Abstract
Background
We explored the prognostic value of the up-regulated carbohydrate antigen (CA19-9) in node-negative patients with gastric cancer as a surrogate marker for micrometastases.
Patients and methods
Micrometastases were determined using reverse transcription quantitative polymerase chain reaction (RT-qPCR) for a subgroup of 30 node-negative patients. This group was used to determine the cut-off for preoperative CA19-9 serum levels as a surrogate marker for micrometastases. Then 187 node-negative T1 to T4 patients were selected to validate the predictive value of this CA19-9 threshold.
Results
Patients with micrometastases had significantly higher preoperative CA19-9 serum levels compared to patients without micrometastases (p = 0.046). CA19-9 serum levels were significantly correlated with tumour site, tumour diameter, and perineural invasion. Although not reaching significance, subgroup analysis showed better five-year survival rates for patients with CA19-9 serum levels below the threshold, compared to patients with CA19-9 serum levels above the cut-off. The cumulative survival for T2 to T4 node-negative patients was significantly better with CA19-9 serum levels below the cut-off (p = 0.04).
Conclusions
Preoperative CA19-9 serum levels can be used to predict higher risk for haematogenous spread and micrometastases in node-negative patients. However, CA19-9 serum levels lack the necessary sensitivity and specificity to reliably predict micrometastases.
Keywords: gastric cancer, micrometastases, CA19-9
Introduction
In addition to local extent tumours, preoperative nodal staging is of the utmost importance when deciding upon lymphadenectomies in gastric cancer. Unfortunately, contemporary imaging modalities struggle with the modest sensitivities and specificities when it comes to nodal staging.1 To make matters even more challenging, the first metastases in early gastric cancer are usually in the form of small tumour-cell deposits, and the metastatic lymph nodes are often not enlarged.2 The average size of lymph nodes with micrometastases has been reported to be < 5 mm, which is below the size that can be reliably detected with preoperative imaging.2
Although the importance of micrometastases has been widely debated, there is some consensus on the prognostic relevance of micrometastases in lymph nodes.3-5 Due to their impact on long-term prognosis; many studies have searched for simple and reliable ways to detect micrometastases in patients with gastric cancer. To date, the only way to determine the presence of micrometastases is the additional analysis of lymph nodes using immunohistochemical or molecular methods.6-9 However, such elaborate and expensive methods used to detect micrometastases cannot be applied to clinical practice in their present form. Additional markers that can indicate the presence of such micrometastases will thus be of immense value.
Serum tumour markers have long been used for early detection and follow-up in patients with gastric cancer.10-15 Elevated serum levels of carbohydrate antigen 19-9 (CA19-9; or the sialyl-Lewis A determinant) have been the focus of investigations because of the reported association of CA19-9 with lymph-node metastases.14 CA19-9 is a tumour-associated carbohydrate determinant. Epigenetic silencing of the sialyltransferase gene early in tumour development leads to reduction in the production of the normally present disialyl-Lewis A determinant. This incomplete synthesis in tumour cells thus results in accumulation of the sialyl-Lewis A determinant (i.e., CA19-9). CA19-9 is a ligand for E-selectin, which is expressed on the surface of endothelial cells. These changes allow tumour cells to invade lymphovascular structures in the setting of low oxygen tissue tension during accelerated growth.16 Patients who show high expressing levels of the CA19-9 antigen have been shown to be at greater risk of developing lymph-node and haematogenous metastases.14,16 The use of CA19-9 to indicate lymph-node metastases is, however, still controversial.10,12,13,17 The low sensitivity and specificity of CA19-9 does not allow for its use in the prediction of lymph-node metastases. Furthermore, CA19-9 serum levels are usually low in early gastric cancer, which precludes its use for the detection of early lymph-node metastases, or even micrometastasis.
In our previous report, we demonstrated significant differences in the subclinical expression of serum levels of CA19-9 in patients with micro-metastases, compared to patients with negative lymph nodes.18 This led us to further explore these differences in patients with node-negative gastric cancer. To confirm the correlation of preoperative CA19-9 serum levels with micrometastases in the lymph nodes, we measured the preoperative CA19-9 serum levels in patients with and without lymph-node micrometastases. We then investigated the correlation between micrometastases and preoperative CA19-9 serum levels to determine the cut-off level for micrometastases detection, along with the respective sensitivities and specificities. Finally, the prognostic value of this cut-off for CA19-9 serum levels was investigated for a group of patients with node-negative gastric cancer.
Patients and methods
Between 1992 and 2013, a total of 1,129 patients underwent surgery for gastric cancer at the University Clinical Centre Maribor, Slovenia. From these, only node-negative patients with complete clinicopathological records and preoperative CA19-9 serum levels were included in this study.
The inclusion criteria were for histologically confirmed node-negative adenocarcinoma of the stomach, D2 lymphadenectomy (as defined by the 3rd English edition of the Japanese Gastric Cancer Association guidelines18), and complete record of preoperative tumour marker levels. All surgical specimens underwent pathological examination according to the guidelines for gastric cancer of the International Union Against Cancer. Patients with missing values were excluded from further analysis. Thus, 187 patients were included in the final study group, with their preoperative CA19-9 cutoff levels tested for clinical significance.
First, in a test group of 30 patients, we prospectively performed with reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis of sentinel lymph nodes for micrometastases, as described in our earlier reports.19,20 The preoperative differences in CA19-9 serum levels in these patients were used to determine the cut-off value of the preoperative CA19-9 serum levels for further analysis.
The study group of 187 patients was then used to determine the correlations between the preoperative CA19-9 serum levels with tumour characteristics, its predictive significance, and the cut-off value. The mean follow-up was 37 ± 49 months (range, 2 days to 241 months). The patients were divided into two groups according to the derived cut-off value for the CA19-9 serum levels.
