Abstract
Background
The saddle shape of the normal mitral annulus has been quantitatively described by several groups. There is strong evidence that this shape is important to valve function. A more complete understanding of regional annular geometry in diseased valves may provide a more educated approach to annuloplasty ring selection and design. We hypothesized that mitral annular shape is markedly distorted in patients with diseased valves.
Methods
Real-time 3-dimensional echocardiography was performed in patients with normal mitral valves (n=20), ischemic mitral regurgitation (IMR, n=10) and myxomatous mitral regurgitation (MMR, n=20). Thirty-six annular points were defined to generate a 3D model of the annulus. Regional annular parameters were measured from these renderings. Left ventricular inner diameter (LVIDd) was obtained from 2D echocardiographic images.
Results
Annular geometry was significantly different between the three groups. The annuli were larger in the MMR and the IMR groups. The annular enlargement was greater and more pervasive in the MMR. Both diseases were associated with annular flattening though the regional distribution of that flattening was different between groups. LVIDd was increased in both groups. However, relative to the LVIDd, the annulus was disproportionately dilated in the MMR group.
Conclusion
Patients with MMR and IMR have enlarged and flattened annuli. In the case of MMR, annular distortions may be the driving factor leading to valve incompetence. These data suggest that the goal of annuloplasty should be the restoration of normal annular saddle shape and that the use of flexible, partial and flat rings may be ill advised.
Keywords: Echocardiography, Mitral Regurgitation, Mitral Valve Repair
Introduction
Levine first reported the three dimensional (3D) saddle shape of the mitral valve annulus in 1987 [1]. For 15 years the finding received little attention until our group described the influence of annular saddle shape on leaflet stress [2, 3]. Since that time we have continued to refine the description of regional mitral annular geometry using 3D echocardiography [4–7]. This information has, to some extent, been useful in guiding annuloplasty ring selection as well as annuloplasty ring design [8–12].
Despite the increasing understanding of the normal annulus, very little has been reported regarding the shape of pathologic valves. Lack of understanding of diseased annuli still hinders annuloplasty ring selection for mitral repair. Many surgeons prefer the use of flexible rings or bands with the idea that such devices preserve annular shape. We hypothesize that regional annular geometry in the two most common forms of mitral valve disease, myxomatous degeneration and ischemic mitral regurgitation, is profoundly abnormal. Therefore, the goal of annuloplasty should be restoration of normal annular systolic saddle shape and not shape preservation.
Patients and Methods
Patients
Intraoperative transesophageal real-time 3D echocardiography (3DE) was performed for 20 patients with myxomatous mitral degeneration (MMR group), 10 patients with ischemic mitral regurgitation (IMR group) and 20 patients with normal mitral valves who were undergoing non-mitral valve cardiac surgery (normal group). Full volume data sets of the annulus were acquired with an iE-33 platform (Philips Medical, Andover, MA) equipped with a X7-2t TEE matrix-array transducer. In the MMR group, 19 patients had posterior leaflet prolapse, and one patient had bi-leaflet prolapse. All patients underwent implantation of an annuloplasty device (complete rigid ring = 15, partial flexible band = 5). Leaflet pathology was addressed using one or combination of the following techniques: triangular resection (n=15), quadrangular resection/ sliding plasty (n=2), leaflet inversion/plication (n=3), chordal transfer or neochord implantation (n=3). All IMR patients had coronary artery disease and discrete LV wall motion abnormality (Inferior = 6, anterolateral = 4). Six patients had significant leaflet restriction. Nine of ten valves were repaired and one valve had to be replaced. Normal patients had no evidence of mitral valvular abnormality. The research protocol was approved by the University of Pennsylvania School of Medicine Institutional Review Board.
Image Segmentation
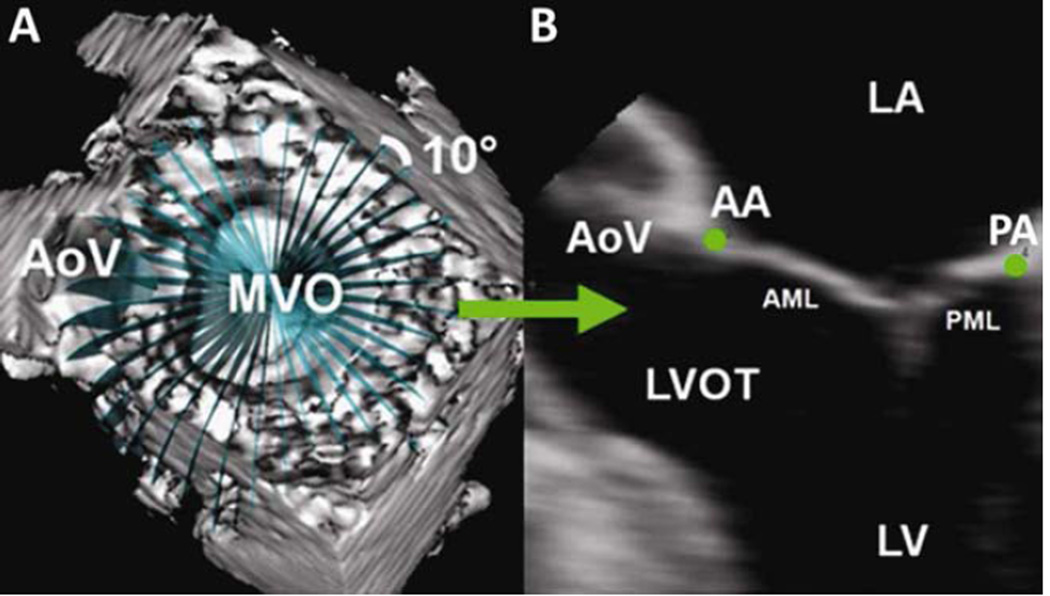
Annular analysis was performed at midsystole as previously described [6]. Each data set was exported to Echo-View 5.4 (TomTec Imaging, Munich, Germany) for image analysis. Two annular points were interactively marked in 18 cross-sectional planes separated by 10 degrees around the annular circumference (Figure 1). Anterior and posterior commissures (AC, PC) were defined at the junction between the anterior and posterior leaflets. The X, Y, Z coordinates were exported from TomTec to Matlab software (The Mathworks, Natick, MA) to perform quantitative reconstruction.
