The injection of tissue homogenates from diseased animals into naïve animals to induce a neurodegenerative phenotype is a feature that defines “prion-like” transmission. Several recent studies have demonstrated that transgenic mice expressing human A53T-α-synuclein (αS; Line M83), respond to injections of CNS tissue homogenates containing abundant αS pathology by accelerated onset of CNS pathology with concurrent accelerated motor impairment [1, 3–5, 9]. Homozygous Line M83+/+ A53T αS mice naturally develop a severe motor phenotype between 8–16 months that is associated with the formation of αS inclusions in the spinal cord, brain stem, thalamus, periaqueductal gray and motor cortex [2]. Hemizyous M83+/− mice do not begin to develop these phenotypes until 21 months or later [2], but disease can be induced earlier by injection of CNS homogenates from affected M83+/+ mice [5, 9]. Although purified αS protein fibrils can reproduce the effects seen with tissue homogenates [1, 3, 6, 8], the identity of the inducing factor in tissue homogenates has not been unequivocally resolved.
To determine whether degenerating tissues might contain non-αS components that could induce pathology in M83+/− mice, we conducted brain injections of young M83+/− mice with spinal cord (SC) homogenates prepared from motor impaired M83+/+ mice, motor impaired transgenic mice expressing the G93A variant of human superoxide dismutase-1 (hSOD-1), and healthy non-transgenic (NTg) mice. The cerebral (hippocampal) injection of SC homogenates from affected M83+/+ mice served as a positive control and these mice were sacrificed at 120 days post injection (DPI), at which time αS pathology was predominantly observed in the forebrain (hippocampus and entorhinal cortex), but also distributed in the midbrain, brainstem and SC (Supplemental Figure 1; Table 1). For the hippocampal injections of SC homogenates from paralyzed G93A hSOD-1 and NTg mice, the study design was to sacrifice mice at 180 DPI to assess pathology levels. Unexpectedly, two of the M83+/− mice injected with SC homogenate from paralyzed G93A hSOD-1 mice developed motor impairments prior to 180 DPI, leading to complete hindlimb paralysis (Table 1). These 2 mice and the other 3 asymptomatic mice, sacrificed at 180 DPI, all had prominent αS inclusion pathology with a distribution typical of aged M83 mice, with additional pathology observed in the hippocampus (Figure 1; Table 1). Even more surprisingly, 4 out of the 5 M83+/− mice injected in the hippocampus with SC homogenates from NTg mice also developed M83-type motor impairment and paralysis (Table 1). M83+/− mice injected with either G93A hSOD-1 or NTg SC homogenates showed similar levels of αS inclusion pathology (Figure 1; Table 1). One pre-symptomatic mouse from each of these cohorts was sacrificed at 105 DPI to survey for earlier pathologic induction and these also presented with αS inclusion pathology (Supplemental Figure 3; Table 1). Sham-injected M83+/− mice did not present any motor phenotype or αS inclusion pathology even at 330 PDI (Figure 1; Table 1). In M83+/− mice that developed pathology from the injection of SC homogenates, the presence of αS inclusion pathology was observed in both neurons and glial cells near the injection site (Supplemental Figure 2 a,b). However, distal from the injection site, most of the induced αS inclusion pathology was present in neurons (Supplemental Figure 2 c,d).
Table 1. Summary of the mice used in the studies.
All mice received bilateral hippocampal injections of the homogenates listed in the left column, except for sham treated mice.
| Inoculums | Number of mice |
Predetermined end point following procedure |
Number of mice that developed paralysis (and their DPIs) |
Distribution of αS pathology |
|---|---|---|---|---|
| SC homogenate from NTg mouse (32ug) |
6 | 180 days* | 4 (112,159,165,154) |
Predominantly thalamus, hypothalamus, periaqueductal gray, brain stem and SC. In some mice also extensive in the hippocampus. |
| SC homogenate from paralyzed G93A hSOD-1 Tg mouse (32ug) |
6 | 180 days* | 2 (153, 153) |
Predominantly thalamus, hypothalamus, periaqueductal gray, brain stem and SC. In some mice also extensive in the hippocampus. |
| SC homogenate from paralyzed M83+/+ mouse (40ug) |
4 | 120 days | None | Predominantly hippocampus and entorhinal cortex with some more sparse throughout the thalamus, hypothalamus periaqueductal gray, brain stem and SC |
| SC homogenate from αS null mouse (40ug) |
3 | 135 days | None | Moderate in the hypothalamus, periaqueductal gray, brain stem and SC. In some mice also in the hippocampus. |
| Cortical brain extract from NTg mouse (40 ug) |
4 | 135 days | None | None |
| Cortical brain extract from αS null mouse (40 ug) |
4 | 135 days | None | None |
| Sham | 5 | 330 days | None | None |
One mouse from each of these cohorts with no overt phenotype was sacrificed at 105 DPI to assess the presentation of αS pathology. The mouse injected with SC homogenate from a NTg mouse presented with widespread αS pathology in the thalamus, periaqueductal gray, brain stem and SC while the mouse treated with SC homogenate from a motor impaired G93A hSOD-1 mouse had sparse αS pathology in the SC and brain stem. DPI = days post-injection.
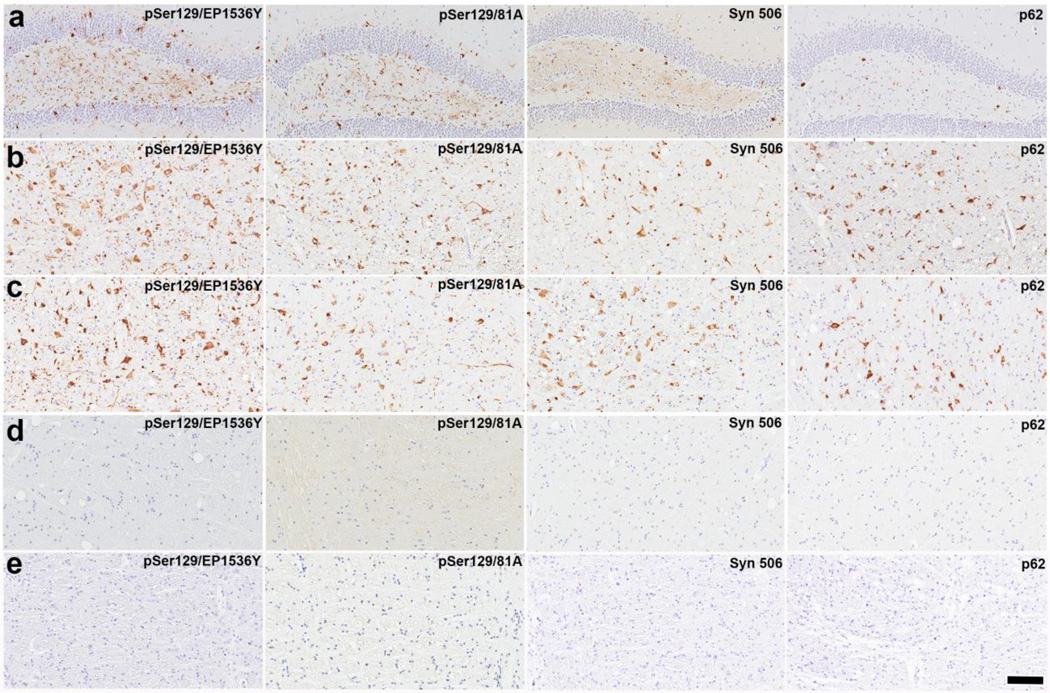
Figure 1. Induction of αS inclusion pathology in M83+/− mice following intracerebral injection of SC homogenates from G93A hSOD-1 and NTg mice.
Representative images showing αS inclusion pathology in the hippocampus (a) and brainstem (b) of M83+/− mice following the hippocampal injection of SC homogenates from G93A hSOD-1 transgenic mice. Similar αS inclusion pathology induced, as shown in the brain stem of M83+/− mice, following the hippocampal injection of SC homogenates from NTg mice (c). Representative images showing the paucity of αS inclusion pathology in the brain stem of M83+/− mice following the hippocampal injection with cortical brain homogenates from NTg mice (d). No inclusion pathology is observed in a sham treated M83+/− mice (e). Tissue sections were stained with antibodies to αS phosphorylated at Ser129 (EP1536Y and /81A), anti-αS (Syn 506) or anti-p62/sequestosome (a general marker of inclusion pathology) as indicated in each panel. Tissue sections were counterstained with hematoxylin. Scale bar = 100 µm.
