Abstract
The impact of widespread and common environmental factors, such as chemical contaminants, on infectious disease risk in amphibians is particularly important because both chemical contaminants and infectious disease have been implicated in worldwide amphibian declines. Here we report on the lone and combined effects of exposure to parasitic cercariae (larval stage) of the digenetic trematode, Acanthostomum burminis, and four commonly used pesticides (insecticides: chlorpyrifos, dimethoate; herbicides: glyphosate, propanil) at ecologically relevant concentrations on the survival, growth, and development of the common hourglass tree frog, Polypedates cruciger Blyth 1852. There was no evidence of any pesticide-induced mortality on cercariae because all the cercariae successfully penetrated each tadpole host regardless of pesticide treatment. In isolation, both cercarial and pesticide exposure significantly decreased frog survival, development, and growth, and increased developmental malformations, such as scoliosis, kyphosis, and also edema and skin ulcers. The combination of cercariae and pesticides generally posed greater risk to frogs than either factor alone by decreasing survival or growth or increasing time to metamorphosis or malformations. The exception was that lone exposure to chlorpyrifos had higher mortality without than with cercariae. Consistent with mathematical models that suggest that stress should increase the impact of generalist parasites, the weight of the evidence from the field and laboratory suggests that ecologically relevant concentrations of agrochemicals generally increase the threat that trematodes pose to amphibians, highlighting the importance of elucidating interactions between anthropogenic activities and infectious disease in taxa of conservation concern.
Keywords: Glyphosate, Chlorpyrifos, Dimethoate, Malformation, Propanil
Introduction
We are in the midst of a sixth global mass extinction event (Wake and Vredenburg 2008). There are many causes for contemporary losses of biodiversity and many researchers have hypothesized that multiple stressors are additively or synergistically increasing the risk of mortality, extirpations, and extinctions (Blaustein and Kiesecker 2002; Rohr et al. 2004; Cahill et al. 2013). Concurrent with widespread biodiversity losses is an unprecedented increase in infectious diseases (Jones et al. 2008), many of which are associated with widespread host extirpations (Stuart et al. 2004; Rohr and Raffel 2010; Fisher et al. 2012). Consequently, it has become urgent to understand how anthropogenic factors affect diseases associated with mass mortality events in taxa of conservation concern. Theory on the impacts of stressors on disease risk suggests that negative or positive associations between disease and stress can occur, emphasizing the need to amass empirical research to evaluate which of these theoretical outcomes are most likely (Lafferty and Holt 2003).
For several reasons, there has been considerable interest in the effects of chemical contaminants on disease risk in amphibians (Rohr et al. 2009a, b; Schotthoefer et al. 2011; McMahon et al. 2013; Rohr et al. 2013). Amphibians are considered the most threatened of all vertebrate taxa and many of their declines have been associated with infectious diseases (Blaustein and Kiesecker 2002; Stuart et al. 2004). Additionally, amphibians are exposed to contaminants that run-off into freshwater ecosystems, have permeable skin that allows chemicals to readily enter their bodies (Rohr et al. 2011), and many chemical contaminants are immunosuppressive and associated with elevated disease endpoints (Voccia et al. 1999; Martin et al. 2010). In particular, trematode infections of amphibians have attracted substantial attention because they are associated with mass mortality events (Johnson et al. 1999; Rohr et al. 2010), are believed to be more common now than historically (Johnson et al. 2003; Rohr et al. 2009a), and can cause grotesque limb and body malformations (Johnson et al. 2012). Additionally, the intensity and severity of these infections have been shown to be quite sensitive to several common agricultural contaminants (Kiesecker 2002; Linzey et al. 2003; Johnson et al. 2007; Rohr et al. 2008a, b; Kelly et al. 2010).
Chemical contaminants have modified both amphibian exposure and susceptibility to trematodes. For instance, fertilizer pollution was shown to increase trematode infections in frogs by increasing the number of trematode-infected snails (Johnson et al.2007; Rohr et al. 2008b). The insecticides malathion and esfenvalerate were associated with elevated trematode infections and limb deformities by reducing frog defenses against cercariae (Kiesecker 2002; Budischak et al. 2008) and the herbicide atrazine increased trematode infections and frog mortality by augmenting both exposure and susceptibility to trematodes (Rohr et al. 2008a, b; Koprivnikar 2010).
Contaminants and parasites can be directly lethal to amphibian hosts, but there are also several sublethal mechanisms by which they can affect host fitness and thus contribute to population declines. For instance, chemical contaminants and trematodes can affect amphibian life history traits, reducing size at metamorphosis and lengthening the time needed to metamorphose (Rohr et al. 2004, 2008a; Raffel et al. 2010). Smaller size at metamorphosis is important because smaller metamorphs often have lower terrestrial survival and fecundity (Smith 1987; Semlitsch et al. 1988; Berven 1990; Rohr and Palmer 2013). Additionally, retarded development can increase the amount of time tadpoles are at trematode-susceptible stages (Raffel et al. 2010), and a longer larval period can increase exposure to both contaminants and aquatic parasites, thus compounding risk (Raffel et al. 2010; Rohr et al. 2011). Finally, both contaminants and trematodes can cause malformations and fluctuating asymmetries (Bridges et al. 2004; Jayawardena et al. 2010b, 2011; Rohr et al.2011; Johnson et al. 2012) that can reduce long-term fitness (Goodman and Johnson 2011).
For years, only the trematode Ribeiroia ondatrae was known to induce limb, axial, and skin deformities in amphibians, but in 2008, another species of trematode, Acanthostomum burminis Bhalerao, 1926, was shown to induce mainly axial and some limb malformations in amphibians (Rajakaruna et al. 2008; Jayawardena et al. 2010a, 2013). Acanthostomum burminis infections also increase mortality and time to metamorphosis and decrease size at metamorphosis (Rajakaruna et al. 2008; Jayawardena et al. 2010a; Jayawardena et al. 2013). Similar to the effects of A. burminis on amphibians, four commonly used agricultural pesticides, two organophosphorous insecticides (chlorpyrifos and dimethoate) and two herbicides (glyphosate and propanil), increased malformations, mortality, and time to metamorphosis in the common hourglass tree frog (Polypedates cruciger) and the Asian common toad (Duttaphrynus melanostictus Schneider, 1799) (Jayawardena et al. 2010b; Jayawardena et al. 2011). Given that A. burminis infections and these pesticides have such similar effects on P. cruciger, we hypothesized that their combination would increase (either additively or synergistically) malformations, time to metamorphosis, and mortality risk and decrease size at metamorphosis relative to either stressor alone. This result would suggest that exposure to this parasite and pesticides could increase the probability of negative population growth for P. cruciger and perhaps populations of other amphibian species.
Materials and Methods
Life Cycle of A. burminis
Before understanding the effects chemical contaminants can have on A. burminis infections, it is important to understand the complex life cycle of this trematode. Adults of A. burminis reproduce sexually in vertebrate definitive hosts (Rajakaruna et al. 2008; Jayawardena et al. 2010a, 2013). Females of A. burminis typically release eggs in the excrement of these hosts and miracidiae, a free-living larval stage, hatch when the eggs encounter water. Miracidiae search for the first intermediate host in the life cycle, which is a snail. The trematode reproduces asexually in the snail and a second free-living larval stage, called a cercaria, is subsequently released. Cercariae search for their next host, which is an amphibian. Upon encountering an amphibian, the cercariae use proteolytic enzymes to encyst subcutaneously, where they can cause deformities in the developing amphibian (Rajakaruna et al. 2008; Jayawardena et al. 2010a, 2013). These encysted cercariae are called metacercariae. If an infected amphibian is consumed by a suitable vertebrate definitive host, the life cycle is completed (Koprivnikar et al. 2012).
Study Animals
Four newly spawned egg masses of P. cruciger were collected from ponds in the Peradeniya University Park (7°15′15′N 80°35′48′E/7.25417°N 80.59667°E) and were brought to the research facility in the Department of Zoology, University of Peradeniya, Sri Lanka. The egg masses were placed in a glass aquarium containing dechlorinated tap water. Hatched tadpoles were fed ground fish flakes twice a day (~ 10% body mass). The debris and feces that collected at the bottom of the aquaria were siphoned out and water level was replenished daily. Separate egg masses were used for each of the four temporal blocks described below. Tadpoles that were five days post-hatching (Gosner stage 25/26) were used in the exposures.
Collection of Cercariae from Snails
Pleurolophocercous cercariae of A. burminis released from the freshwater snail species Thiara scabra (Family: Thiaridae) were used in the infection process. Thiara scabra were collected from the university stream and were kept in plastic vials containing 10–15 mL of dechlorinated tap water, under sunlight to induce cercarial shedding. The snails that were shedding cercariae were kept individually in separate vials to obtain a continuous supply of cercariae for the exposures. One infected snail was used for all the tadpole exposures in a temporal block and a separate infected snail was used for each of the four temporal blocks (see below).
Test Chemicals
We exposed the tadpoles and cercariae to commercial formulations of four widely used agrochemicals; two organophosphorus insecticides (chlorpyrifos and dimethoate) and two herbicides (glyphosate and propanil). Table 1 provides the concentration of the active ingredient (a.i.) tested and any known surfactants in the commercial formulation. The test concentrations of each pesticide were selected based on field concentrations reported in the available literature (Aponso et al. 2003; Wijesinghe et al. 2011) and information from the Pesticide Registrar Office in Peradeniya, Sri Lanka. Hence, each concentration is ecologically relevant (likely to be found in water bodies) based on use in Sri Lanka. Hereafter, each pesticide is referred to based on its a.i.
Table 1.
Active Ingredient, Surfactant, and Commercial Name of the Four Pesticides Used in the Experiment.
| Active ingredient and its concentration | Surfactant/solvent | Trade name |
|---|---|---|
| Chlorpyrifos[O,O-DiethylO-(3,5,6-trichloro-2-pyridyl) phosphorothioate] 400 g/L |
Xylene | Lorsban 40 EC® or Pattas® |
| Dimethoate (O,O-Dimethyl phosphorodithioate) 400 g/L | Water | Dimethoate 40 EC® |
| Glyphosate [N-(Phosphonomethyl) glycine] 360 g/L | POEA | Round Up® or Glyphosate® |
| Propanil [N-(3,4-dichlorophenyl) propanamide] 360 g/L | Cyclohexanone & petroleum solvents | 3, 4-DPA® |
Experimental Design and Exposure of Tadpoles to Cercariae and Pesticides
We employed a 5 × 2 fully factorial randomized block design with one replicate of each treatment combination occurring in each of the four temporal blocks. The five agrochemical levels were water control, chlorpyrifos, dimethoate, glyphosate, or propanil and the two parasite levels were the presence or absence of exposure to 48 cercariae. Two hundred tadpoles from a single clutch (Gosner 25–26, Gosner, 1960) were placed individually in plastic vials each containing 20 mL of the test solution (dechlorinated tap water or pesticide). Half of these were randomly selected to receive cercariae. Within 2 h of being shed from snails, 12 cercariae were transferred to each of the 100 vials containing a tadpole using a dissecting microscope and pipette. This procedure was repeated for four consecutive days so that each tadpole in the cercariae exposure treatment received 48 cercariae in total. We have found that this dose of cercariae induces effects without considerable mortality (Rajakaruna et al. 2008) and is within the range of infection levels found in this frog in the field. Each tadpole was kept in a small volume of test solution to ensure that all cercariae penetrated the tadpole. Cercarial penetration was observed under a dissecting microscope to ensure that no free swimming cercariae remained. After exposure to the cercariae, these 100 tadpoles were randomly assigned to one of five glass aquaria (15 × 15 × 25 cm) containing 2 L of one of the five test solutions (water control, chlorpyrifos, dimethoate, glyphosate, or propanil). Likewise, the 100 tadpoles not exposed to cercariae were assigned to five different glass aquaria as described above.
Given the logistical challenges of individually exposing 400 tadpoles to cercariae in one day, we employed four temporal blocks. Hence, we repeated the procedures described above for three additional times with separate clutches and a separate source snail for cercarial acquisition. Each temporal block was separated by about 20–40 days depending on the availability of egg clutches. This required 800 tadpoles and 40 aquaria in total. The tadpoles were raised in the glass aquaria until metamorphosis. Daytime temperature varied between 27 and 31°C and the tanks were kept under a natural photoperiod of approximately 12:12 h.
In summary, there were 10 aquaria each containing 20 tadpoles and one replicate of each pesticide treatment by cercarial treatment combination in each of four temporal blocks. Clutch and source snail were intentionally the same within each temporal block so there were internal controls within each block. In other words, this design ensured that any variation in infectivity of cercariae among snails or resistance to cercariae among clutches was accounted for by the temporal block and not included in the error term of our statistical models. Hence, our block accounted for several unknown components of variation (time, source snail, and host clutch) for which we do not seek to explain.
Collection of Data
Tadpole mortality, forelimb emergence (stage 42, Gosner 1960), and metamorphosis were assessed daily. When dead tadpoles were noticed, they were removed and preserved in 70% alcohol. Snout-vent length (SVL) to nearest 0.01 cm and body mass to nearest 0.001 g were recorded at metamorphosis. All the tadpoles hatched from their eggs within 2 days, and thus we quantified malformations at approximately 10 days (Gosner stage 27), 30 days post-hatching (Gosner stage 31), and at metamorphosis. We chose 10 days because it allowed enough time for malformations to develop but minimized the impact of treatment-induced developmental difference and mortality, allowing us to compare animals all at a similar age. Malformations were identified and categorized according to Meteyer (2000) and severely malformed frogs (those with ulcers, edemas, and deformed limbs) were euthanized with MS-222 and preserved. All procedures described herein were approved by the Animal Ethical Review Committee at the Postgraduate Institute of Science, University of Peradeniya.
Data Analyses
Data were analyzed using R statistical software. We analyzed survival two ways. To test whether temporal block and the main and interactive effects of pesticide and cercarial treatments affected time of death, we conducted a mixed effects Cox proportional hazards survival analysis (coxme package, coxme function) treating aquarium as a random effect to ensure proper degrees of freedom (because the 20 tadpoles per aquarium are not independent of one another). To test whether these same predictors affected the proportion of frogs that survived in each tank, we used the glm function and a binomial error distribution with frequency of survivors and non-survivors as the response variables (i.e., aquarium was the replicate). This latter analysis was also used to assess how the treatments affected malformation frequency 10 days post-hatching. We used a mixed effects general linear model with aquarium as the random effect to evaluate how temporal block, pesticide treatments, cercarial treatment, and the pesticide-by-cercarial interaction affected days to metamorphosis (log transformed). As an alternative estimate of the effect of these treatments on developmental rate, we ran a general linear model with the day that 50% of the animals in a tank had forelimb emergence (stage 42, Gosner 1960) (TE50) as the response variable.
Even with ad libitum food, high conspecific densities can reduce amphibian growth rates (Rohr et al. 2004); hence, if we do not control for the amount of time tadpoles spent in the aquariums, then the effects of treatment on size could be confounded and thus would be partly redundant with the effects of treatment on mortality. In other words, if we conduct analyses on size at metamorphosis without time tadpoles spent in the aquaria as a covariate, we have no way of knowing if the results are because of the pesticides causing direct mortality that affected growth through competitive effects or if the pesticides had sublethal effects on the growth of survivors independent of changes to competition. So, in an effort to make the effect of treatments on host size (log SVL and log mass) independent of competition and conspecific density, we conducted the exact same analysis as described for days to metamorphosis but included mean log days tadpoles were in each tank as a covariate. Importantly, this covariate captures changes in conspecific densities that occur if an animal dies or metamorphoses.
