Abstract
In this study, the effects of exposure to engineered nickel oxide (NiO 40–60 nm) and cobalt oxide (CoO <100 nm) nanoparticles (NP) were investigated on Artemia salina. Aggregation and stability of the aqueous NP suspensions were characterized by DLS and TEM. Acute exposure was conducted on nauplii (larvae) in seawater in a concentration range from 0.2 to 50 mg/L NPs for 24 h (short term) and 96 h (long term). The hydrodynamic diameters of NiO and CoO NPs in exposure medium were larger than those estimated by TEM. Accumulation rate of NiO NPs were found to be four times higher than that of CoO NPs under the same experimental conditions. Examinations under phase contrast microscope showed that the nanoparticles accumulated in the intestine of artemia, which increased with increasing exposure concentration. Differences were observed in the extent of dissolution of the NPs in the seawater. The CoO NPs dissolved significantly while NiO NPs were relatively more stable. Oxidative stress induced by the NP suspensions was measured by malondialdehyde assay. Suspensions of NiO NPs caused higher oxidative stress on nauplii than those of CoO NPs. The results imply that CoO and NiO NPs exhibit toxicity on artemia (e.g., zooplankton) that are an important source of food in aquatic food chain.
Keywords: NiO nanoparticle, CoO nanoparticle, Exposure, Oxidative stress, Artemia salina
1. Introduction
Nanotechnology enables research and applications at atomic, molecular and macromolecular levels via development of the materials or devices at nanoscale (approximately 1 and 100 nm at least at one dimension). The basic physical and chemical properties of conventional materials drastically change when they are made at nanoscale. Some nanoparticles (NPs), particularly those smaller than 20 nm, possess unique optical, magnetic and chemical properties (Huber, 2005). In comparison to micron-size substances, nanomaterials show much larger surface area to volume ratio which is closely linked to novel properties, such as increased chemical reactivity and physical absorption ability. These properties may also increase toxicological behavior and toxic impact as the NPs interact with biological systems more rapidly. Yet, the effects of size and surface area are not often considered in traditional toxicology approaches (Zhao et al. 2007).
In recent years, various nano-scale metal and metal oxides, including nickel oxide (NiO) and cobalt oxide (CoO) NPs have been manufactured and used in a variety of products. For instance, NiO NPs are utilized in solar cells, lithium-ion batteries, resistive random access memory, light-emitting diodes, electrochemical sensors and biosensors, and as catalyst (Salimi et al. 2007; Rao and Sunandana 2008; Ahamed et al. 2013). NiO NPs may be released to environment by aerial emission of particles, leakages and spills, as well as indirectly storm-water runoff from land to surface waters (Wiesner et al. 2006). As other nanoscale materials, NiO NPs may pose risks for the environmental and human health. NiO NPs have been a subject of passionate research due to finite size effect, weak ferromagnetism and surface effects. Based on in vitro assays, risks from inhalation of NiO NPs have been reported in mammals (Oyabu et al. 2007). Cobalt-based nano size materials are also currently attracting enormous interest due to their unique properties that vary greatly with size and shape, and potential applications in pigments, catalytic processes, sensors, electrochemistry, magnetism, and energy storage (Liu et al. 2005; Papis et al. 2009). Since NiO and CoO NPs are used in many areas of daily life, they will find their ways to aquatic ecosystems and could affect the aquatic species and sustainability of ecosystems. It has already been reported that CoO NPs show toxic effects on microalgae (Navicula sp. and Chetoceros sp.) and bivalve (Meritrixmeritrix) cells (Rebello et al. 2010).
