Abstract
Toward expanding the population of potential BCI users to the many individuals with lateralized cortical stroke, here we examined whether the cortical hemisphere controlling ongoing movements of the contralateral limb can simultaneously generate signals to control a BCI. A monkey was trained to perform a simultaneous BCI and manual control task designed to test whether one hemisphere could effectively differentiate its output and provide independent control of two tasks. Pairs of well-isolated single units were used to control a BCI cursor in one dimension, while isometric wrist torque of the contralateral forelimb controlled the cursor in a second dimension. The monkey could independently modulate cortical units and contralateral wrist torque regardless of the strength of directional tuning of the units controlling the BCI. When the presented targets required explicit decoupling of unit activity and wrist torque, directionally tuned units exhibited significantly less efficient cursor trajectories compared to when unit activity and wrist torque could remain correlated. The results indicate that neural activity from a single hemisphere can be effectively decoupled to simultaneously control a BCI and ongoing limb movement, suggesting that BCIs may be a viable future treatment for individuals with lateralized cortical stroke.
Keywords: stroke, neuroprosthesis, brain-machine interface, motor cortex, hemiparesis
1. Introduction
Brain-computer interfaces (BCIs) have tremendous potential for improving quality of life for individuals with nervous system injury or degeneration. BCIs have advanced from proof-of-concept animal studies showing that neural activity can control biofeedback meter arms (Fetz, 1969), computer cursors,[1-3] robotic arms [4, 5] and muscle stimulation [6-8] to first-in-human studies for individuals with cervical spinal cord injury, brainstem stroke, and spinocerebellar degeneration resulting in paralysis of both hands and arms.[9-12]
With only a few exceptions,[13, 14] BCI studies have utilized neural signals primarily recorded from contralateral cortex for control of robotic arms, computer cursors, or muscle stimulation. BCI technology will benefit a broader population of individuals with motor impairments if it can provide control over paralyzed limbs even if the cortical area which normally controls those movements is damaged, for example, by stroke or traumatic brain injury. In the United States alone, approximately 692,000 ischemic strokes occur each year,[15] and the majority of these involve damage to only a single hemisphere.[16]
Following damage to a single hemisphere, it may be possible to record activity from the undamaged hemisphere and use a BCI to restore control of the paralyzed or paretic limb. In this case control would be derived from the hemisphere ipsilateral to the paralyzed limb. A small subset (< 10%) of neurons in motor and pre-motor cortical areas represent ipsilateral arm movements,[17-19] with somewhat greater representation during bi-manual tasks.[20, 21]
The challenge in implementing a BCI for a patient with cortical damage is that the remaining hemisphere must now provide control over two arms simultaneously and often independently. Thus, the motor areas of this remaining hemisphere must effectively differentiate their output to control both the intact contralateral limb and the ipsilateral limb controlled via the BCI.
This study was designed to determine the optimal tuning of isolated single units for BCI control when the same cortical area must simultaneously and independently control the contralateral limb. We tested the hypothesis that untuned units could most readily decouple from ongoing limb movements and therefore provide the greatest performance in this dual control (limb + BCI) task.
Arbitrary and untuned cortical units can readily be trained to control a BCI,[6, 22-24] and may present an advantage, as they are already independent of ongoing contralateral limb movements. Conversely, directionally tuned units are typically used in decoders to control BCIs, and can demonstrate some independence from ongoing limb movement.[1, 3]
Here we describe a case study in which a monkey simultaneously controlled a BCI and the contralateral limb in a two degrees-of-freedom combined BCI and manual control task. The task was designed to test the independence of brain activity and contralateral limb movement. During each session, we deliberately selected pairs of well isolated units with similar correlations to contralateral limb movement for BCI control. We then compared the performance of directionally-tuned and un-tuned neurons during this task that required dissociation of neural activity from ongoing limb movement.
2. Materials and Methods
One male Macaca nemestrina monkey participated in the experiments. The experiments were approved by the University of Washington Institutional Animal Care and Use Committee. All procedures conformed to the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.”
Sterile surgery was performed with the animal under 1-1.5% isoflurane anesthesia (50:50 O2:N2O). A program of analgesics (buprenorphine 0.15 mg kg−1 IM and ketoprofen 5 mg kg−1 PO) and antibiotics (cephalexin 25 mg kg−1 PO) was given postoperatively. The monkey was implanted with two 96-channel microelectrode arrays (Blackrock Microsystems, Salt Lake City, UT), bilaterally in motor cortex using the standard pneumatic insertion technique.[25, 26] Electrodes were placed over hand and wrist area of the primary motor cortex, on the precentral gyrus and as close as possible to the line extending from the genu of the arcuate sulcus posteriorly to the central sulcus. All data reported here were obtained from the microelectrode array located in the left hemisphere.
The experiment was conducted in a primate behavior booth with a computer monitor (30 cm × 23 cm), tones for audio feedback and a computer-controlled feeder dispensing apple sauce as a reward. The animal's right arm, contralateral to the monitored recording array, was comfortably restrained in a custom 2-DOF isometric manipulandum.
2.1 Behavioral tasks
At the beginning of each session, the monkey was presented with a manual control task: a 2D cursor position task in which isometric wrist torque determined cursor position (Figure 1A). During this manual control, unit responses were recorded and pairs of units were selected for inclusion in the subsequent BCI tasks on the basis of observed correlations with wrist torque production and modulation depth (Figure 1B). For all experiments, units used for control were selected from the hemisphere contralateral to arm movement. We restricted our analysis of single units to those with a mean firing rate above 5 Hz. The firing rates were first smoothed with a truncated Gaussian kernel (σ = 100 ms) and the torque signals were similarly filtered to reduce latency shifts. We calculated the covariance of spike trains and each of the two torque signals, flexion/extension and radial/ulnar torque, over the range of ± 90 s. Covariance was further normalized against spike auto-covariance and the peak correlation was determined. Maximum time shift for admissible peaks was 1s. Tuning strength was calculated as the height of the cross-correlation peak, with a typical range of 0-10.
