Abstract
The dominant roles of the tumor microenvironment in regulating tumor formation, progression, and metastasis have driven the application of tissue engineering strategies in cancer biology. Highly dynamic and reciprocal communication of tumor cells with their surroundings suggests that studying cancer in custom-designed biomaterial scaffolds may lead to novel therapeutic targets and therapeutic regimens more reliably than traditional monolayer tissue culture models. As tissue engineering becomes progressively more successful in recapitulating the native tumor environment, critical insights into mechanisms of tumor resistance may be elucidated, to impact clinical practice, drug development, and biological research. We review here the recent developments in the use of custom-designed biomaterial scaffolds for modeling human tumors.
Keywords: Biomaterials, scaffold, tissue engineering, cancer, tumor models
Introduction
Approximately 40% of men and women in the United States will be diagnosed with at least one form of cancer in their lifetime, with cancer being implicated in one in four deaths [1]. While great strides have been made in early diagnosis and treatment using standard regimens of chemotherapy and radiation, resulting in an overall decrease in cancer mortality, tumor initiation, growth and metastasis continue to evade control. The continued search for effective and targeted drugs has been hindered by the high failure rate of costly clinical trials, illuminating a need for more accurate preclinical models of the disease, not only for pharmaceutical testing, but also for biological research and assay development.
Recent studies have shown that cancer cell behavior and tumor formation, progression, and metastasis are dominated by the tumor microenvironment. The tumor environment has also been shown to be critical in promoting resistance and recurrence of tumors. These findings have inspired the application of tissue engineering strategies in cancer biology, with the expectation that the control over tumor environment will enable identification of therapeutic targets and the development of new therapeutic regimens.
Biomaterial as a Cell Niche
For several decades, tissue engineers have utilized customized biomaterials to direct the differentiation and organization of pluripotent and multipotent cells, with the understanding that phenotypically plastic stem cells communicate in a dynamic and reciprocal manner with their surroundings when adopting a particular cell fate. In the context of tissue regeneration, research has been driven by the need for increasingly complex yet tunable biomaterials that reliably mimic the extracellular matrix (ECM) of specific human organs with respect to biocompatibility, mechanical stiffness, 3D architecture, structural organization, and cell adhesion. Cell signaling molecules that have been shown to improve the yield and maturation of desired cell lineages are often used in concert with engineered scaffolds, either through direct application in cell culture or by incorporation into the biomaterials for controlled release. Indeed, the prevailing wisdom in the field is that in an engineered microenvironment, the cells themselves become the “tissue engineers” via a confluence of cell-cell, cell-ECM, and signaling interactions. Not only do cells respond to their microenvironment, but they also tune their microenvironment to suit their needs, initiating physiological changes that govern tissue formation and function.
Differentiated cells still exhibit some phenotypic plasticity in response to changes in their environment. The most promising tissue regeneration strategies to date have abandoned traditional 2D cell culture on glass or polystyrene in favor of 3D culture systems, including cell spheroids and cells encapsulated in scaffolds, due to the major differences between cells grown in monolayers versus those in native environments. These differences include distinct changes in cell morphology, proliferation, motility, global gene and protein expression, and sensitivity to therapeutic agents. Importantly, these same characteristics of human tumor cells are restored by 3D cell culture on biomimetic matrices, in keeping with the trends observed in stem cell populations. For this reason, tissue engineers have come to rely heavily on biomaterials no matter what their starting cell population. Generation of 3D tumor models involves an integrated use of cancer cells, biomaterial scaffold customized to mimic the particular tissue matrix, supporting non-cancer cells and the culture systems providing cytokines, vascular perfusion and mechanical signals (Figure 1).
Figure 1. Generating a three-dimensional tumor model.
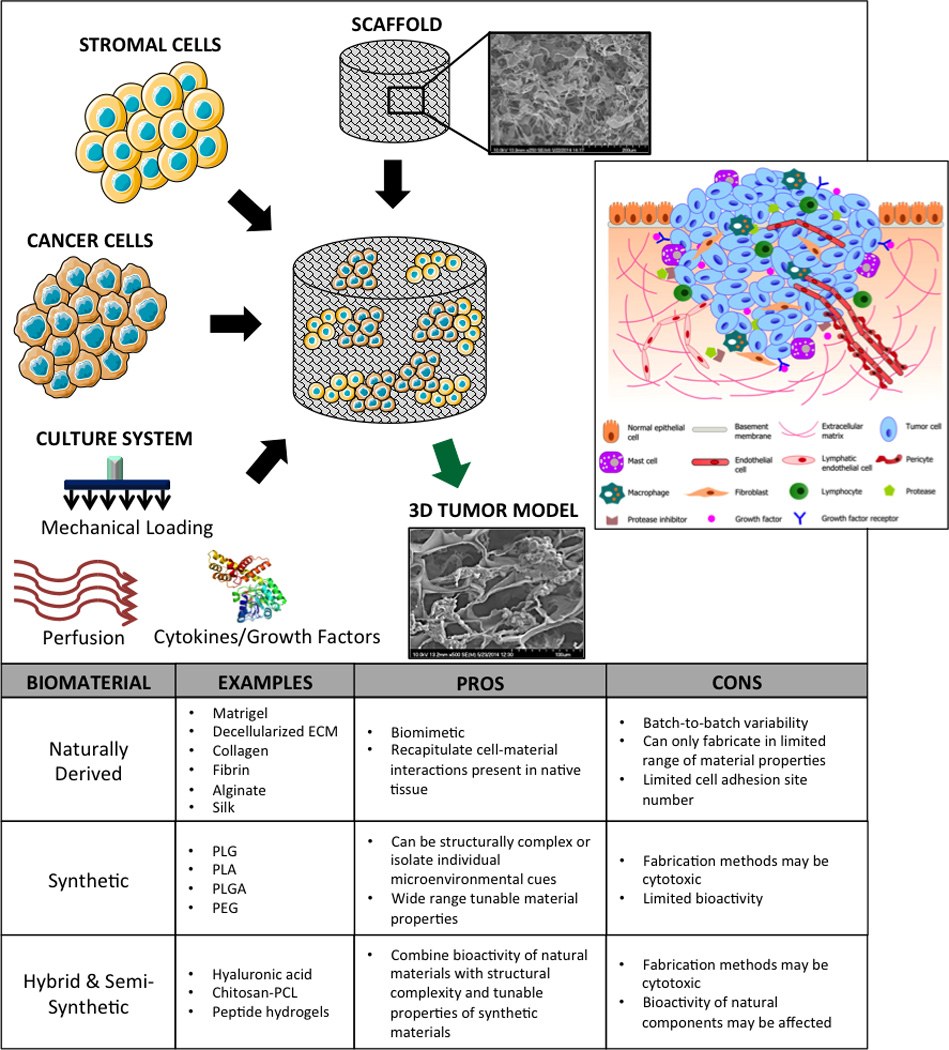
Cancer cells, supporting stromal cells, biomaterial scaffold, molecular and physical signals are used in an integrated way to bioengineer in vitro models of human cancer. Inset: illustration of a typical tumor microenvironment with the supporting cells, ECM, and signaling molecules; reproduced with permission from [2].
