Abstract
4-Hydroxy-2-trans-nonenal (4HNE), one of the major end products of lipid peroxidation (LPO), has been shown to induce apoptosis in a variety of cell lines. It appears to modulate signaling processes in more than one way because it has been suggested to have a role in signaling for differentiation and proliferation. It has been known that glutathione S-transferases (GSTs) can reduce lipid hydroperoxides through their Se-independent glutathione-peroxidase activity and that these enzymes can also detoxify LPO end-products such as 4HNE. Available evidence from earlier studies together with results of recent studies in our laboratories strongly suggests that LPO products, particularly hydroperoxides and 4HNE, are involved in the mechanisms of stress-mediated signaling and that it can be modulated by the alpha-class GSTs through the regulation of the intracellular concentrations of 4HNE. We demonstrate 4HNE induced apoptosis in various cell lines is accompanied with c-Jun-N-terminal kinase (JNK) and caspase-3 activation. Cells exposed to mild, transient heat or oxidative stress acquire the capacity to exclude intracellular 4HNE at a faster rate by inducing GSTA4-4 which conjugate 4HNE to glutathione (GSH), and RLIP76 which mediates the ATP-dependent transport of the GSH-conjugate of 4HNE (GS-HNE). The balance between formation and exclusion promotes different cellular processes – higher concentrations of 4HNE promote apoptosis; whereas, lower concentrations promote proliferation. In this article, we provide a brief summary of the cellular effects of 4HNE, followed by a review of its GST-catalyzed detoxification, with an emphasis on the structural attributes that play an important role in the interactions with alpha-class GSTA4-4. Taken together, 4HNE is a key signaling molecule and that GSTs being determinants of its intracellular concentrations, can regulate stress-mediated signaling, are reviewed in this article.
Keywords: Lipid peroxidation, 4-hydroxynonenal, glutathione S-transferase, glutathione-conjugates, RLIP76
Introduction
Environmental electrophilic chemical carcinogens are detoxified via mercapturic acid pathway (MAP) to be excreted as mercapturic acid derivatives. MAP is also involved in the metabolism of pro-apoptotic and toxic endogenous electrophiles such as 4-hydroxy-2-trans-nonenal (4HNE) (Camandola et al., 2000; Poli et al., 2008; Awasthi et al., 2008a; Kumar et al., 2011). 4HNE is a common denominator in stress-induced signaling and is a pro-apoptotic second messenger that affects cell cycle signaling in a concentration dependent manner. It regulates signaling for apoptosis, differentiation, and gene expression by interacting with transcriptional factors, transcriptional repressors, membrane receptors and other proteins (Awasthi et al., 2003a; 2005a; 2008a; Uchida, 2000; 2003; Yang et al., 2003a; Sharma et al., 2004a; Dwivedi et al., 2007; Chaudhary et al., 2010). First two rate limiting enzymes of the MAP, glutathione S-transferases (GSTs) that conjugate 4HNE to glutathione (GSH), and RLIP76 (RalBP1, Ral-binding protein 1) that excludes GS-HNE conjugate from cells, regulate the intracellular concentration of 4HNE. Thus GSTs and RLIP76 can have a profound effect on cell cycle signaling. Our studies established that increased 4HNE levels in cells promote apoptotic signaling while at decreased levels below its basal constituted levels 4HNE promote proliferation (Sharma et al., 2004b). A major outcome of these findings is that by blocking the MAP mediated detoxification of 4HNE through the inhibition of RLIP76 catalyzed transport of GS-HNE, a complete remission of many human cancer xenografts in mice can be achieved (Singhal et al., 2006; 2007; 2009a–c; Awasthi et al., 2008b; Leake et al., 2012). These studies have opened a new area in the field of reactive oxygen species (ROS)-induced signaling focusing on the regulatory roles of the enzymes involved in the formation and metabolism of 4HNE. Recent studies in this area have shown that enzymes such as GSTs, aldehydes dehydrogenases, aldose reductase (AR), glutathione peroxidase, and RLIP76 that are among the major determinants of intracellular levels of 4HNE can modulate stress-induced signaling for programmed cell death (Awasthi et al., 2005a; Ramana et al., 2006; Singhal et al., 2009d; Balogh and Atkins, 2011).
ROS initiate an autocatalytic chain of lipid peroxidation (LPO) of polyunsaturated fatty acids (PUFAs), resulting in the formation of large amounts of toxic electrophilic species and free radicals that may play important roles in various human diseases, including carcinogenesis (Poli et al., 2008; Esterbauer et al., 1991; Dianzani 2003; Zarkovic et al., 1995; Feng et al., 2004). In previous studies even a small transient exposure of cells to stress agents, such as UV, H2O2, or oxidant chemicals, causes substantial LPO, leading to a significant rise in 4HNE, which is considered to be one of the most abundant cytotoxic aldehydes (Yang et al., 2001; 2003b; Cheng et al., 2001a–c; Zarkovic et al., 1993). Recent studies suggest that 4HNE can induce signaling for apoptosis via multiple pathways, which seem to converge on the activation of JNK and caspase3 (Yang et al., 2001; Sharma et al., 2008a; Ketterer 1998). Furthermore, there is credible evidence that 4HNE plays an important role in membrane receptor-mediated signaling suggests that it can directly interact with transcription factors and transcription repressors. These multiple actions of 4HNE are consistent with its role in regulation of the expression of numerous genes and modulation of various signaling processes (Uchida 2003; Awasthi et al., 2005a; Yang et al., 2003b; Cheng et al., 2001a).
The LPO product 4HNE is a strong electrophile that forms covalent adducts with proteins and, to a lesser extent, nucleic acids and phospholipids. The generation of 4HNE appears to be an inevitable consequence of aerobic metabolism. The metabolism of 4HNE is mainly, although not entirely, conjugative, and proceeds via Michael addition of GSH to the double bond of 4HNE. This reaction is catalyzed by specialized GSTs (Awasthi et al., 2005a; 2008a; Esterbauer et al., 1991; Dianzani, 2003; Zarkovic et al., 1995; Ruef et al., 1998; Srivastava et al., 1998; Uchida et al., 1994). The intracellular concentration of 4HNE appears to be crucial for the nature of cell cycle signaling and may be a determinant for the signaling for differentiation, proliferation, transformation, or apoptosis. The intracellular concentrations of 4HNE are regulated through a coordinated action of GSTA4-4 which conjugate 4HNE to GSH to form the glutathione-conjugate (GS-HNE) and the transporter 76 kDa Ral-binding GTPase activating protein (RLIP76), which catalyze ATP-dependent transport of GS-HNE (Singhal et al., 2009a; Cheng et al., 2001b; Yang et al., 2002a; 2008; Awasthi et al., 2002; 2003b; Sharma et al., 2002; Yadav et al., 2007). These studies and other reports discussed in this article strongly suggest a key role of 4HNE in stress mediated signaling. In this review, we discuss the role of GSTs in the regulation of the intracellular concentrations of 4HNE and in the modulation of oxidative stress and 4HNE-mediated signaling processes including apoptosis. 4HNE is accumulated in numerous oxidative stress-related diseases, such as cardiovascular diseases and metabolic syndrome (Shoeb et al., 2014). 4HNE is also believed to be involved in the mechanisms of diseases such as atherosclerosis (Uchida et al., 1994; Yang et al., 2004), diabetes (Awasthi et al., 2010), Alzheimer’s disease (Sayre et al., 1997; Montine et al., 1997), Parkinson’s disease (Selley 1998), cataract (Awasthi et al., 1996), and cancer (Zarkovic et al., 1995; Hammer et al., 1997).
Glutathione S-transferases (GSTs) regulate 4HNE concentrations
GSTs belong to a family of multifunctional enzymes whose roles in detoxification of electrophilic xenobiotics or electrophilic metabolites is well established (Jakoby, 1978; Mannervik and Danielson, 1989; Awasthi et al., 1994; Hayes and Pulford, 1995; Hayes et al., 2005; Zimniak and Singh, 2006). In general, GSTs catalyze the conjugation of a wide variety of structurally dissimilar compounds containing electrophilic carbon, nitrogen, or sulfur atoms to GSH - the major endogenous low- molecular-weight nonprotein thiol synthesized de novo in mammalian cells (Beutler, 1989; Dickinson and Forman, 2002). All endogenous compounds having α, β-unsaturated carbons (Michael acceptor group) are potential substrates of GSTs (Ketterer et al., 1983; Dickinson and Forman, 2002; Hayes et al., 2005). Disruption of GSH metabolism has a major impact on cellular defenses and normal physiology, and evidence is emerging that GSTs are involved in the regulation of 4HNE-mediated signaling processes (Awasthi et al., 2005a).
GSTs are the major determinants of the intracellular concentration of 4HNE and account for the metabolism of the majority of cellular 4HNE through its conjugation to GSH. Of the multiple cell constituents, 4HNE and other unsaturated aldehydes display the highest reactivity with thiols (Esterbauer et al., 1991). 4HNE readily forms a Michael adduct with GSH, and the reaction is further accelerated by GSTs. The GSTA4-4 isoform is particularly effective conjugating 4HNE to GSH (Alin et al., 1985; Singhal et al., 1994; Nanduri et al., 1996; Hubatsch et al., 1998). This is generally considered a detoxification step, although glutathione-conjugates of α, β-unsaturated aldehydes have been shown to be toxic (Jakoby, 1978).