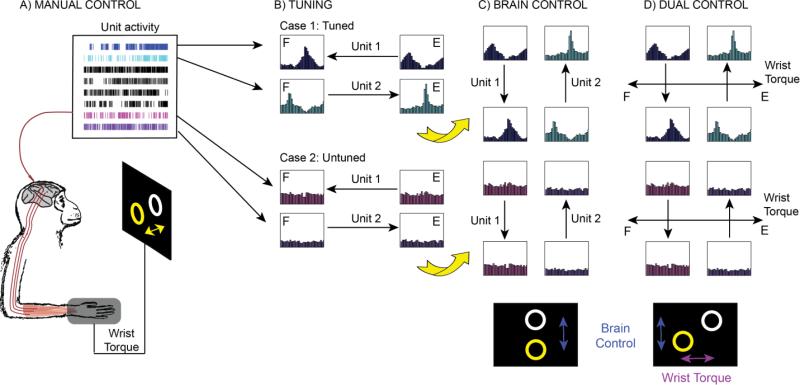
Figure 1.
Experiment overview. (A) The monkey first performed a manual wrist tracking task, while activity from each well-isolated unit was recorded. (B) Pairs of units were then chosen based on both modulation depth and activity correlated with wrist torque. We chose either directionally tuned units (case 1, blue and cyan) or units with little directional tuning (case 2, magenta and purple). Case 1 shows units tuned in the flexor (marked “F”) and extensor (“E”) direction, but experiments were also performed with units tuned in the radial and ulnar direction (not shown). Case 2 shows untuned units where the direction of cursor velocity was arbitrarily assigned. (C) Visual feedback for unit discharge rates were rotated on the screen during all brain control trials so that the cursor moved in a direction orthogonal to each unit's preferred direction. This was done to ensure decoupling from wrist torque in the subsequent dual control task. First the monkey performed a 1D brain control task (C) where velocity of the cursor was determined by aggregate neural modulation of the 2 single units. Increasing discharge rate contributed to cursor velocity in the direction indicated by the arrows. Subsequently the monkey performed a dual control task (D) where 2D cursor velocity was determined by isometric wrist torque in one dimension (e.g., flexion-extension) and by the aggregate neural modulation of 2 single units in the other dimension (as in C).
Experiments were performed with 82 pairs of cortical units. Each pair of units had approximately opposite preferred directions, and were matched for firing rate and tuning strength; the average difference of tuning strength between each of the pairs of units was 1.6 ± 1.36 (mean ± SD), and the difference of firing rates was 5.86 ± 5.51 Hz. The experimenter deliberately selected pairs of units with similar tuning strength for each session, with the goal of exploring the entire range of tuning strength across the experiment. For units with tuning strength greater than 5, each unit's preferred direction was obvious and the experimenter selected a pair of units with opposite direction for those sessions. This resulted in 44 pairs of ‘tuned’ units. When tuning strength was less than 5, preferred directions were less obvious, and for the least tuned units the experimenter had to randomly assign a preferred direction to each unit (see Figure 1). This resulted in 38 pairs of ‘untuned’ units. Offline analysis confirmed that dividing units into ‘tuned’ and ‘untuned’ groups based on tuning strength ranging from 4.2 to 5.8 did not affect the results presented below.
The monkey was then required to serially perform two BCI tasks. In the “brain control” task, neural modulation of two single units was mapped to control the velocity of a cursor moving in 1 dimension (Figure 1C). Each unit contributed to positive cursor velocity in the direction most closely aligned with its preferred direction. Pairs of tuned units were selected with approximately opposite preferred directions. Thus, the contribution of each tuned unit's activity was subtracted from its pair to determine the cursor velocity. Since untuned units do not exhibit clear preferred directions, activity from half of the untuned unit pairs were subtracted (n = 19), whereas the remainder were added to control the cursor. The BCI decoding algorithm was based on a population vector mapping.[27] The algorithm and behavioral tasks were implemented in LabVIEW software (National Instruments), and operated at a sampling rate of 60Hz.
Subsequently, the “dual control” task was implemented to test whether the monkey was able to control two cursor dimensions independently when using neural activity derived from the same hemisphere (Figure 1D). We used the aggregate neural activity of two single units as described above to control the cursor in one dimension, and asked the monkey to independently control the second cursor dimension using isometric wrist torque generated by the contralateral arm.
Mappings of neural activity and wrist torque to cursor velocity were kept consistent across all tasks. In order to ensure decoupling from wrist torque, we rotated the visual feedback for neuron discharge rates on the screen. Therefore, during both brain control and dual control tasks, the unit activity moved the cursor in a direction orthogonal to each unit's preferred direction (Figure 1C–D).
Targets were presented at random positions on the screen, always appearing at least 7 cm away from the present cursor position. The target region was a circle with a radius of 1.8 cm for manual control trials and 2.8 cm for brain control trials. The monkey was required to move to and maintain the cursor center within the target radius for 1s to receive a reward. A 0.5 s time out was provided between trials, during which time the screen was blank. Target display refresh rate was 30 Hz. Targets remained on the screen for up to 40 s, or until acquired by the monkey.
2.2 Performance metrics
To quantify overall performance, we measured the rate of target acquisition during the peak performance as the maximum number of targets acquired in a 5-minute period. The peak performance was compared among pairs of units with different tuning strengths, among the brain and dual control tasks, and to the performance during the initial 5 minutes of practice. Additionally, we analyzed the average amount of practice time the monkey needed for each group of units in order to achieve peak performance in the brain control and dual control tasks.
To provide additional insight into the quality of cursor control, we analyzed properties of the trajectory taken to the target. We assessed the average path efficiency - the shortest path to the target divided by the length of the actual path taken.[28]
We further evaluated cursor control by applying four performance measures, previously adapted for human BCI study [29] from original applications in computer interface design.[30] These measures evaluate the spatio-temporal properties of continuous movements and are capable of providing information on quality of interaction during a trial. We measured relevant aspects of cursor control and movement, including direction changes parallel and orthogonal to the direct path towards the target, movement variability, and error. In addition, we evaluated the two measures which McKenzie et al [30] suggested as most relevant for throughput prediction: target re-entry and movement offset (Figure 2).