Toxic effects of metal-based nanomaterials depend on size, surface area, crystal structure and chemical behavior (stability, dissolution etc.) of the material in the aquatic environment. Artemia salina (zooplankton) constitutes a major link in the food chain and has a particular importance in aquaculture. It is a marine model of aquatic toxicology and a typical filter feeder that consumes organic detritus, microscopic algae, bacteria, and chemicals (Weber 1993). Previously, we have utilized A. salina for investigating the acute toxic effects of TiO2 NPs, Zn and ZnO NPs (Ates et al. 2013a; 2013b) where it has proved to be reliable model for elucidating the impact of nanomaterials. In this study, exposures to aqueous suspensions of NiO and CoO NPs were performed on A. salina larvae to elucidate accumulation, physical and chemical stability of the NPs and potential toxic effects of exposure in aquatic environments. Metal ion concentrations were measured to determine if the observed effects were induced from dissolution of NPs in the exposure medium.
2. Materials and Methods
2.1. Test Organism and Materials
The acute toxicity studies for NiO and CoO NPs were conducted using the Artemia nauplii. The Artemia cysts were purchased from Artemia International LLC, Houston, TX, USA. Procedures for preparation of seawater and test organisms were described in our previous study (Ates et al. 2013a; 2013b). Uncoated nickel oxide (NiO 40–60 nm) and cobalt oxide (CoO 40–60 nm) NPs were obtained from Skyspring Nanomaterials Inc., Houston, TX, USA. All other chemicals were obtained from Sigma–Aldrich (St. Louis, MO, USA). The NP suspensions were also prepared as described in our previous studies (Ates et al. 2013a; 2013b). Standard solutions (1000 μg mL−1) of Co and Ni for elemental measurements by ICP-MS were purchased from SCP Science (Champlain, NY, USA). The concentrations of Ni2+ and Co2+ in experimental suspensions were determined by ultra-filtration. In this procedure, 2 mL from each suspension were taken and centrifuged for 1 h to separate the suspending particulates and NP aggregates. Then, 0.5 mL of the supernatant solution was passed through ultra-filtration filters (VWR International) with a molecular cut-off 3 kDa to separate the Ni and Co ions from the particles. This filter rejects particles greater than 1.3 nm, therefore, the filtrate is assumed to contain Ni2+ and Co2+ predominantly and all NPs and aggregates greater than 1.3 nm are retained on the filter. The filtrate was then analyzed by ICP-MS for Ni and Co ions in the solution.
2.2. Characterization of NPs
The particle size and size distribution of CoO and NiO NPs were measured by transmission electron microscopy using a JEOL-1011 TEM instrument providing a resolution of 0.2 nm lattice with magnification of 50 to 1x106 under the accelerating voltage of 40 to 100 kV). The hydrodynamic diameter and zeta potential of the nanoparticles in the stock solution and exposure medium were characterised by dynamic light scattering (DLS) using a Malvern Nano ZS zetasizer. DLS and zetapotential measurements were made in triplicate for individual suspensions using automated, optimal measurement time and laser attenuation settings. The recorded correlation functions and measured particle mobilities were converted into size distributions and zeta potentials, respectively, using the Malvern Dispersion Software (V5.10; http://www.zetasizer.com/).
2.3. Exposure Studies
A majority of the information concerning aquatic toxicity has been gathered with freshwater species among which daphnia (zooplankton) species, such as Daphnia manga and Ceriodaphnia dubia that are stated in the guidelines for regulatory toxicology and international acute toxicity standards (OECD, ISO and DIN) have been used as test organism. Zooplankton in general is the link in food and energy chain between algae and fish, and thus are ecologically important species. Artemia salina is a zooplankton of marine environments, which is utilized for examining the acute toxicity of CoO and NiO NPs in marine environments according to the Organization for Economic Cooperation and Development (OECD part 202) testing guidelines (OECD, 2004). The exposure experiments were carried out as described in previous study (Ates et al. 2013a). Appropriate amounts of dry powders of NiO and CoO NPs were suspended in deionized water with a resistivity of 18.0 MΩ cm to prepare the stock solutions (1.0 g L−1), which were then sonicated for 10 min in a bath sonicator for complete dispersion prior to adding into exposure medium. A series of experiments were conducted to evaluate the repsonse of Artemia larvae to a concentration range, including 0.1, 1, 10 and 50 mg/L of NiO and CoO NPs under short-term (24 h) and long-term (96 h) exposure. There was no feeding during these experiments. Malondialdehyde (MDA) levels were measured in all groups as described in previous studies Ates et al. (2013a; 2013b).