Figure 1. Technique for Annular Segmentation.
Panel A: 3D echocardiographic volume containing the mitral valve with cross-sectional planes at 10° increments; Panel B: Representative 2D cross-section with green dots representing the selected annular points. AA: anterior mitral annulus; AML: anterior mitral leaflet; AoV: aortic valve; LA: left atrium; LV: left ventricle; LVOT: left ventricular outflow tract; MVO: mitral valve orifice; PA: posterior mitral annulus; PML: posterior mitral leaflet
Annular Analysis
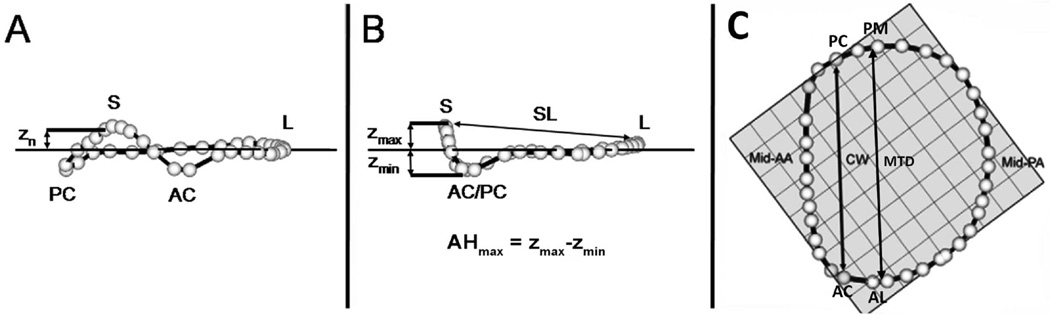
The annulus was reconstructed by interpolation at 1° intervals around the annular circumference. The annular model was rotated such that the valve orifice plane was aligned with the x-y plane. Under these geometric conditions, the z coordinate (zn) of each annular point was equal to its distance to the plane of the mitral valve (Figure 2a). Regional AH for each data point (AHn) was then defined as zn - zmin, where zmin was the lowest point on the mitral annulus. Maximal AH (AHmax) was defined as zmax -zmin, where zmax was the highest point on the mitral annulus.
Figure 2. Annular landmarks.
Oblique (A) Intercommissural (B) and Transvalvular (C) views of a 3D annular model depicting the 36 annular data points (white spheres). Panel A: calculation of annular height at a given annular point (Zn). Panel B: determination of maximum Annular Height (AHmax) and septolateral diameter (SL). Panel C: determination of intercommissural width (CW) and the mitral transverse diameter (MTD). AA = Anterior annulus, AC = Anterior commissure, AL = Anterolateral Annulus, L= Lateral annulus, PA = Posterior annulus, PC = Posterior commissure, PM= Posteromedial annulus, S = Septum, Zmax = Maximumheight, Zmin = Minimum height. The least squares plane is depicted by a horizontal line in panel A, B and the check boxes in panel C.
Several anatomic landmarks were identified (Figure 2 a,b,c). The septum (S) was identified as the anterior horn of the annulus at the aortic valve, corresponding to zmax. AC and PC co-ordinates were superimposed on to the annulus rendering to divide the annulus into anterior and posterior portion. The lateral annulus (L) was located at the middle of the posterior annulus circumference. Finally, with the annular model rotated such that the commissures were aligned on a plane of constant x, the anterolateral and posteromedial (AL and PM) annular points are the locations of maximal and minimal y-value.
Septolateral (SL) diameter was defined as the distance separating data points S and L. Commissural Width (CW) was defined as the distance between commissures. Mitral transverse diameter (MTD) was defined as the distance separating AL and PM. Mitral annular area (MAA) was defined as the area enclosed by the 2D projection of an annular data set onto its corresponding least squares plane. Total mitral annular circumference and the lengths of the anterior and posterior annuli (LAA, LPA) were calculated.
