ABSTRACT
Hereditary spastic paraplegia (HSP) is a set of genetic diseases caused by mutations in one of 72 genes that results in age-dependent corticospinal axon degeneration accompanied by spasticity and paralysis. Two genes implicated in HSPs encode proteins that regulate endoplasmic reticulum (ER) morphology. Atlastin 1 (ATL1, also known as SPG3A) encodes an ER membrane fusion GTPase and reticulon 2 (RTN2, also known as SPG12) helps shape ER tube formation. Here, we use a new fluorescent ER marker to show that the ER within wild-type Drosophila motor nerve terminals forms a network of tubules that is fragmented and made diffuse upon loss of the atlastin 1 ortholog atl. atl or Rtnl1 loss decreases evoked transmitter release and increases arborization. Similar to other HSP proteins, Atl inhibits bone morphogenetic protein (BMP) signaling, and loss of atl causes age-dependent locomotor deficits in adults. These results demonstrate a crucial role for ER in neuronal function, and identify mechanistic links between ER morphology, neuronal function, BMP signaling and adult behavior.
KEY WORDS: Atlastin, ER, Reticulon, Neuron
Highlighted Article: The ER within Drosophila motor nerve terminals forms a network of tubules. Loss of the fusion GTPase atlastin fragments synaptic ER. Alteration of ER structure decreases evoked neurotransmitter release.
INTRODUCTION
The function of intracellular organelles is tightly coordinated with location within the cytoplasm. The endoplasmic reticulum (ER) is an interconnected network of narrow tubes and flattened cisternae or sheets (Hu et al., 2011; Shibata et al., 2009, 2006; Terasaki et al., 2013; Westrate et al., 2015). In most cells, the ER is the most abundant subcellular organelle and extends elaborate processes throughout the cytoplasm. The ER membrane is formed into its tubular architecture by the action of structural proteins within the reticulon, REEP and DP1 family (English and Voeltz, 2013; Hu et al., 2008; Shibata et al., 2009; Voeltz et al., 2006; Yang and Strittmatter, 2007). The members of this diverse family of proteins share a common protein motif called the reticulon homology domain (RHD). The hydrophobic ∼200-amino-acid RHD likely forms a helical hairpin structure that intercalates four hydrophobic helical segments into the outer leaflet of the ER membrane to induce curvature and maintain a tubular shape (Voeltz et al., 2006; Zurek et al., 2011). Many members of the Reticulon, REEP and DP1 family also contain an extended N-terminal segment ranging from a few hundred to a thousand amino acids that likely provides additional functionality (Di Sano et al., 2012). The nature of most of these secondary functions remains to be revealed.
The large ER network also maintains luminal and membrane continuity throughout the cytoplasm. This interconnected nature of the ER network is required for ER function and is maintained by the ER membrane fusion GTPase atlastin (Orso et al., 2009), which is a member of the fusion dynamin-related protein family (fusion DRP) (McNew et al., 2013; Moss et al., 2011; Pendin et al., 2011).
The ER is closely associated with and functionally connected to the plasma membrane. This connection is often associated with the management of Ca2+ stores in the ER lumen. The ER protein STIM1 diffuses through the ER membrane to find binding partners in the plasma membrane including the Orai channel (Jozsef et al., 2014; Soboloff et al., 2012). This set of protein–protein associations works to restore ER Ca2+ through the store-operated Ca2+ channel system. ER–plasma-membrane contact sites are also generated by the association of ER-integral extended synaptotagmins (E-Syt) with phospholipids in the plasma membrane (Fernández-Busnadiego et al., 2015; Giordano et al., 2013; Schauder et al., 2014) as well as proteins like junctophillins in certain cell types (Helle et al., 2013; Stefan et al., 2013).
Most recently, the ER has been found to be stably associated with endosomal structures (Raiborg et al., 2015a; Rowland et al., 2014). In this circumstance, the specific proteins on each surface that interact remain to be precisely defined, but the consequence of the interaction is functional segregation of certain cargoes within the endosome that permits regulated sorting into membrane subdomains prior to an ER-directed membrane fission event (Raiborg et al., 2015a,b; Rowland et al., 2014).
ER structure appears to be crucially important for cell function given that human disease results when components that control this structure are compromised by mutation. The hereditary spastic paraplegias (HSPs) are a group of related genetic disorders caused by mutations in any of more than 70 genes, denoted SPG1 to SPG72 (Lo Giudice et al., 2014; Noreau et al., 2014). Lower limb weakness and spasticity represent two prominent clinical features of these diseases, which occur as a consequence of dysfunction or degeneration of the upper motor neurons (Blackstone et al., 2010). The observation that atlastin 1 (ATL1) and reticulon 2 (RTN2) are HSP genes responsible for SPG3A and SPG12, respectively, implicates ER morphology in the neuronal dysfunction that causes HSPs.
The properties of three additional HSP genes, spartin (SPG20), spastin (SPAST, also known as SPG4) and NIPA1 (also known as spichthyin or SPG6), implicate receptor trafficking through the endocytic system in HSP neuronal dysfunction. For example, loss of spartin attenuates both ligand-stimulated EGF receptor uptake (Bakowska et al., 2007) as well as depolarization-stimulated FM1-43 uptake (Nahm et al., 2013), whereas loss of spastin increases endosome tubule number and alters transferrin receptor sorting (Allison et al., 2013). NIPA1 is also located in endosomes and promotes the endocytosis of receptors for bone morphogenetic protein (BMP) (Tsang et al., 2009).
Phenotypic analysis of mutations in the HSP orthologs of model systems has provided additional clues to the cellular function of these proteins. In Drosophila, loss of spastin, spartin and spichthyin confers a similar, but not identical, set of phenotypes including stabilized microtubules, increased synaptic bouton number and decreased evoked transmitter release at the larval neuromuscular junction (NMJ), age-dependent locomotor deficits and increased BMP signaling at the larval NMJ (Nahm et al., 2013; Ozdowski et al., 2011; Sherwood et al., 2004; Wang et al., 2007). These shared phenotypes might reflect disruption of a common pathway in endocytic receptor trafficking in these mutants. Some of these phenotypes, such as stabilized microtubules, age-dependent locomotor deficits and increased synaptic bouton number, are also observed in flies lacking atlastin (atl) (Lee et al., 2009, 2008).
Here, we extend this phenotypic analysis of altered atl and reticulon-like 1 (Rtnl1) activities in Drosophila. We show that the ER in motor nerve terminals from wild-type larvae forms a network of tubules that resembles a ‘basket’, but is diffuse in larvae lacking atl. We find that neuronal RNA interference (RNAi)-mediated knockdown of either atl or Rtnl1 increases arborization at the larval neuromuscular junction and decreases evoked transmitter release from larval motor nerve terminals, and that elevated bath [Ca2+] fully rescues these transmitter release phenotypes. We also show that atl is required only in motor neurons to affect transmitter release, whereas Rtnl1 is required additionally in the target muscle and peripheral glia. Finally, we show that loss of atl increases BMP signaling in larval motor neurons and causes age-dependent locomotor deficits in adults. Thus, loss of atl and Rtnl1 confers phenotypes similar, but not identical, to each other as well as to mutants defective in spartin, spastin and spichthyin. Our results demonstrate specific mechanistic links between ER morphology and several aspects of neuronal anatomy and function.
RESULTS
Chemical fixation disrupts ER morphology in Drosophila motor neurons, muscles and S2 cells
ER structure has been difficult to examine in Drosophila. To address this issue, we developed improved reagents for in vivo imaging of the ER. First, we utilized a mutant GFP, termed Superfolder GFP (sfGFP) (Aronson et al., 2011; Costantini et al., 2015; Pédelacq et al., 2006), that has been found to fold properly in oxidizing environments such as the ER lumen. This protein traffics to the ER because of addition of the Drosophila BiP signal sequence and is retained by addition of the HDEL ER retention signal from calreticulin (BiP–sfGFP–HDEL). Next, we introduced an ER membrane marker containing Sec61β linked to the fluorescent marker tdTomato. In Fig. 1, we show the ER labeled by BiP–sfGFP–HDEL expressed in the motor neuron cell body (Fig. 1A), the neuromuscular junction (NMJ) (Fig. 1B), larval body wall muscle (Fig. 1C) and cultured S2 cells (Fig. 1D).
Fig. 1.
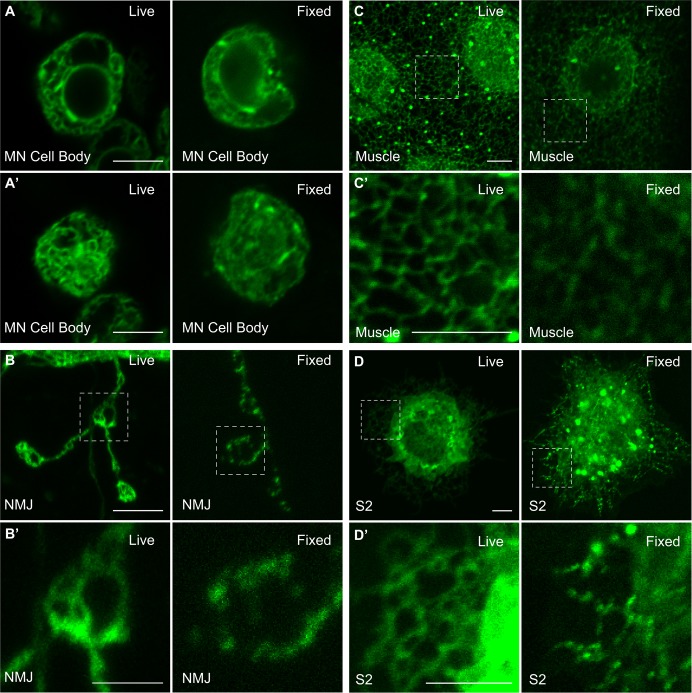
Chemical fixation disrupts the ER network in motor neurons, muscles, and S2 cells. Representative confocal slices through the center (A) and periphery (A′) of live and fixed wild-type motor neuron (MN) cell bodies in the ventral nerve cord. (B) ER in wild-type larval boutons under live and fixed conditions. (B′) Magnification of boxed regions in B. (C) ER in wild-type muscle 6 from segment A3 under live and fixed conditions. (C′) Magnification of boxed regions in C. (D) ER in S2 cells under live and fixed conditions. (D′) Magnification of boxed regions in D. Chemical fixation was achieved by a 5 min exposure to 4% paraformaldehyde. ER was imaged using BiP–sfGFP–HDEL. Scale bars: 5 µm, except B′ (2 µm).
