Abstract
We have observed that in vivo interaction between melanoma and resting T cells promotes suppression of antigen-driven proliferative T cell expansion. We hypothesized that this suppression would affect tumor antigen-specific T cell populations more potently than tumor-unrelated T cell populations. A B16F10 cell line was stably transfected to express low levels of the lymphocytic choriomeningitis virus (LCMV) glycoprotein GP33 (B16GP33). Mice bearing B16F10 or B16GP33 tumors were infected with LCMV, and proliferative expansion of LCMV epitope-specific T cell populations was quantified. In vitro and in vivo assays confirmed low levels of antigenic GP33 expression by B16GP33 tumors. Suppressed expansion of GP33-specific T cells was equivalent between mice bearing B16F10 and B16GP33 tumors. These observations suggest that the ability of growing melanoma tumors to impair antigen-driven proliferative expansion of activated T cells is global and not antigen-specific, and provide further insight into the influence of cancer on activated T cell homeostasis.
Keywords: T cell, Melanoma, Expansion, Antigen, LCMV
1. Introduction
Accumulating evidence indicates the presence of a complex interaction between the immune system and growing cancers, in which the ability of the immune system to mount tumor-specific responses appears to partially or transiently check the growth of cancers in vivo [1-3]. Ultimately, tumor growth is often accompanied by the gradual development and magnification of tumor-induced immune suppression that ultimately overcomes the ability of the immune system to control tumor growth [4,5]. A number of mechanisms with which tumor-specific immune responses are suppressed by cancer growth have been elucidated, and it is evident that experimental immunotherapeutic strategies intended to promote immunological control of cancer will need to be able to overcome these clinically undesirable suppressive phenomena[6-9].
Because of their ability to mediate immunological clearance of tumor cells, many immunotherapeutic strategies have relied on proliferative expansion of tumor-reactive CD8+ cytotoxic T lymphocytes (CTLs) [10]. However, the influence of cancers on T cell proliferation has not been fully explored. We have previously reported that in vivo interaction between growing melanoma tumors and resting T cells promotes a durable suppression of subsequent antigen-driven proliferation of those resting T cells [11]. C57BL/6 mice bearing syngeneic B16F10 tumors responded to lymphocytic choriomeningitis virus (LCMV) infection with weaker expansion of LCMV-specific CD8+ CTL populations compared with tumor-free mice. This suppression was associated with upregulated apoptosis among activated T cells previously exposed to melanoma. Importantly, the lack of antigenic cross-reactivity between B16F10 melanoma and LCMV suggests that this suppression was global and not antigen-specific. In the present report, we describe a series of experiments intended to compare the suppressive influence of melanoma tumors on proliferative expansion of T cells with specificity for tumor antigens to T cells with specificity for tumor-unrelated antigens. We hypothesized that this suppression of antigen-drive proliferative expansion would be strongest for T cells specific for tumor antigens. Using a B16F10 cell line stably transfected to express very low levels of an immunodominant LCMV epitope peptide, we observed that expansion of tumor antigen-specific and non-tumor antigen-specific T cells are comparably suppressed by melanoma.
2. Materials and methods
2.1. Mice
Seven- to eight-week-old female C57BL/6 mice were purchased from Taconic (Hudson, NY) and maintained in pathogen-free conditions. All animal work was performed in strict accordance with the guidelines of the University of Wisconsin and William S. Middleton Memorial VA Hospital Animal Care and Use Committees.
2.2. Tumor cell lines and virus
B16F10, a poorly immunogenic melanoma cell line derived from C57BL/6 mice, was maintained in RPMI-1640 medium (Mediatech, Herndon, VA) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine (Life Technologies, Inc., Grand Island, NY). B16F10 cells were transfected with the β-actin-driven expression vector pActin-IRES-TK-Neo encoding the minigene for the immunodominant LCMV epitope peptide GP33epitope (M-KAVYNFATM) (kindly provided by Dr. Hanspeter Pircher, Department of Immunology, Institute of Medical Microbiology and Hygiene, University of Freiburg, Freiburg, Germany). The resulting transfected cell line (heretofore referred to as B16GP33) was selected using 800 μg/mL G418 and continuously maintained using 200 μg/mL G418. Single inocula of 106 B16F10 or B16GP33 cells suspended in serum-free RPMI1640 media were injected subcutaneously into C57BL/6 mice and tumors were measured using electronic calipers every three days. Tumor volume was estimated as follows:
Mice were infected with 2 × 105 PFU of the Armstrong strain of LCMV by intraperitoneal injection. Levels of LCMV present in explanted tissues were quantified by plaque assay using Vero cells [12].