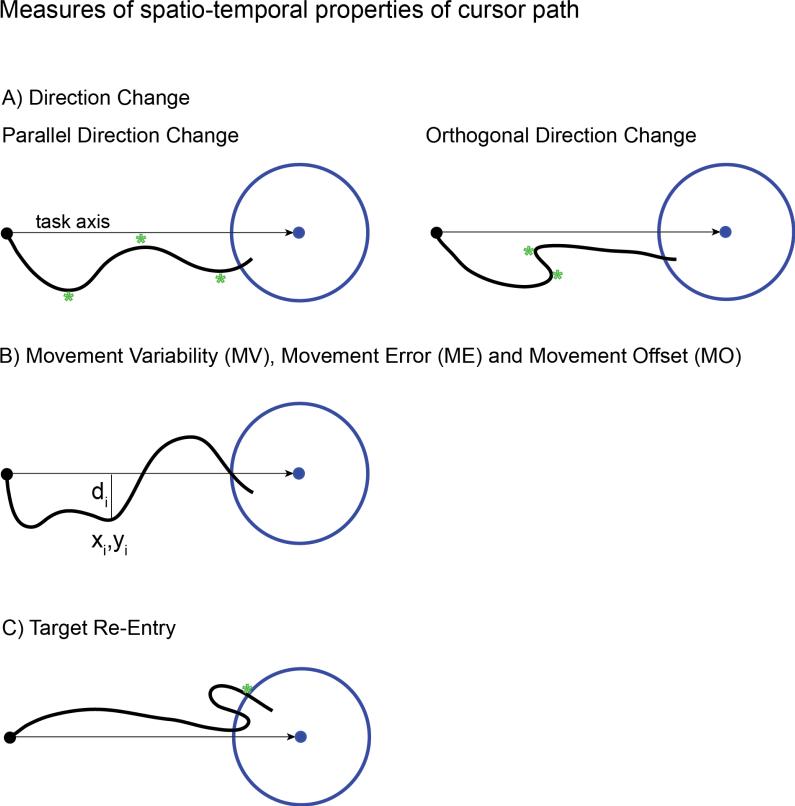
Figure 2.
Illustration of cursor path measures that capture spatio-temporal characteristics of the dual control task. (A) Direction change counts the number of times the cursor changes direction parallel (left) and orthogonal (right) to the task axis (the straight line connecting the cursor and target when the target appears). Direction changes are indicated by a green star. (B) Movement Variability, Movement Error and Movement Offset are calculated by summing the orthogonal distance from each sample point to the task axis as the cursor approaches the target (see equations (1)-(3) in methods). (C) Target re-entry counts the number of times the cursor re-enters the target after the initial entry, marked by a green star in this example.
Direction change measures how many times the cursors path changed direction along the task axis, the straight line between the cursor and target when the target first appeared (Figure 2A). This measure counts direction changes that are parallel to the task axis (movement direction change) or orthogonal to the task axis (orthogonal direction change) and is able to capture the straightness of the path and how consistently the cursor moves toward a target. Movement variability, error and offset are continuous calculated measures derived from the orthogonal distances of the cursor path sample points to the task axis, di (Figure 2B). Movement variability is defined as the standard deviation in the distances di from the mean as:
| (1) |
where d̄ represents the mean distance of the sample points to the task axis and n represents the number of the cursor path samples from the starting point to the target.
Movement error measures how much the cursor path deviates from the ideal straight line, and is defined as the average of di, irrespective of whether the points are above or below the axis:
| (2) |
Movement offset is the average of distances di, which takes into account the direction of offset and represents the tendency of the cursor to remain offset in a particular direction of the task axis during a movement:
| (3) |
Target re-entry counts the number of times the cursor re-enters the target region after entering for the first time (Figure 2C).
2.3 Statistics
Values are reported as mean and standard deviation. Statistics were performed to analyze differences between the tasks and pairs of units following the Lilliefors test for normality. A Student's t-test was applied to normally distributed data sets, otherwise the Wilcoxon nonparametric ranksum test was used.
3. Results
3.1 Influence of unit directional tuning during brain and dual control tasks
The monkey was able to use all tested pairs of cortical units to control cursor movements, regardless of the directional tuning strength. Figure 3 shows the number of targets min−1 acquired during “brain control” of a cursor (A) and “dual control” of the contralateral hand and cursor (B). These points are plotted as a function of the average tuning strength for the pair of neurons controlling the cursor, measured during the preceding manual control task. Linear regressions reveal no relation between the peak performance at each task, quantified during a 5 min period, and tuning strength of the neurons used for control in either task (R2 ≤ 0.02, p ≥ 0.18).
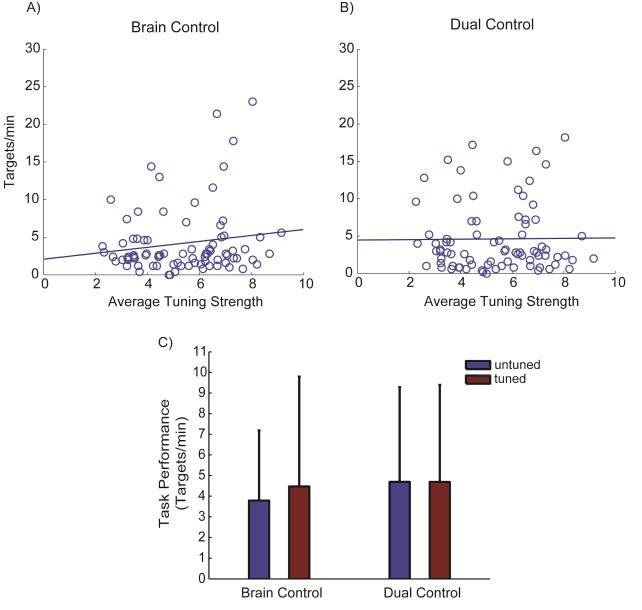
Figure 3.
Task performance relative to unit directional tuning. Regression of tuning strength measured during wrist tracking versus the number of targets per minute acquired during peak performance for (A) brain control and (B) dual control task. Regressions of performance across tuning strength of the units controlling the task were not significant for either task (R2 ≤ 0.02, p ≥ 0.18). (C) The average number of targets per minute acquired by untuned and tuned units during peak performance of each task. The monkey performed both tasks equally well using the untuned and tuned cells during brain control (p = 0.906) as well as during dual control tasks (p = 0.972). Values in (C) are mean + SD.
