Abstract
The evolutionary history of macaques, genus Macaca, has been under debate due to the short times of divergence. In this study, maternal, paternal, and biparental genetic systems were applied to infer phylogenetic relationships among macaques and to trace ancient hybridization events in their evolutionary history. Using a PCR display method, 17 newly phylogenetically informative Alu insertions were identified from M. assamensis. We combined presence/absence analysis of 84 Alu elements with mitochondrial genomes as well as nuclear sequences (five autosomal genes, two Y chromosomal genes, and one X chromosomal fragment) to reconstruct a robust macaque phylogeny. Topologies generated from different inherited markers were similar supporting six well defined species groups and a close relationship of M. assamensis and M. thibetana, but differed in the placing of M. arctoides. Both Alu elements and nuclear genes supported that M. arctoides was close to the sinica group, whereas the mitochondrial data clustered it into the fascicularis/mulatta lineage. Our results reveal that a sex-biased hybridization most likely occurred in the evolutionary history of M. arctoides, and suggest an introgressive pattern of male-mediated gene flow from the ancestors of M. arctoides to the M. mulatta population followed by nuclear swamping. According to the estimation of divergence dates, the hybridization occurred around 0.88~1.77 mya (nuclear data) or 1.38~2.56 mya (mitochondrial data). In general, our study indicates that a combination of various molecular markers could help explain complicated evolutionary relationships. Our results have provided new insights into the evolutionary history of macaques and emphasize that hybridization might play an important role in macaque evolution.
Introduction
Macaques represent one of the most successful primate radiations with 20–23 extant species in genus Macaca. According to Raaum et al. [1], macaques diverged from other members of the tribe Papionini approximately 9–10 million years ago (mya). Fossil data indicated that the genus arose about 7 mya in northern Africa and then the Asian macaque lineage began to appear around 5.5 mya [2,3]. With the exception of M. sylvanus in North Africa and Southern Europe, macaques are widely distributed in southern and eastern Asia [4], ranking second only to the world-wide distribution of humans among the extant primates. With such a variety of habitats and species that differ in ecology and external morphology, macaques are best known as a prime group for studies of species radiation and evolution as well as an important animal model in medical research. Thus it is important to elucidate the phylogeny of the extant taxa of macaques, which will contribute to our understanding of the evolutionary history of the genus as well as other primate radiations.
The relationships among macaques have been the focus of research [2,4–8] (S1 Table). Traditionally, based on male genitalia, Fooden [4] classified the genus into four species groups: silenus-sylvanus group, fascicularis group, arctoides group, and sinica group. Later, Delson [2] made a small modification by placing M. arctoides into the sinica group and M. sylvanus in a group by itself. Meanwhile, Groves [6] divided the genus into six species groups, arguing that Sulawesi macaques separated from the silenus group and formed their own group, and that M. mulatta, M. fuscata and M. cyclopis removed from the fascicularis group to form a new M. mulatta group. Recently, Zinner et al. [7] and Roos et al. [8] proposed seven species groups including three monotypic species groups (M. sylvanus group, M. arctoides group and M. fascicularis group) and four polytypic groups (Sulawesi group, mulatta group, sinica group and silenus group). Recent molecular studies were largely based on mitochondrial DNA (mtDNA) genes [9–14], autosomal genes [15,16], Y chromosomal sequences [16,17], and presence/absence polymorphism of Alu elements [18]. Despite some general consensus, substantial discordances detected on several branches, such as the relationships among closely related species within the same species groups (sinica group and fascicularis group) and the phylogenetic position of M. arctoides, remain unresolved. Furthermore, the short time of divergence and rapid radiation in the genus lead to introgression (i.e., introgressive hybridization) and gene flow among different species/species groups [16–20].
Different genetic markers, such as mobile elements and sequence data from the mitochondrial genome and nuclear genome, have their own unique characteristics in phylogenetic analysis. The complete mitochondrial genome has distinct advantages: a small sequence (~16 kb), a single and no-recombination locus and the maternally inherited, making it a better marker to reconstruct phylogenetic relationships than a single gene or partial genome sequences [21–24]. In addition, the mutation rate of mtDNA in primates was estimated five to ten times higher than that of the nuclear genome [25], indicating it is suitable to resolve relationships even among closely related species. On the other hand, the nuclear loci are essential as well, because they represent neutral and paternal inheritance. Particularly, non-coding intron sequences offer potentially powerful genetic markers, since they have a number of traits that are suitable for molecular phylogenies [26–29]. Another kind of nuclear marker, Alu elements, are thought to be promising tools to uncover phylogenetic relationships. They predominantly possess two remarkable properties: essentially homoplasy-free with identical by descent and unidirectional with the absence of the insertion being the ancestral state [30]. Besides, they are easy to genotype using only a PCR-based approach. Accordingly, Alu elements have been applied to address issues with respect to human population genetics [31–34] as well as numerous controversial primate phylogenetic relationships [35–37]. In addition, SNPs (single nucleotide polymorphisms) and microsatellites are also important nuclear markers that have been widely used for population structure and genetic diversity studies [38–42] due to the unique features of high polymorphism among populations or individuals. Both of them are seldom applied to phylogenetic analyses in previous studies. Given that different genetic markers provide different information, it is suggested that a more robust phylogeny could be derived from the combination of analyses on different genetic systems. Combined analyses of the different molecular markers have been successfully employed to uncover complicated evolutionary relationships and to ascertain reasons that are responsible for incongruent phylogenetic relationships [22,43–45].
Previous studies on macaque phylogenies were merely based on a single genetic system [9–15,18], or a combination of short mitochondrial genes and intron sequences [16,17]. Herein, a PCR display method was applied to M. assamensis to identify newly phylogenetically informative Alu loci for phylogenetic analyses. Then we examined presence/absence pattern of 84 mobile elements and compared the inferred phylogeny with those obtained from mitochondrial data (10,832 bp each species) and nuclear sequence data of autosomes, Y and X chromosomes (9,465 bp each species). By combining these different markers systems, we attempted to resolve the controversial evolutionary relationships in macaque phylogeny, particularly the position of M. arctoides, and to trace ancient hybridization events in the macaque evolutionary history.
Materials and Methods
Samples collection and DNA extraction
Species analyzed in this study are listed in S2 Table including eight macaque species and Papio hamadryas as an outgroup. According to the records in captivity, all macaques have no opportunity to interbreed, and all individuals used in the study did not involve artificial interbreeding or hybridization. The Chengdu Institute of Biology Animal Use Ethics Committee approved this study on the phylogeny of macaques on the basis of the mitochondrial genome and nuclear data. The blood samples were obtained from captive macaques, which solely lived in large steel cages (height: 10m, length: 7.5, and width: 5m). Before drawing blood, these monkeys were anethetised with an intramuscular injection of mixed ketamine (10 mg/kg) and xylazine 0.25–2.0 mg/kg). During the process, animal’s body temperatures, respiration and heart rate were monitored to alleviate suffering. One mL of blood was drawn and stored in disposable vacuum blood vessels with EDTA-K2. These monkeys were not injured throughout the procedure and immediately released after recovery from sedation. All the operations were carried out by professional veterinarians. Total genomic DNA was extracted from whole blood using standard phenol/chloroform methods [46].
Alu insertion loci identification and genotyping
Phylogenetically informative Alu loci from M. assamensis were identified by the PCR assay following the described methods [18,47]. Genomic DNA of M. assamensis was digested by a restriction enzyme NdeI (TaKaRa, China), and then ligated with double stranded linkers (S3 Table). For the first round, ligation products were amplified by using the LNP primer and a rhesus Alu specific primer YbI or YdI in order to obtain partial Alu sequences with the flanking unique sequences. The second round of PCR was performed using Alu YbII or YdII in order to obtain more specific amplicons. The amplicons were cloned into the pMD19-T vector (TaKaRa, China) and then transformed into DH5α competent cells. Positive clones were randomly screened and identified using the primer M13-47, sequencing on ABI 3730 Genetic Analyzer (Applied Biosystems). Sequences with a target Alu element and at least 100 bp of flanking sequences were used as queries in the BLAT [48] searching against rhesus macaque genome to discover the homologous position of insertion. Polymorphic Alu insertions that were present in the Assamese macaque but absent in the rhesus macaque genome were potentially phylogenetically informative and used for further phylogenetic reconstructions.