2.3. Chromium release assay
In vitro assessment of the antigenicity of GP33 expression on B16GP33 cells was assessed using a standard 51Cr-release assay, in which 104 target cells (B16F10, B16GP33, or B16F10 cells loaded with 1 μg/mL GP33 peptide) were labeled with 200 μCi of 51Cr for 2 h, then coincubated with GP33-specific CTLs harvested from C57BL/6 mice 8 days after LCMV infection at the indicated ratios for 4 h at 37 °C. Spontaneous release was measured by incubating target cells with media alone, and maximal release was measured by incubating target cells with the detergent centrimide (Sigma, Inc., St. Louis, MO). Supernatants were transferred to filter paper overnight and percentage of cytotoxicity was calculated based on radioactivity detected by gamma counter for each effector:target ratio as follows:
2.4. Flow cytometry
MHC class I tetramers loaded with various LCMV antigen peptides were prepared as previously described [12]. Single cell suspensions of splenocytes were stained with APC-labeled MHC class I (Db) tetramers loaded with class I-restricted LCMV epitope peptides (NP396, GP33, or GP276), PE-labeled anti-CD8, PECy7-labeled anti-CD62L, and FITC-labeled anti-CD44 antibodies. Alternatively, freshly harvested splenocytes (106 cells/well) were stimulated with or without various LCMV epitope peptides (NP396, GP33, GP34, or GP276) at a concentration of 0.1 μg/mL in the presence of brefeldin A and human recombinant IL-2 (10 U/well) at 37 °C for 5 h in flat-bottomed 96-well plates. Thus, LCMV antigen-specific T cell populations were identified either by their expression of T cell receptors specific for LCMV antigen peptides or by their ability to elaborate inflammatory cytokines in response to LCMV antigen peptide stimulation. In vitro assessment of the antigenicity of GP33 expression on B16GP33 cells was also tested by stimulating splenocytes in cells plated 48 h earlier with 104 B16GP33 or B16F10 cells. Cells were stained with anti-CD8 and anti-CD62L antibodies, then permeabilized and stained for intracellular cytokines using anti-IFNγ, anti-TNFα, and anti-IL-2 antibodies using the Cytofix/Cytoperm kit purchased from BD Biosciences-Pharmingen. Stained cells were acquired on a FACSCalibur flow cytometer (BD Biosciences) and resulting data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR). All reagents and antibodies were purchased from BD Biosciences-Pharmingen (San Diego, CA) with the exception of anti-CD127 and anti-granzyme B antibodies, which were purchased from eBioscience, Inc. (San Diego, CA), and Invitrogen, Inc. (Carlsbad, CA), respectively.
2.5. Statistical analysis
Experimental data were analyzed using SAS statistical software version 9.2 (Cary, NC). Groups were compared using an analysis of variance (ANOVA), and pair-wise comparisons were made using Fisher’s protected least significant difference tests and Tukey’s adjustment. All data were log-transformed prior to analysis in order to better meet the assumptions of ANOVA. All p-values reported are two-sided, and significance was defined as p < 0.05.
