001* |
| Male gender | 0·079 |
| Nodule size | 0·708 |
| > 1 Nodule | 0·529 |
P < 0·05.
TSH and aggressive cancer
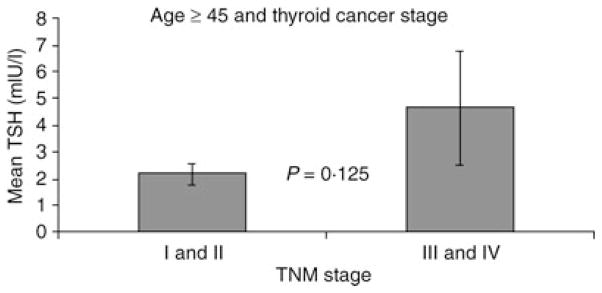
We previously examined the relationship between advanced-stage disease and mean TSH1 but did not control for age, a prognostic indicator. In this current analysis, we only staged patients aged ≥ 45 years. These older patients exhibited a nonsignificant trend for higher mean TSH in those with stage III and IV disease vs. those with stage I and II disease (P = 0·125) (Fig. 3).
Fig. 3.
When only patients over age 45 were staged, there was a trend for higher mean TSH in those with advanced stage disease (P = 0·125). Of the patients over age 45, there were 43 patients with stage III and IV disease and 86 with stage I and II disease. Two patients could not be staged because of incomplete pathology reports.
High-risk features such as age, extrathyroidal extension, tumour size > 4 cm and distant metastases were then analysed in relation to mean TSH in cancer patients. The high-risk feature extrathyroidal extension was associated with higher mean TSH on univariate and multivariate analysis (P=0·004 and 0·002, respectively). When only the patients aged ≥ 45 years were evaluated, again extrathyroidal extension was the only high-risk feature associated with higher TSH (P=0·008) (Tables 3 and 4).
Table 3.
Variation in mean TSH with high-risk features in patients with cancer
| Mean TSH (mIU/l)
|
||||
|---|---|---|---|---|
| High-risk feature present | High-risk feature absent | N | P-value | |
| All patients | ||||
| Extrathyroidal extension | 5·58 ± 2·89 | 1·99 ± 0·22 | 31/247 | 0·004* |
| Tumour size > 4 cm | 2·66 ± 0·45 | 2·43 ± 0·44 | 15/247 | 0·89 |
| Distant metastases | 2·19 ± 0·71 | 2·45 ± 0·43 | 8/247 | 0·913 |
| Patients aged ≥ 45 years | ||||
| Extrathyroidal extension | 7·60 ± 4·45 | 2·13 ± 0·32 | 20/129 | 0·008* |
| Tumour size > 4 cm | 2·61 ± 0·48 | 3·03 ± 0·84 | 14/129 | 0·862 |
| Distant metastases | 2·02 ± 1·12 | 3·02 ± 0·78 | 5/129 | 0·797 |
P < 0·05.
Table 4.
Multivariate analysis with TSH as a dependent variable (cancer patients N = 247)
| Age | Tumour size > 4 cm | Distant metastases | Extrathyroidal extension | |
|---|---|---|---|---|
| P-value | 0·905 | 0·446 | 0·508 | 0·002* |
P < 0·05.
Discussion
The incidence of DTC is rising, and 49% of the increase is due to cancers measuring 1 cm or less.14 Tumours less than 1 cm are more common in older patients.12 Given the increased prevalence of small tumours, the association between higher TSH and increased cancer incidence1,2 and the relationship between rising mean TSH with age,10,11 we examined the relationship between age, cancer and TSH to determine which variable was independently associated with higher TSH.
Multiple population-based studies, including the National Cancer Database Report and the Surveillance, Epidemiology and End Results, have shown age to be an important prognostic indicator for well-differentiated thyroid cancer (WDTC).5,9 Patients over age 45 can have the same degree of disease but a distinctly different prognosis than those under age 45.4 The reason for this discrepancy is not entirely clear, but it does imply that there is either something intrinsic to the cancer or to the treatment that is age dependent. Our previously published study found an association between higher TSH and advanced-stage disease.1 Because advanced-stage disease is age dependent, we wanted to determine whether age, a known prognostic indicator, was the explanation for this relationship.
As illustrated in Table 1, the patient and tumour characteristics in patients less than age 45 vs. age 45 and older are inherently different. Given the higher incidence of thyroid nodules in older patients,15 as a group, older patients are more likely to have benign, large nodules. Thus, the increased incidence of large nodules and surgery for benign nodules in patients aged 45 and older is not surprising. In addition, the increased likelihood of multiple nodules in older patients is also expected. However, despite these marked differences in patient characteristics, the association between higher TSH and cancer incidence persists in every age group. The association is only significant in the two groups with the largest number of patients, aged 20–44 and 45–59 years, but the trend is present in patients aged<20 and ≥ 60 years.