After the computational search, primers for the candidate loci were designed in flanking sequences using Primer3 [49] based on the reference genome. All primers were firstly tested for PCR amplifications with a temperature gradient (48–60°C) on a template consisting of Assamese and rhesus macaques to verify the most appropriate annealing temperature. Then these newly identified Alu loci (S4 Table), together with 67 previously published loci (S5 Table), which were obtained from M. mulatta and M. fuscata [50], and from M. arctoides, M. thibetana, M. nemestrina and M. fascicularis [18,51], were further genotyped for the presence/absence pattern in all specimens. The detailed information on each locus, including primer sequences, chromosomal locations, annealing temperatures, PCR product sizes, and amplification results of studied species was described in S4 and S5 Tables. Presence of an insertion was designated as “1”, absence as “0”, and missing data as “?”. The newly identified Alu loci from M. assamensis were deposited in GenBank with the following accession numbers KU612224-KU612231; KU645892-KU645899; and KU641401.
PCR amplification and sequencing
Complete mitochondrial genomes of the nine investigated species were obtained from GenBank (S6 Table), and the 12 mitochondrial protein coding genes (except for ND6 gene) were used for analyses. We also analyzed nuclear sequence data including five autosomal genes (ALB3, IRBP3, TNP2, TTR1, and vWF11) [22], two Y chromosomal genes (SRY and TSPY) [17], and one X chromosomal fragment (Xq13.3) [52]. We amplified these nuclear genes from each of the macaque species according to the primers in S3 Table and downloaded available nuclear sequences from GenBank (S6 Table). PCR amplification for each gene was set up in a 25μL reaction volume containing at least 25 ng total DNA, 200 nM of each primer, 200 μM dNTPs, 2.5 μL of 10×PCR buffer, and 2.5 U Taq DNA polymerase (TaKaRa, China). PCR amplification was carried out at 94°C for 5 min, 35 cycles of denaturation at 94°C for 1 min, annealing at varying temperatures for 1 min, extension at 72°C for 1 min, and a final extension step at 72°C for 10 min. PCR products were checked on 2% agarose gels, visualized using UV fluorescence (Bio-Rad, Hercules, CA), and sequenced on ABI 3730 Genetic Analyzer (Applied Biosystems). The newly obtained sequences were deposited in GenBank with accession numbers KT356221-KT356259.
Phylogenetic analyses
For the Alu elements, phylogenetic reconstruction was implemented by an exhaustive search with 10,000 bootstrap replications via the PAUP* 4.0b10 software [53] using Dollo parsimony analysis. The sequence data were initially aligned using MEGA 5.2.2 [54] with default settings. Subsequently, poorly aligned positions and indels were removed by eye. Multiple analyses were then performed on the combined 12 mitochondrial protein coding genes and concatenated nuclear dataset. To test whether different nuclear data can be combined, partition homogeneity tests were performed in PAUP with 1000 replicates. Simultaneously, in order to compare the results from different genetic markers, we also performed analyses on five autosomal loci, two Y chromosomal genes, and one X chromosomal region, respectively. Phylogenetic analyses of all datasets were implemented using PAUP* 4.0b10 [53] for MP analyses, using MrBayes v3.1.2 [55] for Bayesian inference (BI) as well as using PHYML[56,57] for ML analyses with 500 bootstrap replications. For MP analyses, the reliability of the clades in phylogenetic trees was assessed by bootstrap probabilities (BSP) computed using 1000 replicates with 20 random additional sequencing replicates for each bootstrap replicate. Alternatively, for ML and Bayesian algorithms, the optimal nucleotide substitution model were chosen using the Akaike Information Criterion (AIC) as implemented in jModeltest 2.1 [58]. For Bayesian reconstructions, relative support of internal nodes was assessed by Bayesian posterior probabilities (BPP) analyses. Two separate runs were performed with four Monte Carlo Markov Chains with the default temperature of 0.1. Four repetitions were run for 1000,000 generations with tree and parameter sampling occurring every 100 generations. The first 25% of samples were discarded as burn-in, leaving 75,001 trees per run. Posterior probabilities for each split were calculated from the posterior density of trees.
Divergence age estimation
A Bayesian MCMC method was applied to estimate divergence times within the genus Macaca by using software BEAST v1.7.5 [59]. As calibration, we used the fossil-based divergence between African and Asian lineages, which split about 5.5 mya [2,3,16] as well as the divergence between Papio and Theropithecus gelada, which split ~ 4 mya [60]. In BEAST, a correlated lognormal model of lineages variation and a Yule process for branching rates were implemented in each analysis. Divergence times were estimated for the nuclear dataset and the combined 12 mitochondrial genes with a mean of 5.5 mya and a standard deviation (SD) of 0.4 mya for the separation of M. sylvanus and Asian macaques as well as with a mean of 4.0 mya and SD of 0.5 mya for the Papio and T. gelada. For the MCMC analysis, two replicates were run for 1000,000 generations with tree and sampling occurring every 1000 generations. The log output files were carried out by the program Tracer [61]. 10% of the trees were discarded as burn-in, and the remaining was assessed by TreeAnnotator v1.7.5 within the BEAST package to obtain a consensus tree, which was visualized with Figtree v1.4.0 [62].
Results
Phylogenetic analyses of Alu elements
Combining a computational approach and a PCR display method, we identified phylogenetically informative Alu loci from M. assamensis. A total of 154 clones were randomly selected for sequencing analyses. The results indicated that 70 sequences contained no repetitive elements or elements beyond the AluY subfamily. The remaining 84 sequences with target AluY elements were further applied to the BLAT search. Among those 84 sequences, we failed to identify the homologous locations of 17 sequences owing to short flanking sequences. After the BLAT search, an additional 40 Alu loci were found to be shared by the rhesus macaque genome and the Assamese macaque genome, indicating they were probably not phylogenetically informative. We identified 27 phylogenetically informative Alu loci in M. assamensis. An additional 10 insertions were rejected because of failures of primer designing or genotyping. Ultimately, 17 novel insertions identified from Assamese macaque were amplified in all samples and were used for further phylogenetic reconstructions.
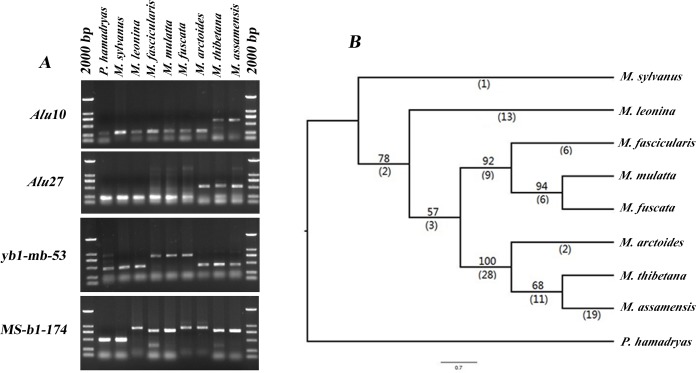
Combined with 67 previously reported Alu loci, a total of 84 loci were genotyped the presence/absence in eight macaque species and one outgroup. Four examples of gel electrophoresis patterns of amplification results are shown in Fig 1A, and the detailed information on gel electrophoresis of all Alu insertions in our study was shown in S1 Fig. Sixty-three of the 84 Alu loci were found to be parsimony informative generating a single most parsimonious tree [Fig 1B, consistency index (CI) = 0.785; homoplasy index (HI) = 0.215; retention index (RI) = 0.770]. The topology of the tree clearly defined four main clades and six species groups: sylvanus group (M. sylvanus), silenus group (M. leonina), arctoides group (M. arctoides), sinica group (M. assamensis and M. thibetana), fascicularis group (M. fascicularis), and mulatta group (M. mulatta and M. fuscata), which were consistent with the most recent classification [7, 8]. M. sylvanus was in a basal position as a sister to all Asian macaques. Within the Asian macaque lineage, thirteen Alu insertion loci supported that M. leonina diverged first forming a sister clade to other four species groups (BSP = 72%). Whereas 28 unambiguous insertions clustered M. assamensis, M. thibetana and M. arctoides together with high bootstrap values (BSP = 100%). Another 11 insertions were shared by M. assamensis and M. thibetana indicating a close relationship between them, while M. arctoides formed a sister clade to them (BSP = 68%). Within the fascicularis/mulatta lineage, six Alu insertion loci supported M. fascicularis diverging first, and M. mulatta and M. fuscata shared a close relationship which was supported by six Alu insertion loci.
