Abstract
Nanosecond electrical pulse (nsEP) exposure activates signaling pathways, produces oxidative stress, stimulates hormone secretion, causes cell swelling and induces apoptotic and necrotic death. The underlying biophysical connection(s) between these diverse cellular reactions and nsEP has yet to be elucidated. Using global genetic analysis, we evaluated how two commonly studied cell types, U937 and Jurkat, respond to nsEP exposure. We hypothesized that by studying the genetic response of the cells following exposure, we would gain direct insight into the stresses experienced by the cell and in turn better understand the biophysical interaction taking place during the exposure. Using Ingenuity Systems software, we found genes associated with cell growth, movement and development to be significantly up-regulated in both cell types 4 h post exposure to nsEP. In agreement with our hypothesis, we also found that both cell lines exhibit significant biological changes consistent with mechanical stress induction. These results advance nsEP research by providing strong evidence that the interaction of nsEPs with cells involves mechanical stress.
Introduction
Cell exposure to high intensity millisecond and microsecond electrical pulses (electroporation) is theorized to cause the formation of membrane pores. These “electro-pores” allow for the transfer of genetic and proteomic material, drugs and chemicals into a cell, for the purpose of inducing a biochemical change [1–6]. Thus, electroporation is a very useful tool for molecular biological research and as such is widely used in many laboratories. Despite the widespread use of electroporation, very little is known how pulsed electric fields in general, affect the molecular processes of cells, especially those associated with gene expression. Our laboratory studies a specific type of electroporation that utilizes nanosecond duration pulses (referred to hereafter as nanosecond electrical pulses or nsEP). The nsEP induced events include swelling [7,8], blebbing [7,8], phospholipid translocation [9,10], prolonged membrane permeablization (nanoporation) [11–13], apoptosis [7,14–17], and necrosis [7,14]. Despite this wealth of evidence, much remains unknown about how a cell reacts genetically to nsEP-delivered stress.
Events associated with nsEP exposure that can cause changes in gene expression have been identified. Using high speed imaging, Beier et al. observed a rapid increase in intracellular calcium originating from membrane regions closest to the electrodes, illustrating a unique directionality to the nsEP response [18]. In agreement with previous studies, they suggested that the rapid increase in intracellular calcium was likely due to several mechanisms, including the formation of nanopores, intracellular calcium release from internal calcium stores such as endoplasmic or sarcoplasmic reticulum, and possible activation of voltage-gated or unspecific cation ion channels [18,19]. One possibility is that calcium enters the cell via mechanically activated channels or through the pore forming subunits of the piezo proteins found in cell membranes. Supporting this hypothesis, work done by Tolstykh et al. has conclusively shown that nsEP exposure activates the intracellular phosphoinositide signaling pathway [20–22], a well-known regulator of mechanically stimulated channel (MSC) activity and IP3 dependent intracellular calcium release [23–25]. The production of reactive oxygen species has also been observed to occur during nsEP exposures, although the connection to the other cellular effects of nsEPs is unknown at this time [26]. Nevertheless, based on these observations, it is clear that cells exposed to nsEP experience an intense stress that would lead to changes in gene expression.
To better characterize and understand this stress, and hopefully shed light on the biophysical mechanisms responsible for nanoporation, we performed a microarray analysis of both U937 and Jurkat cells exposed to 100 nsEPs at a duration of 10 ns and an electric field of 150kV/cm. Real-time quantitative PCR and luminex multiplexing assays were used to confirm the microarray data. The genomics and proteomic data presented in this paper provide the genetic evidence necessary to characterize the nature of the stress endured by both cells types when exposed to nsEP. This is the first time global genetic analysis has been applied to the cells exposed to nsEP.
Materials and Methods
Exposure System
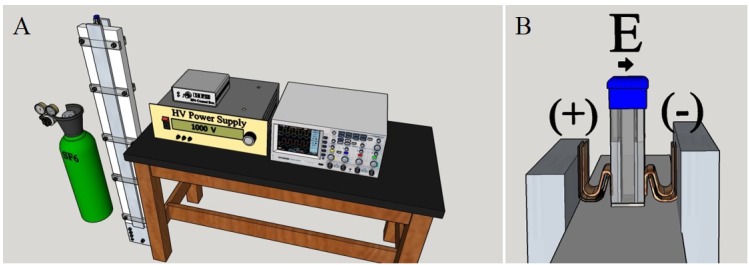
The 10-ns exposure system used in this study has been previously described in great detail [27,28]. In short, a custom pulser was constructed by investigators at Old Dominion University, consisting of a spark gap containing pressurized sulfur hexafluoride (SF6) (Fig 1). This chamber is charged using a high voltage power supply to breakdown the gas within the spark gap generating a pulse. At a constant pressure, increasing the voltage applied to the gap increases the repetition frequency of the pulses, while changing the gas pressure impacts the breakdown voltage resulting in a variable amplitude pulse. A custom controller box was designed that contains a computer-controlled pressure regulator to supply the pulser with SF6 at a controlled pressure. In addition, this box communicates with a power supply and a high speed oscilloscope to initiate pulse generation and count the pulses delivered to the exposure cuvette. This system is controlled using a LabVIEW (National Instruments, Austin, Texas) program that sets the exposure parameters and records the pulse amplitude.
Fig 1. Blumlein line cuvette-based, 10 ns pulser apparatus.
A) Drawing of the complete 10-ns set up, including the Tektronix ocilloscope, Glassman high voltage power supply and custom contol module for regulating the pressure of SF6 in the spark gap chamber. B) This is an enhanced view of the cuvette and its placement/orientation in regards to the pulser.
Cell Culture and Exposure
Both Jurkat (ATCC-TIB-152) and U937 (ATCC-CRL-1593.2) cells were acquired from ATCC (Manassas, VA) and sub-cultured according to supplier’s protocol. All cells were maintained at 37°C/5% CO2/95% humidity. Cells were grown and exposed in complete growth medium (ATCC, RPMI-1640 supplemented with 10% FBS, 1% Penicillin/Streptamycin). All cells were counted using the Countess® Cell Counter from Life Technologies (Grand Island, NY) and the final concentration was adjusted to 1200 cells/μL. The cells were then aliquoted into electroporation cuvettes with a 1mm gap between plates (150 μL volume). The cuvettes were exposed to either 100, 10 ns electrical pulses at 150kV/cm or they were sham exposed. nsEP and sham exposures occurred in a random fashion. The sham control samples were treated identically to the nsEP exposed samples except, when they were placed on the pulser, zero power was applied. Following either sham or nsEP exposure, the cells were transferred into a well plate in triplicate and incubated in the appropriate cell culture conditions for the allotted time necessary for each assay. In order to induce heat shock stress, cells were placed in identical electroporation cuvettes and incubated in a circulating water bath at 44°C for 40 minutes [29].
Viability Assay (MTT)
Cellular viability was evaluated 0.5, 4 and 24 h post-exposure using MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assays, as per manufacturer’s instructions (ATCC). In brief, cells were exposed to nsEP and incubated at 37°C/5% CO2/95% humidity for a predetermined amount of time. 10 μL of MTT reagent was then added to each well and incubated for 2 h. After incubation, 100 μL of detergent was added to each well, the plate was covered in foil, placed on an orbital shaker at 100 rpm and incubated at room temperature overnight. The absorbance was measured at 570 nm with a Synergy HT Plate Reader (BioTek, Winooski, VT). A ladder of serial dilutions of cells (103 to 106 cells) in culture medium was prepared and absorbance measurements were conducted. The absorbance values were plotted versus the cell number and curves were generated and used to determine the number of viable cells in each well. A two-tailed unpaired t-test was performed using GraphPad Prism (GraphPad Software, Inc, La Jolla, CA).
Flow Cytometry Analysis
Cells were prepared and exposed identically as in cell viability experiments (section 2.3). FITC-Annexin V and propidium iodide were added to each sample at 10μL/mL and 2μL/mL respectively. Both reagents were added to the cells within 5 minutes of nsEP exposure and then were allowed to incubate in the exposure media at room temperature (26°C) for 10 minutes to insure proper fluorescent staining. The effects of 10-ns exposures were analyzed using an Accuri Flow Cytometer from BD Biosciences (San Jose, California). A total volume of 75 μL of media was analyzed resulting in typical cellular counts of ~40,000 cells. Cellular expression was measured for each individual channel using sham exposure and digitonin (0.4%) exposures as positive and negative controls. A single threshold was determined and percent of cells expressing each dye was measured. A two-tailed unpaired t-test was performed using GraphPad Prism. Digitonin (Sigma-Aldrich, St. Louis, MO), a non-ionic detergent that is routinely used to permeablize cells, was used as a positive control in this assay.
RNA Isolation
Total RNA was isolated from exposed cells and harvested 4 h after exposure. RNA was isolated with the Qiagen RNeasy Mini Kit and subjected to DNase digestion by the Qiagen RNase-free DNase Kit (QIAGEN Inc. Valencia, CA). RNA quantity was assessed by UV spectrometry at 260 nm / 280 nm absorbance on a NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, DE). RNA quality was assessed on a Agilent Bioanalyzer using the Agilent RNA Nano Chips (Agilent Technologies, Waldbronn Germany).
Microarray
Gene expression analysis was performed in triplicate (three sham, three nsEP exposed and three heat-shock treated) using the Affymetrix GeneChip® Human Genome U133 (HG-U133) plus 2.0 Array that contains 54,675 probe sets. Briefly, two micrograms of RNA were used for preparation of biotin-labeled targets (cRNA) using MessageAmp™-based protocols (Ambion, Inc., Austin, TX). Labeled cRNA was fragmented (0.5 μg/μL per reaction) and used for array hybridization and washing. The cRNA was mixed with a hybridization cocktail, heated to 99°C for 5 min and then incubated at 45°C for 5 min. Hybridization arrays were conducted for 16 h in an Affymetrix Model 640 hybridization oven (45°C, and 60 rpm). Arrays were washed and stained on an FS450 Fluidics station and were scanned on a GeneChip® Scanner 3000 7G. Image signal data, detection calls and annotations were generated for every gene using the Affymetrix Statistical Algorithm MAS 5.0 (GeneChip® Operating Software v1.3). A log2 transformation was conducted and a Student’s t-test was performed for comparison of the nsEP exposed samples to the two control groups (sham and heat-shocked). We conducted multiple testing correction—Benjamini and Hochberg—to determine the false discovery rate, and statistically significant genes were identified using Bonferroni correction procedures.
Microarray Data Analysis
For interpretation of the results, the Ingenuity Pathways Analysis tool (IPA version 8.7, Ingenuity® Systems Inc., Redwood City, CA) was used. IPA is a web-based software application, which enables filtering and dataset comparisons, to identify biological mechanisms, pathways and functions most relevant to experimental datasets or differentially expressed genes. The cut-off criteria for our IPA analysis were: an absolute value of log ratio ≥2 or ≤-2 and a p-value ≤0.05. Other web-based resources, such as the GeneCards® Human Gene Database, the HUGO Gene Nomenclature Committee (HGNC,) and the Gene Ontology Consortium were also used to further supplement the analysis.