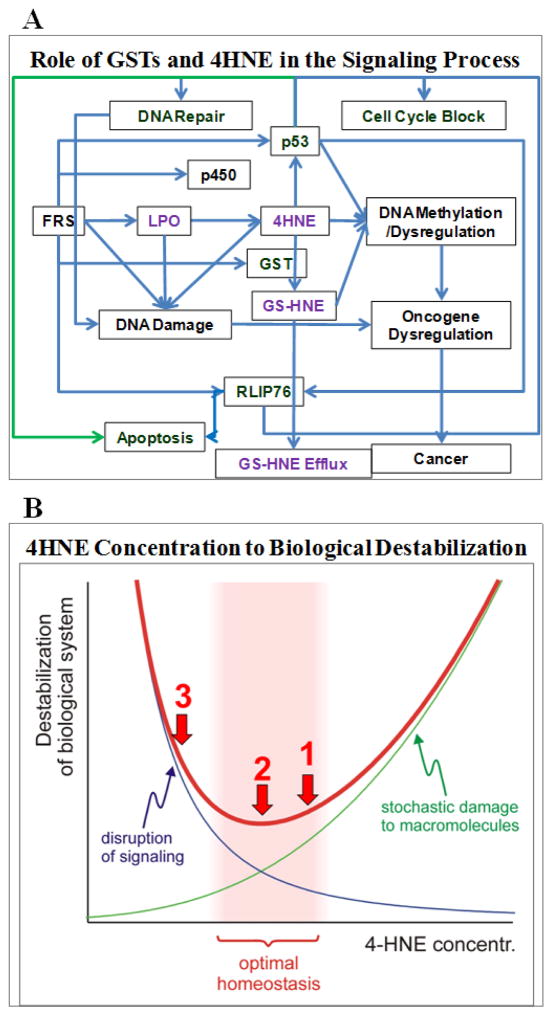
GST catalyzed conjugation of lipid aldehydes; including 4HNE, with GSH and the reduction of those conjugates by AR are the major defense against oxidative stress-induced cytotoxicity (Awasthi et al., 2003a; Sharma et al., 2004a; Ramana et al., 2006). Indeed, GST-catalyzed GS-lipid aldehyde conjugation is the main mechanism of detoxification of endogenous lipid aldehydes, including 4HNE, as well as the metabolism of xenobiotics. GS-lipid aldehyde conjugates such as GS-HNE are readily reduced by AR to corresponding GS-lipid alcohols, such as GS-DHN (Ramana et al., 2006). Both GS-HNE and GS-DHN are actively transported out of the cells (Sharma et al., 2002; Singhal et al., 2009a; 2009d). Thus adduction of toxic aldehydes with GSH, their reduction by AR, and active transport out of the cells may be the major defenses against the oxidative stress-induced cytotoxicity, genotoxicity and a number of related diseases (Knoll et al., 2005). The majority of 4HNE is excluded from cells through its GST catalyzed conjugation to GSH and subsequent transport of the GSH-conjugate (GS-HNE). Therefore, GSTs and the transporter(s) catalyzing the efflux of GS-HNE are likely to play a major role in regulating 4HNE homeostasis in cells (Awasthi et al., 2003a; Sharma et al., 2004a). Consequently, these proteins should be relevant to the mechanisms that regulate 4HNE-mediated signaling for apoptosis, differentiation, proliferation, etc. The major thrust of the article is on the role of GSTs in the regulation of the intracellular concentrations of 4HNE and in the modulation of oxidative stress and 4HNE-mediated signaling processes. 4HNE, a secondary product of lipoperoxidation, can form protein adducts and modifies cell signaling (Fig. 1A).
Figure 1. Proposed model for GST and RLIP76 function.
A model for control of signaling by GST and RLIP76 through regulation of cellular levels of 4HNE and its metabolites such as GS-HNE. RLIP76 is a transporter of glutathione-conjugates of carcinogenic electrophiles (GS-E) arising from xenobiotic compounds as well as endogenously generated electrophilic compounds, particularly metabolites of lipid-peroxidation of ω-6 fatty acids (linoleic, γ-linolenic, arachidonic). 4HNE has been shown to have high toxicity to mammalian cells, can inactivate various enzymes and also inhibit DNA and protein synthesis. 4HNE and or its related bioactive metabolites can damage DNA, leading to formation of pro-mutagenic lesions in inflammation-driven cancers (A). Schematic representation of the non-monotonic relationship between tissue 4HNE concentration and loss of biological homeostasis. Details are provided in the text under the heading “Role of 4HNE in aging” (B).
4-Hydroxynonenal (4HNE)
Being electrophilic in nature, 4HNE (an end product of n-6 PUFAs peroxidation) is a potent alkylating agent that plays a major role in oxidative stress-induced signaling and the toxicity of oxidants. It contributes to toxicity by inducing pro-apoptotic signaling through multiple pathways and also by promoting necrosis (Awasthi et al., 2003a; Sharma et al., 2004a). Since the pioneering work of Esterbauer and his associates in the discovery of 4HNE, studies by his group and various other investigators have demonstrated that 4HNE can cause apoptosis, differentiation, modulate cell growth, and affect various signal transduction pathways (Zarkovic et al., 1993; Yang et al., 2003a; Dianzani, 2003). Some of these studies suggest that 4HNE can differentially affect cell cycle signaling events in a concentration-dependent manner (Ruef et al., 1998; Srivastava et al., 1998; Sharma et al., 2012), implying that the regulation of its intracellular concentrations may be crucial for cells.
Products of LPO such as 4HNE trigger multiple signaling cascades that variably affect cell growth, differentiation, and apoptosis. The expression and function of vascular endothelial growth factor (VEGF) in retinal pigment epithelial (RPE) cells is regulated by 4HNE and GSTA4-4. Vatsyayan et al showed that while inclusion of 0.1 μM 4HNE in the medium caused increased secretion of VEGF, its secretion and expression was significantly suppressed in the presence of >5 μM 4HNE in the media, suggesting a role of 4HNE and GSTA4-4 in oxidative stress induced proliferative retinopathies (Vatsyayan et al., 2012). Similarly, at lower concentrations (as low as 0.1 μM) 4HNE triggered phosphorylation of epidermal growth factor receptor (EGFR) and activation of its downstream signaling components ERK1/2 and Akt that are known to be involved in cell proliferation. These effects of 4HNE on EGFR could be attenuated by the over-expression of GSTA4-4 that reduces intracellular levels of 4HNE. These results also indicated that 4HNE-induced activation of EGFR is a protective mechanism against oxidative stress because EGFR, MEK, and PI3K inhibitors potentiated the toxicity of 4HNE and also inhibited wound healing in a RPE cell model. These studies suggest that as an initial response to oxidative stress, 4HNE induces protective mechanism(s) in RPE cells through EGFR-mediated signaling (Vatsyayan et al., 2011). Chaudhary et al demonstrate that 4HNE induces signaling for apoptosis via both the Fas (CD95)-mediated extrinsic and the p53-dependent intrinsic pathways in HepG2 cells. 4HNE induces a Fas-mediated death-inducing signaling complex (DISC)-independent apoptosis pathway by activating apoptosis signal-regulating kinase 1 (ASK1), JNK, and caspase-3. Parallel treatment of 4HNE to HepG2 cells also induces apoptosis by the p53 pathway through activation of Bax, p21, JNK, and caspase-3. Exposure of HepG2 cells to 4HNE leads to the activation of both Fas and death domain-associated protein (Daxx), promotes the export of Daxx from the nucleus to cytoplasm, and facilitates Fas-Daxx binding (Chaudhary et al., 2010, 2013). These results strongly suggest 4HNE mediates apoptosis through its effects on JNK and caspase 3, and that 4HNE metabolizing GST isozyme(s) may be important in the regulation of this pathway of oxidative-stress-induced apoptosis.
4HNE is formed primarily from the degradation of ω-6 polyunsaturated fatty acids such as arachidonic and linoleic acids. GSTs can regulate intracellular levels of 4HNE by attenuating its formation through their glutathione-peroxidase activity and also by conjugating it to GSH through their transferase activity (Awasthi et al., 2005a). In mammalian tissues, a subgroup of alpha-class GST isozymes has high activity for conjugating 4HNE to GSH (Cheng et al., 1999; Zhao et al., 1999; Yang et al., 2001; 2002b; Awasthi et al., 2005a; 2008a; Balogh et al., 2010; Vatsyayan et al., 2012). Vatsyayan et al, have shown that GSTA4 knock-out mice having impaired 4HNE metabolism and increased 4HNE levels in tissues are more sensitive to the toxicity of oxidant chemicals/oxidative stress suggesting the role of 4HNE in the mechanisms of toxicity of oxidant xenobiotics and a protective role of GSTA4-4 against oxidative stress (Vatsyayan et al., 2012).
Under the normal physiological conditions, the cellular concentration of 4HNE ranges from 0.1 to 3μM. Moreover, under oxidative stress conditions, 4HNE can accumulate in membranes at higher concentrations that range from 10 μM to 5 mM (Esterbauer et al., 1991; Uchida, 2003; Poli et al., 2008). On the other hand, a three-fold decrease in 4HNE concentration has been reported in cells over-expressing GSTA1-1 or GSTA2-2 which suppress LPO and limit 4HNE formation (Zhao et al., 1999; Yang et al., 2002b). These findings suggest that among the defense enzymes, GSTs may be the major determinants of the constitutive 4HNE levels in cells because these enzymes can regulate 4HNE concentration limiting its formation and catalyzing its conjugation to glutathione.
Role of 4HNE in aging
The accumulation of ROS without sufficient antioxidant defenses produces oxidative damage to all macromolecules. Oxidation of lipids, in particular, affects cell membranes and other lipid-containing structures and has important pathological implications due to the high reactivity of its products. Short-lived ROS convert polyunsaturated fatty acids, through a chain reaction via lipid hydroperoxides, into rather stable, but reactive and thus toxic α,β-unsaturated aldehydes, such as 4HNE, acrolein, and malondialdehyde (MDA) (Gutteridge and Halliwell, 1990). As electrophiles, such α,β-unsaturated aldehydes can form Michael adducts on nucleophilic centers of proteins (targeting lysine, cysteine and histidine) and can cause protein cross-linking (Grimsrud et al., 2008). 4HNE is considered to be a signaling molecule that conveys the information that an oxidative event has occurred. The signal then coordinates an appropriate cellular response. We have previously found that conditions that lower the steady-state 4HNE concentration, such as over-expression of 4HNE-metabolizing enzyme GST-10, extend C. elegans lifespan, whereas elevated 4HNE leads to a shorter lifespan (Ayyadevara et al., 2005a; 2005b). Our results suggest (although do not yet conclusively prove) that 4HNE has a dual effect on longevity: at low concentrations 4HNE is pro-homeostatic and extends life span, presumably by carrying out signaling functions. At higher levels, 4HNE causes essentially random and destabilizing damage, thus shortening life span. This is consistent with concentration-dependent, opposing effects of 4HNE in biological settings other than aging (Uchida, 2007), and is schematically represented by the blue and the green lines, respectively, in Fig. 1B. Summation of the two effects results in a U-shaped function that relates 4HNE concentration to biological destabilization, and thus shortened life span (red line in Fig. 1B). When present in relevant target tissues, very low or very high 4HNE levels are detrimental; between these two extremes is an optimal level (shaded area in Fig. 1B). Biological systems probably evolved to occupy a place in the upper range of that optimal area (red arrow 1 in Fig. 1B), thus combining the minimal necessary 4HNE-metabolizing capacity with a lack of overt 4HNE toxicity. Experimentally, a moderate reduction of 4HNE levels further decreases random electrophilic damage without noticeably interfering with 4HNE signaling, resulting in a net protective and life-extending effect (red arrow 2 in Fig. 1B, located at the minimum of the destabilization function). However, a more pronounced depletion of 4HNE in specific target cells disrupts signaling, with detrimental consequences that outweigh the small gain of macromolecular stability (red arrow 3 in Fig. 1B). The non-monotonic dependence of life span on 4HNE concentration resembles the situation seen with ROS, and predicts the existence of an optimal concentration for any chemically reactive signaling molecule.
4HNE and cardiac disease
Oxidative stress and ensuing lipid peroxidation have now been clearly linked to a number of cardiac pathologies, including most significantly myocardial ischemia-reperfusion injury, but also congestive heart failure and diabetic cardiomyopathy (Venardos et al., 2007; Takimoto and Kass, 2007). 4HNE is the most abundant and significant lipid peroxidation product formed during pathological processes in most tissues (Uchida, 2007). Furthermore, 4HNE has been shown to generate still more ROS, thereby contributing a “vicious cycle” to its further accumulation (Murphy, 2006). Given the recent appreciation of its important effect on cell viability, independent of ROS, 4HNE is now recognized as most responsible for so-called “electrophilic stress” (Poli et al., 2008). 4HNE also appears to be the most abundant and significant lipid peroxidation product formed during pathological processes in the mammalian heart and other organs (Uchida, 2007). Formation of 4HNE and the accumulation of 4HNE-modified proteins (carrying specific 4HNE adducts) have been reported in numerous experimental models and appear to be related to the extent of tissue damage (Lefer and Granger, 2000). 4HNE-protein adducts have been frequently detected in cardiac cells undergoing oxidative stress, and particularly as part of ischemia-reperfusion injury (Blasig et al., 1995; Shinmura et al., 2002). During cardiac ischemia/reperfusion, primarily mitochondrial proteins are modified by 4HNE. Usually, such modification of proteins by 4HNE is inhibitory (Lucas and Szweda, 1998).
Fat accumulation in mGSTA4 null mice parallels tissue levels of 4HNE
We generated a mouse lacking mGSTA4-4 (Engle et al., 2004), the sole murine GST with high catalytic efficiency for 4HNE (Zimniak et al., 1994). Knockout mice were maintained in two genetic backgrounds: 129/sv and C57BL. The tissue level of 4HNE is increased in the 129/sv knockout mouse and the mouse is obese, while in the C57BL mouse, the 4HNE level remains unchanged (possibly due to compensatory changes in 4HNE metabolism), and the body weight is normal (Engle et al., 2004). It should be emphasized that common strains of laboratory mice are genetically quite different and, therefore, may respond differently to experimental interventions, including knockouts of individual genes (Linder, 2006). Thus, it is not surprising that the 4HNE accumulation phenotypes of mGSTA4 null mice in the 129/sv and C57BL genetic backgrounds were also dissimilar. This finding does not invalidate the hypothesis that 4HNE has a causative role in obesity but, in fact, provides powerful support to the postulated proportionality of 4HNE and obesity because the comparison is within the same species. In other words, mGSTA4 knockouts in the C57BL background do not accumulate fat not because they fail to respond to 4HNE, but because they do not have more 4HNE in the first place. 129/sv mice are generally considered less prone to develop insulin resistance and diet-induced obesity than C57BL mice (Almind and Kahn, 2004; Linder, 2006; Singh et al., 2008). Therefore, our opposite finding (that mGSTA4 null 129/sv but not C57BL mice develop obesity) is consistent with the hypothesis that obesity is linked to 4HNE levels and is not due to selection of a strain predisposed to show the phenotype under study.
The GSTA4−/− mouse provides a model of constitutive Nrf2 activation
GSTA4-4 is the main enzyme responsible for the removal of 4HNE, an electrophilic product of lipid peroxidation formed in cellular membranes during oxidative stress, by conjugating it with glutathione (Awasthi et al., 2003a; 2005a). We have previously generated and characterized GSTA4-null mice on a 129/sv and C57BL background (Engle et al., 2004). In accordance with their lack of GSTA4-4, these mice show reduced glutathione conjugation of 4HNE in all tissues examined, including the liver, kidney, lung, and heart, and increased levels of 4HNE in the liver, adipose tissue, and skeletal muscle (Engle et al., 2004). Nonetheless, these mice (when on a C57BL genetic background) show an enhanced lifespan compared to wild-type mice, which indicates that they must compensate for the increased levels of 4HNE (Ayyadevara et al., 2005a; 2005b). Indeed, these mice show increased activity of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a transcription factor that is activated by 4HNE and has many antioxidant enzyme target genes (Boerma et al., 2015).
In addition to a prolonged lifespan, GSTA4-null mice also show enhanced resistance to doxorubicin when compared to wild-type animals, both in terms of survival and cardiac function loss. Because doxorubicin is known to be cardiotoxic at least in part via induction of oxidative stress, these results suggest that compensatory mechanisms such as the Nrf2 pathway may have conveyed protection in the GSTA4-null mice. Doxorubicin, however, is administered systemically, and mechanisms outside the heart may have contributed to the protection of the GSTA4-null mice. We also subjected the GSTA4-null mice to local heart irradiation as a means to produce oxidative stress locally in the heart. Since cardiac injury is known to occur late after exposure to ionizing radiation, long-term effects of local heart irradiation on cardiac function, cardiac structure, and mitochondrial oxygen flux were examined (Boerma et al., 2015). Although loss of GSTA4-4 led to enhanced susceptibility of cardiac mitochondria to radiation-induced loss of morphology, cardiac function was preserved in irradiated GSTA4 null mice. We propose that GSTA4 null mice may be protected against radiation-induced cardiac function loss at least in part via an upregulation of the Nrf2 pathway.
Role of 4HNE in carcinogenesis and chemoprevention
Cancer is a major cause of morbidity and mortality throughout the world. Carcinogenesis is a multistep molecular process induced by genetic and epigenetic changes that disrupt pathways controlling cell proliferation, apoptosis, differentiation, and senescence (Hayes and Pulford, 1995; Talalay, 2000). Chemoprevention of cancer via herbal and dietary supplements is a logical approach to combating cancer and currently is an attractive area of investigation (Sharma et al., 2010). Over the years, isothiocyanates, such as sulforaphane (SFN) found in cruciferous vegetables, have been advocated as chemopreventive agents, and their efficacy has been demonstrated in cell lines and animal models. In vivo studies suggest that in addition to protecting normal healthy cells from environmental carcinogens, SFN also exhibits cytotoxicity and apoptotic effects against various cancer cell types (Singh et al., 2004; Xu et al., 2006; Sharma et al., 2010).
Among several mechanisms for the chemopreventive activity of SFN against chemical carcinogenesis, its effect on drug-metabolizing enzymes that cause activation/neutralization of carcinogenic metabolites is well established. Recent studies suggest that SFN exerts its selective cytotoxicity to cancer cells via ROS-mediated generation of LPO products, particularly 4HNE, indicating a major role for 4HNE in the mechanisms of the biological activity of dietary supplements (Sharma et al., 2010). In the past several decades the efficacy of isothiocyanates, particularly those of SFN isolated from the cruciferous vegetables, in cancer chemoprevention has been recognized, and they continue to be extensively studied for their pharmacological effects (Talalay, 2000; Singh et al., 2004; Wang et al., 2004; Xu et al., 2006; Sharma et al., 2010; Sharma et al., 2012). These studies reveal a central role of 4HNE in cellular responses to dietary supplements and reaffirms that 4HNE contributes to oxidative stress-mediated signaling.
4HNE-mediated signaling and its relevance to cancer
The LPO product 4HNE is an electrophilic small molecule and a signaling mediator with wide-ranging biological effects. There is ample evidence that the electrophilic products of LPO, particularly 4HNE, play a crucial role in stress-induced apoptotic signaling (Cheng et al., 2001a–c; Yang et al., 2001; Awasthi et al., 2005a). This is consistent with our studies showing that 4HNE is a common factor in heat, UVA, oxidative stress, and xenobiotic-induced apoptosis of various cell types in culture (Cheng et al., 2001a; Yang et al., 2001; 2003b). Indeed, 4HNE induces apoptosis in all cancer cell lines studied so far in our laboratory (Awasthi et al., 2008a), and recent studies have shown that 4HNE induces Fas-mediated extrinsic, as well as p53-mediated intrinsic, pathways of apoptosis in various cell types (Liu et al., 2000; Sharma et al., 2008a; 2008b; Chaudhary et al., 2010; Vatsyayan et al., 2011). The relevance of 4HNE-mediated signaling to cancer is evident from numerous studies showing that 4HNE induces apoptosis in various cell types and that at low levels it limits its own toxicity by activating mechanisms for cell survival (Sharma et al., 2008a). In recent years, 4HNE has emerged as an important second-messenger molecule involved in signaling for cell proliferation, cell cycle arrest, differentiation, apoptosis, and the regulation of the expression of a multitude of genes in cells of diverse origin (Liu et al., 2000; Sharma et al., 2012). 4HNE has also been shown to modulate survival and death signaling pathways in a concentration-dependent manner by interacting with several signaling proteins (Awasthi et al., 2003a; 2005a; Sharma et al., 2004b; Cheng et al., 2001a).
Role of 4HNE in signaling process appears to be intriguing because its effect is concentration dependent. At increasing concentrations, 4HNE causes cell cycle arrest (Esterbauer et al., 1991), differentiation (Cheng et al., 1999), and apoptosis (Uchida, 2003; Cheng et al., 2001a; 2001b; Sharma et al., 2008b) while at lower concentrations it promotes proliferation in at least some cell types (Zarkovic et al., 1993; Cheng et al., 1999). Accordingly, the regulation of the intracellular concentrations of 4HNE may be crucial for cell cycle signaling. 4HNE also has a biphasic effect on tyrosine kinase receptor (RTK) signaling (Awasthi et al., 2005a) has also been shown to induce stress-responsive prosurvival factors, such as Nrf2, HSF1 and its client heat shock proteins, EGFR, and the transcription repressor Daxx, which can inhibit Fas-mediated apoptosis (Sharma et al., 2008a; Singhal et al., 2008a; Chaudhary et al., 2010).
Because 4HNE is a substrate for GSTs (Alin et al., 1985; Singhal et al., 1994) and the majority of 4HNE in cells may be conjugated to GSH through a GST-catalyzed reaction, it stands to reason that GSTs can affect 4HNE-signalling. If this is the case, the isozymes with highest activity towards 4HNE may play an important regulatory role. Most of the GST isozymes investigated for their activity toward 4HNE show variable activity towards this substrate. Many of the effects of 4HNE on cell signaling have been determined by regulating its intracellular levels with GSTs, which play a major role in regulating the levels of 4HNE in cells (Awasthi et al., 2003a; 2005a; 2008a). Apart from catalyzing the conjugation of carcinogenic electrophiles to GSH, the α-class GSTs (GSTA1-1 and GSTA2-2) attenuate LPO by catalyzing the GSH-dependent reduction of phospholipid hydroperoxides and fatty acid hydroperoxides through its Se-independent glutathione-peroxidase activity, thereby terminating the auto-catalytic chain of LPO reactions resulting in decreased formation of 4HNE (Zhao et al., 1999; Yang et al., 2002a; 2002b). In addition, the isozymes GSTA4-4 can limit 4HNE levels in cells by catalyzing its conjugation of GSH in a highly efficient manner. Several studies demonstrate that 4HNE concentration in mammalian cells is regulated by a coordinated action of GSTA4-4, which conjugates 4HNE to GSH, and RLIP76, which transports the conjugate, GS-HNE, out of cells. It has been shown that elevated 4HNE levels in cells cause apoptosis, and cancer cells can evade apoptosis by the upregulation of GSTs and RLIP76 and consequent lowering of 4HNE levels (Cheng et al., 2001a; Yang et al., 2001; Awasthi et al., 2002; Awasthi et al., 2003b).
The inhibition of RLIP76-mediated ATP-dependent transport of GS-HNE results in apoptosis in all cancer cell types studied so far in our laboratory (Singhal et al., 2009a; 2009d). More importantly, blocking the transport of GS-HNE by RLIP76 inhibition, and thereby increasing intracellular 4HNE, leads to complete and sustained remission of melanoma, colon, lung, prostate, kidney, and pancreas xenografts in nude mice (Singhal et al., 2006; 2007; 2009a–c; Leake et al., 2012). These remarkable findings further emphasize the significance of 4HNE to cancer. Available evidence suggests that a basal constitutive level of 4HNE may be required for normal cellular processes (Awasthi et al., 2005a). It has been suggested that under the conditions of oxidative stress, when the concentration of 4HNE rises above this basal constitutive window, 4HNE induces proapoptotic signaling; whereas, depletion of 4HNE to levels below this physiological window promotes proliferation and transformation (Sharma et al., 2004b). These results are consistent with the idea that 4HNE plays a key role in stress signaling pathways.
The role of the 4HNE in transducing membrane receptor-mediated signaling is further confirmed by our studies which show that the over-expression of RLIP76 in response to the increased level of 4HNE leads to greater endocytosis of the EGF receptor. RLIP76 along with the POB1 (partner of RLIP76) has been shown to be present in signal transduction complexes for membrane tyrosine-kinase receptors (such as EGF-R) and are phosphorylated (Nakashima et al., 1999; Oosterhoff et al., 2003; Yadav et al., 2007; Singhal et al., 2008a). The inhibition of endocytosis in the transport-deficient mutants of RLIP76 further supports the idea that the transport and endocytotic functions of RLIP76 are regulated by the cellular concentration of 4HNE and GS-HNE (Singhal et al., 2011).
Accumulation of 4HNE in cells leads to apoptosis - clinical implications
Genetic alteration and DNA damage during oxidative stress are major risk factors in many disease conditions (Feng et al., 2004; Evans et al., 2004), and electrophilic products of LPO are important contributors to the progression of several pathological states. ROS-induced DNA damage promotes cytotoxicity and also contributes to the pathophysiology of a number of diseases including cardiovascular, cancer, neurodegenerative, autoimmune and diabetic complications (Hayes and Pulford, 1995; Talalay, 2000; Dianzani, 2003; Evans et al., 2004). Normally ROS are readily detoxified by the cellular anti-oxidative system but excessive amounts of ROS causes increased lipid peroxidation and lipid aldehyde formation (Esterbauer, 1993; Li et al., 1996; Hammer et al., 1997; Kruman et al., 1997; Uchida, 2000). One of the most toxic and abundant lipid aldehydes in biological system is 4HNE, formed by the oxidation of ω-6-PUFAs in the plasma membrane (Esterbauer et al., 1991). 4HNE in the nucleus may form adducts with DNA as well as proteins, resulting in cytotoxicity and genotoxicity (Esterbauer, 1993; Knoll et al., 2005), and all four DNA bases are the targets for 4HNE adduct formation (Esterbauer et al., 1991; Feng et al., 2004; Evans et al., 2004). Although previous studies investigated the genotoxic effects of 4HNE, the concentrations of 4HNE (>100 μM) used to study its genotoxic effects are barely attainable in in-vivo conditions during oxidative stress (Knoll et al., 2005).
Overall, 4HNE exerts physiologically beneficial effects depending on its intracellular concentration. Lower intracellular concentrations (< 2 uM) of 4HNE seems to be beneficial to cells as they promote cell survival and proliferation (Vatsyayan et al., 2012). Higher concentrations of 4HNE (10 to 60 μM) have genotoxic effects since they lead to sister-chromatid exchange, micronuclei formation and DNA fragmentation (Brambilla et al., 1986). At concentrations > 100 μM, 4HNE and related aldehydes cause lethal toxicity, and at these concentrations, inhibition of glycolytic enzymes, mitochondrial respiration, DNA and protein synthesis also occurs (Chandra and Srivastava, 1997; Bartsch and Nair, 2006).
4HNE is continually generated in cells due to the peroxidation of unsaturated lipids. Its intracellular concentration is regulated by enzymes such as GSTs, aldehyde dehydrogenases, and aldose reductases in coordination with transporters such as RLIP76 (RalBP1) and MRP1 that catalyze the efflux of its metabolites regulate its intracellular concentrations (Cheng et al., 2001a; Ramana et al., 2006; Awasthi et al., 2008a). GSTs catalyze the conjugation of 4HNE to GSH which is the major pathway for disposition of 4HNE. The glutathione-conjugate of 4HNE (GS-HNE) can be further processed by aldehyde dehydrogenase or AR to yield the corresponding acid or alcohol, respectively (Ramana et al., 2006), but the majority of GS-HNE is transported by RLIP76 for excretion or furthered processed to mercapturic acids (Awasthi et al., 2002; 2003b; 2008b; Singhal et al., 2009d). Blocking the transport of GS-HNE leads to the buildup of intracellular GS-HNE and HNE levels in cell cultures as well as in vivo in mGSTA4 knockout and RLIP76 knockout mice (Engle et al., 2004; Awasthi et al., 2005b; Singhal et al., 2008b; 2008c; Warnke et al., 2008; Chaudhary et al., 2013). Two lines of evidence implicate the potential therapeutic role of buildup of GS-HNE in cancer cells. When cancer cells in culture are coated with RLIP76 antibodies to block the transport of the GSH-conjugates of 4HNE or its metabolites, they undergo apoptosis in the absence of any stressor (drugs, H2O2, UV, etc.). Additionally, the inhibition of the transport functions of RLIP76 by RLIP76-interacting proteins, POB1 and HSF1, also leads to apoptosis of cancer cells, further supporting the role of 4HNE and GS-HNE as regulatory signals for various cellular processes (Singhal et al., 2008a). Induction of apoptosis in cancer cells by blocking the transport of GS-HNE may have major implications for cancer therapy (Fig 1A).
Anti-RLIP76 IgG induce apoptosis by blocking transport of GS-HNE
If stress preconditioned cells acquired resistance to 4HNE and oxidative stress-induced apoptosis by accelerated elimination of 4HNE from cells through an increased formation and transport of GS-HNE, one would expect that this resistance could be overcome by blocking the transport of GS-HNE. Indeed our results show that stress preconditioned cells become sensitive to H2O2 and 4HNE induced apoptosis when coated with anti-RLIP76 IgG which blocks the transport of GS-HNE (Cheng et al., 2001a; Yang et al., 2003b). Coating with pre-immune IgG had no effect and the stress-preconditioned cells remained resistant to H2O2. 4HNE induced apoptosis and the efflux of GS-HNE from cells was not affected. However, when stress-preconditioned cells were coated with anti-RLIP76 IgG and then treated with 4HNE or H2O2, GS-HNE efflux was inhibited and apoptosis was observed in these cells (Cheng et al., 2001a; Yang et al., 2001; 2003b). These results provide strong evidence that 4HNE mediates signaling for apoptosis in cells stressed with oxidants or heat. More importantly, these results demonstrate that RLIP76 mediates the transport of GS-HNE and is one of the major determinants for intracellular concentration of 4HNE (Sharma et al., 2002; Singhal et al., 2009d). Thus, it appears that the maintenance of intracellular 4HNE is crucial for cell cycle signaling.
4HNE is continually formed in cells because of the presence of ROS generated in the metabolic processes and also due to exogenous stimuli (e.g. exposure to xenobiotics). Its disposition therefore, is crucial and a block in this process could lead to apoptosis as indicated by our studies showing that anti-RLIP76 IgG can cause apoptosis without treatment with other apoptotic agents (Awasthi et al., 2003c; 2003d). Our studies suggest that cells mobilize mechanisms to remove of excess 4HNE even quicker than the induction of heat shock proteins and antioxidant enzymes. Even a slight increase in 4HNE levels may influence cell cycle signaling (Yang et al., 2003a; Chaudhary et al., 2013). Exposure of cells to 42 °C for 30 minutes or 50 μM H2O2 for 20 minutes results only in a 50% increase in 4HNE concentrations above its basal levels, but this is accompanied by a transient activation of JNK that quickly subsides as the cells acquire the capacity to exclude 4HNE and bring concentrations to basal ‘physiologic’ levels. These studies suggest that there is a narrow window for the basal levels of 4HNE that is tightly controlled by factors facilitating its metabolism and exclusion from cells (Cheng et al., 2001a–c; Yang et al., 2001; 2003b). Regulation of 4HNE homeostasis therefore, seems to be an important factor in stress-mediated signaling and the intracellular concentration of 4HNE (of which GSTs are primary regulators) may be important in determining whether cells undergo apoptosis, differentiation, or proliferation.
4-Hydroxyhexenal (4HHE)
Lipid peroxidation and its end-product, 4-hydroxyhexenal (4HHE), are known to affect redox balance during aging, which causes various degenerative processes including vascular alterations from endothelial cell deterioration. 4HHE is a reactive byproduct of n-3 fatty acid peroxidation and is structurally similar to 4HNE, which is derived from n-6 fatty acids, but its biological actions and efficacies may vary greatly from 4HHE (Esterbauer et al., 1991). Heme oxygenase-1 (HO-1) is a cyto-protective enzyme with antioxidant and anti-inflammatory properties. 4HHE, an end product of n-3 polyunsaturated fatty acids peroxidation, activate Nrf2/HO-1 pathway in human umbilical vein endothelial cells (HUVECs) and 3T3-L1 adipocytes, and protect cells from oxidative stress in Nrf2-dependent manner. These results strongly suggest the concentration of 4HHE but not the concentration of 4HNE was significantly increased after fish-oil treatment in all tissues of C57BL/6 mice, and the expression of HO-1 mRNA was also significantly increased after fish-oil treatment. In conclusion, the concentration of 4-HHE was closely related to the fatty acid composition of docosahexaenoic acid (DHA) in all tissues and thus DHA content was positively correlated with 4HHE concentration. Therefore, the relationship of DHA content to 4HHE concentration and HO-1 mRNA expression in these tissues suggest that 4HHE derived from DHA may induce HO-1 mRNA expression through activation of Nrf2 (Nakagawa et al., 2014). Another report also shows that 4HHE induces activation of the NFκB pathway in YPEN-1 cells, resulting in elevated expression of inducible nitric oxide synthases (iNOs) and nitric oxide production (Lee et al., 2004; Yang et al., 2008). Hence, increases in HO-1 expression in various tissues by fish-oil supplementation can be considered to be the explanation for the pleiotropic effects of n-3 PUFAs.
Discussion
Reactive aldehydes, such as 4HNE, have been implicated as inducers that generate intracellular ROS which activates stress signaling pathways. These stress pathways integrate with additional signaling pathways to control cellular responses to extracellular stimuli (Awasthi et al., 2008a; Esterbauer et al., 1991; Laurora et al., 2005; Yadav et al., 2008; Dalleau et al., 2013). Conjugation of 4HNE to GSH by GSTA4-4 is a major route of elimination for 4HNE, a toxic LPO product that contributes to numerous diseases and has been suggested to be involved in stress-induced signaling for apoptosis (Sharma et al., 2004a; Balogh et al., 2010). GSTs belong to a supergene family of multifunctional enzymes which are primarily believed to be involved in Phase II biotransformation reactions (Jakoby, 1978; Hayes et al., 2005; Zimniak and Singh, 2006). Exposure of cells and tissues to xenobiotics or physico-chemical sources of oxidative stress (e.g. UV) causes the generation of ROS which, in turn, can damage by interacting with cellular macromolecules such as DNA, proteins, and lipids, as well as form secondary toxic products. ROS-initiated lipid peroxidation is highly damaging to cells because a single oxidative event can start a chain reaction leading to the formation of large amounts of LPO products including toxic electrophiles such as 4HNE (Esterbauer et al., 1991). In recent years, 4HNE has been identified as an important second messenger involved in signaling for cell cycle arrest, differentiation, apoptosis and regulation of the expression of a multitude of genes including p53, in cells of diverse origin (Awasthi et al., 2003a; 2008a; Sharma et al., 2008b). P53 plays an important role in apoptosis, growth arrest, genomic stability, cell senescence, and differentiation (Laurora et al., 2005). It is normally maintained at a low concentration in cells due to its relatively short half-life. Since generation of 4HNE has been suggested to be a common factor in mechanisms of apoptosis caused by diverse forms of oxidative stress (Cheng et al., 2001a; Yang et al., 2003b), it is likely that 4HNE would also affect the expression and activation of p53 (Sharma et al., 2008b). Thus, it is possible that 4HNE-mediated activation of p53 may be one of the mechanisms responsible for 4HNE-induced apoptosis reported in many cell types (Kruman et al., 1997; Liu et al., 2000; Laurora et al., 2005; Sharma et al., 2008b). This would suggest that this effect of 4HNE is not limited to a specific cell line and is perhaps a generalized phenomenon.
Sharma et al, have also shown that the induction of p53 in tissues of mGST-A4 knockout mice correlate with elevated levels of 4HNE due to impaired metabolism (Sharma et al., 2008b). Together, these studies suggest that 4HNE is involved in p53-mediated signaling in in-vitro cell cultures as well as in vivo that can be regulated by GSTs (Engle et al., 2004; Sharma et al., 2008b). Numerous studies by other investigators confirm that 4HNE plays a major role in apoptosis signaling (Awasthi et al., 2003a; Balogh and Atkins, 2011). This implies that the alpha-class GSTs, including GSTA4-4, that regulate LPO and 4HNE levels in cells play an important role in stress-induced signaling. Furthermore, these findings reaffirm that the GSTA4-4 play a key role in regulating LPO by terminating the autocatalytic chain of lipid peroxidation. These findings strongly suggest that LPO products may be a common link amongst signaling mechanisms for apoptosis by oxidative stress, chemical agents, and UV irradiation (Awasthi et al., 2008a). More importantly, these studies strongly indicate that GSTs can influence stress-mediated signaling by regulating the intracellular levels of LPO products. Studies highlighted in this review strongly suggest that 4HNE not only is involved in stress-mediated apoptosis but also can affect cell-cycle signaling events in a concentration-dependent manner.
Conclusions
The role of LPO products, particularly 4HNE in cell cycle signaling is becoming increasingly clear. One of the most plentiful products of LPO is the signaling molecule 4HNE, an α, β-unsaturated carbonyl, which is believed to be responsible for many cytopathological effects observed during inflammatory and oxidative stress (Dianzani, 2003). 4HNE causes DNA damage and G2/M arrest, and over-expression of the alpha-class GSTA4-4 inhibits 4HNE-induced cell arrest (Chaudhary et al., 2013). Hence, 4HNE and GSTA4-4 could play a role in the maintenance of genomic integrity. These studies also suggest that the α-class GSTs can modulate cell survival and death signaling by regulating the intracellular concentrations of 4HNE which reinforces our previous assertion that these enzymes play an important role in the overall regulation of ROS-induced signaling. Thus, we suggest that 1) 4HNE, a secondary product of lipoperoxidation, can form protein adducts and modifies cell signaling, 2) 4HNE causes dose- and time-dependent increases in DNA damage, 3) the genotoxic effect of 4HNE may be ameliorated by modulating the cellular GSH levels, 4) over-expression of 4HNE-detoxifying enzymes such as GSTA4-4 may be a main cellular defense against oxidative stress-induced genotoxicity, 5) 4HNE is accumulated in numerous oxidative stress-related diseases, such as cardiovascular diseases, metabolic syndrome and cancer, and 6) RLIP76 mediates ATP-dependent transport of glutathione-conjugate of 4HNE (GS-HNE). These observations raise the possibility that the glutathione-conjugates of LPO products function as novel mediators of cell signaling and growth. Further studies on specific interactions of 4HNE with various membrane receptors, transcription factors, repressors, and other target proteins may provide clues to the mechanisms by which of 4HNE affects these various cellular processes.
Highlights.