2.4. Instrumental Analysis and Optical Microscopic Examination
Elemental measurements to determine the accumulation of NPs in artemia and free Co and Ni ions in the exposure medium were conducted using Varian 820-MS inductively coupled plasma-mass spectrometry (ICP-MS) (Varian, Australia). Wet artemia samples from short-term and long-term exposures were digested in HNO3 according to protocols described elsewhere (Arslan et al. 2011; Ates et al. 2015b) and were analyzed by ICP-MS. Data collection was performed by ICP–MS Expert software package (version 2.2 b126) using 60Ni and 59Co isotopes of Ni and Co. Germanium (72Ge) was used as an internal standard (IS) element. The IS solution (5.0 μg L−1 Ge in 1% HNO3) was mixed online with the sample solution. Artemia collected from the control and treatments were scrutinized visually under a phase contrast microscope (Micromaster, Model 12-575-252, Fisher Scientific) equipped with a digital camera to elucidate the accumulation of NPs. Images were captured by Micron Imaging software from live Artemia with NPs in petri dishes. All images were taken in 50-μm scale to obtain a clear picture of the artemia.
2.5. Statistics
The data were recorded as the mean and standard deviation. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons was used to detect significant differences in accumulation and elimination rates, and toxicity among the controls and treatments. A p-value of < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Aggregation and Characterization of NPs
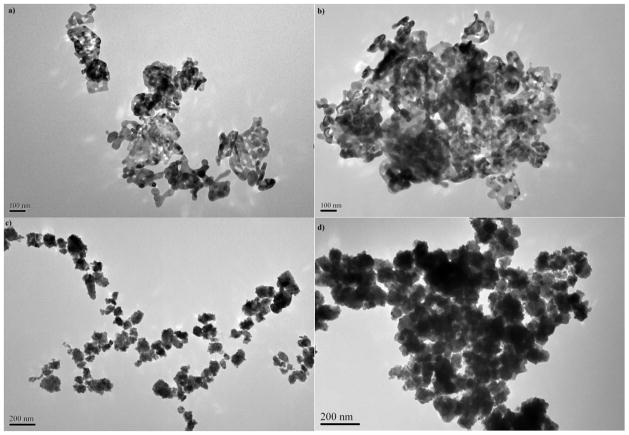
Carbon and metal-based nanoparticles show different characteristics both in seawater and freshwater. The phenomenon is influenced from various factors; in particular the structure, size, shape and the surface charges of the nanoparticles along with environmental variables, such as pH, salinity (e.g., ionic strength) and light exposure. The toxicity of Co and NiO nanoparticles may be caused by the direct uptake of the NPs by fish or the dissolution of the NPs leading to the increased level of Co++ and Ni+ ions in the media, with subsequent effects on fish (Chattopadhyay et al. 2015). Nanoparticles in general tend to form aggregates in aqueous media. In our study both nanoparticles formed aggregates in marine medium. In previous studies, it has been shown that metal and metal oxide NPs form micron size aggregates both in freshwater (Ates et al. 2013a, 2013b) and seawater (Ates et al., 2013c; 2015a). Aggregation also correlates positively with concentration due to increased number of particle-particle interaction per unit volume (Fairbairn et al. 2011; Miller et al. 2010), which in due course results in reduced surface area and dissolution potential of NPs (Baker et al. 2014). In similar studies by Gong et al. (2011) and Oukarroum et al. 2015, NiO NPs also form aggregates with a wide size distribution and about 0.14% ionic Ni was released when NiO NPs were added into seawater. The surface charge of NPs determines particle’s stability in the solvent against agglomeration and thus plays an important role on the interaction with biological systems (Clogston and Patri 2011). Though aggregates are usually smaller in pure water, the surface charge are not found to be sufficiently high to maintain the stability of suspension even in the absence of counter ions (Ates et al. 2013a). In this study, hydrodynamic size distribution and zeta potential of the NPs in stock solution and exposure medium were determined and summarized in Table 1. The results show that surfaces of both NPs were negatively charged. Nevertheless, these surface charges were not sufficient to prevent aggregation. As a result, the hydrodynamic diameters of NiO and CoO NPs measured by DLS in exposure medium were larger than actual sizes estimated by TEM. Nanoparticles in the exposure medium also rapidly aggregated into micrometer size particles (see Fig. 1).