Quantitative Real Time Polymerase Chain Reaction
Each gene selected for validation was validated by quantitative real time polymerase chain reaction (qRT-PCR) using the Applied Biosystems StepOne™ Plus PCR system from ThermoFisher Scientific (Carlsbad, CA). Pre-made, validated TaqMan® Gene Expression Assays were selected for each gene to be validated (ThermoFisher Scientific). Samples were run in triplicate with all reagents from ThermoFisher Scientific including the TaqMan® One-Step RT-PCR Master Mix. Relative quantification (RQ) values were computed using the StepOne™ Plus software.
Protein Isolation and Quantification
Cells were exposed to 10-ns pulse trains, then seeded into three different 12-well plates, and were allowed to incubate for up to twelve hours. One well plate of cells was processed at each time point: 4, 8 and 12 hours post-exposure. The harvested cells were aliquotted in triplicate into microcentrifuge tubes and placed on ice. The tubes were centrifuged at 1,400 rpm for five minutes to pellet the cells. The supernatant fluid was removed and this process was repeated one time using ice-cold PBS to ensure all foreign matter was removed before the cells were lysed by adding Complete Cell Extraction buffer from ThermoFisher Scientific (1% Triton X-100, 20 mM Tris-HCL, pH 7.4, 100 mM NaCl, 0.1 M EDTA, 0.2% SDS, 0.2 mM PMSF and 0.1 mM Leupepsin). The cell lysates were vortexed for two minutes. The lysate was clarified by centrifugation at 10,000 × g for 20 minutes at 4°C. The supernatant was collected and analyzed for protein content using a bicinchoninic acid (BCA) protein assay kit (Pierce™, Rockford, IL) and the analysis of the individual proteins was carried out via Luminex’s bead-based multiplexing immunoassay (EMD Millipore, Billerica, MA) following the manufacturer’s protocol. Briefly, the cell lysates were diluted 1:1 in assay buffer and 5 μg of total protein (25 μl/well) was loaded into the 96-well immunoassay plate along with lysate from positive controls (unstimulated HepG2 cells). Antibody-immobilized beads were added to each well and incubated for 2 h at room temperature (RT) followed by a 1 h incubation with detection antibodies. A streptavidin substrate was then added to each well and incubated for 30 minutes at RT after which the plate was run on a Luminex 200TM. The MILLIPLEX® multiplex detection assay is a rapid alternative to Western blotting and immunoprecipitation. Assays such as this, have the capacity for multiple, conjugated beads to be added to each sample resulting in the ability to obtain multiplexed results from every sample. Two different MILLIPLEX® multiplex detection assay were used, one testing proteins identified in the MAPK/SAPK pathway (MILLIPLEX MAP MAPK/SAPK Signaling 10-Plex Kit—Cell Signaling Multiplex Assay) and the other identifying proteins connected to the oxidative stress response pathway (MILLIPLEX MAP Human Oxidative Stress Magnetic Bead Panel—Cellular Metabolism Multiplex Assay).
Results
Viability
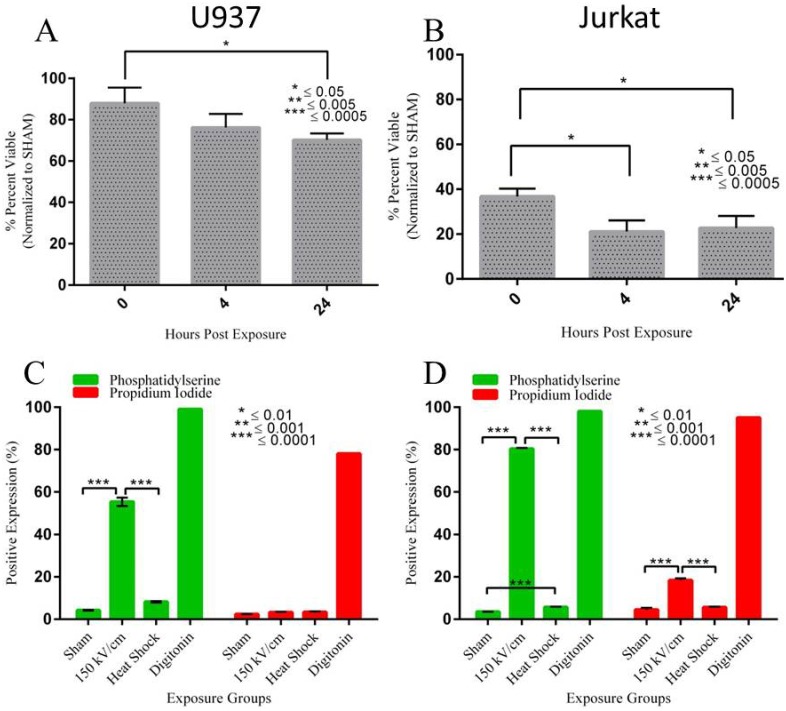
We assessed the viability of both Jurkat and U937 cells at 0, 4 and 24 hours post nsEP exposure. Fig 2A and 2B show the resultant data for both U937 and Jurkat cells from the MTT assay. The values were normalized to the sham absorbance (100%) resulting in percentage of cell survival. U937 viability was only moderately affected by nsEP exposure at any of the time points. Immediately (within 15 minutes) after the nsEP exposure, approximately 88% of cells were viable. This number fell at both 4 and 24 h post exposure, with a viability of 76% and 70% respectively. Jurkat cells appear to have been more susceptible to the detrimental effects of nsEP stress, a finding that agrees well with previously published results [28,30]. Immediately after exposure, only 37% of Jurkat cells were viable. This number fell to 21% and 23% at 4 and 24 h post exposure respectively.
Fig 2. MTT and Flow data for U937 and Jurkat cells exposed to nsEP.
A) Viability of U937 cells exposed to 100 x 10 ns pulses at 150 kV/cm. The lowest level of viability occurred 24 h post exposure. B) Viability of Jurkat cells exposed to 100 x 10 ns pulses at 150 kV/cm. The lowest level of viability occurred 4 h post exposure. C) Phosphatidylserine (PS) and propidium iodide (PI) expression in U937 cells exposed to 100 x 10 ns pulses at 150 kV/cm. D) Phosphatidylserine (PS) and propidium iodide (PI) expression in Jurkat cells exposed to 100 x 10 ns pulses at 150 kV/cm. Heat shock (exposure of cells to 44°C for 40 min) is a stress (apoptosis) control. Digitonin was the positive (necrotic) control.
Membrane Disruption and Nanoporation
FITC-Annexin V and propidium iodide (PI) dyes were used to measure the structure and permeability of the plasma membranes for each cell line exposed to nsEP. FITC-Annexin V dye is used to detect externalization of phosphatidylserine (PS), possibly indicating plasma membrane disruption. We used propidium iodide to detect the formation of nanopores. Fig 2C and 2D show PS/PI flow cytometry data for U937 and Jurkat cells exposed to nsEP. Gating thresholds were set on both fluorescent channels to determine whether a cell was positive or negative in expressing the dye of interest. sham exposure in both cell lines produced little to no expression in either channel, indicating no membrane disruption or nanoporation. Forty-one percent of exposed U937 cells had positive expression of FITC-Annexin V. However, only 5% of exposed U937 cells were positive for PI uptake indicating little to no nanoporation. The majority of exposed Jurkat cells, 79%, displayed positive FITC-Annexin V. Approximately 19% of exposed Jurkat cells were positive for PI expression. This data indicates that both cells experienced membrane disruption, with approximately twice as many Jurkat cells being affected.
Microarray Analysis
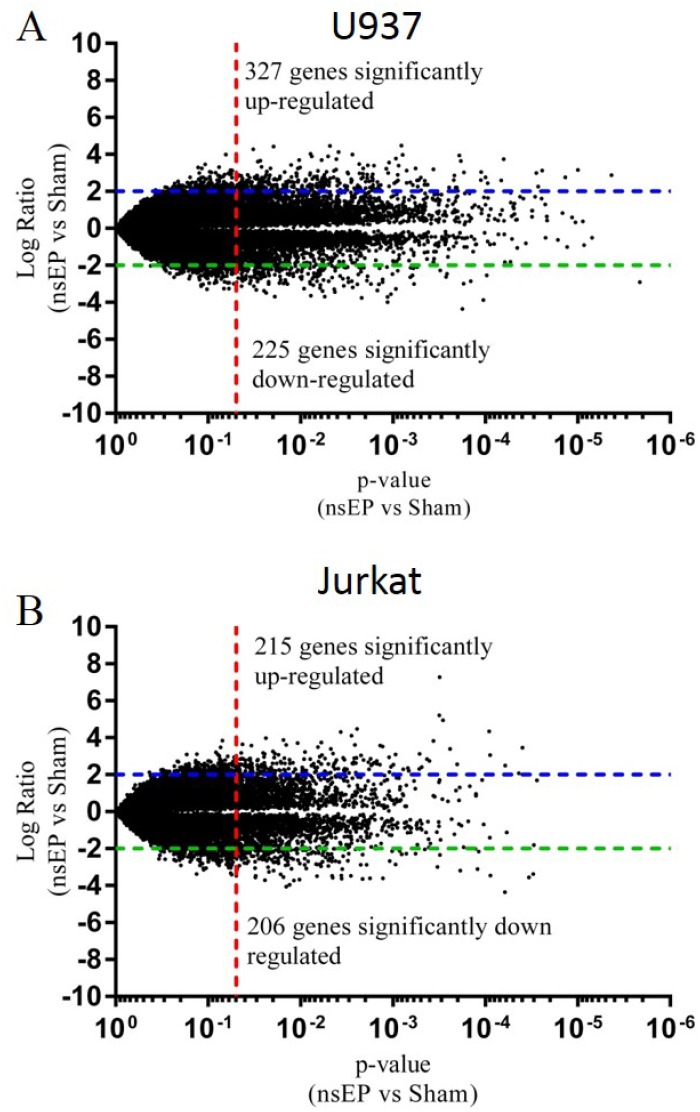
The mRNA from Jurkat and U937 cells exposed to either thermal or nsEP stress was analyzed using standard microarray data analysis techniques. The nsEP exposed samples were compared to sham exposed samples and the log ratio for each gene was plotted with respect to their respective p-values. The resultant volcano plots are displayed in Fig 3A and 3B. The volcano plots for the heat-shocked vs sham-exposed cells can be found in the supplementary information (S1 and S2 Figs). Lines were inserted into the graph at a log ratio of +2 and -2. An additional line was inserted on the X-axis at a p-value of 0.05. Gene expression ratios above the +2 (or below the -2) line and to the right of the p-value line were considered to be significant. Thus, the significant genes were those that had a log ratio ≥2 or ≤-2 and a p-value ≤ 0.05. Of the genes analyzed, 327 were significantly up-regulated and 225 were significantly down-regulated in nsEP exposed U937 cells. Jurkat cells had 215 genes significantly up-regulated and 206 significantly down-regulated. The top 40 responding genes (20 genes with the highest log ratio and the 20 genes with lowest log ratio) for each cell line are listed in Tables 1 and 2. These tables are truncated. The complete tables (S1 and S2 Tables) for U937 and Jurkat cells exposed to nsEP can be found in the supplementary information. The complete tables of gene expression changes for the positive controls (heat shocked) for each cell line are also in the supplementary information (S3 and S4 Tables).
