Abstract
We report the CuI/O2 chemistry of complexes derived from the macrocylic ligands 14-TMC (1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane) and 12-TMC (1,4,7,10-tetramethyl-1,4,7,10-tetraazacyclododecane). While [(14-TMC)CuI]+ is unreactive towards dioxygen, the smaller analog [(12-TMC)CuI(CH3CN)]+ reacts with O2 to give a side-on bound peroxo-dicopper(II) species (SP), confirmed by spectroscopic and computational methods. Intriguingly, 12-TMC as a N4 donor ligand generates SP species, thus in contrast with the previous observation that such species are generated by N2 and N3 ligands. In addition, the reactivity of this macrocyclic side-on peroxodicopper(II) differs from typical SP species, because it reacts only with acid to release H2O2, in contrast with the classic reactivity of Cu2O2 cores. Kinetics and computations are consistent with a protonation mechanism whereby the TMC acts as a hemilabile ligand and shuttles H+ to an isomerized peroxo core.
Keywords: bioinorganic chemistry, copper, dioxygen reduction, macrocyclic ligands, metal–peroxo complexes
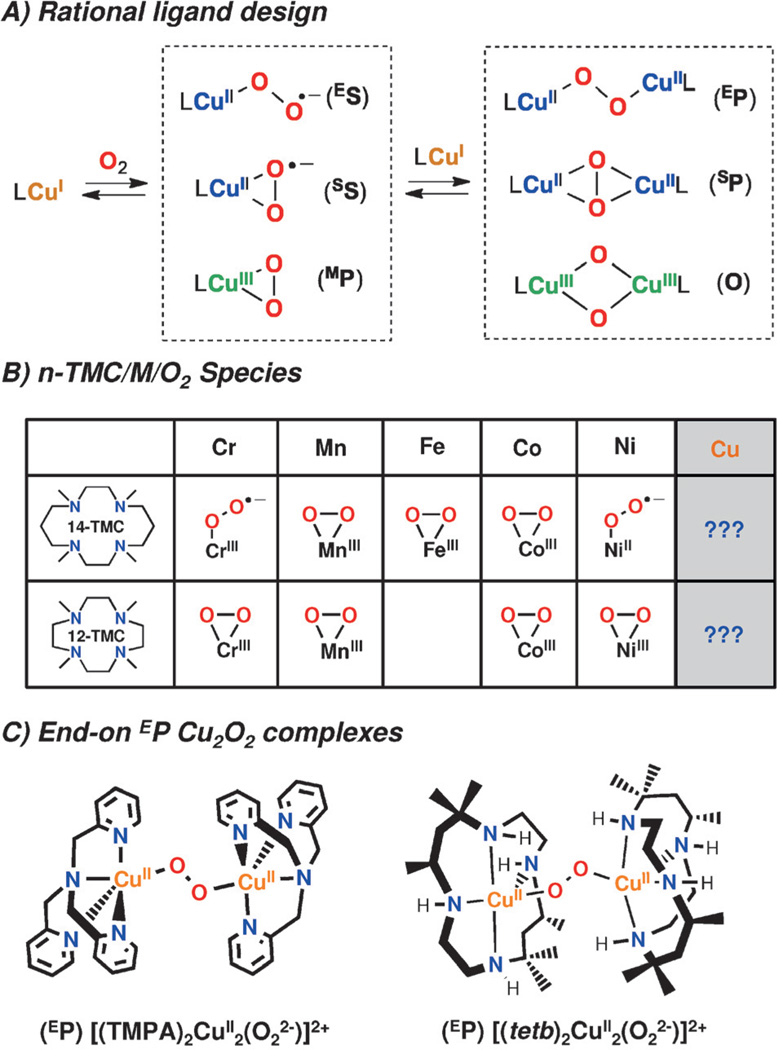
Over the last 3 decades, our research interests have included a focus on the study of transient species derived from the reaction between CuI complexes with dioxygen.[1] Rational design of the ligand employed has allowed our group, among others,[2] to extensively characterize the formation of mononuclear[3] and dinuclear[4] copper–dioxygen assemblies (Figure 1A). Many of our findings are based on the tripodal ligand TMPA (tris((2-pyridyl)methyl)amine) and its derivatives, that have allowed fine tuning of the electronic and steric properties of Cu/O2 derived species.[5] Particularly relevant are our recent findings in the stabilization of mononuclear end-on LCuII(O2•−) species (ES) making use of electron-rich TMPA derivatives[6] or by H-bonding interactions,[7] and our recent reports on the first examples of end-on peroxo-dicopper(II) intermediates (EP) that undergo reversible O–O cleavage via formation of dicopper(III) bis(μ-oxo) species (O).[8] These results show that even after many years of study, new Cu/O2 reactivity patterns are still emerging.