Table 1.
Size distribution, polydispersity index (PI) and surface charge of NiO and CoO NPs estimated from aqueous suspensions. Data expressed as mean, n = 3, where relative standard deviation was < 10%.
| Nanoparticles | TEM/nm | DLS/nm | PI | Zeta value/mV |
|---|---|---|---|---|
| NiO (40 – 60nm) | 20–90 | 1517 | 0.59 | −10.1 |
| CoO (< 100nm) | 40–120 | 1168 | 0.24 | −3.57 |
Fig. 1.
TEM images of NiO (a) and CoO (c) NPs from stock NP suspensions and exposure medium of NiO (b) and CoO (d) NPs.
Size and concentration of the particles affect the dissolution and the tendency of NPs to aggregate. The suspensions in the saltwater exposure medium contained micrometer size aggregates (Adams et al. 2006; Aruoja et al. 2009). Eventually nanoparticles are unlikely to remain at nanoscale in marine environments since counter ions from increasing salinity reduce the electrostatic repulsion among the NPs, thereby leading to agglomeration and reduction in the active surface area.
3.2. Accumulation Profiles of NPs
The accumulation profiles of NiO and CoO NPs are summarized in Table 2 for 24-h and 96-h exposures. The concentrations are based on the wet weight of artemia, reflecting the total body burden across the concentration gradient of 0.2 and 50μg/mL. It was observed that the rate of accumulation increased with increasing exposure dose. Under the same concentration regime, there was a statistically significant increase in the accumulation rate of NiO NPs in the long-term (96 h) exposure compared with that of the short-term (24 h) (p<0.05). Artemia accumulated more CoO NPs during long-term (96 h) exposure, but the increase was not statistically different from that occurred in short-term (24 h) exposure (p≥0.05). The results also show that artemia accumulated NiO NPs substantially higher levels than CoO NPs. The accumulation rate was as high as 4 times in some groups exposed to the same concentrations of NPs. In group D, for instance, that was exposed to 50 μg/mL suspensions of NiO and CoO NPs, the accumulation of NiO was about 19.8 ± 0.95 μg/g for short-term and 47.7 ± 1.18 μg/g for long-term exposure, whereas it was 7.39 ± 0.85 μg/g and 9.78 ± 0.88 μg/g for CoO NPs for short-term and long-term exposures, respectively.
Table 2.
The effect of NP concentration (μg/mL) and exposure time on the accumulation of NiO and CoO NPs (μg/g). The values are mean ± standard deviation (n=3)
| Treatments (NP conc.) | Short term (24 h) | Long term (96 h) | ||
|---|---|---|---|---|
|
| ||||
| NiO (μg/g) | CoO (μg/g) | NiO (μg/g) | CoO (μg/g) | |
| Group A (0.2 μg/mL) | 0.21 ± 0.07 | 0.42 ± 0.09 | 0.39 ± 0.1 | 0.06 ± 0.2 |
| Group B (1 μg/mL) | 0.91 ± 0.6 | 0.90 ± 0.6 | 1.43 ± 0.7 | 0.34 ± 1.1 |
| Group C (10 μg/mL) | 10.3 ± 0.8 | 1.28 ± 0.6 | 17.6 ± 0.9 | 2.72 ± 1.0 |
| Group D (50 μg/mL) | 19.8 ± 0.9 | 7.39 ± 0.8 | 47.7 ± 1.2 | 9.78 ± 0.9 |
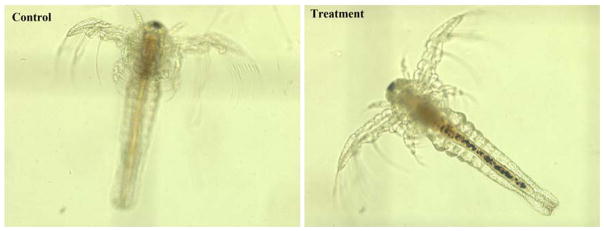
Accumulation profiles of NiO and CoO NPs were visually verified under a phase contrast microscope (PCM) at the conclusion of the exposure experiment. A typical PCM image of control and treatment exposed to NiO NPs is shown in Fig. 2. The images indicated that NPs deposited inside the guts of artemia. The guts contained more NPs as indicated by a tick dark strip in those exposed to higher concentration of the NPs. Also, the accumulation was higher in the intestines of the artemia exposed to NiO NPs than those exposed to CoO NPs. These results were consistent with the quantitative results obtained from the ICP-MS measurements.
Fig. 2.
Phase contrast images of Artemia salina (Control group was not subject to any NiO NP exposure, the treatment group was exposed to 50 μg/mL NiO NPs for 96 hours (The pictures of Artemia salina exposed to CoO NPs are not displayed due to similarity with the one exposed to NiO NPs)
3.3. Dissolution of NPs in the Medium
In order to elucidate the extent of dissolution of NiO and CoO NPs and its contribution on the toxic effects, the concentrations of Ni2+ and Co2+ ions in the experimental suspensions were separated by ultra-filtration and measured by ICP-MS. The results are summarized in Table 3. The data indicated that NiO and CoO NPs had differential dissolution profiles in the marine environment. This phenomenon was also consistent with the accumulation patterns in the artemia as discussed in the previous section. CoO NPs dissolved substantially in seawater in comparison to NiO NPs under long-term exposure regimes (p<0.05) while in short-term regimes the rate of NP dissolution did not show any statistical difference (p>0.05). For example, whereas in the groups exposed to 0.2, 1 and 10 μg/mL there was not too much ionization, in the groups exposed to 50 μg/mL, proportionally more ionization was formed. For long term (96 hr) experimental groups, all CoO NP exposed groups demonstrate 3–4 times more ionization compared to the groups exposed to NiO NPs (see Table 3).
Table 3.
The results for Ni and Co ion concentrations resulting from temporal dissolution patterns of NiO and CoO NPs. The values are mean ± standard deviation (n=3)
| Treatments (NP conc.) | Short term (24 h) | Long term (96 h) | ||
|---|---|---|---|---|
|
| ||||
| NiO (μg/L) | CoO (μg/L) | NiO (μg/L) | CoO (μg/L) | |
| Group A (0.2 μg/mL) | 0.48 ± 0.19 | 2.83 ± 0.65 | 6.80 ± 0.33 | 27.5 ± 0.01 |
| Group B (1 μg/mL) | 0.46 ± 0.20 | 2.52 ± 0.25 | 6.70 ± 0.19 | 37.4 ± 0.52 |
| Group C (10 μg/mL) | 2.55 ± 0.23 | 3.43 ± 0.27 | 6.68 ± 0.15 | 37.1 ± 0.11 |
| Group D (50 μg/mL) | 7.75 ± 0.17 | 10.7 ± 0.05 | 10.1 ± 0.26 | 46.0 ± 0.17 |
3.4. Malondialdehyde Assay
Malondialdehyde (MDA) is a by-product of lipid peroxidation which is an indicator of oxidative stress induced by the toxic chemical on the biological organism or cell line. In this study, the degree of oxidative stress caused by the exposure of NiO and CoO NPs was expressed as total malondialdehyde (MDA) concentration. The MDA levels measured from artemia samples are summarized in Table 4. For 0.2 μg/mL NP suspensions, there was not any significant difference between the controls and treatments. For all other NP suspensions, MDA levels increased during both short-term and long-term exposures as influenced by the concentrations of the suspensions (e.g., 1, 10, and 50 μg/mL). The extent of oxidative stress during long-term (96 h) exposure was significantly higher (ca. 2-fold) than that measured for short-term exposure (p<0.05) for both NP suspensions. Additionally, the MDA levels were relatively higher in treatments exposed to NiO NPs compared to the groups exposed to CoO NPs, indicating that NiO NPs induced more oxidative stress than CoO NPs under similar exposure regimes. Kovrižnych et al. (2013 and 2014) also reported low acute toxicity of NiO NPs on adult zebrafish and also low acute toxicity for eggs of zebrafish, however indicated that longlasting contact with this compound can lead to its accumulation in the tissues and to increased toxicity. Gong et al. (2011) also showed that the NiO NPs had severe impact on algae.