Fig 3. Volcano plots of significant gene expression.
A) U937 cells exposed to nsEP had 327 genes significantly up-regulated as compared to sham (≥2 log ratio and p-value ≤ 0.05). 225 genes were significantly down regulated (≤-2 log ratio and p-value ≤ 0.05). B) Jurkat cells exposed to nsEP had 215 genes significantly up-regulated as compared to sham (≥2 log ratio and p-value ≤ 0.05). 206 genes were significantly down regulated (≤-2 log ratio and p-value ≤ 0.05).
Table 1. Top 20 genes up- and down-regulated in U937 cells exposed to nsEP.
| UniGene ID | Gene name | Symbol | Fold change 150kVnsEP vs. sham | p-Value 150kv nsEP vs. sham |
|---|---|---|---|---|
| Hs.446125 | male germ cell-associated kinase | MAK | 4.483 | 0.00081 |
| Hs.155111 | hepatitis A virus cellular receptor 2 | HAVCR2 | 4.453 | 0.00479 |
| Hs.351316 | transmembrane 4 L six family member 1 | TM4SF1 | 4.417 | 0.01954 |
| Hs.154057 | matrix metalloproteinase 19 | MMP19 | 4.122 | 0.00389 |
| Hs.370036 | chemokine (C-C motif) receptor 7 | CCR7 | 4.022 | 0.00779 |
| Hs.551526 | Brain-specific protein p25 alpha | TPPP | 3.991 | 0.00274 |
| Hs.267038 | premature ovarian failure, 1B | POF1B | 3.974 | 0.00078 |
| Hs.369063 | Zic family member 2 | ZIC2 | 3.944 | 0.00019 |
| Hs.351316 | transmembrane 4 L six family member 1 | TM4SF1 | 3.896 | 0.00504 |
| Hs.315369 | aquaporin 4 | AQP4 | 3.886 | 0.00755 |
| Hs.129794 | spermatogenesis associated 12 | SPATA12 | 3.745 | 0.00410 |
| Hs.414795 | serine (or cysteine) proteinase inhibitor, clade E | SERPINE1 | 3.692 | 0.00591 |
| Hs.362807 | interleukin 7 receptor /// interleukin 7 receptor | IL7R | 3.652 | 0.02727 |
| Hs.436298 | epithelial membrane protein 1 | EMP1 | 3.638 | 0.00018 |
| Hs.436550 | Na2+ channel, voltage gated, type VIII, alpha subunit | SCN8A | 3.606 | 0.02903 |
| Hs.504908 | LIM domain only 3 (rhombotin-like 2) | LMO3 | 3.551 | 0.00110 |
| Hs.91791 | Transmembrane protein 16C | TMEM16C | 3.468 | 0.00232 |
| Hs.249718 | eukaryotic translation initiation factor 4E | EIF4E | 3.417 | 0.01264 |
| Hs.514665 | DLGAP1 antisense RNA 2 | DLGAP1-AS2 | 3.404 | 0.00187 |
| Hs.2258 | matrix metallopeptidase 10 (stromelysin 2) | MMP10 | 3.331 | 0.02632 |
| Hs.388715 | small leucine-rich protein 1 | SMLR1 | -3.009 | 0.02216 |
| Hs.433586 | PPP5 tetratricopeptide repeat domain containing 1 | PPP5D1 | -3.032 | 0.00243 |
| Hs.534859 | Kazal-type serine peptidase inhibitor domain 1 | KAZALD1 | -3.066 | 0.00610 |
| Hs.177193 | synaptotagmin IX | SYT9 | -3.082 | 0.00727 |
| Hs.195298 | sarcoglycan, zeta | SGCZ | -3.114 | 0.00341 |
| Hs.549092 | suppression of tumorigenicity 18, zinc finger | ST18 | -3.199 | 0.00272 |
| Hs.322444 | RAMP2 antisense RNA 1 | RAMP2-AS1 | -3.224 | 0.00613 |
| Hs.4290 | RAB3C, member RAS oncogene family | RAB3C | -3.255 | 0.00912 |
| Hs.208544 | potassium channel, subfamily K, member 1 | KCNK1 | -3.273 | 0.00093 |
| Hs.537383 | olfactory receptor, family 5, subfamily H, member 1 | OR5H1 | -3.290 | 0.00431 |
| Hs.549149 | catenin (cadherin-associated protein), alpha 3 | CTNNA3 | -3.323 | 0.03010 |
| Hs.131152 | long intergenic non-protein coding RNA 643 | LINC00643 | -3.372 | 0.02482 |
| Hs.408453 | Wilms tumor 1 | WT1 | -3.440 | 0.00425 |
| Hs.471162 | Ras association (RalGDS/AF-6) and pleckstrin homology domains 1 | RAPH1 | -3.563 | 0.00105 |
| Hs.274264 | visual system homeobox 1 | VSX1 | -3.633 | 0.00772 |
| Hs.27043 | K+ voltage-gated channel, subfamily H member 5 | KCNH5 | -3.687 | 0.00549 |
| Hs.387367 | cytochrome P450, family 39, subfamily A, polypeptide 1 | CYP39A1 | -3.693 | 0.01892 |
| Hs.147471 | zinc finger protein 749 | ZNF749 | -3.713 | 0.00114 |
| Hs.173536 | protein kinase D3 | PRKD3 | -3.882 | 0.00010 |
| Hs.440722 | zinc finger protein 587 | ZNF417 | -3.996 | 0.00115 |
| Hs.146040 | chromosome 14 open reading frame 105 | C14orf105 | -4.364 | 0.00018 |
Table 2. Top 20 genes up- and down-regulated in Jurkat cells exposed to nsEP.
| UniGene ID | Gene name | Symbol | Fold change 150kVnsEP vs. sham | p-Value 150kv nsEP vs. sham |
|---|---|---|---|---|
| Hs.25647 | v-fos FBJ murine osteosarcoma viral oncogene homolog | FOS | 7.269 | 0.00031 |
| Hs.326035 | Early growth response 1 | EGR1 | 5.213 | 0.00031 |
| Hs.326035 | early growth response 1 | EGR1 | 4.941 | 0.00029 |
| Hs.549031 | early growth response 4 | EGR4 | 4.472 | 0.00244 |
| Hs.326035 | early growth response 1 | EGR1 | 4.349 | 0.00009 |
| Hs.494326 | basic leucine zipper nuclear factor 1 (JEM-1) | BLZF1 | 4.106 | 0.00281 |
| Hs.1395 | early growth response 2 | EGR2 | 3.981 | 0.02375 |
| Hs.536535 | dual specificity phosphatase 16 | DUSP16 | 3.832 | 0.00036 |
| Hs.529512 | zinc finger protein 167 | ZNF167 | 3.700 | 0.01073 |
| Hs.525704 | v-jun sarcoma virus 17 oncogene homolog | JUN | 3.606 | 0.00257 |
| Hs.195398 | oligodendrocyte transcription factor 3 | OLIG3 | 3.604 | 0.01432 |
| Hs.413099 | glycine receptor, alpha 3 | GLRA3 | 3.579 | 0.03397 |
| Hs.75678 | FBJ murine osteosarcoma viral oncogene homolog B | FOSB | 3.577 | 0.00137 |
| Hs.549086 | discs, large (Drosophila) homolog-associated protein 1 | DLGAP1 | 3.523 | 0.00130 |
| Hs.532933 | purinergic receptor P2Y, G-protein coupled, 12 | P2RY12 | 3.499 | 0.00649 |
| Hs.525704 | v-jun sarcoma virus 17 oncogene homolog | JUN | 3.466 | 0.00004 |
| Hs.519601 | Inhibitor of DNA binding 4, dominant negative helix-loop-helix | ID4 | 3.400 | 0.00024 |
| Hs.56247 | inducible T-cell co-stimulator | ICOS | 3.390 | 0.00468 |
| Hs.162246 | transmembrane protein 171 | TMEM171 | 3.265 | 0.01988 |
| Hs.498513 | aldo-keto reductase family 1, member C2 | AKR1C1/AKR1C2 | 3.232 | 0.00894 |
| Hs.369263 | PDS5, regulator of cohesion maintenance, homolog B (S. cerevisiae) | PDS5B | -3.059 | 0.03108 |
| Hs.24115 | miR-17-92 cluster host gene (non-protein coding) | MIR17HG | -3.098 | 0.04827 |
| Hs.510093 | Abelson helper integration site 1 | AHI1 | -3.113 | 0.00354 |
| Hs.551839 | uncharacterized LOC284600 | LOC284600 | -3.155 | 0.04887 |
| Hs.159234 | forkhead box E1 (thyroid transcription factor 2) | FOXE1 | -3.184 | 0.01954 |
| Hs.271791 | ATR serine/threonine kinase | ATR | -3.186 | 0.00019 |
| Hs.525700 | small nuclear ribonucleoprotein polypeptide N | SNRPN | -3.377 | 0.00003 |
| Hs.371903 | glycophorin E (MNS blood group) | GYPE | -3.398 | 0.01643 |
| Hs.72901 | cyclin-dependent kinase inhibitor 2B (p15, inhibits CDK4) | CDKN2B | -3.451 | 0.00363 |
| Hs.2799 | hyaluronan and proteoglycan link protein 1 | HAPLN1 | -3.471 | 0.00454 |
| Hs.44685 | ring finger protein 141 | RNF141 | -3.474 | 0.04947 |
| Hs.549172 | calmodulin-like 4 | CALML4 | -3.495 | 0.00665 |
| Hs.54973 | cadherin 26 | CDH26 | -3.551 | 0.03073 |
| Hs.519523 | serpin peptidase inhibitor, clade B (ovalbumin), member 6 | SERPINB6 | -3.560 | 0.00003 |
| --- | long intergenic non-protein coding RNA 644 | LINC00644 | -3.568 | 0.00450 |
| Hs.76561 | zinc finger protein 404 | ZNF404 | -3.603 | 0.00177 |
| Hs.170849 | coiled-coil domain containing 122 | CCDC122 | -3.614 | 0.00129 |
| Hs.389945 | WD repeat domain 60 | WDR60 | -3.641 | 0.00213 |
| Hs.242520 | uromodulin-like 1 | UMODL1 | -3.680 | 0.01025 |
| Hs.436380 | MAM domain containing glycosylphosphatidylinositol anchor 2 | MDGA2 | -3.703 | 0.02430 |
| Hs.72307 | G protein-coupled receptor 110 | GPR110 | -3.720 | 0.01181 |
Ingenuity Systems IPA Pathway Analysis
The microarray data for each cell line was loaded into Ingenuity Systems IPA pathway analysis software and a core analysis was performed for the top 5000 genes from each set of microarray data. A summary table for each cell line can be found in Tables 3 and 4 respectively. Table 3 contains the top molecular and cellular functions impacted by the gene expression pattern created by nsEP exposure. The functions of cellular movement, cell-to-cell signaling/interaction and cellular development were among the top functions in both cell lines. In the U937 cells exposed to nsEP, 751 molecules associated with cell growth represented the largest number of molecules changing that were associated with a specific cellular function. In the Jurkat cell line, the category of molecular function of cellular development had the largest number of molecules, 575 in all, affected by the nsEP pulse.
Table 3. Pathway Analysis: Top Molecular Functions.
| Molecular and Cellular Functions | ||
|---|---|---|
| Function | p-values | # of Molecules |
| U937 | ||
| Cellular Movement | 5.48E-13–1.32E-03 | 455 |
| Cell-To-Cell Signaling and Interaction | 1.95E-11–1.28E-03 | 533 |
| Cellular Development | 4.35E-08–1.28E-03 | 593 |
| Cellular Growth and Proliferation | 9.37E-08–1.28E-03 | 751 |
| Cell Death and Survival | 2.65E-07–1.36E-03 | 734 |
| Jurkat | ||
| Cellular Movement | 1.52E-14–1.10E-03 | 429 |
| Cell-To-Cell Signaling and Interaction | 8.03E-08–1.17E-03 | 508 |
| Nucleic Acid Metabolism | 1.23E-07–5.33E-05 | 70 |
| Small Molecule Biochemistry | 1.23E-07–1.10E-03 | 283 |
| Cellular Development | 1.50E-07–1.11E-03 | 575 |
Table 4. Top Canonical Pathways.
| Canonical Pathway | ||
|---|---|---|
| Function | p-values | # of Molecules |
| U937 | ||
| VDR/RXR Activation | 4.86E-06 | 33/77 (0.429) |
| B Cell Development | 9.09E-05 | 15/28 (0.536) |
| ILK Signaling | 1.03E-04 | 58/181 (0.32) |
| tRNA Splicing | 1.54E-04 | 17/35 (0.486) |
| Hepatic Fibrosis / Hepatic Stellate Cell Activation | 1.74E-04 | 61/196 (0.311) |
| Jurkat | ||
| Hepatic Fibrosis / Hepatic Stellate Cell Activation | 2.9E-06 | 63/196 (0.321) |
| G-Protein Coupled Receptor Signaling | 1.17E-05 | 75/254 (0.295) |
| LPS/IL-1 Mediated Inhibition of RXR Function | 1.2E-05 | 64/208 (0.308) |
| cAMP-mediated signaling | 8.37E-05 | 63/216 (0.292) |
| Atherosclerosis Signaling | 1.62E-04 | 39/120 (0.325) |
Canonical pathways are “idealized or generalized pathways”; they are considered to be pathways that have previously been well established and classically characterized. The core analysis function in the IPA software identified the top 5 canonical pathways affected by nsEP exposure (Table 4). For the U937 cells, the top pathway affected (based on lowest p-value) was the VDR/RXR Activation pathway with 33 of the 77 (43%) genes changing due to nsEP exposure. For the Jurkat cells, the top canonical pathway was the “Hepatic Fibrosis / Hepatic Stellate Cell Activation”, with a p-value of 2.9E-06 and 63 of 196 molecules (32%) effected. The IPA software core analysis does not suggest which, if any, non-canonical pathways may be effected.
Comparative analysis of the top 5000 genes changing within in each cell line due to nsEP exposure was conducted. Both cell lines shared 1624 common genes changing due to nsEP exposure. However, only 890 of the shared genes had changed in the same manner, i.e. both up-or both down-regulated. Of these 890 genes, only 59 genes had a log ratio ≥1.0 (fold change = 2) and a p-value of ≤0.05 (Table 5).
Table 5. Genes up- or down-regulated in common by nsEP exposure in Jurkat and U937 cells.
| Gene Symbol | Gene name | Jurkat Fold Change | p-Value | U937 Fold Change | p-Value |
|---|---|---|---|---|---|
| JUN | jun proto-oncogene | 3.606 | 0.0026 | 2.444 | 0.0001 |
| VEGFA | vascular endothelial growth factor A | 2.594 | 0.0396 | 1.32 | 0.0146 |
| RORA | RAR-related orphan receptor A | 2.569 | 0.0471 | 1.949 | 0.0072 |
| MXD1 | MAX dimerization protein 1 | 2.354 | 0.0095 | 1.635 | 0.0072 |
| ATF3 | activating transcription factor 3 | 2.301 | 0.0005 | 1.216 | 0.0014 |
| PPP1R15A | protein phosphatase 1, regulatory subunit 15A | 2.298 | 0.0032 | 2.21 | 0.0002 |
| NDUFA10 | NADH dehydrogenase (ubiquinone) 1 alpha, 10, 42kDa | 2.286 | 0.0018 | 1.83 | 0.0424 |
| BHLHE40 | basic helix-loop-helix family, member e40 | 2.235 | 0.0338 | 1.253 | 0.0013 |
| LDLR | low density lipoprotein receptor | 2.218 | 0.0096 | 2.463 | 0.0386 |
| NRP2 | neuropilin 2 | 2.082 | 0.0295 | 3.022 | 0.0031 |
| SASH1 | SAM and SH3 domain containing 1 | 2.081 | 0.0000 | 1.768 | 0.0204 |
| TBX3 | T-box 3 | 2.024 | 0.0420 | 2.052 | 0.0060 |
| DOCK4 | dedicator of cytokinesis 4 | 1.942 | 0.0134 | 1.307 | 0.0303 |
| SLC7A11 | solute carrier family 7 member 11 | 1.875 | 0.0041 | 2.309 | 0.0040 |
| ULK2 | unc-51 like autophagy activating kinase 2 | 1.848 | 0.0293 | 1.38 | 0.0177 |
| S1PR3 | sphingosine-1-phosphate receptor 3 | 1.824 | 0.0463 | 1.18 | 0.0047 |
| DUSP10 | dual specificity phosphatase 10 | 1.821 | 0.0046 | 1.571 | 0.0108 |
| CHD2 | chromodomain helicase DNA binding protein 2 | 1.75 | 0.0034 | 1.511 | 0.0231 |
| TSC22D3 | TSC22 domain family, member 3 | 1.729 | 0.0370 | 1.807 | 0.0408 |
| CACNB2 | calcium channel, voltage-dependent, beta 2 subunit | 1.718 | 0.0290 | 2.412 | 0.0409 |
| EYA3 | EYA transcriptional coactivator and phosphatase 3 | 1.613 | 0.0322 | 2.8 | 0.0480 |
| RNA45S5 | RNA, 45S pre-ribosomal 5 | 1.599 | 0.0003 | 2.887 | 0.0009 |
| CREBRF | CREB3 regulatory factor | 1.578 | 0.0267 | 1.092 | 0.0232 |
| RNF31 | ring finger protein 31 | 1.545 | 0.0488 | 1.72 | 0.0123 |
| CAMK2B | calcium/calmodulin-dependent protein kinase II beta | 1.529 | 0.0252 | 1.706 | 0.0387 |
| KLF6 | Kruppel-like factor 6 | 1.473 | 0.0016 | 1.939 | 0.0029 |
| SESN2 | sestrin 2 | 1.463 | 0.0123 | 1.606 | 0.0000 |
| MASP2 | mannan-binding lectin serine peptidase 2 | 1.448 | 0.0179 | 2.265 | 0.0283 |
| ARG1 | arginase 1 | 1.382 | 0.0441 | 1.386 | 0.0430 |
| NPR1 | natriuretic peptide receptor 1 | 1.362 | 0.0080 | 2.291 | 0.0118 |
| TM4SF1 | transmembrane 4 L six family member 1 | 1.349 | 0.0249 | 4.417 | 0.0195 |
| FAM122C | family with sequence similarity 122C | 1.31 | 0.0341 | 1.058 | 0.0162 |
| JUND | jun D proto-oncogene | 1.308 | 0.0042 | 1.733 | 0.0057 |
| SPATA6 | spermatogenesis associated 6 | 1.27 | 0.0237 | 1.924 | 0.0105 |
| PHLDA1 | pleckstrin homology-like domain, family A, member 1 | 1.142 | 0.0074 | 2.861 | 0.0002 |
| NEU1 | sialidase 1 (lysosomal sialidase) | 1.134 | 0.0296 | 1.201 | 0.0014 |
| CD55 | CD55 molecule, decay accelerating factor for complement | 1.11 | 0.0324 | 1.321 | 0.0062 |
| ABL2 | ABL proto-oncogene 2, non-receptor tyrosine kinase | 1.079 | 0.0455 | 1.267 | 0.0052 |
| SKIL | SKI-like proto-oncogene | 1.073 | 0.0026 | 1.554 | 0.0302 |
| HAO2 | hydroxyacid oxidase 2 (long chain) | 1.042 | 0.0494 | 1.618 | 0.0393 |
| MAFF | v-maf avian musculoaponeurotic fibrosarcoma oncogene F | 1.007 | 0.0170 | 1.653 | 0.0022 |
| MEX3D | mex-3 RNA binding family member D | -1.045 | 0.0318 | -2.098 | 0.0012 |
| EBF3 | early B-cell factor 3 | -1.082 | 0.0122 | -1.047 | 0.0403 |
| ZNF205 | zinc finger protein 205 | -1.083 | 0.0039 | -2.33 | 0.0060 |
| PPIL2 | peptidylprolyl isomerase (cyclophilin)-like 2 | -1.123 | 0.0417 | -2.111 | 0.0041 |
| XIRP2 | xin actin-binding repeat containing 2 | -1.19 | 0.0204 | -2.27 | 0.0255 |
| TEX14 | testis expressed 14 | -1.22 | 0.0002 | -1.113 | 0.0242 |
| CLECL1 | C-type lectin-like 1 | -1.23 | 0.0407 | -2.337 | 0.0097 |
| HNRNPD | heterogeneous nuclear ribonucleoprotein D | -1.307 | 0.0083 | -1.545 | 0.0113 |
| GABBR2 | gamma-aminobutyric acid (GABA) B receptor, 2 | -1.32 | 0.0411 | -1.784 | 0.0319 |
| PPP2R5A | protein phosphatase 2, regulatory subunit B', alpha | -1.38 | 0.0121 | -1.722 | 0.0199 |
| GPR98 | G protein-coupled receptor 98 | -1.515 | 0.0408 | -1.46 | 0.0232 |
| SATB1 | SATB homeobox 1 | -1.97 | 0.0350 | -1.548 | 0.0003 |
| RERE | arginine-glutamic acid dipeptide (RE) repeats | -2.105 | 0.0373 | -2.224 | 0.0488 |
| FERMT1 | fermitin family member 1 | -2.548 | 0.0123 | -1.4 | 0.0182 |
| TSEN54 | TSEN54 tRNA splicing endonuclease subunit | -2.707 | 0.0058 | -1.638 | 0.0389 |
| PHLPP1 | PH domain and leucine rich repeat protein phosphatase 1 | -2.862 | 0.0066 | -1.037 | 0.0132 |
| HAPLN1 | hyaluronan and proteoglycan link protein 1 | -3.471 | 0.0045 | -2.922 | 0.0221 |
qRT-PCR
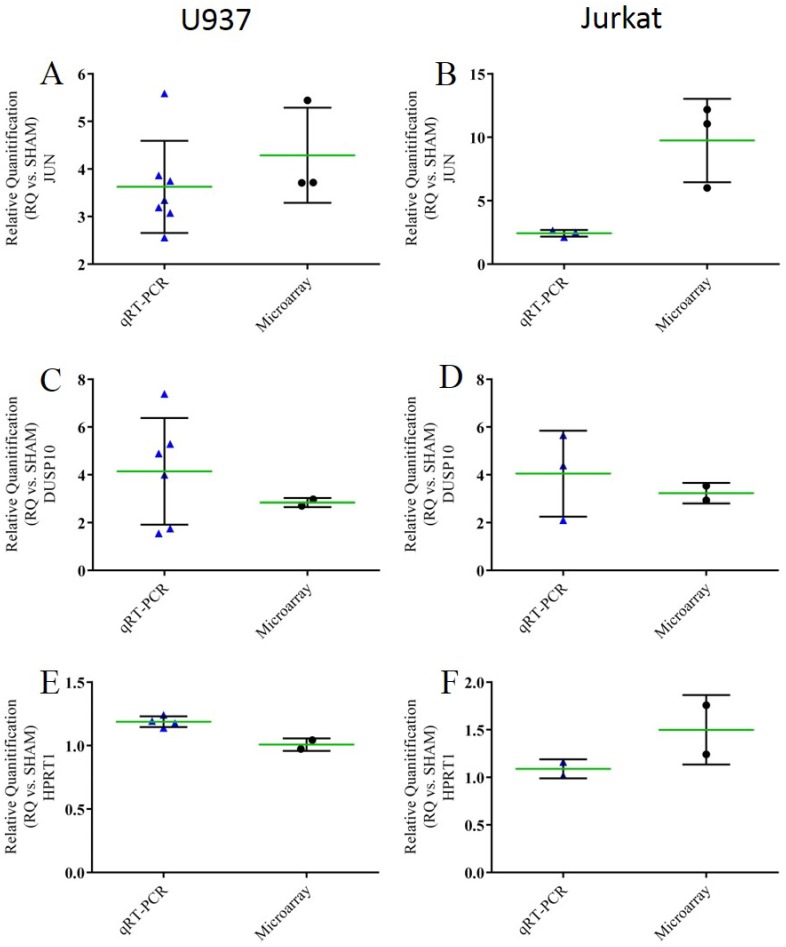
We validated microarray expression for 3 common genes in both U937 and Jurkat cells. qRT-PCR was performed for the putative transforming gene of avian sarcoma virus 17, commonly known as jun proto-oncogene (JUN) in humans. Microarray data indicated JUN expression was increased approximately 4 fold (±1). We confirmed that JUN expression was up-regulated in U937 cells exposed to nsEP; qRT-PCR indicated JUN expression was increased 4 fold (±1) (Fig 4A). The same result was determined with the Jurkat cells, however, the level of JUN expression as determined by microarray was 3x greater than what was determined by qRT-PCR (9-fold change in microarray as compared to 3-fold change in qRT-PCR) (Fig 4B). We validated the microarray expression level for the gene that codes for the dual specificity protein phosphatase, DUSP10. In U937 cells, DUSP10 was significantly up-regulated 5-fold as determined by qRT-PCR and 3-fold as determined by microarray analysis (Fig 4C). In Jurkat cells, DUSP10 was also determined to be significantly up-regulated 5-fold as determined by qRT-PCR and 2.5-fold as determined by microarray analysis (Fig 4D) For the housekeeping gene HPRT1, both qRT-PCR and microarray data agreed in both cell lines, suggesting little to no effect due to nsEP exposure (Fig 4E and 4F). Single reaction qRT-PCR was performed on many other genes of interest for U937 cells. These data can be found in the supplementary information (S3, S4, S5 and S6 Figs).
Fig 4. Scatter dot plot for each qRT-PCR validation sample.
A) Comparison of the expression levels of JUN for U937 cells exposed to nsEP. B) Comparison of the expression levels of JUN for Jurkat cells exposed to nsEP. C) Comparison of the expression levels of DUSP10 for U937 cells exposed to nsEP. D) Comparison of the expression levels of DUSP10 for Jurkat cells exposed to nsEP. E) Comparison of the expression levels of HPRT1 for U937 cells exposed to nsEP. F) Comparison of the expression levels of HPRT1 for Jurkat cells exposed to nsEP. Mean and standard deviation are plotted as the green and black lines respectively.
Luminex Assays
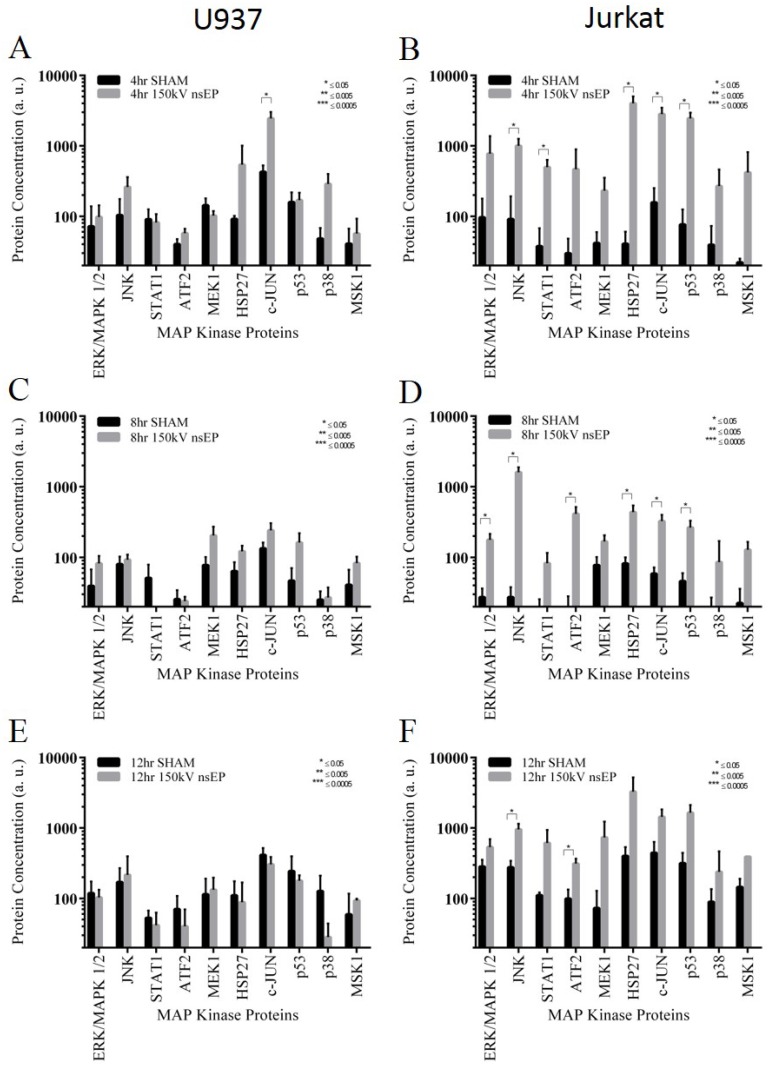
In an effort to link genetic and proteomic data, we performed a bead based multiplexing assay for the MAPK pathway. The bead based kit was used to look for changes in signal transducer and activator of transcription 1 (STAT1), activating transcription factor 2 (ATF2), extracellular signal-regulated kinase (Erk), heat shock 27kDa protein 1 (HSP27), c-Jun N-terminal kinase (JNK), jun proto-oncogene (c-Jun), dual specificity mitogen-activated protein kinase kinase 1 (Map2K1 or MEK1), mitogen- and stress-activated protein kinase-1 (MSK1), p38 mitogen-activated protein kinase (p38) and tumor protein 53 (p53). At the 4 h time point, only the protein c-Jun was significantly increased by nsEP exposure in the U937 cell line (Fig 5A). In stark contrast, the Jurkat cells had 5 of the MAPK pathway proteins significantly increased due to nsEP exposure (Fig 5B). At 8 h post exposure, none of the MAPK pathway proteins were increased due to nsEP exposure in the U937 cells (Fig 5C); however, in the Jurkat cells exposed to nsEP, 6 of the MAPK pathway-associated proteins were significantly increased (Fig 5D). These included ERK1, JNK, ATF2, HSP27, c-JUN, and p53. At 12 h post exposure, none of the MAPK proteins were changed in the U937 cells (Fig 5E), suggesting they had reached a steady state. The Jurkat cells, at 12 h post exposure, still had significantly more JNK and ATF2 proteins compared to the sham samples (Fig 5F).
Fig 5. Levels of MAP Kinase associated proteins post nsEP exposure for U937 and Jurkat Cells.
A) Levels of MAPK proteins for U937 at 4 h post exposure. B) Levels of MAPK proteins for Jurkat at 4 h post exposure. C) Levels of MAPK proteins for U937 at 8 h post exposure. D) Levels of MAPK proteins for Jurkat at 8 h post exposure. E) Levels of MAPK proteins for U937 at 12 h post exposure. F) Levels of MAPK proteins for Jurkat at 12 h post exposure. Error bars represent standard deviation (SD).
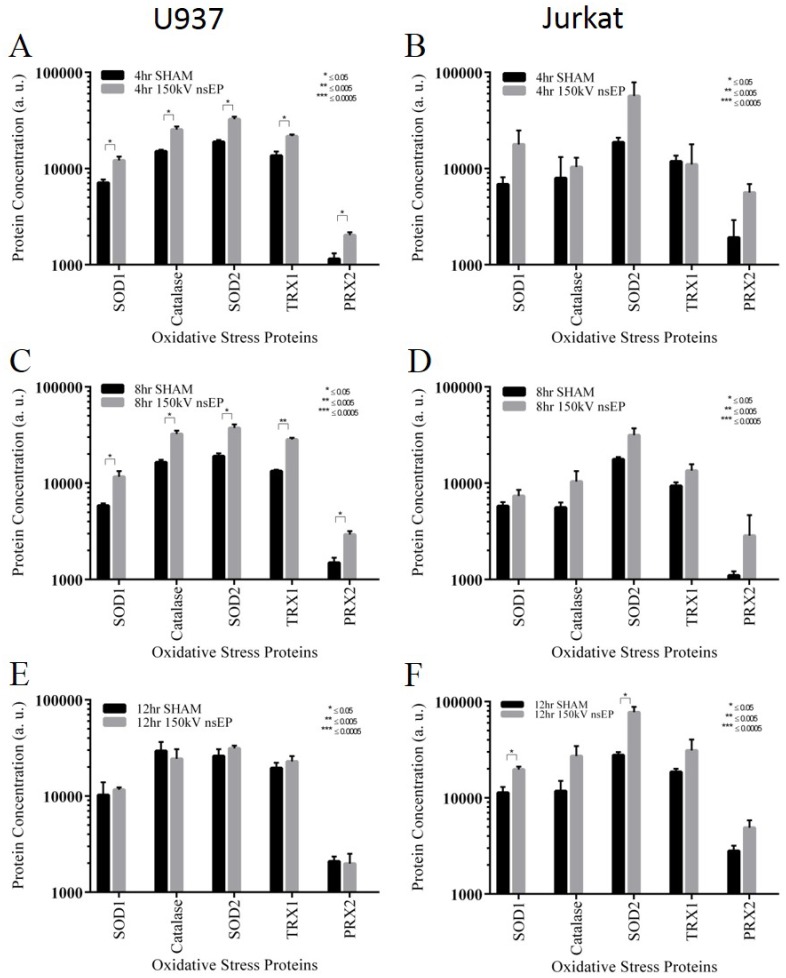
Proteins associated with oxidative stress were also surveyed via Luminex multiplexing bead assay. The human oxidative stress magnetic bead panel was used to measure the amount of Catalase, Peroxiredoxin 2 (PRX2), Superoxide dismutase-1 [Cu-Zn] (SOD1), Superoxide dismutase-2 [Mn] (SOD2), and Thioredoxin (TRX1). At 4 h post nsEP exposure, U937 cells had significant increases in all of the assayed proteins, SOD1, Catalase, SOD2, TRX1 and PRX2 (Fig 6A). SOD1, SOD2 and PRX2, in the Jurkat cells at 4 h post exposure, appear to change in response to nsEP exposure, although not to the point of statistical significance (Fig 6B). At 8 h, all of the proteins increased in the U937 cells remained significantly up-regulated (Fig 6C). At 8 h post, as at 4 h post, changes in the levels of the oxidative stress proteins were not statistically significant; however all of the assayed proteins appear to be increasing in the Jurkat cells (Fig 6D). At 12 h post, no proteins appear to be increased in U937 cells, apparently having returned to a steady state level (Fig 6E). In the Jurkat cells, at 12 h post, the only proteins significantly increased were SOD1 and SOD2 (Fig 6F).