Previous results have suggested that organelle structure in vivo is labile to fixation (Johnson et al., 2015), and therefore an accurate depiction of ER structure might require imaging in live cells. To determine whether ER structures in Drosophila larvae or S2 cells were similarly labile to fixation, we compared ER structure in live cells with the fixed tissue. We found that very mild fixation (4% formaldehyde for 5 min with no permeabilization) significantly altered ER structure in all tissues that were examined (Fig. 1). Upon fixation, ER tubules appeared to fragment into discrete punctae and the uniformly labeled tubules seen in live imaging converted into a ‘beads on a string’ morphology consistent with ruptured tubules. It is possible that the ER tubules are maintained under stress by association with the underlying cytoskeletal network and that chemical fixation disrupts this mechanical tension. Regardless of the mechanism, these observations reveal the disruptive effect of tissue fixation on the ER in vivo. For this reason, all subsequent ER imaging exhibited here was performed on live cells.
BiP–sfGFP–HDEL marks the ER
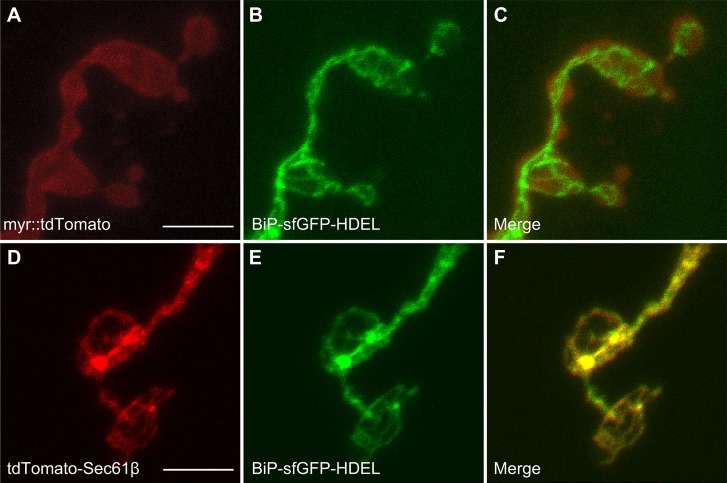
We investigated ER anatomy in larval motor nerve terminals in more detail. To orient the BiP–sfGFP–HDEL signal with the plasma membrane, we co-expressed BiP–sfGFP–HDEL with the plasma membrane marker myr::tdTomato. We found that BiP–sfGFP–HDEL was located immediately adjacent to the myr::tdTomato signal (Fig. 2A–C), indicating that the ER baskets in motor nerve terminals directly underlie the plasma membrane. We also co-expressed BiP–sfGFP–HDEL along with the ER membrane marker tdTomato–Sec61β. As expected, these two markers showed extensive overlap (Fig. 2D–F), thus validating both transgenes as ER markers.
Fig. 2.
The ER lumen marker BiP–sfGFP–HDEL colocalizes with the ER membrane marker tdTomato–Sec61β. Third-instar larval motor nerve terminals from segment A2 muscle 4. (A–C) Representative confocal z-projections showing the plasma membrane marker myr::tdTomato (A), the ER marker BiP–sfGFP–HDEL (B) and the merged signals (C). (D–F) z-projections showing the ER membrane marker tdTomato–Sec61β (D), the ER lumen marker BiP–sfGFP–HDEL (E) and the merged signals (F). All transgenes were driven by the motor neuron driver OK371-Gal4. Scale bars: 5 µm.
The atl2 null mutation alters ER structure in motor axons and presynaptic boutons
atl encodes an ER fusion GTPase (Orso et al., 2009), and thus we anticipated that, by preventing ER fusion, the null mutation atl2 would fragment the ER. To test this prediction, we compared ER morphology in motor neuron cell bodies, motor axon initial segments, and motor nerve terminals in control and atl2 animals (Fig. 3A). We found little effect on ER morphology in cell bodies (Fig. 3Bi versus Fig. 3Bii), but in axon initial segments, we found that the ER crossbridges found in wild-type were almost completely eliminated in atl2 cells, and that the long straight ER tubules observed in wild-type became wavy (Fig. 3Biii,iv). In motor nerve terminals, we found that atl2 eliminated the basket structures and caused a diffuse ER signal, which we attribute to ER fragmentation (Fig. 3Ci,ii).
Fig. 3.
Loss of atl disrupts the tubular ER network in motor axons and motor nerve terminals of third-instar larvae. (A) Schematic of the regions of the motor neuron from which images were collected. (B) Representative central confocal slices of motor neuron cell bodies in control [OK371>BiP–sfGFP–HDEL] (Bi) and atl2 [atl2, OK371>BiP–sfGFP–HDEL] (Bii) larvae. z-projections of motor axons within the ventral nerve cord in wild-type (control) (Biii) and atl2 (Biv) larvae. (C) z-projections of neuromuscular junctions from muscle 4 at segment A6 in wild-type (Ci) and atl2 (Cii) larvae. Average frequency histograms of pixel intensities taken from regions of interest around boutons for control (Ciii) and atl2 (Civ) (w1118 n=5, atl2 n=6; red and blue dashed lines represent the mode and median, respectively, of the histogram and are color coded with the analysis in D). Boxed regions in Ci and Cii are expanded in Ci′ and Cii′, respectively. Dashed lines in Ci′ and Cii′ represent the positions used for the linescans of pixel intensities for wild-type (Cv) and atl2 (Cvi). (D) Mode and median of pixel intensity frequency histograms (mean±s.e.m.) are shown for five control and six atl2 images. P-values shown represent an unpaired Student's t-test performed with KaleidaGraph. Scale bars: 5 µm, except for Ci′ and Cii′ (2 µm).
To quantify the effects of atl2 on ER morphology in nerve terminals, we reasoned that the BiP–sfGFP–HDEL signal in atl2 larvae would be uniformly distributed across the bouton and thus a frequency histogram for the pixel intensities within each terminal would exhibit a Gaussian distribution. In contrast, we anticipated that pixel intensities in wild-type nerve terminals would comprise a small number of bright pixels indicating the tubules, and a larger number of dark pixels indicating voids between tubules. Thus, we predicted that a frequency histogram for the pixel intensities from wild-type would be positively skewed. We compared frequency histograms of pixel intensities, normalized to mean intensity, between wild-type and atl2 and found, as predicted, a strong positive skew in wild-type (Fig. 3Ciii) but a more Gaussian distribution in atl2 (Fig. 3Civ). Furthermore, the mode and median pixel intensities were close to the mean in atl2, consistent with a Gaussian distribution, but were less than the mean in wild-type, consistent with a positive skew (Fig. 3D). In addition, we compared line scans across nerve terminals in atl2 and wild-type. Whereas wild-type line scans showed multiple peaks, corresponding to tubules within the baskets, atl2 line scans were uniform, consistent with a diffuse ER signal (Fig. 3Cv,vi).
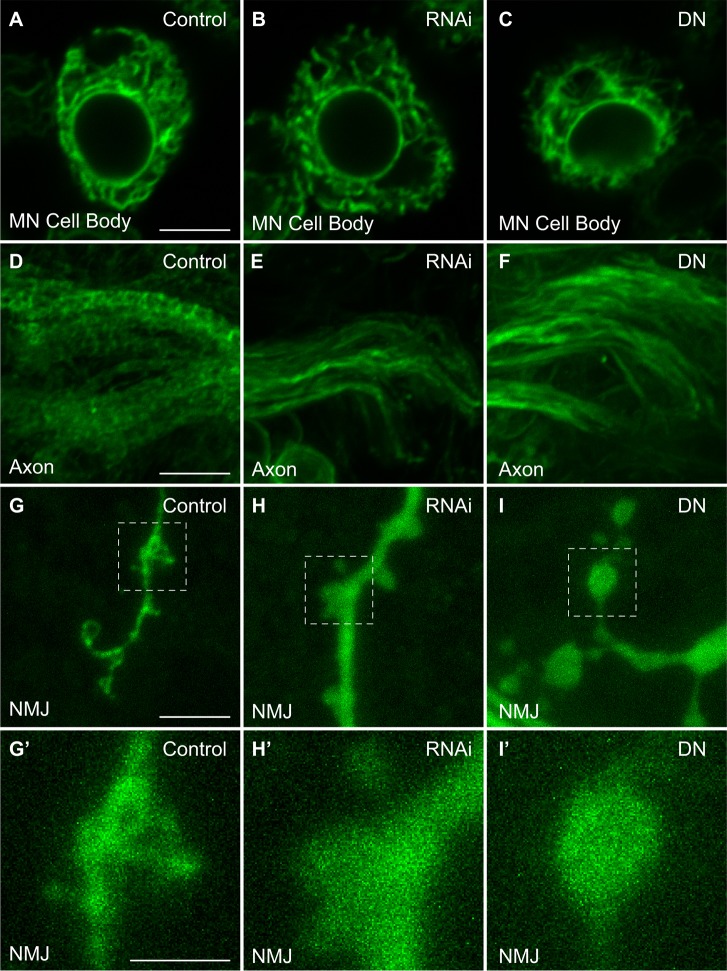
Next, we determined whether the atl2 ER fragmentation could be recapitulated by targeted atl knockdown in motor neurons. Thus, we expressed both an atl RNAi transgene and the dominant-negative atlK51A allele (Orso et al., 2009) in neurons and found a similar decrease in ER tubule crossbridges in axon initial segments (Fig. 4D–F) and a similar diffuse ER signal at motor nerve terminals (Fig. 4G–I). These results demonstrate that maintenance of the correct ER structure in motor neurons requires atlastin activity.
Fig. 4.
Expression of UAS-atlRNAi or UAS-atlK51A disrupts the tubular ER network in motor axons and presynaptic boutons. (A–C) ER in motor neuron (MN) cell bodies for control [nSyb>BiP-sfGFP-HDEL] (A), atl RNAi [nSyb>BiP-sfGFP-HDEL, atlRNAi] (B), and dominant-negative Atl [nSyb>BiP-sfGFP-HDEL, atlK51A] (C). (D–F) ER in motor axons for control (D), atl RNAi (E) and dominant-negative (DN) atlK51A (F). (G–I) ER in presynaptic boutons for control (G), atl RNAi (H) and dominant-negative atlK51A (I). (G′–I′) Magnification of boxed regions for wild-type (G′), atl RNAi (H′), and dominant-negative atlK51A (I′). Scale bars: 5 µm, except for G′,H′ and I′ (2 µm).
In contrast to atl2, the null Rtnl11 allele conferred no detectable abnormalities in ER structure in motor neuron cell bodies, axons or nerve terminals (data not shown).
Impaired evoked transmitter release in atl2 and Rtnl11 null mutants
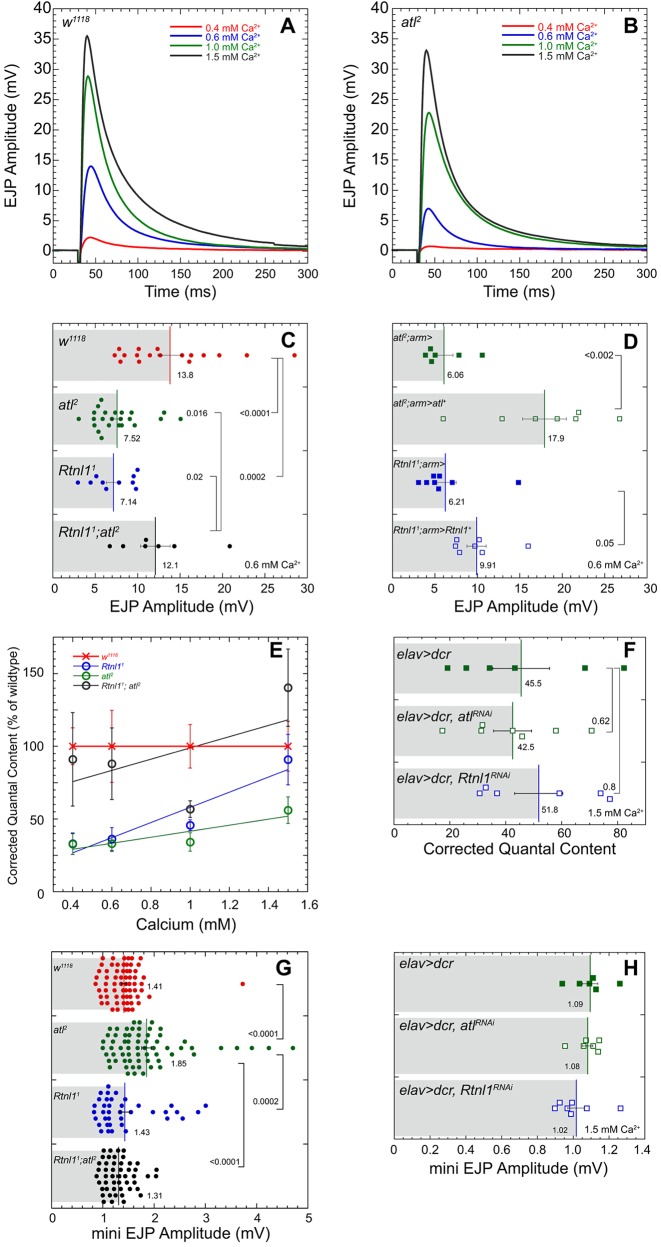
Altered levels of several HSP proteins, including Spartin and Spastin, alter transmitter release from Drosophila motor nerve terminals (Nahm et al., 2013; Ozdowski et al., 2011; Sherwood et al., 2004). We tested the possibility that atl2 and Rtnl11 might similarly affect transmitter release. We used the larval neuromuscular preparation (Jan and Jan, 1976; Stewart et al., 1994) to measure synaptic transmission and found that each mutation indeed decreased evoked transmitter release, using the consequent muscle depolarization termed excitatory junctional potential (EJP) as a readout. Fig. 5A and B show averaged EJPs in wild-type versus atl2, respectively, at the four indicated bath [Ca2+]. At a bath [Ca2+] of 0.6 mM, both atl2 and Rtnl11 decrease transmitter release almost two-fold (Fig. 5C).
Fig. 5.
atl2 and Rtnl11 decrease evoked neurotransmitter release. Average EJP traces for wild-type (w1118) (A) and atl2 (B) larvae at 0.4, 0.6, 1.0, and 1.5 mM Ca2+. In ascending [Ca2+], for w1118 n=7, 17, 7, and 7 and for atl2 n=7, 23, 7, and 7. (C) Mean±s.e.m. EJP amplitude for w1118, atl2, Rtnl11, and Rtnl11; atl2. P-values shown represent a one-way ANOVA using a Fisher's LSD post hoc test performed with KaleidaGraph. (D) Mean±s.e.m. EJP amplitude for atl2; arm>UAS-atl+, Rtnl11; arm>UAS-Rtnl1+, and their respective controls using the ubiquitous arm-Gal4 driver. P-values shown represent an unpaired Student's t-test performed with KaleidaGraph. Recordings for C and D were performed at 0.6 mM Ca2+. (E) Mean±s.e.m. corrected quantal content for w1118, Rtnl11, atl2, and Rtnl11; atl2. In ascending [Ca2+], w1118 n=7, 17, 7, and 7; atl2 n=7, 23, 7, and 7; Rtnl11 n=7, 10, 7, and 7; Rtnl11; atl2 n=7, 7, 7, and 7. Resting membrane potentials (RMPs) were not significantly different among the four genotypes at bath [Ca2+] of 0.4 mM or 1.5 mM. At bath [Ca2+] of 0.6 mM or 1.0 mM, RMPs of wild-type and atl2 were significantly different (P=0.0266, wild-type more hyperpolarized, at 0.6 mM [Ca2+], and P=0.0316, atl2 more hyperpolarized, at 1.0 mM [Ca2+]). RMP of Rtnl11; atl2 was significantly different from other genotypes at a bath [Ca2+] of 1.0 mM, Rtnl11; atl2 more hyperpolarized. (F) Mean±s.e.m. corrected quantal content for elav>dcr, elav>dcr, atlRNAi, and elav>dcr, Rtnl1RNAi at 1.5 mM [Ca2+]. (G) Mean±s.e.m. mEJP amplitudes for w1118, atl2, Rtnl11, and Rtnl11; atl2, pooled from all four [Ca2+]. (H) Mean±s.e.m. mEJP amplitudes for the indicated genotypes collected at 1.5 mM bath [Ca2+]. P-values in D,F,G represent an unpaired Student's t-test performed with KaleidaGraph. Recordings were performed in HL3 from muscle 6 in segment A6. Each scattergram data point is the average of the first 20 EJP responses (successes and failures) recorded for each larva.
To confirm that these transmitter release phenotypes were caused by loss of atl and Rtnl1, we determined whether expression of UAS-atl+ and UAS-Rtnl1+ transgenes would be sufficient to restore wild-type transmitter release to atl2 and Rtnl11. We found that the atl2 transmitter release phenotype was fully rescued by expression of UAS-atl+ driven by the weak ubiquitous Gal4 driver arm-Gal4 (Fig. 5D). In contrast, the Rtnl11 transmitter release phenotype was rescued only partially by arm-Gal4-driven UAS-Rtnl1+; this partial rescue most likely reflected weak expression of the Rtnl1+ transgene because, as shown below, driving UAS-Rtnl1+ expression with the stronger Da-Gal4 driver elicited complete rescue (see Fig. 7B).
Fig. 7.
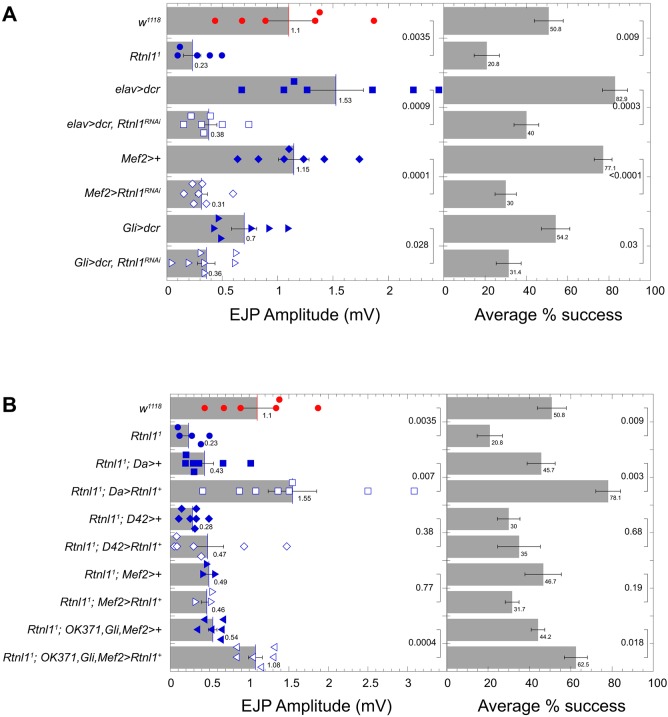
Rtnl1 affects neurotransmitter release from multiple tissues at the NMJ. (A) Mean±s.e.m. EJP amplitude and corresponding percentage success for wild-type (w1118), Rtnl11, and UAS-Rtnl1RNAi expression in neurons, muscles and glia using elav-Gal4, Mef2-Gal4 and Gli-Gal4, respectively. (B) Mean±s.e.m. EJP amplitude and corresponding percentage success for w1118, Rtnl11 and expression of UAS-Rtnl1+ in Rtnl11 mutants. UAS-Rtnl1+ was expressed ubiquitously, in motor neurons and in muscles using da-Gal4, D42-Gal4 and Mef2-Gal4, respectively. OK371, Mef2 and Gli-Gal4 denote concurrent expression of UAS-Rtnl1+ from motor neurons, muscles and glia. Recordings for A and B were made in HL3.1 at 0.1 mM Ca2+ from muscle 6 in segment A6. Each scattergram data point is the average of the first 20 EJP responses (successes and failures) recorded for each larva. P-values shown represent an unpaired Student's t-test performed with KaleidaGraph.
Previous reports have indicated that nerve terminal ER is capable of controlling transmitter release by influencing cytoplasmic [Ca2+] (Emptage et al., 2001; Liang et al., 2002; Llano et al., 2000). We wondered whether the decreased evoked transmitter release in atl2 and Rtnl11 might reflect attenuation of the increased cytoplasmic [Ca2+] caused by nerve stimulation. If so, then we predicted that this decreased transmitter release would be rescued by elevated bath [Ca2+], at which Ca2+ is less limiting for transmitter release (Wong et al., 2014). For both atl2 and Rtnl11, we found that as we increased bath [Ca2+], transmitter release became progressively similar to that in wild type (Fig. 5E, which shows quantal content, corrected for nonlinear summation and normalized to wild type). Elevated bath [Ca2+] completely rescued the Rtnl11 transmitter release phenotype, but rescued the atl2 phenotype only partially. In addition, we found that RNAi-mediated knockdown of either atl or Rtnl1 in neurons similarly failed to decrease transmitter release at elevated bath [Ca2+] (Fig. 5F; efficacy of the atl and Rtnl1 RNAis is demonstrated in experiments described below). It is not known why elevated bath [Ca2+] rescues the atl RNAi knockdown phenotype completely, but atl2 only partially. However, it is possible that atl acts in non-neuronal tissues to affect transmitter release at elevated bath [Ca2+].