Furthermore, there was no significant difference in performance on either task when dividing the neurons into groups of “tuned” (tuning strength ≥ 5) and “untuned” (tuning < 5) units. During brain control, target acquisition rates were not significantly different between directionally tuned (4.47 ± 5.31 targets min−1) and untuned units (3.78 ± 3.40 targets min−1; p = 0.906). Similarly, during the dual control task, tuned units acquired 4.69 ± 4.70 targets min−1 compared to 4.69 ± 4.59 targets min−1 using untuned units (p = 0.972, Figure 3C).
The monkey improved performance in both tasks regardless of the directional tuning of the units selected for cursor control (Figure 4). The initial performance was very similar, regardless of the strength of directional tuning (p > 0.67) and the control task performed (p > 0.32). During the brain control task, the monkey significantly improved with practice compared to the initial performance when using both untuned units (p = 0.03) and directionally tuned units (p = 0.02). Compared to the brain control task, improvement in task performance was significantly greater during the dual control task performed with the untuned units (p = 0.01) and slightly greater (p = 0.057) when directionally tuned units were used. Control with both tuned and untuned units reached similar levels of peak performance in the brain control and dual control tasks (p = 0.98 and p = 0.94, respectively).
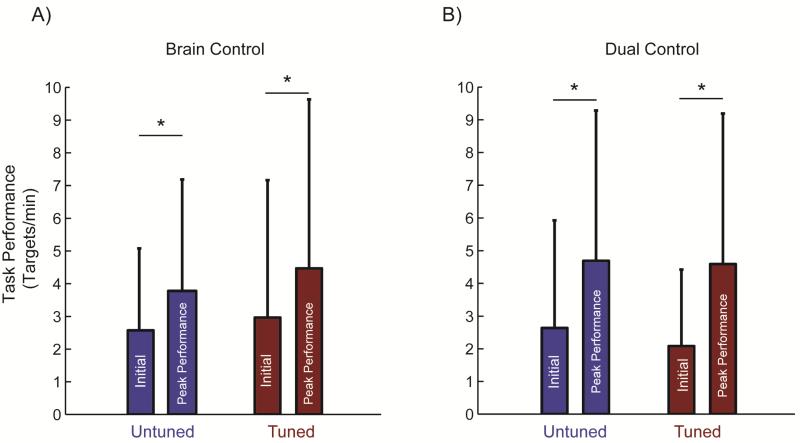
Figure 4.
The number of targets acquired per minute at the beginning of practice and during peak performance during (A) brain control and (B) dual control tasks. During both tasks, the monkey improved significantly with practice when using both untuned (p ≤ 0.03) and tuned units (p ≤ 0.01). Control at the beginning of practice for untuned and tuned cells was not different for either brain control (p = 0.67) or dual control (p = 0.97). Similarly, target acquisition rates during peak performance were similar for both untuned and tuned units in both brain control (p = 0.98) and dual control tasks (p = 0.94). Values are mean + SD.
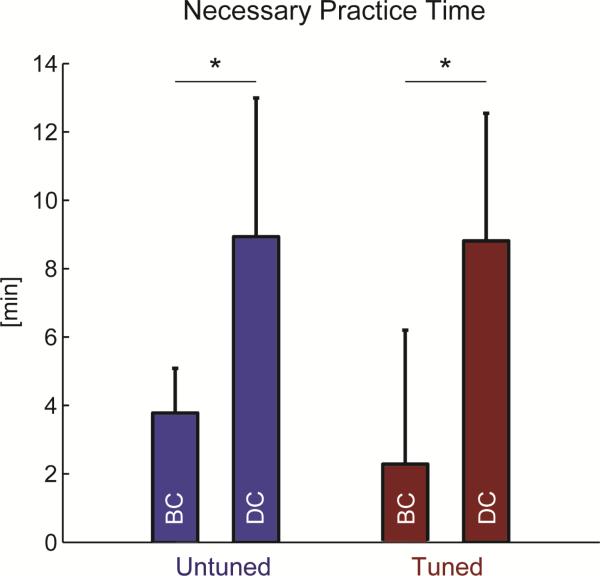
The monkey required similar amounts of practice to achieve peak performance using directionally tuned and untuned units in each task (p = 0.368 for brain control, p = 0.310 for dual control, Figure 5). The dual control task, however, required longer practice time, likely due to greater task complexity. Untuned units required 8.93 ± 4.06 min to reach peak performance in the dual control task compared to 3.78 ± 1.31 min for the brain control task (p = 0.003). Similarly, tuned units required 8.81 ± 3.73 min to reach peak performance in the dual control task compared to 2.29 ± 3.92 min for the brain control task (p = 0.008). The total practice duration with each pair of units averaged 16.03 ± 7.68 min for brain control task and 32.15 ± 9.12 min for dual control task.
Figure 5.
Practice time necessary to achieve peak performance during the Brain Control (BC) and Dual Control (DC) tasks. Both untuned and directionally tuned cells require longer practice time to achieve peak performance in the dual control compared to the brain control task (p ≤ 0.008). There were no differences between time to reach peak performance comparing the tuned and untuned units in either task (p ≥ 0.31). Values are mean + SD.
Directionally tuned units exhibited a slightly higher average firing rate during the manual control task (18 ± 4 Hz) compared to untuned cells (15 ± 5 Hz; p < 0.001). Discharge rate variability, captured by the coefficient of variation of inter-spike intervals, was similar for both tuned (0.9 ± 0.25 Hz) and untuned units (0.9 ± 0.19 Hz; p = 0.65).
