Abstract
Background
Cocaine abuse continues to be a significant public health problem associated with morbidity and mortality. To date, no pharmacotherapeutic approach has proven effective for treating cocaine use disorders. Preclinical and clinical evidence suggests that noradrenergic activity may play a role in mediating some effects of cocaine and may be a rational target for treatment.
Methods
This double blind, placebo-controlled randomized, parallel group, 12-week outpatient clinical trial enrolled cocaine dependent individuals seeking treatment to examine the potential efficacy of the selective norepinephrine reuptake inhibitor, atomoxetine (80 mg/day; p.o.; n=25), compared to placebo (n=25). Subjects were initially stratified on cocaine use (<15 days or ≥15 days of the last 30), age and race using urn randomization. Attendance, medication adherence and study compliance were reinforced with contingency management, and weekly counseling was offered. An array of measures (vital signs, laboratory chemistries, cognitive and psychomotor tests, cocaine craving and urine samples for drug testing) was collected throughout the study and at follow-up.
Results
Survival analysis revealed no differences in study retention between the two groups, with approximately 56% of subjects completing the 12-week study (Cox analysis X2=.72; p=.40; Hazard Ratio 1.48 [CI 0.62–3.39]). GEE analysis of the proportion of urine samples positive for benzoylecgonine, a cocaine metabolite, revealed no differences between the atomoxetine and placebo groups (X2=0.2, p=.66; OR=0.89 [95% CI 0.41 – 1.74). Atomoxetine was generally well tolerated in this population.
Conclusions
These data provide no support for the utility of atomoxetine in the treatment of cocaine dependence.
Keywords: cocaine, norepinephrine, atomoxetine, dependence, treatment, clinical trial
1. Introduction
Cocaine abuse and dependence are associated with increased morbidity and mortality arising from adverse cardiovascular effects, increased transmission of blood-borne illnesses, and increased risks in pregnancy and birth outcomes. Recent estimates suggest that there are 1.5 million current cocaine users in the United States (U.S.; SAMHSA, 2011). According to the DAWN reporting system, cocaine is the most common illicit drug involved in U.S. emergency department visits, which numbered 488,101 in 2010 alone (SAMHSA, 2012). Despite efforts aimed at the development of effective pharmacotherapies for the treatment of cocaine dependence, no agents have demonstrated sufficient efficacy to warrant approval by the Food and Drug Administration.
Cocaine acts in the central nervous system to inhibit monoamine transporters, including dopamine, serotonin and norepinephrine (e.g., see review by Rothman and Baumann, 2003) and it possesses other pharmacological properties. Perhaps it is this complex pharmacological profile that has led to the difficulty in finding an effective pharmacotherapy to treat cocaine dependence. Despite its interaction with multiple central and peripheral targets, the rewarding effects of cocaine are thought to arise primarily from its inhibition of dopamine reuptake, resulting in an increase in synaptic dopamine concentrations in the mesolimbic dopamine system (Di Chiara and Imperato, 1988; Ritz et al., 1987). Moreover, it has been postulated that long-term changes in dopaminergic neurophysiology at the level of the cortex, specifically the orbitofrontal cortex, may underlie cocaine withdrawal symptoms, persistent states of craving, and compulsive drug-seeking behavior (for review see Volkow and Fowler, 2000). Therefore, numerous studies have evaluated agents with dopaminergic activity for efficacy against cocaine in controlled pharmacotherapy trials, including dopamine agonists, such as pergolide (Malcolm et al., 2000), bromocriptine (Handelsman et al., 1997), mazindol (Stine et al., 1995) and levo-dopa (Mooney et al., 2007; Schmitz et al., 2008) and dopamine antagonist-like compounds, such as risperidone (Grabowski et al., 2000; Grabowski et al., 2004a; Loebl et al., 2008) and the dopamine depleting agent, reserpine (Winhusen et al., 2007). The vast majority of these studies have reported no supportive evidence for efficacy, although a few studies of robust stimulant compounds, such as d-amphetamine (Grabowski et al., 2001; Grabowski et al., 2004b; Shearer et al., 2003) and methamphetamine (Mooney et al., 2009) have produced statistically significant signals of efficacy. While preclinical studies suggest a critical role for the serotonergic actions of cocaine (Walsh and Cunningham, 1997), randomized clinical trials of serotonergic agents in primary cocaine dependent individuals, including fluoxetine (Batki et al., 1996; Grabowski et al., 1995; Schmitz et al., 2001; Winstanley et al., 2011), tryptophan (Jones et al., 2004), ritanserin (Johnson et al., 1997), ondansetron (Johnson et al., 2006) and others have largely failed to demonstrate efficacy.
There is preclinical evidence suggesting that targeting the noradrenergic action of cocaine may be a rational approach (Sofuoglu and Sewell, 2009; Weinshenker and Schroeder, 2007). However, only a few clinical trials have been conducted to examine noradrenergic agents for cocaine dependence (for review see Sofuoglu and Sewell, 2009). Atomoxetine (Straterra®) is a potent norepinephrine reuptake inhibitor with little action at dopamine and serotonin transporters (Bolden-Watson and Richelson, 1993; Wong et al., 1982). It is marketed for the treatment of attention deficit hyperactivity disorder (ADHD) in children and adults (Simpson and Plosker, 2004), but, unlike other therapies for ADHD, atomoxetine does not act as a stimulant and does not appear to have abuse potential (Gasior et al., 2005; Jasinski et al., 2008; Lile et al., 2006; Wee and Woolverton, 2004). Furthermore, while atomoxetine does not increase extracellular dopamine concentrations in brain regions involved in reward and reinforcement (e.g., nucleus accumbens), it does increase extracellular dopamine in brain regions thought to be involved in craving and compulsive drug seeking (e.g., prefrontal cortex) (Bymaster et al., 2002). A recent study in rats demonstrated that treatment with acute atomoxetine (1 mg/kg) decreased cue-induced cocaine seeking and relapse to cocaine seeking after abstinence, with minimal effects on sucrose responding and locomotor activity (Economidou et al., 2011). Few human studies have examined atomoxetine for potential efficacy against cocaine. One laboratory study reported that chronic treatment with atomoxetine was safely tolerated but produced minimal attenuation of the subjective effects of acute intranasal cocaine (Stoops et al., 2008). A small (n=20) open-label clinical trial was conducted to study the efficacy of atomoxetine in ADHD patients who also had comorbid cocaine dependence. While modest improvements were observed on attention-related behaviors, atomoxetine did not significantly decrease cocaine use over the course of the 12-week trial (Levin et al., 2009); however, this trial used an open-label design and the co-morbid study population had a high drop-out rate (75%). Thus, the purpose of this study was to conduct a double blind, placebo-controlled, outpatient clinical trial to examine the safety and efficacy of atomoxetine for the treatment of cocaine dependence in a cohort of individuals with primary cocaine dependence.
