Abstract
This study investigates the impacts of n-butylphthalide (NBP) on the expression of vascular endothelial growth factor (VEGF) and transforming growth factor-β1 (TGF-β1) in rats with focal cerebral ischemia. The thread embolization method was used to prepare the rat model of cerebral ischemia-reperfusion (CIR). The animals were divided into a sham operation group, a model control group and NBP treatment group. The NBP group was orally administered 25 mg/kg NBP twice a day after the surgery. The immunohistochemistry and reverse transcription-polymerase chain reaction were performed to observe the protein and mRNA expressions of VEGF and TGF-β 16 hours, 1 day and 2 days after inducing CIR. The mRNA and protein expressions of VEGF and TGF-β1 in the model control group and the NBP treatment group were all increased after CIR, and those of the NBP treatment group at each post-CIR time point were higher than the model control group (p < 0.01). After CIR, the expressions of VEGF and TGF-β1 increased, suggesting that VEGF and TGF-β1 exhibited protective effects towards the ischemic brain injuries, and that NBP could upregulate the expressions of VEGF and TGF-β1 in the peri-infarcted area, thus possibly protecting the ischemic brain tissues through this mechanism.
KEY WORDS: N-butylphthalide, rats with cerebral ischemia and reperfusion, vascular endothelial growth factor, transforming growth factor-β1
INTRODUCTION
Ischemic cerebrovascular disease refers to a class of diseases characterized by the softening of brain tissues, caused by brain blood supply disorders, ischemia and hypoxia [1]. The disease is common within the Chinese elderly population and has a high morbidity and mortality, thus causing a serious social and family burden.
The vascular endothelial growth factor (VEGF) was separated and purified by Ferrara and Henzel [2] from in vitro culture of bovine pituitary follicular astrocytes in 1989, and named VEGF because of its ability to promote the mitosis of vascular endothelial cells (VECs). The studies showed that during the occurrence of cerebral ischemia, VEGF exhibited multiple protective effects towards the nervous system [3,4]. For example, Cui et al. [5] thought that, through the phosphoinositide 3-kinase/Akt signaling pathway, and prior to the formation of new blood vessels, VEGF played a role in neurotrophy and nerve tissue protection, and could help to prolong the survival of cells. The transforming growth factor-β1 (TGF-β1) is a multifunctional protein. Current studies show that TGF-β1 could weaken ischemia-induced cerebral blood vessel dilation, and play an important role in the angiogenic process [6,7]. By knocking off different components of the TGF-β signaling pathway in mice, Goumans et al. [8] confirmed that TGF-β was indispensable for angiogenesis. Investigating the regulation of expression of VEGF and TGF-β1 and their roles in ischemic brain injuries might lead to new treatment modalities. Increasing the expressions of VEGF and TGF-β1 in ischemic brain tissues may become a strategy for treating ischemic cerebrovascular diseases and a theoretical foundation for future clinical treatment.
The n-butylphthalide soft capsule, also known as NBP, has active ingredients extracted from celery seeds; its primary target was the vascular endothelium, and it had the roles of stabilizing the neurovascular units and improving the neurological deficits [9,10]. The related experiments had proved that NBP had the pharmacological effects of reconstructing the microcirculation, reducing the infarcted lesions after focal cerebral ischemia, and reducing the degrees of neurological injuries, although the exact mechanism was still unclear. In this study, we used the focal cerebral ischemia-reperfusion (CIR) rat model to observe the impacts of NBP on protein and mRNA expressions of VEGF and TGF-β1, aiming to further explore the neuroprotective mechanisms of NBP on the ischemia-reperfusion injury.
MATERIALS AND METHODS
Animals and grouping
Male adult Sprague Dawley rats, weighing 220-230 g (provided by the Experimental Animal Center of Zhengzhou University), were divided into three groups: The sham group (n=10), the NBP treatment group (n=30) and the model control group (n=30). The rats in NBP treatment group and model control group were respectively randomly divided into 3 sub-groups according to the time of reperfusion (6 hours, 1 day and 2 days) after 2 hours from cerebral ischemia, 10 rats for each sub-group. 4 rats from each sub-group of NBP group and 4 rats from each sub-group of model control group were taken. The brain was removed to take coronal sections, and 2,3,5-triphenyltetrazolium chloride (TTC) staining was done after removing the olfactory bulb, cerebellum and lower brain stem. We carried out this study in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Zhengzhou University.
Method of administration
NBP, an oily, pale yellow liquid, was provided by the NBP Pharmaceutical Company, Shijiazhuang Pharmaceutical Group, and diluted 10-fold with vegetable oil before administration. The NBP treatment group was fed 25 mg/kg NBP twice a day. The model control group and the sham group were intragastrically administrated the same amount of vegetable oil.
Preparation of focal transient cerebral ischemia model
The animals were fasted 12 hours before the surgery, with free access to water. The rat middle cerebral artery occlusion (MCAO) model was prepared according to the thread embolization method in Longa et al.’s report [11]. The MCAO was performed for 6 hours, 1 day and 2 days respectively after 2 hours from cerebral ischemia in NBP treatment group and model control group. The immunohistochemistry and reverse transcription-polymerase chain reaction methods were performed to observe the protein and mRNA expressions of VEGF and TGF-β1. In the sham group, the neck was exposed, and the vessel was separated, but the left middle cerebral artery was not occluded. If rats died unexpectedly after the surgery, rats from the same batch that had the similar body weights were randomly supplemented.
Perfusion and sampling
The rats of each group were deeply anesthetized with 10% chloral hydrate (800 mg/kg). The thoracotomy was quickly performed to expose the heart, then 200 ml saline was rapidly administered and the cardiac perfusion was done. Next, the rats were decapitated for brain sampling. The brain tissues were fixed for 12-24 hours before the anterior-posterior opticochiasmatic 1 mm thick tissue was sampled for the preparation of a coronal brain slice. The sample was divided into two parts for preservation, with one part preserved in 4% paraformaldehyde-fixation, paraffin-embedding and then pathologically sliced for immunohistochemical staining; the other part was kept in frozen liquid nitrogen for RNA extraction, and the RNA was used for the RT-PCR test.
Neurological examination
The rats of each group were given neurological scores at different time points before killing. We used the neurological scoring method according to Zausinger et al’s study [12]. The higher the 6-grade neurological score was, the more severe the animal’s behavior disorders were.
Determination of the infarcted volume
After CIR, the animals were killed by an overdose of anesthesia, their brains were removed quickly and frozen in the refrigerator to the appropriate hardness, the coronal slices were performed from the frontal pole, with 2 mm thickness, the brains were corona-cut at 1, 3, 5 and 7 mm away from the frontal pole, and were then placed in 2% triphenyl tetrazolium chloride saline for 15-30 minutes incubation at 37°C. After incubation the brain slices were taken out and fixed in 10% formaldehyde solution for 24 hours before being photographed. The computer image analysis software was used to calculate the infarcted area, and the cross products of infarcted area and thickness of brain slices were summed to obtain the infarcted volume.
Immunohistochemistry
The immunohistochemistry used the SP method. The VEGF polyclonal antibody was purchased from Santa Cruz Co., Ltd (with the antibody concentration as 1:100), and the TGF-β1 and SP kits were purchased from Beijing ZSGB-Bio Origene Co., Ltd. The slices were then performed with the DAB staining (Wuhan Boster Biotechnology Co.), hematoxylin re-staining and neutral gum-mounting. The cell counting was performed as follows: 5 slices of brain from each rat were taken, and 6 vision fields of each slice were randomly selected under 40-fold high-magnification microscope. The VEGF- and TGF-β1-immunoreactive cells were stained brown. Both the neurons and the cytoplasm of glial cells were stained. The VEGF and TGF-β1 immunoreactive cells were counted, and the average positive cells were then calculated.
RT-PCR detection
A cryopreserved specimen (0.1 g) was taken and cut into pieces, then added to 1mL of pre-cooled Trizol lysate for full homogenization. We strictly followed the manual for the Trizol RNA extraction kit to extract the total RNA of each group, which was then identified by electrophoresis. The cDNA first-strand synthesis kit was used to reversely transcript the total RNA, and PCR was performed to amplify VEGF and TGF-β1 genes. The primers of VEGF were as follows: Upstream sequence, 5’-ATGAACTTTCTGCTSTCTTGGRTGC-3’; downstream sequence, 5’-TCACCGCCTTGGCTTGTCACA-3’; the product was 645bp. The primers of TGF-β1 were as follows: Upstream sequence, 5’-ACCCGCGTGCTAATGGTGGAC-3’; downstream sequence, 5’-GAGCAGGAAGGGTCGGTTCAT-3’; the product was 453bp. The upstream primer of internal control (β-actin) was 5’-GGGAAATCGTGCGTGACA-3’, and the downstream primer was 5’-TCAGGAGGAGCAATGATC-3’, with the product length as 385bp. All the primers were synthesized by the Beijing SBS Genetech Co. Ltd. The SYNGENE gel analysis software was used to scan the gray value of each strip to analyze the relative gene expression levels among the experimental groups. The experiment was repeated three times.
Statistical analysis
The statistical package for the social sciences version 13.0 statistical software was used for data processing. All data were expressed as mean ± standard deviation (c–±s), the comparison among multiple groups used ANOVA, and the comparison between two groups’ averages used the t test, with α=0.05 considered as the significant test standard.
RESULTS
Neurological scoring
The CIR rats all exhibited obvious symptoms of neurological deficit, while the sham group had no symptoms of neurological deficit. Compared with the model group, the NBP treatment could significantly improve the CIR injury. The neurological score of the NBP treatment group was (1.28±0.46 points) lower than the model control group’s score (2.13±0.71 points), and the difference was statistically significant (t=5.91, p < 0.01).
Infarcted volume
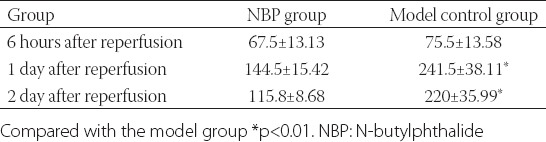
The sham group did not have an infarcted lesion. (Normal brain tissue is dark red, while an infarcted lesion is white.) At the same reperfusion time point, the infarcted volume of the NBP treatment group was less than that of the model group, with the exception of the volumes 6 hours after reperfusion: At this time point there was no significant difference between these groups. The infarcted volumes between the 2 groups at other time points were significantly different (p < 0.01) (Table 1).
TABLE 1.
Comparison of infarcted volume (mm3)
Expressions of VEGF
The brain tissues of the sham group exhibited no positive expression of VEGF, while the expressions of VEGF protein and mRNA in the brain tissues of the model control group and the NBP treatment group reached their peaks 1 day after CIR. The expressions of VEGF protein and mRNA in the NBP treatment group were significantly greater than what occurred in the model control group (p < 0.05) (Table 2). The VEGF protein expressions at different time points were mainly concentrated around the infarcted lesions, while only a small amount of positive expressions were in the center of infarcted lesions. The expressions were mainly inside the neurons and glial cells, followed by the VEC (Figures 1 and 2).
TABLE 2.
Expressions of VEGF protein and mRNA (χ̄±S)
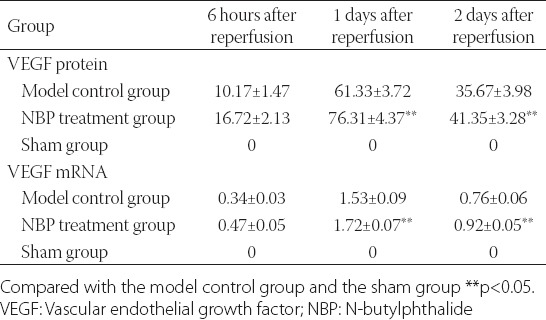
FIGURE 1.
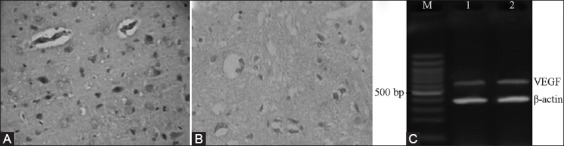
Protein and mRNA expressions of VEGF 6 h after cerebral ischemia-reperfusion. (A) NBP treatment group, (B) Model control group, (C) DNA marker.
FIGURE 2.
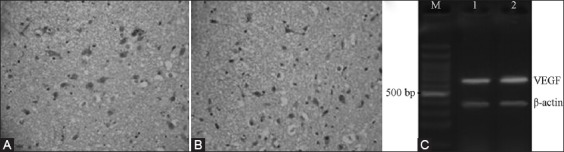
Protein and mRNA expressions of VEGF 1 d after cerebral ischemia-reperfusion. (A) NBP treatment group, (B) Model control group, (C) DNA marker.
Expressions of TGF-β1
The expressions of TGF-β1 protein at different time points were mainly concentrated in the neurons and the cytoplasm of glial cells. The bFGF protein-positive cells were brown. The RT-PCR test results showed that the expression of TGF-β1 mRNA in the NBP treatment group was significantly greater than in the model control group (p < 0.05, Table 3, Figures 3 and 4).
TABLE 3.
Expressions of TGF-β1 protein and mRNA (χ̄±S)
FIGURE 3.
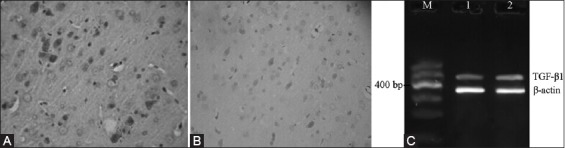
Protein and mRNA expressions of TGF-β1 6 h after cerebral ischemia-reperfusion. (A) NBP treatment group, (B) Model control group, (C) DNA marker.
FIGURE 4.

Protein and mRNA expressions of TGF-β1 1 d after cerebral ischemia-reperfusion. (A) NBP treatment group, (B) Model control group, (C) DNA marker.
DISCUSSION
During the pathophysiological processes of cerebral ischemia, a variety of factors, such as inflammatory reactions, oxygen free radicals and excitatory amino acids, played important roles. As polypeptide nerve growth factors, VEGF and TGF-β1 were important factors in promoting angiogenesis. VEGF was an endothelial cell mitogen with strong in vitro activities, and could specifically act on the endothelial cells and increase the vascular permeability and angiogenesis in vivo. Once the angiogenesis occurred, the blood supply towards the ischemic area was promoted, which would help to prevent the neuronal apoptosis in the ischemic penumbra, reduce the infarcted size, reduce the cerebral ischemic damages and cerebral edema [13]. It was reported that VEGF directly acted on the neurons, the Schwann cells and the astrocytes, promoted the extension of neural synapse, thus exhibiting the effects of neuroprotection and neurogenesis [14,15]. VEGF could directly expand the capillaries [16], thus further improving the perfusion towards the ischemic and surrounding areas, and promoting the recovery of neurological functions after ischemia.
TGF-β1 might antagonize the neurotoxicity and antioxidant effects of excitatory amino acids. The high expression of TGF-β1 in the focal cerebral ischemia exhibited protective effects towards the ischemic neuronal injuries. Recent experimental studies also found that TGF-β1 could regulate the synthesis of fibronectin and promote the proliferation of embryonic stem cells [17]. It could also be involved in the regulation of axonal growth [18] and the proliferation and differentiation of neural stem cells [19]. TGF-β1 could act with the basic fibroblast growth factor and macrophages to induce angiogenesis, which would facilitate the transferring of necrotic tissues and neural remodeling [20]. The animal experiments showed that the exogenous TGF-β1 might reduce the infarcted volume and the degeneration damage to nerve cells caused by the cerebral ischemia. Newborn mice that lacked of TGF-β1 gene expression had extensive neuronal degeneration, and their survival time was significantly shortened; the adult mice were also vulnerable to excitotoxic injuries [21].
The results of this study showed that the neurological score of the NBP treatment group was significantly higher than in the model control group, and the morphological observation revealed that the infarcted volume of the NBP group was smaller, which indicated to some extent that NBP had the protective effect towards the CIR rats. The expression of VEGF and TGF-β1 protein and mRNA indicated that 6 hours after CIR, partial neurons and glial cells in the penumbra around the infarcted lesions of the NBP treatment group and the model control group exhibited positive reactions, and reached their peaks 24 hours after CIR, and then rapidly declined. The expression trends of VEFG and TGF-β1 of both groups were basically the same. The increase of these two factors was a defensive reaction of the body towards the external stimuli, and the mechanism might be through inhibiting apoptosis, reducing the Ca2+ concentration to protect the nerve cells from oxidation, inhibiting the excitatory amino acids and promoting the repair of ischemic tissues. Two days after the ischemia, with the aggravated neuronal damages, the expression activities of VEGF and TGF-β1 by the impaired neurons were also decreased. NBP could promote the expressions of endogenous VEGF and TGF-β1, thus playing a neuroprotective effect.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Tuor UI, Morgunov M, Sule M, Qiao M, Clark D, Rushforth D, et al. Cellular correlates of longitudinal diffusion tensor imaging of axonal degeneration following hypoxic-ischemic cerebral infarction in neonatal rats. Neuroimage Clin. 2014;6:32–42. doi: 10.1016/j.nicl.2014.08.003. http://dx.doi.org/10.1016/j.nicl.2014.08.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161(2):851–8. doi: 10.1016/0006-291x(89)92678-8. http://dx.doi.org/10.1016/0006-291X(89)92678-8 . [DOI] [PubMed] [Google Scholar]
- [3].Won S, Lee JH, Wali B, Stein DG, Sayeed I. Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats:Involvement of the VEGF-MMP pathway. J Cereb Blood Flow Metab. 2014;34(1):72–80. doi: 10.1038/jcbfm.2013.163. http://dx.doi.org/10.1038/jcbfm.2013.163 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tang Y, Wang J, Lin X, Wang L, Shao B, Jin K, et al. Neural stem cell protects aged rat brain from ischemia-reperfusion injury through neurogenesis and angiogenesis. J Cereb Blood Flow Metab. 2014;34(7):1138–47. doi: 10.1038/jcbfm.2014.61. http://dx.doi.org/10.1038/jcbfm.2014.61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cui W, Li W, Han R, Mak S, Zhang H, Hu S, et al. PI3-K/Akt and ERK pathways activated by VEGF play opposite roles in MPP-induced neuronal apoptosis. Neurochem Int. 2011;59(6):945–53. doi: 10.1016/j.neuint.2011.07.005. http://dx.doi.org/10.1016/j.neuint.2011.07.005 . [DOI] [PubMed] [Google Scholar]
- [6].Ponce CC, de Lourdes Lopes Ferrari Chauffaille M, Ihara SS, Silva MR. Increased angiogenesis in primary myelofibrosis:Latent transforming growth factor-ßas a possible angiogenic factor. Rev Bras Hematol Hemoter. 2014;36(5):322–8. doi: 10.1016/j.bjhh.2014.07.010. http://dx.doi.org/10.1016/j.bjhh.2014.07.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Causey MW, Salgar S, Singh N, Martin M, Stallings JD. Valproic acid reversed pathologic endothelial cell gene expression profile associated with ischemia-reperfusion injury in a swine hemorrhagic shock model. J Vasc Surg. 2012;55(4):1096–1103.e51. doi: 10.1016/j.jvs.2011.08.060. http://dx.doi.org/10.1016/j.jvs.2011.08.060 . [DOI] [PubMed] [Google Scholar]
- [8].Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch:A balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med. 2003;13(7):301–7. doi: 10.1016/s1050-1738(03)00142-7. http://dx.doi.org/10.1016/S1050-1738(03)00142-7 . [DOI] [PubMed] [Google Scholar]
- [9].Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38(11):3032–9. doi: 10.1161/STROKEAHA.107.488445. http://dx.doi.org/10.1161/STROKEAHA.107.488445 . [DOI] [PubMed] [Google Scholar]
- [10].Li Y, Lu Z, Keogh CL, Yu SP, Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27(5):1043–54. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- [11].Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. http://dx.doi.org/10.1161/01.STR.20.1.84 . [DOI] [PubMed] [Google Scholar]
- [12].Zausinger S, Hungerhuber E, Baethmann A, Reulen H, Schmid Elsaesser R. Neurological impairment in rats after transient middle cerebral artery occlusion:A comparative study under various treatment paradigms. Brain Res. 2000;863(1-2):94–105. doi: 10.1016/s0006-8993(00)02100-4. http://dx.doi.org/10.1016/S0006-8993(00)02100-4 . [DOI] [PubMed] [Google Scholar]
- [13].Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci. 2013;70(10):1753–61. doi: 10.1007/s00018-013-1282-8. http://dx.doi.org/10.1007/s00018-013-1282-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang ZJ, Bao WL, Qiu MH, Zhang LM, Lu SD, Huang YL, et al. Role of vascular endothelial growth factor in neuronal DNA damage and repair in rat brain following a transient cerebral ischemia. J Neurosci Res. 2002;70(2):140–9. doi: 10.1002/jnr.10380. http://dx.doi.org/10.1002/jnr.10380 . [DOI] [PubMed] [Google Scholar]
- [15].Manoonkitiwongsa PS, Schultz RL, McCreery DB, Whitter EF, Lyden PD. Neuroprotection of ischemic brain by vascular endothelial growth factor is critically dependent on proper dosage and may be compromised by angiogenesis. J Cereb Blood Flow Metab. 2004;24(6):693–702. doi: 10.1097/01.WCB.0000126236.54306.21. http://dx.doi.org/10.1097/01.WCB.0000126236.54306.21 . [DOI] [PubMed] [Google Scholar]
- [16].Hansen TM, Moss AJ, Brindle NP. Vascular endothelial growth factor and angiopoietins in neurovascular regeneration and protection following stroke. Curr Neurovasc Res. 2008;5(4):236–45. doi: 10.2174/156720208786413433. http://dx.doi.org/10.2174/156720208786413433 . [DOI] [PubMed] [Google Scholar]
- [17].Kim YH, Ryu JM, Lee YJ, Han HJ. