Abstract
Background
The data on expression and clinical impact of cancer stem cell markers SOX2, NANOG and OCT4 in lung cancer is still lacking. The aim of our study was to compare SOX2, NANOG and OCT4 mRNA expression levels in whole blood between advanced small-cell lung cancer (SCLC) patients and healthy controls, and to correlate mRNA expression with progression-free survival (PFS) after first-line chemotherapy and overall survival (OS) in advanced SCLC patients.
Patients and methods
50 advanced SCLC patients treated with standard chemotherapy and followed at University Clinic Golnik, Slovenia, between 2009 and 2013 were prospectively included. SOX2, NANOG and OCT4 mRNA expression levels were determined using TaqMan qPCR in whole blood collected prior to chemotherapy. Whole blood of 34 matched healthy individuals with no cancerous disease was also tested.
Results
SOX2 mRNA expression was significantly higher in whole blood of SCLC patients compared to healthy controls (p = 0.006). Significant correlation between SOX2 mRNA expression levels and the number of distant metastatic sites was established (p = 0.027). In survival analysis, patients with high SOX2 expression had shorter OS (p = 0.017) and PFS (p = 0.046). In multivariate Cox analysis, an independent value of high SOX2 expression for shorter OS (p = 0.002), but not PFS was confirmed. No significant differences were observed for NANOG or OCT4 expression levels when comparing SCLC patients and healthy controls neither when analysing survival outcomes in SCLC patients.
Conclusions
SOX2 mRNA expression in whole blood might be a promising non-invasive marker for molecular screening of SCLC and important prognostic marker in advanced chemotherapy-treated SCLC patients, altogether indicating important role of cancer stem-like cell (CSC) regulators in cancer spread. Further evaluation of SOX2 as a possible screening/prognostic marker and a therapeutic target of SCLC is warranted.
Keywords: small-cell lung cancer, cancer stem cell markers, SOX2, OCT4, NANOG, mRNA expression, prognosis
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, with small-cell lung cancer (SCLC) representing approximately 15% of all lung cancer cases.1,2 SCLC represents one of the most aggressive human cancers, with early metastatic dissemination, initiated by cancer cell intravasation into blood, migration and consequent colonisation of sites distant to primary tumour. Despite some advances in therapeutic approaches, most of the advanced SCLC patients still die within the first year after diagnosis.3,4
SCLC is initially chemosensitive disease, with high response rates achieved with first-line chemotherapy regimens. However, majority of SCLC patients relapse within a few months and achieve only modest response rates to second-line chemotherapy, leading to poor survival rates. Platinum-based chemotherapeutic regimens with cisplatin or carboplatin still represent the only effective systemic therapy for SCLC patients. Unfortunately, still, no effective targeted therapy is available in clinical practice to treat SCLC.
Classical clinico-pathological characteristics (e.g. age, gender, performance status, stage, burden of metastatic disease) are still the only ones to predict survival outcome of SCLC patients.5-7 Despite an increased effort in identifying additional prognostic and predictive molecular markers, none of the so far studied molecular markers proved to have prognostic or predictive value in advanced SCLC.6,7
In the last several years, growing body of evidence indicates that cancer stem-like cells (CSCs) behave as crucial actors in cancer development, progression and metastasis.8,9 CSCs have been identified in many human cancer types, including breast cancer10, prostate cancer11, pancreatic cancer12 and lung cancer.13 For detection and identification of lung CSCs several key regulators have been proposed, normally essential for maintenance of pluripotent state of embryonic stem cells and self-renewal of tissue-specific adult stem cells; these regulators include SRY (sex determining region Y)-box 2 (SOX2)14, homeobox protein NANOG (named after Celtic word Tír na nÓg meaning the land of the young)15 and octamer-binding transcription factor 4 (OCT4).16 Cancer stem cells seem to be enriched in tumours resistant to conventional systemic therapy and radiotherapy.17-19 Recent reports also suggest that SOX2, NANOG and OCT4 are potential diagnostic and prognostic markers in lung cancer.20-27 Moreover, as indicated by a recent publications28,29, SOX2 is a commonly activated tumour oncogene that activates ACT28 and EGFR29 signalling pathways in human cancers, altogether indicating its complex biological role in cell faith.
Recent studies mainly conduced in early stage non-small cell lung cancer (NSCLC) after radical surgical therapy correlated SOX2 genomic amplification and/or consequent protein overexpression in primary tumour tissue with better prognosis21,27,30-33; these results were also supported by a meta-analysis, which confirmed significant interaction between high SOX2 expression and improved survival in early NSCLC, regardless of histopathological subtype.22 On the contrary in study evaluating the prognostic value of SOX2 protein expression in primary tumour tissue of early stage SCLC, high SOX2 protein expression was independent prognostic marker for poor survival outcome in SCLC patients.23 So far, only one study quantified the levels of serum SOX2 DNA in patients with different histopathological types and stages of lung cancer.20 In this particular study, serum SOX2 DNA level in lung cancer patients was higher compared to the level in healthy group, and it was closely associated with TNM stage, histopathological type, and tumour size; unfortunately, the association with course of disease and disease prognosis was not assessed in the frame of the later study.
The prognostic value of NANOG and OCT4 has only been evaluated in several retrospective studies with small number of NSCLC patients included.24-26,34-36 Elevated protein expression of both markers, NANOG24,25,34,35 or OCT424,26,36 in primary tumour was correlated with poor survival outcomes in early NSCLC patients treated with radical surgery. According to our knowledge there are no published data on prognostic or predictive value of NANOG or OCT4 expression in either blood or tumour tissue in SCLC patients.
Up to date, various studies have demonstrated that circulating cell-free tumour nucleic acids may reflect the same genetic characteristics as the primary tumour and are therefore attractive for non-invasive biomarkers determination especially during the course of diseases and in patients with no tumour tissue available.37 In lung cancer, previously mentioned study proposed circulating SOX2 DNA levels quantified by fluorescent qPCR as a novel, screening biomarker for lung cancer.20 So far, the prognostic value of SOX2, NANOG or OCT4 mRNA expression in whole blood samples of SCLC patients has not been evaluated yet. Several genetic (e.g. mutations, genomic amplifications) and epigenetic mechanisms, can either decrease or increase the transcription of a particular mRNA38,39; measuring the mRNA expression is therefore an attractive approach in cases where there is no known genetic mechanism affecting gene expression.
The aim of our study was to evaluate the level of SOX2, NANOG and OCT4 mRNA expression in whole blood samples of advanced SCLC patients compared to healthy controls, and to correlate biomarkers expression with overall survival (OS) and progression-free survival (PFS) after first line chemotherapy in advanced SCLC patients.