3.2 Cursor control trajectories as a function of target direction and unit directional tuning
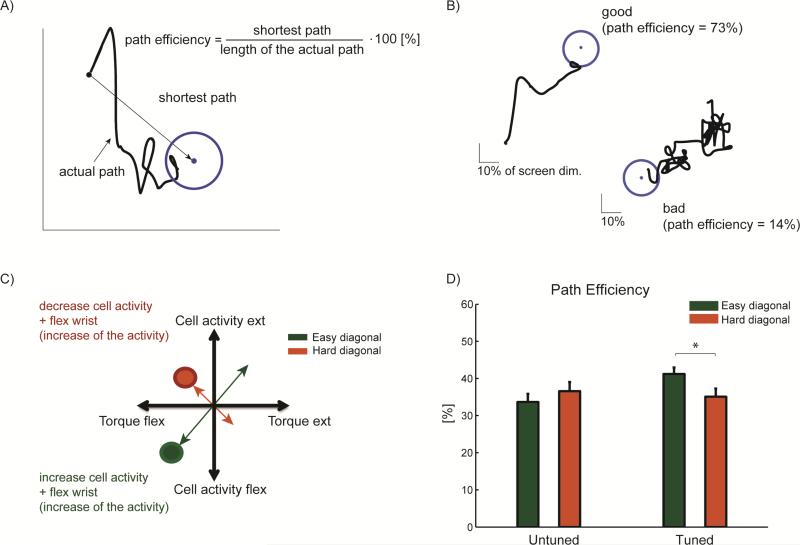
We examined the possibility that the difficulty of the dual control task may not be the same in all directions. Figure 6 illustrates the path efficiency parameter used to investigate any asymmetries in cursor control. Examples of a relatively direct and efficient path and a very inefficient path are shown in Figure 6B.
Figure 6.
Path Efficiency. (A) Definition of path efficiency. (B) Examples of the path efficiency showing both good efficiency (73 %) and poor efficiency (14 %) (C) Diagram of “easy” and “hard” diagonals during dual control. (D) During the dual control task, the path efficiency was significantly higher across easy compared to hard diagonals when the monkey was controlling units with directional tuning (p = 0.04). Path efficiency for untuned units did not differ significantly with target direction (p = 0.40). Values in (D) are mean + SEM.
In the case of directionally tuned units, targets presented in certain directions may be easier to acquire when they simply require a natural combination of discharge rate and wrist torque (e.g., a unit tuned toward wrist flexion and a target that requires simultaneous increases in discharge rate coupled with wrist flexion torque). Conversely, targets may be more difficult to acquire if they require decoupling of the discharge rate and torque. To quantify this potential difference, we defined two diagonals in the task workspace as “easy” and “hard” (Figure 6C). As might be expected, when the monkey was controlling the cursor using the untuned units, the path efficiency was not significantly different across diagonals (34 ± 13 % and 36 ± 15 %, for easy and hard diagonal, respectively, p = 0.40). When using directionally tuned units, however, the monkey acquired targets with significantly greater path efficiency in the “easy” diagonal compared to the “hard” diagonal (41 ± 12 % versus 35 ± 15 %, respectively, p = 0.04).
In order to further explore the differences in cursor control during the dual task, we quantified other relevant aspects of the path of cursor movement, including deviation from the desired trajectory and number of target re-entries. Results are summarized in table 1. Figure 7A shows an example of cursor movements across easy and hard diagonals when the monkey was controlling directionally tuned units. The monkey had difficulty moving straight towards the target in the hard diagonal. Instead, he often deviated from the direct path along the hard diagonal and instead performed a set of indirect movements largely along the easy diagonal.
Table 1.
Additional measures of performance for the analysis of cursor control
| Performance Measure | untuned units | tuned units | ||
|---|---|---|---|---|
| E | H | E | H | |
| Path Efficiency [%] | 34 ± 13 | 36 ± 15 | 41 ± 12 | 35 ± 15* (a) |
| Parallel Direction Change | 41.4 ± 48.1 | 29.1 ± 25.5 | 32.5 ± 30.1 | 37.2 ± 39.9 |
| Orthogonal Direction Change | 39.2 ± 48.5 | 28.71 ± 29.7 | 29.6 ± 31.5 | 31.9 ± 34.3 |
| Movement Variability [%] | 7.4 ± 4 | 6.2 ± 2 | 6.9 ± 4 | 6.4 ± 3 |
| Movement Error [%] | 9.5 ± 4 | 8.9 ± 4 | 8.6 ± 3 | 8.1 ± 4 |
| Movement Offset [%] | 5 ± 5 | 5 ± 3 | 4.4 ± 4 | 5.8 ± 5* (b) |
| Target Re-entry | 1.14 ± 1.1 | 0.94 ± 1.0 | 0.72 ± 0.91 | 0.65 ± 0.47 |
| 1.04 ± 1.05 | 0.68 ± 0.72* (b) | |||
Note: Values are mean ± SD; E, easy diagonal; H, hard diagonal;
p < .05 via Student's t-test or Wilcoxon ranksum when data were nonparametric: (a)– ttest; (b) - ranksum.
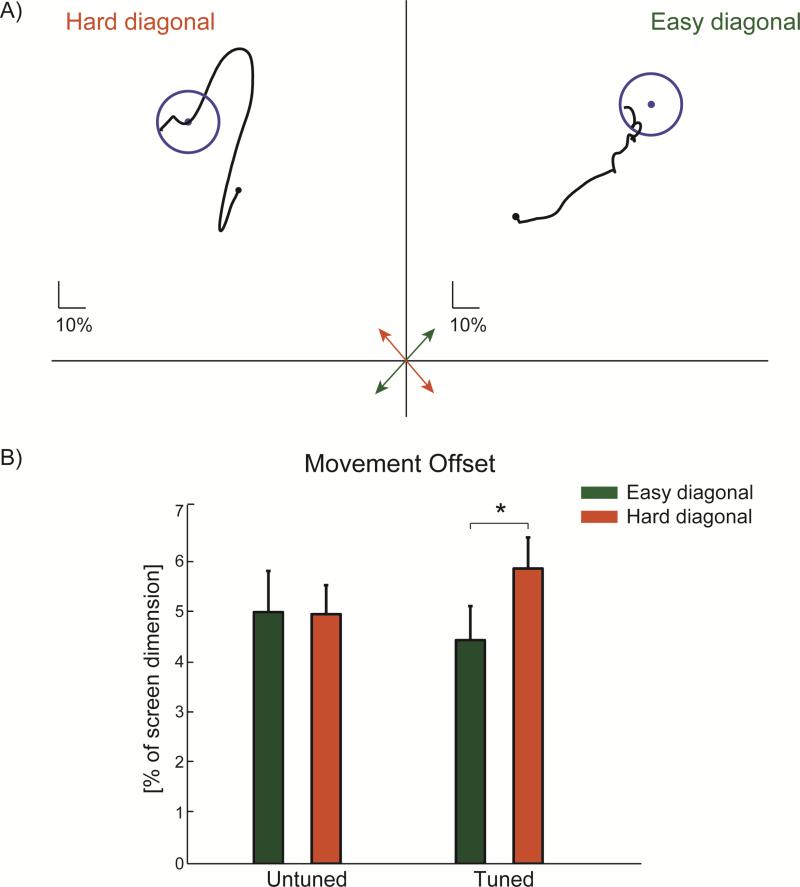
Figure 7.