Dominance of the Tumor Microenvironment
Cancer cell phenotype must be regulated by its microenvironment, given that oncogenic mutations present in the genotype of every cell of a patient only manifests as cancer in specific tissues and regions [3]. It has long been recognized that the ECM surrounding human tumors is characterized by abnormal remodeling, with a variety of ECM components including collagens (I, II, III, V, and IX) and proteoglycans present at higher than normal levels. Additionally, the architecture of these abnormally remodeled or secreted ECM components is also altered; for example, collagen I fibrils become more linear and assume a specific orientation relative to the tumor surface [4,5]. These changes are thought to arise from the pathological interactions of cancer cells and tumor-associated stroma. These interactions are dynamic and reciprocal in nature, similar to the intercommunication between tissue cells and stroma in normal tissues. Furthermore, the angiogenesis and lymph-angiogenesis that typically accompany the maturation of tumors contribute to increases in interstitial fluid pressure [6,7]. Newly formed tumor vasculature is often leaky, increasing fluid flux and shear stress throughout the stroma, and generating gradients of chemokines and growth factors. Taken together, these changes result in ECM that is significantly stiffer than normal. From these and similar findings, it can be concluded that tumor ECM is different from normal tissue ECM, and that the pathology of tumor ECM progresses as the tumor progresses, ultimately leading to metastasis.
Groundbreaking experiments performed by the Dolberg and Bissell in chick embryos inoculated with the Rouse sarcoma virus (RSV) demonstrated the ability of the microenvironment to restrain cancer progression. Previous studies have shown unequivocally that injecting RSV into the wings of chickens induced the formation of large, malignant tumors [8]. Interestingly, however, the injection of RSV into the wings of early chick embryos never produced tumors or even malignantly transformed cells, suggesting that the embryonic microenvironment was able to override the effects of potent oncogenes. When cells from these same embryonic wings were subsequently harvested and cultured in vitro, a malignant phenotype revealed itself overnight. Further experiments in embryos nearer to hatching demonstrated a loss of this regulatory ability, most likely due to the developmental changes in the wing microenvironment [9].
Of greatest clinical importance is the phenomenon of multi-drug resistance (MDR), which is a defining protective function of the tumor microenvironment [10]. The leaky vasculature and increased interstitial fluid pressure in tumor ECM interfere with the delivery of chemotherapeutics and the accumulation of an effective drug concentration. Moreover, the heterogeneous nature of the tumor microenvironment is believed to support a range of cancer cell phenotypes, some of which may be more isolated and resistant to apoptosis, and therefore insensitive to anticancer agents [11]. Other phenotypic variants may lay dormant in a quiescent state and evade chemotherapeutic treatment, providing the cellular basis for primary tumor recurrence and metastasis once tumor-promoting signals are restored [12–16]. These surviving cancer cells remain one of the most significant barriers to effective cancer treatment.
Naturally Derived Biomaterials
In an effort to recapitulate the tumor ECM as faithfully as possible, natural materials have been explored extensively for three-dimensional tumor modeling. While they are ideal for replicating the cell-matrix interactions of the native tumor microenvironment, scaffolds of this type suffer from batch-to-batch variability and can only be fabricated within a limited range of mechanical stiffness, degradation rate, porosity, and cell adhesion site number. Figure 2 shows the three classes of most frequently used naturally derived biomaterials: collagen scaffolds, decellularized human tissue matrix, and fibrin hydrogels.
Figure 2. Naturally derived biomaterials.
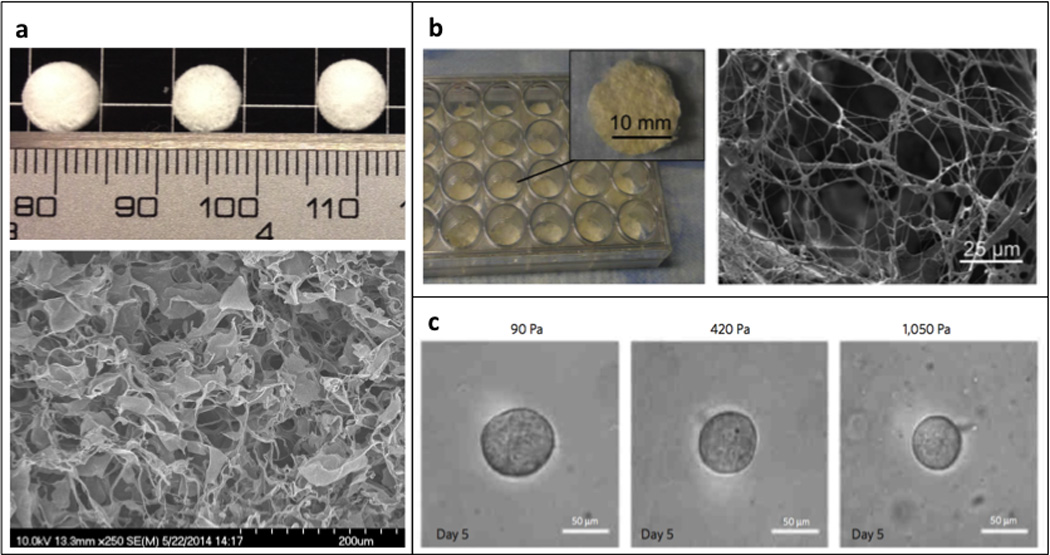
(a) Digital image of a collagen type I sponge (top) and corresponding scanning electron micrograph (bottom); (b) Digital image of decellularized human adipose tissue sponge (left) and a corresponding scanning electron micrograph (right) [21]; (c) Phase contrast images of tumor spheroids grown in 3D fibrin hydrogels of varying stiffness; reproduced with permission from [31].
Early 3D tumor models were generated using Matrigel, a hydrogel derived from Engelbreth-Holm-Swarm (EHS) mouse tumor cell basement membrane, which is primarily composed of collagen and laminin. Several elegant studies performed by the Bissell group compared the effects of conventional 2D tissue culture with 3D Matrigel culture on the phenotype of malignant (T4-2) and non-malignant (S-1) human breast cancer cell lines (HMT-3522), whereby divergent profiles in morphology and proliferation rate could only be observed in the 3D context. The T4-2 line is characterized by aberrantly high expression of β1 and β4 integrins, and disruption of cell-matrix interactions using β1 and β4 integrin inhibitors caused massive apoptosis in the non-malignant cultures and a reversion to a non-malignant phenotype in the malignant cultures. Interestingly, this phenotype reversion was reversible, proven by subsequent “reversion rescue” studies wherein removal of the integrin-inhibiting molecules and return to a fresh 3D Matrigel matrix recovered the malignant phenotype in T4-2 cells [17,18]. Further studies of invasive human breast cancer cell lines including MCF-7 and MDA-MB-231 demonstrated similar phenotypic reversion effects with treatments of epidermal growth factor receptor (EGFR) inhibitor and transfection with an E-cadherin expression vector, respectively [19].
More recently, the Leedman group investigated the influence of tumor microenvironment on microRNA expression in colon cancer cell lines (SW480, SW620). MicroRNAs are small, non-coding RNAs that regulate gene expression and are believed to play a role in cancer cell adhesion, proliferation, and invasion. When the SW480 and SW620 cell lines were cultured on conventional tissue culture plastic versus 3D Matrigel matrices, microarray profiles revealed that expression levels of several miRNAs were significantly altered. The miRNAs exhibiting the most significant Matrigel-induced expression level changes were further probed in different human epithelial cancer cell lines, including HT-29 (colon cancer), A549 (non-small cell lung cancer), and MDA-MB-231 (breast cancer). The same trends in Matrigel-induced upregulation and downregulation of miRNAs were observed in all cancer cell lines, suggesting a consistent pattern in the regulation of miRNA expression by Matrigel across various epithelial cancer cell types [20].