Patients and methods
The present study was conducted and is reported following recommendations for tumour marker prognostic studies (REMARK).40
Patients, healthy volunteers and collection of whole blood samples
50 consecutive patients with pathologically confirmed advanced SCLC, treated with first-line platinum or anthracycline-based chemotherapy and followed at University Clinic Golnik, Slovenia, between December 2009 and June 2013 were prospectively enrolled. For comparison, 50 volunteers with no clinical evidence of cancer disease were also included. Matching criteria were age and gender. 16 volunteers with other chronic pulmonary diseases (chronic obstructive pulmonary disease, asthma) were excluded from this study.
For SOX2, NANOG and OCT4 mRNA expression, whole blood samples (2.5 ml; PAXgene Blood RNA Tubes, which contain proprietary solution that reduces RNA degradation and gene induction; Qiagen) were collected from advanced SCLC patients before the onset of chemotherapy and from healthy controls at health check examinees by peripheral venous puncture. All blood samples were obtained after the first 5 ml of blood were discarded to avoid contamination of the blood sample with skin epithelial cells and stored at −30°C until RNA isolation.
Patients included in this study were treated and followed according to the standard clinical practices in use at the time. All patients received first-line systemic therapy with cisplatin-etoposide or carboplatine-etoposide (PE) or cyclophosphamide–epirubicin–vincristine (CEV) chemotherapy. The dosing schedules, dose modifications and supportive therapy were offered according to the standard practice. The second-line chemotherapy including CEV or PE was offered at clinician’s discretion. Response to chemotherapy was evaluated according to the RECIST1.1 criteria41 at regular time intervals (every 2-3 months) using chest radiography or computerised tomography (CT) scans. Number of distant metastatic sites was defined as the number of the organs or organic systems involved in cancer disease.
mRNA expression analysis
Total RNA was isolated from whole blood using PAXgene Blood miRNA Kit (Qiagen) using the fully automated QIAcube system (Qiagen) to standardize the RNA isolation procedure. Total RNA quantity and purity were assessed using NanoDrop 2000 (ThermoScientific). After isolation and purification of total RNA from blood samples additional step including digestion of genomic DNA with DNaseI (ThermoScientific) was included. Reverse transcription reactions were performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems).
All reagents for RT-PCR were supplied by Applied Biosystems (USA). The expression of stem cell markers was detected by TaqMan RT-qPCR (ABI PRISM 7500 FAST Real-Time PCR System) using gene-specific primers–probe sets (SOX2: Hs01053049_s1, OCT4: Hs00999632_g1, NANOG: Hs02387400_g1) and TaqMan Universal PCR Master Mix II. All measurements were performed in triplicate and relative mRNA expression was determined by the ∆∆Ct method. GAPDH was used as endogenous control and pooled RNA from blood samples of healthy controls was used as a calibrator. All samples with threshold cycle ≥ 38.0 were considered as negative for SOX2, NANOG or OCT4 mRNA expression.
Statistics
Median relative expression values of each analysed stem cell marker were compared between advanced SCLC patients and healthy controls using the Mann-Whitney U-test. The relationship between SOX2, NANOG or OCT4 mRNA expression and patient characteristics was evaluated using the Mann-Whitney U-test or Fisher’s exact test, as appropriate.
Overall survival was defined as the period of time in months from the date of diagnosis to the date of death or last follow-up; the secondary endpoint PFS was defined as the period of time in months from the start of the first-line chemotherapy to the date of progression or death whichever occurred first. Survival probabilities, OS and PFS, were calculated by the Kaplan-Meier method and log-rank test was used to compare different categories, where optimal cut-off value between low and high expression level was set at the median mRNA expression level for each of the three markers analysed in SCLC patients. The independent prognostic value of each individual marker was tested in Cox regression model adjusted for gender, age, PS and the number of distant metastatic sites. A p-value below 0.05 was considered statistically significant. All statistical analyses were carried out using SPSS (version 21, SPSS, Inc., Chicago, IL, USA). All reported p-values are two-tailed.
The study was approved by the Slovenian National Medical Ethics Committee (approval number 135/07/09) before the enrolment of the SCLC patients and healthy controls. The informed consent was obtained before the start of the study from all subjects.
Results
Patient and treatment characteristics
Demographic and treatment characteristics of 50 advanced SCLC patients and 34 healthy volunteers are listed in Table 1. At the time of diagnosis, median age of patients was 65 years (range 46-88 years), majority of the patients were male (35/50; 69%), and in good PS (PS ≤ 1 in 38/50; 76%). As first-line chemotherapy, the majority of patients received platinum-based chemotherapy (42/50; 84%). The second line chemotherapy was offered to 14/50 (28%) patients.
Table 1.
Characteristics of small-cell lung cancer (SCLC) patients and healthy volunteers
| Characteristic | SCLC patients (N = 50) | Healthy volunteers (N = 34) |
|---|---|---|
| Age in years: median (range) | 65 (46-88) | 62 (47-78) |
| Gender, N (%) | ||
| Male | 34 (68) | 24 (71) |
| Female | 16 (32) | 10 (29) |
| PSa N (%) | ||
| 0–1 | 38(76) | |
| ≥ 2 | 12 (24) | |
| Number of distant metastatic sites, N (%) | ||
| < 3 | 34 (68) | |
| ≥ 3 | 16 (32) | |
| Type of first-line chemotherapy, N (%) | ||
| PE | 42 (84) | |
| CEV | 8 (16) |
East Cooperative Oncology Group performance status; CEV = cyclophosphamide-epirubicinvincristine; N = number of SCLC patients/healthy volunteers;
PE = platinum (cisplatin or carboplatin)-etoposide
Evaluation of SOX2, OCT4 and NANOG mRNA expression
SOX2 relative mRNA expression levels were detected in 46/50 (92%) blood samples of advanced SCLC patients and in 25/34 (73%) blood samples of healthy controls. SOX2 mRNA expression levels were significantly higher in whole blood samples of SCLC patients when compared to healthy controls (median: 0.8 (range: 0.0-11.1) vs. 0.6 (range: 0.0-1.8), respectively, p = 0.006; Figure 1). On the other hand, NANOG and OCT4 relative mRNA expression levels were detected in all blood samples of SCLC patients and healthy controls. In addition, no significant differences were observed in NANOG (median: 1.5 (range: 0.4-4.8) vs. 1.2 (range: 0.4-4.4), respectively; p = 0.199, Figure 1) and OCT4 (median: 1.5 (range: 0.4-3.3) vs. 1.2 (range: 0.3-3.4), respectively; p = 0.224; Figure 1) median mRNA levels between SCLC patients and healthy group.
Figure 1.
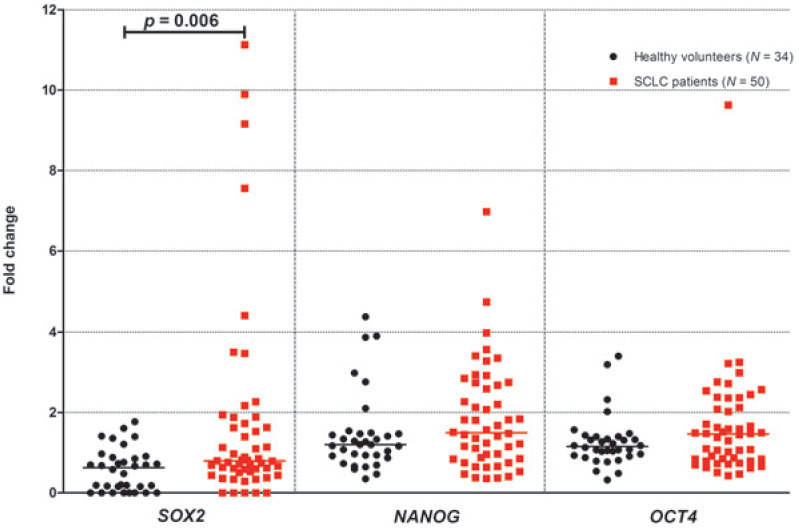
SOX2, NANOG and OCT4 mRNA expression levels in whole blood of healthy volunteers and small-cell lung cancer (SCLC) patients.
N: number of SCLC patients/healthy volunteers; p: p-value
As already mentioned in the methods section, cut-off value between low and high mRNA expression was set at the median expression level for the three markers analysed in SCLC patients. The associations between SOX2, NANOG or OCT4 mR-NA expression and clinical variables are shown in Table 2. High SOX2 mRNA expression was correlated with the higher number of distant metastatic sites (p = 0.027). There were no other significant correlations between SOX2, NANOG or OCT4 mR-NA expression and other clinical variables, such as gender and age.
Table 2.
Association between SOX2, NANOG and OCT4 mRNA expression and patients` characteristics
| Characteristic | SOX2 highb | SOX2 lowb | p-value | NANOG highb | NANOG lowb | p-value | OCT4 highb | OCT4 lowb | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Age in years: median (range) | 63 (46–78) | 66 (47–88) | 0.301c | 65 (47–79) | 65 (46–88) | 0.648 | 64 (47–77) | 66 (46–88) | 0.466 |
| Gender, N (%) | 0.756d | 0.762d | |||||||
| Male | 2 (35) | 22 (65) | 18 (53) | 16 (47) | 16 (47) | 18 (53) | 0.762d | ||
| Female | 7 (44) | 9 (56) | 7 (44) | 9 (56) | 9 (56) | 7 (44) | |||
| PSa, N (%) | 0.332d | 0.742d | 1.000d | ||||||
| 0–1 | 16 (42) | 22 (58) | 20 (53) | 18 (47) | 19 (50) | 19 (50) | |||
| ≥ 2 | 3 (25) | 9 (75) | 5 (42) | 7 (58) | 6 (50) | 6 (50) | |||
| Number of distant metastatic sites, N (%) | 0.027d | 0.762d | 1.000d | ||||||
| < 3 | 9 (26) | 25 (74) | 18 (53) | 16 (47) | 17 (50) | 17 (50) | |||
| ≥ 3 | 10 (62) | 6 (38) | 7 (44) | 9 (56) | 8 (50) | 8 (50 |
N = number of patients
East Cooperative Oncology Group performance status
bmedian mRNA expression levels for each of the three markers analysed were used to stratify patients as either SOX2/NANOG/OCT4 low or high
Mann-Whitney U-test
Fisher’s exact test
Survival analysis
After the median follow-up of 8.5 months (range: 0.5-32.5 months) median PFS was 6.2 months and median OS was 8.4 months in 50 SCLC patients included into analysis.
The level of SOX2 mRNA expression, significantly influenced both PFS after first-line chemotherapy (p = 0.046; Table 3, Figure 2A) and OS (p = 0.017; Table 3, Figure 3A) in our patients, with high SOX2 being associated with poor PFS and OS. Furthermore, in multivariate analysis independent prognostic value of SOX2 expression was confirmed for OS (p = 0.002; Table 3). On the other hand, no significant correlation between NANOG or OCT4 expression and survival outcomes was observed in univariate (Table 3, Figures 2-3) or multivariate analysis (Table 3).
Table 3.
Progression-free survival (PFS) after first-line chemotherapy and overall survival (OS) according to SOX2, NANOG and OCT4 mRNA expression
| mRNA expression | Median PFS (months) | p-value HR (95% CI) | Median OS (months) | p-value HR (95% CI) | ||
|---|---|---|---|---|---|---|
| UV | MV | UV | MV | |||
| All patients (N = 50) | 6.2 | 8.4 | ||||
| SOX2 low | 7.4 | 0.046 | 0.377 | 9.9 | 0.017 | 0.002 |
| SOX2 high | 5.6 | 1.988 (1.011-3.922) | 1.054 (0.938-1.183) | 7.5 | 2.370 (1.164-4.831) | 3.205 (1.536-6.711) |
| NANOG low | 5.7 | 0.221 | 0.299 | 7.4 | 0.347 | 0.376 |
| NANOG high | 6.5 | 0.693 (0.384-1.247) | 0.864 (0.656-1.139) | 8.7 | 0.750 (0.412-1.366) | 0.884 (0.674-1.161) |
| OCT4 low | 5.6 | 0.156 | 0.227 | 7.8 | 0.251 | 0.416 |
| OCT4 high | 7.3 | 0.652 (0.362-1.178) | 0.780 (0.521-1.167) | 9.9 | 0.810 (0.446-1.471) | 0.891 (0.673-1.178) |
95% CI = 95% confidence interval; HR = hazard ratio; MV = multivariate analysis adjusted for age, gender, N = number of patients; PS and the number of distant metastatic sites; relative expression values for each of the three markes analysed were used in MV; UV = univariate analysis; Log-rank test was used to analyse different categories dichotomised according to the median mRNA expression levels for each of the three markers analysed
Figure 2.
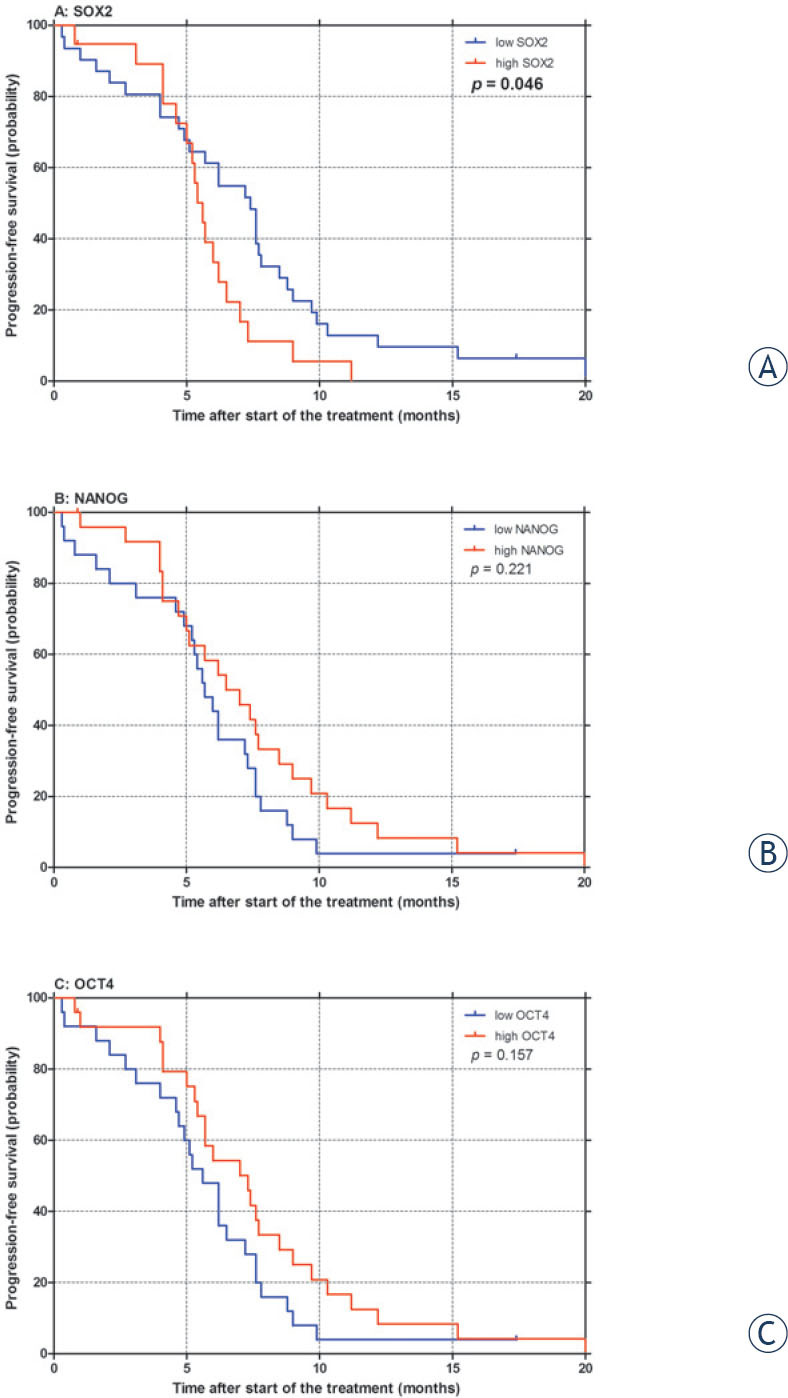
Progression-free survival (PFS) after first-line chemotherapy according to SOX2 (A), NANOG (B) and OCT4 (C) mRNA expression in small-cell lung cancer patients. p: p-value
Figure 3.
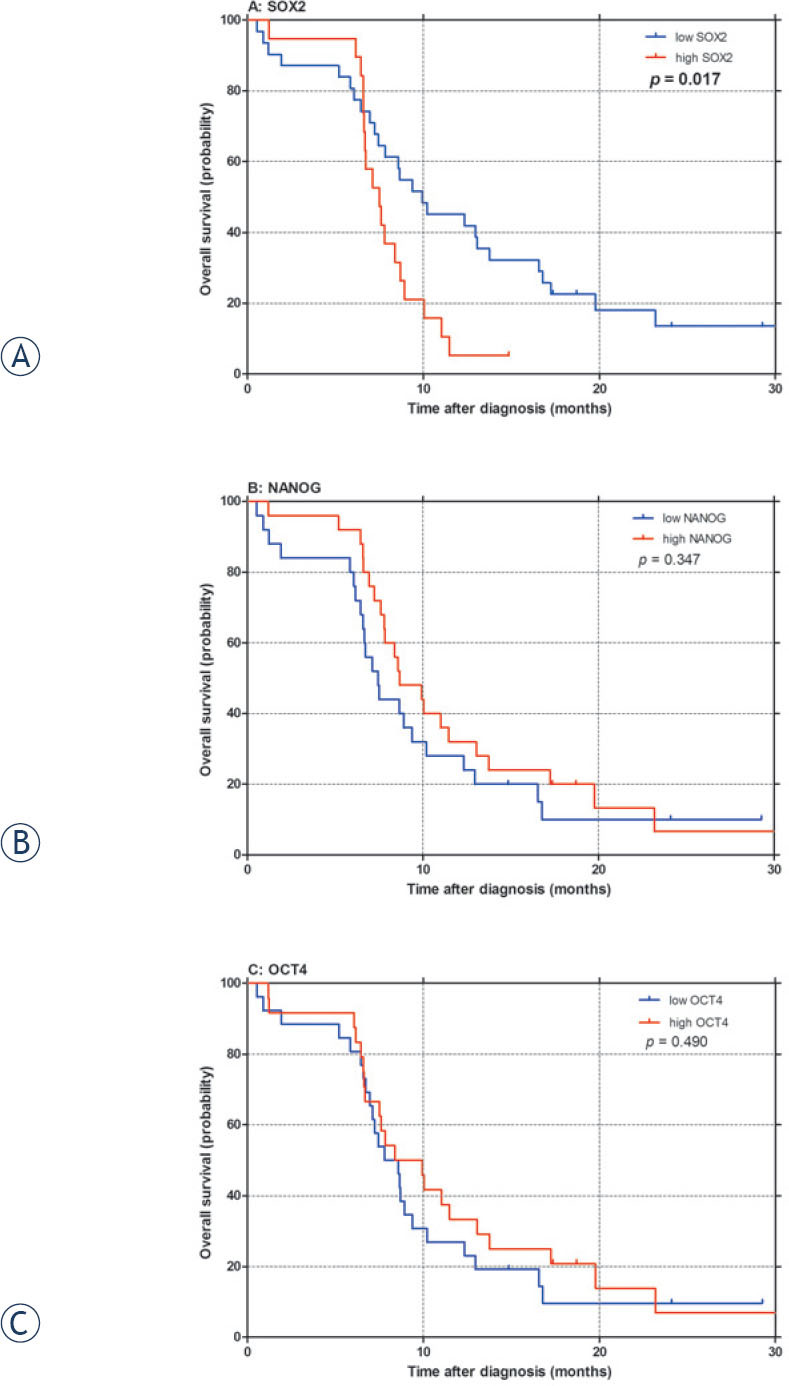
Overall survival (OS) according to SOX2 (A), NANOG (B) and OCT4 (C)mRNA expression in small-cell lung cancer patients. p: p-value.