(A) Example cursor trajectories when the monkey used directionally tuned units to acquire targets appearing in the “easy” and “hard” diagonals during the dual control task (see Figure 6C). When targets appear along the easy diagonal (green arrow), the monkey moved the cursor in relatively straight paths towards the target (Movement offset = 4 % of screen dimension in this example). However, when targets appear along the hard diagonal (red arrow), the monkey combined movements largely along the easy diagonal to indirectly approach the target which resulted in a larger movement offset (6.5 %). (B) Movement offset during cursor control for all units tested in the dual control task. Untuned units control the cursor with equal movement offset when targets appear in either diagonal. Directionally tuned units control the cursor more directly toward targets in the easy diagonal compared to the hard diagonal (p = 0.03). Values in (B) are mean + SEM.
This deviation from the straight line path connecting the cursor starting position to the target position was quantified as the movement offset. The monkey exhibited greater movement offset when using tuned cells to direct the cursor toward targets appearing in the hard diagonal (5.8 ± 5%) compared to the easy diagonal (4.4 ± 4 %; p = 0.03, Figure 7B). There was no difference in movement offset based on target direction when untuned cells controlled the cursor (p = 0.66). In addition, other measures of the cursor movement related to the direction changes and deviation from a straight line were similar across movement directions for both the untuned and tuned units during the dual control task (p > 0.21).
Another indicator of efficient cursor control is the number of times the cursor must reenter a target before successful acquisition. The monkey left and re-entered the target significantly more times prior to success when controlling untuned units (average of 1.04 ± 1.05 re-entries per correct target acquisition) compared to directionally tuned units (0.68 ± 0.72 re-entries; p = 0.008). There were no significant differences in re-entries between the easy and hard diagonals for either the untuned and tuned units (p > 0.19).
4. Discussion
The ability of a single hemisphere to differentiate its output may be required for BCI applications following stroke or traumatic brain injury. Here we explored the capacity of motor cortex in one hemisphere to simultaneously control a BCI and continue to move the contralateral hand. We tested whether directionally-tuned or relatively untuned neurons could more readily be decoupled from ongoing movements of the contralateral arm in this “dual control” task.
In this case report, we show that the overall performance was similar when the monkey used either directionally-tuned or untuned units to perform the dual control task requiring simultaneous hand movements. When performing the task using directionally tuned units, the monkey exhibited less efficient cursor trajectories when moving toward targets that required explicit decoupling from ongoing movement of the contralateral hand. By contrast, the monkey re-entered the target more times when using untuned units to perform the task. When using both tuned and untuned units, the monkey required greater practice time to reach peak performance at the dual control task with ongoing hand movements compared to using the same units for brain-control of a cursor alone. This may be partly explained by the fact that the brain control task utilized a single dimension, whereas the dual control task required acquiring targets in two dimensions.
Although overall performance of the dual control task did not depend on the strength of directional tuning, directionally-tuned units exhibit poorer performance when required to decouple their activity from ongoing contralateral limb movements. When targets appear in a direction that requires tuned unit activity to be unnaturally dissociated from ongoing movements, the monkey exhibited lower path efficiency and cursor trajectories with greater movement offsets. In these cases, the monkey appeared to acquire targets by making a series of orthogonal movements which allowed the unit and arm activity to remain largely correlated (see figure 7A). Conversely, when the activity of directionally tuned units could remain “naturally” correlated with arm movements, the monkey moved more directly to the target resulting in better than average performance. Thus for directionally tuned units, performance at the easy and hard diagonals were offset, such that total performance was similar regardless of the strength of directional tuning during the dual task.
Performance was also independent of the strength of directional tuning for pairs of units used to control the 1-dimensional brain control task. This finding is similar to previous studies from our group using small numbers of units to control a BCI task. While directionally tuned units performed significantly better in a simple 1 degree-of-freedom BCI task,[6, 24] there was no difference between directionally tuned and untuned units when monkeys performed a more complex task of using unit activity to control functional stimulation of paralyzed muscles.[6]
In all of these studies we explicitly trained small populations of one or two neurons to control the BCI task using operant conditioning. An alternative strategy to implementing a BCI for individuals with damage to motor areas of one hemisphere is to decode the intention to move the ipsilateral arm from populations of neurons. Monkeys can learn to control a BCI reflecting ipsilateral kinematics, although representations were more closely correlated with joint angles compared to hand position.[14] That same study also demonstrated that cortical field potentials (ECoG) recorded from human subjects contain representations of ipsilateral arm movement. Although these authors utilized EMG to exclude the possibility of mirror movements, it remains to be tested whether ongoing movement of the contralateral arm will interfere with decoding of ipsilateral activity from spike times or field potentials. The activity of the same neurons recorded from primary motor cortex can be strikingly different when monkeys perform bi-manual vs. unimanual tasks.[19, 21]
Recently, a brain-machine interface was used to provide independent control of two virtual arms in a bi-manual task.[31] To accomplish this, many hundreds of units were recorded from frontal and parietal areas of both hemispheres, potentially limiting the application for users with brain injury. Monkeys can also learn to maintain an isometric force with the hand while controlling a BCI with a majority of units from the contralateral hemisphere.[32] Recordings from posterior parietal cortex within a single hemisphere yield information about bi-manual or contralateral arm movements.[33, 34]. In a recent human trial, some units recorded from posterior parietal cortex demonstrated greater representation of movements of the ipsilateral arm[35], providing another potential location for recording from a single hemisphere following stroke to control movement of the affected limb.
The most direct tests of the concept of using a single hemisphere to control a BCI following brain injuries have utilized non-invasive electroencephalography (EEG) recordings. Ang et al. [36] showed that EEG data recorded from both brain hemispheres could be used to detect motor imagery of the stroke-affected hand. The majority of participants (87%) were able to use motor imagery BCI after stroke. Bundy et al. [13] identified distinct signal features related to the ipsilateral arm recorded over the undamaged hemisphere in human subjects after stroke. Subjects were then able to control a single degree-of-freedom BCI using ipsilateral motor imagery. A parallel study of neurologically intact volunteers demonstrated that subjects could simultaneously control a hand motor imagery signal and contralateral hand movements to complete a two degree-of-freedom combined BCI and manual control task.[37] Similar to the present study, activity originating in a single hemisphere could be decoupled to achieve independent control of the hand and ongoing brain activity.
Perhaps the ultimate test of our approach of using ipsilateral activity to restore function following stroke is to use this cortical activity to control muscle stimulation and re-animate the ipsilateral arm. While initial experiments demonstrate this is possible, the key future test will be a bi-manual task, in order to determine whether a single hemisphere can effectively control both the natural, contralateral arm, as well as stimulation of the ipsilateral, paretic arm. As detailed below, experiments must also take into consideration how the damaged hemisphere will affect activity in the remaining brain regions used for BCI control. Limitations of the present study include the relatively short amount of practice permitted for each pair of units used in the BCI and dual control task. Although on average the monkey achieved peak performance early in each practice session (~ 5 of 15 minutes during brain control and 9 of 32 minutes during dual control), performance at these challenging tasks would probably continue to improve with prolonged practice.[38] Here we strategically chose to sample a large population of units with different degrees of directional tuning, rather than allowing prolonged practice with a smaller sample of units across many days.
A second limitation of this study was in providing visual feedback of unit activity. In order to create a task which explicitly required decoupling of unit activity and hand control, visual feedback of one of these control modalities must be rotated to achieve independent degrees of freedom on the monitor. Here we chose to preserve the relation between wrist torque and cursor movement, as the monkey was well-trained in the manual task. Therefore, visual feedback of unit activity was rotated 90 degrees, similar to previous BCI studies,[39] such that targets could be presented on orthogonal axes when requiring decoupling of unit and arm activity. Such rotation would not be required in future applications of re-animating the ipsilateral arm after brain injury because visual feedback of both arms will provide adequate information.
Finally, it is important to note that in this case study, both hemispheres of the monkey were intact. In individuals with lateralized cortical stroke, the excitability of the spared cortex is increased, presumably due to a decrease in transcollosal inhibition. [40, 41] In the future, it will be important to explicitly test the changes in neural activity in the unaffected hemisphere and demonstrate that observed decoupling is still possible. In order to expand this approach to benefit patients paralyzed by cortical stroke in motor areas, the described paradigm needs to be coordinated with residual motor functions. The observed differentiation must be verified in the context different motor tasks (e.g. more degrees of freedom) and during the performance of various cognitive tasks that may potentially disrupt control.
5. Conclusions
In order to be economically viable, invasive BCIs may need to benefit a large clinical population such as individuals recovering from stroke or traumatic brain injury. In this case a single hemisphere may need to provide control over both the contralateral, unaffected arm as well as the ipsilateral, affected arm. Here we demonstrate that a monkey can learn to control the activity of single units in motor cortex independently of ongoing contralateral limb movements. This flexibility of cortical activity was underscored by the fact that final performance was equivalent whether units were strongly related to contralateral hand movement (i.e., directionally tuned), or relatively unrelated to hand movement. These findings demonstrate independent control of cortical activity and contralateral limb movement, a prerequisite for BCI technology development for individuals with brain injury.
Acknowledgments
The authors thank Paul House for expert array implantations, Charlie Matlack for task design and assistance with data analysis, and Larry Shupe for technical support.
Funding
This work was supported by an American Heart & Stroke Association Scientist Development Grant [NCRP 09SDG2230091]; The Paul G. Allen Family Foundation (Allen Distinguished Investigator Award); National Institutes of Health [NS12542, RR00166]; the Center for Sensorimotor Neural Engineering (CNSE), a National Science Foundation Engineering Research Center [EEC-1028725].
Footnotes
Conflict of interest: NA
References
- 1.Carmena JM, Lebedev MA, Crist RE, O'Doherty JE, Santucci DM, Dimitrov D, Patil PG, Henriquez CS, Nicolelis MA. Learning to Control a Brain-Machine Interface for Reaching and Grasping by Primates. PLoS Biol. 2003;1(2):E42. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002;416(6877):141–2. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- 3.Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296(5574):1829–32. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 4.Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453(7198):1098–101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- 5.Chapin JK, Moxon KA, Markowitz RS, Nicolelis MA. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci. 1999;2(7):664–70. doi: 10.1038/10223. [DOI] [PubMed] [Google Scholar]
- 6.Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456(7222):639–42. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ethier C, Oby ER, Bauman MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature. 2012;485(7398):368–71. doi: 10.1038/nature10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pohlmeyer EA, Oby ER, Perreault EJ, Solla SA, Kilgore KL, Kirsch RF, Miller LE. Toward the restoration of hand use to a paralyzed monkey: brain-controlled functional electrical stimulation of forearm muscles. PLoS One. 2009;4(6):e5924. doi: 10.1371/journal.pone.0005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, McMorland AJ, Velliste M, Boninger ML, Schwartz AB. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2012 doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485(7398):372–5. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442(7099):164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Collinger JL, Degenhart AD, Tyler-Kabara EC, Schwartz AB, Moran DW, Weber DJ, Wodlinger B, Vinjamuri RK, Ashmore RC, Kelly JW, Boninger ML. An electrocorticographic brain interface in an individual with tetraplegia. PLoS One. 2013;8(2):e55344. doi: 10.1371/journal.pone.0055344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bundy DT, Wronkiewicz M, Sharma M, Moran DW, Corbetta M, Leuthardt EC. Using ipsilateral motor signals in the unaffected cerebral hemisphere as a signal platform for brain-computer interfaces in hemiplegic stroke survivors. J Neural Eng. 2012;9(3):036011. doi: 10.1088/1741-2560/9/3/036011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganguly K, Secundo L, Ranade G, Orsborn A, Chang EF, Dimitrov DF, Wallis JD, Barbaro NM, Knight RT, Carmena JM. Cortical representation of ipsilateral arm movements in monkey and man. J Neurosci. 2009;29(41):12948–56. doi: 10.1523/JNEUROSCI.2471-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng YS, Stein J, Ning M, Black-Schaffer RM. Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke. 2007;38(8):2309–14. doi: 10.1161/STROKEAHA.106.475483. [DOI] [PubMed] [Google Scholar]
- 17.Matsunami K, Hamada I. Characteristics of the ipsilateral movement-related neuron in the motor cortex of the monkey. Brain Res. 1981;204(1):29–42. doi: 10.1016/0006-8993(81)90649-1. [DOI] [PubMed] [Google Scholar]
- 18.Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol. 1988;60(1):325–43. doi: 10.1152/jn.1988.60.1.325. [DOI] [PubMed] [Google Scholar]
- 19.Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol. 2003;89(2):922–42. doi: 10.1152/jn.00607.2002. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg O, Donchin O, Gribova A, de Oliveira S Cardosa, Bergman H, Vaadia E. Neuronal populations in primary motor cortex encode bimanual arm movements. Eur J Neurosci. 2002;15(8):1371–80. doi: 10.1046/j.1460-9568.2002.01968.x. [DOI] [PubMed] [Google Scholar]
- 21.Donchin O, Gribova A, Steinberg O, Bergman H, Vaadia E. Primary motor cortex is involved in bimanual coordination. Nature. 1998;395(6699):274–8. doi: 10.1038/26220. [DOI] [PubMed] [Google Scholar]
- 22.Fetz EE. Operant conditioning of cortical unit activity. Science. 1969;163(870):955–8. doi: 10.1126/science.163.3870.955. [DOI] [PubMed] [Google Scholar]
- 23.Fetz EE, Baker MA. Operantly conditioned patterns on precentral unit activity and correlated responses in adjacent cells and contralateral muscles. J Neurophysiol. 1973;36(2):179–204. doi: 10.1152/jn.1973.36.2.179. [DOI] [PubMed] [Google Scholar]
- 24.Moritz CT, Fetz EE. Volitional control of single cortical neurons in a brain-machine interface. J Neural Eng. 2011;8(2):025017. doi: 10.1088/1741-2560/8/2/025017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rousche PJ, Normann RA. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann Biomed Eng. 1992;20(4):413–22. doi: 10.1007/BF02368133. [DOI] [PubMed] [Google Scholar]
- 26.Torab K, Davis TS, Warren DJ, House PA, Normann RA, Greger B. Multiple factors may influence the performance of a visual prosthesis based on intracortical microstimulation: nonhuman primate behavioural experimentation. J Neural Eng. 2011;8(3):035001. doi: 10.1088/1741-2560/8/3/035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233(4771):1416–9. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- 28.Foldes ST, Taylor DM. Speaking and cognitive distractions during EEG-based brain control of a virtual neuroprosthesis-arm. J Neuroeng Rehabil. 2013;10:116. doi: 10.1186/1743-0003-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SP, Simeral JD, Hochberg LR, Donoghue JP, Black MJ. Neural control of computer cursor velocity by decoding motor cortical spiking activity in humans with tetraplegia. J Neural Eng. 2008;5(4):455–76. doi: 10.1088/1741-2560/5/4/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKenzie IS, Kauppinen T, Silfverberg M. Accuracy measures for evaluating computer pointing devices.. Proceedings of the ACM Conference on Human Factors in Computing Systems - CHI 2001; New York. ACM; 2001. [Google Scholar]
- 31.Ifft PJ, Shokur S, Li Z, Lebedev MA, Nicolelis MA. A brain-machine interface enables bimanual arm movements in monkeys. Sci Transl Med. 2013;5(210):210ra154. doi: 10.1126/scitranslmed.3006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orsborn AL, Moorman HG, Overduin SA, Shanechi MM, Dimitrov DF, Carmena JM. Closed-loop decoder adaptation shapes neural plasticity for skillful neuroprosthetic control. Neuron. 2014;82(6):1380–93. doi: 10.1016/j.neuron.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 33.Chang SW, Dickinson AR, Snyder LH. Limb-specific representation for reaching in the posterior parietal cortex. J Neurosci. 2008;28(24):6128–40. doi: 10.1523/JNEUROSCI.1442-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang SW, Snyder LH. The representations of reach endpoints in posterior parietal cortex depend on which hand does the reaching. J Neurophysiol. 2012;107(9):2352–65. doi: 10.1152/jn.00852.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aflalo T, Kellis S, Klaes C, Lee B, Shi Y, Pejsa K, Shanfield K, Hayes-Jackson S, Aisen M, Heck C, Liu C, Andersen RA. Neurophysiology. Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science. 2015;348(6237):906–10. doi: 10.1126/science.aaa5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ang KK, Guan C, Chua KS, Ang BT, Kuah CW, Wang C, Phua KS, Chin ZY, Zhang H. A large clinical study on the ability of stroke patients to use an EEG-based motor imagery brain-computer interface. Clin EEG Neurosci. 2011;42(4):253–8. doi: 10.1177/155005941104200411. [DOI] [PubMed] [Google Scholar]
- 37.Cheung W, Sarma D, Scherer R, Rao RP. Simultaneous brain-computer interfacing and motor control: expanding the reach of non-invasive BCIs. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:6715–8. doi: 10.1109/EMBC.2012.6347535. [DOI] [PubMed] [Google Scholar]
- 38.Ganguly K, Carmena JM. Emergence of a stable cortical map for neuroprosthetic control. PLoS Biol. 2009;7(7):e1000153. doi: 10.1371/journal.pbio.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarosiewicz B, Chase SM, Fraser GW, Velliste M, Kass RE, Schwartz AB. Functional network reorganization during learning in a brain-computer interface paradigm. Proc Natl Acad Sci U S A. 2008;105(49):19486–91. doi: 10.1073/pnas.0808113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144(1-2):160–70. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- 41.Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99(22):14518–23. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]