For all analyses, when there was a significant interaction between the cercarial and pesticide treatments, we tested for an interaction between the cercarial treatment and the presence and absence of each of the four individual pesticides to determine the cause of the interaction. No alpha adjustments were conducted for multiple post hoc tests. This is encouraged by several statisticians because there is no consensus on how to make these adjustments and by not making adjustments, readers can more easily apply an adjustment that they deem appropriate (Gotelli and Ellison 2004).
Results
During tadpole exposure to cercariae, there was no noticeable difference in cercarial activity in vials with than without pesticides. However, this was not quantified. All the cercariae that were introduced readily penetrated the tadpoles and none remained in the vials at the end of the exposure. Hence, attempted infections appear to be the same across pesticide treatments.
Survival
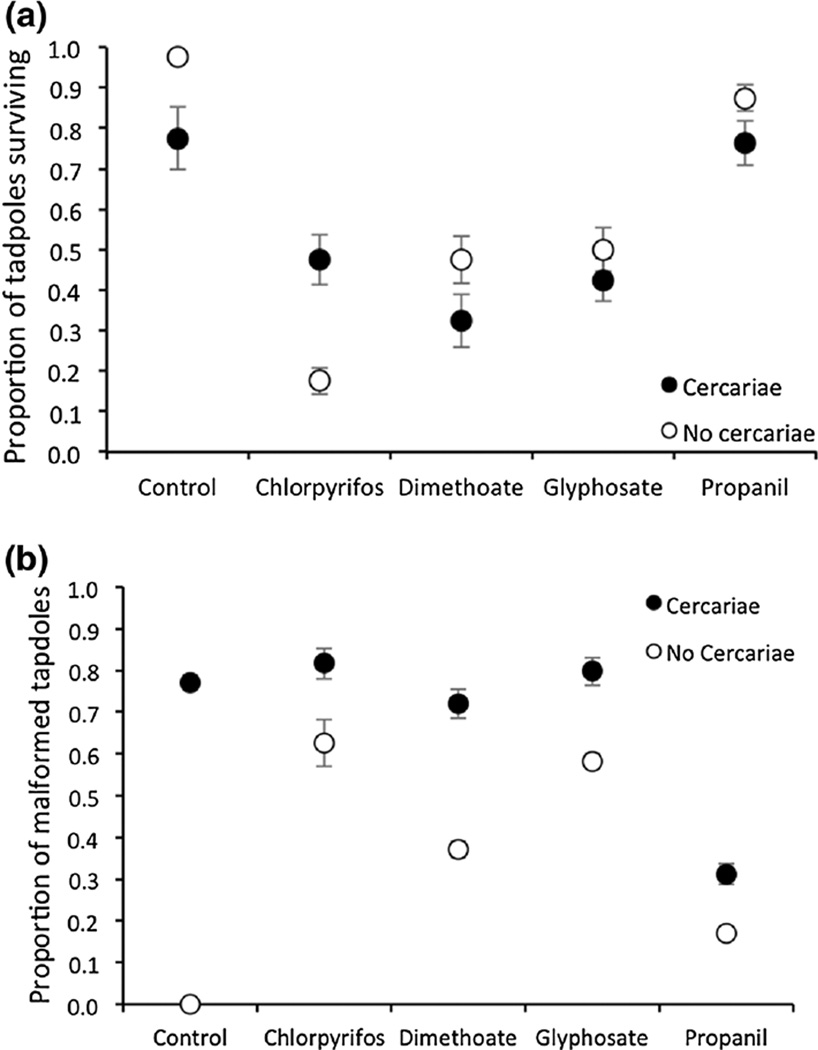
Both the effect of pesticide treatment (Main effect: , P < 0.001) and cercariae (Main effect: , P < 0.001) were significant for time of death because, in general, pesticides and cercariae decreased time to death and increased frog mortality (Fig. 1). In the absence of cercariae, frogs exposed to chlorpyrifos, dimethoate, glyphosate, and propanil had 33, 21, 20, and 5 times the mortality as frogs exposed to the water control (Fig. 2a). In the absence of pesticides, frogs exposed to cercariae had 9 times the mortality as frogs not exposed to cercariae (Fig. 2a). There also was an interaction between pesticide and cercarial treatments for time of death (, P = 0.009, Fig. 1) and proportion of tadpoles that survived to metamorphosis (, P < 0.001, Fig. 2a). Subsequent analyses on each pesticide alone revealed that these interactions were caused by two factors. First, dimethoate, glyphosate, and propanil reduced cercarial-induced mortality relative to the absence of pesticides (Pesticide × cercariae: , P = 0.014; , P = 0.004; , P = 0.039, respectively; Fig. 2a). Second, in the presence of chlorpyrifos, tadpoles had better survival with than without cercariae, whereas in the absence of pesticides, tadpoles had better survival without than with cercariae (Pesticide × cercariae: , P < 0.001; Fig. 2a).
Figure 1.
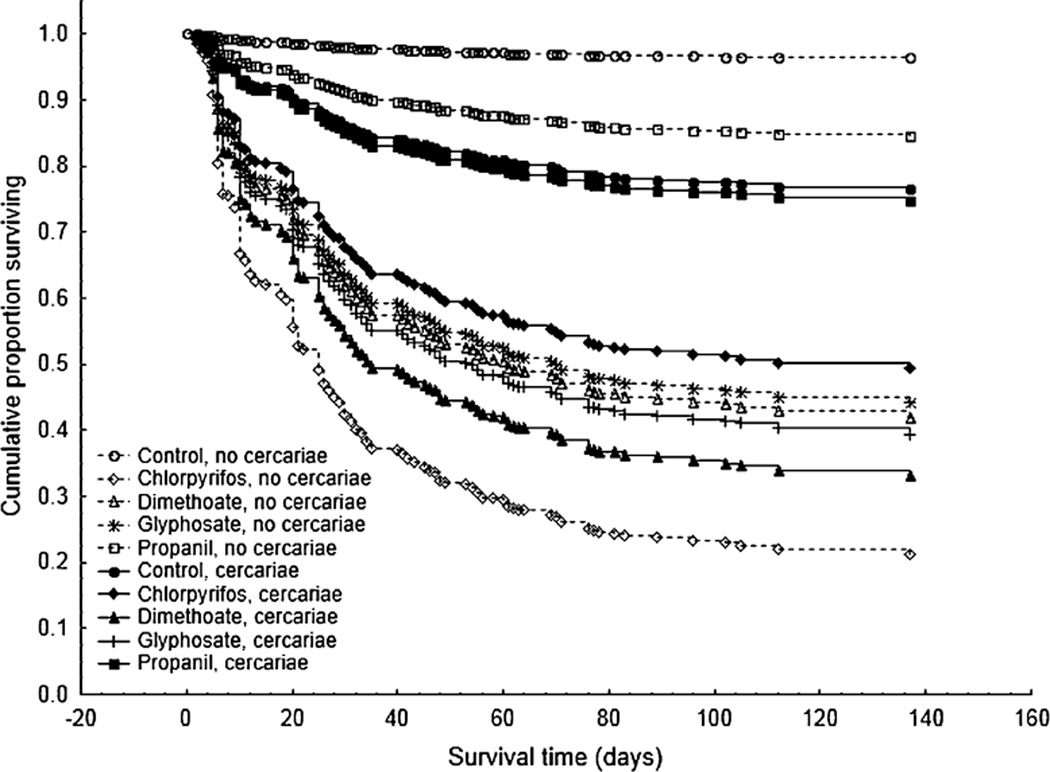
Breslow survivorship curves displaying cumulative proportion of tadpoles of Polypedates cruciger surviving in the 10 treatment groups (5 pesticide by 2 cercariae treatments). Each treatment had four replicate tanks with 20 tadpoles per tank. Chlorpyrifos and dimethoate are insecticides and glyphosate and propanil are herbicides.
Figure 2.
Mean proportion (± SE, n = 4 tanks) of Polypedates cruciger frogs that survived until metamorphosis (a) and that had malformations approximately 10 days post-hatching (b) when exposed to five pesticide treatments (control water, the insecticides chlorpyrifos and dimethoate, and the herbicides glyphosate and propanil) crossed by the presence or absence of exposure to cercariae of the trematode Acanthostomum burminis.
Malformations
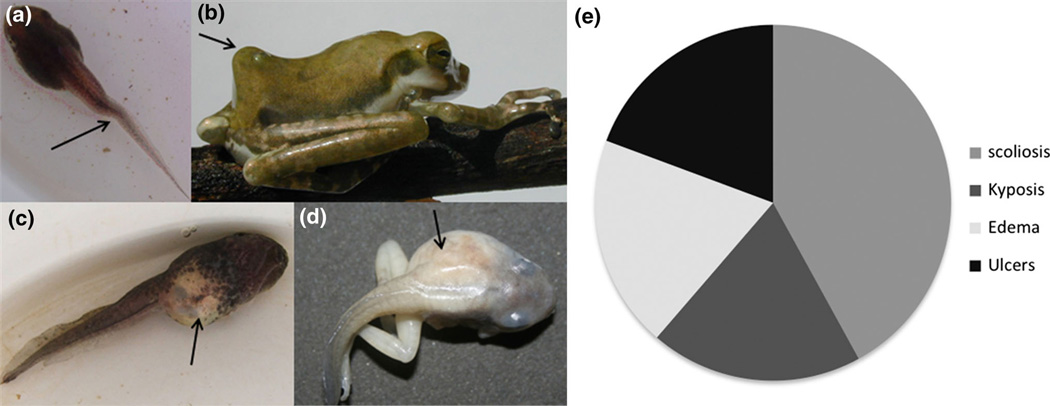
Ten days after metamorphosis, tadpoles had significantly more malformations with than without pesticides (Main effect: , P < 0.001) and with than without cercariae (Main effect: , P < 0.001; Fig. 2b). None of the individuals in the absence of both cercariae and pesticides had malformations (Fig. 2b). However, in the absence of cercariae, chlorpyrifos, glyphosate, dimethoate, and propanil induced malformations in 70%, 62%, 37%, and 17% of the tadpoles, respectively (Fig. 2b). In the absence of pesticides, cercariae induced malformations in 75% of the tadpoles (Fig. 2b). Importantly, the effect of cercariae on malformation incidence depended on the pesticide treatment (Pesticide × cercariae: , P < 0.001). Despite pesticides causing malformations, glyphosate and chlopyrifos increased the incidence of cercarial-induced malformations relative to the absence of pesticide (, P < 0.001; Fig. 2b). Tadpoles exhibited several types of malformations, including scoliosis (tail curvature), kyphosis (hunched back), edema, and skin ulcers, but scoliosis was the most common malformation observed (42%, Fig. 3).
Figure 3.
Different malformations observed in Polypedates cruciger frogs exposed to pesticides or cercariae of the trematode Acanthostomum burminis. Scoliosis (spinal curvature) (a), kyphosis (hunch back) (b), skin ulcer (c), edema at the left side of the body and lack of skin pigmentation (d). Proportion of each malformation type across all treatments (e).
Days to Metamorphosis
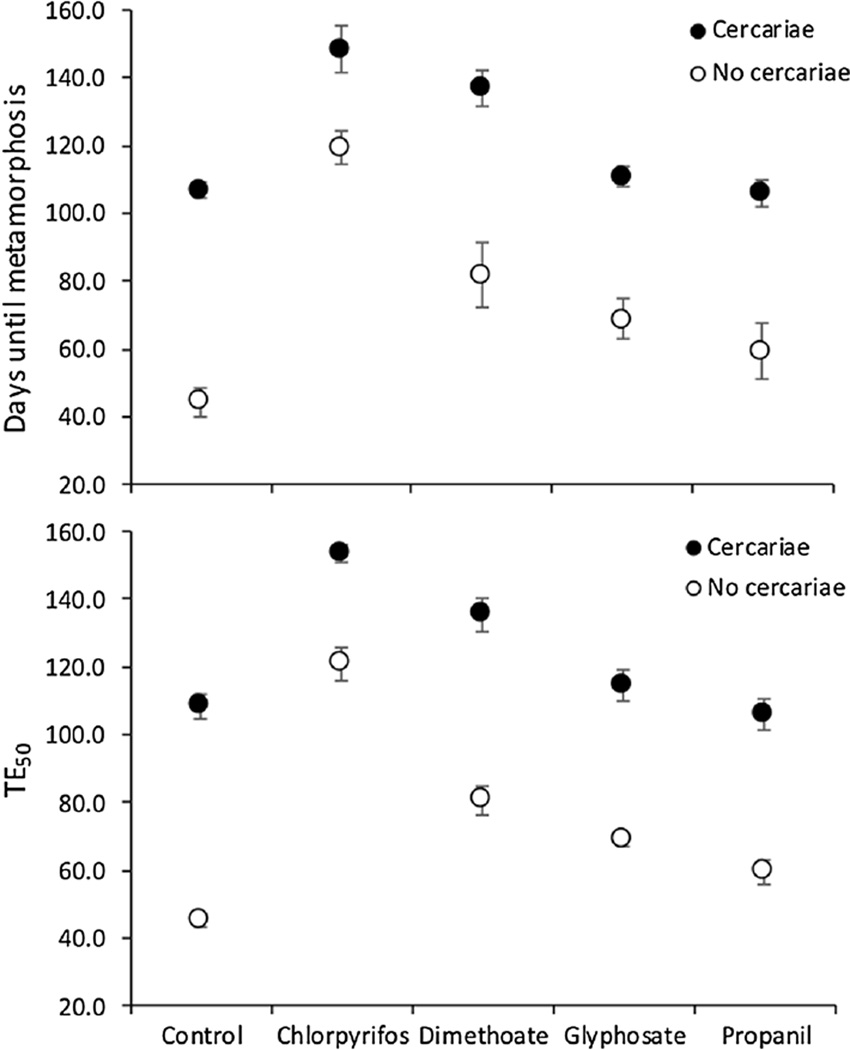
Days to metamorphosis and days until 50% of the frogs had forelimb emergence were significantly increased by both exposure to pesticides (Main effect: , P < 0.001; F4,27 = 36.56, P < 0.001, respectively) and cercariae (Main effect: , P < 0.001; F1,27 = 36.56, P < 0.001, respectively, Fig. 4a, b). In the absence of cercariae, tadpoles exposed to chlorpyrifos, glyphosate, dimethoate, and propanil took 2.70, 1.84, 1.55, and 1.34 times as long to metamorphose, respectively, relative to control tadpoles (Fig. 4a). In the absence of pesticides, tadpoles exposed to cercariae took 2.41 times as long to metamorphose relative to those not exposed to cercariae (Fig. 4a). Additionally, the effect of cercariae on days to metamorphosis and days until 50% of the frogs had forelimb emergence depended on the pesticide treatment (Pesticide × cercariae: , P < 0.001; F1,27 = 6.88, P < 0.001, respectively). This was because cercariae increased development time more in the absence than the presence of any of the pesticides (Fig. 4a, b).
Figure 4.
Mean (±SE, n = 4 tanks) days until Polypedates cruciger frogs metamorphosed (a) and mean experimental day when 50% of these frogs had forelimb emergence (TE50) (b) when exposed to five pesticide treatments (control water, the insecticides chlorpyrifos and dimethoate, and the herbicides glyphosate and propanil) crossed by the presence or absence of exposure to cercariae of the trematode Acanthostomum burminis.
Size
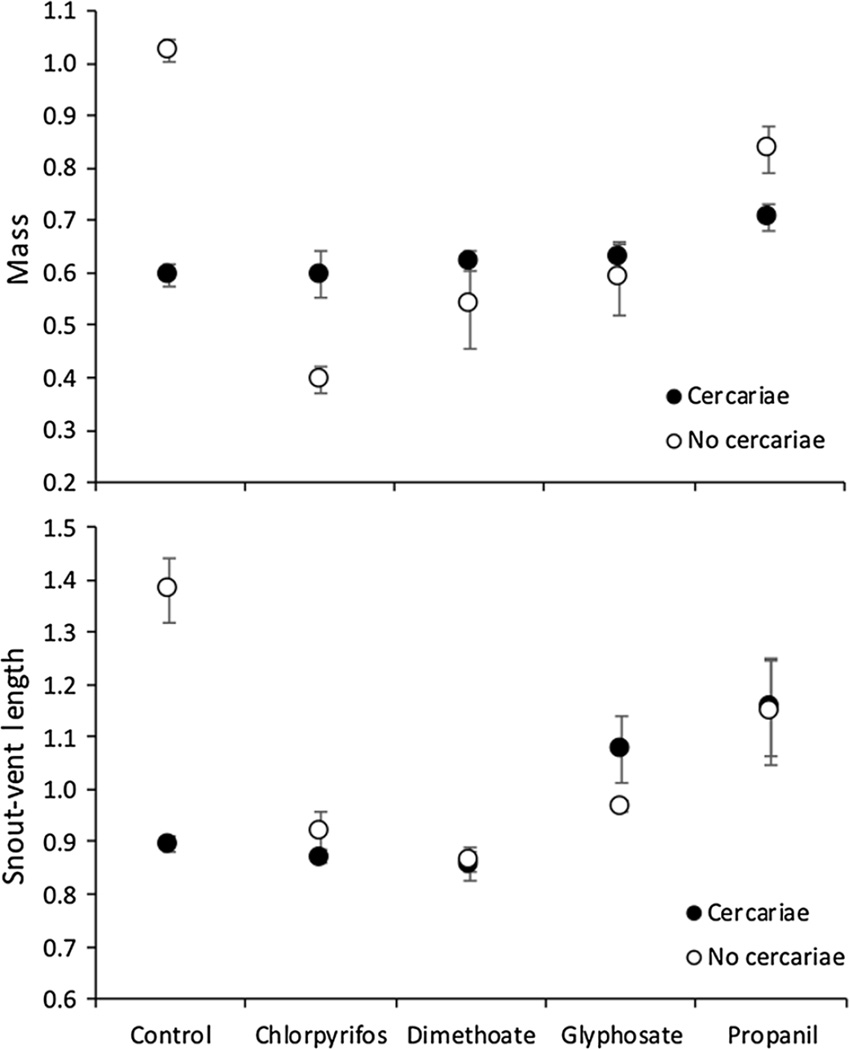
For all size analyses, we controlled for mean log days tadpoles were in each tank to isolate the direct effect of the treatments on growth from the indirect effect on these responses mediated by changes to conspecific densities. Mass and SVL of frogs at metamorphosis were significantly reduced by exposure to pesticides (Main effect: , P < 0.001; , P < 0.001, respectively), whereas mass but not SVL were reduced by cercariae (Main effect: , P < 0.001; , P = 0.140, respectively, Fig. 5a, b). This was despite both pesticides and cercarial treatments extending time to metamorphosis (Fig. 3). In the absence of cercariae, metamorphic frogs exposed to chlorpyrifos, dimethoate, glyphosate, and propanil were 67%, 63%, 70%, and 83% as long and 39%, 53%, 58%, and 82% as heavy as control metamorphs (Fig. 5a, b). In the absence of pesticides, metamorphs previously exposed to cercariae were 65% as long and 58% as heavy as those not exposed to cercariae (Fig. 5a, b). Additionally, there was a significant interaction between cercariae and pesticide treatment on size at metamorphosis (Mass: , P < 0.001; SVL: , P < 0.001). This was because cercariae generally only reduced mass and SVL in the absence of pesticides (Fig. 5a, b).
Figure 5.
Mean (±SE, n = 4 tanks) mass (a) and snout-vent length (SVL) (b) of Polypedates cruciger frogs at metamorphosis when exposed to five pesticide treatments (control water, the insecticides chlorpyrifos and dimethoate, and the herbicides glyphosate and propanil) crossed by the presence or absence of exposure to cercariae of the trematode Acanthostomum burminis.
Discussion
Here, we showed that A. burminis cercariae and four pesticides had significant effects on the survival, development, and growth of the hourglass tree frog. Moreover, the combination of cercariae and pesticides generally posed greater risk to frogs than either factor alone by either decreasing survival or growth or increasing time to metamorphosis or malformations. This result occurred despite earlier findings that cercariae are often sensitive to chemical contaminants (Morley et al. 2003; Pietrock and Marcogliese 2003; Blanar et al. 2009), which did not appear to be the case here because all the cercariae entered the tadpole in both the control and pesticide treatments, indicating that there was no pesticide-induced mortality of the cercariae before they could infect. This is consistent with previous research showing that four pesticides at ecologically relevant concentrations had minimal effects on the survival or infectivity of Echinostoma trivolvis cercariae that infect frogs (Rohr et al. 2008a) and several laboratory studies that suggest that the net effect of pesticides is to enhance amphibian risk from trematode infections (Kiesecker 2002; Budischak et al. 2008, 2009; Rohr et al. 2008a). Moreover, our findings are consistent with empirical work examining the effects of agrochemicals on trematode transmission and abundance in frogs under more natural settings. In experiments conducted in outdoor mesocosms or the field, herbicides, insecticides, and fertilizers all increased trematode infections in frogs (Kiesecker 2002; Johnson et al. 2007; Budischak et al. 2008, 2009; Rohr et al. 2008b). Although mathematical models on the effects of anthropogenic stress on disease risk have found negative, positive, convex, and concave associations between disease and stress (Lafferty and Holt 2003), the weight of the evidence from the field and laboratory suggest that agrochemicals often increase the threat that trematodes pose to amphibians. These same mathematical models suggest that the impact of non-specific diseases or generalist pathogens should increase with stress (Lafferty and Holt 2003), which might be why generalist trematode metacercariae (i.e., capable of infecting any available amphibian species and, in some cases, even non-amphibian hosts) often increase with agrochemical exposure.
Given that cercarial exposure was kept constant in this study (all cercariae entered each tadpole), the likely explanation for the observed enhanced risk when pesticides and cercariae were combined is that the pesticides increased susceptibility to infection, consistent with previous research demonstrating that pesticides can reduce resistance to parasites (Kiesecker 2002; Budischak et al. 2008, 2009; Rohr et al. 2008a, b; Rohr and McCoy 2010). A pesticide-induced increase in susceptibility to parasites can occur through several mechanisms. Pesticide exposure often induces detoxification mechanisms which could result in a trade off in energy allocation with immune defenses (Martin et al. 2010). Alternatively, many pesticides can be directly toxic to immune cells and thus can reduce efficacy of immunity at protecting hosts from infection (Voccia et al. 1999; Martin et al. 2010). Actual metacercarial data (i.e., the proportion of cercariae that entered the host that successfully encsysted and thus were not killed by host immunity) would have been useful to assess the magnitude of each chemical’s effect on susceptibility to cercariae but the Animal Ethics Review Committee at the Postgraduate Institute of Science, University of Peradeniya required us to release the frogs back into the wild precluding the acquisition of these data. Although we do not have these data, this study still provides clear evidence that several of these pesticides enhanced susceptibility to cercariae.