Discussion
The present study aimed to compare SOX2, NANOG and OCT4 mRNA expression in whole blood between advanced SCLC patients and healthy controls, and to assess the prognostic impact of mRNA expression in 50 advanced SCLC patients treated with first-line chemotherapy, either platinum or anthracycline-based. Only SOX2 mR-NA levels were significantly higher in advanced SCLC patients when compared to healthy controls. Moreover, elevated SOX2 expression levels had an independent prognostic value for better OS in advanced chemotherapy-treated SCLC.
Our results indicate significantly higher SOX2 mRNA expression in whole blood of SCLC patients when compared to healthy controls, and are consistent with the only study which observed significantly higher serum SOX2 DNA levels in 94 patients with different histopathological types and stages of lung cancer in comparison to benign lung disease group or healthy group.20 Moreover, we observed a significant positive correlation between high SOX2 mRNA expression in whole blood and the higher number of distant metastatic sites (p = 0.027), suggesting that SOX2 expression might mirror an important oncogenic and metastatic potential in SCLC. Furthermore, correlation between high SOX2 mRNA expression and both poorer PFS after first-line chemotherapy (p = 0.046) and poorer OS (p = 0.017) was observed; association between high SOX2 expression and poor OS (p = 0.002) persisted also in multivariate analysis. Our findings obtained in whole blood of SCLC patients, seem to be consistent with the only study evaluating the prognostic value of SOX2 protein expression in SCLC primary tumours obtained after surgery, where high SOX2 protein expression in the primary tumour proved to be an independent prognostic marker for worse OS and shorter recurrence-free survival in patients with early stage SCLC who underwent surgery.23
In contrast to SCLC, high SOX2 protein expression and SOX2 gene amplification in primary tumours seem to be associated with better prognosis in NSCLC.22 These contradictory results might be due to different methodologies of biomarker determination (e.g. fluorescent in situ hybridization or quantitative PCR for detection of SOX2 genomic amplification, immunohistochemistry for SOX2 protein expression), small number of patients included in our study or may even suggest cancer-specific role of SOX2 in different histopathological types of lung cancer.
Detection of mRNA expression levels of selected biomarkers in whole blood is relatively new concept in lung cancer or any other human cancer that could be developed as an ancillary tool for disease screening and monitoring. Furthermore, it might represent an attractive approach for evaluating gene expression with no known underlying genetic mechanism affecting its expression. Moreover, detection of tumour specific DNA alterations42 and differential mRNA expression43-46 in primary tumours and/or circulating nucleic acids by high-throughput technologies (next-generation sequencing, microarrays) may provide a substantial advance in monitoring disease burden and treatment response in all human cancers.
The cut-off point for SOX2 positivity was set at the median value of SOX2 mRNA expression. Of note, there are no clinically validated cut-off values for SOX2 mRNA expression available in lung cancer, because this is the first study evaluating SOX2 mRNA expression in lung cancer. Studies conducted in other human cancers, including prostate cancer, rectal cancer, and hepatocellular cancer, so far used different, not yet validated thresholds based on the cohort of the included patients.47-49
Our results indicate no difference in NANOG and OCT4 mRNA expression levels in whole blood when comparing SCLC patients and control group. Moreover, no correlation between NANOG and OCT4 expression in whole blood and survival outcomes was observed in our study. To our best knowledge, the diagnostic and prognostic value of NANOG and OCT4 mRNA expression in tumour tissue or whole blood has not yet been evaluated in SCLC. However, several retrospective studies with small number of NSCLC patients included associated high NANOG or high OCT4 protein expression in primary tumour with poor survival outcome of NSCLC patients treated mainly with curative surgical resection.24-26,34-36 Again, these differences might be due to different methodologies of biomarker determination, low number of patients or specific role of these two markers in histopathological types of lung cancer.
The present study has several potential limitations, such as the small sample size that has an impact on statistical power of survival analysis and could therefore greatly limit the accuracy of the results. Furthermore, there are still methodological issues of biomarkers determination that should be appropriately resolved. The methods currently used for the evaluation of SOX2, NANOG or OCT4 expression in lung cancer patients differ greatly among the published reports. Studies conducted in lung cancer mainly used immunohistochemistry (IHC) for SOX2, NANOG and OCT4 protein expression and fluorescence in situ hybridisation (FISH) or quantitative PCR (qPCR) for SOX2 gene amplification detection.21-26,31-33 Besides, there are no clinically validated cut-off values for SOX2, NANOG and OCT4 mRNA expression available in the published literature and major differences still exists regarding the cut-off values of defining the specimens as positive/high for SOX2, NANOG or OCT4 expression determined either by IHC, FISH or qPCR.21-26,31-33 Furthermore, transcription of NANOG and OCT4 pseudogenes was reported in some tumour tissues and their detection by qPCR could give false-positive results.50,51 Unfortunately, direct comparison of SOX2, NANOG and OCT4 mRNA expression between whole blood and primary tumour tissue was not assessed in the frame of our study due to low number of patients with available tumour tissue. Further studies evaluating the correlation between gene expression profiles in whole blood and primary tumour tissue would be of valuable to assess the potential similarity of gene expression characteristics between blood circulation and primary lung tumour.
In conclusion, our prospective observational study found significantly higher mRNA expression levels of SOX2 in whole blood samples of advanced SCLC patients when compared to healthy controls. Equally importantly, a possible prognostic value of SOX2 mRNA expression for overall survival of SCLC patients was observed. No such correlations were found for NANOG or OCT4 expression. Our findings support the emerging oncogenic and metastatic role of SOX2 in SCLC with potential applications as a prognostic CSC marker and therapeutic target in lung cancer. In addition, recently published study highlights a new role of SOX2 biomarker in the regulation of EGFR oncogenic signalling pathway29, for which EGFR oncogene-directed therapies already exist and a number of other therapies are in development.
Nevertheless, possible diagnostic and prognostic value of SOX2 still requires further evaluation in the frame of large-scale prospective trials. However, before embarking on large prospective clinical trials a proper standardization and validation of methodological approaches used for evaluation of selected biomarkers is necessary.