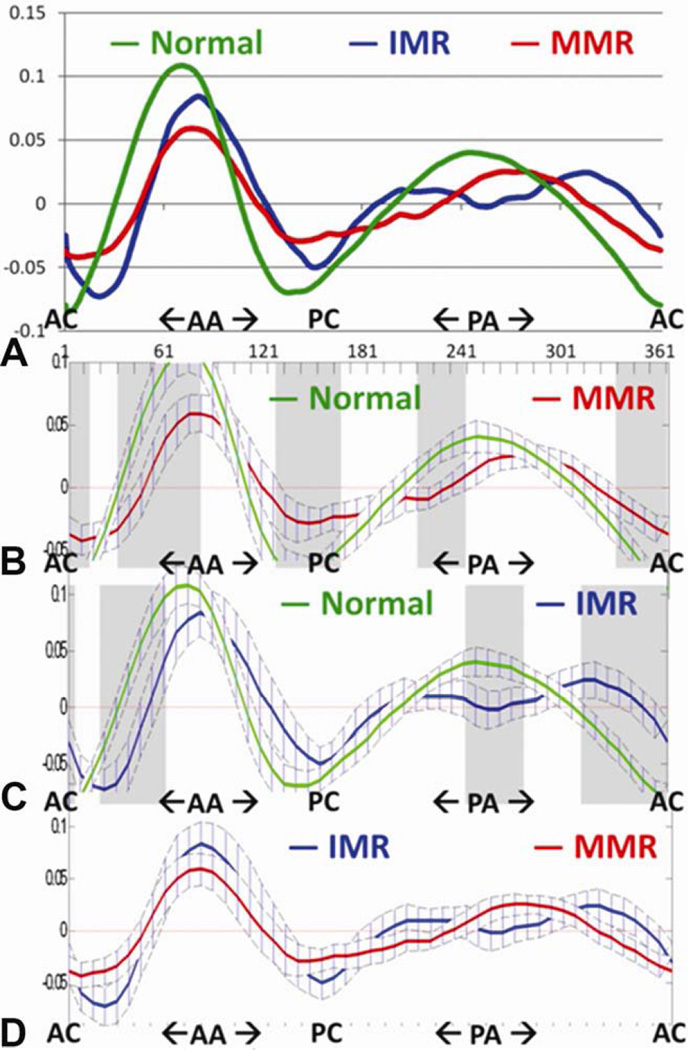
As previously described [2, 4], we define the parameter AHmaxCWR = AHmax/CW *100% to quantify global annular nonplanarity. To facilitate the comparison of regional annular nonplanarity between subjects we define regional AHnCWR = AHn/CW *100%. The Cartesian coordinates for each point in a given annular model were converted to cylindrical coordinates (r, Θ, z), and the data set was translated in the z direction and rotated around the MV axis (Θ direction) so that the anterior commissure was located at Θ = 0, and the center of mass of each annulus was located at z=0. Values of AHn for each point in a given data set were then recalculated in this fixed frame of reference. AHnCWR was plotted as a function of rotational position (Θ) on the mitral annulus for each data set (Figure 3).
Figure 3.
Panel A depicts mean annular height normalized to commissural width (AHCWR) plotted as a function of rotational position around the annulus for each group. The three curves are different in overall shape from each other (p<0.001 by functional ANOVA). The areas where they differ are indicated by the shaded gray bars. (Panel B): Normal vs myxomatous (MMR): Significant differences (p<0.05) at positions 1–15, 30–85, 125–165, 210–240, 330–360. (Panel C): Normal vs. ischemic (IMR): Significant differences (p<0.05) at positions 1–5, 20–60, 240–275, 310–360. (Panel D): IMR vs MMR: No significant difference at any individual position. AA = anterior annulus, AC = Anterior commissure, PA= posterior annulus, PC = Posterior commissure
Creation of Hybrid annular models
The center of gravity of all the annular models within each group were translated to the origin and aligned such that the sum of least square planes coincided with the XY plane. The annuli are rotated such that both commissures for each valve lay on a plane of constant X value (different for each annulus). Radial distance was calculated from the origin to the projection of each annular point on the XY plane. Annular height was calculated as previously described. The radial distance and annular height were interpolated as a function of rotational position on the annulus by repeating this process at 1° interval around the annulus. A mean radial distance and annular height was calculated by averaging these parameters at each rotational position for all annuli within the group. The annular points such obtained were then re-converted to XYZ co-ordinates and rendered in 3D space to generate the hybrid annulus.
Left Ventricle internal dimension and annular ratio
Left Ventricular (LV) internal dimension in diastole (LVIDd) was obtained from 2D-echocardiography images obtained at the same intraoperative exam. To assess degree of annular dilation relative to LV dilation, ratio of annular parameter (MAA, MAC, CW, MTD, SL) to LVIDd was calculated for the IMR and MMR group, and normalized to the normal group.
Statistical Analysis
Regional parameters were compared using pair wise student t-test. Rotational height curves were compared using functional ANOVA.
Results
Patient characteristics
There was no difference in severity of MR in the IMR and the MMR group. The ejection fraction in the IMR group was significantly depressed as compared to the normal and the MMR group (Table 1).
Table 1.