In contrast to the strong effects of altered atl and Rtnl1 on EJP amplitude, the amplitudes of ‘mini’ EJPs, generated by spontaneous release of individual vesicles of transmitter, were not substantially affected in atl2 or Rtnl11 larvae (Fig. 5G). The modest, albeit significant, increase in mini EJP amplitude observed in atl2 is likely a genetic background effect unrelated to the atl genotype, as mini EJP amplitude is not significantly affected by arm-Gal4-driven expression of the atl+ transgene (data not shown), and elav-Gal4-driven expression of atl RNAi does not affect mini EJP amplitude (Fig. 5H).
It has been previously observed that atlastin and reticulon confer opposite effects on ER morphology. In particular, whereas atl2 fragments the ER (Orso et al., 2009), Rtnl1 knockdown by RNAi (O'Sullivan et al., 2012) converts ER tubes into sheets. We found a mutual suppression of the evoked transmitter release phenotype in the Rtnl1; atl2 double mutant (Fig. 5C,E), which supports the possibility that atlastin and reticulon have antagonistic effects on ER morphology.
We examined synapse architecture in atl2 and Rtnl11 mutants by performing immunocytochemistry with antibodies directed at the presynaptic active zone protein Bruchpilot and the postsynaptic neurotransmitter receptor GluRII. We found no obvious structural or organizational defects in the number or size of active zones or in the pattern of GluRII immunoreactivity (data not shown).
atl acts in the motor neuron to control transmitter release
Next, we wished to identify the tissues in which Atl and Rtnl1 are required to control neurotransmitter release. Because atl2 and Rtnl11 exhibited the greatest deficit in neurotransmitter release at the lowest bath [Ca2+] (Fig. 5), we performed the next set of measurements at the low bath [Ca2+] of 0.1 mM, in a solution (HL3.1) conducive for low [Ca2+] recordings.
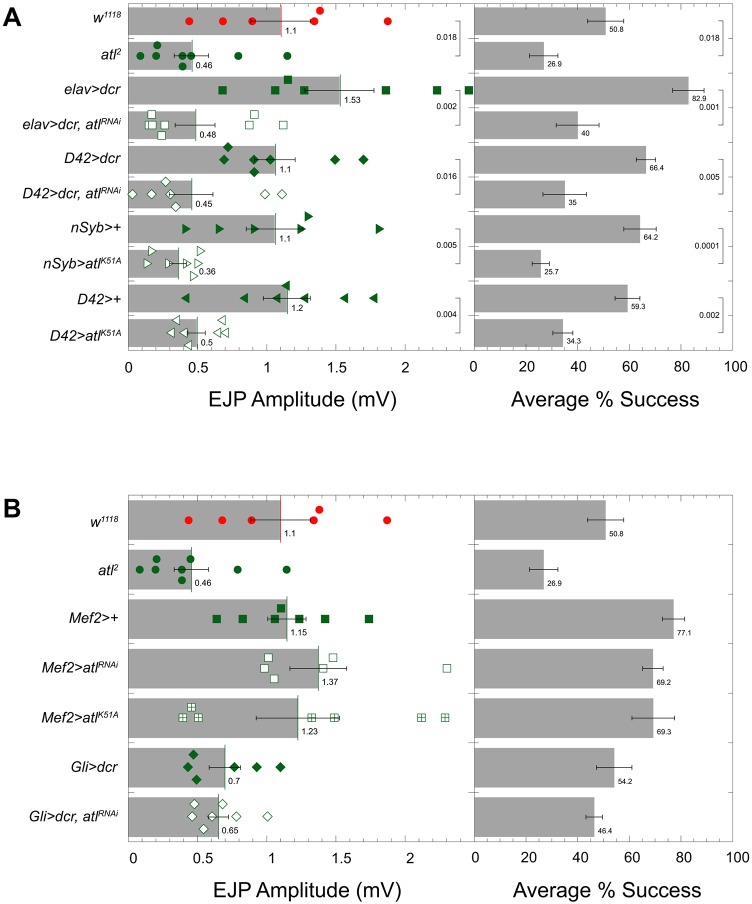
Inhibiting atl by driving atl RNAi expression with the motor neuron driver D42-Gal4 (Fig. 6A, left, green diamonds) or the pan-neuronal driver elav-Gal4 (Fig. 6A, left, green squares) significantly decreased EJP amplitude. Similarly, inhibiting atl by expressing the dominant-negative atlK51A with either D42-Gal4 (Fig. 6A, left, green inverted triangles) or the pan-neuronal driver nSyb-Gal4 (Fig. 6A, left, green triangles) also significantly decreased EJP amplitude. These results indicate that atl activity is required in the motor neuron for wild-type evoked transmitter release.
Fig. 6.
Neuronal expression of UAS-atlRNAi and UAS-atlK51A decreases evoked neurotransmitter release. (A) Mean±s.e.m. EJP amplitude and corresponding percentage success for wild-type (w1118), atl2, UAS-atlRNAi and UAS-atlK51A. Drivers include the pan-neuronal elav-Gal4 and nSyb-Gal4 and the motor neuronal D42-Gal4. (B) Mean±s.e.m. EJP amplitude and percentage success for muscle (Mef2-Gal4) and glial (Gli-Gal4) expression of UAS-atlRNAi and UAS-atlK51A. Recordings for A and B were made in HL3.1 at 0.1 mM Ca2+ from muscle 6 in segment A6. Each scattergram data point is the average of the first 20 EJP responses (successes and failures) recorded for each larva. P-values shown represent an unpaired Student's t-test performed with KaleidaGraph.
We confirmed that the decrease in EJP amplitude represented decreased transmitter release rather than decreased muscle responsiveness to transmitter by analyzing the frequency of synaptic transmission failures. In the low [Ca2+] bath utilized in Fig. 6, the motor neuron responds to nerve stimulation with release of either no transmitter, which is called a failure, or one or two vesicles of transmitter, which is called a success. The decreased transmitter release in the atl knockdown larvae was accompanied by a decreased frequency of successes (Fig. 6A, right), indicating that atl knockdown decreases transmitter release.
Transmitter release at the Drosophila neuromuscular junction can be affected by the target muscle or the neighboring peripheral glia (Huang and Stern, 2002; Kerr et al., 2014; McCabe et al., 2003; Schmidt et al., 2012). RNAi-mediated atl knockdown in muscle or glia did not affect transmitter release or frequency of synaptic failures (Fig. 6B, left, green squares; Fig. 6B, left, green diamonds).
Overexpression of atl in the motor neuron impairs neurotransmitter release
Expression of UAS-atl+ in Drosophila motor neurons causes excessive fusion and expansion of the nuclear envelope and ER membrane, and defects in the secretory pathway in both the neuron cell body and presynaptic terminal (Orso et al., 2009). Using the BiP–sfGFP–HDEL imaging reagent described above, we found that atl+ overexpression caused accumulation of large ER punctae in motor neuron cell bodies and axons (Fig. S1A). To determine whether atl+ overexpression affected transmitter release, we compared EJP amplitudes in atl+ overexpressing and control larvae at both low (0.1 mM) and high (1.5 mM) bath [Ca2+]. We found that atl+ overexpression significantly decreased EJP amplitudes and the frequency of synaptic successes at low bath [Ca2+] (Fig. S1B), and decreased corrected quantal content at high bath [Ca2+] (Fig. S1C). Rtnl1+ overexpression also decreased transmitter release but to a lesser extent than atl+. We conclude that maximum levels of evoked transmitter release require specific ratios of Atl and Rtnl1 protein.
Rtnl1 regulates transmitter release from multiple tissues at the NMJ
We used both tissue-specific transgenic rescue and RNAi-mediated knockdown to identify the tissue(s) within which Rtnl1 functions to control transmitter release. First, we found that driving Rtnl1 RNAi expression with the pan-neuronal driver elav-Gal4 (Fig. 7A, left, blue squares) significantly decreased both evoked EJP amplitude and percentage of synaptic successes (Fig. 7A, right). This result demonstrates that Rtnl1 is required in the motor neuron to affect transmitter release. We then determined whether Rtnl1 is required in the other cell types of the tripartite synapse and found that RNAi-mediated Rtnl1 knockdown in either muscle (Fig. 7A, left, blue diamonds) or glia (Fig. 7A, left, blue triangles) also significantly decreased evoked EJP amplitude and synaptic success rate (Fig. 7A, right). Thus, Rtnl1 activity is required in all three cell types of the tripartite synapse for proper evoked transmitter release.
We next determined whether Rtnl1 activity in these three cell types was sufficient for proper evoked transmitter release. First, we verified effectiveness of the Rtnl1+ rescue construct by determining that ubiquitous expression of the Rtnl1+ transgene, driven by the Da-Gal4 driver, was sufficient to rescue both evoked EJP amplitude (Fig. 7B, left) and frequency of synaptic successes of Rtnl11 (Fig. 7B, right). As expected from the RNAi results described above, expression of Rtnl1+ driven pan-neuronally, in the motor neuron alone, the muscle alone, or both the motor neuron and muscle, was not sufficient to rescue the EJP amplitude or synaptic success phenotypes of Rtnl11 (Fig. 7B and data not shown). However, simultaneous expression of Rtnl1+ in the motor neuron, muscle and peripheral glia was sufficient to significantly restore proper EJP amplitude and synaptic success frequency to Rtnl11 (Fig. 7B). We conclude that Rtnl1 activity in the motor neuron, target muscle and neighboring peripheral glia is sufficient as well as necessary to maintain proper synaptic transmission.
atl2 increases BMP signaling in larval motor neurons
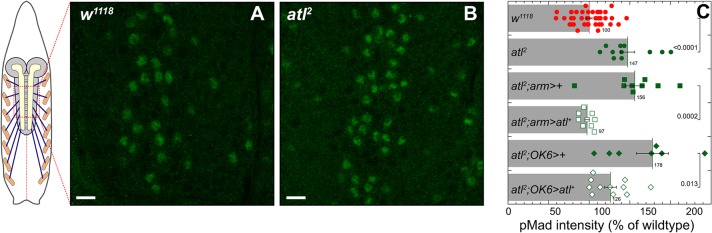
BMPs are a family of secreted ligands that control a wide variety of organismal functions. Following binding to membrane receptors, BMPs trigger the phosphorylation and activation of the Mad transcription factor (Miyazono et al., 2010), which is assayed with antibodies specific to the active phosphorylated (p)Mad. In addition to developmental functions, Drosophila BMP ligands released from muscle, peripheral glia or the motor neuron itself regulate transmitter release and synaptic growth in motor neurons (Fuentes-Medel et al., 2012; McCabe et al., 2003). Many HSP proteins, including Drosophila Spichthyin and Spartin, mammalian spastin and zebrafish atlastin, inhibit BMP signaling (Fassier et al., 2010; Nahm et al., 2013; Wang et al., 2007), possibly through altered BMP receptor trafficking. To determine whether Atl might similarly inhibit BMP signaling, we measured nuclear pMad within motor neuron nuclei of atl2 third-instar larvae. We observed a significant increase in nuclear pMad in atl2, which was rescued by both weak ubiquitous (Fig. 8C, green squares) or motor neuron-specific expression of atl+ (Fig. 8C, green diamonds). Thus, Atl behaves similarly to other HSP proteins as an inhibitor of BMP signaling.