Figure 1.
Controlled generation of different LCu–O2 species by rational ligand design A) First row metal/O2 species bearing macrocyclic 14-TMC and 12-TMC ligands. B) Crystallographically characterized EP species (C).[5a,10]
One of the most extensively employed ligand types in bioinspired O2-activation chemistry is the macrocycle 14-tetramethylcyclam (14-TMC), along with its contracted analogue 12-TMC. Nam and co-workers have extensively characterized M-(O2•−) and M-(O2(H)) complexes of the 1st row of transition metals (Figure 1B); associated O2 activation levels and substrate reactivity are dependent on both metal identity and TMC ring size.[9] However, the reactivity of analogous TMC/Cu/O2 systems has remained elusive. Schindler and co-workers[10] recently reported the first example of end-on peroxo Cu2O2 species (EP) bearing a macrocylic ligand tetb (rac-5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane), a EP complex with a distorted square-pyramidal (τ = 0.3) geometry and equatorial bound bent bridging O22− ligand (Figure 1C). This geometry differs from the TMPA-based EP species, where the O22− ligand is found to be coordinated in an axial position of a trigonal bipyramidal (τ = 0.9)[11] geometry (Figure 1C), and suggests macrocyclic ancillary ligands can form Cu/O2 species with unique structure. Herein, we report the first study on the Cu–O2 chemistry of the 14-TMC and 12-TMC systems and the first example of a side-on (i.e., μ-η2 :η2) peroxodicopper(II) complex (SP) bearing the 12-TMC ligand.
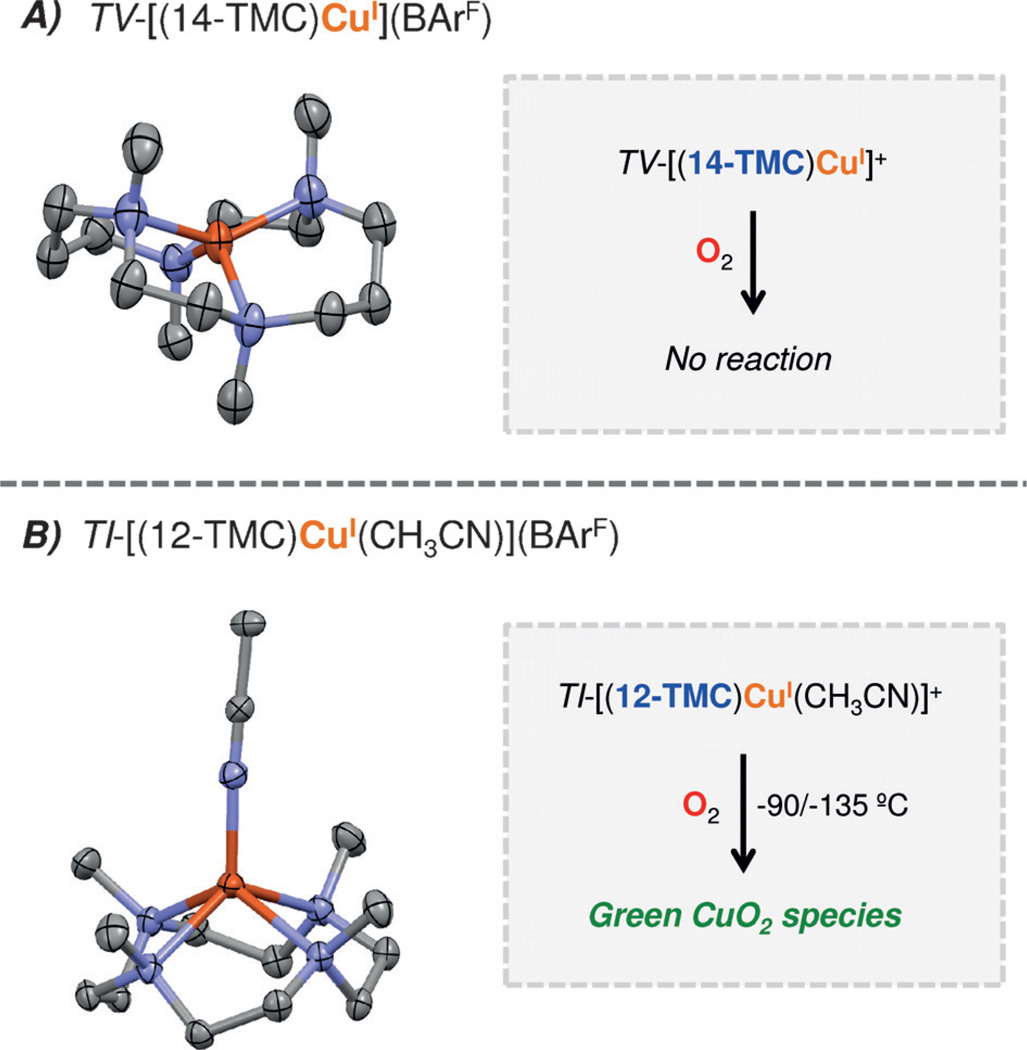
Mixing equimolar amounts of the 14-TMC ligand with [CuI(CH3CN)4](BArF) (BArF: B(C6F5)4−) in Et2 O at room temperature under Ar allows the growth of colorless crystals, which were analyzed by single crystal X-ray diffraction (Figure 2A); the 14-TMC ligand shows an unusual type-V (TV) conformation (TV, where 1,8-Me groups are oriented up; 4,11-Me groups down)[12] in the CuI complex TV-[(14-TMC)CuI]+,[13] where the Cu ion adopts a flattened tetrahedral geometry. Surprisingly, this macrocyclic type-V [(14-TMC)CuI]+ complex is completely unreactive towards O2 (see the Supporting Information for details).[14]
Figure 2.
Displacement ellipsoid plots (50% probability level) of TV-[(14-TMC)CuI]+. A) (un-reactive towards O2) and TI-[(12-TMC)CuI(CH3CN)]+ B) (reactive with O2). For clarity, the BArF anions and the H atoms are not depicted. See the Supporting Information for synthetic details and structural features.
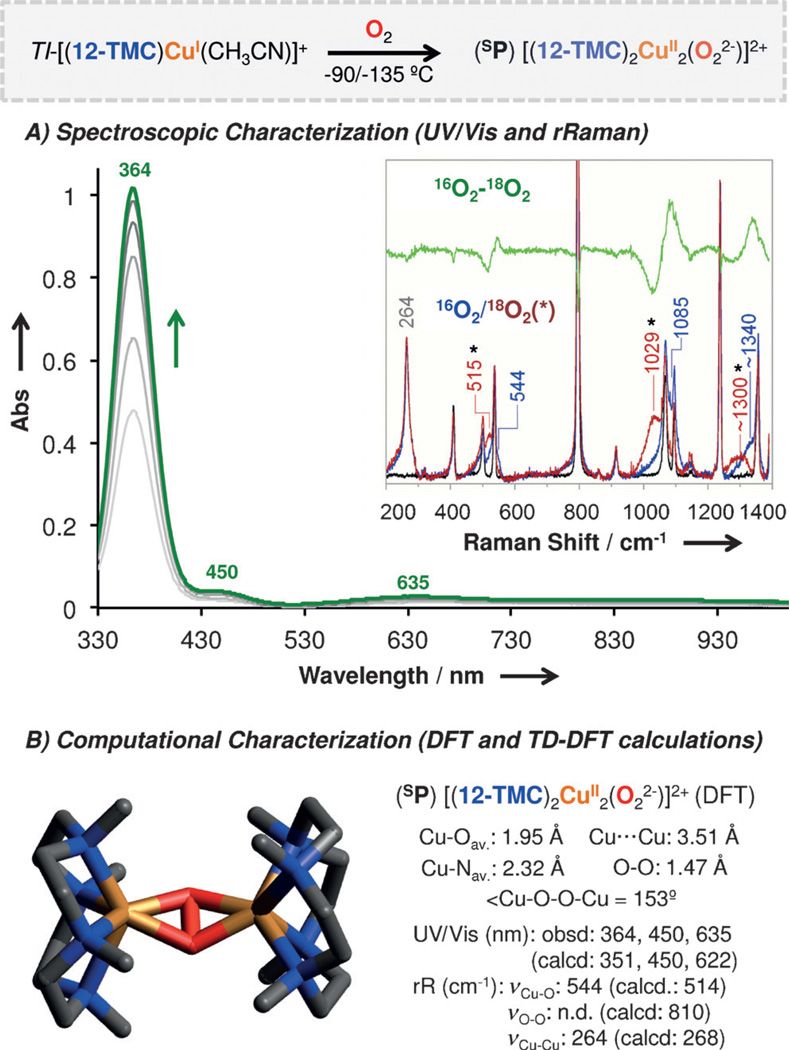
The 12-TMC cuprous complex was synthesized similarly and the X-ray structural determination disclosed an N5Cu(I) center in which the 12-TMC ligand adopts a type-I conformation (with all Me groups up) in TI-[(12-TMC)CuI(CH3CN)](BArF) with a tightly coordinated acetonitrile molecule (d(Cu-NCH3CN) = 1.92 Å) (Figure 2B). Unlike for TV-[(14-TMC)CuI]+, TI-[(12-TMC)CuI(CH3-CN)]+ reacts with O2 at −90 to −135 °C, forming a new green species in acetone or MeTHF (2-methyltetrahydrofuran). UV/Vis spectroscopy (Figure 3A) reveals an intense high-energy spectral feature at 364 nm (ε = 20 mm−1 cm−1) and two weak transitions at 450 and 635 nm, which are characteristic of a complex with a side-on peroxodicopper(II) coordination (SP).[2a] [(12-TMC)2CuII2(O22−)]2+ is the first example of a SP complex bearing a tetradentate ligand; N4 ligands typically provide for an end-on peroxo (EP) coordination.
Figure 3.
A) Spectroscopic characterization (UV/Vis) of green [(12-TMC)2CuII2(O22−)]2+ (λmax = 364 nm) generated by the oxygenation of TI-[(12-TMC)CuI(CH3CN)]+. Inset: rR spectra (λ = 380 nm) of [(12-TMC)2CuII2(O22−)]2+ generated from 16O2 and 18O2 (*), and 16O2–18O2 subtraction. B) DFT optimized structure of SP [(12-TMC)2CuII2(O22−)]2+ and TD-DFT calculated spectroscopic features (rR and UV/Vis).
Additional proof for the side-on [(12-TMC)2CuII2(O22−)]2+ formulation is provided by resonance Raman (rR) spectroscopy (Figure 3A, inset). Laser excitation (λ = 380 nm) of frozen samples of [(12-TMC)2CuII2(O22−)]2+ generated in both MeTHF and acetone led to observation of an isotope-sensitive feature at νCu–O = 544 cm−1 (Δ(18O2) = −29 cm−1) plus a νCu–Cu = 264 cm−1 isotope-insensitive feature that are typical of SP complexes.[15] Higher energy features are observed at 1085 cm−1 and around 1340 cm−1, and are consistent with assignments as an overtone (2νCu–O) and combination band (2νCu–O+νCu–Cu), respectively. Since the antisymmetric νCu–O mode is not Raman active in a planar core, the unusually high intensity of νCu–O in [(12-TMC)2CuII2(O22−)]2+ suggests the Cu2O2 core adopts a butterfly-distorted geometry (see below). This is consistent with the perpendicular π*v →Cu LMCT being observed (450 nm), which is a feature that has no intensity in planar Cu2(O22−) cores.[15b]
DFT calculations of the [(12-TMC)2CuII2(O22−)]2+ intermediate led to the SP species as the lowest energy isomer, with both Cu centers presenting a distorted octahedral geometry around a butterflied Cu2O2 core (Figure 3 B), as suggested by the experimental UV/Vis and rR spectroscopies.[16] The calculated Cu–O, O–O and Cu–Cu distances are consistent with other well-characterized SP complexes.[17] The optimized Cu–Nav distance (2.32 Å) was found to be slightly longer than the ones found for other SP-type species (2.0–2.2 Å), due to the elongation of two of the Cu–N distances per Cu ion (2.39–2.56 Å). These weak Cu–N bonds have also been observed by Tolman and co-workers in the Cu/O2 species bearing tridentate macrocyclic systems, inducing the formation of O-type species over SP.[18] In our case, we propose that these weak, pseudoaxial, Cu–N bonds are a key factor to explain the unusual reactivity of the macrocyclic SP core (vide infra). DFT and TD-DFT calculations also reproduce the experimental rR data (calcd νCu–O = 514 cm−1 [exptl: 544 cm−1]; calcd νCu–Cu = 268 cm−1 (exptl: 264 cm−1); and absorption spectrum (calcd: 351, 450 and 622 nm; exptl: 364, 450 and 635 nm, see Figure S9).