Disclosure: No potential conflicts of interest were disclosed.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA Cancer J Clin 2014; 64: 9-29. [DOI] [PubMed]; Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JWW, Comber H, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013; 49: 1374-403. [DOI] [PubMed]; Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JWW, Comber H. et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3. Planchard D, Le Péchoux C. Small cell lung cancer: new clinical recommendations and current status of biomarker assessment. Eur J Cancer 2011; 47(Suppl 3): S272-83. [DOI] [PubMed]; Planchard D, Le Péchoux C. Small cell lung cancer: new clinical recommendations and current status of biomarker assessment. Eur J Cancer. 2011;47(Suppl 3):S272–83. doi: 10.1016/S0959-8049(11)70173-3. [DOI] [PubMed] [Google Scholar]
- 4. Kalemkerian GP. Advances in the treatment of small-cell lung cancer. Semin Respir Crit Care Med 2011; 32: 94-101. [DOI] [PubMed]; Kalemkerian GP. Advances in the treatment of small-cell lung cancer. Semin Respir Crit Care Med. 2011;32:94–101. doi: 10.1055/s-0031-1272873. [DOI] [PubMed] [Google Scholar]
- 5. Foster NR, Mandrekar SJ, Schild SE, Nelson GD, Rowland KM Jr, Deming RL, et al. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer 2009; 115: 2721-31. [DOI] [PMC free article] [PubMed]; Foster NR, Mandrekar SJ, Schild SE, Nelson GD, Rowland KM, Deming RL. et al. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2009;115:2721–31. doi: 10.1002/cncr.24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knez L, Sodja E, Kern I, Košnik M, Cufer T. Predictive value of multidrug resistance proteins, topoisomerases II and ERCC1 in small cell lung cancer: a systematic review. Lung Cancer 2011; 72: 271-9. [DOI] [PubMed]; Knez L, Sodja E, Kern I, Košnik M, Cufer T. Predictive value of multidrug resistance proteins, topoisomerases II and ERCC1 in small cell lung cancer: a systematic review. Lung Cancer. 2011;72:271–9. doi: 10.1016/j.lungcan.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 7. Sodja E, Knez L, Kern I, Ovčariček T, Sadikov A, Cufer T. Impact of ERCC1 expression on treatment outcome in small-cell lung cancer patients treated with platinum-based chemotherapy. Eur J Cancer 2012; 48: 3378-85. [DOI] [PubMed]; Sodja E, Knez L, Kern I, Ovčariček T, Sadikov A, Cufer T. Impact of ERCC1 expression on treatment outcome in small-cell lung cancer patients treated with platinum-based chemotherapy. Eur J Cancer. 2012;48:3378–85. doi: 10.1016/j.ejca.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 8. Berns A. Stem cells for lung cancer? Cell 2005; 121: 811-3. [DOI] [PubMed]; Berns A. Stem cells for lung cancer? Cell. 2005;121:811–3. doi: 10.1016/j.cell.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 9. Pine SR, Marshall B, Varticovski L. Lung cancer stem cells. Dis Markers 2008; 24: 257-66. [DOI] [PMC free article] [PubMed]; Pine SR, Marshall B, Varticovski L. Lung cancer stem cells. Dis Markers. 2008;24:257–66. doi: 10.1155/2008/396281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 2005; 65: 5506-11. [DOI] [PubMed]; Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D. et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 11. Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006; 25: 1696-708. [DOI] [PubMed]; Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S. et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 12. Li C, Heidt DG, Dalerba P, Burnat CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res 2007; 67: 1030-7. [DOI] [PubMed]; Li C, Heidt DG, Dalerba P, Burnat CF, Zhang L, Adsay V. et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 13. Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 2008; 15: 504-14. [DOI] [PubMed]; Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A. et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–14. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 14. Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BLM, et al. Evidence that SOX2 overexpression is oncogenic in the lung. PLos One 2010; 5: e11022. [DOI] [PMC free article] [PubMed]; Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BLM. et al. Evidence that SOX2 overexpression is oncogenic in the lung. PLos One. 2010;5:e11022. doi: 10.1371/journal.pone.0011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gialmanidis IP, Bravou V, Petrou I, Kourea H, Mathioudakis A, Lilis I, et al. Expression of Bmi1, FoxF1, Nanog, and γ-catenin in relation to hedgehog signaling pathway in human non-small-cell lung cancer. Lung 2013; 191:511-21. [DOI] [PubMed]; Gialmanidis IP, Bravou V, Petrou I, Kourea H, Mathioudakis A, Lilis I. et al. Expression of Bmi1, FoxF1, Nanog, and γ-catenin in relation to hedgehog signaling pathway in human non-small-cell lung cancer. Lung. 2013;191:511–21. doi: 10.1007/s00408-013-9490-4. [DOI] [PubMed] [Google Scholar]
- 16. Chen YC, Hsu HS, Chen YW, Tsai TH, How CK, Wang CY, et al. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133positive cells. PLos One 2008; 3: e2637. [DOI] [PMC free article] [PubMed]; Chen YC, Hsu HS, Chen YW, Tsai TH, How CK, Wang CY. et al. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133positive cells. PLos One. 2008;3:e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sullivan JP, Minna JD, Shay JW. Evidence for self-renewing lung cancer stem cells and their implications in tumor initiation, progression, and targeted therapy. Cancer Metastasis Rev 2010; 29: 61-72. [DOI] [PMC free article] [PubMed]; Sullivan JP, Minna JD, Shay JW. Evidence for self-renewing lung cancer stem cells and their implications in tumor initiation, progression, and targeted therapy. Cancer Metastasis Rev. 2010;29:61–72. doi: 10.1007/s10555-010-9216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bertolini G, Roz L, Perego P, Tortoeto M, Fontanella E, Gatti L, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci 2009; 106: 16281-6. [DOI] [PMC free article] [PubMed]; Bertolini G, Roz L, Perego P, Tortoeto M, Fontanella E, Gatti L. et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci. 2009;106:16281–6. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stewart DJ. Tumor and host factors that may limit efficacy of chemotherapy in non-small cell and small cell lung cancer. Crit Rev Oncol Hematol 2010; 75: 173-234. [DOI] [PMC free article] [PubMed]; Stewart DJ. Tumor and host factors that may limit efficacy of chemotherapy in non-small cell and small cell lung cancer. Crit Rev Oncol Hematol. 2010;75:173–234. doi: 10.1016/j.critrevonc.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Y, Du X, Xue C, Li D, Zheng Q, Li X, et al. Quantification of serum SOX2 DNA with FQ-PCR potentially provides a diagnostic biomarker for lung cancer. Med Oncol 2013; 30: 737. [DOI] [PubMed]; Wu Y, Du X, Xue C, Li D, Zheng Q, Li X. et al. Quantification of serum SOX2 DNA with FQ-PCR potentially provides a diagnostic biomarker for lung cancer. Med Oncol. 2013;30:737. doi: 10.1007/s12032-013-0737-y. [DOI] [PubMed] [Google Scholar]