Fig 1. Phylogenetic relationships among macaques based on Alu elements.
(A) PCR amplification analysis of Alu insertion polymorphisms in Macaca. The locus Alu 10 is an Alu insertion specific to the sinica group. The locus Alu 27 is an Alu insertion shared by the sinica and the arctoides groups. The locus yb1-mb-53 is an Alu insertion shared by the fascicularis and the mulatta groups. The locus MS-b1-174 is an Alu insertion clustering the sinica/arctoides and the fascicularis/mulatta lineages. (B) Macaque phylogenetic tree derived from 84 Alu insertion loci polymorphisms. The amplification patterns of the Alu insertions were used to construct a Dollo parsimony tree of macaque phylogenetic relationships using P. hamadryas as outgroup in PAUP*4.0b10. The numbers above the branches indicate the percentage of bootstrap replicates (1000 iterations) producing trees including that node. The numbers below the branches indicate the number of unambiguous insertions supporting each node.
Phylogenetic analyses of nuclear dataset
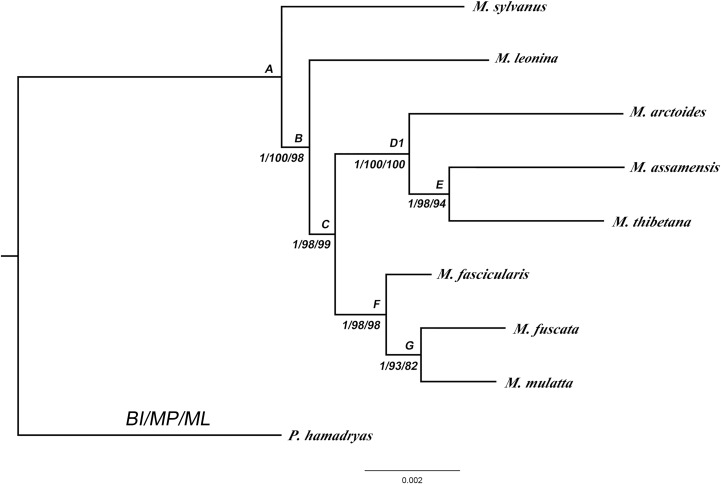
Next, phylogenetic reconstructions were performed on the combined nuclear sequences of five autosomal loci, two Y chromosomal genes and one X chromosomal region. Partition homogeneity tests revealed no significant difference in their evolutionary history (Y chromosomal loci combined: P = 0.2471; autosomal loci combined: P = 0.1305; all nuclear loci combined: P = 0.3021). The concatenated nuclear datasets were consisted of 9,465 nucleotide positions. The best fit model of nucleotide substitution for the concatenated dataset was TVM+I (Table 1). The CI (0.9636) and RI (0.8171) of the nuclear dataset were higher than those of Alu elements and mitochondrial data, which indicated less homoplasy of the nuclear genes. Phylogenetic trees obtained from BI, MP and ML analyses yielded identical and significantly supported branching patterns (BPP = 1, BSP> 93%, BSP>94%) (Fig 2). Only the M. fuscata/mulatta clade gained relatively weak bootstrap values (ML: 82%). In principal, the resultant tree topology was identical to the Alu element-based phylogeny.
Table 1. Characterization of nuclear loci and mitochondrial genome for phylogenetic analyses.
| Locus | Length | Inform-ative | Variable | CI | RI | Best model | Alpha |
|---|---|---|---|---|---|---|---|
| ALB3 | 809 | 2 | 29 | 0.9688 | 0.8750 | HKY | N/A |
| IRBP3 | 1561 | 19 | 76 | 0.9796 | 0.9545 | TVM | N/A |
| TNP2 | 815 | 10 | 79 | 0.9663 | 0.8000 | GTR+G | 0.1464 |
| TTR1 | 743 | 1 | 29 | 0.9818 | 0.7857 | TVM+I | N/A |
| vWF11 | 945 | 3 | 42 | 1.0000 | 1.0000 | TrN | N/A |
| Com autosome | 4973 | 35 | 255 | 0.9597 | 0.7105 | TVM+I+G | 0.1459 |
| X chromosome | 1498 | 6 | 21 | 1.0000 | 1.0000 | TVM+I+G | N/A |
| SRY | 770 | 6 | 23 | 1.0000 | 1.0000 | TVM | N/A |
| TSPY | 2224 | 23 | 75 | 0.9870 | 0.9688 | GTR | N/A |
| Com Y chromosome | 2994 | 29 | 98 | 0.9892 | 0.9722 | GTR+I+G | N/A |
| Com nuclear genome | 9465 | 70 | 374 | 0.9636 | 0.8171 | TVM+I+G | 0.0774 |
| ATP6 | 678 | 132 | 258 | 0.6797 | 0.4739 | TrN+G | 0.3200 |
| ATP8 | 198 | 42 | 81 | 0.7016 | 0.5375 | TrN+I | N/A |
| COX1 | 1539 | 241 | 436 | 0.6539 | 0.4404 | HKY+G | 0.1900 |
| COX2 | 681 | 108 | 189 | 0.6472 | 0.4577 | TIM+G | 0.1330 |
| COX3 | 783 | 116 | 243 | 0.7378 | 0.5309 | TPM2uf+G | 0.3310 |
| CYTB | 1141 | 184 | 344 | 0.6619 | 0.4388 | TrN+G | 0.1710 |
| ND1 | 948 | 142 | 277 | 0.6857 | 0.4923 | HKY+G | 0.2270 |
| ND2 | 1038 | 155 | 325 | 0.7231 | 0.5092 | TrN+G | 0.2600 |
| ND3 | 345 | 59 | 121 | 0.7500 | 0.5591 | TrN+I | N/A |
| ND4 | 1378 | 252 | 477 | 0.6576 | 0.4325 | TrN+I | N/A |
| ND4L | 294 | 47 | 100 | 0.7034 | 0.4756 | TrN+I | N/A |
| ND5 | 1809 | 281 | 585 | 0.7064 | 0.4667 | TPM2uf+G | 0.3100 |
| Com Mt | 10832 | 1759 | 3436 | 0.6822 | 0.4610 | TIM2+G | 0.2470 |
| Total datasets | 20297 | 1829 | 3810 | - | - | - |
CI: consistency index; RI: retention index; Best model: best fitting model under the Akaike information criterion; Alpha: gamma distribution shape parameter; Mt: mitochondrial genome.
Fig 2. Molecular phylogenetic tree derived from nuclear data using Bayesian, MP and ML analysis.
The numbers are Bayesian posterior probabilities (BPP) and bootstrap support (BSP). The A-G besides the nodes refers to divergence times shown as in Table 2.
We also performed phylogenetic analyses based on autosomal loci, Y chromosomal genes, and X chromosomal fragment. The best fit models of nucleotide substitution for the three datasets were TVM+G, GTR+I and TVM (Table 1), respectively. The trees estimated based on different molecular markers are shown in S2 Fig. The results appeared similar to the Alu element-based tree and the combined nuclear tree, but differed in several nodes. First, M. fuscata was closely related to M. mulatta in the Alu elements tree, Y and X chromosomal trees, and the combined nuclear genes tree, while, in the autosomal tree, it formed a sister clade to other four species groups with margin of support values (BPP = 0.86, BSP = 65%, BSP = 66%). Second, the position of M. arctoides was unstable in different nuclear trees. Both autosomal and Y chromosomal trees supported that M. arctoides diverged earlier than the split of M. thibetana and M. assamensis, which was consistent with the combined nuclear tree and the Alu element-based tree, while X chromosomal tree showed M. arctoides was closer to M. assamensis than to M. thibetana. Unexpectedly, the Y chromosomal tree nested M. sylvanus within the Asian macaques, and then clustered with the sinica/arctoides lineage (BPP = 0.21, BSP = 51%, BSP = 70%) contradicting to other nuclear genes, Alu elements, and mitochondrial topologies.