Fig. 8.
atl2 increases BMP signaling in motor neurons. Representative confocal images of w1118 (A) and atl2 (B) motor neuron nuclei from third-instar larvae labeled with anti-pMad antibody. Scale bar: 10 µm. (C) Mean±s.e.m. pMad levels for w1118, atl2, atl2; arm>+, atl2; arm>atl+, atl2; OK6>+, and atl2; OK6>atl+ in motor neuron nuclei. Each scattergram data point is the average nuclear signal intensity per area for a single larva. P-values shown represent an unpaired Student's t-test performed with KaleidaGraph.
Neuronal loss of atl and Rtnl1 causes axon terminal overgrowth
Mutations in the Drosophila HSP orthologs described above increase synaptic bouton number at the third-instar larval NMJ. In addition, Lee et al. (2009) have reported that bouton number is also increased in atl2 NMJs, and that this increase is rescued by muscle but not neuronal atl+ expression. We found that pan-neuronal knockdown of either atl or Rtnl1 significantly increased bouton number, and that this increase was suppressed when both genes were knocked down simultaneously (Fig. S2). These results suggest that Atl and Rtnl1 are required within the motor neuron to restrain axon outgrowth.
Neuronal knockdown of atl causes age-dependent locomotor deficits
HSP patients share a characteristic increase in spasticity and weakening of the lower extremities with age. Drosophila mutant for any of several HSP orthologues similarly display behavioral deficits (Lee et al., 2008; Nahm et al., 2013; Sherwood et al., 2004). We used two assays to determine whether pan-neuronal atl inhibition generated locomotor deficits. First, we measured the time required for elav>Dcr, atlRNAi adults to climb 6 cm, and second, we used the iFly tracking system (Kohlhoff et al., 2011) to measure walking velocity. We observed age-dependent locomotor deficits with each assay (Fig. S3). It is unlikely that these locomotor deficits result directly from the deficits in larval synaptic transmission that we have documented. Rather, these locomotor deficits might reflect age-dependent degeneration of specific populations of neurons as has been reported previously for an atl hypomorph (Lee et al., 2008).
DISCUSSION
The role of ER morphology in nervous system function and anatomy
Mutations in two genes that affect ER morphology, atlastin (ATL1) and reticulon 2 (RTN2), cause two forms of hereditary spastic paraplegia (HSP), which result in progressive limb weakness, spasticity and degeneration of the longest motor axons (Blackstone et al., 2010). These observations suggest that altered ER morphology is causal for motor axon dysfunction, but the mechanisms underlying these dysfunctions are unclear. Here, we use Drosophila to evaluate the nervous system deficits caused by altered atl and Rtnl1 activity. Using a new fluorescent ER imaging reagent, we show that the ER in wild-type motor nerve terminals is present as a network of tubules, termed ‘baskets’, underlying the plasma membrane, and that these baskets are eliminated in larvae lacking or overexpressing atl. We also show that loss of either atl or Rtnl1 increases arborization and decreases evoked transmitter release, and that evoked release is restored to normal by elevated bath [Ca2+]. Finally, we show that atl loss increases signaling through the bone morphogenetic protein (BMP) pathway and causes age-dependent declines in adult locomotion. This set of phenotypes is also exhibited by Drosophila mutant for the HSP orthologs of spartin, spastin and spichthyin, as well as for spinster and nervous wreck, which encode regulators of receptor trafficking through endosomes (Nahm et al., 2013; O'Connor-Giles et al., 2008; Ozdowski et al., 2011; Sherwood et al., 2004; Sweeney and Davis, 2002; Wang et al., 2007). The possible involvement of the ER in this receptor trafficking pathway will be discussed.
Effects of atl loss or overexpression on ER morphology in motor neurons
We made two adjustments to improve visualization of the ER. First, we introduced into flies a transgene that encoded an ER-localized superfolder GFP, which was optimized for efficient folding in the ER. Second, based on previous results indicating that the ER as well as the lysosomal tubule network is labile to fixation (Johnson et al., 2015), we imaged ER in live tissues. Using these approaches, we showed that the ER is present within the axon initial segments of motor neurons as a polygonal structure with numerous crossbridges (three-way junctions), and in motor nerve terminals as a network of tubules that we term ‘baskets’, which underlie the plasma membrane. We also showed that these structures are disrupted by either loss of or overexpression of atl. In particular, atl overexpression causes the aberrant appearance of large punctae in motor neuron cell bodies or axon initial segments. In contrast, atl loss decreases the number of crossbridges in the axon initial segment, leading to excessively long tubules. A similar appearance was noted previously (Hu et al., 2009; Orso et al., 2009) and attributed to deficits in fusion of orthogonal ER membranes. Loss of atl also disrupts nerve terminal baskets and appears to cause ER fragmentation. It is possible that the transition from tubules to baskets as the ER moves from the interbouton region to boutons occurs through ER fragmentation followed by Atl-dependent reassembly. In this view, loss of atl would prevent this reassembly, thus causing the fragmented ER that we observe.
Deficits in evoked transmitter release in larvae lacking atl or Rtnl1 are rescued by elevated bath [Ca2+]
Evoked transmitter release deficits in both atl2 and Rtnl11 mutants, and in pan-neuronal atl or Rtnl1 knockdown larvae, were rescued partially or completely by elevated bath [Ca2+]. These results indicate that loss of atl or Rtnl1 decreases evoked transmitter release at low bath [Ca2+] through causing deficits in evoked increases in cytoplasmic [Ca2+]. Insufficient Ca2+ influx could result from attenuated action potentials, which would decrease the opening of voltage-gated Ca2+ channels, decreases in number of plasma membrane Ca2+ channels or decreased Ca2+ release from the ER. Given the role of atlastin and the reticulons as ER-shaping molecules, effects on ER Ca2+ release would be the most direct explanation for this Ca2+ phenotype. ER-localized Ca2+ release channels such as the inositol 1,4,5-trisphosphate (IP3) receptor, the ryanodine receptor and the TRPV1 channel play key roles in evoked neurotransmitter release (Emptage et al., 2001; Liang et al., 2002; Llano et al., 2000; Wong et al., 2014). In addition, dominant-negative mutations in the Drosophila ER-localized Ca2+ pump SERCA decrease evoked transmitter release by ∼50% (Sanyal et al., 2005), which is consistent with the possibility that ER-derived Ca2+ contributes significantly to the Ca2+ required to trigger transmitter release.
Rtnl1 affects evoked neurotransmitter release from multiple tissues
Unlike Atl, which appears to affect evoked transmitter release from neurons alone, Rtnl1 is required in neurons, muscles and peripheral glia for correct evoked transmitter release. This finding is consistent with previous data demonstrating that proper synaptic transmission requires intercellular signaling among these three cell types. In particular, loss of activity within the peripheral glia of the kinesin heavy chain gene or the inebriated-encoded neurotransmitter transporter alters evoked transmitter release (Huang and Stern, 2002; Schmidt et al., 2012). In addition, the peripheral glia secrete at least two proteins, the TGF-β ligand Maverick and Wingless/Wnt, that regulate synaptic function (Fuentes-Medel et al., 2012; Kerr et al., 2014). The muscle, in turn, secretes the BMP ligand Gbb to regulate both evoked transmitter release and motor neuron arborization (McCabe et al., 2003). It is possible that loss of Rtnl1 affects transmitter release from glia or muscle by perturbing secretion of these or other regulators.
Pan-neuronal atl knockdown causes progressive adult locomotor deficits during aging
The most prominent clinical symptom in HSP patients is progressive, age-dependent locomotor difficulties. Drosophila mutant for any of several HSP orthologs, including spartin, spastin, atl and Rtnl1, as well as spinster, exhibit similar age-dependent locomotor deficits or lifespan deficits (Dermaut et al., 2005; Lee et al., 2008; Nahm et al., 2013; O'Sullivan et al., 2012; Orso et al., 2009; Sweeney and Davis, 2002). Here, we show locomotor impairment in adults with neuronal-specific atl knockdown. These results indicate a requirement for atl in neurons for proper locomotion but do not rule out crucial roles for atl in other tissues as well.
A potential role for the ER in endocytic receptor trafficking
Mutants in Drosophila orthologs of several HSP genes, including spartin, spastin and spichthyin, and the additional related genes spinster and nervous wreck share a common set of nervous system phenotypes, including increased arborization and BMP signaling at the larval NMJ, decreased evoked transmitter release and locomotor deficits (Nahm et al., 2013; O'Connor-Giles et al., 2008; Ozdowski et al., 2011; Sherwood et al., 2004; Sweeney and Davis, 2002; Wang et al., 2007) (note that not all phenotypes have been reported for each mutant). The encoded proteins localize to various compartments within the endocytic receptor trafficking pathway (Allison et al., 2013; Edwards et al., 2009; O'Connor-Giles et al., 2008; Sweeney and Davis, 2002; Wang et al., 2007). In fact, the increased BMP signaling in several of these mutants has been attributed to trafficking defects of the BMP receptor Wishful thinking (Wit). We have shown that atl loss confers these same phenotypes, raising the possibility that atl acts in the endocytic receptor trafficking pathway as well. Although the ER is not known to play prominent roles in this pathway, a recent report has demonstrated that the ER is required for endosome fission in COS cells, and, in fact, the ER selects the location of fission (Rowland et al., 2014). In addition, it has been found that this process is inhibited by overexpression of Rtn4a, which, similarly to atl loss, elongates ER tubules and inhibits formation of crossbridges (Rowland et al., 2014). Thus, loss of atl could impact on the receptor trafficking pathway in Drosophila nerve terminals by similarly preventing endosome fission.