Table 4.
Malondialdehyde levels (nmol/g) measured from Artemia salina larvae exposed to suspensions of NiO and CoO NPs for 24 h and 96 h. The values are mean ± standard deviation (n=3)
| Treatments | NiO NPs | CoO NPs | ||
|---|---|---|---|---|
|
| ||||
| Short-term | Long-term | Short-term | Long-term | |
| Control | 11.5 ± 0.1 | 10.2 ± 0.2 | 9.5 ± 0.1 | 9.1 ± 0.5 |
| Group A (0.2 μg/mL) | 13.5 ± 0.1 | 14.7 ± 0.3 | 10.4 ± 0.4 | 13.0 ± 1.3 |
| Group B (1 μg/mL) | 13.9 ± 0.1 | 31.1 ± 0.9 | 13.0 ± 1.0 | 29.2 ± 1.7 |
| Group C (10 μg/mL) | 19.0 ± 1.1 | 39.1 ± 0.3 | 18.3 ± 1.0 | 37.7 ± 1.4 |
| Group D (50 μg/mL) | 21.0 ± 1.1 | 49.0 ± 2.1 | 20.0 ± 1.2 | 43.0 ± 1.3 |
4. Conclusions
In this study, the effects of NiO and CoO NPs were investigated upon exposure in aquatic systems. The results showed that both NiO and CoO NPs form micrometer size aggregates in seawater. Despite the formation of large particles, artemia as a filter feeder accumulated the particle readily into the guts. This information was also confirmed with phase contrast microscopy images. Accumulation of NiO NPs was higher compared with CoO. This phenomenon was thought to associate with formation of larger aggregates of NiO NPs so that the elimination of the NiO aggregates was slower than that of CoO. It was also found that CoO NPs dissolves to larger extent in seawater, which could also affect the differences in the accumulation patterns of the NPs. Despite a higher dissolution, on the other hand, the toxic effects from NiO NPs were relatively higher. The result could indicate that the oxidative stress experienced by artemia was mostly from the particles rather than the free ions released from NPs. Based on the results, it is concluded that micro-organisms constituting important links in the aquatic food chain are particularly at risk of exposure to nanoparticles. The impact of nanoparticles in environmental ecosystems and species would be more noticeable with the current growth trends in nanotechnology and the use of products formulated with nanomaterials; therefore, extensive studies ought to be conducted to fully understand the fate and toxicological properties of nanomaterials to avoid catastrophic effects on environmental and human health.
Acknowledgments
This project is funded in part by grants from the National Institutes of Health (NIH) through Research Centers in Minority Institutions (RCMI) Program (Grant No: G12RR013459) and the U.S. Department of Defense (DOD) through the Engineer, Research and Development Center (Vicksburg, MS); (Contract #W912HZ-10-2-0045). The views expressed herein are those of authors and do not necessarily represent the official views of the funding agencies, and any of their sub-agencies.