Apart from Matrigel, ECM-derived scaffolds have been used to generate 3D models of human tumors. These include the use of decellularized matrices harvested from human adipose and small intestinal submucosal tissues, as well as decellularized rat lung matrices [21–23]. In each of these studies, the authors found that the 3D ECM scaffolds more closely mimic the in vivo microenvironment when compared with conventional 2D culture, as determined by the metrics of cell morphology, migration, adhesion molecule expression, and chemosensitivity. More notably, MCF7, BT474, and SKBR3 human breast cancer cell lines cultured on 3D decellularized adipose tissue more closely resembled in vivo mouse xenografts than when cultured on 3D Matrigel scaffolds [21].
A compelling variation on ECM-derived scaffold fabrication was explored by the Hutmatcher group, by harvesting primary human osteoblasts from patients undergoing knee replacement surgery and seeding them in high density on tissue culture flasks. Following 28 days in culture, the osteoblasts had deposited a highly mineralized matrix, which was then decellularized and seeded with LNCaP and PC3 prostrate cancer cells, yielding a biomimetic model of the prostate cancer bone metastasis observed in 90% of prostate cancer patients [24].
A similar approach was taken by the Cheng group, which used NIH-3T3 cells overexpressing fibroblast activation protein (FAP) to deposit a tumor-associated stroma-like ECM onto tissue culture plates. Following eight days in culture and decellularization, these 3D fibroblast-derived matrices were seeded with metastatic (Capan-1, Panc-1) and less aggressive (AsPC-1, HPAF-II) pancreatic cancer cell lines, as well as benign (MCF-10A) and malignant (MCF-7, MDA-MB-231) human breast cancer cell lines. The FAP+ matrices were shown to promote the invasiveness of both pancreatic and breast cancer cell lines [25].
Finally, Hoshiba and Tanaka modeled staged tumorigenesis by culturing human breast cancer cell lines of increasing malignancy and invasiveness (MCF-10A, MCF-7, MDA-MB-231) at high concentration for one week on tissue culture polystyrene. The resultant cancer cell-derived matrices were decellularized and re-seeded with each individual cell type to evaluate the effect of increasingly pathological tumor microenvironments on cell attachment, proliferation, and chemoresistance. It was found that cell attachment decreased and cell proliferation increased with increasingly malignant matrices, most prominently on those derived from MDA-MB-231 cells. Chemoresistance to 5-fluorouracil (5-FU) and doxorubicin (Dox) was variable amongst all cell types and staged matrices, suggesting a more complex mechanism of drug resistance [26].
Collagen is the most abundant ECM protein found in humans, and for that reason collagen hydrogels and sponges have been used extensively for tumor modeling and anti-cancer drug testing. A chemically cross-linked collagen I scaffold was used by the Dai group to simulate in vivo conditions and study changes in cellular properties of MCF-7 cells with respect to 2D culture. The secretion of angiogenic factors (VEGF, bFGF, IL-8) and matrix metalloproteinases (MMPs) increased with cells in 3D culture, and an enhanced cancer stem cell (CSC)-like profile was simultaneously observed, as evidenced by the increased expression of breast cancer stem cell markers (OCT4A, SOX2, SOX4, JAG1, CD49F). These findings were further substantiated by the higher tumorigenicity of 3D–cultured MCF-7 cells in in vivo mouse models, which formed significantly larger tumors than 2D–cultured cells [27].
Using the same material, the McGuigan group designed a 3D tumor model that could be rapidly disassembled for easy cell isolation from specific tumor locations, allowing for spatial mapping of cellular metabolism in response to hypoxia. Porous cellulose membrane strips were infiltrated with a concentrated cancer cell suspension into collagen type I solution, then rolled around an impermeable 6 mm-diameter metallic core to yield a layered configuration that recapitulated the length scales over which oxygen and nutrient gradients occur in vivo. This clever design proved superior to homogenous 2D or suspension cultures for mimicking and analyzing metabolic adaptations in cells from spatially defined regions within the tumor microenvironment [28].
Of note are tumor models incorporating multiple cell types, including those associated with tumor angiogenesis that were generated by the Rylander group using a bi-layered collagen I hydrogel with encapsulated MCF-7 or MDA-MB-231 human breast cancer cells. Telomerase-immortalized human microvascular endothelial (TIME) cells co-cultured with cell-encapsulated hydrogels across an acellular collagen I hydrogel barrier exhibited a significant increase in cell number, elongated morphology, and invasive sprouting into the acellular layer. This neovascular formation due to paracrine signaling was only observed in co-cultures with the more highly aggressive MDA-MB-231 cell line [29].
Finally, clever addition of paramagnetic beads and fibronectin into a collagen I hydrogel allowed for modeling of the effects of mechanical stimulation on HT1080 human fibrosarcoma cells and B16F10 mouse melanoma cells, as compared to a non-invasive mouse embryonic fibroblast (MEF) controls. Mechanical stimulation applied via a rotating rare earth magnet beneath the cultures enhanced invasiveness of the cancerous cells, but only when fibronectin was present within the scaffold [30].
Other naturally derived materials worth mentioning include fibrin [31], alginate [32], and silk fibroin protein [33]. 3D models generated using these materials have shown superiority over 2D culture as defined by the same metrics applied to 3D Matrigel, decellularized ECM, and collagen scaffolds, and have also yielded co-culture models of tumor angiogenesis [34]. Interestingly, these materials were also useful for isolating specific cancer cell populations that are necessary for developing more effective anti-cancer drugs. For example, a porous 3D chitosan-alginate scaffold was used by the Zhang group to select for and proliferate CD133+ cancer stem cell (CSC)-like cells found within U-87 and U-118 MG glioblastoma cell lines. CSCs, believed to be a foundation of MDR, are present in very low concentrations and are typically isolated using time-consuming and costly methods. The comparative ease of CSC enrichment using 3D scaffold culture is an unexpected but intriguing development [35]. In yet another application, the same chitosan-alginate scaffold was used to study tumor-associated fibroblast (TAF)-mediated suppression of breast tumor specific T cells. TAFs were shown to induce potent T cell suppression via transforming growth factor beta (TGF-beta) and interleukin-10 (IL-10) [36].
Synthetic Biomaterials
To overcome the limitations of natural materials, many groups have been developing novel biocompatible materials with a superior capacity for structural complexity and tunable physical properties, within a much wider range. While these materials are synthetic, they provide a “blank slate” onto which the desired ECM characteristics can be engineered depending on cell or tumor type, allowing for the study of individual microenvironmental cues in a highly controlled manner. Nonetheless, factors that still limit their use include fabrication methods that may be cytotoxic, and the limited bioactivity of many synthetic materials. In contrast to natural materials, cell adhesion to synthetic polymers often depends on non-specific protein adsorption from serum in culture media. Figure 3 shows the synthetic and semi-synthetic biomaterials used in modeling tumors: porous and electrospun polymer scaffold, peptide nanofiber scaffolds and hyaluronic acid-methacrylate hydrogels.
Figure 3. Synthetic and semi-synthetic biomaterials.