Phylogenetic analyses of mitochondrial dataset
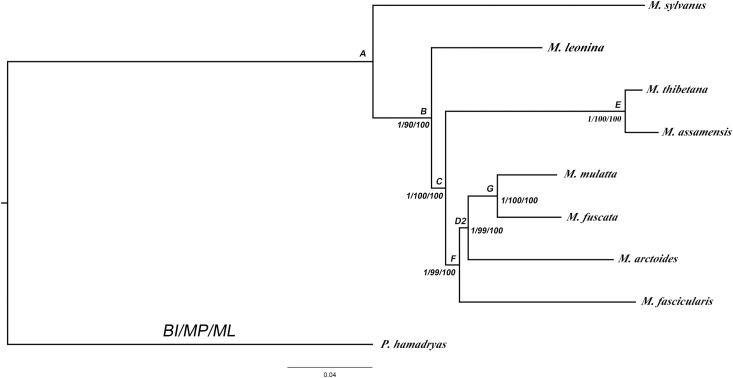
We did not combine mitochondrial and nuclear data due to differences in effective population size, dispersal rate, and mode of inheritance [63,64]. The combination of 12 mitochondrial protein coding genes included 10,832 aligned nucleotide positions. The best fit model of nucleotide substitution was TIM2+G (Table 1). The topology of mitochondrial data tree (Fig 3) was congruent with those obtained from mobile elements and nuclear sequence data with the exception of the position of M. arctoides. The mitochondrial data tree unambiguously clustered M. arctoides into the fascicularis/mulatta lineage. Particularly, it showed a closer relationship to the mulatta group than to the fascicularis group (BPP = 1, BSP = 99%, BSP = 100%), whereas both the Alu elements and the nuclear genes supported a close relationship of M. arctoides to the sinica group (M. thibetana and M. assamensis).
Fig 3. Molecular phylogenetic tree obtained from mitochondrial genome using Bayesian, MP and ML analysis.
The numbers are Bayesian posterior probabilities (BPP) and bootstrap support (BSP). The A-G besides the nodes refers to divergence age shown as in Table 2.
Divergence age estimation
Divergence age estimation based on nuclear genes and the mitochondrial genome was summarized in Table 2 and S3 Fig. The nuclear data estimated that the African and Asian lineages separated ~5.36 mya. In the Asian lineage, an initial split occurred at ~4.32 mya, which led to a clade containing M. leonina and a clade consisting of the fascicularis/mulatta lineage and the sinica/arctoides lineage. These two lineages successively diverged from each other around 3.08 mya. Within the fascicularis/mulatta lineage, M. fascicularis diverged first around 1.79 mya, and then M. fuscata separated from M. mulatta at ~0.88 mya. Speciation and divergence of the sinica/arctoides lineage occurred almost at the same time with the fascicularis/mulatta lineage. Within the sinica group, M. arctoides represented an early divergence at ~1.77 mya in this lineage, while M. thibetana and M. assamensis represented the most recent separation at ~0.58 mya. However, the divergence estimations based on the mitochondrial genome generally were earlier than the estimations based on nuclear data. The mitochondrial tree inferred that the divergence date between fascicularis/mulatta lineage and sinica lineage was ~3.81 mya. Within the fascicularis/mulatta lineage, M. fascicularis diverged first at ~2.92 mya. The mitochondrial tree placed M. arctoides in the fascicularis/mulatta lineage and indicated that the divergence of M. arctoides occurred ~2.56 mya, which was earlier than the split of M. mulatta and M. fuscata (~1.38 mya). Both the nuclear and mitochondrial data supported that the most recent divergence occurred between M. thibetana and M. assamensis at no more than 1.0 mya.
Table 2. Divergence date estimations based on the nuclear genes and mitochondrial genome.
| Node | Divergence dates (MYA) | |
|---|---|---|
| nuclear genes | mitochondrial genome | |
| Macaca—Papio/Theropithecus | 6.58 (4.91–10.6) | 9.35 (4.75–11.4) |
| Papio—Theropithecus | 3.83 (2.45–4.76) | 3.89 (3.14–4.66) |
| (A) African—Asian macaque | 5.36 (4.84–6.01) | 5.38 (4.69–6.04) |
| (B) silenus group | 4.32 (1.98–5.79) | 4.83 (4.24–5.55) |
| (C) sinica—fascicularis/mulatta lineage | 3.08 (0.99–5.11) | 3.81 (3.23–4.55) |
| (D1) M. arctoides—M. thibetana/M. assamensis | 1.77 (0.27–3.75) | - |
| (D2) M. arctoides—mulatta group | - | 2.56 (1.75–3.51) |
| (E) M. thibetana—M. assamensis | 0.58 (0.02–2.45) | 0.54 (0.01–0.71) |
| (F) fascicularis—mulatta group | 1.79 (0.40–3.82) | 2.92 (0.18–3.60) |
| (G) M. mulatta—M. fuscata | 0.88 (0.13–2.40) | 1.38 (0.04–1.80) |
Discussion
Combination of different genetic systems to infer macaque phylogeny
Different types of genetic markers are subjected to a unique stress from biological and behavioral conditions reflecting different evolutionary signals. The mitochondrial genome, Y chromosome, and autosome represent maternal, paternal, and bi-parental lineage, respectively. Single genetic marker can only reflect one kind of evolutionary pattern, making it difficult to discuss comprehensive analysis. However, multiple genetic systems can provide helpful information in phylogenetic analyses [16,22,43–45]. By combining presence/absence pattern of Alu elements with autosomal, Y chromosomal, X chromosomal sequences, and the mitochondrial genome, our study provides comprehensive insights into the evolutionary history of macaque species. The phylogenetic trees and the estimated divergence ages from different datasets are broadly in line with those reported in previous studies [16,18,23]. Compared with previous macaque phylogenetic studies, most relationships have been resolved and obtained stronger supported by Alu elements and sequence data in our study. Although we did not include the Sulawesi macaques, other six species groups (sylvanus, silenus, sinica, arctoides, mulatta, and fascicularis) has been clearly defined in the genus Macaca, which are generally in agreement with the results of Zinner et al. [7] and Roos et al. [8]. M. sylvanus is a sister taxon to all the Asian lineages, Within the Asian macaques, M. leonina in the silenus group separated first followed by the two sister clades, the sinica/arctoides and the fascicularis/mulatta lineages. They finally divided into the extant four species groups. Our study further supports two close relationships within this genus: M. assamensis and M. thibetana as well as M. mulatta and M. fuscata. The study also revealed several significant discrepancies among genetic systems and inferred sex-biased introgression or hybridization among ancestral macaque lineages such as the observed phylogenetic incongruences with respect to M. arctoides (see below for more details). In conclusion, our study suggested that the combination of maternally, paternally, and bi-parentally inherited markers along with the combination of sequence data with presence/absence patterns of mobile elements proved to be an adequate and reliable phylogenetic approach, particularly for revealing hybridization events.