References
- Adams LK, Lyon DY, Alvarez PJJ. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water research. 2006;40:3527–3532. doi: 10.1016/j.watres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Ahamed M, Ali D, Alhadlaq HA, Akhtar MJ. Nickel oxide nanoparticles exert cytotoxicity via oxidative stress and induce apoptotic response in human liver cells (HepG2) Chemosphere. 2013;93:2514–2522. doi: 10.1016/j.chemosphere.2013.09.047. [DOI] [PubMed] [Google Scholar]
- Arslan Z, Ates M, McDuffy W, Agachan MS, Farah IO, Yu WW, Bednar AJ. Probing metabolic stability of CdSe nanoparticles: alkaline extraction of free cadmium from liver and kidney samples of rats exposed to CdSe nanoparticles. Journal of Hazardous Materials. 2011;192:192–199. doi: 10.1016/j.jhazmat.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoja V, Dubourguier HC, Kasemets K, Kahru A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Science of the Total Environment. 2009;407:1461–8. doi: 10.1016/j.scitotenv.2008.10.053. [DOI] [PubMed] [Google Scholar]
- Ates M, Demir V, Arslan Z, Daniels J, Farah IO, Bogatu C. Evaluation of alpha and gamma aluminum oxide nanoparticle accumulation, toxicity and depuration in artemia salina larvae. Environmental Toxicology. 2015a;30:109–118. doi: 10.1002/tox.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates M, Daniels J, Arslan Z, Farah IO. Comparative study of accumulation, toxicity and trophic transfer of CuO and ZnO nanoparticles through waterborne and dietary exposure of goldfish (Carassius auratus) Environmental Toxicology. 2015b;30:119–128. doi: 10.1002/tox.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates M, Daniels J, Arslan Z, Farah IO. Uptake and toxicity of titanium dioxide (TiO2) nanoparticles to brine shrimp (Artemia salina) Environmental Monitoring and Assessment. 2013a;185:3339–3348. doi: 10.1007/s10661-012-2794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates M, Daniels J, Arslan Z, Farah IO, Félix-Rivera H. Comparative evaluation of impact of Zn and ZnO nanoparticles on Brine shrimp (Artemia salina) larvae: effects of particle size and solubility on toxicity. Environmental Science: Processes & Impacts. 2013b;15:225–233. doi: 10.1039/c2em30540b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates M, Demir V, Adiguzel R, Arslan Z. Bioaccumulation, subacute toxicity, and tissue distribution of engineered titanium dioxide nanoparticles in goldfish (carassius auratus) Journal of Nanomaterials. 2013c doi: 10.1155/2013/460518. org/10.1155/2013/460518. [DOI] [PMC free article] [PubMed]
- Baker TJ, Tyler CR, Galloway TS. Impacts of metal and metal oxide nanoparticles on marine organisms. Environmental Pollution. 2014;186:257–271. doi: 10.1016/j.envpol.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Dash SK, Tripathy S, Das B, Mandal D, Pramanik P, Roy S. Toxicity of cobalt oxide nanoparticles to normal cells; an in vitro and in vivo study. Chemico-Biological Interactions. 2015;226:58–71. doi: 10.1016/j.cbi.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Clogston JD, Patri AK. Zeta potential measurement. Methods in Molecular Biology. 2011;697:63–70. doi: 10.1007/978-1-60327-198-1_6. [DOI] [PubMed] [Google Scholar]
- Fairbairn EA, Keller AA, Madler L, Zhou D, Pokhrel S, Cherr GN. Metal oxide nanomaterials in seawater: linking physicochemical characteristics with biological response in sea urchin development. Journal of Hazardous Materials. 2011;192:1565–1571. doi: 10.1016/j.jhazmat.2011.06.080. [DOI] [PubMed] [Google Scholar]