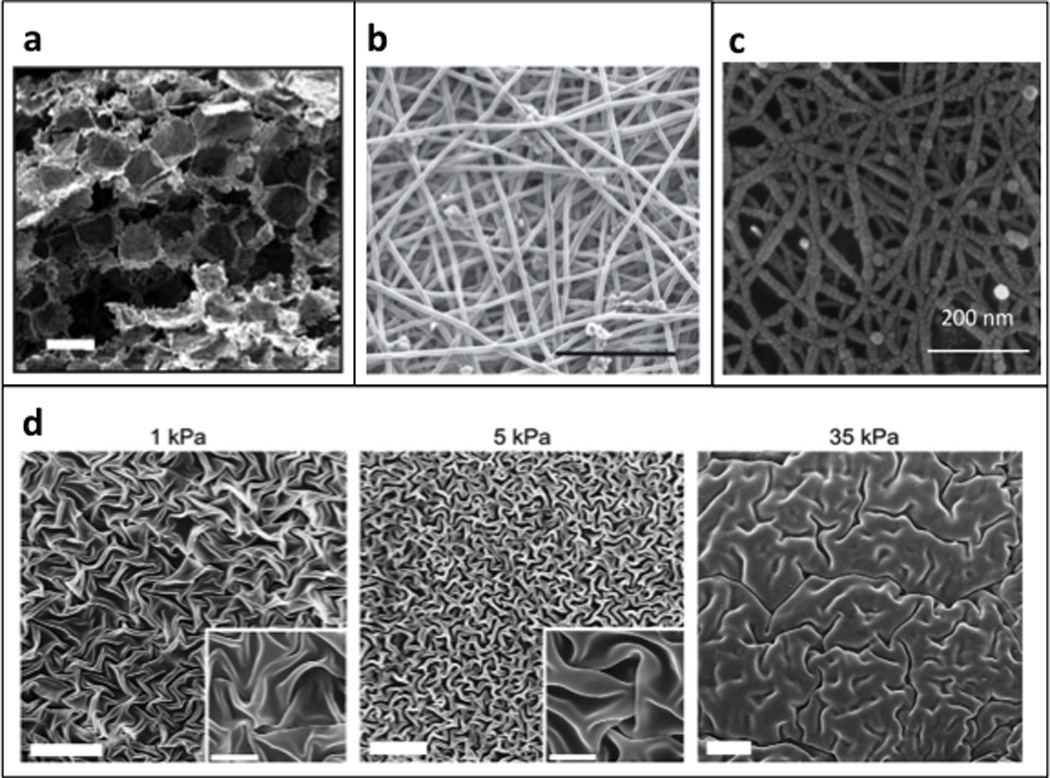
Scanning electron micrographs of (a) porous poly-lactic-glycolic scaffolds; reproduced with permission from [37] and (b) electrospun polycaprolactone scaffold; scale bar = 200 µm; reproduced with permission from [39]. (c) Scanning electron micrograph of a self-assembling peptide hydrogel; reproduced with permission from [49]. (d) Scanning electron micrograph of dehydrated hyaluronic acid gels; scale bar = 20 µm; inset scale bar = 5 µm; reproduced with permission from [45].
The Mooney group generated a 3D model of human oral cancer by culturing oral squamous cell carcinoma (OSCC-3) cells on synthetic poly(lactide-co-glycolide) (PLG) scaffolds with defined porosity. The angiogenic capacity, invasive potential, and chemosensitivty of OSCC-3 cells in 3D PLG culture were enhanced comparable to what is observed in vivo when compared with cells cultured in 2D and 3D Matrigel. Notably, the PLG-cultured cells exhibited more significant upregulation of angiogenic factor IL-8 expression than Matrigel-cultured cells, suggesting that PLG restores microenvironmental cues specific to the material rather than simple 3D architecture. Similar experiments were performed with additional tumor cell lines including MCF-7 and the U87 glioblastoma cell line, yielding similar results and establishing the wide applicability of PLG for tumor modeling [37].
Subsequent studies incorporating hydroxyapatite (HA), the main mineral component of bone, into the PLG scaffolds provided a bone-like microenvironment in which to study breast cancer metastasis [38]. Also, poly(ε-caprolactone) (PCL), another polymer popular in tissue engineering applications, was electrospun into a porous, fibrous scaffold and seeded with TC-71 Ewing’s sarcoma cells. This tumor model engineered by the Ludwig group exhibited increased chemoresistance and a distinct gene expression profile when compared with cells cultured in 2D [39].
An important feature of synthetic materials is the relative ease with which they can be functionalized in a controlled manner to reestablish bioactivity. Incorporating cell-binding sites (e.g. arginine-glycine-aspartate (RGD) peptide sequences) and MMP-degradable regions (e.g. GGGPQGIWGQGK (PQ) peptide) within the 3D architecture of scaffolds facilitates normal cell-matrix interactions such as matrix remodeling. For example, polyethylene glycol) (PEG) hydrogels functionalized with RGD and MMP-sensitive peptides were used to generate models of epithelial ovarian cancer (EOC) and lung adenocarcinoma by the Rizzi and West groups, respectively. The Rizzi group used the functionalized PEG matrices to culture OV-MZ-6 and SKOV-3 cells in biomimetic conditions, and observed distinct proliferation profiles, multicellular spheroid formation, and enhanced chemoresistance from cells cultured in 2D. Specific integrin activation was also implicated in EOC progression [40]. The West group encapsulated a murine metastatic lung adenocarcinoma cell line (344SQ) on comparable PEG hydrogels of varying stiffness to study the influence of cell-matrix interactions on microRNA-200 (miR-200)-dependent epithelial-mesenchymal transition (EMT) and metastasis. They concluded that matrix stiffness and the concentration of cell adhesion peptides significantly influenced the expression of miR-200 and EMT marker genes [41].
The Lin group developed a novel PEG-peptide hydrogel system comprised of 8-arm PEG-octa-norbornene (PEG8NB) units with integrated protease-sensitive or MMP-degradable regions (dithiothreitol (DTT), KCGPQGIWGQCK) using thiol-ene photoclick chemistry. A known epidermal growth factor receptor (EGFR) peptide inhibitor (NYQQNC) was also incorporated during fabrication to probe the effect of EGFR inhibiton on pancreatic ductal adenocarcinoma (PDAC) cells (Panc-1) encapsulated in scaffolds of varying stiffness. Epidermal growth factor (EGF) expression is a known hallmark of the epithelial-to-mesenchymal transition (EMT), and PDAC metastasis is characterized by EGFR overexpression. Interestingly, the expected apoptotic effects of EGFR inhibition were only observed in stiff matrices, indicating that anti-cancer drug efficacy can be governed by matrix properties [42].
On account of their highly tunable degradation profiles, several groups have also designed synthetic matrices for controlled drug or bioactive molecule delivery. Depending on the delivery profile desired, polymeric scaffolds can be tailored to produce a burst (fast-degrading) or controlled (slow-degrading) release. To investigate the role of the inflammatory response in melanoma cancer metastasis, the Tang group loaded poly(lactic-co-glycolic acid) (PLGA) scaffolds with chemokines (SDF-1α, erythropoietin) and implanted them in a murine model of metastasis. The combination of local inflammatory response and chemokine release resulted in the significantly higher accumulation of melanoma cells at the implant site, and approximately 30% extension in mouse lifespan. The authors postulated that the chemokine-releasing scaffolds acted as “traps” for circulating cancer cells that would have otherwise formed metastases and accelerated animal mortality [43].