3. Results
3.1. The stably transfected B16GP33 cell line expresses GP33 at low levels of antigenicity
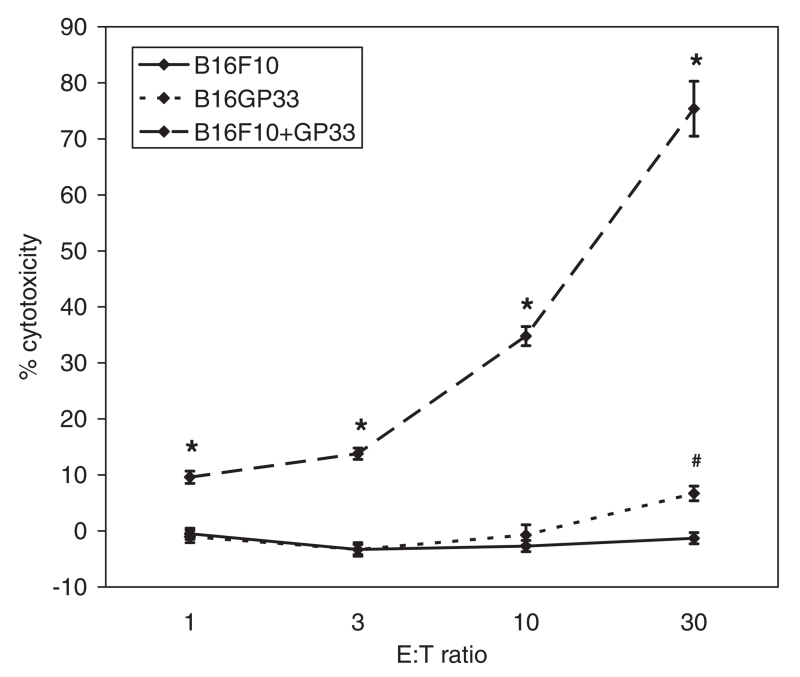
Transfected clones were selected in media containing 800 μg/mL G418, then passaged over 10 times in media containing 200 μg/mL G418 to confirm stable transfection. Chromium release assays identified statistically significantly greater lysis of transfected B16GP33 cells than parental B16F10 cells by activated splenocytes harvested from C57BL/6 mice 8 days after LCMV infection (Fig. 1). However, significantly lower levels of B16GP33 cell lysis were observed as compared with B16F10 cells exogenously supplemented with 1 μg/mL GP33 peptide, suggesting that the B16GP33 cell line expresses very low levels of GP33.
Fig. 1.
Chromium release assays were performed by incubating splenocytes from C57BL/6 mice 8 days after LCMV infection (effector cells) with B16F10 cells, B16GP33 cells, or B16F10 cells exogenously supplemented with 1 μg/mL GP33 peptide (“B16F10 + GP33”) (target cells) previously labeled with 200 μCi 51Cr at the indicated effector:target (“E:T”) ratios at 37 °C for 4 h. Percent cytotoxicity was calculated based on experimental release of 51Cr under experimental conditions, spontaneous release when target cells were incubated with media alone, and maximal release when target cells were incubated with centrimide (see Section 2). This experiment was repeated two times with identical results. (Error bars denote standard error of the mean; * denotes p < 0.05 of B16F10 + GP33 compared with B16F10; # denotes p < 0.05 of B16GP33 compared with B16F10).
Splenocytes were harvested from C57BL/6 mice 8 days after LCMV infection and incubated in the presence of B16F10, B16GP33, and B16F10 tumors supplemented with 0.0001 μg/mL or 0.001 μg/mL GP33 peptide in the presence of brefeldin and IL-2 for 5 h (Fig. 2). As was observed with the chromium release assay, coincubation with B16GP33 cells induced low levels of expression of IFNγ among splenocytes that was significantly greater than that observed by coincubation with B16F10 alone. The extent of GP33-specific T cell activation by B16GP33 cells was intermediate between that observed by coincubation with B16F10 exogenously supplemented with 0.0001 μg/mL and 0.001 μg/mL GP33 peptide.
Fig. 2.
Splenocytes harvested from C57BL/6 mice 8 days after LCMV infection were coincubated with B16F10 cells, B16GP33 cells, and B16F10 cells exogenously supplemented with the indicated concentration of GP33 peptide in the presence of brefeldin A and 10U IL-2 at 37 °C for 5 h. Splenocytes were then collected and CD8+ T cells were analyzed for intracellular levels of IFNγ expression by flow cytometry. This experiment was repeated three times with identical results. (Error bars denote standard error of the mean; * denotes p < 0.05 of B16GP33 compared with B16F10; # denotes p < 0.05 of B16GP33 compared with B16F10 + 10−4 μg/mL GP33 peptide, + denotes p < 0.05 of B16GP33 compared with B16F10 + 10−3 μg/mL GP33 peptide; NS denotes p > 0.05 between indicated groups).