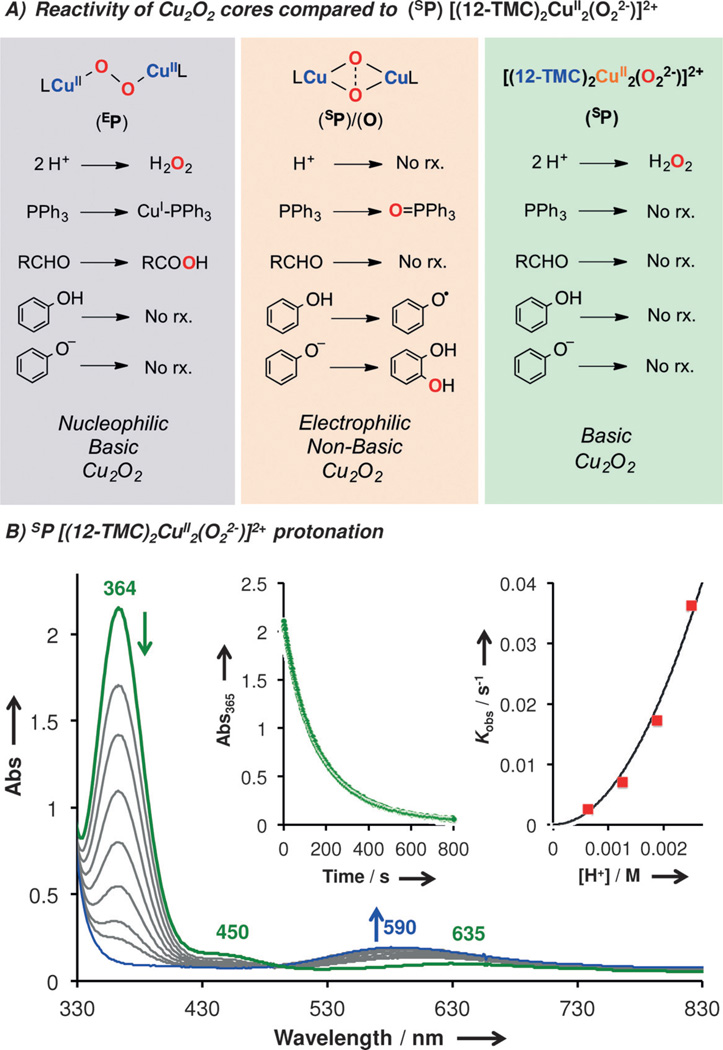
The study of the reactivity of the different Cu2O2 centers (EP, SP, O) towards external substrates has led to some general rules which depend on the core formulation.[19] EP complexes are considered basic/nucleophilic species, while SP and O-type complexes are considered non-basic/electrophilic.[19] However, this SP [(12-TMC)2CuII2(O22−)]2+ species is found to be unreactive towards external substrates usually oxidized by electrophilic species (Figure 4A): phenols (2,4-tBu2-PhOH/2,6-tBu2-4-MeO-PhOH, for H• abstraction), sodium phenolates (for ortho-hydroxylation) and PPh3 (for O-atom transfer) are each unreactive when added in excess (up to 50 equiv, see SI). The 12-TMC-based SP species is also found to be stable when it was exposed to substrates that can be oxidized by nucleophilic M(O22−) species such as 4-substituted benzaldehydes (oxidation to benzoic acids) or cyclohexanecarboxaldehyde (decarboxylation to cyclohexene).[9a]
Figure 4.
A) Reactivity towards external substrates shown by SP [(12-TMC)2CuII2(O22−)]2+ (right) compared to generic reactivity of EP and SP/O cores. B) UV/Vis changes and kinetic analysis (inset) for the reaction of [(12-TMC)2CuII2(O22−)]2+ (λmax = 364 nm) with acid to generate 2 equivalents of [(12-TMC)CuII]2+ (λmax = 590 nm) and H2O2.
On the other hand, when [(12-TMC)2CuII2(O22−)]2+ was exposed to a strong acid (H+•DMF•CF3SO3−), decay of its UV/Vis features was observed (Figure 4B) with concomitant formation of new weak features centered at 590 nm, corresponding to the mononuclear [(12-TMC)CuII(L)]2+ complex (for CuII quantification and EPR characterization see Figure S7). H2O2 formation was confirmed by iodometric titration of the final reaction solution (95% based on [Cu2O2]0, see Figure S6). To the best of our knowledge, this is one of the first examples of a SP species that can be protonated quantitatively to release H2O2, a reaction that is typical of EP complexes.[20]
In order to obtain mechanistic insight, kinetic analysis on the reaction of [(12-TMC)2CuII2(O22−)]2+ with (H+•DMF•CF3SO3−) was conducted under pseudo-first-order conditions (5–20 equiv of acid). The decrease of the SP UV/Vis features over time was fit using pseudo-first-order exponential decays to obtain the kinetic constants (kobs) at different [H+] (Figure 4B, inset). The kobs increased parabolically with increasing acid concentration (rate law: d[Cu2O2]/dt = −kH+[Cu2O2][H+]2), suggesting an unusual involvement of 2 molecules of acid during the rate determining step (r.d.s.). The same kinetic behavior was observed with mild acids such as substituted acetic acids (RCO2H, R: Cl3C, Cl2HC, CF3, CH3). The kobs values were found to be independent on the H+ strength and an inverse kinetic isotope effect (KIE) was observed (kCF3CO2H/kCF3CO2D = 0.8, see Figure S5 in the Supporting Information).