The variety of phenotypes exhibited in common by the mutants described above raises the possibility that certain phenotypes might have causal relationships with others. The subcellular locations of these proteins suggest that they might directly affect receptor trafficking. If so, then the increased BMP signaling, as a consequence of altered Wit trafficking, might be the direct cause of the increased arborization and locomotor deficits. The phenotypes conferred by direct activation of the BMP pathway in neurons are consistent with this possibility (McCabe et al., 2003; Nahm et al., 2013). However, the increased BMP signaling is unlikely to cause the decreased transmitter release, as decreased BMP signaling, rather than increased BMP signaling, decreases evoked transmitter release (McCabe et al., 2003). We suggest that trafficking of receptors in addition to Wit are altered in the mutants described above, and it is the altered signaling of these additional receptors that is at least partly responsible for the transmitter release phenotype. Drosophila motor nerve terminals express a cholecystokinin-like receptor (CCKLR), a toll-like receptor, a metabotropic glutamate receptor (mGluRA) and likely the insulin receptor (Ballard et al., 2014; Bogdanik et al., 2004; Chen and Ganetzky, 2012; Howlett et al., 2008). Loss of mGluRA increases evoked transmitter release (Bogdanik et al., 2004; Howlett et al., 2008), raising the possibility that increased mGluRA signaling might decrease transmitter release, which could explain the decreased transmitter release observed in these receptor trafficking mutants.
MATERIALS AND METHODS
Drosophila stocks and medium
The following lines were obtained from the Bloomington Drosophila Stock Center at Indiana University: w1118 (#3605), arm-Gal4 (#1560), elav-Gal4 (#458), elav-Gal4; UAS-Dcr-2 (#25750), nSyb-Gal4 (#51635), OK371-Gal4 (#26160), Mef2-Gal4 (#27390), UAS-Dcr-2 (#24650), UAS-Dcr-2 (#24651), and UAS-myr::tdTomato (#32221). The da G32 (#108252) Gal4 driver line was provided by the Drosophila Genetic Resource Center. UAS-Rtnl1RNAi (#7866) was obtained from the Vienna Drosophila Resource Center. OK6-Gal4 (Aberle et al., 2002) was provided by Hermann Aberle (Heinrich Heine University, Düsseldorf, Germany). D42-Gal4 (Yeh et al., 1995) was provided by Thomas Schwarz (Children's Hospital Boston, F. M. Kirby Neurobiology Center, Boston, MA). Gli-Gal4 (Sepp and Auld, 1999) was provided by Vanessa Auld (The University of British Columbia, Department of Zoology, Vancouver, British Columbia, Canada). UAS-atl+, UAS-atlK51A, and UAS-atlRNAi were as described previously (Orso et al., 2009). atl2 (Lee et al., 2009) and Rtnl11 (Wakefield and Tear, 2006) were as described previously. UAS-Rtnl1+ was provided by Andrea Daga.
All fly stocks were maintained on cornmeal and agar medium (6% dextrose, 6.8% cornmeal, 1.2% yeast, 0.72% agar, 2% methyl 4-hydroxybenzoate) at room temperature (∼23°C) or 25°C.
Plasmid construction
To construct pJM952 (pAc5/BiP-sfGFP-HDEL), Superfolder GFP (sfGFP) in pEGFP-N1 (Aronson et al., 2011)] was provided by Erik Snapp (Albert Einstein College of Medicine, Department of Anatomy & Structural Biology, Bronx, NY). The Drosophila BiP signal sequence (MKLCILLAVVAFVGLSLG-RS) was then fused to sfGFP with a C-terminal ER retention signal (-HDEL) from Drosophila Calreticulin (Smith, 1992) in pAc5.1/V5-His A (Invitrogen). To construct pJM1033 (UAS-BiP-sfGFP-HDEL), pUASTattB (Bischof et al., 2007) was provided by Konrad Basler (University of Zürich, Institute of Molecular Life Sciences, Zürich, Switzerland). The BiP-sfGFP-HDEL cassette from pJM952 was then moved to pUASTattB. To construct pJM1072 (UAS-tdTomato-dSec61β), tdTomato was fused to Drosophila Sec61β (Drosophila Genomics Resource Center cDNA clone #RE18615) in a 20X-UAS-IVS vector derived from pJFRC7 (Addgene #26220).
Drosophila stock construction
pJM1033 and pJM1072 were injected into attP2 and VK37 embryos by Genetivision (Houston, TX). Injected flies were backcrossed twice to w1118 flies and stocks carrying insertions on chromosome two (VK37) and three (attP2) were established. The UAS-Rtnl1 line was produced with the Rtnl1-PB isoform cDNA (LD14068). The cDNA was subcloned in-frame with an HA tag in the pUAST vector, and transgenic lines were generated by standard microinjection.
Live larval imaging
Wandering third-instar larvae, reared at 25°C, were dissected in HL6 buffer (Macleod et al., 2002), a complex buffer designed for imaging metabolically active fly larvae, with 0 mM CaCl2 and 7 mM monosodium glutamate on 35-mm petri dish lids completely filled with Sylgard 184 (Electron Microscopy Sciences). Minutien pins (0.1-mm diameter, Fine Science Tools), bent to 90° and trimmed, were used to secure larvae to the Sylgard. Round 25-mm coverslips (#1 thickness) were cut in half with a diamond knife and attached to the Sylgard on either side of dissected larvae to form a channel. Square 22-mm coverslips (#1.5 thickness) were placed over the larvae and secured to the bottom coverslips with nail polish. Larvae were imaged on a Zeiss LSM 710 inverted confocal microscope using a Plan-Apochromat 63× (1.40 NA) oil immersion objective. GFP was excited with a 488-nm argon laser and emitted light between 493 and 522 nm was collected. tdTomato was excited with a 543-nm helium neon laser and emitted light between 552 and 691 nm was collected. Images were adjusted for brightness and contrast in ImageJ. Regions of interest (ROIs) were manually drawn around boutons in ImageJ. ROI pixel intensities were extracted and processed with a custom Python script (available upon request). Pixel intensities were scaled by dividing each value by the mean pixel intensity. These data were binned into 50 equally spaced bins ranging from 0 to 8 to generate a frequency histogram. The median and mode of pixel intensities were calculated for each image and graphed with KaleidaGraph.
Fixed larval imaging
Wandering third-instar larvae were reared and dissected as described for live imaging. Larvae were then fixed with 4% formaldehyde in HL6 buffer for 5 min, washed with HL6 and mounted in Vectashield (Vector Labs). Slides were imaged as described for live larvae.
S2 cell imaging
pJM952 was transfected into S2R+ cells (Drosophila Genomics Resource Center) using Fugene HD (Promega) according to the manufacturer's protocols. Cells were adhered to Concanavalin-A-coated glass-bottom dishes (Mat-tek) overnight. Fixed cells were exposed to 4% formaldehyde in HL3 for 5 min and washed with PBS. Cells were imaged on a Nikon A1-Rsi laser scanning confocal microscope using a CFI Plan Apo VC 60× (1.4 NA) oil immersion objective. GFP was excited with a 488-nm argon laser and emitted light between 500 and 550 nm was collected.
Electrophysiology
Wandering third-instar larvae were dissected in HL3 (Stewart et al., 1994) for data collected at 0.4 mM, 0.6 mM, 1.0 mM and 1.5 mM [Ca2+], or HL3.1 (Feng et al., 2004) for data collected at 0.1 mM [Ca2+]. Recordings were performed as described previously (Howlett et al., 2008). Evoked response traces for larvae were recorded for at least 20 s. Quiescent traces of 60 s were recorded for mEJP frequency and amplitude analysis. EJP and mEJP analysis was accomplished using Synaptosoft Mini Analysis Program. Frequency and amplitude of mEJPs were analyzed using the ‘non-stop analysis’ function. For this analysis, the mEJP amplitude threshold was set at 0.7 mV with a LoPass Butterworth filter and a cut-off frequency of 200 Hz. EJP amplitude was determined by manually selecting the peaks, which corresponded to the first 20 stimulations within a trace. Failures within the first 20 stimulations of a trace were denoted as zeroes and contributed toward average EJP data. No threshold minimum was employed for EJPs. Corrected quantal content was calculated using m=ν/ν1(1-ν/V0)−1, where ν=EJP amplitude, ν1=mEJP amplitude and V0=resting potential minus a correction factor of 15 mV (Martin, 1955).
pMad quantification
Wandering third-instar larvae, reared at room temperature, were dissected in S2 medium (Invitrogen) and fixed immediately (4% formaldehyde in S2 medium) for 15 min. Larvae were washed with PBS-T (PBS plus 0.1% Triton X-100) and transferred to Sylgard-coated 24-well plates. Larvae were incubated with anti-p-Smad1/5 (Ser463/465) rabbit monoclonal antibody (Cell Signaling, 41D10, 1:50 in PBS-T) overnight (4°C) with gentle orbital shaking, washed with PBS-T, incubated with goat Alexa-Fluor-488-conjugated anti-rabbit IgG antibody (Invitrogen, A-11008, 1:1000 in PBS-T) for 90 min at room temperature, washed with PBS, and mounted in vectashield. For each experiment, at least three w1118 larvae were included, and all larvae were exposed to the same antibody dilution. Ventral ganglia from at least eight larvae from two independent experiments were analyzed. Larvae were imaged as described for live larvae. ROIs were drawn around motoneuronal nuclei in the ventral ganglia using ImageJ. Mean pixel intensity was divided by area. ROIs were summed and averaged for each larva, and normalized to the w1118 internal control.
Arborization
Wandering third-instar larvae reared at 25°C were dissected in HL3, fixed in 4% formaldehyde (in HL3) and washed with PBS-T. Pelts were placed in sylgard-coated 24-well plates and exposed to goat Alexa-Fluor-488-conjugated anti-horseradish-peroxidase antibody (Jackson ImmunoResearch, 123-545-021, 1:500) overnight at 4°C with gentle orbital shaking, washed in PBS, and mounted in Vectashield. Boutons at muscles 6 and 7 in segment A3 were imaged as described for live larvae. Boutons were counted in ImageJ.
Climb tests
Crosses were made with ten mating pairs in half-pint bottles at 25°C. Progeny were collected 2 days after eclosion, and flies were aged for 0 and 2 days post eclosion. Males were placed into vials in groups of 10–12 flies and aged at 25°C. Adult male flies were separated into individual empty vials, tapped to the bottom and timed to see how long it took them to climb 6 cm vertically. Flies experienced three time trials with 5 min of rest between trials. If a fly failed to cross the 6-cm mark in 60 s, the time was recorded as 60 s. The minimum time for each fly was compiled and averaged as the time for that day. Results represent a minimum of ten flies.
Velocity tests
Adult flies that were 0–2 days old were reared at 25°C and anesthetized with carbon dioxide. Groups of 6–10 males were aged until 3, 10, 15, 25 and 30 days (±1 day). Flies were transferred, without anesthesia, into vials with 10 ml of 1% agar and allowed to rest for 10 min. Flies were placed in a white, translucent Plexiglas chamber with a digital video camera focused on the vial. Two mirrors placed behind the vial at 40° angles produce two reflections visible in the video. Vials were banged to knock flies to the agar surface and climbing-behavior videos were captured. Three trials of 30 s each were recorded in succession for each group. Average velocity for each vial was measured using the iFly system (Jahn et al., 2011; Kohlhoff et al., 2011).
