Abstract
The alteration of craniofacial structures has been associated with obstructive sleep apnea (OSA). We hypothesized that 1) a smaller mandible is a risk factor for OSA; and 2) the previously observed inferiorly positioned hyoid bone in apneics is associated with enlarged tongue volume.
This is a case-control study using three-dimensional MRI cephalometry. 55 apneics and 55 controls were matched for age, gender and race. The analysis was stratified by gender and controlled for age, race, height, neck visceral fat, skeletal type and tongue volume.
We found that a 1-SD increase in mandibular length and depth were associated with decreased risk of sleep apnea (odds ratio[OR]=0.52, 95% confidence interval[CI]:0.28-0.99, OR=0.46, 95%CI:0.23-0.91, respectively) in men but not in women. Greater hyoid to nasion (OR=2.64, 95%CI:1.19-5.89 in men; OR=5.01, 95%CI:2.00-12.52 in women) and supramentale-to-hyoid (OR=2.39, 95%CI:1.12-5.14 in men; OR=3.38, 95%CI:1.49-7.68 in women) distances were associated with increased risk of OSA. The difference between apneics and controls for hyoid position was lost after controlling for tongue volume.
Enlargement of tongue is likely to be the pathogenic factor for inferior-posterior positioning of hyoid. A small and shallow mandible is an independent risk factor for obstructive sleep apnea in men but not in women.
Keywords: cephalometrics, hyoid bone, mandible, obstructive sleep apnea, three-dimensional magnetic resonance imaging
Obstructive sleep apnea (OSA) is a serious public health disorder that affects at least 4% of middle-aged men and 2% of middle-aged women (1) in its symptomatic form, i.e., with excessive sleepiness. Accumulating evidence has suggested that OSA is an independent risk factor for hypertension, ischemic heart disease, stroke, heart failure, atrial fibrillation and sudden death (2, 3) neuro-physiologic morbidity (4, 5), and insulin resistance (6, 7). However, we presently possess only limited knowledge about the pathogenesis and, in particular, craniofacial risk factors for this complex disorder.
OSA is mediated by several intermediate traits such as obesity, increased soft tissue volume, altered craniofacial structure, as well as neuromuscular and ventilatory control (8). A critical abnormality in patients with OSA is narrowing of the upper airway that is detected even during wakefulness (9, 10). Enlargement of the upper airway soft tissue structures, increased upper airway adipose tissue and reduction in the size of craniofacial structures have all been shown to be risk factors for OSA (10-12). All of these factors can be examined by upper airway magnetic resonance imaging (MRI).
Cephalometry, a standardized lateral radiograph of the head and neck, has been utilized in the past to study craniofacial structures in patients with sleep apnea. In general these studies have shown that apneics have small retroposed mandibles, narrow posterior airway spaces, enlargement of the tongue and soft palate, an inferiorly positioned hyoid bone, and retroposition of the maxilla compared to normals (13-17). These craniofacial risk factors for sleep apnea are more commonly reported in non-obese apneics than in obese apneics (18). Although cephalometric studies have provided insights into craniofacial abnormalities in patients with obstructive sleep apnea, a meta-analysis evaluating these studies found that only one cephalometric variable, mandibular body length, demonstrated a clinically significant association with sleep apnea (17).
There are several significant limitations to conventional cephalometrics. In addition to problems associated with standardizing the radiographic equipment, technique and interpretative skills, it is difficult/impossible to perform volumetric analysis; patients are exposed to radiation; it can not be performed in the supine position; it is a two-dimensional representation of a three-dimensional object (therefore, magnification and distortion are problems); and it only provides limited information about soft tissues lateral structures (an important disadvantage since numerous studies show that the airway is primarily narrowed in the lateral dimension in OSA subjects) (9, 10).
There are significant advantages to MRI. MRI provides excellent resolution of upper airway soft tissue structures (including adipose tissue); accurately measures cross-sectional airway area and volume; allows multiplanar imaging (axial, sagittal, coronal); provides data for three dimensional upper airway reconstructions; can be performed in the supine position during wakefulness and sleep; and does not expose patients to radiation.
We have developed MRI cephalometric techniques to study upper airway anatomical structures in two and three dimensions with MRI. These techniques allow us not only to assess standard cephalometric measurements but also provide a wealth of craniofacial information not available on standard cephalometry. We have already successfully utilized similar techniques to identify several important upper airway soft tissue structural risk factors for sleep apnea (9, 10, 19, 20). We demonstrated that increased volume of the tongue, lateral pharyngeal walls and total soft tissue significantly increase the risk for sleep apnea (10). Although we showed that the upper airway soft tissue structures are enlarged in patients with sleep apnea, we did not previously examine craniofacial structures.
Recently, similar MRI algorithms have been utilized to quantify craniofacial structures and in particular the mandible in a study of 31 Japanese men with OSA and 20 normal controls (21). A smaller mandibular internal length, a wider mandibular divergence and a smaller area at the mandibular base plane was found in apneic men compared to normals (21). However, in this investigation, important confounders such as age, height, BMI or craniofacial subtypes, were not considered.
Inferior positioning of the hyoid bone has also been documented to be associated with sleep apnea using cephalometric techniques (13, 22-25). It has been proposed that the position of the hyoid bone has an impact on tongue shape and posture, thereby affecting the patency of the hypopharyngeal airway (26). However, to our knowledge, no study has examined the actual relationship between hyoid position and tongue, as well as total upper airway soft tissue using a three-dimensional analytic approach.
In the present study, we used MRI to identify the alterations in craniofacial structures that increase the risk for obstructive sleep apnea utilizing a case control design in normal subjects and patients with sleep apnea. Subjects with sleep apnea and control subjects were matched for sex, ethnicity and age (within 5 years). We also controlled for obesity (parapharyngeal fat pad or body mass index [BMI]) as a covariate in our analyses. We specifically hypothesized that: 1) smaller mandibular body length, width, and depth are independent risk factors for OSA; and 2) the previously observed inferior-posteriorly positioned hyoid bone in apneics is associated with enlarged tongue volume. Other analyses were exploratory. Some of the results of this study have been previously reported in the form of an abstract (27).
METHODS
Subjects
The present study used a case-control design and studied 55 cases (apneics) and 55 matched controls. The University of Pennsylvania institutional review board for human studies approved the protocol, and informed written consent was obtained from each patient. Cases were recruited primarily from the Penn Center for Sleep Disorders (Philadelphia, PA) outpatient practice and were newly diagnosed as having sleep apnea. They had symptoms of sleep apnea. To qualify as a “case” such patients had to have an apnea–hypopnea index (AHI) greater than 15 episodes/hour. Control (normal) subjects were recruited through local advertisements in the neighborhood (same school district) of the cases. Ethnicity was determined by self-report. Controls were of the same sex and ethnic background as the cases. To qualify as controls, individuals needed to be free of sleep apnea. This was confirmed by a sleep study and all controls recruited had an AHI of less than 5 episodes/hour. Hence, we specifically examined two ends of the spectrum and excluded subjects with mild sleep apnea. Subjects with AHI greater than 5 but less than or equal to 15 episodes/hour were considered indeterminate and were not studied further (these subjects were not in the analysis since they did not undergo a MRI).
Polysomnography
Standard polysomnography techniques were used (28). (see online supplement for additional information).
Magnetic Resonance Imaging
In the present study, upper airway imaging was performed identically in normal subjects and subjects with sleep apnea, using a 1.5-Tesla magnetic resonance imaging scanner (see online supplement for additional information).
Anatomic Definitions and Measurements
The primary craniofacial measurements included: 1) axial measurements: mandibular length and depth measured in three-dimensions and mandibular width measured in two-dimensions (see Figure 1); 2) midsagittal cephalometric measurements: the distance from hyoid to nasion, sella, and supramentale (Figure 2A); and 3) skeletal type classifications according to relationship of maxilla and mandible (29) (Figure 2B). Detailed explanations of three-dimensional (inter-slice) measurements and two-dimensional (intra-slice) measurements can be found in the online supplement in Figure E3. Secondary measurements included maxillary measurements, and other mandibular and cephalometric measurements (please refer to online supplement). The three-dimensional volumetric measurements of parapharyngeal fat pad, tongue volume and total upper airway soft tissue volume were assessed as described in previous studies (10).
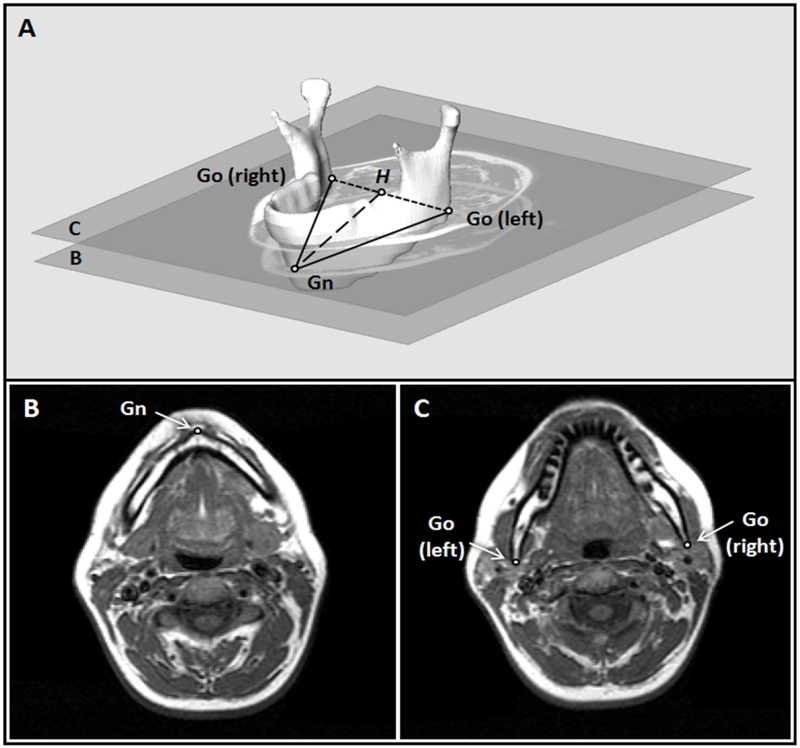
Figure 1.
Definition of mandibular measurements and MR correlates. 1A: Three-dimensional reconstruction of the mandible with craniofacial points and distance measurements. Points: (1) Gnathion (Gn): the most anterior–inferior point of the mandible; (2) Gonion (Go): The left and right Go are the most posterior and inferior points of the mandible. Distances measured: (1) Mandibular body length (solid lines): the distance from Gn to the left or right Go; (2) Mandibular width (dotted line): the 2-dimentional measurement of the width of the lower portion of the mandible. It is the distance between the left and right Go; (3) Mandibular depth (dashed line): the distance from Gn to H, where H is the midpoint between the left and right Go. 1B: The axial MR slice containing the Gn. 1C: The axial MR slice containing the left and right Go.
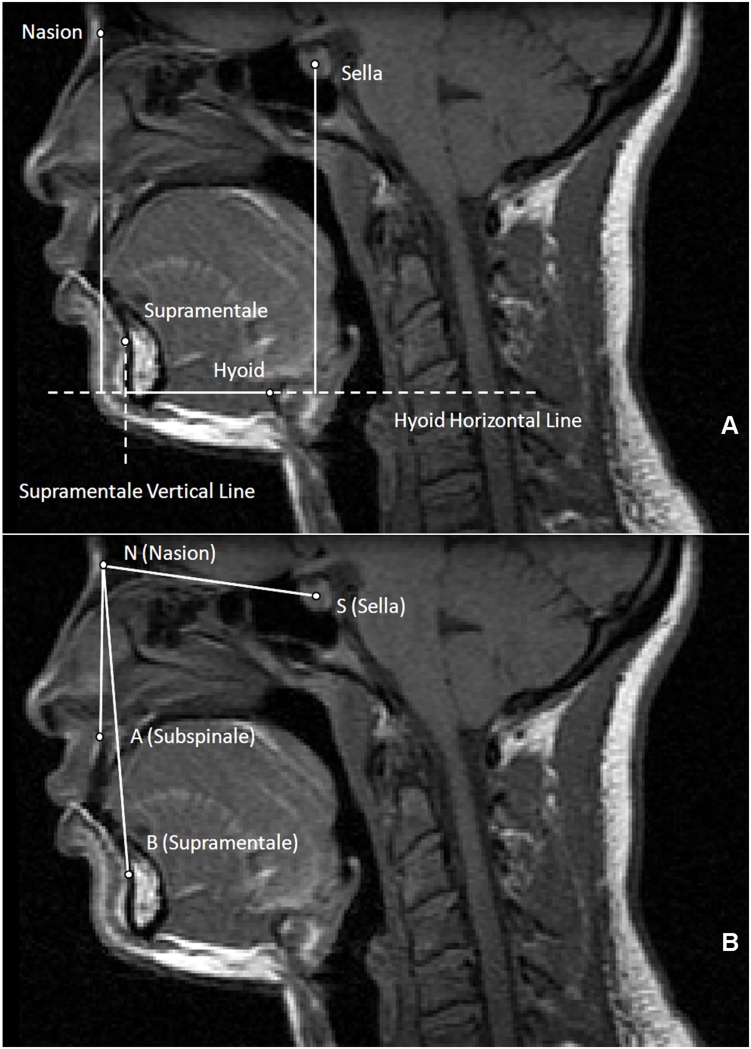
Figure 2.
A. Midsagittal MR slice with cephalometric hyoid measurements. Points: 1) Nasion: the most anterior aspect of the frontonasal suture; 2) Sella: the most anterior-inferior point of the sella turcica; 3) Supramentale: the deepest point on the curvature of the facial surface of the mandibular symphysis; 4) Hyoid bone: the most superior and anterior point on the body of the hyoid bone. Lines: 1) Hyoid horizontal line: the horizontal line that passes through the hyoid point and extends beyond the posterior wall of the airway; 2) Supramentale vertical line: the vertical line that passes through supramentale and intersects with the hyoid horizontal line. Measurements: 1) nasion-to-hyoid distance: the vertical line segment from nasion to the hyoid horizontal line; 2) sella-to-hyoid distance: the vertical line segment from sella to the hyoid horizontal line; 3) supramentale-to-hyoid distance: the horizontal line segment from hyoid to the supramentale vertical line. All lines and distances were measured either perpendicular or parallel to the true horizontal.
B. MRI cephalometric angular measurements. Points: 1) Nasion (N); 2) Sella (S); 3) Supramentale (B); 4) Subspinale (A): the deepest point on the curvature of the facial surface of the maxilla (premaxilla). Angles: 1) SNA: sella-nasion-A point (subspinale) angle; 2) SNB: sella-nasion-B point (supramentale) angle; 3) ANB: A point-nasion-B point angle (the difference between SNA and SNB).
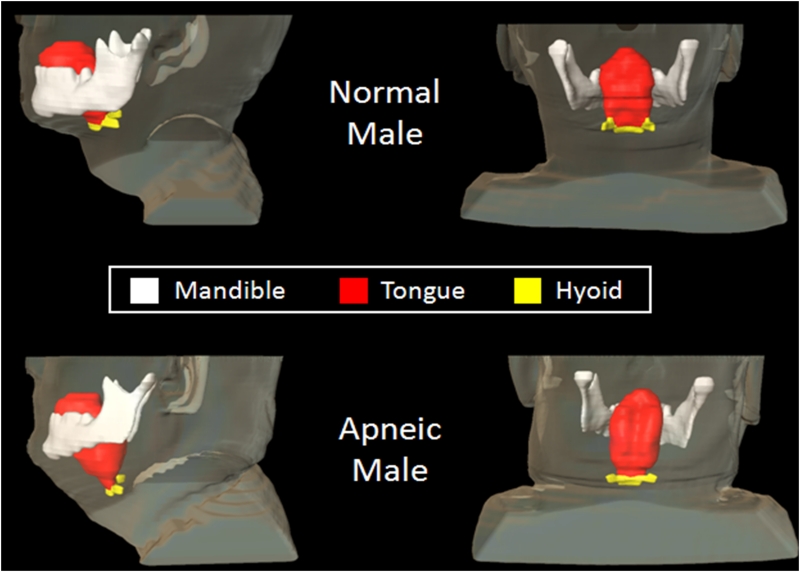
Figure 3.
Illustration of inferior-posterior positioning of hyoid and enlarged tongue volume. Three-dimensional reconstruction of hyoid (yellow), tongue (red), and mandible (white) in a patient with sleep apnea (bottom; AHI: 86 events/hour, BMI: 31 kg/m2, 49 years old, male) and a normal subject (top; AHI: 5 events/hour, BMI: 25 kg/m2, 44 years old, male). Note that the hyoid is more inferior-posteriorly positioned in the apneic subject than in the normal subject; tongue volume is greater in the apneic subject (115.7 cm3) than in the normal subject (100.0 cm3).
Primary Mandibular Measurements
Three mandibular measurements were collected to determine the dimensions of the lower portion of the mandible. The coordinates for the most anterior-inferior point of the mandible (gnathion) and the most posterior-inferior points of the mandible (left and right gonion) were identified on the corresponding MR slices (Figure 1B and 1C). Mandibular body length was measured as the average distance from the gnathion to the left and right gonion, and mandibular width was measured as the distance between the left and right gonion. Mandibular depth was calculated as the distance from the gnathion to the midpoint between the left and right gonion (Figure 1A).
Mid-Sagtital Hyoid Measurements
Hyoid position is typically determined by measuring the distance between the hyoid to the mandibular plane (13). Since the mandibular plane is not seen on the MR midsagittal images, we did not use this measurement. We utilized, instead, vertical and horizontal measurements to characterize the position of the hyoid as described previously to determine the caudal and/or inferior position of the hyoid bone (23, 24).
Angular Measurements and Craniofacial Skeletal Subtype Classifications
To assess the position of mandible and maxilla relative to the cranial bases, the SNA (sella [S] – nasion [N] – subspinale [A]), SNB (sella [S] – nasion [N] – supramentale [B]) and ANB (subspinale [A] – nasion [N] – supramentale [B]) angles were determined (Figure 2B) (29). The ANB angle, the difference between SNA and SNB angle, assessed the anterior-posterior relationship of the maxilla and the mandible in reference to the cranial base. We used the ANB value to determine the skeletal type as proposed by Steiner (29). Each subject was subgrouped according to the following norms: skeletal type class I: a normal anteroposterior relationship between maxilla and mandible (ANB angle between 0° - 4° for Caucasians, 4° – 8° for African Americans and 0.5° – 4.5° for Asians); class II, a retrognathic mandible in reference to the maxilla (ANB > 4° for Caucasians and > 6° for African Americans and > 4.5° for Asians); class III: a prognathic mandible with the normal maxilla or a retrognathic maxilla with a normal mandible or a combination of a retrognathic maxilla and a prognathic mandibule (ANB < 0° for Caucasians and Asians, ANB < 4 for African Americans) (29).
Other 3-dimensional and 2-dimensional mandibular, maxillary and standard MRI cephalometric measurements were examined in order to provide a more complete assessment of craniofacial risk factors for OSA. However, these a priori exploratory analyses lacked power to detect small effects especially given multiple testing and subgroup analysis. The results of these exploratory analyses are provided in the online supplement.
Measurement reliability study
In order to demonstrate reliability, the 6 primary variables (mandibular length, width, depth and 3 hyoid bone position variables were the primary measurements in the analysis) in the present study were analyzed in 10 randomly selected subjects by two readers. Each variable was measured 3 times over three separate days to reduce recall bias by the two different readers. The order of images analyzed on a given day was randomized. Intra-reader and inter-reader reliability were then analyzed.
Statistical analysis
Baseline characteristics of case and control subjects were compared using chi-square tests for categorical variables and t tests for continuous variables (or rank-based procedures for non-parametric data, if specified). Mandibular length, width, depth and 3 hyoid bone position variables were the primary measurements in the analysis. Secondary analysis focused on maxillary and other madibular and hyoid variables.
We matched cases and controls on age (within five years). We controlled for age, height, parapharyngeal fat pad volume or BMI, and tongue volume or total soft tissue volume (10) in each gender stratum, using linear regression models to determine the association of those craniofacial risk factors for OSA. We controlled for fat pad volume, a measure of the relevant visceral adiposity in the neck, since BMI may be influenced by non-fat mass as well as adipose deposits in other subcutaneous and central locations (10). Nonetheless we performed the analysis controlling for each of these variables. Mandibular body length, width and depth may be correlated with each other; therefore, a multivariable logistic regression analysis was performed, which encompassed all the a priori factors (mandibular length, width and depth or hyoid bone measurements) as well as confounding factors. This model took into account any correlation between the measurements and independently examined the increase risk of sleep apnea for each of these factors. Within each gender stratum, multivariable logistic regression models were used to obtain adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the effects of a 1-SD change in the size of the craniofacial measurements. Since multiple regression models were fit to the data, the conservative Bonferroni multiple testing correction was applied to all primary hypotheses tests.
Intra-reader reliability for MRI measurements was analyzed by the intra-class correlation coefficient (ICC). To compute ICC, we used mixed effect models for each variable using a compound symmetric correlation structure. Inter-reader reliability was analyzed by two-way analysis of variance with main random effects for variable and reader. All analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC).
We based power calculations on the smallest gender stratum available to provide the lower bound on power. The only available volumetric MRI study (21) demonstrated a 3.5 mm mandibular body length difference between male cases and controls with an SD of 4.2 mm. Hence we based our calculations on this effect size. Using a two sided t-test with significance level of 0.05, 27 male apneics, and 27 male nonapneics, with SD = 4.2 mm, our study had 85% power to detect this demonstrated effect size. The Power and Sample Size Calculation Program version 2.1.31 was used for power calculations.
RESULTS
Demographics of Case Subjects and Control Subjects
Pertinent characteristics of the study sample are summarized in Table 1. There were no significant differences between the height of apneics and controls. Cases tended to be older than the controls, but this difference was not statistically significant. The BMI of cases was significantly greater than that of controls, but many controls were overweight. As expected by design, patients with sleep apnea had significantly greater mean AHI than nonapneic controls (Table 1A). There were no differences in skeletal types between apneics and controls in the full sample or within gender strata (Table 1B). There were no differences in marital status and education between controls and apneics (see Table E1 in online supplement). More of the female cases and controls were African American than males.
TABLE 1A. Demographics of apneic and normal subjects.
| Cases (n = 55) |
Controls (n = 55) |
p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Med | Min | Max | Mean | SD | Med | Min | Max | ||
| Age, years | 44.5 | 9.7 | 47.0 | 27.0 | 63.0 | 41.0 | 10.2 | 42.0 | 24.0 | 66.0 | 0.07* |
| Height, cm | 171.0 | 10.6 | 170.2 | 149.9 | 193.0 | 171.7 | 9.2 | 170.2 | 152.4 | 190.5 | 0.72* |
| Weight, kg | 101.6 | 19.4 | 104.8 | 56.2 | 138.6 | 80.4 | 16.1 | 72.6 | 48.1 | 127.9 | <0.0001* |
| BMI, kg/m2 | 35.5 | 8.5 | 32.9 | 20.6 | 52.9 | 25.9 | 4.5 | 24.8 | 19.4 | 42.8 | <0.0001* |
| AHI, events/hour | 46.8 | 33.5 | 33.5 | 15.1 | 142.0 | 2.2 | 1.7 | 1.8 | 0.0 | 6.3 + | <0.0001† |
|
| |||||||||||
|
Cases
(n) |
% |
Controls
(n) |
% | p | |||||||
| Gender (male) | 27.0 | 49.0 | 27.0 | 49.0 | 1.0000 | ||||||
| Race | . | ||||||||||
| White | 25 | 45.5 | 25 | 45.5 | . | ||||||
| African American | 27 | 49.1 | 27 | 49.1 | . | ||||||
| Asian | 2 | 3.6 | 2 | 3.6 | . | ||||||
| Hispanic | 1 | 1.8 | 1 | 1.8 | . | ||||||
Definition of abbreviations: AHI = apnea-hypopnea index; BMI = body mass index; SD: standard deviation; Max = maximum; Med = median; Min = minimum.
p values from Student’s t-test.
p values from Wilcoxon rank test.
n = number of subjects. Significant differences (p < 0.05) presented in bold.
Four control subjects had an AHI between 5 and 6 events/hour (5.3, 5.4, 5.7 and 6.3). Although they did not quite meet the protocol guidelines for a control subject (AHI ≤ 5 events/hour), they met all other criteria, so we did not exclude them due to this slight elevation. Results shown here remained the same when these subjects were excluded from the analysis (data not shown).
TABLE 1B. Population of apneic and normal subjects according to craniofacial skeletal type and gender.
| Total |
Men |
Women |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skeletal Type |
Total n = 107* |
Apneic n = 52* |
Normal n = 55 |
p† | Apneic n = 24 |
Normal n = 27 |
p† | Apneic n = 28 |
Normal n = 28 |
p† |
| Class I | 60 (56%) | 31 (59%) | 29 (53%) |
0.67 | 14 (58%) | 13 (48%) | 0.55 | 17(61%) | 16 (57%) | 0.96 |
| Class II | 34 (32%) | 16 (31%) | 18(33%) | . | 8 (33%) | 9 (33%) | . | 8(28%) | 9(32%) | . |
| Class III | 13 (12%) | 5(10%) | 8(14%) | . | 2(9%) | 5(19%) | . | 3(11 %) | 3(11 %) | . |
SNA, SNB and ANB angles were not able to be obtained in three subjects due to artifacts on the midsagittal MRI image.
p values from Chi-square test.
Polysomnography
As shown in Table E2 (see online supplement), sleep efficiency was not different between cases and controls (p = 0.91), and there were no significant differences between the mean time spent in stage 1 sleep (p = 0.21) and stage 2 sleep (p = 0.61). Cases had significantly less rapid eye movement (REM) sleep than did controls (p = 0.01) and tended to have less delta sleep (p = 0.06). There were significantly more nocturnal arousals among cases than controls (p < 0.0001).
Measurement Reliability Analysis
In our intra-reader reliability study, the inter-class correlation efficient was above 0.98 for the MRI craniofacial measurements (see online supplement Table E3a). In our inter-reader reliability study, the percentage of total variance attributable to between reader variations was below 1% (see online supplement Table E3b). Therefore, our MRI measurements are highly reproducible based on our excellent agreement between and within the two readers.
MRI Mandibular measurements
Unadjusted mean mandibular comparisons between apneics and controls are shown in Table 2. Before the stratification for gender, the mandibular length and depth were smaller, and the mandibular width was larger in cases than in controls, but these differences did not reach a significant level (p values = 0.24, 0.26, and 0.12, respectively). After stratification for gender (also shown in Table 2), the unadjusted means of mandibular length and depth were significantly smaller in cases than in controls in men (p = 0.04 and p = 0.02, respectively), but not in women.
TABLE 2. Mean comparison of mandibular measurements between apneic and normal subjects.
| Mandibular Measurements (mm) | Apneic |
Normal |
% Diff. | p* | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Total (Apneic n = 54 †, Normal n = 55) | ||||||
| Mandibular Body Length | 86.79 | 4.55 | 88.01 | 5.95 | −1.4 | 0.25 |
| Mandibular Width | 90.21 | 6.12 | 88.12 | 7.26 | 2.3 | 0.12 |
| Mandibular Depth | 71.75 | 5.85 | 73.08 | 6.13 | −1.9 | 0.26 |
| Men (Apneic n = 26†, Normal n = 27) | ||||||
| Mandibular Body Length | 88.42 | 4.78 | 91.29 | 4.58 | −3.2 | 0.04 |
| Mandibular Width | 94.83 | 3.70 | 92.50 | 6.99 | 5.4 | 0.17 |
| Mandibular Depth | 70.35 | 5.43 | 73.98 | 4.89 | −5.2 | 0.02 |
| Women (Apneic n = 28, Normal n = 28) | ||||||
| Mandibular Body Length | 85.52 | 4.00 | 84.84 | 5.42 | 0.8 | 0.60 |
| Mandibular Width | 84.59 | 5.14 | 82.14 | 4.54 | 2.9 | 0.04 |
| Mandibular Depth | 72.85 | 6.02 | 72.21 | 7.12 | 0.9 | 0.72 |
Significant differences (p < 0.05) presented in bold. Mandibular body length = average left and right mandibular body length. % Diff. = % Difference = [(apneic - normal)/apneic]*100. n = number of subjects. SD = standard deviation.
p value for student’s t-test.
mandibular measurements were unable to be obtained in one subject due to MRI artifacts.
Table 3A shows differences in means of mandibular measurements between cases and controls, adjusting for important confounding risk factors. After adjusting for ethnicity, age, height, skeletal type (ANB angle) and parapharyngeal fat pad volume, mandibular length and depth were significantly smaller in cases than in controls in men (p = 0.01, p < 0.01 respectively). The smaller mandibular length and depth remained significant even after using stringent Bonferroni corrections for multiple testing. However, the differences in mandibular length, width and depth between apneic and normal women were not statistically significant in any of the models.
TABLE 3A. Comparison of adjusted mean differences in mandibular measurements between apneic and normal subjects, stratified by gender.
| Mandibular Measurements (mm) | Adjusted Model-1 (Race, Age, Height, Fat Pad Volume, ANB Angle) |
Adjusted Model-2 (Model-1 + Tongue Volume) |
||||
|---|---|---|---|---|---|---|
| Diff. | SE | p | Diff. | SE | p | |
| Men | ||||||
| Mandibular Body Length | −3.36 | 1.26 | <0.01* | −3.54 | 1.38 | 0.01 |
| Mandibular Width | 2.12 | 1.71 | 0.22 | 1.43 | 1.87 | 0.45 |
| Mandibular Depth | −3.74 | 1.42 | <0.01* | −3.78 | 1.30 | <0.01* |
| Women | ||||||
| Mandibular Body Length | 1.01 | 1.39 | 0.47 | 0.74 | 1.83 | 0.69 |
| Mandibular Width | 2.11 | 1.42 | 0.15 | −0.22 | 1.80 | 0.90 |
| Mandibular Depth | 1.55 | 1.61 | 0.34 | 1.52 | 2.12 | 0.48 |
Significant differences (p < 0.05) presented in bold. Definition of abbreviations: Diff. = adjusted difference (apneic - normal); SE = standard error.
Significant after Bonferroni correction for multiple testing for all hypothesis tests.
These findings remained significant after further controlling for tongue volume (Table 3A). The analysis was also performed including total upper airway soft tissue volume in place of tongue volume (see online supplement Table E4a), which showed similar results. We adjusted for neck fat rather than BMI since fat surrounding the upper airway may be much more important in mediating the pathogenesis of sleep apnea than BMI which is a global measure of fat. However, we repeated the analyses using BMI in place of fat pad volume as a covariate and the results were similar (see online supplement Table E4b).
In men, the unadjusted odds ratios for 1-SD increase in mandibular body length and mandibular depth were significantly smaller than one, indicating increased risk of sleep apnea among subjects with a shorter mandibular body length, and a shallower mandibular depth (Table 3B). Results were similar after adjusting for race, age, height, skeletal type (ANB angle) and parapharyngeal fat pad volume. Further controlling for tongue volume did not alter the magnitude of odds ratios. Replacing the volume of the parapharyngeal fat pad and tongue volume with total soft tissue volume showed the same results (see online supplement Table E5a) or including BMI in place of fat pad as a confounder did not alter the results (see online supplement Table E5a and E5b). In women, however, mandibular length and depth were not associated with increased risk for OSA before and after above adjustments. Mandibular width was not associated with increased risk for sleep apnea in either men or women. There was a trend that increased mandibular width was a predictive of OSA in women (OR from 1.09 to 1.9); however, these findings were not statistically significant, as indicated by all 95%CIs including 1.0 (Table 4).
TABLE 3B.
Comparison of odds ratios in mandibular measurements between apneic and normal subjects, stratified by gender
| Mandibular Measurements (mm) |
SD | Unadjusted | Adjusted Model-1 (Race, Age, Height, Fat Pad Volume, ANB Angle) |
Adjusted Model-2 (Model-1 + Tongue Volume) |
|||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| OR | 95% CI | OR* | 95% CI | OR* | 95% CI | ||
| Men | |||||||
| Mandibular Body Length | 4.84 | 0.52 | 0.27-0.99 | 0.38 | 0.16-0.85 | 0.33 | 0.13-0.85 |
| Mandibular Width | 5.82 | 1.54 | 0.84-2.84 | 1.65 | 0.78-3.42 | 1.34 | 0.61-2.95 |
| Mandibular Depth | 5.40 | 0.46 | 0.23-0.91 | 0.19 | 0.06-0.64 | 0.20 | 0.05-0.76 |
| Women | |||||||
| Mandibular Body Length | 4.73 | 1.16 | 0.68-1.97 | 1.27 | 0.65-2.50 | 1.11 | 0.41-2.97 |
| Mandibular Width | 4.99 | 1.81 | 1.00-3.82 | 1.75 | 0.90-3.73 | 1.09 | 0.37-3.24 |
| Mandibular Depth | 6.54 | 1.10 | 0.65-1.88 | 1.55 | 0.69-3.50 | 1.07 | 0.31-3.74 |
Definition of abbreviations: OR = odds ratio for 1-SD increase; OR* = adjusted OR; 95% CI = 95% confidence interval. Significant differences (95% CI not including 1.0) are presented in bold.
TABLE 4. Mean comparison of hyoid measurements between apneic and normal subjects before and after stratification for gender.
| Hyoid Measurements (mm) | Apneic |
Normal |
% Diff. |
p* | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Total (Apneic n = 51†, Normal n = 55) | ||||||
| Hyoid to Sella | 110.4 | 9.7 | 105.1 | 10.1 | 4.7 | <0.01 |
| Hyoid to Nasion | 131.7 | 9.7 | 123.5 | 9.5 | 6.2 | <0.0001 |
| Hyoid to Supramentale | 58.8 | 7.4 | 52.7 | 7.1 | 10.4 | <0.0001 |
| Men (Apneic n = 23†, Normal n = 27) | ||||||
| Hyoid to Sella | 117.1 | 8.2 | 112.9 | 6.4 | 3.6 | 0.047 |
| Hyoid to Nasion | 136.7 | 9.6 | 129.2 | 8.0 | 5.4 | <0.01 |
| Hyoid to Supramentale | 60.0 | 6.2 | 54.6 | 7.1 | 9.0 | <0.01 |
| Women (Apneic n = 28, Normal n = 28) | ||||||
| Hyoid to Sella | 102.1 | 7.1 | 97.7 | 6.9 | 4.3 | <0.001 |
| Hyoid to Nasion | 127.5 | 7.8 | 118.1 | 7.5 | 7.4 | <0.0001 |
| Hyoid to Supramentale | 57.9 | 8.2 | 50.9 | 6.7 | 12.0 | 0.001 |
Significant differences (p < 0.05) presented in bold. % Diff. = % Difference = [(apneic - normal)/apneic]*100. n = number of subjects. SD = standard deviation.
p value for student’s t-test.
hyoid measurements were unable to obtained in 4 subjects due to MRI artifacts.
Hyoid measurements using MRI
Figure 3 demonstrates enlarged tongue volume and inferior-posterior positioning of the hyoid in a case compared to a control. Unadjusted analyses revealed a significant inferior-posterior displacement of the hyoid bone in cases with sleep apnea compared to controls in the full sample and after stratifying by gender (p value ranging from < 0.0001 to 0.047) (Table 4).
The differences in mean hyoid measurements between apneics and normal subjects after adjusting for confounders are shown in Table 5A. The vertical distance between sella or nasion to a horizontal line passing through the hyoid is greater in apneics in both men and women than in controls. The increase in this distance indicates the inferior positioning of the hyoid. Increase of the horizontal distance between hyoid to a vertical line passing through supramentale indicates posterior positioning of the hyoid. After adjusting for ethnicity, age, height, skeletal type (ANB angle) and parapharyngeal fat pad volume, inferior-posterior positioning of hyoid remained significantly associated with risk of OSA in men (p < 0.01 for hyoid to nasion, p = 0.03 for hyoid-to-supramentale) and women (p < 0.001 for hyoid to sella, p < 0.0001 for hyoid-to-nasion and p = 0.001 for hyoid-to-supramentale). However, after controlling for total tongue volume in addition to the above adjustments, the differences between apneics and normal subjects became insignificant overall and also in men and women separately.
TABLE 5A. Comparison of adjusted mean differences in hyoid measurements between apneic and normal subjects, stratified by gender.
| Hyoid Measurements (mm) | Adjusted Model-1 (Race, Age, Height, Fat Pad Volume) |
Adjusted Model-2 (Model-1 + Tongue Volume) |
||||
|---|---|---|---|---|---|---|
| Diff. | SE | p | Diff. | SE | p | |
| Men | ||||||
| Hyoid to Sella | 4.10 | 2.22 | 0.07 | 2.96 | 2.42 | 0.23 |
| Hyoid to Nasion | 7.37 | 2.72 | <0.01* | 5.47 | 2.88 | 0.06 |
| Hyoid to Supramentale | 4.72 | 2.0 | 0.02 | 3.37 | 2.11 | 0.12 |
| Women | ||||||
| Hyoid to Sella | 7.70 | 2.0 | <0.001* | 4.42 | 2.57 | 0.09 |
| Hyoid to Nasion | 9.55 | 2.23 | <0.0001* | 3.44 | 2.62 | 0.20 |
| Hyoid to Supramentale | 6.86 | 2.31 | <0.01* | 3.15 | 2.92 | 0.29 |
Significant differences (p < 0.05) presented in bold. Definition of abbreviations: Diff. = adjusted difference (apneic - normal); SE = standard error.
Significant after Bonferroni correction for multiple testing for all hypothesis tests.
Adjusting for upper airway total soft tissue volume in place of total tongue volume (see online supplement Table E6a) or including BMI in place of fat pad as a confounder led to identical conclusions (see online supplement Table E6b).
The unadjusted odds ratios for the majority of hyoid measurements were significantly greater than one (Table 5B), indicating that an inferior-posterior positioning of the hyoid bone was associated with an increased risk of sleep apnea. After adjusting for race, age, height and parapharyngeal fat pad volume: 1) hyoid to sella vertical distance was not associated with increased risk of sleep apnea in men; but it was associated increased risk in women; 2) hyoid to nasion vertical distance was associated with increased risk in both men and women; 3) supramentale to hyoid horizontal distance was also associated with increased risk in both men and women. The associations of all measures (except hyoid to nasion in men and this OR was reduced in magnitude) of hyoid position were no longer significant after additional adjustment for tongue volume.
TABLE 5B. Comparison of odds ratios in hyoid measurements between apneic and normal subjects, stratified by gender.
| Hyoid Measurements (mm) |
SD | Unadjusted Model |
Adjusted Model-1 (Race, Age, Height, Fat Pad Volume) |
Adjusted Model-2 (Adjusted Model-1 + Tongue Volume) |
|||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR* | 95% CI | OR* | 95% CI | ||
| Men | |||||||
| Hyoid to Sella | 7.54 | 1.88 | 0.98-3.60 | 1.94 | 0.92-4.10 | 1.72 | 0.77-3.87 |
| Hyoid to Nasion | 9.44 | 2.68 | 1.25-5.75 | 2.91 | 1.21-7.00 | 2.57 | 1.02-6.51 |
| Hyoid to Supramentale | 7.16 | 2.42 | 1.22-4.80 | 2.63 | 1.15-6.00 | 2.27 | 0.93-5.56 |
| Women | |||||||
| Hyoid to Sella | 7.79 | 2.99 | 1.53-5.87 | 3.95 | 1.69-9.22 | 2.54 | 0.68-9.50 |
| Hyoid to Nasion | 8.99 | 4.08 | 1.59-8.98 | 5.47 | 1.96-15.26 | 1.62 | 0.46-5.78 |
| Hyoid to Supramentale | 8.21 | 3.10 | 1.45-6.64 | 3.08 | 1.30-7.34 | 2.68 | 0.58-12.33 |
Definition of abbreviations: OR = odds ratio for 1-SD increase; OR* = adjusted OR; 95% CI = 95% confidence interval. Significant differences (95% CI not including 1.0) are presented in bold.
Adjusting for upper airway total soft tissue volume in place of total tongue volume (see online supplement Table E7a) or including BMI in place of fat pad as a confounder in the models adjusting for tongue volume showed similar results (see online supplement Table E7b).
Moreover, a correlation analysis was performed in order to identify the relationship between hyoid position and tongue volume or AHI. The data showed that tongue volume was closely related to AHI and hyoid displacement. Hyoid displacement (which was secondary to increased tongue volume) was also correlated with AHI (Table 6).
Table 6. Correlations between AHI or Tongue Volume to Hyoid Position.
| AHI* |
Tongue Volume |
|||
|---|---|---|---|---|
| Correlation coefficient |
p value | Correlation coefficient |
p value | |
| Tongue Volume | 0.47 | <0.0001 | - | - |
| Hyoid to Sella | 0.18 | 0.07 | 0.59 | <0.0001 |
| Hyoid to Nasion | 0.37 | 0.002 | 0.62 | <0.0001 |
| Hyoid to Supramentale | 0.52 | <0.0001 | 0.48 | <0.0001 |
Spearman correlation. AHI=apnea hypopnea index (events/hour)
In exploratory analyses, we did not identify additional mandibular or maxillary measurements that were risk factors for sleep apnea either in men or in women after adjusting for confounders and controlling for multiple testing using Bonferroni corrections. They are included in the online supplement in Tables E8-E19.
DISCUSSION
This is the first study that has used two- and three-dimensional MRI in a large population to examine the differences of entire the craniofacial structure between normals and apneics. After controlling for important confounders, we found the following: 1) a smaller and shallower mandible was an independent risk factor for OSA in men, but not for women; 2) we confirmed the inferior displacement of the hyoid in apneics compared to controls; the enlargement of the tongue appears to be the pathogenic factor for inferior-posterior hyoid bone position in apneics since when controlling for tongue volume there were no differences between normals and apneics in the position of the hyoid; 3) the size of the mandible (length and depth) was a much more important risk factor for OSA than maxillary measures or angular measurements (SNA, SNB, etc) in our cohort.
Potential bias and limitations of this study
There are several potential limitations that need to be discussed. We had more Caucasian male apneics than African American males and fewer Caucasian female apneics than African American females (see online supplement Table E1). However, in our study, cases and controls were exactly matched on gender and race separately (see Table 1A), and the joint distribution of gender and race was also exactly balanced between apneics and nonapneics. Hence, overall differences between cases and controls are unbiased with respect to race and gender. However, our female sample was largely African American (74%), while our male sample was largely Caucasian (66%). Ideally, the analysis should be conducted to investigate race specific differences within each gender. However, our sample size in race-gender subgroups did not provide the required power to make these within strata comparisons. We have, as a first step in this investigation, included subjects from different ethnic backgrounds and controlled for race as a covariate. This is a fairly standard initial approach. Future studies will need to address these questions in different relatively homogenous ethnic groups to evaluate ethnic specific effects.
Measurement error could also have been a problem in this study. However, we followed identical protocols and used the same MRI machine to obtain measurements on both cases and controls. We also assessed the reliability of our measures of mandible length etc. We did so by performing repeated measures on 10 subjects and found that the intraclass correlation was very high indicating that measurement error is low. We found that our reliability coefficients for the variables we assessed were extremely high.
Another concern was related to the precision of our MRI measurements (5 mm slices) with the differences in mandibular length between groups being on average 4 mm. 5 mm slices were used to reconstruct the complete mandible and other upper airway structures. The reconstructions interpolate between adjacent slices and hence the structures are reconstructed in a continuous fashion. Hence, the measurements are continuous rather than in 5 mm intervals. We also found that our reliability coefficients for mandibular length were extremely high. Given the high reliability, the differences in mandibular length between cases and controls which were 4 mm on average can be determined. Indeed, the mean difference was 3.5-3.8 mm and the standard error was 1.3-1.4 mm, the observed effect size was 2.5 for mandibular length after adjusting for important confounders, indicating a statistical difference from zero and therefore the measurement error should not be an issue. Moreover, we examined the standard error of our measurements. If the standard error is low then the issue of 5 mm MRI slices vs. 4 mm measurement differences is not a concern. Our data show that the standard error of the differences were quite small (less than 1.3 mm) demonstrating that our results (plus or minus the standard error) were within the 5 mm range.
We performed the craniofacial MR image analysis in the same patients that we had used in our previous study of soft tissue structures (10). However, the measurements that we obtained and the hypotheses that we tested in the current study are different from those previously reported (10). Hence, we only corrected for multiple testing within the context of the current study. We did however use a stringent and conservative multiple testing correction (Bonferroni correction) in this study to account for the multiple variables we tested.
We did not exclude four control subjects who met all other criteria but had a slightly elevated AHI between 5.3 to 6.3 events/hour (5.3, 5.4, 5.7 and 6.3). Our results remain the same when these four subjects were excluded from the analysis (data not shown), although they did not quite meet the protocol guidelines for a control subject (AHI less than 5 events/hour).
MR cephalometrics is a powerful imaging modality to study the craniofacial structure in two and three dimensions. However, conventional cephalometric radiographs have been the primary imaging modality to examine the craniofacial structure in apneics. Unfortunately there are several significant limitations to conventional cephalometric radiographs (see introduction). We have compared MRI to conventional cephalometric radiographs in 10 subjects to confirm the validity of our technique. These data showed there were no significant differences between several standard cephalometric measurements made on conventional cephalometric radiographs and those same measurements made on MRI. The intraclass correlation coefficients were very high (0.77-0.99) (see online supplement Table E3a-c). Therefore, these data indicate that MRI is a valid and robust technique to detect standard cephalometric landmarks.
Craniofacial Risk Factors for OSA
Alterations in craniofacial structure are known to be an important risk factor for sleep apnea (13-18, 21-26), since reduction in size of the craniofacial skeleton can lead to reductions in airway caliber. Several cephalometric studies have demonstrated craniofacial abnormalities in patients with OSA compared to age and gender matched controls (17, 26). A study by Okubo and colleagues examined mandibular dimensions in normals and apneics using MRI. A significantly smaller mandibular internal length, mandibular divergence (the angle between the spina mentalis to the left internal gonion line and the spina mentalis to the right internal gonion line) and mandibular enclosed area measured at mandibular plane in subjects with sleep apnea than in control subjects were noted. These results may imply a smaller mandibular depth in patients with OSA compared with controls (21). Our data support the findings from this study by showing the size of the mandible rather than the maxilla or angular measurements may be a more important risk factor for OSA. But in addition, we also showed a gender effect in these craniofacial risk factors for OSA. We demonstrated that smaller mandibular body length and mandibular depth are independent risk factors for OSA in men but not women. However, most of the women were African American hence these differences could also be related to ethnicity.
Etiology of Hyoid Displacement
An important finding of our study is that the hyoid bone is displaced in a more posterior and caudal position, in apneics as compared to controls, as has been shown in previous studies (30-32). Our results confirm this observation but provide new information as to the etiology of the hyoid displacement. It has been speculated that the position of hyoid bone is crucial to pharyngeal patency and therefore a risk factor for OSA because an inferiorly placed hyoid bone places the geniohyoid muscle at a mechanical disadvantage by its angulation (26). Several factors have been hypothesized to influence the position of the hyoid bone. Sforza et al suggested that obesity, through the deposition of fat around the neck, could be the cause of the downward displacement of the hyoid bone (24). Ferguson et al. reported that the distance between the hyoid bone and the mandibular plane increases in proportion to the circumference of the neck (33). Tangugsorn et al. (25) and Nelson et al. (18) found that the hyoid bone is in a lower position in obese patients, hypothesizing that the hyoid movement is an adaptation to the increased size of the tongue that moves the tongue base lower in the hypopharynx in turn reducing the patency of the upper airway. Other hypotheses for the downward and posterior migration of the hyoid bone with increased BMI include imbalance between action of the suprahyoid and infrahyoid muscles, chronic snoring, repetitive chemical and mechanical stimuli, pharyngeal neuropathy, and neuromuscular factors (34, 35). Finally, Paoli and colleagues (23) hypothesized that the low position of the hyoid bone is more likely to be a consequence of OSA than a pre-existing anatomical abnormality; they posited that overtime the repeated apneas during the night might cause a lengthening of the hyoid ligaments.
Our data provide strong support for the hypothesis of Tangugsorn et al (25) and Nelson et al (18) that the enlarged tongue displaces the hyoid inferiorly and posteriorly. Specifically, we found that if we introduce tongue volume into our models as a covariate there was no longer any difference between apneics and controls in hyoid position. Thus, we provide support for the concept that it is the enlarged tongue in apneics that leads to the alteration in the hyoid position.
This is clinically important because surgical procedures (i.e., hyoid advancement/hyoid suspension) have been developed to normalize the position of the hyoid. Such procedures are not addressing the fundamental issue. Thus surgical procedures that alter the position/size of the tongue may be more beneficial. Surgical procedures to reduce tongue volume have been introduced (36-38) but in these studies the effect on hyoid position was not addressed.
CONCLUSIONS
Analysis of three-dimensional MRI allowed us to accurately quantify craniofacial structures. The present study is the first study that has used MR cephalometrics to examine the entire craniofacial structure in a large population. In our study population, the size of the mandible rather than the maxilla or angular measurements (SNA, SNB) was the most important risk factor for OSA. Alterations in mandibular structure including shorter mandibular length, and smaller mandibular depth are independent risk factors for sleep apnea in men, but not in the women. In both men and women, apneics have an inferiorly displaced hyoid as compared to controls. This difference was no longer found when tongue volume was introduced as a covariate. Thus, the inferior-posterior positioning of the hyoid bone in apneics is likely the direct result of the enlarged tongue volumes in the patients directly displacing the hyoid bone. These findings have important implications for the pathogenesis and treatment of sleep apnea.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Eugene Kim for the three-dimensional reconstruction MRI images of mandible, tongue and hyoid.