The one exception to the general finding that the combination of pesticides and cercariae generally posed equal to or greater risk to frogs than either factor in isolation was the combination of chlorpyrifos and cercariae on frog survival. This combination surprisingly enhanced survival relative to chlorpyrifos alone (Figs. 1 and 2a). Chlorpyrifos can induce an inflammatory response in vertebrates associated with a Th1 immune response (i.e., proinflammatory responses responsible for killing intracellular parasites) (Monnet-Tschudi et al. 2007), whereas many helminths trigger a Th2 immune response that is fundamentally anti-inflammatory (Sears et al. 2011). Hence, by shifting energy allocation to Th2 responses, trematodes could potentially redirect or reduce immune-associated inflammation induced by chlorpyrifos increasing tolerance to both pesticides and cercariae (Sears et al. 2011). However, this is strictly a hypothesis that remains to be tested. Although chlorpyrifos was an exception to the rule for survival, amphibians exposed to cercariae plus chlorpyrifos metamorphosed later (Fig. 4) and were shorter (Fig. 5) than those exposed to either factor alone, consistent with the general pattern of pesticides and cercariae being more adverse than either factor alone.
Cercariae and pesticides increased time to forelimb emergence and metamorphosis. However, the magnitude of the effect of pesticides on development was greater in the absence than presence of cercariae (i.e., a statistical interaction), suggesting that there might be an upper bound on time to metamorphosis that prevented the two factors from having additive effects (Fig. 4). Propanil had the smallest effect, increasing time to metamorphosis by 15 days whereas chlorpyrifos had the largest effect, increasing time to metamorphosis by 75 days (Fig. 4). The effect of chlorpyrifos was comparable to cercarial exposure, which increased time to metamorphosis by 62.5 days (Fig. 4). Exposure to contaminants often causes reduced growth and development presumably because energy is reallocated to detoxification (Rohr and McCoy 2010). Even a 15-day increase in time to metamorphosis should substantially increase mortality risk from pond drying (Rohr et al. 2004) and would increase exposure to aquatic abiotic and biotic stressors, such as chemical contaminants and cercariae (Rohr et al. 2004, 2010; Rohr et al. 2011). Moreover, in nature, retarded development increases the amount of time tadpoles spend at cercarial-susceptible early-life stages (Raffel et al. 2010). Hence, under more natural conditions, pesticide-induced delays in metamorphosis could increase the adverse effects of trematodes and increase mortality risk from pond drying.
Even though, exposure to pesticides and cercariae increased time to metamorphosis, they did not increase size at metamorphosis. In fact, in the absence of cercariae, amphibians exposed to any of the four pesticides were significantly smaller at metamorphosis than those not exposed to pesticides (Fig. 5). The insecticides seemed to have more adverse effects on size at metamorphosis than herbicides; in fact, in the absence of cercariae, frogs exposed to chlorpyrifos or dimethoate were less than 2/3 the length of control frogs at metamorphosis (Fig. 5b). However, these effects of pesticides were largely neutralized in the presence of cercariae (Fig. 5). This might be a product of there being a well-documented minimum size for metamorphosis (Wilbur and Collins 1973) that likely resulted in the convergence of the stressor combination on this lower bound. Importantly, small size at metamorphosis can have significant effects on amphibian fitness because smaller metamorphs often have lower terrestrial survival and fecundity (Smith 1987; Semlitsch et al.1988; Berven 1990; Rohr and Palmer 2013).
In addition to decreasing development and growth, both the pesticides and cercariae increased malformations. None of the frogs exhibited malformations in the absence of cercariae or pesticides, but in the presence of cercariae, approximately 80% of the frogs were malformed across all the pesticide treatments except propanil, and in the absence of cercariae, pesticides induced malformation frequencies ranging from 17% to 62% (Fig. 2). Axial deformities, such as scoliosis and kyphosis, accounted for more than half the observed deformities, which is consistent with the types of deformities previously reported for this frog species exposed separately to A. burminis cercariae (Rajakaruna et al. 2008; Jayawardena et al. 2010a, 2013) and these pesticides (Jayawardena et al. 2010b, 2011). The active or inactive ingredients of several pesticides, such as the insecticides carbaryl, chlorpyrifos, malathion, and endosulfan, the fungicide chlorothalonil, and the surfactant octylphenol, have also induced malformations or organ damage in amphibians (Bridges 2000; Rohr et al. 2003; Budischak et al. 2008; McMahon et al. 2011; Wijesinghe et al. 2011). Frogs that exhibited malformations induced by the trematode R. ondatrae exhibited significantly shorter jumping distances, slower swimming speeds, reduced endurance, and lower foraging success relative to infected frogs without malformations. This reduced performance of malformed frogs resulted in a 22% higher biweekly predation rate than normal frogs and very little recruitment to the adult subpopulation (Goodman and Johnson 2011). The cercarial- and pesticide-induced axial malformations observed here likely have similar effects on frog performance, foraging, and predator avoidance, suggesting that we might be underestimating the effects of cercarial and pesticide exposure on hourglass tree frog survival in the wild and that these effects could have population-level impacts by reducing recruitment to the adult subpopulation. However, this would need to be demonstrated.
It is disconcerting that chlorpyrifos alone reduced survival from 98% to 18% and the combination of cercariae and dimethoate and cercariae and glyphosate reduced survival from 78% (cercariae only) to 33% and 78% to 43%, respectively (Fig. 1, 2a). These levels of mortality are comparable to other studies on amphibians and these pesticides (Relyea 2005a, b; Wacksman et al. 2006; Jayawardena et al. 2010b, 2011). Moreover, such substantial drops in survival are unlikely to be overcome by negative density dependence (Vonesh and De la Cruz 2002; Rohr et al. 2006). If this mortality is representative of what occurs in the wild, then A. burminis and at least three of these pesticides have the potential to contribute to amphibian population declines. Given that many amphibians are likely exposed to A. burminis and these chemicals, cross-species studies would be valuable to better understand which amphibian species are at greatest risk (e.g., Sears et al. 2015).
Finally, our findings suggest that some agrochemicals might serve as surrogates for others to improve amphibian populations (Halstead et al. 2014). For instance, in this study, propanil was the only pesticide that did not significantly increase mortality relative to controls (Figs. 1 and 2a) and it had the least adverse effects on development and growth (Figs. 2b, 4, and 5). Thus, propanil might be a viable alternative to glyphosate or other herbicides that increase amphibian mortality or infection risk. Additionally, providing buffer strips of vegetation around wetlands and around sites of agrochemical applications could help to reduce levels of pesticides that enter aquatic ecosystems. Reducing factors that enhance snail abundance, such as nutrients, enhancing factors that reduce snail and cercarial abundance, such as snail and cercarial predators, and increasing amphibian habitat might also be effective at reducing risk (Halstead et al. 2014; Civitello et al. 2015; Halstead et al. 2015; Rohr et al. 2015). Importantly, this work highlights the importance of quantifying the combined effects of common environmental stressors on taxa of conservation concern to enhance management strategies.
Acknowledgments
Authors thank V. Imbuldeniya and Y.G. Ariyaratne for technical assistance. Financial support was provided by the National Science Foundation of Sri Lanka (NSF/2005/EB/02 to R.S.R.) and the US National Science Foundation (EF-1241889 to J.R.R.), the US Department of Agriculture (NRI 2006-01370 and 2009-35102-0543 to J.R.R), the US Environmental Protection Agency grant (CAREER 83518801 to J.R.R), and the US National Institute Of General Medical Sciences of the National Institutes of Health (R01GM 109499 to J.R.R.).