Patient Demographics
| Normal (n=20) |
MMR (n=20) |
IMR (n=10) |
|
|---|---|---|---|
| Age | 64.7 ± 15.8 | 58.3 ± 9.3 | 67.4 ± 11.3 a |
| Ejection Fraction (%) | 60.6 ± 12.4 | 58.9 ± 9.1 | 26.5 ± 17.2 a,b |
| Mitral regurgitation grade | 0.6 ± 0.4 | 3.6 ± 0.6 c | 3.3 ± 0.8 b |
Mean ± SD
= p<0.05 IMR vs. MMR
= p<0.05 Normal vs. IMR
= p<0.05 Normal vs. MMR
Annular size and shape
Annular parameters are summarized in Table 2. MAA is increased in both the IMR and the MMR group as compared to normal group, however interesting differences are noted between the IMR and the MMR group. First, only the septolateral diameter is enlarged in the IMR group, but the intercommissural diameter is similar to the normal group. In the MMR group both the septolateral and the intercommissural diameters are increased, resulting in significantly larger annular area, not only when compared to the normal group, but also when compared to the IMR group. Secondly, both the anterior and posterior annular circumference is increased in the MMR group. Only the posterior annular circumference is increased in the IMR group.
Table 2.
Annular Parameters
| Normal (n=20) |
MMR (n=20) |
IMR (n=10) |
|
|---|---|---|---|
| Annular height (mm) | 7.18 ± 1.65 | 6.0 ± 1.14 a | 7.6 ± 1.8 b |
| Commissural width (mm) | 31.44 ± 3.45 | 44.50 ± 6.00 a | 33.0 ± 3.6 b |
| Annular height: Commissural width ratio |
22.90 ± 5.06 | 12.90 ± 3.65 a | 23.2 ± 5.9 b |
| Septolateral dimension (mm) |
28.62 ± 3.29 | 37.39 ± 6.05 a | 34.6 ± 5.0 c |
| Maximum transverse diameter (mm) |
34.55 ± 4.00 | 45.25 ± 6.15 a | 38.9 ± 3.0 b,c |
| Mitral annular area (mm2) | 784.49 ± 159.34 | 1344.47 ± 367.68 a | 1067.7 ± 178.5 b,c |
| Anterior circumference (mm) | 39.44 ± 5.18 | 61.17 ± 9.59 a | 41.1 ± 5.0 b |
| Posterior circumference (mm) |
63.01 ± 9.06 | 72.81 ± 11.83 a | 81.6 ± 7.4 b,c |
| Total circumference (mm) | 102.45 ± 10.80 | 133.98 ± 17.84 a | 122.8 ± 9.4 c |
Mean ± SD
= p<0.05 Normal vs. MMR
= p<0.05 IMR vs. MMR
= p<0.05 IMR vs. MMR
The abnormal shape of the annulus in the MMR and the IMR groups is illustrated by the analysis of regional annular height (Figure 3). Regions of annular flattening are prominent in both disease process but more diffuse in the cases of MMR. The MMR annuli are flatten most prominently at the “anterior horn” near the junction of the A1 and A2 regions. Additionally the MMR annuli are flatter at both commisures and the junction of the P2–P3 segments. The IMR annuli are flatten at the AC, A1 region as well as the mid segment of P2 and all of P3.
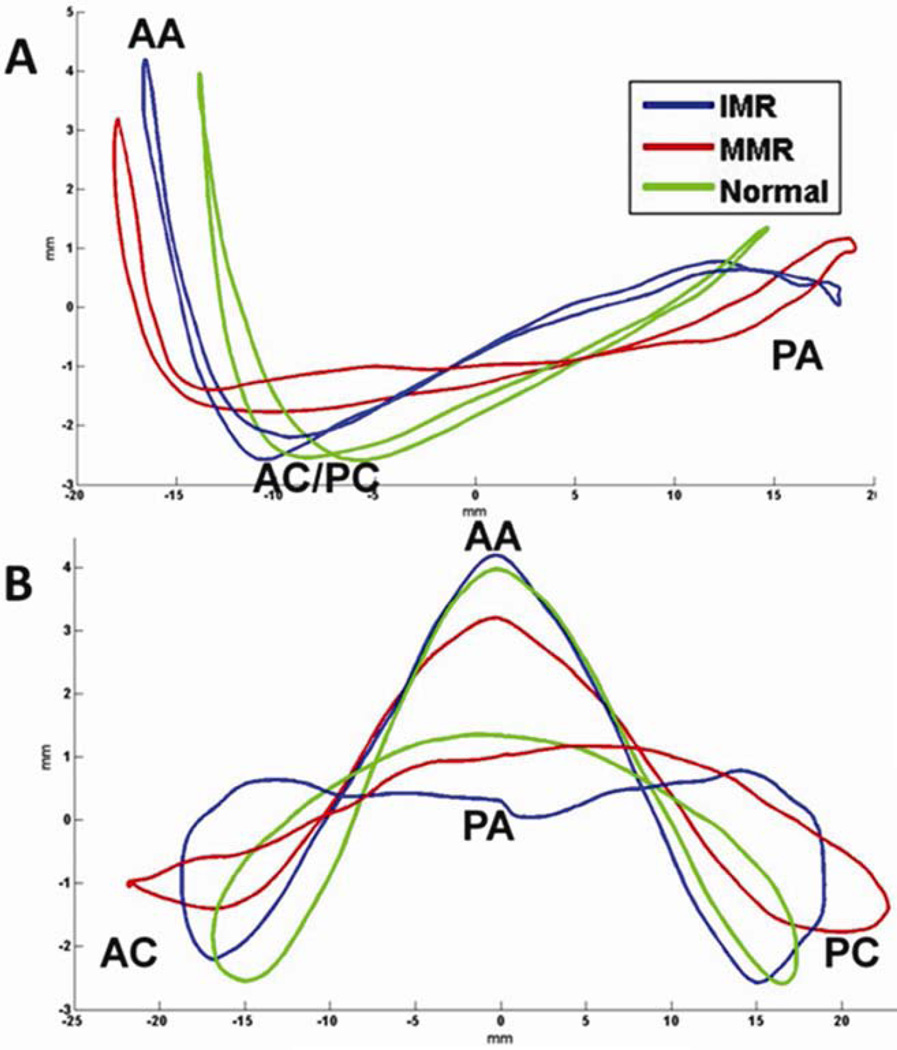
AHmax and AHmaxCWR were markedly reduced in the MMR patients when compared to normal. Interestingly these global assessments of annular nonplanarity were not significantly different from normal in the IMR group. This finding underscores the value of the regional assessment of mitral annular nonplanarity in assessing the geometrical distortions of pathologic valves. If only the global parameters had been assessed the conclusion of this study would have been completely different. These annular distortions are also evident in the hybrid annular models as illustrated in Figure 4.