3.2. In vivo growth of B16GP33 tumors is comparable to B16F10
In order to evaluate the immunogenicity of B16GP33 tumors in vivo, C57BL/6 mice were inoculated with subcutaneous injections of 106 B16F10 or B16GP33 cells, and tumor growth was measured. No differences in tumor growth kinetics were observed between B16F10 and B16GP33 tumors, suggesting that B16GP33 tumors did not promote a spontaneous immunological reaction capable of altering tumor growth (Fig. 3).
Fig. 3.
106 B16F10 or B16GP33 cells were suspended in serum-free media and administered as subcutaneous inocula into C57BL/6 mice (n = 6 mice per group). Tumor size was measured every three days. This experiment was repeated four times with identical results. (Error bars denote standard error of the mean).
3.3. GP33 expression on B16GP33 tumors exhibits in vivo antigenicity
In order to verify that antigenic levels of GP33 peptide were expressed by B16GP33 tumors in vivo, C57BL/6 mice were infected with intraperitoneal injections of 2 × 105 PFU Armstrong strain LCMV. On post-infection day 8 (coinciding with the point of viral clearance and maximal LCMV-specific T cell expansion), mice were inoculated with subcutaneous injections of 106 B16F10 or B16GP33 cells, and tumor growth was measured. B16F10 tumor growth occurred in a typically exponential manner, but no B16GP33 tumor growth was observed after LCMV infection, suggesting that the induction of large populations of activated GP33-specific T cells after LCMV infection was capable of preventing growth of B16GP33 tumors (Fig. 4).
Fig. 4.
106 B16F10 or B16GP33 cells were suspended in serum-free media and administered as subcutaneous inocula into C57BL/6 mice 8 days after LCMV infection (n = 5 mice per group). Tumor size was measured every three days. This experiment was repeated four times with identical results. (Error bars denote standard error of the mean; * denotes p < 0.05 of B16GP33 compared with B16F10).
3.4. Expansion of GP33-specific CTL populations after LCMV infection is comparably suppressed by B16F10 and B16GP33
We have previously observed that the ability of C57BL/6 mice to expand LCMV antigen-specific populations of CD8+ and CD4+ T cells is markedly suppressed in the presence of growing B16F10 tumors. To test our hypothesis this melanoma-induced suppression would affect the proliferative expansion of tumor antigen-specific T cells more potently than that of non-tumor antigen-specific T cells, we inoculated C57BL/6 mice with subcutaneous injections of media, 106 B16F10 cells, or 106 B16GP33 cells, then infected mice with intraperitoneal injections of 2 × 105 PFU Armstrong strain LCMV 10 days later. The kinetics of B16GP33 tumor growth decelerated between post-infection days 5 and 8, suggesting that massive expansion of GP33-specific CTL populations was capable of inhibiting the growth of GP33-expressing tumors (Fig. 5). Splenocytes were harvested on post-infection day 8 (coinciding with the point of viral clearance and maximal LCMV-specific T cell expansion [12]) and LCMV antigen-specific T cell populations were quantified based on TCR expression using MHC tetramer-based flow cytometric assays (Fig. 6A and B). As we have previously observed [11], peak expansion of LCMV antigen-specific T cell populations was smaller in mice bearing B16F10 tumors as compared with tumor-free controls. Despite minor inhibition of B16GP33 tumor growth after LCMV infection, expansion of GP33-specific CD8+ T cells was suppressed to a similar degree in mice bearing B16F10 and B16GP33 tumors, suggesting that suppressed expansion of tumor antigen-specific T cell populations in the presence of tumor was not more potent than that of non-tumor antigen-specific T cells in our model. When LCMV antigen-specific T cells were identified by culturing splenocytes and measuring intracellular expression of inflammatory cytokines after in vitro stimulation with LCMV antigen peptides and IL-2 for 5 h, a similar suppression of LCMV-induced T cell expansion was observed in tumor-bearing mice (Fig. 6C). Interestingly, larger populations of T cells expressing IFNγ in response to GP33 and GP34 stimulation were observed in splenocytes harvested from B16GP33 tumor-bearing mice than in B16F10 tumor-bearing mice, although these differences were not statistically significant.
Fig. 5.
106 B16F10 or B16GP33 cells were suspended in serum-free media and administered as subcutaneous inocula into C57BL/6 mice (n = 5 mice per group). On day 10, all mice were infected with LCMV. Tumor size was measured every three days. This experiment was repeated four times with identical results. (Error bars denote standard error of the mean; * denotes p < 0.05 of B16GP33 compared with B16F10).
Fig. 6.
(A) C57BL/6 mice were inoculated with serum-free media (“no tumor”) or 106 B16F10 or B16GP33 cells, then infected with LCMV on day 10, and euthanized on day 18 for splenocyte harvest (n = 6 mice per group). Splenocytes were stained with anti-CD8 antibodies and Db MHC class I tetramers loaded with LCMV epitope peptides, then analyzed by flow cytometry (upper panel). Alternatively, splenocytes were stimulated in vitro with LCMV epitope peptides, or left unstimulated as a negative control, for 5 h in the presence of brefeldin A and IL-2, stained with antibodies against CD8 and intracellular IFNγ, and analyzed by flow cytometry (lower panel). These representative plots are gated on total viable splenocytes and the highlighted populations indicate the percentage of CD8+ T cells specific for the immunodominant LCMV epitope peptide NP396. (B) Absolute numbers of CD8+ T cells (percent of tetramer-positive cells multiplied by total numbers of splenocytes) specific for NP396, GP33, and GP276 present 8 days after LCMV infection in control (“no tumor”), B16F10-bearing and B16GP33-bearing mice are shown. (C) Absolute numbers of CD8+ T cells expressing IFNγ in response to in vitro stimulation with NP396, GP33, GP34, and GP276 8 days after LCMV infection in control (“no tumor”), B16F10-bearing and B16GP33-bearing mice are shown. This experiment was repeated four times with identical results. (Error bars denote standard error of the mean; * denotes p < 0.05 of no tumor compared with B16F10; # denotes p < 0.05 of no tumor compared with B16GP33; NS denotes p > 0.05 between indicated groups; US denotes unstimulated negative controls).
4. Discussion
The ability of CD8+ cytotoxic T lymphocytes to mediate tumor recognition and killing makes this population of immune cells appealing for immunotherapy [10]. Adoptive immunotherapy strategies attempt to promote enough in vivo expansion of tumor antigen-specific CTLs in patients with cancer to induce tumor clearance [8,9,14,15]. To date, the precise influence of cancer on T cell expansion has not been comprehensively explored. It is possible that antigen-driven T cell proliferative expansion may be impaired in the setting of advanced malignancy. Indeed, recent trials in adoptive immunotherapy suggest that adjunctive strategies like host lymphodepletion may be necessary to permit a level of in vivo CTL expansion capable of exerting a therapeutic effect [14,15]. Our laboratory has previously demonstrated that growing melanoma tumors exert a suppressive influence on resting T cells that significantly inhibits their ability to undergo subsequent antigen-driven proliferative expansion [11]. This suppressive influence appears to be rather antigen-nonspecific, as the presence of B16F10 melanoma tumors inhibited the expansion of T cell populations specific to all LCMV epitopes after LCMV infection.
Given the well-documented ability of cancer to promote functional suppression of tumor antigen-specific T cell responses [4-9], we hypothesized that the global tumor-induced suppression of T cell expansion we observed would be particularly pronounced for activated T cells specific for tumor antigens. By inoculating mice with B16F10 tumors transfected to express the LCMV epitope peptide GP33, we expected that mice previously exposed to GP33 in the form of a tumor antigen would exhibit particularly marked suppression of GP33-specific T cell expansion in response to subsequent LCMV infection. In order to model the poor immunogenicity of human malignancies, experimental tumor cell lines must optimally exhibit very low levels of tumor antigen expression. This low level of antigenicity was confirmed using in vitro assays that suggested an approximate level of antigenicity equivalent to an in vitro GP33 peptide concentration of 5 × 10−4 μg/mL. In addition, the kinetics of B16GP33 tumor growth in vivo were comparable to those exhibited by the poorly immunogenic parental B16F10 tumors. Moreover, we were unable to identify GP33-specific T cell populations in splenocytes harvested from mice 18 days after B16GP33 tumor inoculation in the absence of LCMV infection (data not shown). However, these tumors retained some level of in vivo antigenicity, as evidenced by the inability of B16GP33 tumors to grow in mice bearing large quantities of acutely expanded activated GP33-specific CD8+ T cell populations after LCMV infection. This discrepancy of GP33 antigenicity evidenced in vitro versus in vivo is likely due to the massive numbers of activated GP33-specific CTLs present in mice in vivo following LCMV infection (that are not replicated in vitro), and the fact that CTLs and B16GP33 are coincubated for only 4 and 5 h in the chromium release and cytokine release assays shown in Figs. 1 and 2, respectively. Indeed, we have recently observed that at least 24-48 h of co-incubation of large quantities of activated GP33-specific CTLs with B16GP33 cells are needed in order to measure inhibited proliferative activity of B16GP33 cells (data not shown).
Contrary to our hypothesis, when quantifying antigen-specific T cell populations based on TCR specificity, we observed that the suppressed expansion of GP33-specific T cell populations by tumor was no more pronounced in mice bearing B16GP33 tumors than in mice bearing B16F10 tumors. LCMV antigen-specific T cell populations can also be identified by their ability to express inflammatory cytokines in response to brief in vitro stimulation with LCMV epitope peptides. When quantifying LCMV-specific T cell populations in this manner, we again observed smaller populations of activated T cells specific for the viral peptides NP396 and GP276 in B16F10 and B16GP33 tumor-bearing mice. In contrast, expression of IFNγ in response to GP33 and GP34 was reduced among activated T cells harvested from mice bearing B16F10 tumors but not from mice bearing B16GP33 tumors. This discrepancy between peptide-specific TCR expression and peptide-induced cytokine expression suggests that the expansion of GP33-specific T cells in response to LCMV infection was similarly suppressed in mice bearing B16F10 and B16GP33 tumors, but that presentation of GP33 as a tumor antigen by B16GP33 may differ from presentation of GP33 as a viral antigen by LCMV. Specifically, it is likely that presentation of GP33 as a tumor antigen may have primed Db-restricted T cell responses against GP33 as well as Kb-restricted T cell responses against GP34. GP33 and GP34 are overlapping 11-mer and 10-mer peptide sequences, respectively that share 10 common amino acids, with GP33 being largely Db-restricted and GP34 being Kb-restricted [13]. Stimulation with GP33 and GP34 peptides induced cytokine expression in distinct T cell populations in control and B16F10 tumor-bearing mice; in contrast, stimulation with either peptide may have induced cytokine expression in both T cell populations in B16GP33 tumor-bearing mice, suggesting that altered presentation of the GP33 peptide by B16GP33 tumor cells may have primed both cell populations in vivo. Indeed, the number of T cells expressing IFNγ in response to GP33 or GP34 alone in mice bearing B16GP33 tumors closely approximated the sum of T cells expressing IFNγ in response to GP33 and GP34 together in mice bearing B16F10 tumors. Alternatively, it is possible that exposure to GP33 as a tumor antigen prior to LCMV infection may have sensitized GP33-specific (and, by virtue of their antigenic overlap, GP34-specific) T cell activity against LCMV infection. We have previously reported that the suppressive influence of growing tumors in our model affects proliferative expansion but not individual cellular function, as evidenced by the absence of T cell anergy among activated T cells from tumor-bearing mice [11]. It is unlikely that anergy was induced among tumor antigen-specific T cell populations in the present experiment, as evidenced by the partial inhibition of B16GP33 (but not B16F10) tumor growth after LCMV infection. On the contrary, it remains possible that the low level of in vivo exposure to GP33 as a tumor antigen in our model might have potentiated GP33-specific T cell responsiveness in response to the high level of in vivo exposure to GP33 as a viral antigen.