Blood samples were obtained by peripheral venous puncture before surgery. CA19-9 serum levels were determined using commercial enzyme immunoassay kits (CA19-9; Dainabbot, Tokyo, Japan). The cut-off value was determined through receiver operating characteristics (ROC) analysis of the expression profiles from the RT-qPCR analysis for micrometastases.
The sentinel lymph nodes were extracted as described in our previous reports.19,21 In brief, preoperative risk assessment for the metastatic involvement of lymph nodes was carried out according to the Maruyama computer program, preoperative staging, and intraoperative dye navigation (Patente Blue V Dye; Guerbet Patent Blue V Sodium 2.5%; Guerbet, Roissy, France). The sentinel lymph nodes were harvested for RT-qPCR analysis. The total RNA was extracted using RNeasy Mini Plus kits (Qiagen, Hilden, Germany), and reverse transcribed with High Capacity cDNA Reverse
Transcription kits (Applied Biosystems, Carlsbad, CA, USA). Q-PCR was performed on an ABI Prism SDS 7500 PCR machine (Applied Biosystems), using TaqMan chemistry in a 96-well format. TaqMan Universal PCR Master Mix (Applied Biosystems) and the following Gene Expression Assays (Applied Biosystems) were used: for CEACAM5 Hs 00237075_m1; for KRT20 Hs00300643_m1; and for GAPDH 4333764. Thirty-five cycles were selected as the Ct threshold values for CEACAM5 and CK-20 expression, as determined in our sensitivity and specificity studies.19
All continuous data are expressed as means ± standard deviations, and the categorical data are expressed in percentages. Continuous variables were compared with Student’s t tests, and χ-squared tests were used for the comparison of discrete variables. Linear correlations were accessed by calculation of Pearson’s correlation coefficients. The ROC curves were used to identify potential cut-off values for the CA19-9 serum levels, along with the
sensitivities and specificities. The Kaplan-Maier method was used for the survival analysis. Survival time was calculated from surgery to death or the date of the last follow-up visit. The overall survival differences between the groups were determined using log-rank tests. Cox regression models were used to determine the factors related to the overall survival of node-negative patients. The final model was calculated with backward stepwise selection.
A p value < 0.05 was defined as the limit of significance. SPSS v.20 for Windows 8 was used for the statistical analyses. The probability of lymph-node involvement was estimated with WinEstimate (version 2.5; München, Germany).
Results
Micrometastases were detected in eight patients (26.7%) from the 30 histologically node-negative patients. These patients with micrometastases had significantly higher preoperative CA19-9 serum levels (15.8 ±13 IU/ml) than those without micro-metastases (6.9 ± 9 IU/ml; p = 0.046). With the ROC analysis, the cut-off value for CA19-9 serum levels of 3.5 IU/ml was selected as a predictor for micrometastases deposits in lymph nodes. With this threshold value, patients with micrometastases were determined with a sensitivity of 87.5% and a specificity of 50% (AUC, 0.724; p = 0.064).
The mean CA19-9 serum level of the patients with node-negative gastric cancer was 27.8 ± 185 IU/ml. Out of the 187 patients, 114 (61%) were above the threshold CA19-9 serum level of 3.5 IU/ ml. There was significant linear correlation between the preoperative CA19-9 serum levels and tumour sites (p = 0.035), tumour diameters (p = 0.012), and perineural infiltration (p = 0.007). There were significant differences in the preoperative CA19-9 serum levels between patients with different tumour sites, as seen by one-way analysis of variance (ANOVA) tests. The patients with Bormann type IV tumour (i.e., whole stomach involvement) had the highest preoperative CA19-9 serum levels (i.e., lesser curvature: 15.9 ± 48 IU/ml; greater curvature: 15.1 ± 52 IU/ml; anterior wall: 11.7 ± 20 IU/ml; whole circumference: 633.7 ± 1227 IU/ml; posterior wall: 9.7 ± 7 IU/ml; p < 0.0001). The preoperative CA19-9 serum levels of the patients with a tumour involving the entire stomach were significantly greater than those where the tumour was confined to one location, irrespective of the TNM stage (p < 0.0001). Also, the patients with perineural infiltration had significantly higher preoperative CA19-9 serum levels (143.4 ± 526 IU/ml vs. 14.5 ± 43 IU/ml; p = 0.007). There were no statistically significant correlations between the cut-off value for the CA19-9 serum levels and the clinicopathological characteristics of the patients.
These clinicopathological characteristics of the patients with CA19-9 serum levels above and below the cut-off of 3.5 IU/ml are shown in Table 1. Between these groups, there were no significant differences in age, gender, grade, Lauren histological type, TNM stage, tumour diameter, lymphangial infiltration, vascular infiltration, perineural invasion, extranodal infiltration, or extent of lymphadenectomy distribution.
Table 1.
Patient demographic and tumor characteristics according to their positive and negative Ca19-9 serum levels around the cut-off of 3.5 IU/ml
| Characteristic | Ca19-9 negative | Ca19-9 positive |
|---|---|---|
| Gender [male (%)] | 63 | 57 |
| Age (years ± SD) | 64 ± 12.2 | 64 ± 11.9 |
| ASA (%) | ||
| I | 41.8 | 37.1 |
| II | 37.3 | 37.1 |
| III | 20.9 | 25.7 |
| Lymphadenectomy (%) | ||
| D1 | 19.2 | 23.9 |
| D2 | 80.8 | 76.1 |
| Tumou r site (%) | ||
| Lesser curvature | 33.3 | 38.6 |
| Greater curvature | 38.9 | 40.4 |
| Anterior wall | 25.0 | 15.8 |
| Posterior wall | 1.4 | 2.6 |
| Circumferential | 1.4 | 2.6 |
| Differentiation (%) | ||
| Well | 27.4 | 21.0 |
| Moderate | 25.8 | 33.0 |
| Poor | 46.8 | 46.0 |
| Lauren (%) | ||
| Intestinal | 63.9 | 47.2 |
| Diffuse | 19.7 | 31.5 |
| Mixed | 16.4 | 21.3 |
| Lymphangial invasion [yes (%)] | 52.9 | 54.7 |
| Vascular invasion[yes (%)] | 7.3 | 11.1 |
| Perineural invasion[yes (%)] | 15.7 | 14.4 |
| T stage (%) | ||
| 1 | 31.5 | 43.8 |
| 2 | 38.4 | 28.9 |
| 3 | 26.0 | 18.4 |
| 4 | 4.1 | 8.8 |
| UICC (%) | ||
| Ia | 31.5 | 45.6 |
| Ib | 38.4 | 28.9 |
| IIa | 26.0 | 16.7 |
| IIb | 1.4 | 5.3 |
| IIIb | 2.7 | 3.5 |
| Tumour diameter (mm ± SD) | 52 ± 33.8 | 50 ± 32.4 |
| Number of extractedlymph nodes (n ± SD) | 21 ± 11.2 | 20 ± 10.7 |
ASA = American Society of Anesthesiologists physical status classification system; UICC = Union for International Cancer Control
The cumulative 5 year survival of the node-negative patient group was 67.4% ± 4%, with a median survival of 130.9 months. The cumulative 5 year overall survival rates by T stage for T1, T2, T3, T4a and T4b were 77% ± 6%, 69% ± 7%, 56% ± 9%, 25% ± 22% and 31% ± 24%, respectively.
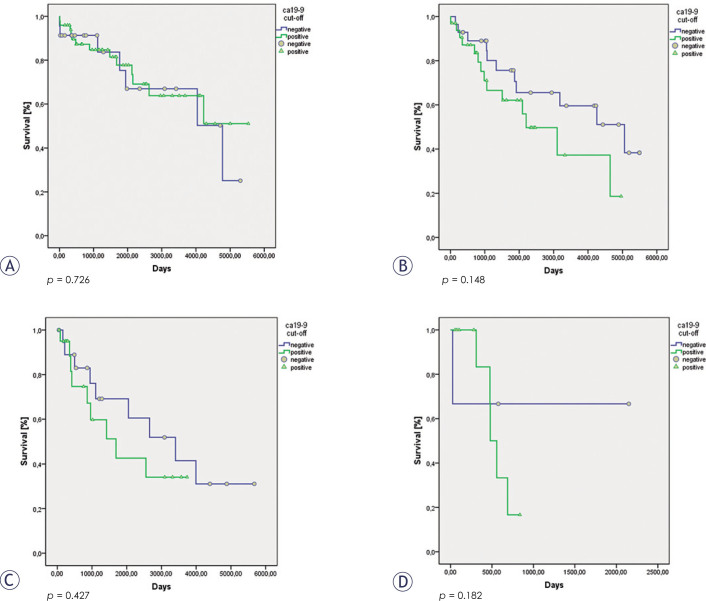
There were no significant differences in the cumulative 5 year overall survival rates between groups with different cut-off values of the CA199 serum levels (CA19-9 negative group: 73% ± 6%; CA19-9 positive group: 63% ± 5%; p = 0.305). However, if we excluded the patients with stages T1a and T1b from the analysis, a significant difference was seen between the overall survival of the patients with CA19-9 serum levels above and below our cut-off of 3.5 IU/ml (CA19-9 negative group: 72% ± 7%; CA19-9 positive group: 50% ± 8%; p = 0.04). Subgroup analysis failed to show significant differences in the 5 year overall survival rates for the individual stages of T1 to T4 between these CA19-9 negative and positive groups. Even so, the patients with stages T2 to T4 with CA19-9 serum levels above the set cut-off of 3.5 IU/ml had consistently worse overall survival rates than the patients below this cut-off value (Table 2, Figures 1, 2).
Table 2.
Median survival rates of patients with T1 to T4 N0 tumours according to their positive and negative Ca19-9 serum levels around the cut-off of 3.5 IU/ml
| Tumour stage | Ca19-9 cut-off | Median survival (months ± SD |
|---|---|---|
| T1N0 | Negative | 121 ± 15.7 |
| Positive | 126 ± 12.2 | |
| T2N0 | Negative | 121 ± 13.9 |
| Positive | 89 ± 13.1 | |
| T3N0 | Negative | 103 ± 18 |
| Positive | 65 ± 12.4 | |
| T4N0 | Negative | 47 ± 18.9 |
| Positive | 18 ± 2.2 |
Figure 1.
Survival of patients with T1 N0 (A), T2 N0 (B), T3 N0 (C) and T4 N0 (D) gastric cancer according to their positive and negative Ca19-9 serum levels around the cut-off of 3.5 IU/ml.
Figure 2.
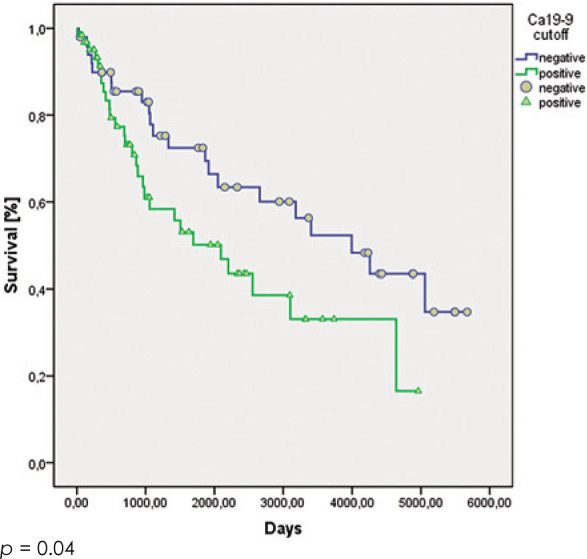
Survival of patients with T2 to T4 N0 gastric cancer according to their positive and negative Ca19-9 serum levels around the cut-off of 3.5 IU/ml.
The factors identified as significant predictors through univariate analysis were: preoperative CA19-9 serum level, tumour site, tumour diameter, T stage, number of extracted lymph nodes and the CA19-9 cut-off value. These were included in the Cox proportional hazard regression model. The multivariate analysis identified the significant prognostic factors in node-negative patients as T stage (hazard ratio, 1.755; 95% confidence interval, 1.321–2.33; p < 0.001) and number of extracted lymph nodes (hazard ratio, 0.972; 95% confidence interval, 0.948–0.997; p = 0.026) (Table 3).
Table 3.
Results of the multivariate regression model analysis
| Factor | Hazard ratio | 95% confidence interval | p | |
|---|---|---|---|---|
| Lower | Upper | |||
| Perineural invasion | 1.337 | 0.612 | 2.921 | NS |
| Tumour site | 1.151 | 0.881 | 1.502 | NS |
| T stage | 1.755 | 1.321 | 2.330 | < 0.0001 |
| Extracted lymph nodes | 0.972 | 0.948 | 0.997 | 0.026 |
| Preoperative Ca19-9 serum level | 1 | 1.000 | 1.001 | NS |
| Ca19-9 cut-off (3.5 IU/ml) | 1.045 | 0.528 | 2.068 | NS |
NS = not significant
The procedures described in this study were in accordance with the Helsinki declaration. All patients gave their written informed consent before being included in the present study. This study was approved by the National Ethics Committee (No. 153/02/0).
Discussion
According to previous reports, micrometastases in the lymph nodes have significant impact on patient survival.5,22-24 As the incidence of micrometastases is said to be even as high as 30% in node-negative patients25-27, it appears that these patients should be correctly staged at least intraoperatively. While it might be possible to reliably detect micrometastases with immunohistochemical or molecular techniques6-9, these techniques are time and labour intensive, and the results are usually available only after the operation. To identify a preoperative tool for micrometastases prediction, we explored the prognostic value of CA19-9 serum levels in node-negative patients.
Since the introduction of CA19-9 serum levels in clinical practice, numerous publications have confirmed that elevated CA19-9 serum levels are a predictor for lymph-node metastases and indicate worse prognosis for patients with advanced gastric cancer.10-15 To the best of our knowledge, the preoperative CA19-9 serum levels have never been used to predict micrometastases in patients with gastric cancer. As the up-regulated sialyl-Lewis A determinant (i.e., the CA19-9 antigen) in tumour cells has been shown to predispose patients with adenocarcinoma and squamous cell carcinoma to haematogenous metastases16, it can be seen that patients with elevated CA19-9 serum levels are at greater risk for micrometastatic lymph-node involvement. The aim of our study was to determine whether there is a correlation between early elevation of CA19-9 serum levels and the presence of micrometastases in patients with gastric cancer. We therefore studied the correlations of CA19-9 serum levels with the pathological properties of these tumours and the impact on the long-term survival of patients with node-negative gastric cancer.
As previously reported by our group, a significant difference was noted in the preoperative subclinical (< 37 IU/ml) CA19-9 serum levels in patients with node-negative gastric cancer with micrometastases, compared to patients without micrometastases.18 Patients with micrometastases were seen to have preoperative CA19-9 serum levels that were almost twice as high as those for patients without micrometastases. This observation led us to believe that CA19-9 serum levels can be used as a predictor for micrometastatic lymph-node involvement in patients with node-negative gastric cancer.
Cut-off values for CA19-9 serum levels as a marker for micrometastases were determined on a subgroup of 30 patients where their sentinel lymph nodes were subjected to RT-qPCR analysis in addition to routine histology.19-21 For further analysis, the patients in the study group were divided into two groups according to the derived cut-off value of CA19-9 serum level of 3.5 IU/ml. This cut-off value was used as a surrogate marker for micro-metastases. Based on this cut-off, 61% of node-negative patients were shown to have elevated CA19-9 serum levels. Assuming that these patients were at high risk of harbouring micrometastases, the incidence of micrometastases was significantly higher than the 30% usually reported.24-27 In contrast with the present study, T4 patients are usually excluded from micrometastases studies, due to the high proportion of early tumour recurrence in the peritoneal cavity.28,29 The T4 patients included in the present study with a probability of lymph-node deposits of > 80%, explain a much higher micro-metastatic involvement in the patient cohort in the present study compared to other reports.
To determine whether CA19-9 serum levels were elevated in patients with micrometastases, a group of node-negative patients was retrospectively analysed. We selected patients from our database with TNM stages T1 to T4N0. As it would have been too time consuming to retrospectively look for micrometastases in the paraffin blocks of the lymph nodes of 187 patients, we instead searched for correlations of CA19-9 serum levels with the pathological features usually associated with node-negative patients with micrometastases, as indirect markers for the presence of micrometastases. In the present study, a significant correlation was observed between the preoperative CA19-9 serum levels and tumour size, perineural invasion, and type Borman IV tumours. All of these pathological features are signs of more aggressive and invasive tumour behavior30-35, and patients with tumours that show such features were found to be at greater risk for haematogenic spread and micro-metastases in the lym ph nodes.
To determine whether the cut-off value of the CA19-9 serum levels has a similar prognostic impact on node-negative patients as described for micrometastases, we compared the survival of the node-negative patients stratified into two groups according to the derived CA19-9 cut-off level. While there was no difference in the cumulative survival rate, a significant difference was found when the T1N0 patients were excluded. Patients with a tumour limited to the mucosa (T1a) have an excellent long-term survival rate, and when a D2 lymphadenectomy is performed, only a modest survival benefit is achieved compared to patients without micrometastases.28,29,36 Reports of the impact of micrometastases on survival are usually restricted to stages T1b, T2 and T3.28,29,36,37 These findings coincide with our data here that indicate a survival benefit for the T2 to T4N0 patients with lower CA19-9 serum levels, and hence a lower probability of micrometastases. Assuming that preoperative CA19-9 serum levels are indeed a marker for micrometastases in node-negative patients, we can see that the stratification of patients according to our CA19-9 cut-off level had the same impact on survival as would be expected in patients with micrometastases. However, although this cut-off of CA19-9 serum levels of 3.5 IU/ml was identified as a significant predictor with univariate analysis, it failed to reach the limit of significance with multivariate analysis. Thus, multivariate analysis identified only T stage and the number of extracted lymph nodes as significant prognostic factors for overall survival.
Although tumour markers have been extensively used for the follow-up of oncological patients, their preoperative prognostic value remains to be determined. Based on our data, we show here that tumours with elevations in CA19-9 serum levels above 3.5 IU/ml share similar pathological properties as seen in patients with micrometastases. This would identify the patients with higher likelihood for haematogenic dissemination. Whether CA19-9 serum levels can serve as a surrogate marker for micrometastases in patients with gastric cancer remains a matter of debate, but we have shown here that our CA19-9 cut-off of 3.5 IU/ml has prognostic significance in some node-negative patients, and in the future, this might be one of the preoperative screening tests that can be used to guide surgical and multimodal treatments of patients with node-negative gastric cancer.
Disclosure: No potential conflicts of interest were disclosed.
References
- 1. Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 2009; 12: 6-22. [DOI] [PubMed]; Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6–22. doi: 10.1007/s10120-008-0492-5. [DOI] [PubMed] [Google Scholar]
- 2. Arai K, Iwasaki Y, Takahashi T. Clinicopathological analysis of early gastric cancer with solitary lymph node metastasis. Brit J Surg 2002; 89: 1435-7. [DOI] [PubMed]; Arai K, Iwasaki Y, Takahashi T. Clinicopathological analysis of early gastric cancer with solitary lymph node metastasis. Brit J Surg. 2002;89:1435–7. doi: 10.1046/j.1365-2168.2002.02204.x. [DOI] [PubMed] [Google Scholar]
- 3. Yasuda K, Adachi Y, Shiraishi N, Inomata M, Takeuchi H, Kitano S. Prognostic effect of lymph node micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol 2002; 9: 771-4. [DOI] [PubMed]; Yasuda K, Adachi Y, Shiraishi N, Inomata M, Takeuchi H, Kitano S. Prognostic effect of lymph node micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol. 2002;9:771–4. doi: 10.1007/BF02574499. [DOI] [PubMed] [Google Scholar]
- 4. Huang J, Xu Y, Li M, Sun Z, Zhu Z, Song Y, et al. The prognostic impact of occult lymph node metastasis in node-negative gastric cancer: A systemic review and meta-analysis. Ann Surg Oncol 2013; 20: 3927-34. [DOI] [PubMed]; Huang J, Xu Y, Li M, Sun Z, Zhu Z, Song Y. et al. The prognostic impact of occult lymph node metastasis in node-negative gastric cancer: A systemic review and meta-analysis. Ann Surg Oncol. 2013;20:3927–34. doi: 10.1245/s10434-013-3021-7. [DOI] [PubMed] [Google Scholar]
- 5. Cai J, Ikeguchi M, Kaibara N, Sakatani T. Clinicopathological value of immunohistochemical detection of occult involvement in pT3N0 gastric cancer. Gastric Cancer 1999; 2: 95-100. [DOI] [PubMed]; Cai J, Ikeguchi M, Kaibara N, Sakatani T. Clinicopathological value of immunohistochemical detection of occult involvement in pT3N0 gastric cancer. Gastric Cancer. 1999;2:95–100. doi: 10.1007/s101200050030. [DOI] [PubMed] [Google Scholar]
- 6. Yanagita S, Natsugoe S, Uenosono Y, Arigami T, Arima H, Kozono T, et al. Detection of micrometastases in sentinel node navigation surgery for gastric cancer. Surg Oncol 2008; 17: 203-10. [DOI] [PubMed]; Yanagita S, Natsugoe S, Uenosono Y, Arigami T, Arima H, Kozono T. et al. Detection of micrometastases in sentinel node navigation surgery for gastric cancer. Surg Oncol. 2008;17:203–10. doi: 10.1016/j.suronc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 7. Kubota K, Nakanishi H, Hiki N, Shimizu N, Tsuji E, Yamaguchi H, et al. Quantitative detection of micrometastases in the lymph nodes of gastric cancer patients with Real-time RT-PCR: A comperative study with immunohistochemistry. Int J Cancer 2003; 105: 136-43. [DOI] [PubMed]; Kubota K, Nakanishi H, Hiki N, Shimizu N, Tsuji E, Yamaguchi H. et al. Quantitative detection of micrometastases in the lymph nodes of gastric cancer patients with Real-time RT-PCR: A comperative study with immunohistochemistry. Int J Cancer. 2003;105:136–43. doi: 10.1002/ijc.11031. [DOI] [PubMed] [Google Scholar]
- 8. Osaka H, Yashiro M, Sawada T, Katsuragi K, Hirakawa K. Is a lymph node detected by the dye-guided method a true sentinel node in gastric cancer? Clin Cancer Res 2004; 10: 6912-8. [DOI] [PubMed]; Osaka H, Yashiro M, Sawada T, Katsuragi K, Hirakawa K. Is a lymph node detected by the dye-guided method a true sentinel node in gastric cancer? Clin Cancer Res. 2004;10:6912–8. doi: 10.1158/1078-0432.CCR-04-0476. [DOI] [PubMed] [Google Scholar]
- 9. Shimizu Y, Takeuchi H, Sakakura Y, Saikawa Y, Nakahara T, Mukai M, et al. Molecular detection of sentinel node micrometastases in patients with clinical N0 gastric carcinoma with real-time multiplex reverse transcription-polymerase chain reaction assay. Ann Surg Oncol 2012; 19: 469-77. [DOI] [PubMed]; Shimizu Y, Takeuchi H, Sakakura Y, Saikawa Y, Nakahara T, Mukai M. et al. Molecular detection of sentinel node micrometastases in patients with clinical N0 gastric carcinoma with real-time multiplex reverse transcription-polymerase chain reaction assay. Ann Surg Oncol. 2012;19:469–77. doi: 10.1245/s10434-011-2122-4. [DOI] [PubMed] [Google Scholar]
- 10. Ishigami S, Natsugoe S, Hokita S, Che X, Tokuda K, Nakajo A, et al. Clinical importance of preoperative carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer. J Clin Gastroenterol 2001; 32: 41-4. [DOI] [PubMed]; Ishigami S, Natsugoe S, Hokita S, Che X, Tokuda K, Nakajo A. et al. Clinical importance of preoperative carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer. J Clin Gastroenterol. 2001;32:41–4. doi: 10.1097/00004836-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 11. Dilege E, Mihmanli M, Demir U, Özer K, Bostancu Ö, Kaya C, et al. Prognostic value of preoperative CEA and CA 19-9 levels in resectable gastric cancer. Hepatogastroenterology 2010; 57: 674-7. [PubMed]; Dilege E, Mihmanli M, Demir U, Özer K, Bostancu Ö, Kaya C. et al. Prognostic value of preoperative CEA and CA 19-9 levels in resectable gastric cancer. Hepatogastroenterology. 2010;57:674–7. [PubMed] [Google Scholar]
- 12. Kodera Y, Yamamura Y, Torii A, Uesaka K, Hirai T, Yasui K, et al. The prognostic value of preoperative serum level of CEA and CA19-9 in patients with gastric cancer. Am J Gastroent 1996; 91: 49-53. [PubMed]; Kodera Y, Yamamura Y, Torii A, Uesaka K, Hirai T, Yasui K. et al. The prognostic value of preoperative serum level of CEA and CA19-9 in patients with gastric cancer. Am J Gastroent. 1996;91:49–53. [PubMed] [Google Scholar]
- 13. Mihmanli M, Dilege E, Demir U, Coskun H, Eroglu T, Uysalol MD. The use of tumor markers as predictors of prognosis in gastric cancer. Hepatogastroenterology 2004; 51: 1544-7. [PubMed]; Mihmanli M, Dilege E, Demir U, Coskun H, Eroglu T, Uysalol MD. The use of tumor markers as predictors of prognosis in gastric cancer. Hepatogastroenterology. 2004;51:1544–7. [PubMed] [Google Scholar]
- 14. Tsirlis TD, Kostakis A, Papastratis G, Masselou K, Vlachos I, Papachristodoulou A, et al. Predictive significance of preoperative serum VEGF-C and VEGF-D, independently and combined with Ca19-9, for the presence of malignancy and lymph node metastasis in patients with gastric cancer. J Surg Oncol 2010; 102: 699-703. [DOI] [PubMed]; Tsirlis TD, Kostakis A, Papastratis G, Masselou K, Vlachos I, Papachristodoulou A. et al. Predictive significance of preoperative serum VEGF-C and VEGF-D, independently and combined with Ca19-9, for the presence of malignancy and lymph node metastasis in patients with gastric cancer. J Surg Oncol. 2010;102:699–703. doi: 10.1002/jso.21677. [DOI] [PubMed] [Google Scholar]
- 15. Mattar R, Alves de Andrade CR, DiFavero GM, Gama-Rodrigues JJ, Laudanna AA. Preoperative serum levels of CA 72-5, CEA, CA 19-9 and Alpha-fetoprotein in patents with gastric cancer. Rev Hosp Clin Fac Med S Paulo 2002; 57: 89-92. [DOI] [PubMed]; Mattar R, Alves de Andrade CR, DiFavero GM, Gama-Rodrigues JJ, Laudanna AA. Preoperative serum levels of CA 72-5, CEA, CA 19-9 and Alpha-fetoprotein in patents with gastric cancer. Rev Hosp Clin Fac Med S Paulo. 2002;57:89–92. doi: 10.1590/s0041-87812002000300001. [DOI] [PubMed] [Google Scholar]
- 16. Kannagi R. Carbohydrate antigen Sialyl Lewis a – Its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med J 2007; 30: 189-209. [PubMed]; Kannagi R. Carbohydrate antigen Sialyl Lewis a – Its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med J. 2007;30:189–209. [PubMed] [Google Scholar]
- 17. Duraker N, Celik AN. The prognostic significance of preoperative serum CA 19-9 in patients with resectable gastric carcinoma: Comparison with CEA. J Surg Oncol 2001; 76: 266-71. [DOI] [PubMed]; Duraker N, Celik AN. The prognostic significance of preoperative serum CA 19-9 in patients with resectable gastric carcinoma: Comparison with CEA. J Surg Oncol. 2001;76:266–71. doi: 10.1002/jso.1044. [DOI] [PubMed] [Google Scholar]
- 18. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14: 113-23. [DOI] [PubMed]; Japanese Gastric Cancer Association. Gastric Cancer. 2011;14:113–23. Japanese gastric cancer treatment guidelines 2010 (ver. 3) [Google Scholar]
- 19. Jagric T, Potrc S, Ivanecz A, Horvat M, Plankl M, Mars T. Evaluation of focused sentinel lymph node RT-qPCR screening for micrometastases with the use of the Maruyama computer program. Eur Surg 2013; 45: 270-6. [DOI] [PMC free article] [PubMed]; Jagric T, Potrc S, Ivanecz A, Horvat M, Plankl M, Mars T. Evaluation of focused sentinel lymph node RT-qPCR screening for micrometastases with the use of the Maruyama computer program. Eur Surg. 2013;45:270–6. doi: 10.1007/s10353-013-0226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jagric T, Plakl M, Ivanecz A, Horvat M, Gajzer B, Grubic Z, et al. The prognostic value of micrometastases found intraoperatively in the first drainig lymph node in gastric cancer patients. Zdrav Vestn 2012; 81: 775-83.; Jagric T, Plakl M, Ivanecz A, Horvat M, Gajzer B, Grubic Z. et al. The prognostic value of micrometastases found intraoperatively in the first drainig lymph node in gastric cancer patients. Zdrav Vestn. 2012;81:775–83. [Google Scholar]
- 21. Jagric T, Ivanecz A, Horvat M, Plankl M, Kavalar R, Potrc S, et al. Evaluation of a focused sentinel lymph node protocol in node-negative gastric cancer patients, Hepatogastroenterol 2013; 60: 1231-6. [DOI] [PubMed]; Jagric T, Ivanecz A, Horvat M, Plankl M, Kavalar R, Potrc S. et al. Evaluation of a focused sentinel lymph node protocol in node-negative gastric cancer patients. Hepatogastroenterol. 2013;60:1231–6. doi: 10.5754/hge121167. [DOI] [PubMed] [Google Scholar]
- 22. Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Higashi H, Watanabe T, et al. Clinical impact of micrometastasis of lymph node in gastric cancer. Am Surg 2003; 69: 573-7. [PubMed]; Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Higashi H, Watanabe T. et al. Clinical impact of micrometastasis of lymph node in gastric cancer. Am Surg. 2003;69:573–7. [PubMed] [Google Scholar]
- 23. Nakajo A, Natsugoe S, Ishigami S, Matsumoto M, Nakashima S, Hokita S, et al. Detection and prediction of micrometastasis in the lymph nodes of patients with pN0 gastric cancer. Ann Surg Oncol 2000; 8: 158-62. [DOI] [PubMed]; Nakajo A, Natsugoe S, Ishigami S, Matsumoto M, Nakashima S, Hokita S. et al. Detection and prediction of micrometastasis in the lymph nodes of patients with pN0 gastric cancer. Ann Surg Oncol. 2000;8:158–62. doi: 10.1007/s10434-001-0158-6. [DOI] [PubMed] [Google Scholar]
- 24. Sievert JR, Kestlmaier R, Busch R, Böttcher K, Roder JD, Müller J, et al. Benefits of D2 lymph node dissection for patients with gastric cancer and pN0 and pN1 lymph node metastases. Brit J Surg 1996; 83: 1144-7. [DOI] [PubMed]; Sievert JR, Kestlmaier R, Busch R, Böttcher K, Roder JD, Müller J. et al. Benefits of D2 lymph node dissection for patients with gastric cancer and pN0 and pN1 lymph node metastases. Brit J Surg. 1996;83:1144–7. doi: 10.1002/bjs.1800830836. [DOI] [PubMed] [Google Scholar]
- 25. Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, Ohro S, et al. Recurrence in early gastric cancer – Presence of micrometastases in lymph node negative early gastric cancer patient with recurrence. Hepatogastroenterol 2007; 54: 620-4. [PubMed]; Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, Ohro S. et al. Recurrence in early gastric cancer – Presence of micrometastases in lymph node negative early gastric cancer patient with recurrence. Hepatogastroenterol. 2007;54:620–4. [PubMed] [Google Scholar]
- 26. Otsuji E, Toma A, Kobayashi S, Okamoto K, Hagiwara A, Yamagishi H. Outcome of profilactic Radical lymphadenectomy with gastrectomy in patients with early gastric carcinoma without lymph node metastases. Cancer 2000; 89: 1425-30. [PubMed]; Otsuji E, Toma A, Kobayashi S, Okamoto K, Hagiwara A, Yamagishi H. Outcome of profilactic Radical lymphadenectomy with gastrectomy in patients with early gastric carcinoma without lymph node metastases. Cancer. 2000;89:1425–30. [PubMed] [Google Scholar]
- 27. Nakajo A, Natsugoe S, Ishigami S, Matsumoto M, Nakashima S, Hokita S, et al. Detection and prediction of micrometastases in the lymph nodes of patients with pN0 gastric cancer. Ann Surg Oncol 2001; 8: 158-62. [DOI] [PubMed]; Nakajo A, Natsugoe S, Ishigami S, Matsumoto M, Nakashima S, Hokita S. et al. Detection and prediction of micrometastases in the lymph nodes of patients with pN0 gastric cancer. Ann Surg Oncol. 2001;8:158–62. doi: 10.1007/s10434-001-0158-6. [DOI] [PubMed] [Google Scholar]
- 28. Fukagawa T, Sasako M, Ito S, Nakanishi H, Iinuma H, Natsugoe S. The prognostic signifficance of isolated tumor cells in the lymph nodes of gastric cancer patients. Gastric Cancer 2010; 13: 191-6. [DOI] [PubMed]; Fukagawa T, Sasako M, Ito S, Nakanishi H, Iinuma H, Natsugoe S. The prognostic signifficance of isolated tumor cells in the lymph nodes of gastric cancer patients. Gastric Cancer. 2010;13:191–6. doi: 10.1007/s10120-010-0556-1. [DOI] [PubMed] [Google Scholar]
- 29. Fukagawa T, Sasako M, Mann GB, Sano T, Katai H, Maruyama K, et al. Immunohistochemically detected micrometastases of the lymph nodes in patients with gastric carcinoma. Cancer 2001; 92: 753–60. [DOI] [PubMed]; Fukagawa T, Sasako M, Mann GB, Sano T, Katai H, Maruyama K. et al. Immunohistochemically detected micrometastases of the lymph nodes in patients with gastric carcinoma. Cancer. 2001;92:753–60. doi: 10.1002/1097-0142(20010815)92:4<753::aid-cncr1379>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30. Cao L, Hu X, Zhang Y, Huang G. Adverse prognosis of clustered-cell versus single-cell micrometastases in pN0 early gastric cancer. J Surg Oncol 2011; 103: 53-6. [DOI] [PubMed]; Cao L, Hu X, Zhang Y, Huang G. Adverse prognosis of clustered-cell versus single-cell micrometastases in pN0 early gastric cancer. J Surg Oncol. 2011;103:53–6. doi: 10.1002/jso.21755. [DOI] [PubMed] [Google Scholar]
- 31. Kim JJ, Song KY, Hur H, Hur JI, Park SM, Park CH. Lymph node micrometastasis in node negative early gastric cancer. Eur J Surg Oncol 2009; 35: 409-14. [DOI] [PubMed]; Kim JJ, Song KY, Hur H, Hur JI, Park SM, Park CH. Lymph node micrometastasis in node negative early gastric cancer. Eur J Surg Oncol. 2009;35:409–14. doi: 10.1016/j.ejso.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 32. Chou HH, Kuo CJ, Hsu JT, Chen TH, Lin CJ, Tseng JH, et al. Clinicopatologic study of node-negative advanced gastric cancer and analysis of factors predicting its recurrence and prognosis. Am J Surgery 2013; 101:623-30. [DOI] [PubMed]; Chou HH, Kuo CJ, Hsu JT, Chen TH, Lin CJ, Tseng JH. et al. Clinicopatologic study of node-negative advanced gastric cancer and analysis of factors predicting its recurrence and prognosis. Am J Surgery. 2013;101:623–30. doi: 10.1016/j.amjsurg.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 33. Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, et al. Phase II study of preoperative chemotherapy with S-1 and Cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol 2013; 107: 741-5. [DOI] [PubMed]; Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H. et al. Phase II study of preoperative chemotherapy with S-1 and Cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210) J Surg Oncol. 2013;107:741–5. doi: 10.1002/jso.23301. [DOI] [PubMed] [Google Scholar]
- 34. Kodera Y, Nakanishi H, Ito S, Mochizuki Y, Yamamura Y, Fujiwara M, et al. Detection of disseminated cancer cells in linitis plastic-type gastric carcinoma. Jpn J Clin Oncol 2004; 34: 525-31. [DOI] [PubMed]; Kodera Y, Nakanishi H, Ito S, Mochizuki Y, Yamamura Y, Fujiwara M. et al. Detection of disseminated cancer cells in linitis plastic-type gastric carcinoma. Jpn J Clin Oncol. 2004;34:525–31. doi: 10.1093/jjco/hyh097. [DOI] [PubMed] [Google Scholar]
- 35. Endo K, Sakurai M, Kusumoto E, Uehara H, Yamaguchi S, Tsutsumi N, et al. Biological significance of localized type IV scirrhous gastric carcinoma. Oncol Letters 2012; 3: 94-9. [DOI] [PMC free article] [PubMed]; Endo K, Sakurai M, Kusumoto E, Uehara H, Yamaguchi S, Tsutsumi N. et al. Biological significance of localized type IV scirrhous gastric carcinoma. Oncol Letters. 2012;3:94–9. doi: 10.3892/ol.2011.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miwa K, Miyazaki I, Sahara H, Fujimura T, Yonemura Y, Noguchi M, et al. Rationale for extensive lymphadenectomy in early gastric carcinoma. Brit J Cancer 1995; 72: 1518-24. [DOI] [PMC free article] [PubMed]; Miwa K, Miyazaki I, Sahara H, Fujimura T, Yonemura Y, Noguchi M. et al. Rationale for extensive lymphadenectomy in early gastric carcinoma. Brit J Cancer. 1995;72:1518–24. doi: 10.1038/bjc.1995.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maehara Z, Oshiro T, Endo K, Baba H, Oda S, Ichiyoshi Y, et al. Clinical significance of occult micrometastasis lymph nodes from patients with early gastric cancer who died of recurrence. Surgery 1996, 119: 397-402. [DOI] [PubMed]; Maehara Z, Oshiro T, Endo K, Baba H, Oda S, Ichiyoshi Y. et al. Clinical significance of occult micrometastasis lymph nodes from patients with early gastric cancer who died of recurrence. Surgery. 1996;119:397–402. doi: 10.1016/s0039-6060(96)80138-3. [DOI] [PubMed] [Google Scholar]