Figure 4.
Transcommissural (Panel A) and anteroposterior (Panel B) views of hybrid annular models for the normal, ischemic mitral regurgitation (IMR), and myxomatous mitral regurgitation (MMR) groups. AA= Anterior annulus, AC = Anterior commissure, PA = posterior annulus, PC = Posterior commissure
LV- Annular size relationship
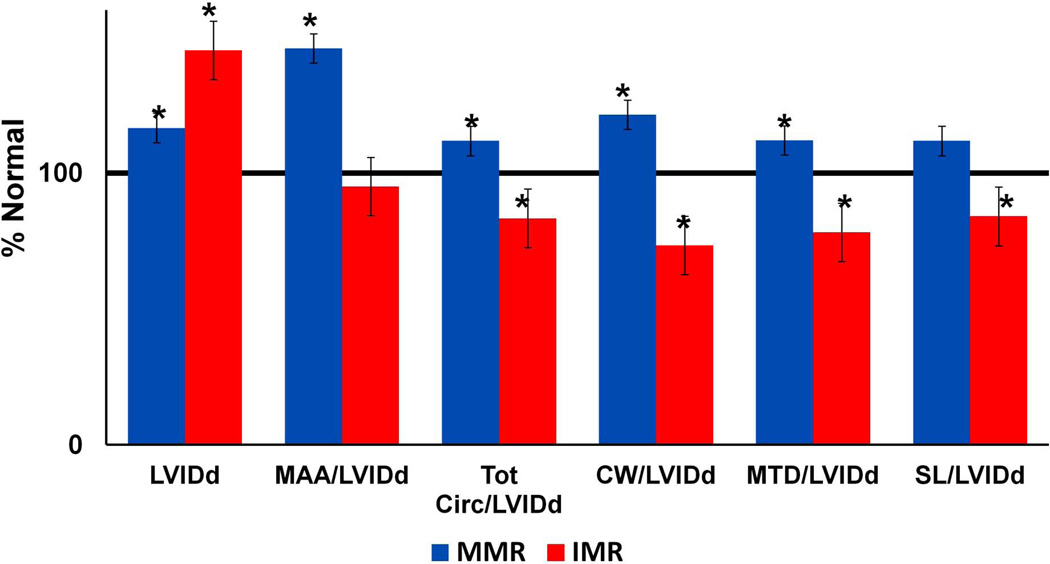
Compared to the normal group, diastolic left ventricular internal dimension (LVIDd) was increased in both the IMR and the MMR group. However, the degree of annular enlargement relative to the LVIDd was significantly higher in the MMR group as compared to the normal group. On the contrary, in the IMR group the ratio of annular size to LV size was less compared to normal values (Figure 5) indicating the LV had undergone a greater degree of dilatation.
Figure 5.
Left ventricle diameter (LVIDd) and ratio of left ventricle diameter to various annular size parameters for the ischemic (IMR) and the myxomatous (MMR) groups are depicted as a percent of the normal group measurements (horizontal black line). Error bars = standard error.
Comment
When taken together the reported annular and LV distortions shed light on the pathogenesis of both diseases. In cases of IMR the annulus is dilated to a lesser degree relative to the LV when compared to normal. The annulus also undergoes significant regional flattening. In the IMR group the annular distortions (enlargement and flattening) were more pronounced along the posterior portion of the annulus. These findings are consistent with what is known about the pathogenesis of IMR. That is, a regional infarct produces regional LV distortion which subsequentenly causes regional annular distortions and leaflet tethering which results in MR [8,13–15].
In MMR patients, the annular enlargement and flattening are both greater and more pervasive when compared to the IMR group. The annular enlargement is also out of proportion to the LV enlargement, suggesting the annular distortions occur prior to and independent of the ventricular enlargement. It raises the possibility that the MMR annuli are congenitally enlarged and flat, and these annular distortions maybe the primary cause of the MMR. Annular flattening and enlargement has been shown to decrease leaflet curvature resulting in increased leaflet stress [2, 3, 16] and strain [17, 18], which in MMR annuli over time, may cause myxomatous tissue degeneration, chordal rupture and MR. A recent study by Lee et.al. supports this theory [19]. Using similar 3D echocardiographic techniques this group evaluated patients with normal valves, mitral valve prolapse with varying degrees of MR as well as patients with MR and no leaflet prolapse. They found that annular flattening in MMR patients had a strong association with progressive leaflet billowing, chordal rupture and MR. In their study patients with AHmaxCWR of < 15% were 7 times more likely to have chordal rupture and MR.