Finally, the Mohapatra group developed 3D nanofibrous scaffold by electrospinning a mixture of PLGA, polylactic acid (PLA), and monomethoxypolyethylene glycol (mPEG). When seeded onto these scaffolds, the MCF-10A and MCF-7 breast cancer, PC3 prostate cancer, B16 melanoma, BG1 ovarian, and LLC1 Lewis lung cancer cells aggregated to form irregularly-shaped tumoroids and underwent EMT, upregulating vimentin expression and downregulating E-cadherin expression. As expected, the tumoroids exhibited increased chemoresistance and increased sensitivity to known EMT inhibitors when compared with monolayer culture controls, resembling some aspects of the in vivo tumor behavior. Notably, the authors demonstrated that the scaffold could be used to culture tumor biopsies (fine-needle aspirates of implanted mouse tumors) and evaluate host-specific drug sensitivity. LLC1 cells were injected into C57BL/6 mice and allowed to form subcutaneous tumors over a two-week period. Single-cell suspensions obtained from the implanted tumors formed tumoroids with higher drug resistance than LLC1 tumoroids, indicating that these scaffolds may be used as models of cancer [44].
Hybrid and Semi-Synthetic Biomaterials
To leverage the best features of both natural and synthetic materials, tissue engineers began developing semi-synthetic matrices by chemically modifying natural materials in order to engender the control over scaffold fabrication and design typically achieved only with synthetic materials. Alternatively, composite matrices with integrated natural and synthetic components were also explored to augment the bioactivity of synthetic materials.
One prominent semi-synthetic material used extensively for tissue engineering is the modified hyaluronic acid (HA). HA is a ubiquitous glycosaminoglycan found in both normal tissue and tumor ECM that has been implicated in tumor progression. Most commonly, HA chains are modified with acrylate or methacrylate to yield a crosslinkable hydrogel with tunable mechanical and degradation properties that can also be functionalized to enhance cell engagement. A study performed by the Kumar group established the applicability of HA gels in tumor modeling by engineering brain-mimetic hydrogel scaffolds of varying stiffness and RGD peptide density to recapitulate normal and tumorigenic brain ECM. U373-MG and U87-MG glioblastoma multiforme (GBM) cells were cultured as multicellular spheroids and embedded within functionalized HA gels, and compared with parallel cultures on HA-coated tissue culture plates and in 3D collagen hydrogels. GBM spheroids embedded in brain-mimetic scaffolds exhibited invasive capacity and morphological patterns similar to what is seen in human brain slices, in contrast to glioma cells in the 2D and 3D collagen contexts [45]. Similar studies carried out by the Harley group using U87-MG cells encapsulated in a gelatin and PEG-based hydrogels grafted with a HA hydrogel network produced corroborating results, while also identifying CD44 as a key driver of glioma malignancy [46].
Alternatively, the Zhang group reported chitosan-PCL polyblend nanofibrous mats made by electrospinning to use as a model of brain ECM and study the effect of nanotopography on GBM cell invasion. Aligned and unaligned nanofibers in the 200 nm - 1.1 µm diameter range were seeded with U87 MG cells. Cells cultured on aligned substrates were elongated and aligned with the orientation of the fibers, and upregulated expression of invasion-related markers when cultured on small-diameter (200 nm and 400 nm) aligned fibers. Importantly, GBM cells showed similar migration profiles to those observed in vivo. Conversely, cells cultured on randomly oriented fibers and aligned substrates with large diameter fibers exhibited less phenotypic changes. These highly controlled and reproducible fibrous substrates were validated for application to high-throughput testing of anti-invasion tumor therapies [47].
In a more elaborate system, the Jia group modeled the characteristic multi-drug resistance (MDR) of prostate cancer using the LNCaP prostate cancer cell line encapsulated in 3D HA hydrogels. A copolymer mixture of PEG and PCL was used to fabricate Doxorubicin (Dox)-loaded nanoparticles (d = 54 ± 1 nm), reflective of novel drug delivery methods currently under development. As expected, the 3D microenvironment upregulated expression of MDR proteins, enhancing chemoresistance to Dox applied freely in the culture medium. Interestingly, however, the Dox-loaded nanoparticles were able to bypass the resistive functions of the MDR proteins by homogenously permeating throughout the scaffold and getting taken up directly into the cytoplasm of LNCaP cells, and therefore increased cytotoxic effects of the drug. This study clearly demonstrated the broad applicability of synthetic and semi-synthetic materials as tumor scaffolds and drug delivery vehicles [48].
Finally, advances in peptide engineering technology have spawned a new class of nanofibrous hydrogels derived from self-assembling peptides. The Nguyen group synthesized h9e peptides (FLIVIGSIIGPGGDGPGGD), capable of hydrogelation at room temperature at concentrations as low as 1mM, for a 3D model of human breast cancer. Features particular to this fibrous scaffold (d = 20 nm) include its fast assembly (15 min, concentration-dependent), high deformability, and simple conversion back to liquid by shearing for cell isolation. MCF-7 cells were encapsulated in peptide gels of varying concentrations, and hence stiffness, within which they formed tumor-like clusters. Evaluation of cell morphology, proliferation, and apoptosis revealed that cell behavior in the hydrogels more closely approximated that of in vivo tumors when compared with 2D cultures [49]. Similarly, the Zhao group used commercially available RADA16-I peptides, capable of generating nanofibrous (d = 10 nm) hydrogels with pore size ranging from 5 to 200 nm, for a 3D tumor model of ovarian cancer (A2780, A2780/DDP, SK-OV-3) [50].
Microfabricated 3D Substrates
Advances in materials science have made microfabrication techniques invaluable to tissue engineers for designing substrates with highly detailed and reproducible features on the microscale, beyond the scope of what can be achieved using traditional scaffold fabrication techniques, regardless of material type. These same advantages have made microfabrication ideal for investigating the relationship between matrix properties (e.g. geometry, stiffness) and cancer cell behavior in a more controlled manner than what has been described previously. Microfabricated 3D substrates for cancer research are generated by a variety of photolithography and microlithography techniques (Figure 4).
Figure 4. Microfabricated 3D substrates.
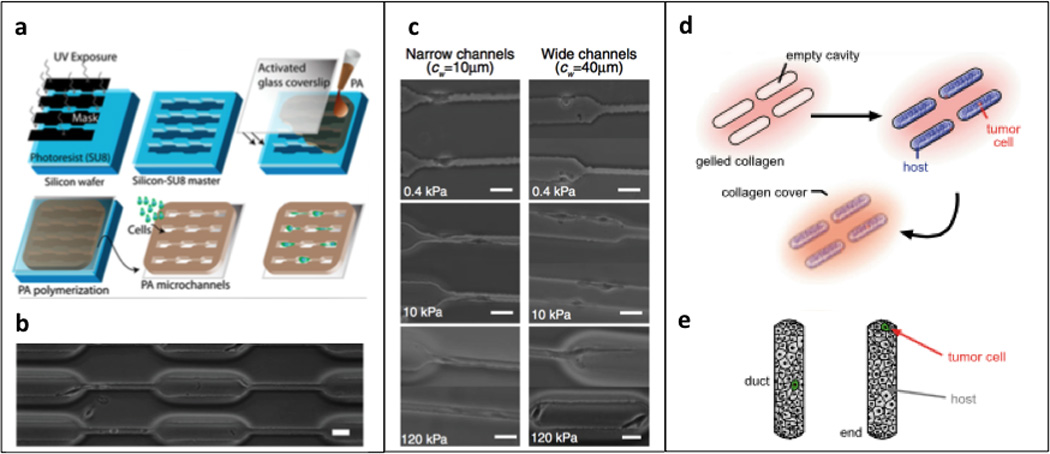
(a, b) Photolithography technique for generating polyacrylamide microchannels; reproduced with permission from [51]. (c) Phase contrast images of U373-MG human glioma cells migrating inside channels of varying stiffness and width, scale bar = 40 µm; reproduced with permission from [51]. (d) 3D microlithography approach to engineering mammary epithelial duct tissue; reproduced with permission from [53]. (e) schematic depicting “duct” versus “end” locations in epithelial host tissue; reproduced with permission from [53].