The in vivo antigenicity of B16GP33 tumors was also evidenced by the late retardation of B16GP33 tumor growth seen between days 5 and 8 after LCMV infection. We have previously observed that the magnitude of suppressed T cell expansion is directly proportional to the extent of tumor burden. However, in light of the comparable numbers of LCMV-specific T cell populations seen between mice bearing B16F10 and B16GP33 tumors, it appears that the minor differences in tumor burden between these tumor groups did not strongly impact the degree of tumor-induced suppression of T cell proliferation.
Further elucidation of the mechanisms underlying this global melanoma-induced suppression of activated T cell expansion is needed. We have previously reported that this suppression is not associated with significant induction of regulatory T cells or myeloid-derived suppressor cells. Rather, we have observed that this suppression is associated with upregulated apoptotic activity across all activated T cell populations in melanoma-bearing mice. In addition, by using adoptive transfer experiments, we have found that this suppression is mediated not at the moment of T cell activation by antigen encounter, but on resting T cells prior to activation [11]. Our laboratory is currently investigating potential mechanisms by which in vivo exposure of resting T cells to growing melanoma tumors may induce upregulated activation-induced apoptosis.
5. Conclusion
These observations suggest that the negative influence of growing tumors on in vivo T cell expansion does not affect tumor antigen-specific responses more profoundly than non-tumor antigen-specific responses. This finding further supports the likelihood that growing cancers exert a globally suppressive influence on activated T cell homeostasis. Clinical strategies in adoptive cancer immunotherapy rely on the ability to induce profound expansion of tumor-specific CTLs in vivo; further elucidation of this fundamentally suppressive influence of growing tumors will be needed to actualize the enormous potential of these oncologic strategies.
Acknowledgments
This work was supported by grant support from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Science Research and Development Service, Career Development Award (CDA-2), American College of Surgeons Faculty Research Fellowship, and Central Surgical Association Foundation Grant to CSC.
Footnotes
Disclosure/disclaimer of potential conflicts of interest
There are no potential conflicts of interest; the contents of this work do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- [1].Dunn GP, Old LJ, Schreiber RD. The three E’s of cancer immunoediting. Ann. Rev. Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- [2].Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- [4].Shankaran V, Ikeda H, Bruce AT, et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- [5].Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Ann. Rev. Immunol. 2007;25:267–295. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bai XG, Liu J, Li O, et al. Antigenic drift as a mechanism for tumor evasion of destruction by cytolytic T lymphocytes. J. Clin. Invest. 2003;111:1487–1496. doi: 10.1172/JCI17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marincola F, Wang E, Herlyn M, et al. Tumors as elusive targets of T-cell-based active immunotherapy. Trends Immunol. 2003;24:335–342. doi: 10.1016/s1471-4906(03)00116-9. [DOI] [PubMed] [Google Scholar]
- [8].Lizee G, Cantu MA, Hwu P. Less yin, more yang: confronting the barriers to cancer immunotherapy. Clin. Cancer Res. 2007;13:5250–5255. doi: 10.1158/1078-0432.CCR-07-1722. [DOI] [PubMed] [Google Scholar]
- [9].Zou W. Regulatory T cells, tumor immunity, and immunotherapy. Nat. Rev. Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- [10].Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- [11].Russ AJ, Wentworth L, Xu K, et al. Suppression of T cell expansion by melanoma is exerted on resting cells. Ann. Surg. Oncol. 2011 doi: 10.1245/s10434-011-1667-6. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Murali-Krishna K, Altman JD, Suresh M, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- [13].Hudrisier D, Oldstone MB, Gairin JE. The signal sequence of lymphocytic choriomeningitis virus contains an immunodominant cytotoxic T cell epitope that is restricted by both H-2D(b) and H-2K(b) molecules. Virology. 1997;21:62–73. doi: 10.1006/viro.1997.8627. [DOI] [PubMed] [Google Scholar]
- [14].Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr. Opin. Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]