The work by Lee et. al. is also very interesting in that its mean measurements for most annular parameters in all groups (normal, MMR and IMR) are within millimeters (for some parameters fractions of millimeters) of the measurements reported in this study. The two studies taken together bear strongly on annuloplasty ring selection.
For almost 45 years there has been little consensus regarding the most appropriate annuloplasty ring design. Early on the major debate was whether the ring should be rigid [20, 21] or flexible [22, 23]. As more centers became experienced with repair, the debate expanded to include the relative efficacy of complete [24, 25] vs. partial rings [26, 27]. During the early 2000s, the concept of disease specific annuloplasty briefly became vogue [28, 29]. Despite all the conjecture and opinion there was no quantitative support for the use of any device over another. In 2002 our group was the first to propose the efficacy of saddle shape annuloplasty in reducing leaflet stress and potentially increasing repair durability [2]. Other groups have expanded our work to demonstrate that saddle shape preservation decreases leaflet [16, 17] and annular strain [13, 30] as well as increasing leaflet coaptation [31].
Our results and the results of Lee et. al. indicate that in cases of MMR and IMR the annulus is significantly abnormal. This finding argues against the use of flexible and partial rings which were conceived to maintain “normal” annular geometry and function. In these disease processes there is no normal geometry to maintain. The goal of annuloplasty is to restore not maintain annular geometry.
Even more profoundly, the results of the two studies argue strongly against the use of flat annuloplasty rings. If it is true that an AHmaxCWR of less than 15% predesposes to leaflet billowing, chordal rupture and MR what does forcing an annulus to an AHCWR of 0% with a flat annuloplasty ring do to the potential for further tissue degeneration after repair?
This question becomes even more compelling in light of the growing body of evidence indicating that mitral valve repair durability is far less robust than initially reported. Longer term follow-up has revealed that between 10% and 16% of patients undergoing mitral repair for MMR will require reoperation for severe MR within 10 years [32– 34]. Even more concerning are the reports of an unexpectedly high incidence of recurrent moderate MR after repair. Several studies from experienced centers indicate that the recurrence of 2+ or greater MR is between 2% and 4% per year [35–37]. A significant number of these failures result from chordal, leaflet and suture line disruption, suggesting mechanical stress and strain to be the cause [38].
The proposed efficacy of saddle shape annuloplasty on mitral valve repair durability is speculative but compelling in light of a growing body of work by our group and others that supports the positive influence of annular saddle shape on valve stress/strain profiles and leaflet coaptation.
Table 3.
Annular dilation relative to the left ventricular dilation
| Normal (n=20) |
MMR (n=20) |
IMR (n=10) |
|
|---|---|---|---|
| Left Ventricular diastolic diameter (LVIDd) (cm) |
4.86 ± 0.61 | 5.66 ± 0.71 a | 7.05 ± 1.11 b,c |
| Mitral annular area/LVIDd | 1.63 ± 0.37 | 2.38 ± 0.58 a | 1.55 ± 0.42 c |
| Mitral annular circumference/LVIDd | 2.14 ± 0.35 | 2.39 ± 0.35 a | 1.78 ± 0.34 b,c |
| Commisural width/LVIDd | 0.65 ± 0.09 | 0.79 ± 0.12 a | 0.48 ± 0.10 b,c |
| Maximum transverse diameter/LVIDd | 0.72 ± 0.12 | 0.81 ± 0.12 a | 0.56 ± 0.10 b,c |
| Septolateral dimension/LVID | 0.60 ± 0.10 | 0.67 ± 0.12 | 0.50 ± 0.12b,c |
Mean ± SD
= p<0.05 Normal vs. MMR,
= p<0.05 Normal vs. IMR,
= p<0.05 IMR vs. MMR
Acknowledgments
This study was supported by grants from the National Heart, Lung and Blood Institute, Bethesda, MD, (HL63954, HL73021, HL103723, HL108330). R. Gorman and J. Gorman are supported by individual Established Investigator Awards from the American Heart Association, Dallas, TX. A. Jassar was supported by AHA post-doctoral fellowship.
Abbreviations and Acronyms
- AC
Anterior Commissure
- AH
Annular Height
- AHCWR
Annular height: Commissural width ratio
- CW
Commissural width
- IMR
Ischemic mitral regurgitation
- L
Lateral annulus
- LAA
Length of anterior annulus
- LPA
Length of posterior annulus
- LVIDd
Left ventricle inner diameter (diastolic)
- MAA
Mitral annular area
- MMR
Myxomatous mitral regurgitation
- MTD
Maximum transverse diameter
- PC
Posterior commissure
- PM
Posteromedial annulus
- S
Septum
- SL
Septolateral dimension
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levine RA, Triulzi MO, Harrigan P, Weyman AE. The relationship of mitral annular shape to the diagnosis of mitral valve prolapse. Circulation. 1987;75:756–767. doi: 10.1161/01.cir.75.4.756. [DOI] [PubMed] [Google Scholar]
- 2.Salgo IS, Gorman JH, 3rd, Gorman RC, et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation. 2003;106:711–717. doi: 10.1161/01.cir.0000025426.39426.83. [DOI] [PubMed] [Google Scholar]
- 3.Ryan LP, Jackson BM, Hamamoto H, et al. The Influence of Annuloplasty Ring Geometry on Mitral Leaflet Curvature. Annals of Thoracic Surgery. 2008;86:749–760. doi: 10.1016/j.athoracsur.2008.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan LP, Jackson BM, Enomoto Y, et al. Description of Regional Mitral Annular Non-planarity in Healthy Human Subjects: A Novel Methodology. Journal of Thoracic and Cardiovascular Surgery. 2007;134:644–648. doi: 10.1016/j.jtcvs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Ryan LP, Jackson BM, Eperjesi TJ, et al. Quantitative Description of Mitral Valve Geometry by Means of Real-Time Three-Dimensional Echocardiography. Innovations: Technology and Techniques in Cardiothoracic and Vascular Surgery. 2007;2:237–244. doi: 10.1097/IMI.0b013e31815bdbdf. [DOI] [PubMed] [Google Scholar]
- 6.Ryan LP, Jackson BM, Eperjesi TJ, et al. Regional Assessment of Human Mitral Leaflet Curvature Using Real-Time Three-Dimensional Echocardiography. Journal of Thoracic and Cardiovascular Surgery. 2008;136:726–734. doi: 10.1016/j.jtcvs.2008.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmood F, Karthik S, Subramaniam B, et al. Intraoperative application of geometric three-dimensional mitral valve assessment package: a feasibility study. J Cardiothorac Vasc Anesth. 2008;22:292–298. doi: 10.1053/j.jvca.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Gorman JH, 3rd, Gorman RC, Jackson BM, Enomoto Y, St John-Sutton MG, Edmunds LH., Jr Annuloplasty ring selection for chronic ischemic mitral regurgitation: lessons from the ovine model. Ann Thorac Surg. 2003;76:1556–1563. doi: 10.1016/s0003-4975(03)00891-9. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MO, Jensen H, Smerup M, et al. Saddle-shaped mitral valve annuloplasty rings experience lower forces compared with flat rings. Circulation. 2008;118(14 Suppl):S250–S255. doi: 10.1161/CIRCULATIONAHA.107.746776. [DOI] [PubMed] [Google Scholar]
- 10.Mahmood F, Subramanian B, Gorman JH, III, et al. Three-Dimensional Echocardiographic Assessment of Changes in Mitral Valve Geometry after Valve Repair. Annals of Thoracic Surgery. 2009;88:1838–1844. doi: 10.1016/j.athoracsur.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahmood F, Gorman JH, III, Subramanian B, et al. Changes in Mitral Valve Annular Geometry After Repair Saddle Shaped vs. Flat Annuloplasty Rings. Annals of Thoracic Surgery. 2010;90:1212–1220. doi: 10.1016/j.athoracsur.2010.03.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levack MM, Jassar AS, Shang EK, et al. 3D-Echocardiographic Analysis of Mitral Annular Dynamics: Implication for Annuloplasty Selection. Circulation. 2012;126:S183–S188. doi: 10.1161/CIRCULATIONAHA.111.084483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorman JH, III, Jackson BM, Enomoto Y, Gorman RC. The Effect of Regional Ischemia on Mitral Valve Annular Saddle Shape. Annals of Thoracic Surgery. 2004;77:544–548. doi: 10.1016/S0003-4975(03)01354-7. [DOI] [PubMed] [Google Scholar]
- 14.Ryan LP, Jackson BM, Parish LM, et al. Regional and Global Patterns of Annular Remodeling in Ischemic Mitral Regurgitation. Annals of Thoracic Surgery. 2007;84:553–559. doi: 10.1016/j.athoracsur.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Vergnat M, Jassar AS, Jackson BM, et al. Ischemic Mitral Regurgitation: A Quantitative Three-Dimensional Echocardiographic Analysis. Annals of Thoracic Surgery. 2011;91:157–164. doi: 10.1016/j.athoracsur.2010.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pouch AM, Xu C, Yushkevich PA, et al. Semi-Automated Mitral Valve Morphometry and Computational Stress Analysis Using 3D Ultrasound. Journal of Biomechanics. 2012;45:903–907. doi: 10.1016/j.jbiomech.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padala M, Hutchison RA, Croft LR, et al. Saddle Shape of the Mitral Annulus Reduces Systolic Strain on the P2 Segment of the Posterior Mitral Leaflet. Annals of Thoracic Surgery. 2009;88:1499–1505. doi: 10.1016/j.athoracsur.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimenez JH, Liou SW, Padala M, et al. A Saddle Shaped Annulus Reduces Systolic Strain on the Central Region of the Mitral Valve Anterior Leaflet. Journal of Thoracic and Cardiovascular Surgery. 2007;134:1562–1568. doi: 10.1016/j.jtcvs.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Lee AP, Hsiung MC, Salgo IS, et al. Quantitative analysis of mitral valve morphology in mitral valve prolapse with real-time 3-dimensional echocardiography: importance of annular saddle shape in the pathogenesis of mitral regurgitation. Circulation. 2013;127:832–841. doi: 10.1161/CIRCULATIONAHA.112.118083. [DOI] [PubMed] [Google Scholar]