References
- Aponso GLM, Magamage C, Ekanayake WM, Manuweera GK. Analysis of water for pesticides in two major agricultural areas of the dry zone. Annals of the Sri Lanka Department of Agriculture. 2003;5:7–22. [Google Scholar]
- Berven KA. Factors affecting population fluctuations in larval and adult stages of the Wood Frog (Rana sylvatica) Ecology. 1990;71:1599–1608. [Google Scholar]
- Blanar CA, Munkittrick KR, Houlahan J, MacLatchy DL, Marcogliese DJ. Pollution and parasitism in aquatic animals: A meta-analysis of effect size. Aquatic Toxicology. 2009;93:18–28. doi: 10.1016/j.aquatox.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Kiesecker JM. Complexity in conservation: lessons from the global decline of amphibian populations. Ecology Letters. 2002;5:597–608. [Google Scholar]
- Bridges C, Little E, Gardiner D, Petty J, Huckins J. Assessing the toxicity and teratogenicity of pond water in north-central Minnesota to amphibians. Environmental Science and Pollution Research. 2004;11:233–239. doi: 10.1007/BF02979631. [DOI] [PubMed] [Google Scholar]
- Bridges CM. Long-term effects of pesticide exposure at various life stages of the southern leopard frog (Rana sphenocephala) Archives of Environmental Contamination and Toxicology. 2000;39:91–96. doi: 10.1007/s002440010084. [DOI] [PubMed] [Google Scholar]
- Budischak SA, Belden LK, Hopkins WA. Effects of malathion on embryonic development and latent susceptibility to trematode parasites in ranid tadpoles. Environmental Toxicology and Chemistry. 2008;27:2496–2500. doi: 10.1897/08-018.1. [DOI] [PubMed] [Google Scholar]
- Budischak SA, Belden LK, Hopkins WA. Relative toxicity of malathion to trematode-infected and noninfected Rana palustris tadpoles. Archives of Environmental Contamination and Toxicology. 2009;56:123–128. doi: 10.1007/s00244-008-9167-9. [DOI] [PubMed] [Google Scholar]
- Cahill AE, Aiello-Lammens ME, Fisher-Reid MC, Hua X, Karanewsky CJ, Ryu HY, et al. How does climate change cause extinction? Proceedings of the Royal Society B-Biological Sciences. 2013;280:20121890. doi: 10.1098/rspb.2012.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitello DJ, Cohen J, Fatima H, Halstead NT, Liriano J, McMahon TA, et al. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8667–8867. doi: 10.1073/pnas.1506279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484:186–194. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman BA, Johnson PTJ. Disease and the extended phenotype: parasites control host performance and survival through induced changes in body plan. PLoS One. 2011;6:e20193. doi: 10.1371/journal.pone.0020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotelli NJ, Ellison AM. A Primer of Ecological Statistics. Massachusetts: Sinauer Associates Inc; 2004. p. 348. [Google Scholar]
- Halstead NT, Civitello DJ, Rohr JR. Comparative toxicities of organophosphate and pyrethroid insecticides to aquatic macroarthropods. Chemosphere. 2015;135:265–271. doi: 10.1016/j.chemosphere.2015.03.091. [DOI] [PubMed] [Google Scholar]
- Halstead NT, McMahon TA, Johnson SA, Raffel TR, Romansic JM, Crumrine PW, et al. Community ecology theory predicts the effects of agrochemical mixtures on aquatic biodiversity and ecosystem properties. Ecology Letters. 2014;17:932–941. doi: 10.1111/ele.12295. [DOI] [PubMed] [Google Scholar]
- Jayawardena UA, Navaratne AN, Amerasinghe PH, Rajakaruna RS. Acute and chronic toxicity of four commonly used agricultural pesticides on the Asian common toad, Bufo melanostictus Schneider. Journal of the National Science Foundation of Sri Lanka. 2011;39:267–276. [Google Scholar]
- Jayawardena UA, Rajakaruna RS, Navaratne AN, Amerasinghe PH. Monostome cercariae induced malformations in amphibians: effect of infection at the pre-limb-bud stage tadpoles of Polypedates cruciger Blyth. Journal of the National Science Foundation of Sri Lanka. 2010;38:241–248. [Google Scholar]
- Jayawardena UA, Rajakaruna RS, Navaratne AN, Amerasinghe PH. Toxicity of agrochemicals to common hourglass tree frog (Polypedates cruciger) in Acute and chronic exposure. International Journal of Agriculture and Biology. 2010;12:641–648. [Google Scholar]
- Jayawardena UA, Tkach VV, Navaratne AN, Amerasinghe PH, Rajakaruna RS. Malformations and mortality in the Asian Common Toad induced by exposure to pleurolophocercous cercariae (Trematoda: Cryptogonimidae) Parasitology International. 2013;62:246–252. doi: 10.1016/j.parint.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Chase JM, Dosch KL, Hartson RB, Gross JA, Larson DJ, et al. Aquatic eutrophication promotes pathogenic infection in amphibians. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15781–15786. doi: 10.1073/pnas.0707763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Lunde KB, Ritchie EG, Launer AE. The effect of trematode infection on amphibian limb development and survivorship. Science. 1999;284:802–804. doi: 10.1126/science.284.5415.802. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Lunde KB, Zelmer DA, Werner JK. Limb deformities as an emerging parasitic disease in amphibians: Evidence from museum specimens and resurvey data. Conservation Biology. 2003;17:1724–1737. [Google Scholar]
- Johnson PTJ, Rohr JR, Hoverman JT, Kellermanns E, Bowerman J, Lunde KB. Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecology Letters. 2012;15:235–242. doi: 10.1111/j.1461-0248.2011.01730.x. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–994. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DW, Poulin R, Tompkins DM, Townsend CR. Synergistic effects of glyphosate formulation and parasite infection on fish malformations and survival. Journal of Applied Ecology. 2010;47:498–504. [Google Scholar]
- Kiesecker JM. Synergism between trematode infection and pesticide exposure: A link to amphibian limb deformities in nature? Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9900–9904. doi: 10.1073/pnas.152098899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivnikar J. Interactions of environmental stressors impact survival and development of parasitized larval amphibians. Ecological Applications. 2010;20:2263–2272. doi: 10.1890/09-1558.1. [DOI] [PubMed] [Google Scholar]
- Koprivnikar J, Marcogliese DJ, Rohr JR, Orlofske SA, Raffel TR, Johnson PTJ. Macroparasite infections of amphibians: what can they tell us? EcoHealth. 2012;9:342–360. doi: 10.1007/s10393-012-0785-3. [DOI] [PubMed] [Google Scholar]
- Lafferty KD, Holt RD. How should environmental stress affect the population dynamics of disease? Ecology Letters. 2003;6:654–664. [Google Scholar]
- Linzey DW, Burroughs J, Hudson L, Marini M, Robertson J, Bacon JP, et al. Role of environmental pollutants on immune functions, parasitic infections and limb malformations in marine toads and whistling frogs from Bermuda. International Journal of Environmental Health Research. 2003;13:125–148. doi: 10.1080/0960312031000098053. [DOI] [PubMed] [Google Scholar]
- Martin LB, Hopkins WA, Mydlarz LD, Rohr JR. The effects of anthropogenic global changes on immune functions and disease resistance. Annals of the New York Academy of Sciences. 2010;1195:129–148. doi: 10.1111/j.1749-6632.2010.05454.x. [DOI] [PubMed] [Google Scholar]
- McMahon T, Boughton R, Crumrine P, Halstead N, Johnson S, Martin LB, et al. The fungicide chlorothalonil is nonlinearly associated with corticosterone levels, immunity, and mortality in amphibians. Environmental Health Perspectives. 2011;119:1098–1103. doi: 10.1289/ehp.1002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon TA, Romansic JM, Rohr JR. Nonmonotonic and monotonic effects of pesticides on the pathogenic fungus Batrachochytrium dendrobatidis in culture and on tadpoles. Environmental Science & Technology. 2013;47:7958–7964. doi: 10.1021/es401725s. [DOI] [PubMed] [Google Scholar]