- Gong N, Shao K, Feng W, Lin Z, Liang C, Sun Y. Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. 2011 doi: 10.1016/j.chemosphere.2010.12.059. [DOI] [PubMed] [Google Scholar]
- Huber D. Synthesis, properties, and applications of iron nanoparticles. Small. 2005;1:482–501. doi: 10.1002/smll.200500006. [DOI] [PubMed] [Google Scholar]
- Kovrižnych JA, Sotníková R, Zeljenková D, Rollerová E, Szabová E, Wimmerová S. Acute toxicity of 31 diff erent nanoparticles to zebrafish (Danio rerio) tested in adulthood and in early life stages – comparative study. Interdisciplinary Toxicology. 2013;6:67–73. doi: 10.2478/intox-2013-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovrižnych JA, Sotníková R, Zeljenková D, Rollerová E, Szabová E. Long-term (30 days) toxicity of NiO nanoparticles for adult zebrafish Danio rerio. Interdisciplinary Toxicology. 2014;7(1):23–26. doi: 10.2478/intox-2014-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Qiu G, Li X. Shape-controlled synthesis and properties of uniform spinel cobalt oxide nanotubes. Nanotechnology. 2005;16:3035–3040. [Google Scholar]
- Miller RJ, Lenihan HS, Muller EB, Tseng N, Hanna SK, Keller AA. Impacts of metal oxide nanoparticles on marine phytoplankton. Environmental Science & Technology. 2010;44:7329–7334. doi: 10.1021/es100247x. [DOI] [PubMed] [Google Scholar]
- OECD. Guideline for testing of chemicals- Daphnia sp. acute immobilisation test. 2004;202 [Google Scholar]
- Oukarroum A, Barhoumi L, Samadani M, Dewez D. Toxic Effects of Nickel Oxide Bulk and Nanoparticles on the Aquatic Plant Lemna gibba L. BioMed Research International. 2015;2015:1–7. doi: 10.1155/2015/501326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyabu T, Ogami A, Morimoto Y, Shimada M, Lenggoro W, Okuyama K, Tanaka I. Biopersistence of inhaled nickel oxide nanoparticles in rat lung. Inhalation Toxicology. 2007;19(Suppl 1):55–58. doi: 10.1080/08958370701492995. [DOI] [PubMed] [Google Scholar]
- Papis E, Rossi F, Raspanti M, Dalle-Donne I, Colombo G, Milzani A, Bernardini G, Gornati R. Engineered cobalt oxide nanoparticles readily enter cells. Toxicology Letters. 2009;189:253–259. doi: 10.1016/j.toxlet.2009.06.851. [DOI] [PubMed] [Google Scholar]
- Rao KV, Sunandana CS. Effect of fuel to oxidizer ratio on the structure, micro structure and EPR of combustion synthesized NiO nanoparticles. Journal of Nanoscience & Nanotechnology. 2008;8:4247–4253. doi: 10.1166/jnn.2008.an59. [DOI] [PubMed] [Google Scholar]
- Rebello V, Shaikh S, Desa PV. Toxicity of Cobalt Oxide Nanoparticles. International Conference on Environmental Engineering and Applications (ICEEA 2010) 2010:195–199. [Google Scholar]
- Salimi A, Sharifi E, Noorbakhsh A, Soltanian S. Direct electrochemistry and electrocatalytic activity of catalase immobilized onto electrodeposited nanoscale islands of nickel oxide. Biophysical Chemistry. 2007;125:540–548. doi: 10.1016/j.bpc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Weber CI. Methods for Measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 4. Environmental Monitoring Systems Laboratory; Cincinnati, OH: 1993. p. 167. EPA/600/4-90/027F. [Google Scholar]
- Wiesner MR, Lowry GV, Alvarez P, Dionysiou D, Biswas P. Assessing the risks of manufactured nanomaterials. Environmental Science & Technology. 2006;40:4336–4345. doi: 10.1021/es062726m. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Meng H, Chen Z, Feng Z, Chai Z. Dependence of nanotoxicity on nanoscale characteristics and strategies for reducing and eliminating nanotoxicity. In: Zhao Y, Singh NH, editors. Nanotoxicology. American Scientific Publisher; Valencia, CA, USA: 2007. pp. 265–280. [Google Scholar]