GSTs are the major determinants of the intracellular concentration of 4HNE
Higher concentrations of 4HNE promote apoptosis whereas lower promote proliferation
Stress-mediated signaling can be modulated by the α-class glutathione S-transferase
Genotoxic effect of 4HNE may be ameliorated by modulating the cellular GSH levels
RLIP76 (RalBP1) mediates ATP-dependent transport of GSH-conjugate of 4HNE (GSHNE)
Acknowledgments
This work was supported in part by the National Institutes of Health grant (CA 77495 to SA and AG032643 to SPS), grant from the American Heart Association (14GRNT18890084 to SPS), and funds from the Perricone Family Foundation (to SA), Los Angeles, CA. Funding from the Beckman Research Institute of the City of Hope is also acknowledged. We thank Prof. Arthur Riggs for critical reading of the manuscript. The funding agencies had no role in the study design, data collection, analysis or interpretation of data; or in the writing of the report or in the decision to submit the article for publication. We apologize to all colleagues whose work we could not cite due to space constraints.
The abbreviations used are
- AR
aldose reductase
- EGFR
epidermal growth factor receptor
- GSH
glutathione
- GS-HNE
glutathionyl 4-hydroxynonanal
- GS-DHN
glutathionyl 1,4-dihydroxynonanol
- GSTs
glutathione S-transferases
- 4HHE
4-hydroxyhexenal
- 4HNE
4-hydroxynonenal
- JNK
c-Jun-N-terminal kinase
- LPO
lipid peroxidation
- MAP
mercapturic acid pathway
- MDA
malondialdehyde
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- p53
tumor protein 53
- PUFA
polyunsaturated fatty acid
- RLIP76
76-kDa Ral-binding GTPase activating protein (RalBP1)
- ROS
reactive oxygen species
- RPE
retinal pigment epithelial
- VEGF
vascular endothelial growth factor
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alin P, Danielson UH, Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985;179:267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004;53:3274–3285. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Dandapat A, Singh SP, Benes H, Zimniak L, et al. Lifespan extension in hypomorphic daf-2 mutants of Caenorhabditis elegans is partially mediated by glutathione transferase CeGSTP2-2. Aging Cell. 2005a;4:299–307. doi: 10.1111/j.1474-9726.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Engle MR, Singh SP, Dandapat A, Lichti CF, et al. Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell. 2005b;4:257–271. doi: 10.1111/j.1474-9726.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Sharma R, Singhal SS. Human glutathione S-transferases. Int J Biochem. 1994;26:295–308. doi: 10.1016/0020-711x(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Srivastava SK, Piper JT, Singhal SS, Chaubey M, et al. Curcumin protects against 4-hydroxy-2-trans-nonenal-induced cataract formation in rat lenses. Am J Clin Nutr. 1996;64:761–766. doi: 10.1093/ajcn/64.5.761. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Sharma R, Singhal SS, Zimniak P, Awasthi YC. RLIP76, A novel transporter catalyzing ATP-dependent efflux of xenobiotics. Drug Metab Disp. 2002;30:1300–1310. doi: 10.1124/dmd.30.12.1300. [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Sharma R, Cheng JZ, Yang Y, Sharma A, et al. Role of 4- hydroxynonenal in stress-mediated apoptosis signaling. Mol Aspects Med. 2003a;24:219–230. doi: 10.1016/s0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Singhal SS, Sharma R, Zimniak P, Awasthi YC. Transport of glutathione-conjugates and chemotherapeutic drugs by RLIP76 (RALBP1): a novel link between G-protein and tyrosine kinase signaling and drug resistance. Int J cancer. 2003b;106:635–646. doi: 10.1002/ijc.11260. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Singhal SS, Singhal J, Cheng J, Zimniak P, et al. Role of RLIP76 in lung cancer doxorubicin resistance: Doxorubicin transport in lung cancer by RLIP76. Int J Oncol. 2003c;22:713–720. [PubMed] [Google Scholar]
- Awasthi S, Singhal SS, Singhal J, Yang Y, Zimniak P, et al. Role of RLIP76 in lung cancer doxorubicin resistance: Anti-RLIP76 antibodies trigger apoptosis in lung cancer cells and synergistically increase doxorubicin cytotoxicity. Int J Oncol. 2003d;22:721–732. [PubMed] [Google Scholar]
- Awasthi YC, Ansari GA, Awasthi S. Regulation of 4-hydroxynonenal mediated signaling by glutathione S-transferases. Methods Enzymol. 2005a;401:379–407. doi: 10.1016/S0076-6879(05)01024-4. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Singhal SS, Yadav S, Singhal J, Drake K, et al. RALBP1 is a major determinant of radiation sensitivity. Cancer Res. 2005b;65:6022–6028. doi: 10.1158/0008-5472.CAN-05-0968. [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Sharma R, Sharma A, Yadav S, Singhal SS, et al. Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death. Free Radic Biol Med. 2008a;45:111–118. doi: 10.1016/j.freeradbiomed.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi S, Singhal SS, Awasthi YC, Martin B, Woo JH, et al. RLIP76 and Cancer. Clin Cancer Res. 2008b;14:4372–4377. doi: 10.1158/1078-0432.CCR-08-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi S, Singhal SS, Yadav S, Singhal J, Vatsyayan R, et al. A central role of RLIP76 in regulation of glycemic control. Diabetes. 2010;59:714–725. doi: 10.2337/db09-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh LM, Le Trong I, Kripps KA, Shireman LM, Stenkamp RE, et al. Substrate specificity combined with stereopromiscuity in glutathione transferase A4-4-dependent metabolism of 4-hydroxynonenal. Biochemistry. 2010;49:1541–1548. doi: 10.1021/bi902038u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh LM, Atkins WM. Interactions of glutathione transferases with 4hydroxynonenal. Drug Metab Rev. 2011;43:165–178. doi: 10.3109/03602532.2011.558092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- Beutler E. Nutritional and metabolic aspects of glutathione. Annual Rev Nutr. 1989;9:287–302. doi: 10.1146/annurev.nu.09.070189.001443. [DOI] [PubMed] [Google Scholar]
- Blasig IE, Grune T, Schönheit K, Rohde E, Jakstadt M, et al. 4-Hydroxynonenal, a novel indicator of lipid peroxidation for reperfusion injury of the myocardium. Am J Physiol. 1995;269:H14–22. doi: 10.1152/ajpheart.1995.269.1.H14. [DOI] [PubMed] [Google Scholar]
- Boerma M, Singh P, Sridharan V, Tripathi P, Sharma S, et al. Effects of local heart irradiation in a glutathione s-transferase alpha 4-null mouse model. Radiat Res. 2015;183:610–619. doi: 10.1667/RR13979.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla G, Sciabà L, Faggin P, Maura A, Marinari UM, et al. Cytotoxicity, DNA fragmentation and sister-chromatid exchange in chinese hamster ovary cells exposed to the lipid peroxidation product 4-hydroxynonenal and homologous aldehydes. Mutat Res. 1986;171:169–176. doi: 10.1016/0165-1218(86)90051-0. [DOI] [PubMed] [Google Scholar]
- Camandola S, Poli G, Mattson MP. The lipid peroxidation product 4-hydroxy-2,3-nonenal increases AP-1-binding activity through caspase activation in neurons. J Neurochem. 2000;74:159–168. doi: 10.1046/j.1471-4159.2000.0740159.x. [DOI] [PubMed] [Google Scholar]
- Chandra A, Srivastava SK. A synthesis of 4-hydroxy-2- trans -nonenal and 4-(3H) 4-hydroxy-2-trans –nonenal. Lipids. 1997;32:779–782. doi: 10.1007/s11745-997-0100-6. [DOI] [PubMed] [Google Scholar]
- Chaudhary P, Sharma R, Sharma A, Vatsyayan R, Yadav S, et al. Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling. Biochemistry. 2010;49:6263–6275. doi: 10.1021/bi100517x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary P, Sharma R, Sahu M, Vishwanatha JK, Awasthi S, et al. 4-Hydroxynonenal induces G2/M phase cell cycle arrest by activation of the ataxia telangiectasia mutated and Rad3-related protein (ATR)/checkpoint kinase 1 (Chk1) signaling pathway. J Biol Chem. 2013;288:20532–20546. doi: 10.1074/jbc.M113.467662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JZ, Singhal SS, Saini M, Singhal J, Piper JT, et al. Effects of mGSTA4 transfection on 4-hydroxynonenal-mediated apoptosis and differentiation of K562 human erythroleukemia cells. Arch Biochem Biophys. 1999;372:29–36. doi: 10.1006/abbi.1999.1479. [DOI] [PubMed] [Google Scholar]
- Cheng JZ, Sharma R, Yang Y, Singhal SS, Sharma A, et al. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J Biol Chem. 2001a;276:41213–41223. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- Cheng JZ, Singhal SS, Sharma A, Saini M, Yang Y, et al. Transfection of mGSTA4 in HL-60 cells protects against 4-hydroxynonenal-induced apoptosis by inhibiting JNK-mediated signaling. Arch Biochem Biophys. 2001b;392:197–207. doi: 10.1006/abbi.2001.2452. [DOI] [PubMed] [Google Scholar]
- Cheng JZ, Yang Y, Singh SP, Singhal SS, Awasthi S, et al. Two distinct 4-hydroxynonenal metabolizing glutathione S-transferase isozymes are differentially expressed in human tissues. Biochem Biophys Res Commun. 2001c;282:1268–1274. doi: 10.1006/bbrc.2001.4707. [DOI] [PubMed] [Google Scholar]
- Dalleau S, Baradat M, Guéraud F, Huc L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Diff. 2013;20:1615–1630. doi: 10.1038/cdd.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianzani MU. 4-Hydroxynonenal from pathology to physiology. Mol Aspects Med. 2003;24:263–272. doi: 10.1016/s0098-2997(03)00021-9. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- Dwivedi S, Sharma A, Patrick B, Sharma R, Awasthi YC. Role of 4hydroxynonenal and its metabolites in signaling. Redox Rep. 2007;12:4–10. doi: 10.1179/135100007X162211. [DOI] [PubMed] [Google Scholar]
- Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, et al. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: generation and analysis of mGsta4 null mouse. Toxicol Appl Pharmacol. 2004;194:296–308. doi: 10.1016/j.taap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr. 1993;57:779S–786S. doi: 10.1093/ajcn/57.5.779S. [DOI] [PubMed] [Google Scholar]
- Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Feng Z, Hu W, Tang MS. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells. A possible mechanism for lipid peroxidation-induced carcinogenesis. Proc Natl Acad Sci USA. 2004;101:8598–8602. doi: 10.1073/pnas.0402794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge JM, Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990;15:129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Hammer A, Ferro M, Tillian HM, Tatzber F, Zollner H, et al. Effect of oxidative stress by iron on 4-hydroxynonenal formation and proliferative activity in hepatomas of different degrees of differentiation. Free Radic Biol Med. 1997;23:26–33. doi: 10.1016/s0891-5849(96)00630-2. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase super gene family: regulation of GST and contribution of the isozymes to cancer chemoprevention and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Hubatsch I, Ridderström M, Mannervik B. Human glutathione transferase A4-4: an alpha class enzyme with high catalytic efficiency in the conjugation of 4-hydroxynonenal and other genotoxic products of lipid peroxidation. Biochem J. 1998;330:175–179. doi: 10.1042/bj3300175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby WB. The isozymes of glutathione transferase. Adv Enzymol Related Areas Mol Biol. 1978;57:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Ketterer B, Coles B, Meyer DJ. The role of glutathione in detoxication. Environ Health Perspect. 1983;49:59–69. doi: 10.1289/ehp.834959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterer B. Glutathione S-transferases and prevention of cellular free radical damage. Free Radic Res. 1998;28:647–658. doi: 10.3109/10715769809065820. [DOI] [PubMed] [Google Scholar]
- Knoll N, Ruhe C, Veeriah S, Sauer J, Glei M, et al. Genotoxicity of 4-hydroxy-2-nonenal in human colon tumor cells is associated with cellular levels of glutathione and the modulation of glutathione S-transferase A4 expression by butyrate. Toxicol Sci. 2005;86:27–35. doi: 10.1093/toxsci/kfi171. [DOI] [PubMed] [Google Scholar]
- Kruman I, Bruce-Keller AJ, Bredesen D, Waeg G, Mattson MP. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J Neurosci. 1997;17:5089–5100. doi: 10.1523/JNEUROSCI.17-13-05089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kokate RA, Sahu M, Chaudhary P, Sharma R, et al. Inhibition of mercapturic acid pathway-mediated disposal of 4-hydroxynonenal causes complete and sustained remission of human cancer xenografts in nude mice. Indian J Exp Biol. 2011;49:817–825. [PubMed] [Google Scholar]
- Laurora S, Tamagno E, Briatore F, Bearding P, Pizzimenti S, et al. 4-Hydroxynonenal modulation of p53 family gene expression in the SK-N-BE neuroblastoma cell line. Free Radic Biol Med. 2005;38:215–225. doi: 10.1016/j.freeradbiomed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Leake K, Singhal J, Nagaprashantha LD, Awasthi S, Singhal SS. RLIP76 regulates PI3K/Akt signaling and chemo-radio-therapy resistance in pancreatic cancer. PLoS ONE. 2012;7(4):e34582. doi: 10.1371/journal.pone.0034582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Je JH, Jung KJ, Yu BP, Chung HY. Induction of endothelial iNOS by 4-hydroxyhexenal through NFκB activation. Free Radic Biol Med. 2004;37:539–548. doi: 10.1016/j.freeradbiomed.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Lefer DJ, Granger DN. Oxidative stress and cardiac disease. Am J Med. 2000;109:315–323. doi: 10.1016/s0002-9343(00)00467-8. [DOI] [PubMed] [Google Scholar]
- Li L, Hamilton RF, Kirichenko A, Holian A. 4-Hydroxynonenal-induced cell death in murine alveolar macrophages. Toxicol Appl Pharmacol. 1996;139:135–143. doi: 10.1006/taap.1996.0152. [DOI] [PubMed] [Google Scholar]
- Linder CC. Genetic variables that influence phenotype. ILAR Journal. 2006;47:132–140. doi: 10.1093/ilar.47.2.132. [DOI] [PubMed] [Google Scholar]
- Liu W, Kato M, Akhand AA, Hayakawa A, Suzuki H, et al. 4-hydroxynonenal induces a cellular redox status-related activation of the caspase cascade for apoptotic cell death. J Cell Sci. 2000;113:635–641. doi: 10.1242/jcs.113.4.635. [DOI] [PubMed] [Google Scholar]
- Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc Natl Acad Sci U S A. 1998;95:510–514. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B, Danielson UH. Glutathione S-transferases-structure and catalytic activity. CRC Crit Rev Biochem Mol Biol. 1989;23:283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Montine KS, Olson SJ, Amarnath V, Whetsell WO, Graham DG, et al. Immuno-histochemical detection of 4-hydroxy-2-nonenal adducts in Alzheimer’s disease is associated with inheritance of APOE4. Am J Pathol. 1997;150:437–443. [PMC free article] [PubMed] [Google Scholar]
- Murphy MP. Induction of mitochondrial ROS production by electrophilic lipids: a new pathway of redox signaling? Am J Physiol Heart Circ Physiol. 2006;290:H1754–1755. doi: 10.1152/ajpheart.00040.2006. [DOI] [PubMed] [Google Scholar]
- Nakagawa F, Morino K, Ugi S, Ishikado A, Kondo K, et al. 4-Hydroxy hexenal derived from dietary n-3 polyunsaturated fatty acids induces anti-oxidative enzyme heme oxygenase-1 in multiple organs. Biochem Biophys Res Commun. 2014;443:991–996. doi: 10.1016/j.bbrc.2013.12.085. [DOI] [PubMed] [Google Scholar]
- Nakashima S, Morinaka K, Koyama S, Ikeda M, Kishida M, et al. Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. EMBO J. 1999;18:3629–3642. doi: 10.1093/emboj/18.13.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri B, Hayden JB, Awasthi YC, Zimniak P. Amino acid residue 104 in an alpha-class glutathione S-transferase is essential for the high selectivity and specificity of the enzyme for 4-hydroxynonenal. Arch Biochem Biophys. 1996;335:305–310. doi: 10.1006/abbi.1996.0511. [DOI] [PubMed] [Google Scholar]
- Oosterhoff JK, Penninkhof F, Brinkmann AO, Grootegoed JA, Blok LG. POB1 is down-regulated during human prostate cancer progression and inhibits growth factor signaling in prostate cancer cells. Oncogene. 2003;22:2920–2925. doi: 10.1038/sj.onc.1206397. [DOI] [PubMed] [Google Scholar]
- Poli G, Schaur RJ, Siems WG, Leonarduzzi G. 4-hydroxynonenal: A membrane lipid oxidation product of medicinal interest. Med Res Rev. 2008;28:569–631. doi: 10.1002/med.20117. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, et al. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- Ruef J, Rao GN, Li F, Bode C, Patterson C, et al. Induction of rat aortic smooth muscle cell growth by the lipid peroxidation product 4-hydroxy-2-nonenal. Circulation. 1998;97:1071–1078. doi: 10.1161/01.cir.97.11.1071. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, et al. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Selley ML. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic Biol Med. 1998;25:169–174. doi: 10.1016/s0891-5849(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Sharma R, Sharma A, Yang Y, Awasthi S, Singhal SS, et al. Functional reconstitution of Ral-binding GTPase activating protein, RLIP76, in proteoliposomes catalyzing ATP-dependent transport of glutathione conjugate of 4-hydroxynonenal. Acta Biochim Pol. 2002;49:693–701. [PubMed] [Google Scholar]
- Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal. 2004a;6:289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- Sharma R, Brown D, Awasthi S, Yang Y, Sharma A, et al. Transfection with 4-hydroxynonenal-metabolizing glutathione S-transferase isozymes leads to phenotypic transformation and immortalization of adherent cells. Eur J Biochem. 2004b;271:1690–1701. doi: 10.1111/j.1432-1033.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- Sharma R, Sharma A, Dwivedi S, Zimniak P, Awasthi S, et al. 4-Hydroxynonenal self-limits fas-mediated DISC-independent apoptosis by promoting export of Daxx from the nucleus to the cytosol and its binding to Fas. Biochemistry. 2008a;47:143–156. doi: 10.1021/bi701559f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Sharma R, Chaudhary P, Vatsyayan R, Pearce V, et al. 4-Hydroxynonenal induces p53-mediated apoptosis in retinal pigment epithelial cells. Arch Biochem Biophys. 2008b;480:85–94. doi: 10.1016/j.abb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Sharma A, Chaudhary P, Vatsyayan R, Pearce V, et al. Role of lipid peroxidation in cellular responses to D,L-sulforaphane, a promising cancer chemopreventive agent. Biochemistry. 2010;49:3191–3202. doi: 10.1021/bi100104e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Sharma A, Chaudhary P, Sahu M, Jaiswal S, et al. Role of 4-hydroxynonenal in chemopreventive activities of sulforaphane. Free Radic Biol Med. 2012;52:2177–2185. doi: 10.1016/j.freeradbiomed.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Bolli R, Liu SQ, Tang XL, Kodani E, et al. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ Res. 2002;91:240–246. doi: 10.1161/01.res.0000029970.97247.57. [DOI] [PubMed] [Google Scholar]
- Shoeb M, Ansari NH, Srivastava SK, Ramana KV. 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr Med Chem. 2014;21:230–237. doi: 10.2174/09298673113209990181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in-vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- Singh SP, Niemczyk M, Saini D, Awasthi YC, Zimniak L, et al. Role of the electrophilic lipid peroxidation product 4-hydroxynonenal in the development and maintenance of obesity in mice. Biochemistry. 2008;47:3900–3911. doi: 10.1021/bi702124u. [DOI] [PubMed] [Google Scholar]
- Singhal SS, Zimniak P, Awasthi S, Piper JT, He NG, et al. Several closely related glutathione S-transferase isozymes catalyzing conjugation of 4-hydroxynonenals are differentially expressed in human tissues. Arch Biochem Biophys. 1994;311:242–250. doi: 10.1006/abbi.1994.1233. [DOI] [PubMed] [Google Scholar]
- Singhal SS, Awasthi YC, Awasthi S. Regression of melanoma in a murine model byRLIP76 depletion. Cancer Res. 2006;66:2354–2360. doi: 10.1158/0008-5472.CAN-05-3534. [DOI] [PubMed] [Google Scholar]
- Singhal SS, Singhal J, Yadav S, Dwivedi S, Boor P, et al. Regression of lung and colon cancer xenografts by depleting or inhibiting RLIP76 (RALBP1) Cancer Res. 2007;67:4382–4389. doi: 10.1158/0008-5472.CAN-06-4124. [DOI] [PubMed] [Google Scholar]
- Singhal SS, Yadav S, Drake K, Singhal J, Awasthi S. Hsf-1 and POB1 induce drug-sensitivity and apoptosis by inhibiting Ralbp1. J Biol Chem. 2008a;283:19714–19729. doi: 10.1074/jbc.M708703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal J, Singhal SS, Yadav S, Warnke M, Yacoub A, et al. RLIP76 in defense of radiation poisoning. Int J Rad Oncol Biol Phys. 2008b;72:553–561. doi: 10.1016/j.ijrobp.2008.06.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal SS, Yadav S, Singhal J, Sahu M, Sehrawat A, et al. Diminished drug transport and augmented radiation sensitivity caused by loss of RLIP76. FEBS Lett. 2008c;582:3408–3414. doi: 10.1016/j.febslet.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal SS, Yadav S, Roth C, Singhal J. RLIP76: A novel glutathione-conjugate and multi-drug transporter. Biochem Pharmacol. 2009a;77:761–769. doi: 10.1016/j.bcp.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal SS, Singhal J, Yadav S, Sahu M, Awasthi YC, et al. RLIP76: A target for kidney cancer therapy. Cancer Res. 2009b;69:4244–4251. doi: 10.1158/0008-5472.CAN-08-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal SS, Roth C, Leake K, Singhal J, Yadav S, et al. Regression of prostate cancer xenografts by RLIP76 depletion. Biochem Pharmacol. 2009c;77:1074–1083. doi: 10.1016/j.bcp.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal SS, Sehrawat A, Metha A, Sahu M, Awasthi S. Functional reconstitution of RLIP76 catalyzing ATP-dependent transport of glutathione-conjugate. Int J Oncol. 2009d;34:191–199. [PMC free article] [PubMed] [Google Scholar]
- Singhal SS, Wickramarachchi D, Yadav S, Singhal J, Leake K, et al. Glutathione- conjugate transport by RLIP76 is required for clathrin-dependent endocytosis and chemical carcinogenesis. Mol Cancer Therapeu. 2011;10:16–28. doi: 10.1158/1535-7163.MCT-10-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Chandra A, Wang LF, Seifert WE, DaGue BB, et al. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J Biol Chem. 1998;273:10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- Uchida K, Toyokuni S, Nishikawa K, Kawakishi S, Oda H, et al. Michael addition-type 4-hydroxy-2-nonenal adducts in modified low-density lipoproteins: markers for atherosclerosis. Biochemistry. 1994;33:12487–12494. doi: 10.1021/bi00207a016. [DOI] [PubMed] [Google Scholar]
- Uchida K. Cellular response to bioactive lipid peroxidation products. Free Radic Res. 2000;33:731–737. doi: 10.1080/10715760000301251. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal. A product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Uchida K. Future of toxicology-lipid peroxidation in the future: from biomarker to etiology. Chem Res Toxicol. 2007;20:3–5. doi: 10.1021/tx600304n. [DOI] [PubMed] [Google Scholar]
- Vatsyayan R, Chaudhary P, Sharma A, Sharma R, Rao Lelsani PC, et al. Role of 4-hydroxynonenal in epidermal growth factor receptor-mediated signaling in retinal pigment epithelial cells. Exp Eye Res. 2011;92:147–154. doi: 10.1016/j.exer.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsyayan R, Lelsani PC, Chaudhary P, Kumar S, Awasthi S, et al. The expression and function of vascular endothelial growth factor in retinal pigment epithelial (RPE) cells is regulated by 4-hydroxynonenal (HNE) and glutathione S-transferaseA4-4. Biochem Biophys Res Commun. 2012;417:346–351. doi: 10.1016/j.bbrc.2011.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venardos KM, Perkins A, Headrick J, Kaye DM. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: a review. Curr Med Chem. 2007;14:1539–1549. doi: 10.2174/092986707780831078. [DOI] [PubMed] [Google Scholar]
- Wang LI, Giovannucci EL, Hunter D, Neuberg D, Su L, et al. Dietary intake of cruciferous vegetables, glutathioneS-transferase (GST) polymorphisms and lung cancer risk in a Caucasian population. Cancer Causes Control. 2004;15:977–985. doi: 10.1007/s10552-004-1093-1. [DOI] [PubMed] [Google Scholar]
- Warnke MM, Wanigasekara E, Singhal SS, Singhal J, Awasthi S, et al. The determination of glutathione-4-hydroxynonenal (GS-HNE), E-4-hydroxynonenal (HNE), and E-1-hydroxynon-2-en-4-one (HNO) in mouse liver tissue by LC-ESI-MS. Analyt Bioanalyt Chem. 2008;392:1325–1333. doi: 10.1007/s00216-008-2383-3. [DOI] [PubMed] [Google Scholar]
- Xu C, Huang MT, Shen G, Yuan X, Lin W, et al. Inhibition of 7,12-dimethylbenz (a) anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- Yadav S, Zajac E, Singhal SS, Awasthi S. Linking stress-signaling, glutathione metabolism, signaling pathways and xenobiotic transporters. Cancer Metas Rev. 2007;26:59–69. doi: 10.1007/s10555-007-9043-5. [DOI] [PubMed] [Google Scholar]
- Yadav UC, Ramana KV, Awasthi YC, Srivastava SK. Glutathione level regulates HNE-induced genotoxicity in human erythroleukemia cells. Toxicol Appl Pharmacol. 2008;227:257–264. doi: 10.1016/j.taap.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cheng JZ, Singhal SS, Saini M, Pandya U, et al. Role of glutathione S-transferases in protection against lipid peroxidation. Over-expression of hGSTA2-2 in K562cells protects against hydrogen peroxide-induced apoptosis and inhibits JNK and caspase 3 activation. J Biol Chem. 2001;276:19220–19230. doi: 10.1074/jbc.M100551200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sharma R, Zimniak P, Awasthi YC. Role of Alpha class glutathione S-transferases as antioxidant enzymes in rodent tissues. Toxicol Appl Pharmacol. 2002a;182:105–115. doi: 10.1006/taap.2002.9450. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sharma R, Cheng JZ, Saini MK, Ansari NH, et al. Protection against hydrogen peroxide and naphthalene induced lipid peroxidation and apoptosis by transfection with hGSTA1 and hGSTA2. Invest Ophthalmol Vis Sci. 2002b;43:434–445. [PubMed] [Google Scholar]
- Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol. 2003a;50:319–336. [PubMed] [Google Scholar]
- Yang Y, Sharma A, Sharma R, Patrick B, Singhal SS, et al. Cells preconditioned with mild, transient UVA irradiation acquire resistance to oxidative stress and UVA-induced apoptosis: role of 4-hydroxynonenal in UVA-mediated signaling for apoptosis. J Biol Chem. 2003b;278:41380–41388. doi: 10.1074/jbc.M305766200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yang Y, Trent MB, He N, Lick SD, et al. Glutathione-S-transferase A4-4 modulates oxidative stress in endothelium: possible role in human atherosclerosis. Atherosclerosis. 2004;173:211–221. doi: 10.1016/j.atherosclerosis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yang Y, Xu Y, Lick SD, Awasthi YC, et al. Endothelial glutathione-S-transferase A4-4 protects against oxidative stress and modulates iNOS expression through NFκB translocation. Toxicol Appl Pharmacol. 2008;230:187–196. doi: 10.1016/j.taap.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkovic N, Ilic Z, Jurin M, Schaur RJ, Puhl H, et al. Stimulation of HeLa cell growth by physiological concentrations of 4-hydroxynonenal. Cell Biochem Funct. 1993;11:279–286. doi: 10.1002/cbf.290110409. [DOI] [PubMed] [Google Scholar]
- Zarkovic N, Tillian MH, Schaur J, Waeg G, Jurin M, et al. Inhibition of melanoma B16-F10 growth by lipid peroxidation product 4-hydroxynonenal. Cancer Biother. 1995;10:153–156. doi: 10.1089/cbr.1995.10.153. [DOI] [PubMed] [Google Scholar]
- Zhao T, Singhal SS, Piper JT, Cheng J, Pandya U, et al. The role of human glutathione S-transferases hGSTA1-1 and hGSTA2-2 in protection against oxidative stress. Arch Biochem Biophys. 1999;367:216–224. doi: 10.1006/abbi.1999.1277. [DOI] [PubMed] [Google Scholar]
- Zimniak P, Singhal SS, Srivastava SK, Awasthi S, Sharma R, et al. Estimation of genomic complexity, heterologous expression, and enzymatic characterization of mouse glutathione S-transferase mGSTA4-4 (GST 5.7) J Biol Chem. 1994;269:992–1000. [PubMed] [Google Scholar]
- Zimniak P, Singh SP. Families of glutathione transferases. In: Awasthi YC, editor. Toxicology of glutathione transferases. CRC Press; Boca Raton, FL: 2006. pp. 11–26. [Google Scholar]