The Kumar group has made great strides in investigating the relationship between matrix stiffness, niche size and cancer cell behavior using PA substrates. A microchanneled polydimethylsiloxane (PDMS) master was generated using standard photolithography techniques to control the geometry of polymerized PA hydrogels of defined size and shape, while independently varying mechanical stiffness (0.4 – 120 kPa) and channel width (10–40 µm). The authors found that human glioma cells (U373-MG) migrated more quickly in narrow channels when compared with wide channels or unconstrained 2D surfaces, possibly due to increased polarization and cell-ECM traction forces, and this observed migration rate increased with an increase in matrix stiffness [51]. This platform represents a unique model for studying the regulation of cancer cell invasion by matrix confinement and stiffness.
In a recent study, the Moeinzadeh group encapsulated human MCF-7 and MDA-MB-231 breast cancer cells, HCT-116 colorectal carcinoma cells, AGS gastric carcinoma cells, and U2OS osteosarcoma cells in micropatterned polyethylene glycol) diacrylate (PEGDA) gels of varying compressive moduli (2 – 70 kPa), and compared them against non-tumorigenic epithelial cells (CRL-10317). The authors sought to investigate matrix regulation of cancer stem cell (CSC) phenotype, a subpopulation of cells with elevated anticancer drug resistance believed to promote tumor recurrence. An acrylamide-terminated CD44 binding peptide (involved in cell-cell adhesion of CSCs) was synthetized and patterned into the PEGDA hydrogels to control the size of cell spheroid formation (50 – 250 µm). The authors found that cells encapsulated in PEGDA hydrogels exhibited CSC-like phenotypic markers most significantly when encapsulated in hydrogels of compressive moduli equivalent to their native tissues. Furthermore, this phenotype was most pronounced in all cell lines at the optimum niche size of 50 µm, in the corresponding optimum stiffness gel [52].
The Nelson group used 3D microlithography to examine how breast cancer cell invasiveness was affected by host epithelial geometry. Briefly, a normal human epithelial mammary duct was engineered using a PDMS stamp coated with a BSA and collagen type I solution. Following gelation of the BSA and collagen I onto a glass coverslip, normal human epithelial cells (D920) in suspension were seeded over the stamped regions, with the addition of a single invasive (SCg6, 4T1, MDA-MB-231, Hs578T) or non-invasive (SKBR3, MDA-MB-361, MDA-MB-453) breast cancer cell at either the “end” or “duct” region of the engineered epithelial tissue. Using this system, the authors were able to conclude that cancer cell phenotype was dictated by the initial position within the engineered duct, with proliferation and invasion being enhanced only at the “end” regions, and inhibited elsewhere. These “end” regions were characterized by high mechanical stress, suggesting that the mechanical tone of normal host epithelium can regulate cancer cell phenotype [53].
Engineered Tissues as Substrates
Tissue engineering methods continue to shape cancer research, with an emphasis on mimicking the tumor microenvironment with increasing fidelity. An excellent example is the work reported by the Hutmacher group. An engineered bone construct composed of human osteoblasts isolated from explanted human bone and seeded onto a PCL-tricalcium phosphate (PCL-TCP) scaffold was co-cultured with PC3 and LNCaP human prostate cancer cells. The physiological relevance of this model for understanding mechanisms of bone metastasis was made clear when intercellular and cell-engineered bone interactions reflected data from in vivo tumor studies [54].
More recently, the Vunjak-Novakovic group devised an elegant model of human Ewing’s sarcoma within a bone niche using natural materials. Human mesenchymal stem cells (hMSCs) were seeded onto decellularized bovine bone matrices and cultured in osteogenic medium for four weeks. Tumor spheroids derived from human Ewing’s sarcoma cancer cell lines (RD-ES, SK-N-MC) were then infused into the engineered bone and cultured for an additional two to four weeks. Analysis of gene expression profiles with an emphasis on hypoxic and glycolytic phenotypes demonstrated that the tissue-engineered Ewing’s sarcoma model more closely recapitulated native tumor profiles than their monolayer counterparts. The same conclusions were drawn following evaluation of the angiogenic ability, vasculogenic mimicry, and apoptotic behavior of the tissue-engineered model when compared with monolayer cultures [55].
Concluding remarks
As the dominant role of the tumor microenvironment in the initiation, progression, metastasis, and therapeutic resistance of cancer becomes increasingly clear, cancer biologists continue to find that custom biomaterials provide a 3D niche for in vitro tumor modeling that is superior to traditional monolayer tissue culture models. Tissue-engineered tumors recapitulate the behavior of native tumors more reliably than simple cell culture settings, and are of increasing clinical relevance, with applicability to disease modeling and pharmaceutical testing.
It is clear that the field of cancer research is advancing towards more complex, heterotypic culture systems, and with this shift the techniques and conceptual paradigms that are fundamental to tissue regeneration are being adopted and adapted for tumor modeling.
Accordingly, it the challenges associated with regenerating complex, heterogeneous tissues will also need to be addressed within the context of cancer biology. First and foremost, while the studies noted in this review clearly demonstrate the utility of biomaterials and engineering techniques in tumor modeling, the materials themselves – due to their potentially cytotoxic fabrication, limited material properties, and variable bioactivity - will dictate how clinically relevant and patient-specific these models can become.
Notably, such concerns have generated great interest in the applicability of cutting-edge 3D printing technologies to biological systems, with promising early results. Advanced fabrication methods including stereolithography and laser-based direct-write techniques, bioplotting or inkjet bioprinting, and fused deposition modeling promise exceptional control over 3D micro-and nano-architectures as well as the material properties of the scaffold, while retaining the capacity to incorporate bioactive molecules. Furthermore, the ability to print cells in controlled spatial arrangements, in multi-material composites and via computer-assisted fabrication, ensures a level of precision and reproducibility previously not achievable [56,57].
The introduction of multiple cell types to recapitulate the tumor stroma, vasculature, and immune response in 3D models is expected to reveal some of the key insights into cancer progression and metastasis that are otherwise missed in simple cell culture settings. Similarly, incorporation of external stimuli (e.g. mechanical compression, fluid shear) using novel bioreactor designs will allow for more faithful recapitulation of native tumor microenvironments. Indeed, preliminary studies have already shown that the integration of supporting cells and external stimuli enhances the native-like characteristics of engineered tumors [58,59]. While the relationship between the complexity of the tumor model and its ultimate clinical relevance has yet to be defined, it is evident that the fields of tissue engineering and cancer biology will continue to converge.
Highlights.
The tumor microenvironment regulates tumor initiation, progression, and metastasis.
We summarize the use of biomaterials in tumor modeling in vitro.
Engineered tumor models are becoming increasingly complex and heterotypic.
Insights into drug resistance may inform the development of novel therapeutic targets.