Acknowledgements
The authors would like to thank various members of the Drosophila community as well as the Bloomington Stock Center for providing fly lines. Damian Crowther and Eleonora Khabirova provided software and assistance with the iFly system. Enrique Martinez assisted with the S2 imaging, Justin Lee assisted in determining EJP amplitudes with Synaptosoft, Zach Wright assisted with imaging active zone proteins, Miguel Betancourt-Solis assisted with the sfGFP constructions and Justin Vincent assisted with analysis of synaptic bouton number.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
J.A.M., M.S., J.B.S. and J.E.F. designed the experiments. M.S., J.B.S. and J.E.F. generated reagents, D.P. constructed the UAS-Rtnl1 line while in the lab of A.D., J.B.S. and J.E.F. performed the experiments. J.B.S., J.E.F., E.F. and J.F. analyzed data. J.A.M., M.S., J.B.S. and J.F. wrote the manuscript.
Funding
E.F. was supported by a George J. Schroepfer, Jr. Undergraduate Summer Fellowship; M.S. was supported by a grant from the Hamill Foundation; J.A.M. is supported by the National Institutes of Health [grant number GM101377]; the Simmons Family Foundation; and the Hamill Foundation. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.184929/-/DC1
References
- Aberle H., Haghighi A. P., Fetter R. D., McCabe B. D., Magalhães T. R. and Goodman C. S. (2002). wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33, 545-558. 10.1016/S0896-6273(02)00589-5 [DOI] [PubMed] [Google Scholar]
- Allison R., Lumb J. H., Fassier C., Connell J. W., Ten Martin D., Seaman M. N. J., Hazan J. and Reid E. (2013). An ESCRT-spastin interaction promotes fission of recycling tubules from the endosome. J. Cell Biol. 202, 527-543. 10.1083/jcb.201211045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson D. E., Costantini L. M. and Snapp E. L. (2011). Superfolder GFP is fluorescent in oxidizing environments when targeted via the Sec translocon. Traffic 12, 543-548. 10.1111/j.1600-0854.2011.01168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowska J. C., Jupille H., Fatheddin P., Puertollano R. and Blackstone C. (2007). Troyer syndrome protein spartin is mono-ubiquitinated and functions in EGF receptor trafficking. Mol. Biol. Cell 18, 1683-1692. 10.1091/mbc.E06-09-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard S. L., Miller D. L. and Ganetzky B. (2014). Retrograde neurotrophin signaling through Tollo regulates synaptic growth in Drosophila. J. Cell Biol. 204, 1157-1172. 10.1083/jcb.201308115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F. and Basler K. (2007). An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104, 3312-3317. 10.1073/pnas.0611511104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone C., O'Kane C. J. and Reid E. (2010). Hereditary spastic paraplegias: membrane traffic and the motor pathway. Nat. Rev. Neurosci. 1, 31-42. 10.1038/nrn2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanik L., Mohrmann R., Ramaekers A., Bockaert J., Grau Y., Broadie K. and Parmentier M.-L. (2004). The Drosophila metabotropic glutamate receptor DmGluRA regulates activity-dependent synaptic facilitation and fine synaptic morphology. J. Neurosci. 24, 9105-9116. 10.1523/JNEUROSCI.2724-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. and Ganetzky B. (2012). A neuropeptide signaling pathway regulates synaptic growth in Drosophila. J. Cell Biol. 196, 529-543. 10.1083/jcb.201109044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini L. M., Baloban M., Markwardt M. L., Rizzo M., Guo F., Verkhusha V. V. and Snapp E. L. (2015). A palette of fluorescent proteins optimized for diverse cellular environments. Nat. Commun. 6, 7670 10.1038/ncomms8670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermaut B., Norga K. K., Kania A., Verstreken P., Pan H., Zhou Y., Callaerts P. and Bellen H. J. (2005). Aberrant lysosomal carbohydrate storage accompanies endocytic defects and neurodegeneration in Drosophila benchwarmer. J. Cell Biol. 170, 127-139. 10.1083/jcb.200412001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sano F., Bernardoni P. and Piacentini M. (2012). The reticulons: guardians of the structure and function of the endoplasmic reticulum. Exp. Cell Res. 318, 1201-1207. 10.1016/j.yexcr.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Edwards T. L., Clowes V. E., Tsang H. T. H., Connell J. W., Sanderson C. M., Luzio J. P. and Reid E. (2009). Endogenous spartin (SPG20) is recruited to endosomes and lipid droplets and interacts with the ubiquitin E3 ligases AIP4 and AIP5. Biochem. J. 423, 31-39. 10.1042/BJ20082398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage N. J., Reid C. A. and Fine A. (2001). Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron 29, 197-208. 10.1016/S0896-6273(01)00190-8 [DOI] [PubMed] [Google Scholar]
- English A. R. and Voeltz G. K. (2013). Endoplasmic reticulum structure and interconnections with other organelles. Cold Spring Harb. Perspect. Biol. 5, a013227 10.1101/cshperspect.a013227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassier C., Hutt J. A., Scholpp S., Lumsden A., Giros B., Nothias F., Schneider-Maunoury S., Houart C. and Hazan J. (2010). Zebrafish atlastin controls motility and spinal motor axon architecture via inhibition of the BMP pathway. Nat. Neurosci. 13, 1380-1387. 10.1038/nn.2662 [DOI] [PubMed] [Google Scholar]
- Feng Y., Ueda A. and Wu C.-F. (2004). A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J. Neurogenet. 18, 377-402. 10.1080/01677060490894522 [DOI] [PubMed] [Google Scholar]
- Fernández-Busnadiego R., Saheki Y. and De Camilli P. (2015). Three-dimensional architecture of extended synaptotagmin-mediated endoplasmic reticulum-plasma membrane contact sites. Proc. Natl. Acad. Sci. USA 112, E2004-E2013. 10.1073/pnas.1503191112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Medel Y., Ashley J., Barria R., Maloney R., Freeman M. and Budnik V. (2012). Integration of a retrograde signal during synapse formation by glia-secreted TGF-beta ligand. Curr. Biol. 22, 1831-1838. 10.1016/j.cub.2012.07.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano F., Saheki Y., Idevall-Hagren O., Colombo S. F., Pirruccello M., Milosevic I., Gracheva E. O., Bagriantsev S. N., Borgese N. and De Camilli P. (2013). PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell 153, 1494-1509. 10.1016/j.cell.2013.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle S. C. J., Kanfer G., Kolar K., Lang A., Michel A. H. and Kornmann B. (2013). Organization and function of membrane contact sites. Biochim. Biophys. Acta 1833, 2526-2541. 10.1016/j.bbamcr.2013.01.028 [DOI] [PubMed] [Google Scholar]
- Howlett E., Lin C. C.-J., Lavery W. and Stern M. (2008). A PI3-kinase-mediated negative feedback regulates neuronal excitability. PLoS Genet. 4, e1000277 10.1371/journal.pgen.1000277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Shibata Y., Voss C., Shemesh T., Li Z., Coughlin M., Kozlov M. M., Rapoport T. A. and Prinz W. A. (2008). Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science 319, 1247-1250. 10.1126/science.1153634 [DOI] [PubMed] [Google Scholar]
- Hu J., Shibata Y., Zhu P.-P., Voss C., Rismanchi N., Prinz W. A., Rapoport T. A. and Blackstone C. (2009). A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell 138, 549-561. 10.1016/j.cell.2009.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Prinz W. A. and Rapoport T. A. (2011). Weaving the Web of ER Tubules. Cell 147, 1226-1231. 10.1016/j.cell.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. and Stern M. (2002). In vivo properties of the Drosophila inebriated-encoded neurotransmitter transporter. J. Neurosci. 22, 1698-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn T. R., Kohlhoff K. J., Scott M., Tartaglia G. G., Lomas D. A., Dobson C. M., Vendruscolo M. and Crowther D. C. (2011). Detection of early locomotor abnormalities in a Drosophila model of Alzheimer's disease. J. Neurosci. Methods 197, 186-189. 10.1016/j.jneumeth.2011.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y. and Jan Y. N. (1976). Properties of the larval neuromuscular junction in Drosophila melanogaster. J. Physiol. 262, 189-214. 10.1113/jphysiol.1976.sp011592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. E., Shu H., Hauswirth A. G., Tong A. and Davis G. W. (2015). VCP-dependent muscle degeneration is linked to defects in a dynamic tubular lysosomal network in vivo. Elife 4, e07366 10.7554/eLife.07366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozsef L., Tashiro K., Kuo A., Park E. J., Skoura A., Albinsson S., Rivera-Molina F., Harrison K. D., Iwakiri Y., Toomre D. et al. (2014). Reticulon 4 is necessary for endoplasmic reticulum tubulation, STIM1-Orai1 coupling, and store-operated calcium entry. J. Biol. Chem. 289, 9380-9395. 10.1074/jbc.M114.548602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr K. S., Fuentes-Medel Y., Brewer C., Barria R., Ashley J., Abruzzi K. C., Sheehan A., Tasdemir-Yilmaz O. E., Freeman M. R. and Budnik V. (2014). Glial wingless/Wnt regulates glutamate receptor clustering and synaptic physiology at the Drosophila neuromuscular junction. J. Neurosci. 34, 2910-2920. 10.1523/JNEUROSCI.3714-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhoff K. J., Jahn T. R., Lomas D. A., Dobson C. M., Crowther D. C. and Vendruscolo M. (2011). The iFly tracking system for an automated locomotor and behavioural analysis of Drosophila melanogaster. Integr. Biol. 3, 755-760. 10.1039/c0ib00149j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Paik D., Bang S., Kang J., Chun B., Lee S., Bae E., Chung J. and Kim J. (2008). Loss of spastic paraplegia gene atlastin induces age-dependent death of dopaminergic neurons in Drosophila. Neurobiol. Aging 29, 84-94. 10.1016/j.neurobiolaging.2006.09.004 [DOI] [PubMed] [Google Scholar]
- Lee M., Paik S. K., Lee M.-J., Kim Y.-J., Kim S., Nahm M., Oh S.-J., Kim H.-M., Yim J., Lee C. J. et al. (2009). Drosophila Atlastin regulates the stability of muscle microtubules and is required for synapse development. Dev. Biol. 330, 250-262. 10.1016/j.ydbio.2009.03.019 [DOI] [PubMed] [Google Scholar]