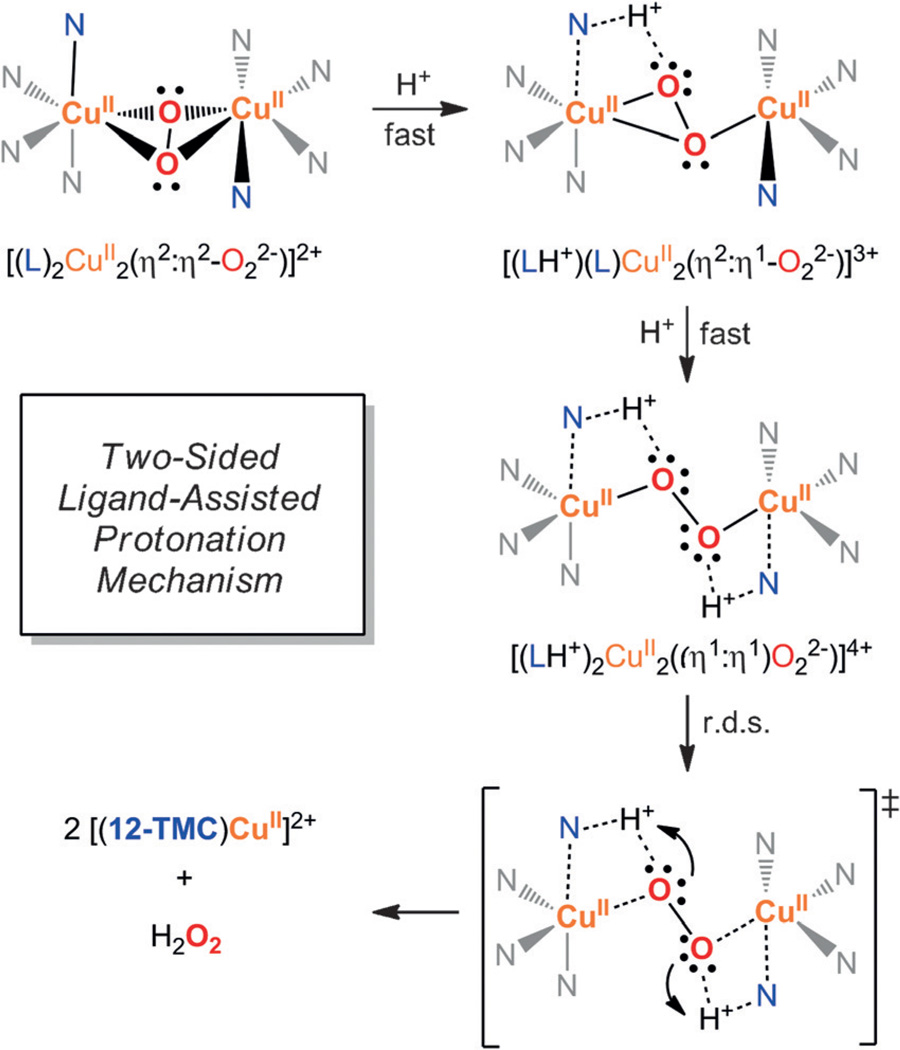
Protonation of Cu2O2 species to generate H2O2 is characteristic of EP species; SP cores are usually stable under acidic conditions. However, the basicity of this [(12-TMC)2CuII2(O22−)]2+ complex is not matched with a nucleophilic character. We propose that the unique reactivity of [(12-TMC)2CuII2(O22−)]2+ is dictated by the ligand, since the 6-coordinate Cu ions have no coordination positions available in the Cu2O2 core for incoming substrates. The lack of a pKa effect in the O22− protonation indicates the r.d.s. involves protonation by a common intermediate, regardless of the identity of added acid. This suggests the TMC ligand is initially protonated by acid, and subsequently transfers H+ intramolecularly to the peroxide. DFT calculations support this idea, showing that two sequential TMC protonation events lead first to isomerization of the SP to a mono-protonated η2 :η1-Cu2O2 and subsequently a di-protonated EP species (Figure 5), both stabilized by H-bonding interactions. We propose that this nucleophilic EP core is now activated towards intramolecular H+ transfer from the protonated N atoms of TMC (see Figures S10–S13 in the Supporting Information).
Figure 5.
Proposed reaction mechanism for the protonation of [(12-TMC)2CuII2(O22−)]2+ based on experimental findings and DFT calculations.
The experimental observations (2nd order in [H+] with no pKa effect) and DFT calculations point towards a mechanism where a first fast protonation to 12-TMC (uphill)[21], enabled by the weak Cu–N bonds, is followed by a second rate-determining protonation. Rate-limiting proton transfer from 12-TMC to the peroxo moiety is also consistent with the inverse KIE since the O–H bond being formed is stronger than the N–H bond being broken. We propose that the macrocyclic ligand has a dual role upon protonation: the flexibility to accommodate different Cu2O2 isomers (control of the primary coordination sphere) and the ability to act as a proton shuttle (control of the secondary coordination sphere by H-bonding interactions). A similar duality is observed in the reactivity of selected metalloenzymes (e.g. Cu,Zn-SOD[22]) and bio-inspired catalysts (e.g. Ni-catalyzed H2 formation[23]).