The phylogenetic position and evolutionary history of M. arctoides
Based on morphological characters, such as reproductive organs [65,66], hair growth patterns [5,66,67], dentition and cranium structure [2,66], and allozyme frequencies [65,66,68], previous studies suggested that M. arctoides was closely associated with the sinica group. However, the molecular data with respect to the position of M. arctoides is less concordant. Phylogenetic analyses based on autosomal loci [15–17], Y chromosomal genes [16,17], and Alu elements [18] consistently agreed with the morphological studies in assigning M. arctoides into the sinica group. However, mitochondrial genes indicated that M. arctoides should be ascribed to the fascicularis group [9–14]. Even within the same species group, the evolutionary relationship of M. arctoides to other macaques remained unstable. In the present study, 28 Alu insertions were shared by M. arctoides, M. thibetana, and M. assamensis, strongly confirming a close relationship among them. The close relationship of M. arctoides to the sinica group was further supported by the autosomal, Y chromosomal, and X chromosomal trees. With respect to the evolutionary relationship among the three macaques, both the nuclear genes and Alu elements consistently inferred that M. arctoides was a distinct species, which diverged earlier than the split of M. thibetana and M. assamensis. And the inferred relationship was strongly supported with high bootstrap values, whereas the X chromosomal tree lumps M. arctoides with M. assamensis, to the exclusion of M. thibetana with a marginal support value. According to similar morphological characteristics such as a relatively short tail and big body shape, many previous studies have suggested that M. arctoides was probably derived from a M. thibetana-like ancestor [18,66] or an ancestor closely related to proto-M. thibetana/assamensis [15–17]. However, our results disagreed with these hypotheses suggesting an early divergence of M. arctoides, and the molecular clock estimated that the divergence of M. arctoides occurred at ~1.77 mya. Further investigation should be employed with more sufficient samples from other sinica group species and including more genetic markers from the whole genomes to ascertain the evolutionary relationships among these closely related species.
Similar to previous mitochondrial studies, our mitochondrial genome tree contradicted the nuclear genes and the Alu elements-based tree on the position of M. arctoides. The complete mitochondrial genome demonstrated convincingly a close association of the stump-tailed macaque to the mulatta group instead of the sinica group. Based on one or several mtDNA genes, Morales and Melnick [11] and Li and Zhang [12,13] suggested M. arctoides was closer to M. fascicularis than to M. mulatta, whereas Tosi et al. [17] inferred it was closer to M. mulatta than to M. fascicularis. Our results associated M. arctoides with the mulatta group, which was consistent with Tosi et al. [17]. The estimated divergence age of M. arctoides was around 2.56 mya, after M. fascicularis had separated (~2.92 mya) and before the split of M. fuscata and M. mulatta (~1.38 mya). The divergence age estimations from the mitochondrial genome were slightly earlier than that from the nuclear dataset, most likely due to the relatively faster evolutionary rate of mitochondrial genes than nuclear genes [25].
Incongruent phylogenetic relationships among genes are common in phylogenetic analyses and could be explained by insufficient data, homoplasy, and incomplete lineage sorting (ILS) or hybridization [69–72]. In order to resolve which of these possibilities have been responsible for the discordances on the position of M. arctoides, we combined phylogenetic analyses based on maternal, paternal, and biparental genetic systems. First of all, inadequate data are not likely a problem, since both the base pairs of the nuclear dataset and mitochondrial genome are more than 9000 pairs. Similarly, homoplasy is also unlikely, because CI and RI of the Alu elements (0.785, 0.770), the nuclear dataset (0.964, 0.817) and the mitochondrial genome (0.682, 0.461) are relatively high. In addition, ILS cannot be the explanation for the incongruent phylogenetic relationships among genes because such limited divergence failed to account for the extensive similarities. Furthermore, mtDNA is less likely to show ILS, since it has a smaller effective population size which argues strongly against an ILS hypothesis. Although we cannot completely rule out that ILS may have had an effect by the combination of analyses on different genetic systems, a more likely reason for the detected discordances from nuclear and mitochondrial phylogenies is ancient hybridization between the closely related macaque species.
We further conducted comparative analysis of phylogenies generated from different genetic systems to reveal the potential hybridization pattern in M. arctoides. Tosi et al. [16,17] pointed out that M. arctoides was probably a hybrid taxon, originating from interbreeding proto-sinica species and proto-fascicularis species. Based on a large-scale application of Alu elements, the study of Li et al. [18] allied with the hybrid origin hypothesis. Based on the more sufficient datasets from maternal, paternal, and biparental genetic systems, we confirm the previous studies finding an ancient hybridization in M. arctoides. Nevertheless, M. arctoides is not of hybrid origin, and the hybridization occurred after it diverged from other sinica species. In particular, our results suggested that the hybridization in M. arctoides was not bidirectional but was more likely a male-mediated introgression followed by nuclear swamping.
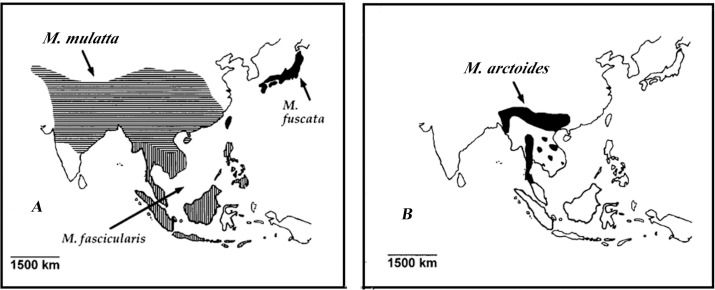
Given the high levels of male dispersal and extreme female philopatry in macaques, the most likely outcome of hybridization might be male-mediated introgression. According to the distribution and speciation of M. arctoides and M. mulatta (Fig 4), we assume that after the speciation of M. arctoides, the ancestors of M. arctoides pushed into South Asia because of the invasion of savanna and dry grassland [73] where the males of M. arctoides dispersed into the M. mulatta population, thus hybridization between them could have happened. The studies of Tosi et al. [16,17] also suggested that extensive hybridization events could have occurred between early sinica and fascicularis group members in a Pleistocene forest refugium. If the male-mediated gene flow continued over generations, and the hybrid offspring had backcrossed with male M. arctoides, the frequency of M. mulatta genetic signals on the sex chromosomes and autosomes would have been significantly reduced. The nuclear genome of M. mulatta thus have been ‘‘swamped” by that of M. arctoides in the hybrid population until it was completely replaced. In contrast, the original M. mulatta mitochondrial genome would stay in the population (Fig 5). The hybrid population gave rise to a unique genetic entity of the extant M. arctoides retaining a mitochondrial genome of the origin of rhesus macaque but autosomes, Y and X chromosomes of the sinica group. The divergence date estimation indicated that the introgression occurred 0.88~1.77 mya based on nuclear genes, or 1.38~2.56 mya based on mitochondrial genome, before the split of M. mulatta/fuscata and the split of M. thibetana/assamensis.
Fig 4. Distribution maps of M. mulatta and M. arctoides.
A refers to M. mulatta, and B represents M. arctoides. Distribution contours of individual species are according to Corbet and Hill [74].
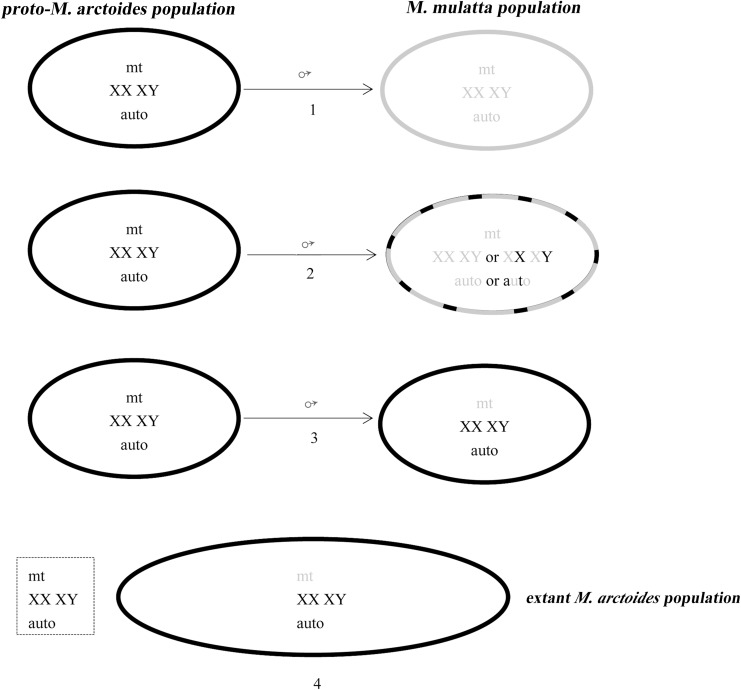
Fig 5. Male introgression and nuclear swamp.