Fig 6. Oxidative stress related protein levels in U937 and Jurkat cells exposed to nsEP.
A) Levels of oxidative stress proteins for U937 at 4 h post exposure. B) Levels of oxidative stress proteins for Jurkat at 4 h post exposure. C) Levels of oxidative stress proteins for U937 at 8 h post exposure. D) Levels of oxidative stress proteins for Jurkat at 8 h post exposure. E) Levels of oxidative stress proteins for U937 at 12 h post exposure. F) Levels of oxidative stress proteins for Jurkat at 12 h post exposure. Error bars represent standard deviation (SD).
Discussion
The viability (MTT) and cell flow data clearly show that both cells lines are affected by the nsEP exposure parameters used in this paper. Jurkat cells have been used extensively in nsEP research [31–34] and have been shown to be sensitive to these types of ultrashort pulses [28]. Our group speculated that Jurkat sensitivity to nsEP could be based on “inherent susceptibilities.” Thus, we believed the most efficient method for identifying these “inherent susceptibilities” was to perform a microarray analysis thus leading to this current body of work. Given the viability data, we feel confident that the exposure parameters were sufficient to elicit a survival/stress response requiring the up/down regulation of specific genes. Further evidence that the exposure parameters used were sufficient to cause a known effect associated with nsEP can be seen with the flow cytometry data. Vernier was among the first researchers to identify PS rearrangement as a direct effect associated with nsEP exposure [9,34–36]. Our data suggests that the nsEP parameters we used were not only effective at causing a biochemical response (loss of cell viability) but also a physical response (rearrangement of phosphatidylserine residuals in the plasma membrane).
The microarray data presented here is a mere snapshot of the molecular changes that occurred within these cells 4 h post exposure. Both the Jurkat and U937 cells have approximately the same number of genes changing (400–500 genes each) despite their different responses to nsEP exposure (viability and PS expression). This number of genes is low in comparison to a heat shock stress which causes approximately 1200 genes to have a significant response (S1 and S2 Figs). With the filter criteria relaxed to a log ratio of ≥1 or ≤-1 and a p-value of ≤0.05, only 59 genes are equally up- or down-regulated in both cell lines due to nsEP exposure. With the small number of genes being shared by both cell lines, it is difficult to pinpoint the activation of a specific, dominant pathway; however, IPA core analysis suggests many common stress pathways are shared, suggesting a common, generalized physiological response, although the genetic response appears to be tailored to each cell line.
The most striking finding of this entire study is the indication by the IPA software (based on the gene profiles) that the major cellular/molecular function affected by nsEP exposure is cellular growth/development/movement. This is in stark contrast to the MTT data. The viability data suggests the Jurkat cells are quite susceptible to the effects of nsEP with only 23% of the cells surviving at 4 h post exposure. Based on the loss of viability, we expected the genetic profile to indicate possibly necrotic or apoptotic gene pathway up-regulation, but instead, cellular growth development/movement functions are indicated as the top functions up-regulated in each cell line following nsEP exposure.
Despite these findings, identification of a dominant pathway was not possible. The increase in cellular growth development/movement functions is not the result of an individual pathway, but rather is the result of an intricate network of many pathways. Therefore, given this level of complexity, we analyzed individual genes, their response to stress and their associated pathways. Analysis of the top 20 genes changing in both cell lines indicated many of these genes play important roles in the cellular response to mechanical stress (Table 6). Of the top 20 genes from both cell lines, 25% have specific functions associated with either MAPK signaling or directly in cellular growth. The majority of these genes are found in the Jurkat genetic profile. Another 25% of the top 20 genes up-regulated in these cell lines by nsEP are associated with the disruption of the plasma membrane, with the majority of these genes being present in the U937 cells. This finding directly correlates to the level of PS detected in flow cytometry, directly tying an observed bioeffect associated with nsEP to a specific genetic response.
Table 6. Mechanical stress-associated genes Up-Regulated by nsEP.
| Gene Name | Symbol | Function (from NCBI Resources: Gene) | Cell |
|---|---|---|---|
| Mechanical: Increases in MAP Kinase Signaling Pathway//increases in cell growth | |||
| male germ cell-associated kinase | MAK | serine/threonine protein kinase related to kinases involved in cell cycle regulation | U937 |
| v-jun sarcoma virus 17 oncogene homolog | JUN | interacts directly with specific target DNA sequences to regulate gene expression, part of the JNK pathway | U937/ Jurkat |
| FBJ murine osteosarcoma viral oncogene homolog | FOS | regulator of cell proliferation, differentiation, and transformation. | Jurkat |
| Early Growth Response 1 | EGR1 | induces the expression of growth factors, growth factor receptors, extracellular matrix proteins, proteins involved in the regulation of cell growth or differentiation, and proteins involved in apoptosis, growth arrest, and stress responses | Jurkat |
| Early Growth Response 4 | EGR4 | cell proliferation by increased expression of potassium chloride cotransporter 2b (KCC2b) | Jurkat |
| basic leucine zipper nuclear factor 1 | BLZF1 | regulation of cell growth | Jurkat |
| Early Growth Response 2 | EGR2 | mediates NFκB and MAPK signaling | Jurkat |
| dual specificity phosphatase 10 | DUSP10 | regulates the c-Jun amino-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) pathways | U937 Jurkat |
| dual specificity phosphatase 16 | DUSP16 | regulates the c-Jun amino-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) pathways | Jurkat |
| Mechanical: Calcium release from the endoplasmic reticulum | |||
| flavin containing monooxygenase 3 | FMO3 | transmembrane protein localizes to the endoplasmic reticulum | U937 |
| Mechanical: Disruption of the plasma membrane, extracellular matrix and cytoskeleton | |||
| transmembrane 4 L six family member 1 | TM4SF1 | molecular organizer that interacts with membrane and cytoskeleton-associated proteins | U937 |
| matrix metalloproteinase 19 | MMP19 | breakdown of extracellular matrix | U937 |
| premature ovarian failure, 1B | POF1B | binds non-muscle actin filaments | U937 |
| Brain-specific protein p25 alpha | TPPP | binds to tubulin and microtubules and induces aberrant microtubule assemblies | U937 |
| aquaporin 4 | AQP4 | function as water-selective channels in the plasma membranes | U937 |
| Anoctamins | TMEM16C | phospholipid scrambling | U937 |
| matrix metalloproteinase 10 (stromelysin 2) | MMP10 | breakdown of extracellular matrix | U937 |
| Annexin A1 | ANXA1 | membrane-localized protein that binds phospholipids | U937 |
| glycine receptor, alpha 3 | GLRA3 | encodes a member of the ligand-gated ion channel protein family | Jurkat |
| Mechanical: Increased IP3 production | |||
| chemokine (C-C motif) receptor 7 | CCR7 | member of the G protein-coupled receptor family | U937 |
| Mechanical: Increases in GPCR | |||
| regulator of G-protein signaling 1 | RGS1 | attenuates the signaling activity of G-proteins by binding to activated, GTP-bound G alpha subunit | U937 |
| polycystic kidney disease 1 like 1 | PKD1L1 | novel G-protein-binding protein. Ca2+-permeable pore-forming subunits and receptor-like integral membrane proteins | U937 |
| purinergic receptor P2Y, G-protein coupled, 12 | P2RY12 | belongs to the family of G-protein coupled receptor | Jurkat |
Given the level of gene expression associated with cellular growth and the apparent activation of the MAPK pathway, we correlated gene expression with protein levels using a quantitative Luminex assay. The MAPK pathway can be activated by mitogens or through certain stress pathways. Activation of the MAPK pathway in response to nsEP has been shown before, however, it was not linked to a specific genetic response [37,38]. The Luminex data suggests that the MAPK pathway is activated within the Jurkat cells for several hours, eventually returning to a quasi-normal state at 12 h post exposure. These data are reflected by the associated gene data shown in Table 6. The U937 cells do not appear to have MAPK proteins significantly expressed in response to nsEP at the time points examined. Nevertheless, genetic evidence suggests that the MAPK pathway may be activated in the U937 cells, as shown by the increase in the dual specificity protein phosphatase (DUSP) genes. The DUSP genes essentially “turn off” the MAPK pathway, and several of these genes are up-regulated in the U937 cells following nsEP.
Of the top 10 significantly changing genes in response to nsEP, EGR1 appears 3 times, with EGR4 and EGR2 appearing once each. ERG genes have been associated with MAPK pathway activation, but also, they have also been found to be up-regulated as part of the antioxidative response [39]. It is unclear if the ERG genes are up-regulated in response to the MAPK pathway or another pathway, however the production of reactive oxygen species (ROS) have been observed with nsEP exposures [40]. The source of the ROS is not clear, however it is possible that both electrochemistry and mitochondria stimulation contribute to the increased levels. Beebe has published a great deal concerning the effect of nsEP on mitochondria and the subsequent flooding of calcium into cells post exposure [15,19,32,41–43]. The mitochondria appear to be both directly (increased membrane permeability) and indirectly (storage of free calcium) affected by nsEP. Given that mitochondria appear to be a target of nsEP and the possible role of EGRs as initiators of an antioxidant response, we looked at oxidative stress proteins for both Jurkat and U937 cells exposed to nsEP. Proteins associated with oxidative stress were observed as not being statistically significant, but appear to be slightly increased in the Jurkat cells. This was somewhat expected, given the increased levels of EGR gene expression in Jurkat cells. Increased expression of EGR genes give cells an ability to initiate an antioxidant response. On the contrary, the U937 cells, which had no significant genetic increases in EGR genes, had many proteins associated with oxidative stress significantly up-regulated.