- Liang Y., Yuan L. L., Johnston D. and Gray R. (2002). Calcium signaling at single mossy fiber presynaptic terminals in the rat hippocampus. J. Neurophysiol. 87, 1132-1137. [DOI] [PubMed] [Google Scholar]
- Llano I., González J., Caputo C., Lai F. A., Blayney L. M., Tan Y. P. and Marty A. (2000). Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat. Neurosci. 3, 1256-1265. 10.1038/81781 [DOI] [PubMed] [Google Scholar]
- Lo Giudice T., Lombardi F., Santorelli F. M., Kawarai T. and Orlacchio A. (2014). Hereditary spastic paraplegia: clinical-genetic characteristics and evolving molecular mechanisms. Exp. Neurol. 261, 518-539. 10.1016/j.expneurol.2014.06.011 [DOI] [PubMed] [Google Scholar]
- Macleod G. T., Hegström-Wojtowicz M., Charlton M. P. and Atwood H. L. (2002). Fast calcium signals in Drosophila motor neuron terminals. J. Neurophysiol. 88, 2659-2663. 10.1152/jn.00515.2002 [DOI] [PubMed] [Google Scholar]
- Martin A. R. (1955). A further study of the statistical composition of the end-plate potential. J. Physiol. 130, 114-122. 10.1113/jphysiol.1955.sp005397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe B. D., Marqués G., Haghighi A. P., Fetter R. D., Crotty M. L., Haerry T. E., Goodman C. S. and O'Connor M. B. (2003). The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39, 241-254. 10.1016/S0896-6273(03)00426-4 [DOI] [PubMed] [Google Scholar]
- McNew J. A., Sondermann H., Lee T., Stern M. and Brandizzi F. (2013). GTP-dependent membrane fusion. Annu. Rev. Cell Dev. Biol. 29, 529-550. 10.1146/annurev-cellbio-101512-122328 [DOI] [PubMed] [Google Scholar]
- Miyazono K., Kamiya Y. and Morikawa M. (2010). Bone morphogenetic protein receptors and signal transduction. J. Biochem. 147, 35-51. 10.1093/jb/mvp148 [DOI] [PubMed] [Google Scholar]
- Moss T. J., Daga A. and McNew J. A. (2011). Fusing a lasting relationship between ER tubules. Trends Cell Biol. 21, 416-423. 10.1016/j.tcb.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm M., Lee M.-J., Parkinson W., Lee M., Kim H., Kim Y.-J., Kim S., Cho Y. S., Min B.-M., Bae Y. C. et al. (2013). Spartin regulates synaptic growth and neuronal survival by inhibiting BMP-mediated microtubule stabilization. Neuron 77, 680-695. 10.1016/j.neuron.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreau A., Dion P. A. and Rouleau G. A. (2014). Molecular aspects of hereditary spastic paraplegia. Exp. Cell Res. 325, 18-26. 10.1016/j.yexcr.2014.02.021 [DOI] [PubMed] [Google Scholar]
- O'Connor-Giles K. M., Ho L. L. and Ganetzky B. (2008). Nervous wreck interacts with thickveins and the endocytic machinery to attenuate retrograde BMP signaling during synaptic growth. Neuron 58, 507-518. 10.1016/j.neuron.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orso G., Pendin D., Liu S., Tosetto J., Moss T. J., Faust J. E., Micaroni M., Egorova A., Martinuzzi A., McNew J. A. et al. (2009). Homotypic fusion of ER membranes requires the dynamin-like GTPase Atlastin. Nature 460, 978-983. 10.1038/nature08280 [DOI] [PubMed] [Google Scholar]
- O'Sullivan N. C., Jahn T. R., Reid E. and O'Kane C. J. (2012). Reticulon-like-1, the Drosophila orthologue of the Hereditary Spastic Paraplegia gene reticulon 2, is required for organization of endoplasmic reticulum and of distal motor axons. Hum. Mol. Genet. 21, 3356-3365. 10.1093/hmg/dds167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdowski E. F., Gayle S., Bao H., Zhang B. and Sherwood N. T. (2011). Loss of Drosophila melanogaster p21-activated kinase 3 suppresses defects in synapse structure and function caused by spastin mutations. Genetics 189, 123-135. 10.1534/genetics.111.130831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pédelacq J. D., Cabantous S., Tran T., Terwilliger T. C. and Waldo G. S. (2006). Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 24, 79-88. 10.1038/nbt1172 [DOI] [PubMed] [Google Scholar]
- Pendin D., McNew J. A. and Daga A. (2011). Balancing ER dynamics: shaping, bending, severing, and mending membranes. Curr. Opin. Cell Biol. 23, 435-442. 10.1016/j.ceb.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C., Wenzel E. M., Pedersen N. M., Olsvik H., Schink K. O., Schultz S. W., Vietri M., Nisi V., Bucci C., Brech A. et al. (2015a). Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature 520, 234-238. 10.1038/nature14359 [DOI] [PubMed] [Google Scholar]
- Raiborg C., Wenzel E. M. and Stenmark H. (2015b). ER-endosome contact sites: molecular compositions and functions. EMBO J. 34, 1848-1858. 10.15252/embj.201591481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland A. A., Chitwood P. J., Phillips M. J. and Voeltz G. K. (2014). ER contact sites define the position and timing of endosome fission. Cell 159, 1027-1041. 10.1016/j.cell.2014.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S., Consoulas C., Kuromi H., Basole A., Mukai L., Kidokoro Y., Krishnan K. S. and Ramaswami M. (2005). Analysis of conditional paralytic mutants in Drosophila sarco-endoplasmic reticulum calcium ATPase reveals novel mechanisms for regulating membrane excitability. Genetics 169, 737-750. 10.1534/genetics.104.031930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder C. M., Wu X., Saheki Y., Narayanaswamy P., Torta F., Wenk M. R., De Camilli P. and Reinisch K. M. (2014). Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature 510, 552-555. 10.1038/nature13269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt I., Thomas S., Kain P., Risse B., Naffin E. and Klambt C. (2012). Kinesin heavy chain function in Drosophila glial cells controls neuronal activity. J. Neurosci. 32, 7466-7476. 10.1523/JNEUROSCI.0349-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp K. J. and Auld V. J. (1999). Conversion of lacZ enhancer trap lines to GAL4 lines using targeted transposition in Drosophila melanogaster. Genetics 151, 1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood N. T., Sun Q., Xue M., Zhang B. and Zinn K. (2004). Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol. 2, e429 10.1371/journal.pbio.0020429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y., Voeltz G. K. and Rapoport T. A. (2006). Rough sheets and smooth tubules. Cell 126, 435-439. 10.1016/j.cell.2006.07.019 [DOI] [PubMed] [Google Scholar]
- Shibata Y., Hu J., Kozlov M. M. and Rapoport T. A. (2009). Mechanisms shaping the membranes of cellular organelles. Annu. Rev. Cell Dev. Biol. 25, 329-354. 10.1146/annurev.cellbio.042308.113324 [DOI] [PubMed] [Google Scholar]
- Smith M. J. (1992). Nucleotide sequence of a Drosophila melanogaster gene encoding a calreticulin homologue. DNA Seq. 3, 247-250. 10.3109/10425179209034025 [DOI] [PubMed] [Google Scholar]
- Soboloff J., Rothberg B. S., Madesh M. and Gill D. L. (2012). STIM proteins: dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol. 13, 549-565. 10.1038/nrm3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan C. J., Manford A. G. and Emr S. D. (2013). ER-PM connections: sites of information transfer and inter-organelle communication. Curr. Opin. Cell Biol. 25, 434-442. 10.1016/j.ceb.2013.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart B. A., Atwood H. L., Renger J. J., Wang J. and Wu C.-F. (1994). Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A 175, 179-191. 10.1007/BF00215114 [DOI] [PubMed] [Google Scholar]
- Sweeney S. T. and Davis G. W. (2002). Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron 36, 403-416. 10.1016/S0896-6273(02)01014-0 [DOI] [PubMed] [Google Scholar]
- Terasaki M., Shemesh T., Kasthuri N., Klemm R. W., Schalek R., Hayworth K. J., Hand A. R., Yankova M., Huber G., Lichtman J. W. et al. (2013). Stacked endoplasmic reticulum sheets are connected by helicoidal membrane motifs. Cell 154, 285-296. 10.1016/j.cell.2013.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang H. T. H., Edwards T. L., Wang X., Connell J. W., Davies R. J., Durrington H. J., O'Kane C. J., Luzio J. P. and Reid E. (2009). The hereditary spastic paraplegia proteins NIPA1, spastin and spartin are inhibitors of mammalian BMP signalling. Hum. Mol. Genet. 18, 3805-3821. 10.1093/hmg/ddp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz G. K., Prinz W. A., Shibata Y., Rist J. M. and Rapoport T. A. (2006). A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124, 573-586. 10.1016/j.cell.2005.11.047 [DOI] [PubMed] [Google Scholar]
- Wakefield S. and Tear G. (2006). The Drosophila reticulon, Rtnl-1, has multiple differentially expressed isoforms that are associated with a sub-compartment of the endoplasmic reticulum. Cell. Mol. Life Sci. 63, 2027-2038. 10.1007/s00018-006-6142-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Shaw W. R., Tsang H. T. H., Reid E. and O'Kane C. J. (2007). Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat. Neurosci. 10, 177-185. 10.1038/nn1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrate L. M., Lee J. E., Prinz W. A. and Voeltz G. K. (2015). Form follows function: the importance of endoplasmic reticulum shape. Annu. Rev. Biochem. 84, 791-811. 10.1146/annurev-biochem-072711-163501 [DOI] [PubMed] [Google Scholar]
- Wong C.-O., Chen K., Lin Y. Q., Chao Y., Duraine L., Lu Z., Yoon W. H., Sullivan J. M., Broadhead G. T., Sumner C. J. et al. (2014). A TRPV channel in Drosophila motor neurons regulates presynaptic resting Ca(2+) levels, synapse growth, and synaptic transmission. Neuron 84, 764-777. 10.1016/j.neuron.2014.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong N. X., Pu J. Z., Zhao H. Y. and Zhang F. C. (2008). Effect of Nogo-A gene inhibition on dopamine release in PC12 cells. Neuro. Endocrinol. Lett. 29, 884-888. [PubMed] [Google Scholar]
- Yang Y. S. and Strittmatter S. M. (2007). The reticulons: a family of proteins with diverse functions. Genome Biol. 8, 234 10.1186/gb-2007-8-12-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E., Gustafson K. and Boulianne G. L. (1995). Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc. Natl. Acad. Sci. USA 92, 7036-7040. 10.1073/pnas.92.15.7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek N., Sparks L. and Voeltz G. (2011). Reticulon short hairpin transmembrane domains are used to shape ER tubules. Traffic 12, 28-41. 10.1111/j.1600-0854.2010.01134.x [DOI] [PMC free article] [PubMed] [Google Scholar]