- Meteyer CU. Field guide to malformations of frogs and toads with radiographic interpretations. USGS/BRD/BSR-2000-0005, U.S. Geological Survey. 2000 [Google Scholar]
- Monnet-Tschudi F, Zurich MG, Honegger P. Neurotoxicant-induced inflammatory response in three-dimensional brain cell cultures. Human & Experimental Toxicology. 2007;26:339–346. doi: 10.1177/0960327107074589. [DOI] [PubMed] [Google Scholar]
- Morley NJ, Irwin SW, Lewis JW. Pollution toxicity to the transmission of larval digeneans through their molluscan hosts. Parasitology. 2003;126:S5–S26. doi: 10.1017/s0031182003003755. [DOI] [PubMed] [Google Scholar]
- Pietrock M, Marcogliese DJ. Free-living endohelminth stages: at the mercy of environmental conditions. Trends in Parasitology. 2003;19:293–299. doi: 10.1016/s1471-4922(03)00117-x. [DOI] [PubMed] [Google Scholar]
- Raffel TR, Hoverman JT, Halstead NT, Michel P, Rohr JR. Parasitism in a community context: Trait-mediated interactions with competition and predation. Ecology. 2010;91:1900–1907. doi: 10.1890/09-1697.1. [DOI] [PubMed] [Google Scholar]
- Rajakaruna RS, Piyatissa P, Jayawardena UA, Navaratne AN, Amerasinghe PH. Trematode infection induced malformations in the common hourglass treefrogs. Journal of Zoology. 2008;275:89–95. [Google Scholar]
- Relyea RA. The lethal impact of roundup on aquatic and terrestrial amphibians. Ecological Applications. 2005;15:1118–1124. [Google Scholar]
- Relyea RA. The lethal impacts of roundup and predatory stress on six species of North American tadpoles. Archives of Environmental Contamination and Toxicology. 2005;48:351–357. doi: 10.1007/s00244-004-0086-0. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Civitello DJ, Crumrine PW, Halstead NT, Miller AD, Schotthoefer AM, et al. Predator diversity, intraguild predation, and indirect effects drive parasite transmission. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:3008–3013. doi: 10.1073/pnas.1415971112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Elskus AA, Shepherd BS, Crowley PH, McCarthy TM, Niedzwiecki JH, et al. Lethal and sublethal effects of atrazine, carbaryl, endosulfan, and octylphenol on the stream-side salamander, Ambystoma barbouri. Environmental Toxicology and Chemistry. 2003;22:2385–2392. doi: 10.1897/02-528. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Elskus AA, Shepherd BS, Crowley PH, McCarthy TM, Niedzwiecki JH, et al. Multiple stressors and salamanders: Effects of an herbicide, food limitation, and hydroperiod. Ecological Applications. 2004;14:1028–1040. [Google Scholar]
- Rohr JR, McCoy KA. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environmental Health Perspectives. 2010;18:20–32. doi: 10.1289/ehp.0901164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Palmer BD. Climate change, multiple stressors, and the decline of ectotherms. Conservation Biology. 2013;27:741–751. doi: 10.1111/cobi.12086. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8269–8274. doi: 10.1073/pnas.0912883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Hall CA. Developmental variation in resistance and tolerance in a multi-host-parasite system. Functional Ecology. 2010;24:1110–1121. [Google Scholar]
- Rohr JR, Raffel TR, Halstead NT, McMahon TA, Johnson SA, Boughton RK, et al. Early-life exposure to a herbicide has enduring effects on pathogen-induced mortality. Prooceedings of the Royal Society B-Biological Sciences. 2013;280:20131502. doi: 10.1098/rspb.2013.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Sessions SK. Digenetic trematodes and their relationship to amphibian declines and deformities. In: Heatwole H, editor. Amphibian Biology; vol. 8, Amphibian Decline: Diseases, Parasites, Maladies, and Pollution. Chipping Norton: Surrey Beatty & Sons; 2009. pp. 3067–3088. [Google Scholar]
- Rohr JR, Raffel TR, Sessions SK, Hudson PJ. Understanding the net effects of pesticides on amphibian trematode infections. Ecological Applications. 2008;18:1743–1753. doi: 10.1890/07-1429.1. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Sager T, Sesterhenn TM, Palmer BD. Exposure, postexposure, and density-mediated effects of atrazine on amphibians: Breaking down net effects into their parts. Environmental Health Perspectives. 2006;114:46–50. doi: 10.1289/ehp.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Schotthoefer AM, Raffel TR, Carrick HJ, Halstead N, Hoverman JT, et al. Agrochemicals increase trematode infections in a declining amphibian species. Nature. 2008;455:1235–1239. doi: 10.1038/nature07281. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Sesterhenn TM, Stieha C. Will climate change reduce the effects of a pesticide on amphibians?: Partitioning effects on exposure and susceptibility to pollution. Global Change Biology. 2011;17:657–666. [Google Scholar]
- Rohr JR, Swan A, Raffel TR, Hudson PJ. Parasites, info-disruption, and the ecology of fear. Oecologia. 2009;159:447–454. doi: 10.1007/s00442-008-1208-6. [DOI] [PubMed] [Google Scholar]
- Schotthoefer AM, Rohr JR, Cole RA, Koehler AV, Johnson CM, Johnson LB, et al. Effects of wetland vs. landscape variables on parasite communities of Rana pipiens: links to anthropogenic factors. Ecological Applications. 2011;21:1257–1271. doi: 10.1890/10-0374.1. [DOI] [PubMed] [Google Scholar]
- Sears BF, Rohr JR, Allen JE, Martin LB. The economy of inflammation: when is less more? Trends in Parasitology. 2011;27:382–387. doi: 10.1016/j.pt.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Sears BF, Snyder PW, Rohr JR. Host life history and host-parasite syntopy predict behavioural resistance and tolerance of parasites. Journal of Animal Ecology. 2015;84:625–636. doi: 10.1111/1365-2656.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlitsch RD, Scott DE, Pechmann JHK. Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology. 1988;69:184–192. [Google Scholar]
- Smith DC. Adult recruitment in chorus frogs: Effects of size and date at aetamorphosis. Ecology. 1987;68:344–350. [Google Scholar]
- Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Voccia I, Blakley B, Brousseau P, Fournier M. Immunotoxicity of pesticides: a review. Toxicology and Industrial Health. 1999;15:119–132. doi: 10.1177/074823379901500110. [DOI] [PubMed] [Google Scholar]
- Vonesh JR, De la Cruz O. Complex life cycles and density dependence: assessing the contribution of egg mortality to amphibian declines. Oecologia. 2002;133:325–333. doi: 10.1007/s00442-002-1039-9. [DOI] [PubMed] [Google Scholar]
- Wacksman MN, Maul JD, Lydy MJ. Impact of atrazine on chlorpyrifos toxicity in four aquatic vertebrates. Archives of Environmental Contamination and Toxicology. 2006;51:681–689. doi: 10.1007/s00244-005-0264-8. [DOI] [PubMed] [Google Scholar]
- Wake DB, Vredenburg VT. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesinghe MR, Bandara M, Ratnasooriya WD, Lakraj GP. Chlorpyrifos-induced toxicity in Duttaphrynus melanostictus (Schneider 1799) larvae. Archives of Environmental Contamination and Toxicology. 2011;60:690–696. doi: 10.1007/s00244-010-9577-3. [DOI] [PubMed] [Google Scholar]
- Wilbur HM, Collins JP. Ecological aspects of amphibian metamorphosis. Science. 1973;182:1305–1314. doi: 10.1126/science.182.4119.1305. [DOI] [PubMed] [Google Scholar]