In conclusion, we have succeeded in generating and describing previously elusive LCu/O2 chemistry of the macrocyclic ligands 14-TMC and 12-TMC. The dramatic effects on structure and chemistry previously known for differing TMC ring-size,[9] along with the finding of a 12-TMC CuI/O2 adduct with distinctive structure and behavior, are manifest by the new findings. Current studies are focused on understanding the Cu-dioxygen chemistry of other TMC isomers generated in the in situ reactions of 14-TMC/CuI,[14a] along with exploring other macrocyclic systems beyond 12-TMC and 14-TMC likely to provide Cu/O2 derived species with new structural, spectroscopic and reactivity features.
Supplementary Material
Acknowledgments
This research was supported by the U.S. NIH (GM28962 to K.D.K., DK31450 to E.I.S., NRSA postdoctoral fellowship F32-GM105288 to R.E.C.) and by the NRF of Korea through CRI (NRF-2012R1A3A2048842) and GRL (NRF-2010-00353) (to W.N.). I.G.-B. thanks the E.C. for a Marie Curie IOF Fellowship.
Footnotes
Supporting information for this article can be found under http://dx.doi.org/10.1002/chem.201600551.
Contributor Information
Dr. Isaac Garcia-Bosch, Email: igarciabosch@smu.edu.
Prof. Wonwoo Nam, Email: wwnam@ewha.ac.kr.
Prof. Edward I. Solomon, Email: edward.solomon@stanford.edu.
Prof. Kenneth D. Karlin, Email: karlin@jhu.edu.
References
- 1.a) Himes RA, Karlin KD. Curr. Opin. Chem. Biol. 2009;13:119–131. doi: 10.1016/j.cbpa.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hatcher LQ, Karlin KD. Adv. Inorg. Chem. 2006;58:131–184. [Google Scholar]
- 2.a) Mirica LM, Ottenwaelder X, Stack TDP. Chem. Rev. 2004;104:1013–1045. doi: 10.1021/cr020632z. [DOI] [PubMed] [Google Scholar]; b) Lewis EA, Tolman WB. Chem. Rev. 2004;104:1047–1076. doi: 10.1021/cr020633r. [DOI] [PubMed] [Google Scholar]
- 3.a) Aboelella NW, Lewis EA, Reynolds AM, Brennessel WW, Cramer CJ, Tolman WB. J. Am. Chem. Soc. 2002;124:10660–10661. doi: 10.1021/ja027164v. [DOI] [PubMed] [Google Scholar]; b) Fujisawa K, Tanaka M, Morooka Y, Kitajima N. J. Am. Chem. Soc. 1994;116:12079–12080. [Google Scholar]
- 4.a) Halfen JA, Mahapatra S, Wilkinson EC, Kaderli S, Young VG, Jr, Que L, Jr, Zuberbühler AD, Tolman WB. Science. 1996;271:1397–1400. doi: 10.1126/science.271.5254.1397. [DOI] [PubMed] [Google Scholar]; b) Mirica LM, Vance M, Rudd DJ, Hedman B, Hodgson KO, Solomon EI, Stack TDP. Science. 2005;308:1890–1892. doi: 10.1126/science.1112081. [DOI] [PubMed] [Google Scholar]
- 5.a) Jacobson RR, Tyeklár Z, Karlin KD, Liu S, Zubieta J. J. Am. Chem. Soc. 1988;110:3690–3692. [Google Scholar]; b) Zhang CX, Kaderli S, Costas M, Kim E-i, Neuhold Y-M, Karlin KD, Zuberbühler AD. Inorg. Chem. 2003;42:1807–1824. doi: 10.1021/ic0205684. [DOI] [PubMed] [Google Scholar]
- 6.Lee JY, Peterson RL, Ohkubo K, Garcia-Bosch I, Himes RA, Woertink J, Moore CD, Solomon EI, Fukuzumi S, Karlin KD. J. Am Chem. Soc. 2014;136:9925–9937. doi: 10.1021/ja503105b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson RL, Himes RA, Kotani H, Suenobu T, Tian L, Siegler MA, Solomon EI, Fukuzumi S, Karlin KD. J. Am. Chem. Soc. 2011;133:1702–1705. doi: 10.1021/ja110466q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Kieber-Emmons MT, Ginsbach JW, Wick PK, Lucas HR, Helton ME, Lucchese B, Suzuki M, Zuberbühler AD, Karlin KD, Solomon EI. Angew Chem. Int. Ed. 2014;126:5035–5039. doi: 10.1002/anie.201402166. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kim S, Ginsbach JW, Billah AI, Siegler MA, Moore CD, Solomon EI, Karlin KD. J. Am. Chem. Soc. 2014;136:8063–8071. doi: 10.1021/ja502974c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Cho J, Sarangi R, Nam W. Acc. Chem. Res. 2012;45:1321–1330. doi: 10.1021/ar3000019. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rohde J-U, In J-H, Lim MH, Brennessel WW, Bukowski MR, Stubna A, Münck E, Nam W, Que L., Jr Science. 2003;299:1037–1039. doi: 10.1126/science.299.5609.1037. [DOI] [PubMed] [Google Scholar]; c) Ray K, Pfaff FF, Wang B, Nam W. J. Am. Chem. Soc. 2014;136:13942–13958. doi: 10.1021/ja507807v. [DOI] [PubMed] [Google Scholar]
- 10.Hoppe T, Schaub S, Becker J, Würtele C, Schindler S. Angew. Chem. Int. Ed. 2013;52:870–873. doi: 10.1002/anie.201205663. Angew. Chem.2013,125, 904–907. [DOI] [PubMed] [Google Scholar]
- 11.For square-pyramidal (τ = 0) and trigonal bypyramidal (τ = 1) geometry analysis described by: Addison AW, Rao TN, Reedijk J, van Rijn J, Verschoor GC. J. Chem. Soc., Dalton Trans. 1984:1349–1356.
- 12.Bosnich B, Poon CK, Tobe M. Inog. Chem. 1965;4:1102–1108. [Google Scholar]
- 13.Cyclic voltammetry experiments using TV-[(14-TMC)CuI](BArF) as starting material matched the 1 e– redox behavior (E0 = −0.36 V vs. Fc+/0) of the unidentified 14-TMC/CuI product observed in previous electrochemical studies (see the Supporting Information). Bucher C, Duval E, Espinosa E, Barbe J-M, Verpeaux J-N, Amatore C, Guilard R. Eur. J. Inorg. Chem. 2001:1077–1079. doi: 10.1021/ic001472h.
- 14.a) Current experiments are carried out where in situ generation of other than type-V 14-TMC/CuI isomers leads to new 14-TMC/Cu/O2 derived species. These results will be published elsewhere. b) CCDC 1446257, 1446258, and 1446259 contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.
- 15.The ν(O–O) was not observed by rR, however the ν(Cu–O) and ν(Cu–Cu) belong unambiguously to a SP species: Henson MJ, Vance MA, Zhang CX, Liang H-C, Karlin KD, Solomon EI. J. Am. Chem. Soc. 2003;125:5186–5192. doi: 10.1021/ja0276366. Pidcock E, Obias HV, Abe M, Liang H-C, Karlin KD, Solomon EI. J. Am. Chem. Soc. 1999;121:1299–1308.
- 16. Karlin KD, Tyeklár Z, Farooq A, Haka MS, Ghosh P, Cruse RW, Gultneh Y, Hayes JC, Toscano PJ, Zubieta J. Inorg. Chem. 1992;31:1436–1451. b) The DFT optimized structure of the SP complex where the 12-TMC ligand is acting as a tridentate ligand was found to be 16 kcal mol−1 higher in energy.
- 17.Kodera M, Katayama K, Tachi Y, Kano K, Hirota S, Fujinami S, Suzuki M. J. Am. Chem. Soc. 1999;121:11006–11007. [Google Scholar]
- 18.Lam BMT, Halfen JA, Young VG, Jr, Hagadorn JR, Holland PL, Lledos A, Cucurull-Sanchez L, Novoa JJ, Alvarez S, Tolman WB. Inorg. Chem. 2000;39:4059–4072. doi: 10.1021/ic000248p. [DOI] [PubMed] [Google Scholar]
- 19.Paul PP, Tyeklár Z, Jacobson RR, Karlin KD. J. Am. Chem. Soc. 1991;113:5322–5332. [Google Scholar]
- 20.Santagostini L, Gullotti M, Monzani E, Casella L, Dillinger R, Tuczek F. Chem. Eur. J. 2000;6:519–522. doi: 10.1002/(sici)1521-3765(20000204)6:3<519::aid-chem519>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.a) A mechanistic scenario where a concerted diprotonation of the Cu2O2 center is also plausible. Additional studies are necessary to distinguish between these two reaction pathways. b) The inverse KIE indicates the N–H/O–H bonding interaction at the TS is stronger than the N–H bond in the reactant. This is consistent with the higher frequency O-H stretch in the O-protonated product (calcd 3597 cm−1) than the N–H stretch in the N-protonated reactant (calcd 2721 cm−1), see the Supporting Information
- 22.Hart PJ, Balbirnie MM, Ogihara NL, Nersissian AM, Weiss MS, Valentine JS, Eisenberg D. Biochemistry. 1999;38:2167–2178. doi: 10.1021/bi982284u. [DOI] [PubMed] [Google Scholar]
- 23.Helm ML, Stewart MP, Bullock RM, DuBois MR, DuBois DL. Science. 2011;333:863–866. doi: 10.1126/science.1205864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.