Footnotes
This article has an online data supplement.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: Current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–178. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 4.Ellen RL, Marshall SC, Palayew M, Molnar FJ, Wilson KG, Man-Son-Hing M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2006;2:193–200. [PubMed] [Google Scholar]
- 5.George CF. Sleep apnea, alertness, and motor vehicle crashes. Am J Respir Crit Care Med. 2007;176:954–956. doi: 10.1164/rccm.200605-629PP. [DOI] [PubMed] [Google Scholar]
- 6.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 7.Seicean S, Kirchner HL, Gottlieb DJ, Punjabi NM, Resnick H, Sanders M, Budhiraja R, Singer M, Redline S. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: The sleep heart health study. Diabetes Care. 2008;31:1001–1006. doi: 10.2337/dc07-2003. [DOI] [PubMed] [Google Scholar]
- 8.White DP. Sleep apnea. Proc Am Thorac Soc. 2006;3:124–128. doi: 10.1513/pats.200510-116JH. [DOI] [PubMed] [Google Scholar]
- 9.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152:1673–1689. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 10.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Arens R, Maislin G, Pack AI. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric mri. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 11.Haponik EF, Smith PL, Bohlman ME, Allen RP, Goldman SM, Bleecker ER. Computerized tomography in obstructive sleep apnea. Correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983;127:221–226. doi: 10.1164/arrd.1983.127.2.221. [DOI] [PubMed] [Google Scholar]
- 12.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:462–466. doi: 10.1164/ajrccm/148.2.462. [DOI] [PubMed] [Google Scholar]
- 13.Guilleminault C, Riley R, Powell N. Obstructive sleep apnea and abnormal cephalometric measurements. Implications for treatment. Chest. 1984;86:793–794. doi: 10.1378/chest.86.5.793. [DOI] [PubMed] [Google Scholar]
- 14.Lowe AA, Fleetham JA, Adachi S, Ryan CF. Cephalometric and computed tomographic predictors of obstructive sleep apnea severity. Am J Orthod Dentofacial Orthop. 1995;107:589–595. doi: 10.1016/s0889-5406(95)70101-x. [DOI] [PubMed] [Google Scholar]
- 15.Lowe AA, Santamaria JD, Fleetham JA, Price C. Facial morphology and obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1986;90:484–491. doi: 10.1016/0889-5406(86)90108-3. [DOI] [PubMed] [Google Scholar]
- 16.Lyberg T, Krogstad O, Djupesland G. Cephalometric analysis in patients with obstructive sleep apnoea syndrome. I. Skeletal morphology. J Laryngol Otol. 1989;103:287–292. doi: 10.1017/s0022215100108734. [DOI] [PubMed] [Google Scholar]
- 17.Miles PG, Vig PS, Weyant RJ, Forrest TD, Rockette HE. Craniofacial structure and obstructive sleep apnea syndrome—a qualitative analysis and meta-analysis of the literature. Am J Orthod Dentofacial Orthop. 1996:163–172. doi: 10.1016/s0889-5406(96)70177-4. [DOI] [PubMed] [Google Scholar]
- 18.Nelson S, Hans M. Contribution of craniofacial risk factors in increasing apneic activity among obese and nonobese habitual snorers. Chest. 1997;111:154–162. doi: 10.1378/chest.111.1.154. [DOI] [PubMed] [Google Scholar]
- 19.Schwab RJ, Pack AI, Gupta KB, Metzger LJ, Oh E, Getsy JE, Hoffman EA, Gefter WB. Upper airway and soft tissue structural changes induced by cpap in normal subjects. Am J Respir Crit Care Med. 1996;154:1106–1116. doi: 10.1164/ajrccm.154.4.8887615. [DOI] [PubMed] [Google Scholar]
- 20.Schwab RJ, Pasirstein M, Kaplan L, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med. 2006;173:453–463. doi: 10.1164/rccm.200412-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okubo M, Suzuki M, Horiuchi A, Okabe S, Ikeda K, Higano S, Mitani H, Hida W, Kobayashi T, Sugawara J. Morphologic analyses of mandible and upper airway soft tissue by mri of patients with obstructive sleep apnea hypopnea syndrome. Sleep. 2006;29:909–915. doi: 10.1093/sleep/29.7.909. [DOI] [PubMed] [Google Scholar]
- 22.Iked N, Hazime N, Dekeister C, Folia M, Tiberge M, Paoli JR. Comparison of the cephalometric characteristics of snoring patients and apneic patients as a function of the degree of obesity. Apropos of 162 cases. Rev Stomatol Chir Maxillofac. 2001;102:305–311. [PubMed] [Google Scholar]
- 23.Paoli JR, Lauwers F, Lacassagne L, Tiberge M, Dodart L, Boutault F. Craniofacial differences according to the body mass index of patients with obstructive sleep apnoea syndrome: Cephalometric study in 85 patients. Br J Oral Maxillofac Surg. 2001;39:40–45. doi: 10.1054/bjom.2000.0551. [DOI] [PubMed] [Google Scholar]
- 24.Sforza E, Bacon W, Weiss T, Thibault A, Petiau C, Krieger J. Upper airway collapsibility and cephalometric variables in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:347–352. doi: 10.1164/ajrccm.161.2.9810091. [DOI] [PubMed] [Google Scholar]
- 25.Tangugsorn V, Krogstad O, Espeland L, Lyberg T. Obstructive sleep apnoea: Multiple comparisons of cephalometric variables of obese and non-obese patients. J Craniomaxillofac Surg. 2000;28:204–212. doi: 10.1054/jcms.2000.0147. [DOI] [PubMed] [Google Scholar]
- 26.Lowe AA, Ozbek MM, Miyamoto K, Pae EK, Fleetham JA. Cephalometric and demographic characteristics of obstructive sleep apnea: An evaluation with partial least squares analysis. Angle Orthod. 1997;67:143–153. doi: 10.1043/0003-3219(1997)067<0143:CADCOO>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Pierson RW, Pack AI, Maislin G, Schwab RJ. Identification of craniofacial risk factors for obstructive sleep apnea using novel MR cephalometric techniques. Am J Respir Crit Care Med. 2003;167:A601. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Kales A, editors. A manual of standardized terminology: Techniques and scoring system for sleep stages of human subjects. Brain Information Service/Brain Research Institute, University of California; Los Angeles: 1968. [Google Scholar]
- 29.Steiner CC. The use of cephalometrics as an aid to planning and assessing orthodontic treatment. Am J orthod. 1960;46:721–735. [Google Scholar]
- 30.Banabilh SM, Suzina AH, Dinsuhaimi S, Singh GD. Cranial base and airway morphology in adult males with obstructive sleep apnoea. Aust Orthod J. 2007;23:89–95. [PubMed] [Google Scholar]
- 31.Guilleminault C, Quera-Salva MA, Partinen M, Jamieson A. Women and the obstructive sleep apnea syndrome. Chest. 1988;93:104–109. doi: 10.1378/chest.93.1.104. [DOI] [PubMed] [Google Scholar]
- 32.Lowe AA, Ono T, Ferguson KA, Pae EK, Ryan CF, Fleetham JA. Cephalometric comparisons of craniofacial and upper airway structure by skeletal subtype and gender in patients with obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 1996;110:653–664. doi: 10.1016/s0889-5406(96)80043-6. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson KA, Ono T, Lowe AA, Ryan CF, Fleetham JA. The relationship between obesity and craniofacial structure in obstructive sleep apnea. Chest. 1995;108:375–381. doi: 10.1378/chest.108.2.375. [DOI] [PubMed] [Google Scholar]
- 34.Friberg D, Ansved T, Borg K, Carlsson-Nordlander B, Larsson H, Svanborg E. Histological indications of a progressive snorers disease in an upper airway muscle. Am J Respir Crit Care Med. 1998;157:586–593. doi: 10.1164/ajrccm.157.2.96-06049. [DOI] [PubMed] [Google Scholar]
- 35.Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med. 1996;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- 36.Steward DL. Effectiveness of multilevel (tongue and palate) radiofrequency tissue ablation for patients with obstructive sleep apnea syndrome. Laryngoscope. 2004;114:2073–2084. doi: 10.1097/01.mlg.0000149438.35855.af. [DOI] [PubMed] [Google Scholar]
- 37.Stuck BA, Maurer JT, Verse T, Hormann K. Tongue base reduction with temperature-controlled radiofrequency volumetric tissue reduction for treatment of obstructive sleep apnea syndrome. Acta Otolaryngol. 2002;122:531–536. doi: 10.1080/00016480260092354. [DOI] [PubMed] [Google Scholar]
- 38.Terris DJ, Kunda LD, Gonella MC. Minimally invasive tongue base surgery for obstructive sleep apnoea. J Laryngol Otol. 2002;116:716–721. doi: 10.1258/002221502760238028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.