The proto-M. arctoides population is characterized by a black mitochondrial genome (mt), and black sex chromosomes (XY) and autosomes (auto). The M. mulatta population covers a grey mitochondrial (mt), grey sex chromosomes (XX), and autosome. (1) Given male migration and female philopatry, the males from population proto-M. arctoides invade population M. mulatta and produce hybrid offspring carrying grey mt but a half black sex chromosome and autosome. (2) The female hybrids from population M. mulatta backcrossed with males of population proto-M. arctoides. (3) If this continued over generations, the frequency of population M. mulatta genetic signals on the grey sex chromosome and autosome would significantly decrease. The grey nuclear genome will be swamped by black nuclear genome until it is completely replaced. In contrary, the grey mitochondrial genome will remain in population M. mulatta. (4) The extant M. arctoides population only has one grey mitochondrial haplotype and one black nuclear genome. The black mitochondrial genome (dotted line in the square) disappeared probably due to bottleneck during glaciations period. (The Fig imitates Zinner et al. [75]).
Although the male-mediated introgression hypothesis was in agreement with our extensive analyses on maternal, paternal, and bi-parental molecular markers, there exists another hybridization hypothesis. For instance, a mitochondrial capture from M. mulatta to M. arctoides also could result in a similar discrepancy between the mitochondrial genome and nuclear genome. This hypothesis assumes that females of M. mulatta dispersed into the population of M. arctoides and hybridized with males of M. arctoides, whereas no backcrossing with the invading M. mulatta took place. If the process continued for generations, the nuclear genome of the invaded M. arctoides population would barely change but absorb the mitochondrial genome of M. mulatta. However, mitochondrial capture has never been suggested in macaque species due to the female philopatry and male dispersal characteristics. Thus we suggest that the hybridization hypothesis in M. arctoides would benefit from more extensive genome-wide investigations.
Other gene tree discordances in macaque phylogeny
Except for M. arctoides, we also detected other discordances in the positions of several macaque species among the gene trees. First, the Alu elements, concatenated nuclear data and mitochondrial data consistently supported a close relationship of M. fuscata and M. mulatta, while the autosomal tree (S2 Fig) suggested an outgroup clade of M. fuscata to the sinica/arctoides and the fascicularis/mulatta lineages. Tosi et al. [16] also detected a similar phylogenetic relationship of M. fuscata based on an intron sequence of the C4 gene. M. fuscata was usually considered to have diverged recently from the continental subspecies, M. mulatta tcheliensis, on the basis of fossil, morphological, and molecular evidence [4,5,76]. As an island macaque species endemic to Japan, the chance of introgression or hybridization with other macaque species is rare. Furthermore, both the X and Y chromosomal data did not show conflicting phylogenies with Alu elements and mitochondrial data with respect to the M. fuscata’s position. It is more likely that the incongruent position of M. fuscata relative to the autosomal tree and other gene trees resulted from differentially selected autosomal genes or ILS. Second, the Y-chromosomal tree (S2 Fig) nested M. sylvanus within the Asian macaques as a sister clade to the sinica/arctoides clade, which is in conflict with the Alu elements, concatenated nuclear data, and the mitochondrial genome based phylogenies as well as previous macaque phylogenetic studies [12,13,16,17]. M. sylvanus, the only species distributed in Africa, is isolated from the Asian macaques. Therefore, gene flow or hybridization among them can be excluded. In addition, the fossil evidence supported the earliest divergence of M. sylvanus in the genus Macaca [2,3]. We speculate that the conflicting position between the Y-chromosome and other genetic systems is due to insufficient informative sites of our Y-chromosomal genes or ILS. Another discordant case is that of M. assamensis. We applied PCR display methods to identify phylogenetically informative Alu insertions from M. assamensis for phylogenetic analysis. A total of 28 Alu insertions were identified and characterized. Seventeen of those loci were phylogenetically informative, which provided important information for the position of M. assamensis. Alu elements-based phylogeny was congruent with concatenated nuclear data and mitochondrial data, which showed support for M. assamensis and M. thibetana as the most closely related species, while the X-chromosome tree (S2 Fig) suggested M. assamensis was closer to M. arctoides than to M. thibetana. Introgression or hybridization resulted in discordant relationships with respect to M. assamensis, which cannot be rejected. However, the strong evidence from the Alu elements, nuclear genes, and mitochondrial genomes indicated that insufficient informative sites in the X-chromosomal data might have contributed to the discordance among gene trees.
Supporting Information
The M1 and M2 refer to 100bp ladder and 2000bp marker, respectively. The numbers 1–8 represent P. hamadryas, M. sylvanus, M. leonina, M. thibetana, M. arctoides, M. fascicularis, M. fuscata, M. mulatta, and M. assamensis, respectively.
(PDF)
The numbers under the branches are Bayesian posterior probabilities (BPP) and bootstrap support (BSP). Panels refer to combined autosomal sequence data (A), X chromosomal fragment (B), and Y chromosomal loci (C).
(PDF)
Panels refer to nuclear genes (A) and mitochondrial genome (B). The numbers above branches are Bayesian posterior probabilities (BPP). The A-G besides the nodes refers to divergence times shown as in Table 2. The horizontal blue rectangles indicate the estimated 95% credibility intervals of divergence times.
(PDF)
(DOCX)
(DOCX)
(DOCX)
(XLS)
(XLSX)
(XLSX)
Acknowledgments
We are grateful to workers of the Chengdu Zoo for the DNA sample collections and Prof. Timothy Moermond for English revision. The research was funded by the National Natural Science Foundation of China (No. 31270431and 31530068), and partly supported by the Sichuan Application Foundation Project (2015JY0268).
Data Availability
All sequence files are available from the Genbank database (accession number(s): KT356221-KT356259).
Funding Statement
The research was funded by the National Natural Science Foundation of China (No. 31270431and 31530068), and partly supported by the Sichuan Application Foundation Project (2015JY0268). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Raaum RL, Sterner KN, Noviello CM, Stewart CB, Disotell TR. Catarrhine primate divergence dates estimated from complete mitochondrial genomes: concordance with fossil and nuclear DNA evidence. J Hum Evol. 2005; 48: 237–257. [DOI] [PubMed] [Google Scholar]
- 2.Delson E. Fossil macaques phyletic relationships and a scenario of development In: Lindburg D.G. (Ed.), The Macaques: Studies in Ecology Behavior and Evolution. Van Nostrand Reinhold Co., New York; 1980. pp. 10–30. [Google Scholar]
- 3.Delson E. The oldest monkeys in Asia In: Takenaka O. (Ed.), Abstracts, International Symposium: Evolution of Asian Primates. Freude and Kyoto University, Primate Research Institute, Inuyama, Aichi, Japan; 1996, pp. 40. [Google Scholar]
- 4.Fooden J. Provisional classification and key to living species of macaques (Primates: Macaca). Folia primatologica 1976; 25:225–236. [DOI] [PubMed] [Google Scholar]
- 5.Fa JE. The genus Macaca: a review of taxonomy and evolution. Mammal Rev. 1989; 19:45–81. [Google Scholar]
- 6.Groves CP. Primate Taxonomy Smithsonian Institution Press, Washington DC; 2001. [Google Scholar]
- 7.Zinner D, Fickenscher GH, Roos C. Family Cercopithecidae (Old World Monkeys). In: Mittermeier RA, Rylands AB, Wilson DE, editors. Handbook of the Mammals of the World. Volume 3, Primates. Barcelona, Lynx Edicions, 2013, pp. 550–627.