2. Methods
2.1 Subject Recruitment and Screening
Adult volunteers ages 18–60 reporting cocaine use in the preceding 30 days who met DSM-IV criteria for and were seeking treatment for cocaine dependence were recruited through advertisements and word-of-mouth. Exclusion criteria included dependence on any drug requiring detoxification (i.e., benzodiazepines, barbiturates, alcohol or opioids), current Axis I disorder other than substance use, significant ongoing medical problems (e.g., seizure disorders, uncontrolled hypertension, abnormal ECG), pregnant or lactating females, and recent use of CYP2D6 inhibitors/inducers, MAO-inhibitors or selective serotonin reuptake inhibitors. Individuals enrolled in other drug treatment programs or required to provide urine samples for parole/probation were excluded. The study took place at the Robert Straus Behavioral Research Science Building in Lexington, KY. This study was conducted in accordance with the Helsinki guidelines for ethical human research and was approved by the University of Kentucky (UK) Institutional Review Board. A Certificate of Confidentiality was obtained from the National Institutes of Health, and all subjects gave written informed consent. The study was registered at www.clinicaltrials.gov (NCT00617201).
Screening lasted up to 2 weeks (a minimum of 4 clinic visits were required separated by ≥ 48 hours) during which study eligibility was determined. At each visit, breath alcohol level (Alcomate Prestige; AK Solutions, U.S.A, Palisades Park, NJ) and vital signs were obtained. Observed urine samples were collected and tested for the presence of drugs (methamphetamine, cocaine, tetrahydrocannabinol, methadone, benzodiazepines, barbiturates, morphine-like opioids, phencyclidine, oxycodone, methylenedioxymethamphetamine; Redwood Toxicology Laboratory, Santa Rosa, CA). To qualify, subjects were required to provide at least one urine sample positive for cocaine during screening but were not informed of the enrollment criteria. Urine pregnancy tests were conducted weekly. Subjects were paid $15 for each visit except the day of their physical examination when they received $25.
An extensive medical and psychiatric evaluation was completed that included substance use and drug abuse treatment history, licit medication usage, electrocardiogram (ECG), blood and urine chemistries (including pregnancy testing for females), physical exam, and structured interviews including the Addiction Severity Index (ASI; (McLellan et al., 1992) and Structured Clinical Interview for DSM-IV diagnoses (First et al., 1996). Additional assessments included weight, demographics, the NEO personality inventory (Costa and McCrae, 1985), the Beck Depression Inventory (Beck et al., 1961), the Profile of Mood States (McNair et al., 1971), the Conners Adult ADHD Rating Scale Short Version (CAARS-S:S; Connors et al., 1999), a cocaine-use timeline follow-back (TLFB; adapted from (Sobell and Sobell, 1992) and a Cocaine Craving Scale (Sussner et al., 2006). The TLFB used a calendar to record the days subjects used cocaine, how much cocaine was used, how much time and money was spent using cocaine and the route of cocaine administration. The Cocaine Craving Scale asked subjects to rate the following statements on a 7-point scale: “I want cocaine so bad I can almost taste it.” “I have an urge for cocaine.” “I am going to use cocaine as soon as possible.” “I think that I could resist using ‘coke’ now.” “I crave ‘coke’ right now.” “All I want to use now is cocaine.” “I have no desire for cocaine right now.” “Using cocaine now would make things seem just perfect.” “I will use cocaine as soon as I get the chance.” “Nothing would be better than using ‘coke’ right now.”
Because atomoxetine is approved for the treatment of ADHD, four psychomotor/cognitive tasks were incorporated into the trial. During screening, these were administered on each of four visits for practice and to establish baseline responding. They included a symbol test and a math task (both described in Vansickel et al., 2006), a modified repeated acquisition task (see Rush and Griffiths, 1996), and the digit-symbol substitution task (DSST; McLeod et al., 1982).
2.2 Clinical Trial Procedures
2.2.1 Study Design and Outcomes
This 12-week outpatient study employed a placebo-controlled, double blind, randomized, parallel group design. Once qualified, urn randomization was used to stratify the groups on age, gender and severity of cocaine use (the latter employing a dichotomous variable of self-reported cocaine use < than or ≥ 15 of the past 30 days from the TLFB). Subjects were randomized into one of two groups: placebo (n=25) or atomoxetine (n=25; 80 mg/day) by the investigational pharmacists who were off site and had no interaction with volunteers; investigator, medical and study staff as well as participants were blind to the assignments. Subjects were required to attend the clinic every Monday, Wednesday and Friday for 12 weeks. An a priori study criteria included that a subject take at least one dose of medication to be included in the intent-to-treat analysis. Drop-out criteria were defined as subjects who voluntarily withdrew from the study, subjects who missed three consecutive visits following the date of their last available medication dose or subjects who were administratively discharged.
A set of core measures was collected at each visit (breath alcohol level, urine drug testing, vital signs, TLFB, and pill counts), while others were collected once weekly (weight, pregnancy, medication side effects, adverse events and concomitant medications [prescription and over-the-counter], the DSST, BDI and Cocaine Craving Scale]) or monthly (the POMS, the psychomotor task battery (symbol copying, math task and repeated acquisition), an abbreviated ASI, a physical exam, an ECG, and liver function tests). Urine specimens were divided into two samples with one tested immediately and the other frozen (−80°C) for potential later analyses.
2.2.2 Medication
Atomoxetine hydrochloride (Strattera®) and matched placebos were obtained from Eli Lilly and Company (Indianapolis, IN). Study medication was packaged by the UK Investigational Pharmacy into sealed blister packs (2 capsules per blister) with a maximum 9-day supply. Atomoxetine was initiated at 40 mg/day for the first 4 days (the recommended starting dose for ADHD in adults) and was increased to 80 mg/day thereafter. At the time of study initiation, the prescribing information recommended a starting dose of 40 mg/day to increase within three days to 80 mg/day (with potential maximum dosing at 100 mg/day but only after 3–4 weeks of dosing). Thus, we chose the recommended starting dose to ensure maximum toleration and escalated to a final therapeutic dose of 80 mg in order to rapidly achieve steady state with a therapeutic dose. Subjects were instructed to take their medication once daily at the same time each day and to bring their medication to each clinic visit where staff observed medication ingestion and recorded the number of capsules in the blister pack to enhance and monitor adherence. Subjects also reported the number of capsules taken on non-clinic days. If subjects missed a dose, they were instructed to leave it in the package; empty blister packs were returned to the clinic prior to provision of the next one.
2.2.3 Contingency Management and Counseling
A contingency management procedure was employed to reinforce clinic attendance and study compliance for all participants (DSST; McLeod et al., 1982). In order to receive the financial incentive, subjects were required to attend the clinic visit, provide a urine and a breath sample, and ingest their study medication under supervision (urine test outcomes had no bearing on the delivery of reinforcement). On the first visit, $1.75 could be earned; this increased by $0.65/day for each consecutive day of attendance. Three consecutive visits yielded a $10 bonus payment. Any missed visits led to the daily payment being reset back to the initial first payment amount ($1.75). If subjects then returned for 3 consecutive visits, the value of the incentive was returned to the value prior to the reset. Subjects also received a $25 bonus for completing their physical examination during weeks 4, 8 and 12. Payment could be obtained at each visit or money could accrue in an online account with payment later depending upon subject preference. Perfect attendance and compliance would yield $667.50.
Subjects were offered non-mandatory once weekly cognitive behavioral therapy aimed at relapse prevention through provision of coping skills. A trained and licensed therapist delivered the therapy using a manual-driven 12-week approach developed for cocaine dependent patients (Carroll et al., 1998, 2004.
2.2.4 Follow-Up
All participants, regardless of completion status, were invited to return for a follow-up visit 12 weeks after their last day of medication and paid $25 for this visit. Data collection included the ASI, DSST, POMS, breath alcohol screen, urine drug test, pregnancy test (if female), vital signs, weight, BDI, psychomotor testing, the TLFB and Cocaine Craving Scale. Any adverse events that had not been resolved during the study period were queried at the follow-up.
2.3 Statistical Data Analysis
Independent t-tests and Chi square (X2) were employed to compare baseline characteristics and responses between the two groups. These analyses incorporated Levene’s test for equality of variance to assess and correct for instances of non-homogeneity of variance (modified from Silverman, 2004); corrected T values are reported where appropriate. Trial retention was compared between groups using Cox proportional hazards model (Levene, 1960). An “event” was defined as the day of the last clinic visit with data censored at visit 36. Chi square values are reported along with hazard ratios and 95% confidence intervals (CI).
Generalized estimating equation (GEE) analyses with an exchangeable correlation structure were used to assess for significant differences between groups on the probability of positive and negative benzoylecgonine urine outcomes, counseling attendance, and medication adhererence. Results are reported as odds ratios (OR) with 95% CIs (Cox, 1972). Mixed models with an AR(1) covariance structure in Proc Mixed (Zeger and Liang, 1986) were employed to analyze longitudinal data collected over the course of the 12-week trial (e.g., laboratory results, psychomotor performance measures, vital signs, etc.). All analyses were run with SAS 9.3 (SAS Institute, Inc., Cary, NC) for Windows® and were considered significant when p<.05.
3. Results
3.1 Subjects
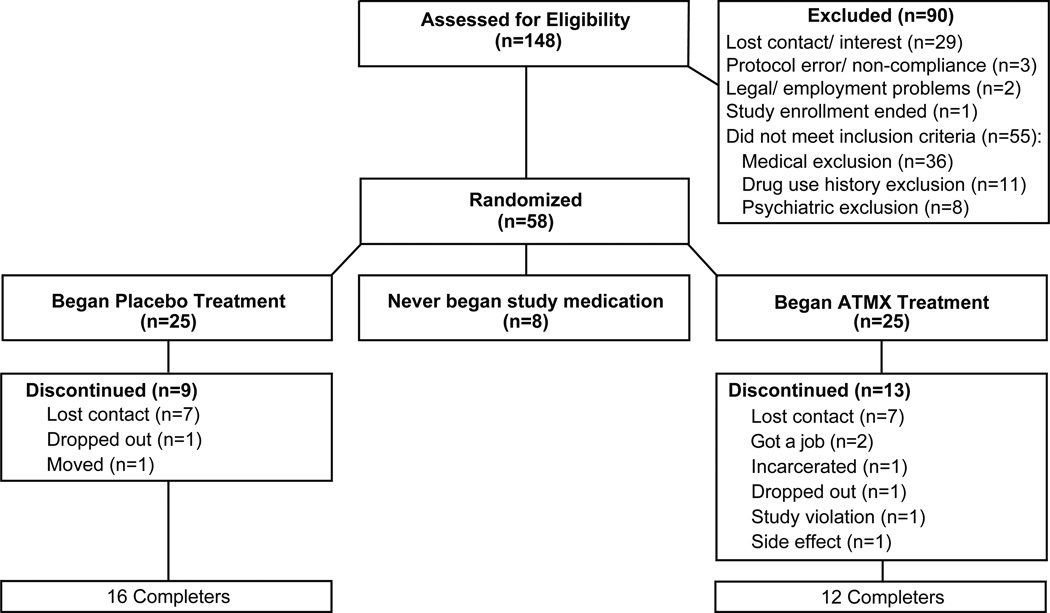
A total of 148 individuals presented for in-person study screening after the initial phone pre-screen, and 58 completed the entire screening process and were qualified to participate. Eight either failed to take their first dose of medication or never returned after completing the screening; thus, a total of 50 subjects were randomized (n=25 in each of two groups; see Figure 1). Subject demographic and substance use characteristics are shown in Table 1. The study population was primarily male (72%) and African-American (68%) with an average age of 43.1 years. Nearly 80% of all subjects reported current use of tobacco, and the groups did not differ. The majority of subjects reported smoking “crack” cocaine as their preferred cocaine route of administration (92%) with the remainder reporting snorting/insufflation; the cohort reported using cocaine an average of 16.8 days of the past 30 days. There were no significant differences between the groups on key demographic and drug use characteristics (t-tests with Levene’s; p>.05; Table 1) other than frequency of any alcohol use (but no difference in reports of using alcohol to intoxication). No past 30-day use was reported for heroin, methadone, barbiturates, inhalants, amphetamines or hallucinogens.
Figure 1.
CONSORT diagram for disposition of subjects.
Table 1.
Demographic and drug use characteristics of subjects by group assignment
| Placebo | Atomoxetine | |
|---|---|---|
| Age (years) | 41.6 (1.6) | 44.5 (1.6) |
| Race (N [%]) | ||
| African-American | 19 | 15 |
| Caucasian | 6 | 10 |
| Gender | ||
| Male | 17 | 19 |
| Female | 8 | 6 |
| Education (years) | 12.7 (0.5) | 12.4 (0.4) |
| Employment status | ||
| Employed | 5 | 7 |
| Unemployed | 20 | 18 |
| Age at first cocaine use (years) | 23.7 (1.5) | 26.0 (1.6) |
| Past 30-day cocaine use (days) | 17.5 (1.8) | 16.0 (1.7) |
| Primary route of use for cocaine | ||
| Smoking | 23 | 23 |
| Intranasal | 2 | 2 |
| Lifetime cocaine use (years) | 15.0 (1.6) | 15.1 (1.7) |
| Past 30-day use (days) | ||
| Alcohol | 11.2 (2.1) | 5.0 (1.6)* |
| Alcohol to Intoxication | 3.0 (1.0) | 1.8 (1.0) |
| Other Opiates/Analgesics | 1.4 (1.0) | 0.5 (0.5) |
| Sedatives/Hypnotics | 0.1 (0.1) | 0.8 (0.8) |
| Cannabis | 7.4 (2.3) | 2.7 (1.3) |
| Prior cocaine treatment episodes | 1.2 (0.3) | 1.0 (0.3) |
Means values are shown (±1 SEM) or counts are shown. Subjects were stratified using urn randomization for age, race and past 30-day cocaine use. Drug use data were derived from the ASI interviews. No significant differences were found for any variables except for average days reported for any alcohol use (*t-test; p<.05).
3.2 Trial Retention and Participation
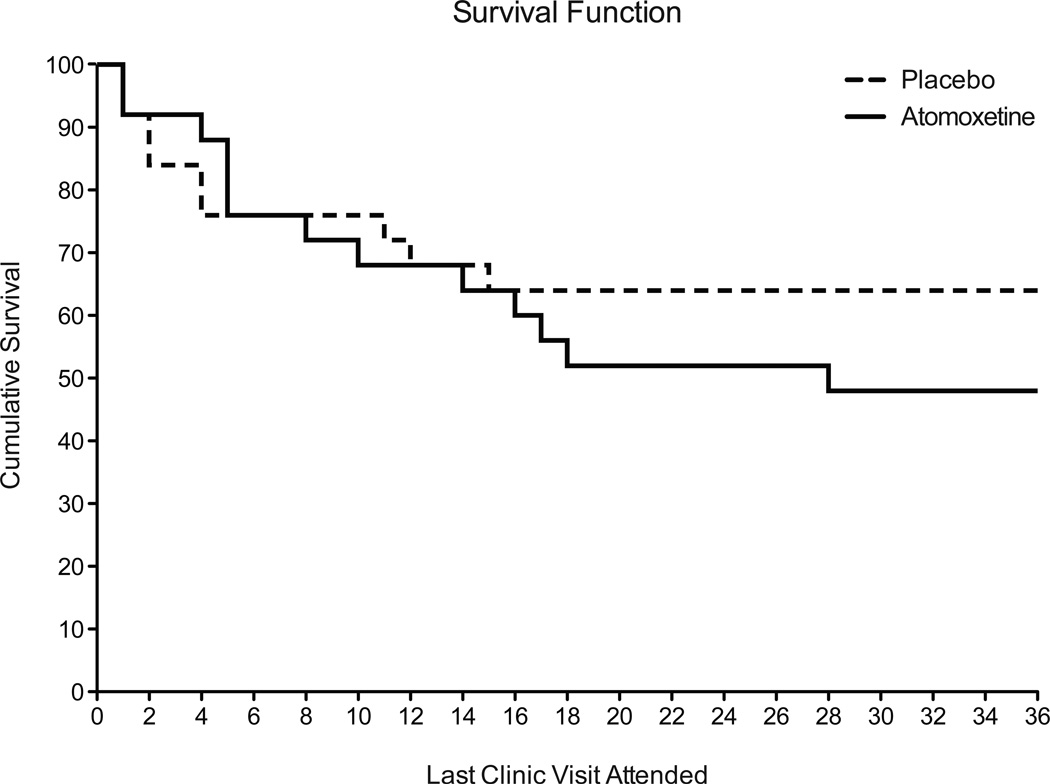
Figure 2 illustrates the survival curve for retention in the study. By the end of 12 weeks, 64% were retained in the placebo group and 48% were retained in the atomoxetine group. Only one subject was administratively discharged from the study, and this was due to theft and forgery of a check from the clinic; he was assigned to the atomoxetine group. The survival functions for the two groups did not differ significantly (Cox analysis X2=.72; p=.40; Hazard Ratio 1.48 [CI 0.62–3.39]). The average number of days completed in the trial was 57.4 (±7) for the placebo group and 51.6 (±6.7) for the atomoxetine group. Examination of the contingency management outcomes (as a proxy for attendance and study compliance) revealed that the placebo group earned an average of $372.23 and the atomoxetine group earned an average of $334.30 for the 12-week trial period (from total possible earnings of $667.50); there was no group difference in earnings (t [df=48]=0.49; p=.63).
Figure 2.
Line functions illustrate the Kaplan-Meijer survival curves for retention in the 12-week trial (with 3 weekly visits for a total of 36 visits over 12 weeks) for the placebo (dotted line) and the atomoxetine-treated (solid line) groups. No significant differences in retention were found between the groups (Cox analysis X2=.72; p=.40; Hazard Ratio 1.48 [CI 0.62–3.39]).
With regard to adherence, the groups did not differ in any meaningful manner. For example, pill counts were accurate for 98% (556 of 595) and 97% (509 of 524) of the total visits attended for the placebo- and atomoxetine-assigned subjects (OR=1.48: CI 0.57–3.82). There was no statistically significant difference in the proportion of counseling sessions attended by patients in the two study arms (X2 =0.74, p=0.39; OR 1.499 [CI95% 0.62–3.63]); however, the atomoxetine-assigned subjects participated in 12% fewer sessions overall.
3.3 Cocaine Urinalysis
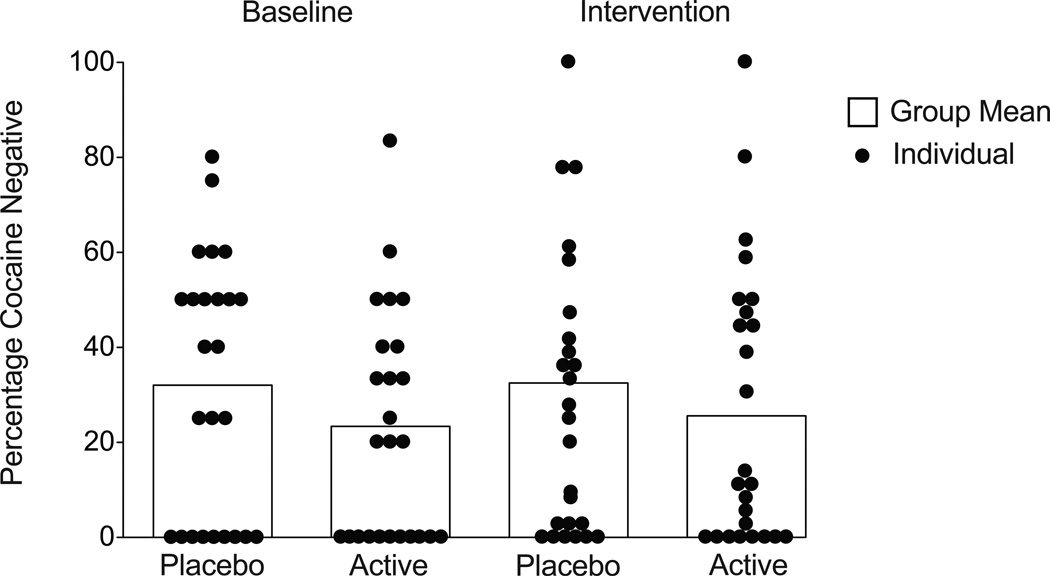
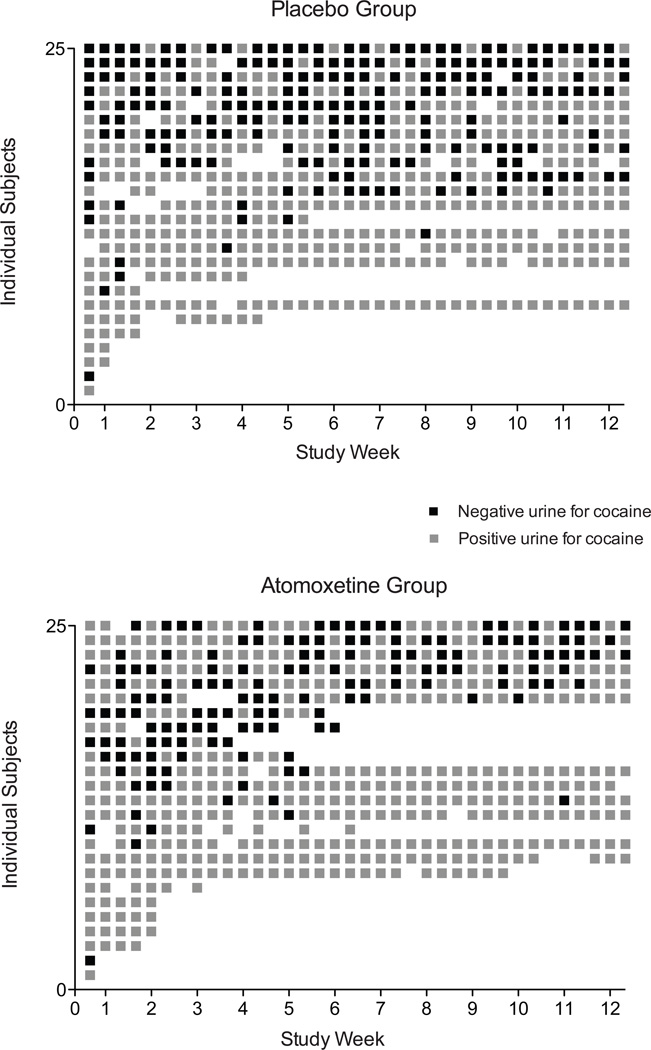
Urine samples collected during the baseline period revealed substantial cocaine use; a total of 72% of samples tested positive for benzoylecgonine. GEE analysis of the proportion of cocaine negative urine samples provided at baseline did not differ between the groups (X2=1.49, p=.22; OR=1.52 [CI 0.76 – 3.23]); data shown in Figure 3 (left side of the figure) illustrate these data as percentage of samples negative for cocaine in the placebo (32%) and atomoxetine (24%) groups. Figure 4 provides a more detailed view of the individual outcomes for urinalysis results and clinic attendance during the intervention phase. GEE analysis of cocaine urine outcomes for cocaine over the 12-week trial period revealed no significant difference between the placebo and atomoxetine groups. This was true regardless of whether missing urine samples were counted as missing (X2=0.17, p=.68; OR=0.86 [CI 0.42 – 1.77) or counted as positive (X2=0.2, p=.66; OR=0.89 [95% CI 0.41 – 1.74). The overall averages for cocaine positive urine samples during the intervention period (when counting missing as positive) were 67% for the placebo group and 74% for the atomoxetine group (Figure 3, right side). There were no differences between the groups on the amount of missing data for urinalysis outcomes.
Figure 3.
The figure illustrates benzoylecgonine negative urine samples provided during the 2-week screening period (left side) and during the 12-week intervention period (right side). The histogram illustrates the mean number of benzoylecgonine negative urines for the two groups, and the circles represent the data for each individual subject. There were no significant differences between the groups with respect to provision of negative urine samples during the baseline period, and the intervention with atomoxetine treatment did not alter this pattern.
Figure 4.
Data shown are for the placebo (upper panel) and atomoxetine (lower panel) treatment groups with individual subjects plotted along the y-axis and study week (with each of the three study visits/week) plotted along the x-axis. Individual visits (squares) in black designate provision of a cocaine negative sample, and those in gray designate provision of a cocaine positive sample. Missing squares represent missed clinic visits. There were no differences between the groups regardless of whether missing samples were counted as missing (X2=0.17, p=.68; OR=0.86 [95% CI 0.42 – 1.77) or counted as positive (X2=0.2, p=.66; OR=0.89 [95% CI 0.41 – 1.74).
3.4 Behavioral Outcome Measures
The groups did not differ on subscale scores for the CAARS including Subscale E: ADHD index at baseline. Similarly, no differences between groups were observed for the NEO subscales at baseline or the BDI scores at baseline and during the 12-week trial. There were no group differences at baseline on any of the cocaine craving visual analog scales. During the trial, there was a significant group×time interaction (F[11, 310]=1.99; p=.03) for “All I want to use now is cocaine” and for “Nothing would be better than using coke right now” (F= 2.37; p= .008). Examination of the data suggest that these findings were not due to systematic changes over time in craving for either group; rather the placebo group reported slightly higher scores reliably throughout the trial and during one week (Week 11) the atomoxetine group reported higher scores (creating the interaction) which declined the following week. No other differences on craving items were observed. While no baseline differences were found on the POMS, there were some significant group and group × time interactions, whereby the placebo group showed increasing scores on the Tension-Anxiety scale over the 12-week course of the trial while the atomoxetine group scores declined (F[2, 50]=4.37; p=.02). This finding was driven by significant increases on single items from that scale, including tense, uneasy and anxious (p<.05); other POMS scale results did not differ between the groups.
The psychomotor performance battery revealed no meaningful differences between the groups; the primary statistically significant findings were related to changes over time during the trial on performance of the repeated acquisition task (total errors and total percent errors) and no significant findings for the DSST, symbol test and math test.
3.5 Safety Outcomes
Atomoxetine was well tolerated overall and there were few problems with the initial dose escalation during the lead-in period. There were significant group differences for both systolic (F [1, 48] = 6.2; p=.02) and diastolic blood pressure (F[1,48]=40.8; p<.0001) over the course of the trial characterized by statistically significant elevations in blood pressure for the atomoxetine group (an average of ~ 4 mmHg for systolic and diastolic values) compared to the placebo group. Moreover, a modest but significant increase in core temperature for both groups was observed during the trial (group×time F [35, 1014]= 1.9; p=.002). No between-group differences at baseline or over the course of the intervention were observed for heart rate, respiratory rate, body weight, or liver function tests (p>0.05).
Weekly side effect questionnaires revealed that the most common side effects reported with atomoxetine treatment (each in 10 or more of the subjects) included nausea, dry mouth, trouble sleeping, and fatigued/tired; however, analysis of the dichomotized response (i.e., presence or absence of response reported during the trial) to each of these items did not statistically differ between the active and placebo groups.
4. Discussion
This study explored the potential efficacy of atomoxetine for the treatment of cocaine dependence using a double-blind, randomized, placebo-controlled design coupled with contingency management and counseling. Overall, 56% of randomized subjects were retained in the 12-week study, and there were no differences in retention between the two groups. Analysis of urine drug tests for the presence of benzoylecgonine, regardless of method used for treatment of missing data (i.e., missing as missing, missing as positive), revealed no statistical difference between the placebo and atomoxetine groups. Cocaine use was substantial over the course of the trial, with smoking cocaine base as the predominant route of use, with approximately 67% and 74% of samples positive for the cocaine metabolite in the placebo and atomoxetine groups, respectively.
Atomoxetine was generally well tolerated by this population. Weekly collection of adverse event reports did not reveal significant differences between atomoxetine and placebo. However, one male subject from the atomoxetine treatment group discontinued participation after five days because of complaints of urinary hesitancy, which is of note given some interest in atomoxetine for the treatment of enuresis (Singer, 1998). Modest, but statistically significant, increases in both systolic and diastolic blood pressure were observed in the atomoxetine group over time in comparison to the placebo group. This finding has been reported previously after acute (Sumner et al., 2006) and chronic (Heil et al., 2002) dosing with atomoxetine in adults. There was no evidence of change in liver function (AST, ALT or alkaline phosphate) in this study, which has been reported previously but only as an infrequent occurrence (Stiefel and Besag, 2010).
The study provides no evidence that atomoxetine will have any utility in treating cocaine dependence. Although one limitation of the study is the small sample size, the two groups were stratified and well matched on key demographic characteristics, reported substantial rates of cocaine use at the start of the study, and expressed interest in treatment. Clinic attendance was generally comparable between groups (as were contingency management earnings for adherence and attendance). Greater than one-half of the subjects were retained and completed the 12-week trial. This is generally consistent with, and in some cases slightly better than, pharmacotherapy studies conducted in primary cocaine abusers for studies of this same duration in other regions, where retention has ranged, for example, from 20 to 60% (Bangs et al., 2008). There were no differences in urinalysis results of cocaine use; rates were high at baseline and remained so over the course of the study (Figure 3). Thus, with no differences on retention or urinalysis outcomes, the data suggest no benefit of atomoxetine treatment on cocaine use.
Recent reports have advocated for noradrenergic agents, including atomoxetine, as treatments for stimulant dependence specifically because of their role as cognitive enhancers as this effect may facilitate and improve the efficacy of behavioral interventions (Anderson et al., 2009; Bisaga et al., 2010; Carroll et al., 1998; Malcolm et al., 2005; Schmitz et al., 2010). This study did not examine that question directly but did collect data that could help inform the hypothesis. First, because atomoxetine is an effective treatment for ADHD, the CAARS (Sofuoglu et al., 2012; Sofuoglu and Sewell, 2009), a validated instrument used for screening for ADHD with adults, was collected at baseline to assess the study population. In the event that differential responses were observed between the groups, the CAARS scores could have been employed as a covariate to assess improvement in attention as a contributing factor to the cocaine dependence outcomes. However, no differences in response to treatment were observed nor were there differences between the groups on the CAARS scores at baseline. Moreover, a battery of cognitive measures (including repeated acquisition, symbol and math tests) were collected over the course of the study but no group differences emerged between the two groups on these measures either. Thus, whether cognitive enhancement through noradrenergic stimulation may alter outcomes for the treatment of psychostimulant dependence is uncertain.
In summary, this outpatient study examining the effects of atomoxetine in cocaine dependent treatment-seeking individuals found no evidence that cocaine use was significantly altered in comparison to placebo based on urinalysis outcomes. The two groups were comparable on key demographic and drug use characteristics, retention and study compliance/adherence were generally favorable and similar in the two groups, and atomoxetine was well tolerated. However, the present data suggest no direct effect of atomoxetine on cocaine use behaviors and provide no support for further evaluation of atomoxetine as a treatment for cocaine dependence.
Acknowledgments
The authors are grateful to Jaclyn Miller, Anthony Fluty, Marie Bate, R.N. and Steve Johnson, M.S.W. for their professional contributions to the study and to Dr. Steve Sitzlar for providing expert pharmacy services.
Role of Funding Source
This project was supported by a grant from the National Institute on Drug Abuse (NIDA) R01 DA022191 (SLW). We are grateful to Eli Lilly & Co., Indianapolis, Indiana for their gratis provision of study medication (atomoxetine and matched-placebos). Neither NIDA nor Eli Lilly & Co. had any role in the study design, collection, analysis and interpretation of the data or in the decision to submit the paper. The initial draft was shared with staff from Eli Lilly & Co. for their review, and no changes resulted from this process.
Footnotes
Contributors
SLW, CJW, PAN, CRR and MRL contributed to the design of the study. SLW and CJW wrote the protocol. SLW, LSM, CJW, PAN and MRL provided oversight for the day-to-day operations of the clinical trial. CLC and MRL provided medical expertise and care for the volunteers during screening and enrollment in the study. PAN conducted the statistical analyses. SLW wrote the first draft of the manuscript. All authors have contributed to and approved the final manuscript.
Contributor Information
Sharon L. Walsh, Center on Drug and Alcohol Research, Department of Behavioral Science, University of Kentucky College of Medicine, 515 Oldham Court, Lexington, Kentucky 40502, Phone: 859-257-6485, Fax: 859-257-5232, sharon.walsh@uky.edu
Lisa S. Middleton, Center on Drug and Alcohol Research, Department of Behavioral Science, University of Kentucky College of Medicine, 515 Oldham Court, Lexington, Kentucky 40502
Conrad J. Wong, Early Phase Regulatory – Neuroscience Lilly Corporate Center Indianapolis IN 46285
Paul A. Nuzzo, Center on Drug and Alcohol Research, University of Kentucky College of Medicine, 515 Oldham Court, Lexington, Kentucky 40502
Charles L. Campbell, Department of Internal Medicine, Division of Cardiology, University of Kentucky College of Medicine, 326 Charles T. Wethington Building, Lexington, Kentucky 40536-0200
Craig R. Rush, Department of Behavioral Science, University of Kentucky College of Medicine, 204B 265 E. High Street, Lexington, Kentucky 40507
Michelle R. Lofwall, Center on Drug and Alcohol Research, Department of Psychiatry, University of Kentucky College of Medicine, 515 Oldham Court, Lexington, Kentucky 40502
References
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs ME, Hazell P, Danckaerts M, Hoare P, Coghill DR, Wehmeier PM, Williams DW, Moore RJ, Levine L. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics. 2008;121:e314–e320. doi: 10.1542/peds.2006-1880. [DOI] [PubMed] [Google Scholar]
- Batki SL, Washburn AM, Delucchi K, Jones RT. A controlled trial of fluoxetine in crack cocaine dependence. Drug Alcohol Depend. 1996;41:137–142. doi: 10.1016/0376-8716(96)01233-1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;54:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Cheng WY, Levin FR, Mariani JJ, Raby WN, Nunes EV. A placebo-controlled trial of memantine for cocaine dependence with high-value voucher incentives during a pre-randomization lead-in period. Drug Alcohol Depend. 2010;111:97–104. doi: 10.1016/j.drugalcdep.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden-Watson C, Richelson E. Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci. 1993;52:1023–1029. doi: 10.1016/0024-3205(93)90194-8. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsaville BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–728. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Connors CK, Erhardt D, Sparrow EP. Conners' Adult ADHD Rating Scales Technical Manual. North Tonawanda, New York: Multi-Health Systems Inc.; 1999. [Google Scholar]
- Costa PT, McCrae RR. The NEO Personality Inventory Manual. Odessa, Florida: Psychological Assessment Resources; 1985. [Google Scholar]
- Cox DR. Regression models and life-tables. J. R. Stat. Soc. Series B, Statistical Methodology. 1972;34:187–220. [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Dalley JW, Everitt BJ. Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol. Psychiatry. 2011;69:266–274. doi: 10.1016/j.biopsych.2010.09.040. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. Structured clinical interview for DSM-IV Axis I disorders. New York, New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- Gasior M, Bergman J, Kallman MJ, Paronis CA. Evaluation of the reinforcing effects of monoamine reuptake inhibitors under a concurrent schedule of food and i.v. drug delivery in rhesus monkeys. Neuropsychopharmacology. 2005;30:758–764. doi: 10.1038/sj.npp.1300593. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Elk R, Schmitz J, Davis C, Creson D, Kirby K. Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: two placebo-controlled double-blind trials. J. Clin. Psychopharmacol. 1995;15:163–174. doi: 10.1097/00004714-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz JM, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence: A double-blind randomized clinical trial. J. Clin. Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades HM, Silverman K, Schmitz JM, Stotts A, Creson D, Bailey R. Risperidone for the treatment of cocaine dependence: Randomized, double-blind trial. J. Clin. Psychopharmacol. 2000;20:305–310. doi: 10.1097/00004714-200006000-00003. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades HM, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz JM. Agonist-like or antagonist-like tratment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004a;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict. Behav. 2004b;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Rosenblum A, Palij M, Magura S, Foote J, Lovejoy M, Stimmel B. Bromocriptine for cocaine dependence: A controlled clinical trial. Am. J. Addict. 1997;6:54–64. [PubMed] [Google Scholar]
- Heil SH, Holmes HW, Bickel WK, Higgins ST, Badger GJ, Laws HF, Faries DE. Comparison of the subjective, physiological and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend. 2002;67:149–156. doi: 10.1016/s0376-8716(02)00053-4. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Faries DE, Moore RJ, Schuh LM, Allen AJ. Abuse liability assessment of atomoxetine in a drug-abusing population. Drug Alcohol Depend. 2008;95:140–146. doi: 10.1016/j.drugalcdep.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Chen YR, Swann AC, Schmitz J, Lesser J, Ruiz P, Johnson P, Clyde C. Ritanserin in the treatment of cocaine dependence. Biol. Psychiatry. 1997;42:932–940. doi: 10.1016/S0006-3223(96)00490-8. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Javors MA, Harrison JM, Elkashef A, Mojsiak J, Li SH, Bloch DA. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of cocaine dependence. Drug Alcohol Depend. 2006;84:256–263. doi: 10.1016/j.drugalcdep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Jones HE, Johnson RE, Bigelow GE, Silverman K, Mudric T, Strain EC. Safety and efficacy of L-tryptophan and behavioral incentives for treatment of cocaine dependence: a randomized clinical trial. Am. J. Addict. 2004;13:421–437. doi: 10.1080/10550490490512753. [DOI] [PubMed] [Google Scholar]
- Levene H. Contributions to Probability and Statistics: Essays in honor of Harold Hotelling. Stanford University Press; 1960. [Google Scholar]
- Levin FR, Mariani JJ, Secora A, Brooks D, Cheng WY, Bisaga A, Nunes E, Aharonovich E, Raby W, Hennessy G. Atomoxetine Treatment for Cocaine Abuse and Adult Attention-Deficit Hyperactivity Disorder (ADHD): A Preliminary Open Trial. J. Dual Diagn. 2009;5:41–56. doi: 10.1080/15504260802628767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Durell TM, Glaser PE, Rush CR. Discriminative-stimulus, self-reported, performance, and cardiovascular effects of atomoxetine in methylphenidate-trained humans. Exp. Clin. Psychopharmacol. 2006;14:136–147. doi: 10.1037/1064-1297.14.2.136. [DOI] [PubMed] [Google Scholar]
- Loebl T, Angarita GA, Pachas GN, Huang KL, Lee SH, Nino J, Logvinenko T, Culhane MA, Evins AE. A randomized, double-blind, placebo-controlled trial of long-acting risperidone in cocaine-dependent men. J. Clin. Psychiatry. 2008;69:480–486. doi: 10.4088/jcp.v69n0321. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Kajdasz DK, Herron J, Anton RF, Brady KT. A double-blind, placebo-controlled outpatient trial of pergolide for cocaine dependence. Drug Alcohol Depend. 2000;60:161–168. doi: 10.1016/s0376-8716(99)00151-9. [DOI] [PubMed] [Google Scholar]
- Malcolm R, LaRowe S, Cochran K, Moak D, Herron J, Brady K, Hedden S, Woolson R, Halushka P. A controlled trial of amlodipine for cocaine dependence: a negative report. J. Subst. Abuse Treat. 2005;28:197–204. doi: 10.1016/j.jsat.2004.12.006. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grisson G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. J. Subst. Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLeod D, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Beh. Res. Meth. Instru. 1982;14:433–436. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. EITS Manual for the Profile of Mood States. San Diego, California: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Schmitz JM, Moeller FG, Grabowski J. Safety, tolerability and efficacy of levodopa-carbidopa treatment for cocaine dependence: two double-blind, randomized, clinical trials. Drug Alcohol Depend. 2007;88:214–223. doi: 10.1016/j.drugalcdep.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, editor. SAMHSA. The DAWN Report: Highlights of the 2010 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. Rockville, MD: 2012. [PubMed] [Google Scholar]
- Schmitz JM, Averill P, Stotts AL, Moeller FG, Rhoades HM, Grabowski J. Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug Alcohol Depend. 2001;63:207–214. doi: 10.1016/s0376-8716(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Lindsay JA, Stotts AL, Green CE, Moeller FG. Contingency management and levodopa-carbidopa for cocaine treatment: a comparison of three behavioral targets. Exp. Clin. Psychopharmacol. 2010;18:238–244. doi: 10.1037/a0019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Mooney ME, Moeller FG, Stotts AL, Green C, Grabowski J. Levodopa pharmacotherapy for cocaine dependence: choosing the optimal behavioral therapy platform. Drug Alcohol Depend. 2008;94:142–150. doi: 10.1016/j.drugalcdep.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98:1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Silverman K. Exploring the limits and utility of operant conditioning in the treatment of drug addiction. Behavior Anal. 2004;27:209–230. doi: 10.1007/BF03393181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D, Plosker GL. Atomoxetine: a review of its use in adults with attention deficit hyperactivity disorder. Drugs. 2004;64:205–222. doi: 10.2165/00003495-200464020-00005. [DOI] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J. Educ. Behav. Stat. 1998;24:323–355. [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Measuring Alcohol Consumption: Psychosocial and biological methods. Rockville, MD: The Humana Press, Inc.; 1992. pp. 207–224. [Google Scholar]
- Sofuoglu M, Devito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.06.021. Epub 2012 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict. Biol. 2009;14:119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiefel G, Besag FM. Cardiovascular effects of methylphenidate, amphetamines and atomoxetine in the treatment of attention-deficit hyperactivity disorder. Drug Saf. 2010;33:821–842. doi: 10.2165/11536380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Stine SM, Krystal JH, Kosten TR, Charney DS. Mazindol treatment for cocaine dependence. Drug Alcohol Depend. 1995;39:245–252. doi: 10.1016/0376-8716(95)01174-4. [DOI] [PubMed] [Google Scholar]
- Sumner CR, Schuh KJ, Sutton VK, Lipetz R, Kelsey DK. Placebo-controlled study of the effects of atomoxetine on bladder control in children with nocturnal enuresis. J. Child Adolesc. Psychopharmacol. 2006;16:699–711. doi: 10.1089/cap.2006.16.699. [DOI] [PubMed] [Google Scholar]
- Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83:233–237. doi: 10.1016/j.drugalcdep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Hays LR, Rush CR. Discriminative-stimulus effects of triazolam in women and men. Am. J. Drug Alcohol Abuse. 2006;32:329–349. doi: 10.1080/00952990500479266. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb. Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology. 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. Evaluation of the reinforcing effects of atomoxetine in monkeys: Comparison with methylphenidate and desipramine. Drug Alcohol Depend. 2004;75:271–276. doi: 10.1016/j.drugalcdep.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Somoza E, Sarid-Segal O, Goldsmith RJ, Harrer JM, Coleman FS, Kahn R, Osman S, Mezinskis J, Li SH, Lewis D, Afshar M, Ciraulo DA, Horn P, Montgomery MA, Elkashef A. A double-blind, placebo-controlled trial of reserpine for the treatment of cocaine dependence. Drug Alcohol Depend. 2007;91:205–212. doi: 10.1016/j.drugalcdep.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley EL, Bigelow GE, Silverman K, Johnson RE, Strain EC. A randomized controlled trial of fluoxetine in the treatment of cocaine dependence among methadone-maintained patients. J. Subst. Abuse Treat. 2011;40:255–264. doi: 10.1016/j.jsat.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DT, Threlkeld PG, Best KL, Bymaster FP. A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J. Pharmacol. Exp. Ther. 1982;222:61–65. [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]