The mean TSH in patients aged ≥ 45 years is significantly higher than in those aged < 45. However, when we subdivide patients into cancer vs. no cancer, the predictive value of age weakens. Similar to data from the National Health and Nutrition Examination Survey (NHANES III) on a healthy population with no known thyroid disease, 10 our data from the benign subgroups show a trend for rising mean TSH with age. Yet when patients with thyroid cancer are compared to those with benign nodular disease, cancer patients have a higher mean TSH within every age group. The mean TSH in the cancer population rises in parallel, but is consistently higher than the mean TSH in the benign nodular population. These data suggest that the association between higher TSH and increased thyroid cancer incidence cannot be explained by age alone. Further supporting the independent relationship between TSH and thyroid cancer incidence, on multivariate analysis, thyroid cancer, not age, has a significant association with higher TSH. Although the trend we observe supports the NHANES data on rising TSH with age, the relationship between cancer and high TSH dominates in the studied population.
Our previous publication showed that advanced-stage disease was associated with higher mean TSH.1 Our current analysis shows this trend persists when patients aged ≥ 45 are selected, again suggesting that age is not the explanation. If age cannot explain the relationship between higher TSH and advanced-stage disease, what can? In multiple staging systems, including TNM, AMES, AGES and MACIS, extrathyroidal extension, distant metastases, large tumour size and advanced age are associated with poor prognosis.3 For our study, the definition of extrathyroidal extension is based on pathology report. When we examine the previously mentioned high-risk features in relationship to TSH, extrathyroidal extension is the only risk factor with a significant association with higher TSH both on univariate and multivariate analysis.
A limitation of this current study is that all patients studied were status post-thyroid surgery and this population may not be representative of the population at large. Another limitation is that this analysis is a retrospective cohort study and not all patients had preoperative TSH levels drawn. Five different surgeons performed thyroid surgery during this 13·5-year time-span. Preoperative TSH measurement was not available for all patients because some surgeons did not routinely order this laboratory test. In addition, six patients had an incomplete pathology report. These patients either had reports that stated ‘papillary thyroid cancer’ but then did not give details on the tumour or they had reports of ‘benign thyroid’ but no further characterization and classification of the nodule.
However, as this is a study group post-surgery, this patient population has pathology confirming thyroid cancer and Hashimoto’s in addition to providing information about high-risk features such as tumour size and extrathyroidal extension. Thus, although being a population post-surgery is a limitation in regard to selection bias, it is also a strength in regard to access to complete pathology data. Despite the limitations in design, this study has a large sample of patients and analysis reveals an independent relationship between thyroid cancer and higher TSH. More importantly, this study offers an explanation for the association between advanced-stage disease and higher TSH. Based on our data, there is a clear relationship between higher mean TSH and extrathyroidal extension, a known high-risk feature in thyroid cancer patients.
The pathogenesis behind mean TSH being elevated in cancer patients across all age groups is unclear. It is well known that individual thyroid hormone concentrations are maintained within narrow limits16 and that the heritable contribution to serum TSH is as high as 65%.17 One possibility is that patients with higher TSH have a genetic predisposition to both higher TSH and cancer.18 However, if the increased cancer incidence was secondary to a TSH receptor polymorphism, similar to the TSHR-Glu727 allele associated with lower TSH,19 or a polymorphism in the thyroid hormone pathway genes,20 a clear genetic predisposition for thyroid cancer would be expected. Instead, only 3–6% of nonmedullary thyroid cancer is familial.21,22
There are alternative explanations for the association between higher TSH and thyroid cancer. There may be resistance to TSH at the level of the malignancy due to TSH receptor silencing in the setting of aberrant DNA methylation,23 but even in this situation the remaining healthy thyrocytes would be expected to compensate, and for the relationship between higher TSH and cancer to not exist. If TSH was truly carcinogenic, as suggested in a mouse model,24,25 we would expect the absolute TSH to be consistently elevated to a threshold, not to rise with age parallel to benign thyroid disease. The likelihood of early end-organ damage leading to relative TSH resistance seems reasonable, but the absence of a clear relationship between pathologic Hashimoto’s, higher TSH and cancer weakens this hypothesis.1,26 In the current study, thyroid cancer was independently associated with higher TSH whereas the association between age and higher TSH weakened when variables such as gender and Hashimoto’s were included in the multivariate analysis.
Perhaps the most probable explanation for the relationship between higher TSH and thyroid cancer incidence is that TSH, a growth factor, leads to increased cancer detection. More than one-third of patients have occult microcarcinomas on thorough autopsy reports,27 and yet only a small number of individuals are diagnosed with thyroid cancer each year.28 TSH stimulation may cause occult microcarcinomas to grow to a detectable size.
Our data also suggest that TSH facilitates extrathyroidal extension of disease. Previous in vitro studies have indicated a relationship between TSH stimulation and cancer invasion.29,30 In vitro, TSH stimulates invasion and growth of human thyroid cancer cell lines by protein kinase C (PKC) stimulation.29 Activation of matrix metalloproteinases plays a key role in this invasion,31 and the impact of TSH on the metalloproteinases in healthy surrounding thyroid tissue and the cancer foci has not been determined.32 Further studies are needed to define this relationship as understanding it may be pivotal in understanding the pathogenesis of this disease.
Despite unclear pathogenesis, independent of age, there is an association between thyroid cancer and higher TSH. When evaluating specific high-risk features, extrathyroidal extension correlates with higher TSH on both univariate and multivariate analyses.
Acknowledgments
We thank Anna Bargren and Dan Repplinger for their assistance with data collection.
References
- 1.Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumour stage. Journal of Clinical Endocrinology and Metabolism. 2008;93:809–814. doi: 10.1210/jc.2007-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boelaert K, Horacek J, Holder RL, et al. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. Journal of Clinical Endocrinology and Metabolism. 2006;91:4295–4301. doi: 10.1210/jc.2006-0527. [DOI] [PubMed] [Google Scholar]
- 3.Dean DS, Hay ID. Prognostic indicators in differentiated thyroid carcinoma. Cancer Control: Journal of the Moffitt Cancer Center. 2000;7:229–238. doi: 10.1177/107327480000700302. [DOI] [PubMed] [Google Scholar]
- 4.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M, editors. AJCC Cancer Staging Manual. 6. Springer-Verlag; New York: 2002. Thyroid; pp. 77–87. No authors listed. [Google Scholar]
- 5.Gilliland FD, Hunt WC, Morris DM, et al. Prognostic factors for thyroid carcinoma: a population-based study of 15 698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997;79:564–573. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Jung TS, Kim TY, Kim KW, et al. Clinical features and prognostic factors for survival in patients with poorly differentiated thyroid carcinoma and comparison to the patients with the aggressive variants of papillary thyroid carcinoma. Endocrine Journal. 2007;54:265–274. doi: 10.1507/endocrj.k06-166. [DOI] [PubMed] [Google Scholar]
- 7.Sampson E, Brierley JD, Le LW, et al. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer. 2007;110:1451–1456. doi: 10.1002/cncr.22956. [DOI] [PubMed] [Google Scholar]
- 8.Lin JD, Liou MJ, Chao TC, et al. Prognostic variables of papillary and follicular thyroid carcinoma patients with lymph node metastases and without distant metastases. Endocrine-Related Cancer. 1999;6:109–115. doi: 10.1677/erc.0.0060109. [DOI] [PubMed] [Google Scholar]
- 9.Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53 856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Surks MI, Hollowell JG. Age-specific distribution of serum TSH and antithyroid antibodies in the united states population: implications for the prevalence of subclinical hypothyroidism. Journal of Clinical Endocrinology and Metabolism. 2007;92:4575–4582. doi: 10.1210/jc.2007-1499. [DOI] [PubMed] [Google Scholar]
- 11.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988–1994): National Health and Nutrition Examination Survey (NHANES III) Journal of Clinical Endocrinology and Metabolism. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 12.Miccoli P, Minuto MN, Ugolini C, et al. Papillary thyroid cancer: pathological parameters as prognostic factors in different classes of age. Journal of Otolaryngology – Head and Neck Surgery. 2008;138:200–203. doi: 10.1016/j.otohns.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Mazzaferri EL, Kloos RT. Current approaches to primary therapy for papillary and follicular thyroid cancer. Journal of Clinical Endocrinology and Metabolism. 2001;86:1447–1463. doi: 10.1210/jcem.86.4.7407. [DOI] [PubMed] [Google Scholar]
- 14.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. Journal of the American Medical Association. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 15.Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Annals of Internal Medicine. 1997;126:226–231. doi: 10.7326/0003-4819-126-3-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 16.Anderson S, Pederson KM, Bruun NH, et al. Narrow individual variations in serum T4 and T3 in normal subjects: a clue to the understanding of subclinical thyroid disease. Journal of Clinical Endocrinology and Metabolism. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 17.Panicker V, Wilson SG, Spector TD, et al. Heritability of serum TSH, free T4 and free T3 concentrations: a study of a large UK twin cohort. Clinical Endocrinology. 2008;66:652–659. doi: 10.1111/j.1365-2265.2007.03079.x. [DOI] [PubMed] [Google Scholar]
- 18.Panicker V, Wilson SG, Spector TD, et al. Genetic loci linked to pituitary-thyroid axis set points: a genome-wide scan of a large twin cohort. Journal of Clinical Endocrinology and Metabolism. 2008;93:3519–3523. doi: 10.1210/jc.2007-2650. [DOI] [PubMed] [Google Scholar]
- 19.Hansen PS, van der Deure WM, Peeters RP, et al. The impact of a TSH receptor gene polymorphism on thyroid related phenotypes in a healthy Danish twin population. Clinical Endocrinology. 2007;66:827–832. doi: 10.1111/j.1365-2265.2007.02820.x. [DOI] [PubMed] [Google Scholar]
- 20.Peeters RP, van Toor H, Klootwijk W, et al. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. Journal of Clinical Endocrinology and Metabolism. 2003;88:2880–2888. doi: 10.1210/jc.2002-021592. [DOI] [PubMed] [Google Scholar]
- 21.Sippel RS, Caron NR, Clark OH. An evidence-based approach to familial nonmedullary thyroid cancer: screening, clinical management, and follow-up. World Journal of Surgery. 2007;31:924–933. doi: 10.1007/s00268-006-0847-1. [DOI] [PubMed] [Google Scholar]
- 22.Pal T, Vogl FD, Chappius PO, et al. Increased risk for nonmedullary thyroid cancer in the first-degree relatives of prevalent cases of nonmedullary thyroid cancer: a hospital-based study. Journal of Clinical Endocrinology and Metabolism. 2001;86:5307–5312. doi: 10.1210/jcem.86.11.8010. [DOI] [PubMed] [Google Scholar]
- 23.Xing M, Usadel H, Cohen Y, et al. Methylation of the thyroid-stimulating hormone receptor gene in epithelial thyroid tumors: a marker of malignancy and a cause of gene silencing. Cancer Research. 2003;63:2316–2321. [PubMed] [Google Scholar]
- 24.Brewer C, Yeager N, Di Cristofano A. Thyroid-stimulating hormone-initiated proliferative signals converge in vivo on the mTOR kinase without activating AKT. Cancer Research. 2007;67:8002–8006. doi: 10.1158/0008-5472.CAN-07-2471. [DOI] [PubMed] [Google Scholar]
- 25.Yeager N, Klein-Szanto A, Kimura S, et al. Pten loss in mouse thyroid causes goiter and follicular adenomas:insights into thyroid function and Cowden disease pathogenesis. Cancer Research. 2007;67:959–966. doi: 10.1158/0008-5472.CAN-06-3524. [DOI] [PubMed] [Google Scholar]
- 26.Repplinger D, Bargren A, Zhang Y, et al. Is Hashimoto’s thyroiditis a risk factor for papillary thyroid cancer? Journal of Surgical Research. 2008;150:49–52. doi: 10.1016/j.jss.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A ‘normal’ finding in Finland. A systematic autopsy study. Cancer. 1985;56:531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.American Cancer Society, Inc. [last accessed 25 November 2008];What are the key statistics about thyroid cancer? 2008 [updated 3 October 2007] Available from http://www.cancer.org.
- 29.Hoelting T, Tezelman S, Siperstein AE, et al. Thyrotropin stimulates invasion and growth of follicular thyroid cancer cells via PKC- rather than PKA-activation. Biochemical and Biophysical Research Communications. 1993;195:1230–1236. doi: 10.1006/bbrc.1993.2176. [DOI] [PubMed] [Google Scholar]
- 30.Hoelting T, Tezelman S, Siperstein AE, et al. Biphasic effects of thyrotropin on invasion and growth of papillary and follicular thyroid cancer in vitro. Thyroid. 1995;5:35–40. doi: 10.1089/thy.1995.5.35. [DOI] [PubMed] [Google Scholar]
- 31.Yeh MW, Rougier JP, Park JW, et al. Differentiated thyroid cancer cell invasion is regulated through epidermal growth factor receptor-dependent activation of matrix metalloproteinase (MMP)-2/gelatinase A. Endocrine-Related Cancer. 2006;13:1173–1183. doi: 10.1677/erc.1.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldini E, Tollen M, Graziano FM, et al. Expression of matrix metalloproteinases and their specific inhibitors in normal and different human thyroid tumour cell lines. Thyroid. 2004;14:881–888. doi: 10.1089/thy.2004.14.881. [DOI] [PubMed] [Google Scholar]