Early pathology could also be induced in M83+/− mice (135 DPIs) by hippocampal injections of SC homogenates from αS null mice (Supplemental Figure 3; Table 1), demonstrating that the presence of αS was not required for SC homogenates to induce pathology. By comparison, hippocampal injections of cortical brain homogenates from NTg or αS null mice did not induce αS inclusion pathology in a similar interval of time (Figure 1, Supplemental Figure 3; Table 1).
Our studies demonstrate that intracerebral injection of SC homogenates from paralyzed G93A hSOD-1 mice, asymptomatic NTg mice, and asymptomatic mice lacking αS expression contains an entity that triggers the accelerated formation of αS inclusion pathology in M83+/− mice. By comparison, cortical brain homogenates from NTg and αS null mice do not contain such entities at levels sufficient to induce pathology rapidly. Whether cortical brain homogenates from asymptomatic mice contain such entities at any level will require further investigation. Importantly, the inability of the brain homogenates to induce αS pathology in M83 mice indicates that cross-contamination is an unlikely explanation for our results.
There is little doubt that prion-like induction of αS pathology can occur because purified recombinant αS protein fibrilized in vitro can induce accelerated pathology when injected either peripherally or in the brain of M83 mice [1, 3, 6–8]. However, our study demonstrates that experiments in which tissue homogenates are used as the source of αS aggregates to induce αS pathology in M83 mice may require greater diligence in assigning an effect to any αS entities in the tissue. At this time, the identity of the component of the SC homogenates in NTg mice that accelerates the appearance of αS pathology in M83+/− mice is unknown. Since SC homogenates contain much higher levels of myelinated white matter than brain cortical extracts, which did not induce αS inclusion pathology, it is likely that some component of the white matter is responsible for the accelerated pathology in the M83 mouse model. Collectively, our findings demonstrate that intracerebral injection of homogenates from SC tissue can induce pathologic changes that mimic the prion-like pathologic changes that occur in the M83 mice when purified αS fibrils are injected to accelerate disease onset [1, 3, 6, 8].
Supplementary Material
Acknowledgments
This study was supported by NINDS R01-NS089622 (BG) and R01-NS092788 (DB).
Footnotes
The author(s) declare that they have no other competing interests.
References
- 1.Betemps D, Verchere J, Brot S, Morignat E, Bousset L, Gaillard D, Lakhdar L, Melki R, Baron T. Alpha-synuclein spreading in M83 mice brain revealed by detection of pathological alpha-synuclein by enhanced ELISA. Acta Neuropathol Commun. 2014;2:29. doi: 10.1186/2051-5960-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 3.Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mougenot AL, Nicot S, Bencsik A, Morignat E, Verchere J, Lakhdar L, Legastelois S, Baron T. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging. 2012;33:2225–2228. doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, et al. Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1514475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutherford NJ, Sacino AN, Brooks M, Ceballos-Diaz C, Ladd TB, Howard JK, Golde TE, Giasson BI. Studies of lipopolysaccharide effects on the induction of alpha-synuclein pathology by exogenous fibrils in transgenic mice. Mol Neurodegener. 2015;10:32. doi: 10.1186/s13024-015-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacino AN, Brooks M, Thomas MA, McKinney AB, Lee S, Regenhardt RW, McGarvey NH, Ayers JI, Notterpek L, Borchelt DR, et al. Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci USA. 2014;111:10732–10737. doi: 10.1073/pnas.1321785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacino AN, Brooks M, Thomas MA, McKinney AB, McGarvey NH, Rutherford NJ, Ceballos-Diaz C, Robertson J, Golde TE, Giasson BI. Amyloidogenic alpha-synuclein seeds do not invariably induce rapid, widespread pathology in mice. Acta Neuropathol. 2014;127:645–665. doi: 10.1007/s00401-014-1268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, DeArmond SJ, Prusiner SB. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci USA. 2013;110:19555–19560. doi: 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.