Acknowledgments
The authors gratefully acknowledge funding support by the National Institutes of Health (grants EB002520 and UH3EB017103).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Xu X, Farach-Carson MC, Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol Adv. 2014;32:1256–1268. doi: 10.1016/j.biotechadv.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38–54. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Z-D, Ji X-Y, Qazi H, Tarbell JM. Interstitial flow promotes vascular fibroblast, myofibroblast, and smooth muscle cell motility in 3D collagen I via upregulation of MMP-1. Am J Physiol Heart Circ Physiol. 2009;297:H1225–H1234. doi: 10.1152/ajpheart.00369.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields J, Emmett M, Dunn D, Joory K, Sage L, Rigby H, Mortimer P, Orlando A, Levick J, Bates D. Chemokine-mediated migration of melanoma cells towards lymphatics - a mechanism contributing to metastasis. Oncogene. 2007;26:2997–3005. doi: 10.1038/sj.onc.1210114. [DOI] [PubMed] [Google Scholar]
- 8.Martin GS. Rous sarcoma virus: A function required for the maintenance of the transformed state. Nature. 1970;227:1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- 9.Dolberg D, Bissell MJ. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309:552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- 10.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat. 2012;15:39–49. doi: 10.1016/j.drup.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Nefedova Y, Landowski T, Dalton W. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17:1175–1182. doi: 10.1038/sj.leu.2402924. [DOI] [PubMed] [Google Scholar]
- 13.Korah R, Boots M, Wieder R. Integrin α5β1 promotes survival of growth-arrested breast cancer cells: An in vitro paradigm for breast cancer dormancy in bone marrow. Cancer Res. 2004;64:4514–4522. doi: 10.1158/0008-5472.CAN-03-3853. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Lu Y, Parant JM, Lozano G, Bacher G, Beckers T, Fan Z. Differential roles of p21(Waf1) and p27(Kip1) in modulating chemosensitivity and their possible application in drug discovery studies. Mol Pharmacol. 2001;60:900–906. doi: 10.1124/mol.60.5.900. [DOI] [PubMed] [Google Scholar]
- 15.Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VH, Groom AC, Chambers AF. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res Treat. 2003;82:199–206. doi: 10.1023/B:BREA.0000004377.12288.3c. [DOI] [PubMed] [Google Scholar]
- 16.Goodison S, Kawai K, Hihara J, Jiang P, Yang M, Urquidi V, Hoffman RM, Tarin D. Prolonged dormancy and site-specific growth potential of cancer cells spontaneously disseminated from nonmetastatic breast tumors as revealed by labeling with green fluorescent protein. Clin Cancer Res. 2003;9:3808–3814. [PubMed] [Google Scholar]
- 17.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, Bissell MJ. B1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. Beta4 integrin-dependent formation of polarized three- dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Peterson OW, Turley EA, Bissell MJ. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price KJ, Tsykin A, Giles KM, Sladic RT, Epis MR, Ganss R, Goodall GJ, Leedman PJ. Matrigel basement membrane matrix influences expression of microRNAs in cancer cell lines. Biochem Biophys Res Commun. 2012;427:343–348. doi: 10.1016/j.bbrc.2012.09.059. [DOI] [PubMed] [Google Scholar]
- 21.Dunne LW, Huang Z, Meng W, Fan X, Zhang N, Zhang Q, An Z. Human decellularized adipose tissue scaffold as a model for breast cancer cell growth and drug treatments. Biomaterials. 2014;35:4940–4949. doi: 10.1016/j.biomaterials.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Mishra DK, Thrall MJ, Baird BN, ott HC, Blackmon SH, Kurie JM, Kim MP. Human lung cancer cells grown on acellular rat lung matrix create perfusable tumor nodules. Ann Thorac Surg. 2012;93:1075–1081. doi: 10.1016/j.athoracsur.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stratmann AT, Fecher D, Wangorsch G, Gottlich C, Walles T, Walles H, Dandekar T, Dandekar G, Nietzer SL. Establishment of a human 3D lung cancer model based on a biological tissue matrix combined with a boolean in silico model. Mol Oncol. 2014;8:351–365. doi: 10.1016/j.molonc.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichert JC, Quent VM, Burke LJ, Stansfield SH, Clements JA, Hutmacher DW. Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment. Biomaterials. 2010;31:7928–7936. doi: 10.1016/j.biomaterials.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 25.Lee H-O, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, Cheng JD. Fap-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer. 2011;11:245. doi: 10.1186/1471-2407-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoshiba T, Tanaka M. Breast cancer cell behaviors on staged tumorigenesis-mimicking matrices derived from tumor cells at various malignant stages. Biochem Biophys Res Commun. 2013;439:291–296. doi: 10.1016/j.bbrc.2013.08.038. Using human breast cancer cell lines of increasing malignancy and invasiveness, staged tumorigenesis-mimicking matrices were generated to demonstrate the effect of increasingly pathological tumor microenvironments on cancer cell attachment, proliferation, and chemoresistance. While cancer cell attachment decreased and proliferation increased with increasingly malignant matrices, chemoresistance was variable suggesting a more complex mechanism of drug resistance.
- 27.Chen L, Xiao Z, Meng Y, Zhao Y, Han J, Su G, Chen B, Dai J. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials. 2012;33:1437–1444. doi: 10.1016/j.biomaterials.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 28. Rodenhizer D, Gaude E, Cojocari D, Mahadevan R, Frezza C, Wouters BG, McGuigan AP. A three-dimensional engineered tumour for spatial snapshot analysis of cell metabolism and phenotype in hypoxic gradients. Epub ahead of print. 2015 Nov;23 doi: 10.1038/nmat4515. Nat Mater A layered 3D engineered tumor was generated from porous cellulose strips, infiltrated with a cancer cell and collagen type I solution, and rolled around an impermeable metallic core. This model recapitulated the length scales over which oxygen and nutrient gradients occur in vivo and could be rapidly disassembled to study cell metabolism in hypoxic conditions, in spatially defined regions of the tumor.
- 29.Szot CS, Buchana CF, Freeman JW, Rylander MN. In vitro angiogenesis induced by tumor-endothelial cell co-culture in bilayered, collagen I hydrogel bioengineered tumors. Tissue Eng Part C Methods. 2013;19:864–874. doi: 10.1089/ten.tec.2012.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menon S, Beningo KA. Cancer cell invasion is enhanced by applied mechanical stimulation. PLoS One. 2011;6:e17277. doi: 10.1371/journal.pone.0017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Tan Y, Zhang H, Zhang Y, Xu P, Chen J, Poh Y-C, Tang K, Wang N, Huang B. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat Mater. 2012;11:734–741. doi: 10.1038/nmat3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Prot Natl Acad Sci. 2009;106:399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talukdar S, Kundu SC. A non-mulberry silk fibroin protein based 3d in vitro tumor model for evaluation of anticancer drug activity. Adv Funct Mater. 2012;22:4778–4788. [Google Scholar]
- 34.Verbridge SS, Choi NW, Zheng Y, Brooks DJ, Stroock AD, Fischbach C. Oxygen-controlled three-dimensional cultures to analyze tumor angiogenesis. Tissue Eng Part A. 2010;16:2133–2141. doi: 10.1089/ten.tea.2009.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kievit FM, Florczyk SJ, Leung MC, Wang K, Wu JD, Silber JR, Ellenbogen RG, Lee JSH, Zhang M. Proliferation and enrichment of CD133+ glioblastoma cancer stem cells on 3D chitosan-alginate scaffolds. Biomaterials. 2014;35:9137–9143. doi: 10.1016/j.biomaterials.2014.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phan-Lai V, Florczyk SJ, Kievit FM, Wang K, Gad E, Disi ML, Zhang M. Three-dimensional scaffolds to evaluate tumor associated fibroblast-mediated suppression of breast tumor specific T cells. Biomacromolecules. 2013;14:1330–7. doi: 10.1021/bm301928u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–860. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 38.Pathi SP, Kowalczewski C, Tadipatri R, Fischbach C. A novel 3D mineralized tumor model to study breast cancer bone metastasis. PLoS One. 2010;5:e8849. doi: 10.1371/journal.pone.0008849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fong ELS, Lamhamedi-Cherradi S-E, Burdett E, Ramamoorthy V, Lazar AJ, Kasper FK, Farach-Carson MC, Vishwamitra D, Demicco EG, Ludwig JA. Modeling Ewing’s sarcoma tumors in vitro with 3D scaffolds. Proc Natl Acad Sci USA. 2013;110:6500–6505. doi: 10.1073/pnas.1221403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 2010;31:8494–8506. doi: 10.1016/j.biomaterials.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 41.Gill BJ, Gibbons DL, Roudsari LC, Saik JE, Rizvi ZH, Roybal JD, Kurie JM, West JL. A synthetic matrix with independently tunable biochemistry and mechanical properties to study epithelial morphogenesis and EMT in a lung adenocarcinoma model. Cancer Res. 2012;72:6013–6023. doi: 10.1158/0008-5472.CAN-12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ki CS, Shih H, Lin C-C. Effect of 3D matrix compositions on the efficacy of EGFR inhibition in pancreatic ductal adenocarcinoma cells. Biomacromolecules. 2015;14:3017–3026. doi: 10.1021/bm4004496. [DOI] [PubMed] [Google Scholar]
- 43.Ko C-Y, Wu L, Nair AM, Tsai Y-T, Lin VK, Tang L. The use of chemokine-releasing tissue engineering scaffolds in a model of inflammatory response-mediated melanoma cancer metastasis. Biomaterials. 2012;33:876–885. doi: 10.1016/j.biomaterials.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Girard YK, Wang C, Ravi S, Howell MC, Mallela J, Alibrahim M, Green R, Hellermann G, Mohapatra SS, Mohapatra S. A 3D fibrous scaffold inducing tumoroids: A platform for anticancer drug development. PLoS One. 2013;8:e75345. doi: 10.1371/journal.pone.0075345. 3D nanofibrous scaffolds electrospun from a mixture of PLGA, PLA, and mPEG were seeded with breast, prostate, melanoma, ovarian, and lung cancer cell lines to form tumoroids undergoing EMT. The tumoroids exhibited increased anticancer drug resistance and sensitivity to EMT inhibitors. Furthermore, cells obtained from fine-needle aspirates of implanted tumors were seeded onto the scaffolds and displayed higher drug resistance when compared with in vitro tumoroids, suggesting host-specific drug sensitivities can be recapitulated on this culture platform.
- 45. Ananthanarayanan B, Kim Y, Kumar S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials. 2011;32:7913–7923. doi: 10.1016/j.biomaterials.2011.07.005. Brain-mimetic hydrogels of varying stiffness were engineered from modified HA and functionalized to enhance cell attachment to serve as models of normal and tumorigenic brain ECM. Glioblastoma multiforme cell spheroids exhibited an invasive capacity and morphological patterns more similar to what is observed in human brain slices when encapsulated within the brain-mimetic scaffolds, as compared with cells cultured on 2D and 3D collagen.
- 46.Pedron S, Becka E, Harley BA. Regulation of glioma cell phenotype in 3D matrices by hyaluronic acid. Biomaterials. 2013;34:7408–7417. doi: 10.1016/j.biomaterials.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 47.Kievit FM, Cooper A, Jana S, Leung MC, Wang K, Edmondson D, Wood D, Lee JSH, Ellenbogen RG, Zhang M. Aligned chitosan-polycaprolactone polyblend nanofibers promote the migration of glioblastoma cells. Adv Healthc Mater. 2013;2:1651–1659. doi: 10.1002/adhm.201300092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu X, Sabanayagam CR, Harrington DA, Farach-Carson MC, Jia X. A hydrogel-based tumor model for the evaluation of nanoparticle-based cancer therapeutics. Biomaterials. 2014;35:3319–3330. doi: 10.1016/j.biomaterials.2013.12.080. Prostate cancer cells encapsulated in HA hydrogels were used to investigate the efficacy of Dox-loaded PEG -PCL nanoparticles as a drug delivery system. The 3D HA hydrogels upregulated the expression of MDR proteins in the tumor models, but were more effectively penetrated and treated by Dox-loaded nanoparticles when compared with Dox applied freely in the culture medium.
- 49.Huang H, Ding Y, Sun XS, Nguyen TA. Peptide hydrogelation and cell encapsulation for 3D culture of MCF-7 breast cancer cells. PLoS One. 2013;8:e59482. doi: 10.1371/journal.pone.0059482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Z, Zhao X. A 3D model of ovarian cancer cell lines on peptide nanofiber scaffold to explore the cell-scaffold interaction and chemotherapeutic resistance of anticancer drugs. Int J Nanomedicine. 2011;2011:303–310. doi: 10.2147/IJN.S15279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci USA. 2012;109:10334–10339. doi: 10.1073/pnas.1118073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jabbari E, Sarvestani SK, Daneshian L, Moeinzadeh S. Optimum 3D matrix stiffness for maintenance of cancer stem cells is dependent on tissue origin of cancer cells. PLoS One. 2015;10:e0132377. doi: 10.1371/journal.pone.0132377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boghaert E, Gleghorn JP, Lee K, Gjorevski N, Radisky DC, Nelson CM. Host epithelial geometry regulates breast cancer cell invasiveness. Proc Natl Acad Sci USA. 2012;109:19632–19637. doi: 10.1073/pnas.1118872109. 3D microlithography techniques were used to engineer a mammary duct with a collagen type I matrix. Normal human epithelial cells seeded onto the engineered ducts with a single breast cancer cell modeled the effect of host epithelial geometry on cancer cell phenotype, demonstrating that regions of high mechanical stress enhanced cancer cell proliferation and invasion.
- 54.Sieh S, Lubik AA, Clements JA, Nelson CC, Hutmacher DW. Interactions between human osteoblasts and prostate cancer cells in a novel 3D in vitro model. Organogenesis. 2010;6:181–188. doi: 10.4161/org.6.3.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Villasante A, Marturano-Kruik A, Vunjak-Novakovic G. Bioengineered human tumor within a bone niche. Biomaterials. 2014;35:5785–5794. doi: 10.1016/j.biomaterials.2014.03.081. Engineered bone derived from hMSCs cultured on decellularized bovine bone matrices were used to study human Ewing’s sarcoma within its natural bone niche. The model generated more closely recapitulated native tumor profiles than their typical monolayer counterparts with respect to angiogenic ability, vasculogenic mimicry, and apoptotic behavior.
- 56.O’Brien CM, Holmes B, Faucett S, Zhang LG. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng Part B. 2015;21:103–114. doi: 10.1089/ten.teb.2014.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koch L, Gruene M, Unger C, Chichkov B. Laser assisted cell printing. Curr Pharm Biotechnol. 2013;14:91–97. [PubMed] [Google Scholar]
- 58.Chwalek K, Bray LJ, Werner C. Tissue-engineered 3D tumor angiogenesis models: Potential technologies for anti-cancer drug discovery. Adv Drug Deliv Rev. 2014;79–80:30–39. doi: 10.1016/j.addr.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Yates C, Shepard CR, Papworth G, Dash A, Beer Stolz D, Tannenbaum S, Griffith L, Wells A. Novel three-dimensional organotypic liver bioreactor to directly visualize early events in metastatic progression. Adv Cancer Res. 2007;97:225–246. doi: 10.1016/S0065-230X(06)97010-9. [DOI] [PubMed] [Google Scholar]