Based on the genetic response of both cell lines, it appears that these cells respond to nsEP as a mechanical stress. It has been reported in other studies that osteoblasts undergoing mechanical stretch, preferentially up-regulated FOS, JUN, and EGR1, 2, and 3 genes [44]. The authors of the osteoblast study go on to suggest that EGR2 is actually a “mechanically sensitive gene” [44]. It is important to remember that Jurkat cells are not osteoblasts and any mechanical stress associated with nsEP is most likely due to acute and not tension like stress. The MAPK pathway has also been shown to be specifically up-regulated by mechanical stress [45–47]. Although these genes are associated with mechanical stress, they are and can be up-regulated by other factors, and thus they alone are not indicative of mechanical stress being the dominate force acting upon the cells.
Although circumstantial, other data suggests that nsEP exposure can induce mechanical stress on cells. Biomarkers of mechanical stress and the observed bioeffects of nsEP exposure are strikingly similar. Markers of mechanical stress include calcium release from the endoplasmic reticulum [48], disruption of the extracellular matrix and cytoskeleton [49–52], increased IP3 production [48], increases in GPCR and MAPK pathway signaling [39,53–55], and the production of reactive oxygen species resulting in oxidative stress [56]. These markers of mechanical stress can be directly and indirectly linked to specific changes in gene expression. Evidence suggests that cells in vitro interpret mechanical stress as a mitogen or as a signal to grow/enter the cell cycle [45–47].
The findings presented in this paper provide strong evidence that cells exposed to nsEP experience a stress that is interpreted as being mechanical in nature. It is important to note that we did not control for swelling in these experiments. However, it is unlikely that colloid osmotic swelling of these cells is responsible for the specific changes in mechanical stress associated gene expression. These exposures were performed in full growth medium, and analysis of the forward scattering (FSC-H) data collected from the flow cytometry analysis indicated that swelling did not occur (S7 Fig).
We feel that these findings are important in the field of bioelectrics, because it suggests for the first time that the cells exposed to nsEP experience a mechanical stress of significant amplitude to elicit a specific genetic response. Although it is not explicitly mentioned by or accounted for by other nsEP researchers, mechanical stress, whether caused by electrodeformation [1,5,57], cell swelling [7,8], or through the generation of an acoustic pressure transients generated by nsEP [58], could be responsible for the specific gene expression profiles identified in this study. Pakhomov et al. suggested that future research should focus on many likely mechanisms, including “mechanical stress due to thermoelastic expansion effect.”[59]. The rearrangement of the plasma membrane and possible formation of nanopores witnessed in nsEP is consistent with what is seen in sonoporation. Sonoporation uses ultrasonic waves (i.e. mechanical force) to create holes in the biomembranes of cells and vesicles for the purposes of either delivering or releasing compounds, biomolecules, drugs, etc. [60] Sonoporation causes the cavitation microbubbles, leading to poration by one of the following mechanisms: acoustic micro-streaming, bubble oscillations, or inertial cavitation shock waves [60]. Inertial cavitation shock waves, impart mechanical stress on the plasma membranes of nearby cells leading to poration. Our group has identified and characterized acoustic pressure transients generated by nsEP exposure very similar to those used in sonoporation[58].
Further work is underway to identify the source of the mechanical stress and quantify the amount of force generated by each of the previously identified sources of mechanical stress. The overall goal of future work will be to determine how and to what degree each source of mechanical stress contributes to the nanoporation phenomena or to other cellular reactions. Understanding the mechanisms responsible for the bioeffects associated with nsEP exposure is critical to the continued development and application of this technology.
Supporting Information
U937 cells exposed to thermal stress had 1058 genes significantly up-regulated as compared to sham (≥2 log ratio and p-value ≤ 0.05). 101 genes were significantly down regulated (≤-2 log ratio and p-value ≤ 0.05).
(TIF)
Jurkat cells exposed to thermal stress had 1004 genes significantly up-regulated as compared to sham (≥2 log ratio and p-value ≤ 0.05). 158 genes were significantly down regulated (≤-2 log ratio and p-value ≤ 0.05).
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(JPG)
Genes were selected based on log ratio (≥2, or ≤ -2) and with a p-value of ≤ 0.05.
(DOCX)
Genes were selected based on log ratio (≥2, or ≤ -2) and with a p-value of ≤ 0.05.
(DOCX)
Genes were selected based on log ratio (≥2, or ≤ -2) and with a p-value of ≤ 0.05.
(DOCX)
Genes were selected based on log ratio (≥2, or ≤ -2) and with a p-value of ≤ 0.05.
(DOCX)
Acknowledgments
Mr. Roth is a SMART Scholar and is supported by the OSD-T&E (Office of Secretary Defense-Test and Evaluation), Defense–Wide / PE0601120D8Z National Defense Education Program (NDEP) / BA-1, Basic Research.
Data Availability
The data discussed in this paper have been deposited in the NCBI Gene Expression Omnibus and are accessible through GEO Series accession number GSE77907 and GSE77908 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE77907 and http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE77908).
Funding Statement
Mr. Roth is a SMART Scholar and is supported by the OSD-T&E (Office of Secretary Defense-Test and Evaluation), Defense –Wide / PE0601120D8Z National Defense Education Program (NDEP) / BA-1, Basic Research. This research was supported by intramural funds from the Air Force Surgeon General's Office, Medical Research Program and the Air Force Office of Scientific Research LRIR 13RH08COR. Mr. Erick Moen was funded by the Repperger Fellowship Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chen C, Smye S, Robinson M, Evans J (2006) Membrane electroporation theories: a review. Medical and Biological Engineering and Computing 44: 5–14. [DOI] [PubMed] [Google Scholar]
- 2.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider P (1982) Gene transfer into mouse lyoma cells by electroporation in high electric fields. The EMBO journal 1: 841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenbach K, Barker R, Liu S (1999) Nonthermal medical/biological treatments using electromagnetic fields and ionized gases. Pulsed Power Conference, 1999. Digest of Technical Papers. 12th IEEE International. Vol. 1. pp. 497–501.
- 4.Sugar IP, Neumann E (1984) Stochastic model for electric field-induced membrane pores electroporation. Biophysical chemistry 19: 211–225. [DOI] [PubMed] [Google Scholar]
- 5.Teissie J, Golzio M, Rols M (2005) Mechanisms of cell membrane electropermeabilization: a minireview of our present (lack of?) knowledge. Biochimica et Biophysica Acta (BBA)-General Subjects 1724: 270–280. [DOI] [PubMed] [Google Scholar]
- 6.Weaver JC (2000) Electroporation of cells and tissues. Plasma Science, IEEE Transactions on 28: 24–33. [Google Scholar]
- 7.Pakhomova ON, Gregory BW, Semenov I, Pakhomov AG (2013) Two Modes of Cell Death Caused by Exposure to Nanosecond Pulsed Electric Field. PLoS One 8: e70278 10.1371/journal.pone.0070278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincelette RL, Roth CC, McConnell MP, Payne JA, Beier HT, Ibey BL (2013) Thresholds for Phosphatidylserine Externalization in Chinese Hamster Ovarian Cells following Exposure to Nanosecond Pulsed Electrical Fields (nsPEF). PLoS One 8: e63122 10.1371/journal.pone.0063122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vernier PT, Sun Y, Marcu L, Craft CM, Gundersen MA (2004) Nanoelectropulse-induced phosphatidylserine translocation. Biophysical journal 86: 4040–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vernier PT, Sun Y, Wang J, Thu MM, Garon E, Valderrabano M, et al. (2005) Nanoelectropulse intracellular perturbation and electropermeabilization technology: phospholipid translocation, calcium bursts, chromatin rearrangement, cardiomyocyte activation, and tumor cell sensitivity. Conf Proc IEEE Eng Med Biol Soc 6: 5850–5853. 10.1109/IEMBS.2005.1615820 [DOI] [PubMed] [Google Scholar]
- 11.Ibey BL, Mixon DG, Payne JA, Bowman A, Sickendick K, Wilmink GJ, et al. (2010) Plasma membrane permeabilization by trains of ultrashort electric pulses. Bioelectrochemistry 79: 114–121. 10.1016/j.bioelechem.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pakhomov AG, Kolb JF, White JA, Joshi RP, Xiao S, Schoenbach KH (2007) Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF). Bioelectromagnetics 28: 655–663. 10.1002/bem.20354 [DOI] [PubMed] [Google Scholar]
- 13.Pakhomov AG, Shevin R, White JA, Kolb JF, Pakhomova ON, Joshi RP, et al. (2007) Membrane permeabilization and cell damage by ultrashort electric field shocks. Archives of biochemistry and biophysics 465: 109–118. [DOI] [PubMed] [Google Scholar]
- 14.Estlack LE, Roth CC, Thompson GL III, Lambert WA III, Ibey BL (2014) Nanosecond pulsed electric fields modulate the expression of Fas/CD95 death receptor pathway regulators in U937 and Jurkat Cells. Apoptosis 19: 1755–1768. 10.1007/s10495-014-1041-9 [DOI] [PubMed] [Google Scholar]
- 15.Beebe SJ, Sain NM, Ren W (2013) Induction of cell death mechanisms and apoptosis by nanosecond pulsed electric fields (nsPEFs). Cells 2: 136–162. 10.3390/cells2010136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, Sain NM, Harlow KT, Chen Y-J, Shires PK, Heller R, et al. (2014) A protective effect after clearance of orthotopic rat hepatocellular carcinoma by nanosecond pulsed electric fields. Eur J Cancer 50: 2705–2713. 10.1016/j.ejca.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 17.Ren W, Sain NM, Beebe SJ (2012) Nanosecond pulsed electric fields (nsPEFs) activate intrinsic caspase-dependent and caspase-independent cell death in Jurkat cells. Biochemical and biophysical research communications 421: 808–812. 10.1016/j.bbrc.2012.04.094 [DOI] [PubMed] [Google Scholar]
- 18.Beier HT, Roth CC, Tolstykh GP, Ibey BL (2012) Resolving the spatial kinetics of electric pulse-induced ion release. Biochem Biophys Res Commun 423: 863–866. 10.1016/j.bbrc.2012.06.055 [DOI] [PubMed] [Google Scholar]
- 19.Beebe SJ (2015) Considering effects of nanosecond pulsed electric fields on proteins. Bioelectrochemistry 103: 52–59. 10.1016/j.bioelechem.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 20.Tolstykh GP, Beier HT, Roth CC, Thompson GL, Ibey BL (2014) 600 ns pulse electric field-induced phosphatidylinositol4,5-bisphosphate depletion. Bioelectrochemistry 100: 80–87. 10.1016/j.bioelechem.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 21.Tolstykh GP, Beier HT, Roth CC, Thompson GL, Payne JA, Kuipers MA, et al. (2013) Activation of intracellular phosphoinositide signaling after a single 600 nanosecond electric pulse. Bioelectrochemistry 94: 23–29. 10.1016/j.bioelechem.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 22.Tolstykh GP, Tarango M, Roth CC, Ibey BL (2014) Dose dependent translocations of fluorescent probes of PIP2 hydrolysis in cells exposed to nanosecond pulsed electric fields.