- 8.Roos C, Boonratana R, Supriatna J, Fellowes J, Groves C, Nash SD, et al. An updated taxonomy and conservation status review of Asian primates. Asian Primates Journal 2014; 4: 2–38. [Google Scholar]
- 9.Zhang YP, Shi LM. Phylogenetic relationships of macaques as inferred from restriction endonuclease analysis of mitochondrial DNA. Folia Primatologica 1993; 60: 7–17. [DOI] [PubMed] [Google Scholar]
- 10.Hayasaka K, Fujii K, Horai S. Molecular phylogeny of macaques: implications of nucleotide sequences from an 896-base pair region of mitochondrial DNA. Mol Biol Evol. 1996; 13: 1044–1053. [DOI] [PubMed] [Google Scholar]
- 11.Morales JC, Melnick DJ. Phylogenetic relationships of the macaques (Cercopithecidae: Macaca), as revealed by high resolution restriction site mapping of mitochondrial ribosomal genes. J Hum Evol. 1998; 34: 1–23. [DOI] [PubMed] [Google Scholar]
- 12.Li QQ, Zhang YP. A molecular phylogeny of Macaca based on mitochondrial control region sequences. Zool Research 2004; 25: 385–390. [Google Scholar]
- 13.Li QQ, Zhang YP. Phylogenetic relationships of the macaques (Cercopithecidae: Macaca), inferred from mitochondrial DNA sequences. Biochem Genet. 2005; 43:375–386. [DOI] [PubMed] [Google Scholar]
- 14.Rahim NAA, Gani M, Abdullah MT. Phylogenetic relationships of macaques (Cercopithecidae: Macaca) Inferred from partial mitochondrial DNA (mtDNA) cytochrome oxidase II (COII) gene. Faculty of Reource Science and Technology 2014; 4: 42–51. [Google Scholar]
- 15.Deinard A, Smith DG. Phylogenetic relationships among the macaques: evidence from the nuclear locus NRAMP1. J Hum Evol. 2001; 41: 45–59. [DOI] [PubMed] [Google Scholar]
- 16.Tosi AJ, Morales JC, Melnick DJ. Paternal, maternal, and biparental molecular markers provide unique windows onto the evolutionary history of macaque monkeys. Evolution 2003; 57: 1419–1435. [DOI] [PubMed] [Google Scholar]
- 17.Tosi AJ, Morales JC, Melnick DJ. Comparison of Y chromosome and mtDNA phylogenies leads to unique inferences of macaque evolutionary history. Mol Phylogenet Evol. 2000;17: 133–144. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Han K, Xing J, Kim HS, Rogers J, Ryder OA, et al. Phylogeny of the macaques (Cercopithecidae: Macaca) based on Alu elements. Gene 2009; 448: 242–249. 10.1016/j.gene.2009.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan G, Zhang G, Fang X, Zhang Y, Li C, Ling F, et al. Genome sequencing and comparison of two nonhuman primate animal models, the cynomolgus and Chinese rhesus macaques. Nat Biotechnol. 2011; 29: 1019–1023. 10.1038/nbt.1992 [DOI] [PubMed] [Google Scholar]
- 20.Fan Z, Zhao G, Li P, Osada N, Xing J, Yi Y, et al. Whole-genome sequencing of Tibetan macaque (Macaca thibetana) provides new insight into the macaque evolutionary history. Mol Biol Evol. 2014; 10.1093/molbev/msu104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen YY, Liang L, Sun YB, Yue BS, Yang XJ, Murphy RW, et al. A mitogenomic perspective on the ancient, rapid radiation in the Galliformes with an emphasis on the Phasianidae. BMC Evol Biol. 2010; 10: 132 10.1186/1471-2148-10-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roos C, Zinner D, Kubatko LS, Schwarz C, Yang M, Meyer D, et al. Nuclear versus mitochondrial DNA: evidence for hybridization in colobine monkeys. BMC Eevol Biol. 2011; 11: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liedigk R, Roos C, Brameier M, Zinner D. Mitogenomics of the Old World monkey tribe Papionini. BMC Evol Biol. 2014; 14: 176 10.1186/s12862-014-0176-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liedigk R, Kolleck J, Böker KO, Meijaard E, Md-Zain BM, Abdul-Latiff MAB, et al. Mitogenomic phylogeny of the common long-tailed macaque (Macaca fascicularis fascicularis). BMC Genomics 2015; 16: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown WM, Prager EM, Wang A, Wilson AC. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982; 18: 225–239. [DOI] [PubMed] [Google Scholar]
- 26.Matthee CA, Eick G, Willows-Munro S, Montgelard C, Pardini AT, Robinson TJ, et al. Indel evolution of mammalian introns and the utility of non-coding nuclear markers in eutherian phylogenetics. Mol Phylogenet Evol. 2007; 42: 827–837. [DOI] [PubMed] [Google Scholar]
- 27.Yu L, Luan PT, Jin W, Ryder OA, Chemnick LG, Davis HA, et al. Phylogenetic utility of nuclear introns in interfamilial relationships of Caniformia (Order Carnivora). Syst Biol. 2011; 60: 175–187. 10.1093/sysbio/syq090 [DOI] [PubMed] [Google Scholar]
- 28.Haddrath O, Baker AJ. Multiple nuclear genes and retroposons support vicariance and dispersal of the palaeognaths, and an Early Cretaceous origin of modern birds. Proc Biol Sci. 2012; 279: 4617–4625. 10.1098/rspb.2012.1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen D, Liu Y, Davison GWH, Dong L, Chang J, Gao S, et al. Revival of the genusTropicoperdix Blyth 1859 (Phasianidae, Aves) using multilocus sequence data. Zool J Linn Soc-Lond. 2015; 175: 429–438. [Google Scholar]
- 30.Batzer MA, Deininger PL. Alu repeats and human genomic diversity. Nat Rev Genet. 2002; 3: 370–379. [DOI] [PubMed] [Google Scholar]
- 31.Watkins WS, Rogers AR, Ostler CT, Wooding S, Bamshad MJ, Brassington AME, et al. Genetic variation among world populations: inferences from 100 Alu insertion polymorphisms. Genome Res. 2003; 13: 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordaux R, Lee J, Dinoso L, Batzer MA. Recently integrated Alu retrotransposons are essentially neutral residents of the human genome. Gene 2006; 373: 138–144. [DOI] [PubMed] [Google Scholar]
- 33.Cordaux R, Srikanta D, Lee J, Stoneking M, Batzer MA. In search of polymorphic Alu insertions with restricted geographic distributions. Genomics 2007; 90: 154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart C, Kural D, Stromberg M, Walker JA, Konkel MK, Stütz AM, et al. A comprehensive map of mobile element insertion polymorphisms in humans. PLoS Genet. 2011; 7 10.1371/journal.pgen.1002236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roos C, Schmitz J, Zischler H. Primate jumping genes elucidate strepsirrhine phylogeny. Proceedings of the National Academy of Sciences of the United States of America, 2004; 101: 10650–10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz J, Roos C, Zischler H. Primate phylogeny: molecular evidence from retroposons. Cytogenet Genome Res. 2005; 108: 26–37. [DOI] [PubMed] [Google Scholar]
- 37.Xing J, Wang H, Han K, Ray DA, Huang CH, Chemnick LG, et al. A mobile element based phylogeny of Old World monkeys. Mol Phylogenet Evol. 2005; 37: 872–880. [DOI] [PubMed] [Google Scholar]
- 38.Lin BZ, Sasazaki S, Mannen H. Genetic diversity and structure in Bos taurus and Bos indicus populations analyzed by SNP markers. J Anim Sci. 2010; 1: 281–289. [DOI] [PubMed] [Google Scholar]
- 39.Van Inghelandt D, Melchinger AE, Lebreton C, Stich B. Population structure and genetic diversity in a commercial maize breeding program assessed with SSR and SNP markers. Theor Appl Genet. 2010; 120: 1289–1299. 10.1007/s00122-009-1256-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia XD, Yang BD, Yue BS, Yin HL, Wang HX, Zhang YP, et al. Isolation and characterization of twenty-one polymorphic microsatellite loci in the Tibetan macaque (Macaca thibetana). RussJ Genet. 2011; 47: 884–887. [PubMed] [Google Scholar]
- 41.Emanuelli F, Lorenzi S, Grzeskowiak L, Catalano V, Stefanini M, et al. Genetic diversity and population structure assessed by SSR and SNP markers in a large germplasm collection of grape. BMC Plant Biol. 2013; 13: 39 10.1186/1471-2229-13-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filippi CV, Aguirre N, Rivas JG, Zubrzycki J, Puebla A. Population structure and genetic diversity characterization of a sunflower association mapping population using SSR and SNP markers. BMC Plant Biol. 2015; 15: 52 10.1186/s12870-014-0360-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ting N, Tosi AJ, Li Y, Zhang YP, Disotell TR. Phylogenetic incongruence between nuclear and mitochondrial markers in the Asian colobines and the evolution of the langurs and leaf monkeys. Mol Phylogenet Evol. 2008; 46: 466–474. 10.1016/j.ympev.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 44.Liedigk R, Thinh VN, Nadler T, Walter L, Roos C. Evolutionary history and phylogenetic position of the Indochinese grey langur (Trachypithecus crepusculus). Vietnamese J Primatol. 2009; 1: 1–8. [Google Scholar]
- 45.Liedigk R, Yang M, Jablonski NG, Momberg F, Geissmann T, Lwin N, et al. Evolutionary history of the odd-nosed monkeys and the phylogenetic position of the newly described Myanmar snub-nosed monkey Rhinopithecus strykeri. PLoS One 2012; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook J, Russell DW. Molecular Cloning: a Laboratory Manual, third ed. 2002. Science Press, Beijing. [Google Scholar]
- 47.Roy AM, Carroll ML, Kass DH, Nguyen SV, Salem AH, Batzer MA, et al. Recently integrated human Alu repeats: finding needles in the haystack. Genetica 1999; 107: 149–161. [PubMed] [Google Scholar]
- 48.Kent WJ. BLAT—the BLAST-like alignment tool. Genome Res. 2002; 12: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rozen S, Skaletsky HJ. 1998. Primer3. Code available at http://www-genomewimitedu/genome_software/other/primer3html.