- 21. Velcheti V, Schalper K, Yao X, Cheng H, Kocoglu M, Dhodapkar K, et al. High SOX2 levels predict better outcome in non-small cell lung carcinomas. PLoS One 2013; 8: e61427. [DOI] [PMC free article] [PubMed]; Velcheti V, Schalper K, Yao X, Cheng H, Kocoglu M, Dhodapkar K. et al. High SOX2 levels predict better outcome in non-small cell lung carcinomas. PLoS One. 2013;8:e61427. doi: 10.1371/journal.pone.0061427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y, Huang Y, Huang Y, Chen J, Wang S, Zhou J. The prognostic value of SOX2 expression in non-small cell lung cancer: a meta-analysis. PLoS One 2013; 8: e71140. [DOI] [PMC free article] [PubMed]; Chen Y, Huang Y, Huang Y, Chen J, Wang S, Zhou J. The prognostic value of SOX2 expression in non-small cell lung cancer: a meta-analysis. PLoS One. 2013;8:e71140. doi: 10.1371/journal.pone.0071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang F, Gao Y, Geng J, Qu D, Han Q, Qi J, et al. Elevated expression of SOX2 and FGFR1 in correlation with poor prognosis in patients with small cell lung cancer. Int J Clin Exp Pathol 2013; 6: 2846-54. [PMC free article] [PubMed]; Yang F, Gao Y, Geng J, Qu D, Han Q, Qi J. et al. Elevated expression of SOX2 and FGFR1 in correlation with poor prognosis in patients with small cell lung cancer. Int J Clin Exp Pathol. 2013;6:2846–54. [PMC free article] [PubMed] [Google Scholar]
- 24. Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, et al. Coexpression of oct4 and nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res 2010; 70: 10433-44. [DOI] [PubMed]; Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ. et al. Coexpression of oct4 and nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–44. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 25. Li XQ, Yang XL, Zhang G, Wu SP, Deng XB, Xiao SJ, et al. Nuclear β-catenin accumulation is associated with increased expression of Nanog protein and predicts poor prognosis of non-small cell lung cancer. J Transl Med 2013; 11: 114. [DOI] [PMC free article] [PubMed]; Li XQ, Yang XL, Zhang G, Wu SP, Deng XB, Xiao SJ. et al. Nuclear β-catenin accumulation is associated with increased expression of Nanog protein and predicts poor prognosis of non-small cell lung cancer. J Transl Med. 2013;11:114. doi: 10.1186/1479-5876-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li XL, Jia LL, Shi MM, Li X, Li ZH, Li HF, et al. Downregulation of KPNA2 in non-small-cell lung cancer is associated with Oct4 expression. J Transl Med 2013; 11: 232. [DOI] [PMC free article] [PubMed]; Li XL, Jia LL, Shi MM, Li X, Li ZH, Li HF. et al. Downregulation of KPNA2 in non-small-cell lung cancer is associated with Oct4 expression. J Transl Med. 2013;11:232. doi: 10.1186/1479-5876-11-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilbertz T, Wagner P, Petersen K, Stiedl AC, Scheble VJ, Maier S, et al. SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Mod Pathol 2011; 24: 944-53. [DOI] [PubMed]; Wilbertz T, Wagner P, Petersen K, Stiedl AC, Scheble VJ, Maier S. et al. SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Mod Pathol. 2011;24:944–53. doi: 10.1038/modpathol.2011.49. [DOI] [PubMed] [Google Scholar]
- 28. Tian Y, Jia X, Wang S, Li Y, Zhao P, Cai D, et al. SOX2 oncogenes amplified and operate to activate AKT signaling in gastric cancer and predict immunotherapy responsiveness. J Cancer Res Clin Oncol 2014; 140: 1117-24. [DOI] [PMC free article] [PubMed] [Retracted]; Tian Y, Jia X, Wang S, Li Y, Zhao P, Cai D. et al. SOX2 oncogenes amplified and operate to activate AKT signaling in gastric cancer and predict immunotherapy responsiveness. J Cancer Res Clin Oncol. 2014;140:1117–24. doi: 10.1007/s00432-014-1660-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Chou YT, Lee CC, Hsiao SH, Lin SE, Lin SC, Chung CH, et al. The emerging role of SOX2 in cell proliferation and survival and its crosstalk with oncogenic signaling in lung cancer. Stem Cells 2013; 31: 2607-19. [DOI] [PubMed]; Chou YT, Lee CC, Hsiao SH, Lin SE, Lin SC, Chung CH. et al. The emerging role of SOX2 in cell proliferation and survival and its crosstalk with oncogenic signaling in lung cancer. Stem Cells. 2013;31:2607–19. doi: 10.1002/stem.1518. [DOI] [PubMed] [Google Scholar]
- 30. Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembelé D, et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One 2010; 5: e8960. [DOI] [PMC free article] [PubMed]; Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembelé D. et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One. 2010;5:e8960. doi: 10.1371/journal.pone.0008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toschi L, Finocchiaro G, Nguyen TT, Skokan MC, Giordano L, Gianoncelli L, et al. Increased SOX2 gene copy number is associated with FGFR1 and PIK3CA gene gain in non-small cell lung cancer and predicts improved survival in early stage disease. PLoS One 2014; 9: e95303. [DOI] [PMC free article] [PubMed]; Toschi L, Finocchiaro G, Nguyen TT, Skokan MC, Giordano L, Gianoncelli L. et al. Increased SOX2 gene copy number is associated with FGFR1 and PIK3CA gene gain in non-small cell lung cancer and predicts improved survival in early stage disease. PLoS One. 2014;9:e95303. doi: 10.1371/journal.pone.0095303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cai YR, Zhang HQ, Zhang ZD, Mu J, Li ZH. Detection of MET and SOX2 amplification by quantitative real-time PCR in non-small cell lung carcinoma. Oncol Lett 2011; 2: 257-64. [DOI] [PMC free article] [PubMed]; Cai YR, Zhang HQ, Zhang ZD, Mu J, Li ZH. Detection of MET and SOX2 amplification by quantitative real-time PCR in non-small cell lung carcinoma. Oncol Lett. 2011;2:257–64. doi: 10.3892/ol.