- 23.Nishitani WS, Alencar AM, Wang Y (2015) Rapid and Localized Mechanical Stimulation and Adhesion Assay: TRPM7 Involvement in Calcium Signaling and Cell Adhesion. PLoS One 10: e0126440 10.1371/journal.pone.0126440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishitani WS, Saif TA, Wang Y (2011) Calcium signaling in live cells on elastic gels under mechanical vibration at subcellular levels. PLoS One 6: e26181 10.1371/journal.pone.0026181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickson EJ, Jensen JB, Hille B (2014) Golgi and plasma membrane pools of PI(4)P contribute to plasma membrane PI(4,5)P2 and maintenance of KCNQ2/3 ion channel current. Proc Natl Acad Sci USA 111: E2281–90. 10.1073/pnas.1407133111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker K, Pakhomova ON, Kolb J, Schoenbach KS, Stuck BE, Murphy MR, et al. (2006) Oxygen enhances lethal effect of high-intensity, ultrashort electrical pulses. Bioelectromagnetics 27: 221–225. [DOI] [PubMed] [Google Scholar]
- 27.Kolb JF, Kono S, Schoenbach KH (2006) Nanosecond pulsed electric field generators for the study of subcellular effects. Bioelectromagnetics 27: 172–187. 10.1002/bem.20185 [DOI] [PubMed] [Google Scholar]
- 28.Ibey BL, Roth CC, Pakhomov AG, Bernhard JA, Wilmink GJ, Pakhomova ON (2011) Dose-dependent thresholds of 10-ns electric pulse induced plasma membrane disruption and cytotoxicity in multiple cell lines. PLoS One 6: e15642 10.1371/journal.pone.0015642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilmink GJ. Using Optical Imaging Methods to Assess Laser-Tissue Interactions. Doctoral Dissertation, Vanderbilt University. 2007. Available: http://etd.library.vanderbilt.edu/available/etd-11062007-161955/unrestricted/Wilmink.pdf
- 30.Ibey BL, Pakhomov AG, Gregory BW, Khorokhorina VA, Roth CC, Rassokhin MA, et al. (2010) Selective cytotoxicity of intense nanosecond-duration electric pulses in mammalian cells. Biochimica et Biophysica Acta (BBA)-General Subjects 1800: 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beebe SJ, Blackmore PF, White J, Joshi RP, Schoenbach KH (2004) Nanosecond pulsed electric fields modulate cell function through intracellular signal transduction mechanisms. Physiol Meas 25: 1077–1093. [DOI] [PubMed] [Google Scholar]
- 32.Beebe SJ, Fox PM, Rec LJ, Willis ELK, Schoenbach KH (2003) Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J 17: 1493–1495. 10.1096/fj.02-0859fje [DOI] [PubMed] [Google Scholar]
- 33.Frey W, White JA, Price RO, Blackmore PF, Joshi RP, Nucitelli R, et al. (2006) Plasma membrane voltage changes during nanosecond pulsed electric field exposure. Biophysical journal 90: 3608–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vernier PT, Sun Y, Marcu L, Craft CM, Gundersen MA (2004) Nanosecond pulsed electric fields perturb membrane phospholipids in T lymphoblasts. FEBS Lett 572: 103–108. 10.1016/j.febslet.2004.07.021 [DOI] [PubMed] [Google Scholar]
- 35.Vernier PT, Sun Y, Marcu L, Salemi S, Craft CM, Gundersen MA (2003) Calcium bursts induced by nanosecond electric pulses. Biochemical and biophysical research communications 310: 286–295. [DOI] [PubMed] [Google Scholar]
- 36.Vernier PT, Ziegler MJ, Sun Y, Gundersen MA, Tieleman DP (2006) Nanopore-facilitated, voltage-driven phosphatidylserine translocation in lipid bilayers-in cells and in silico. Phys Biol 3: 233–247. 10.1088/1478-3975/3/4/001 [DOI] [PubMed] [Google Scholar]
- 37.Morotomi-Yano K, Akiyama H, Yano K (2011) Nanosecond pulsed electric fields activate MAPK pathways in human cells. Archives of Biochemistry and Biophysics 515: 99–106. 10.1016/j.abb.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 38.Morotomi-Yano K, Uemura Y, Katsuki S, Akiyama H, Yano K (2011) Activation of the JNK pathway by nanosecond pulsed electric fields. Biochemical and Biophysical Research Communications 408: 471–476. 10.1016/j.bbrc.2011.04.056 [DOI] [PubMed] [Google Scholar]
- 39.Cui W, Bryant MR, Sweet PM, McDonnell PJ (2004) Changes in gene expression in response to mechanical strain in human scleral fibroblasts. Exp Eye Res 78: 275–284. [DOI] [PubMed] [Google Scholar]
- 40.Pakhomova ON, Khorokhorina VA, Bowman AM, Rodaite-Rivsevivciene R, Saulis G, Xiao S, et al. (2012) Oxidative effects of nanosecond pulsed electric field exposure in cells and cell-free media. Archives of biochemistry and biophysics 527: 55–64. 10.1016/j.abb.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beebe SJ (2013) Cell responses without receptors and ligands, using nanosecond pulsed electric fields (nsPEFs). Int J Nanomedicine 8: 3401 10.2147/IJN.S51357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beebe SJ, Ford WE, Ren W, Chen X, Schoenbach KH (2009) Non-ionizing radiation with nanosecond pulsed electric fields as a cancer treatment: in vitro studies. Engineering in Medicine and Biology Society, 2009. EMBC 2009. Annual International Conference of the IEEE. pp. 6509–6512. [DOI] [PubMed] [Google Scholar]
- 43.Beebe SJ, White J, Blackmore PF, Deng Y, Somers K, Schoenbach KH (2003) Diverse effects of nanosecond pulsed electric fields on cells and tissues. DNA Cell Biol 22: 785–796. 10.1089/104454903322624993 [DOI] [PubMed] [Google Scholar]
- 44.Ott C-E, Bauer S, Manke T, Ahrens S, Rӧdelsperger C, Grünhagen J, et al. (2009) Promiscuous and Depolarization-Induced Immediate-Early Response Genes Are Induced by Mechanical Strain of Osteoblasts. Journal of Bone and Mineral Research 24: 1247–1262. 10.1359/jbmr.090206 [DOI] [PubMed] [Google Scholar]
- 45.Pereira AM, Tudor C, Pouille P-A, Shekhar S, Kanger JS, Subramaniam V, et al. (2014) Plasticity of the MAPK Signaling Network in Response to Mechanical Stress. PLoS One 9: e101963 10.1371/journal.pone.0101963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiegand C, White R (2013) Microdeformation in wound healing. Wound Repair and Regeneration 21: 793–799. 10.1111/wrr.12111 [DOI] [PubMed] [Google Scholar]
- 47.Vandenburgh HH (1992) Mechanical forces and their second messengers in stimulating cell growth in vitro. Am J Physiol 262: R350–5. [DOI] [PubMed] [Google Scholar]
- 48.Compton JL, Luo JC, Ma H, Botvinick E, Venugopalan V (2014) High-throughput optical screening of cellular mechanotransduction. Nat Photonics 8: 710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, et al. (2012) Mechanical stress-activated integrin α5β1 induces opening of connexin 43 hemichannels. Proceedings of the National Academy of Sciences 109: 3359–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross TD, Coon BG, Yun S, Baeyens N, Tanaka K, Ouyang M, et al. (2013) Integrins in mechanotransduction. Curr Opin Cell Biol 25: 613–618. 10.1016/j.ceb.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinha B, Kӧster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, et al. (2011) Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144: 402–413. 10.1016/j.cell.2010.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang W, Wei P, Chen Y, Yang L, Jiang C, Jiang P, et al. (2014) Down-regulated expression of vimentin induced by mechanical stress in fibroblasts derived from patients with ossification of the posterior longitudinal ligament. Eur Spine J. 10.1007/s00586-014-3394-8 [DOI] [PubMed] [Google Scholar]
- 53.Adam RM, Eaton SH, Estrada C, Nimgaonkar A, Shih S-C, Smith LE, et al. (2004) Mechanical stretch is a highly selective regulator of gene expression in human bladder smooth muscle cells. Physiol Genomics 20: 36–44. [DOI] [PubMed] [Google Scholar]
- 54.Chen BP, Li Y, Zhao Y, Chen K, Li S, Lao J, et al. (2002) DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics 7: 55–96. [DOI] [PubMed] [Google Scholar]
- 55.De Araujo R, Oba Y, Moriyama K (2007) Identification of genes related to mechanical stress in human periodontal ligament cells using microarray analysis. J Periodontal Res 42: 15–22. [DOI] [PubMed] [Google Scholar]
- 56.Ward CW, Prosser BL, Lederer WJ (2013) Mechanical stretch induced activation of ROS/RNS signaling in striated muscle. Antioxid Redox Signal. 10.1089/ars.2013.5517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho S, Mittal G (1996) Electroporation of cell membranes: a review. Critical reviews in biotechnology 16: 349–362. [DOI] [PubMed] [Google Scholar]
- 58.Roth CC, Barnes RA, Ibey BL, Beier HT, Mimun LC, Maswadi SM, et al. (2015) Characterization of Pressure Transients Generated by Nanosecond Electrical Pulse (nsEP) Exposure. Sci Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pakhomov AG, Phinney A, Ashmore J, Walker K, Kolb JF, Kono S, et al. (2004) Characterization of the cytotoxic effect of high-intensity, 10-ns duration electrical pulses. Plasma Science, IEEE Transactions on 32: 1579–1586. [Google Scholar]
- 60.Wrenn SP, Dicker SM, Small EF, Dan NR, Mleczko M, Schmitz G, et al. (2012) Bursting bubbles and bilayers. Theranostics 2: 1140 10.7150/thno.4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
U937 cells exposed to thermal stress had 1058 genes significantly up-regulated as compared to sham (≥2 log ratio and p-value ≤ 0.05). 101 genes were significantly down regulated (≤-2 log ratio and p-value ≤ 0.05).
(TIF)
Jurkat cells exposed to thermal stress had 1004 genes significantly up-regulated as compared to sham (≥2 log ratio and p-value ≤ 0.05). 158 genes were significantly down regulated (≤-2 log ratio and p-value ≤ 0.05).
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(JPG)
Genes were selected based on log ratio (≥2, or ≤ -2) and with a p-value of ≤ 0.05.
(DOCX)
Genes were selected based on log ratio (≥2, or ≤ -2) and with a p-value of ≤ 0.05.
(DOCX)
Genes were selected based on log ratio (≥2, or ≤ -2) and with a p-value of ≤ 0.05.
(DOCX)
Genes were selected based on log ratio (≥2, or ≤ -2) and with a p-value of ≤ 0.05.
(DOCX)
Data Availability Statement
The data discussed in this paper have been deposited in the NCBI Gene Expression Omnibus and are accessible through GEO Series accession number GSE77907 and GSE77908 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE77907 and http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE77908).