- 50.Han K, Konkel MK, Xing J, Wang H, Lee J, Meyer TJ, et al. Mobile DNA in Old World monkeys: a glimpse through the rhesus macaque genome. Science 2007; 316: 238–240. [DOI] [PubMed] [Google Scholar]
- 51.Guo HW, Jiang J, Cui YY, Yi Y, Jia XD, Wang HX, et al. Identification and characterization of polymorphic Alu insertions in the Tibetan macaque (Macaca thibetana). Eur J Wildlife Res. 2015; 61: 143–149. [Google Scholar]
- 52.Tosi AJ, Melnick DJ, Disotell TR. Sex chromosome phylogenetics indicate a single transition to terrestriality in the guenons (tribe Cercopithecini). J Hum Evol. 2004; 46: 223–237. [DOI] [PubMed] [Google Scholar]
- 53.Swofford DL. 2003. PAUP*: Phylogenetic analysis using parsimony (*and other methods), version 4 Sunderland, MA: Sinauer Associates. [Google Scholar]
- 54.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28: 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003; 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 56.Farris JS, Kluge AG, Eckardt MJ. A numerical approach to phylogenetic systematics. Syst Biol. 1970; 19: 172–189. [Google Scholar]
- 57.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003; 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 58.Posada D. Selection of models of DNA evolution with jModelTest. Meth Mol Biol. 2009; 537: 93–112. [DOI] [PubMed] [Google Scholar]
- 59.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007; 7: 214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delson E. Cercopithecinae In Encyclopedia of Human Evolution and Prehistory. Edited by: Delson E, Tattersall I, Van Couvering JA, Brooks AS. New York: Garland Publishing; Inc 2000: 166–171. [Google Scholar]
- 61.Rambaut A, Suchard M, Xie D, Drummond A. Tracer v1. 6 http://beast.bio.ed.ac.uk. Tracer; 2014.
- 62.Rambaut A. http://tree.bio.ed.ac.uk/software/figtree/FigTree, a graphical viewer of phylogenetic trees; 2008.
- 63.Moore WS. Inferring phylogenies from mtDNA variation: mitochondrial-gene trees versus nuclear-gene trees. Evolution 1995; 718–726. [DOI] [PubMed] [Google Scholar]
- 64.Ruvolo M. Molecular phylogeny of the hominoids: inferences from multiple independent DNA sequence data sets. Mol Biol Evol. 1997; 14: 248–265. [DOI] [PubMed] [Google Scholar]
- 65.Fooden J, Lanyon SM (1989) Blood-protein allele frequencies and phylogenetic relationships in Macaca: A review. American Journal of Primatology 17: 209–241. [DOI] [PubMed] [Google Scholar]
- 66.Fooden J. The bear macaque, Macaca arctoides: a systematic review. J Hum Evol. 1990; 19: 607–686. [Google Scholar]
- 67.Inagaki H. Some hair characteristics of Macaca monkeys and an attempt to group them based on those features. Variation in the Asian Macaques (Shotake T, Wada K, eds)1996; 89–96. [Google Scholar]
- 68.Cronin J, Cann R, Sarich V. Molecular evolution and systematics of the genus Macaca In: Lindburg D.G. (Ed.), The Macaques: Studies in Ecology Behavior and Evolution. Van Nostrand Reinhold Co., New York, 1980, pp. 31–51. [Google Scholar]
- 69.Nichols R (2001) Gene trees and species trees are not the same. Trends in Ecology & Evolution 16: 358–364. [DOI] [PubMed] [Google Scholar]
- 70.McCracken KG, Sorenson MD. Is homoplasy or lineage sorting the source of incongruent mtDNA and nuclear gene trees in the stiff-tailed ducks (Nomonyx-Oxyura)? Syst Biol. 2005; 54: 35–55. [DOI] [PubMed] [Google Scholar]
- 71.Pollard DA, Iyer VN, Moses AM, Eisen MB. Widespread discordance of gene trees with species tree in Drosophila: evidence for incomplete lineage sorting. PLoS Genet. 2006; 2: e173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koblmüller S, Duftner N, Sefc KM, Aibara M, Stipacek M, Blanc M, et al. Reticulate phylogeny of gastropod-shell-breeding cichlids from Lake Tanganyika–the result of repeated introgressive hybridization. BMC Evol Biol. 2007;7: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eudey AA. Pleistocene glacial phenomena and the evolution of Asian macaques in D.G. Lindburg, ed. The macaques: studies in ecology, behavior, and evolution. Van Nostrand Reinhold, New York, 1980, pp. 52–83.
- 74.Corbet GB, Hill JE. The mammals of the Indomalayan region: a systematic review: Oxford University Press; Oxford; 1992. [Google Scholar]
- 75.Zinner D, Arnold ML, Roos C. The strange blood: natural hybridization in primates. Evol Anthropol. 2011; 20: 96–103. 10.1002/evan.20301 [DOI] [PubMed] [Google Scholar]
- 76.Kawamoto Y, Shotake T, Nozawa K, Kawamoto S, Tomari KI, Kawai S. et al. Postglacial population expansion of Japanese macaques (Macaca fuscata) inferred from mitochondrial DNA phylogeography. Primates 2007; 48: 27–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The M1 and M2 refer to 100bp ladder and 2000bp marker, respectively. The numbers 1–8 represent P. hamadryas, M. sylvanus, M. leonina, M. thibetana, M. arctoides, M. fascicularis, M. fuscata, M. mulatta, and M. assamensis, respectively.
(PDF)
The numbers under the branches are Bayesian posterior probabilities (BPP) and bootstrap support (BSP). Panels refer to combined autosomal sequence data (A), X chromosomal fragment (B), and Y chromosomal loci (C).
(PDF)
Panels refer to nuclear genes (A) and mitochondrial genome (B). The numbers above branches are Bayesian posterior probabilities (BPP). The A-G besides the nodes refers to divergence times shown as in Table 2. The horizontal blue rectangles indicate the estimated 95% credibility intervals of divergence times.
(PDF)
(DOCX)
(DOCX)
(DOCX)
(XLS)
(XLSX)
(XLSX)
Data Availability Statement
All sequence files are available from the Genbank database (accession number(s): KT356221-KT356259).