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sasaki H, Yokota K, Hikosaka Y, Moriyama S, Yano M, Fujii Y. Increased Sox2 copy number in lung squamous cell carcinomas. Exp Ther Med 2012; 3:44-8. [DOI] [PMC free article] [PubMed]; Sasaki H, Yokota K, Hikosaka Y, Moriyama S, Yano M, Fujii Y. Increased Sox2 copy number in lung squamous cell carcinomas. Exp Ther Med. 2012;3:44–8. doi: 10.3892/etm.2011.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen WJ, Ho CC, Chang YL, Chen HY, Lin CA, Ling TY, et al. Pulmonary adenocarcinoma in malignant pleural effusion enriches cancer stem cell properties during metastatic cascade. PLoS One 2013; 8: e54659. [DOI] [PMC free article] [PubMed]; Chen WJ, Ho CC, Chang YL, Chen HY, Lin CA, Ling TY. et al. Pulmonary adenocarcinoma in malignant pleural effusion enriches cancer stem cell properties during metastatic cascade. PLoS One. 2013;8:e54659. doi: 10.1371/journal.pone.0054659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Du Y, Ma C, Wang Z, Liu Z, Liu H, Wang T. Nanog, a novel prognostic marker for lung cancer. Surg Oncol 2013; 22: 224-9. [DOI] [PubMed]; Du Y, Ma C, Wang Z, Liu Z, Liu H, Wang T. Nanog, a novel prognostic marker for lung cancer. Surg Oncol. 2013;22:224–9. doi: 10.1016/j.suronc.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 36. Li X, Wang J, Xu Z, Ahmad A, Li E, Wang Y, et al. Expression of sox2 and oct4 and their clinical significance in human non-small-cell lung cancer. Int J Mol Sci 2012; 13: 7663-75. [DOI] [PMC free article] [PubMed]; Li X, Wang J, Xu Z, Ahmad A, Li E, Wang Y. et al. Expression of sox2 and oct4 and their clinical significance in human non-small-cell lung cancer. Int J Mol Sci. 2012;13:7663–75. doi: 10.3390/ijms13067663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011; 11: 426-37. [DOI] [PubMed]; Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–37. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 38. Croce CM. Oncogenes and cancer. N Engl J Med 2008; 358: 502-11 [DOI] [PubMed]; Croce CM. Oncogenes and cancer. N Engl J Med. 2008;358:502–11. doi: 10.1056/NEJMra072367. [DOI] [PubMed] [Google Scholar]
- 39. Jones PA, Baylin SB. The epigenomics of cancer. Cell 2007; 128: 683-92. [DOI] [PMC free article] [PubMed]; Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and Elaboration. PLoS Med 2012; 9: e1001216. [DOI] [PMC free article] [PubMed]; Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and Elaboration. PLoS Med. 2012;9:e1001216. doi: 10.1371/journal.pmed.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228-47. [DOI] [PubMed]; Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 42. Sequist LV, Heist RS, Shaw AT, Fidias P, Rosovsky R, Temel JS, et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol 2011; 22: 2616-24. [DOI] [PMC free article] [PubMed]; Sequist LV, Heist RS, Shaw AT, Fidias P, Rosovsky R, Temel JS. et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol. 2011;22:2616–24. doi: 10.1093/annonc/mdr489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Y, Elashoff D, Oh M, Sinha U, St John MA, Zhou X, et al. Serum circulating human mRNA profiling and its utility for oral cancer detection. J Clin Oncol 2006; 24: 1754-60. [DOI] [PubMed]; Li Y, Elashoff D, Oh M, Sinha U, St John MA, Zhou X. et al. Serum circulating human mRNA profiling and its utility for oral cancer detection. J Clin Oncol. 2006;24:1754–60. doi: 10.1200/JCO.2005.03.7598. [DOI] [PubMed] [Google Scholar]
- 44. Beane J, Spira A, Lenburg ME. Clinical impact of high-throughput gene expression studies in lung cancer. J Thorac Oncol 2009; 4: 109-18. [DOI] [PMC free article] [PubMed]; Beane J, Spira A, Lenburg ME. Clinical impact of high-throughput gene expression studies in lung cancer. J Thorac Oncol. 2009;4:109–18. doi: 10.1097/JTO.0b013e31819151f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci U S A 2001; 98: 13784-9. [DOI] [PMC free article] [PubMed]; Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M. et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci U S A. 2001;98:13784–9. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci U S A 2001; 98: 13790-5. [DOI] [PMC free article] [PubMed]; Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P. et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci U S A. 2001;98:13790–5. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, et al. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol 2009; 16: 3488-98. [DOI] [PubMed]; Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y. et al. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16:3488–98. doi: 10.1245/s10434-009-0617-z. [DOI] [PubMed] [Google Scholar]
- 48. Fujimura T, Takahashi S, Urano T, Takayama K, Sugihara T, Obinata D, et al. Expression of androgen and estrogen signaling components and stem cell markers to predict cancer progression and cancer-specific survival in patients with metastatic prostate cancer. Clin Cancer Res 2014; 20: 4625-35. [DOI] [PubMed]; Fujimura T, Takahashi S, Urano T, Takayama K, Sugihara T, Obinata D. et al. Expression of androgen and estrogen signaling components and stem cell markers to predict cancer progression and cancer-specific survival in patients with metastatic prostate cancer. Clin Cancer Res. 2014;20:4625–35. doi: 10.1158/1078-0432.CCR-13-1105. [DOI] [PubMed] [Google Scholar]
- 49. Huang P, Qiu J, Li B, Hong J, Lu C, Wang L, et al. Role of Sox2 and Oct4 in predicting survival of hepatocellular carcinoma patients after hepatectomy. Clin Biochem 2011; 44: 582-9. [DOI] [PubMed]; Huang P, Qiu J, Li B, Hong J, Lu C, Wang L. et al. Role of Sox2 and Oct4 in predicting survival of hepatocellular carcinoma patients after hepatectomy. Clin Biochem. 2011;44:582–9. doi: 10.1016/j.clinbiochem.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 50. Zhao S, Yuan Q, Hao H, Guo Y, Liu S, Zhang Y, et al. Expression of OCT4 pseudogenes in human tumours: lessons from glioma and breast carcinoma. J Pathol 2011; 223: 672-82. [DOI] [PubMed]; Zhao S, Yuan Q, Hao H, Guo Y, Liu S, Zhang Y. et al. Expression of OCT4 pseudogenes in human tumours: lessons from glioma and breast carcinoma. J Pathol. 2011;223:672–82. doi: 10.1002/path.2827. [DOI] [PubMed] [Google Scholar]
- 51. Uchino K, Hirano G, Hirahashi M, Isobe T, Shirakawa T, Kusaba H, et al. Human Nanog pseudogene8 promotes the proliferation of gastrointestinal cancer cells. Exp Cell Res 2012; 318: 1799-807. [DOI] [PubMed]; Uchino K, Hirano G, Hirahashi M, Isobe T, Shirakawa T, Kusaba H. et al. Human Nanog pseudogene8 promotes the proliferation of gastrointestinal cancer cells. Exp Cell Res. 2012;318:1799–807. doi: 10.1016/j.yexcr.2012.04.011. [DOI] [PubMed] [Google Scholar]