- 20.Carpentier A, Chauvaud S, Fabiani JN, et al. Reconstructive surgery of mitral valve incompetence: Ten-year appraisal. J Thorac Cardiovasc Surg. 1980;79:338–348. [PubMed] [Google Scholar]
- 21.Carpentier A. Cardiac valve surgery: The “French correction”. J Thorac Cardiovasc Surg. 1983;86:323–337. [PubMed] [Google Scholar]
- 22.Duran CMG, Pomar JL, Cucchiara G. A flexible ring for atrioventricular heart valve reconstruction. J. Cardiovasc. Surg. 1978;19:417–420. [PubMed] [Google Scholar]
- 23.Castro LJ, Moon MR, Rayhill SC, et al. Annuloplasty with flexible or rigid ring does not alter left ventricular systolic performance, energetics, or ventricular-arterial coupling in conscious, closed-chest dogs. J Thorac Cardiovasc Surg. 1993;105:643–659. [PubMed] [Google Scholar]
- 24.Carpentier A. Mitral Valve Annuloplasty. Ann Thorac Surg. 1990;49:508–509. doi: 10.1016/0003-4975(90)90277-d. [DOI] [PubMed] [Google Scholar]
- 25.Carpentier AF, Lessana A, Relland JYM. The Physio-Ring” An advanced concept in mitral valve annuloplasty. Ann. Thorac. Surg. 1995;60:1177–1186. doi: 10.1016/0003-4975(95)00753-8. [DOI] [PubMed] [Google Scholar]
- 26.Cosgrove DM, Arcidi JM, Rodriguez L, Steward WJ, Powell K, Thomas JD. Initial experience with the Cosgrove-Edwards annuloplasty system. Ann Thorac Surg. 1995;60:499–504. doi: 10.1016/0003-4975(95)00458-W. [DOI] [PubMed] [Google Scholar]
- 27.David TE, Komeda M, Pollick C, Burns RJ. Mitral valve annuloplasty: the effect of the type on left ventricular function. Ann Thorac Surg. 1989;(47):524–528. doi: 10.1016/0003-4975(89)90426-8. [DOI] [PubMed] [Google Scholar]
- 28.Maisano F, Redaelli A, Soncini M, Votta E, Arcobasso L, Alfieri O. An annular prosthesis for the treatment of functional mitral regurgitation: finite element model analysis of a dog bone-shaped ring prosthesis. Ann Thorac Surg. 2005;79:1268–1275. doi: 10.1016/j.athoracsur.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Votta E, Maisano F, Bolling SF, Alfieri O, Montevecchi FM, Redaelli A. The Geoform Disease-Specific Annuloplasty System: A Finite Element Study. Ann Thorac Surg. 2007;84:92–102. doi: 10.1016/j.athoracsur.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 30.Jensen MO, Jensen H, Nielsen SL, et al. What forces act on a flat rigid mitral annuloplasty ring? J Heart Valve Dis. 2008;17:267–275. [PubMed] [Google Scholar]
- 31.Jensen MO, Jensen H, Levine RA, et al. Saddle-shaped mitral valve annuloplasty rings improve leaflet coaptation geometry. JThorac Cardiovasc Surg. 2011;142:697–703. doi: 10.1016/j.jtcvs.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braunberger E, Deloche A, Berrebi A, et al. Very long-term results (more than 20 years) of valve repair with Carpentier’s techniques in nonrheumatic mitral valve insufficiency. Circulation. 2001;104(12 suppl 1):I8–I11. [PubMed] [Google Scholar]
- 33.Chauvaud S, Fuzellier JF, Berrebi A, Deloche A, Fabiani JN, Carpentier A. Long-term (29 years) results of reconstructive surgery in rheumatic mitral valve insufficiency. Circulation. 2001;104(12 Suppl 1):I12–I15. doi: 10.1161/hc37t1.094707. [DOI] [PubMed] [Google Scholar]
- 34.David TE, Armstrong S, Sun Z, Daniel L. Late results of mitral valve repair for mitral regurgitation due to degenerative disease. Ann Thorac Surg. 1993;56:7–12. doi: 10.1016/0003-4975(93)90396-y. [DOI] [PubMed] [Google Scholar]
- 35.Gillinov AM, Cosgrove DM, 3rd, Shiota T, et al. Cosgrove-Edwards Annuloplasty System: midterm results. Ann Thorac Surg. 2000;69:717–721. doi: 10.1016/s0003-4975(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 36.Flameng W, Herijgers P, Bogaerts K. Recurrence of mitral valve regurgitation after mitral valve repair in degenerative valve disease. Circulation. 2003;107:1609–1613. doi: 10.1161/01.CIR.0000058703.26715.9D. [DOI] [PubMed] [Google Scholar]
- 37.Flameng W, Meuris B, Herijgers P, Herregods MC. Durability of mitral valve repair in Barlow disease versus fibroelastic deficiency. J Thorac Cardiovasc Surg. 2008;135(2):274–282. doi: 10.1016/j.jtcvs.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 38.Zegdi R, Sleilaty G, Latremouille C, et al. Reoperation for failure of mitral valve repair in degenerative disease: a single-center experience. Ann Thorac Surg. 2008;86:1480–1484. doi: 10.1016/j.athoracsur.2008.07.020. [DOI] [PubMed] [Google Scholar]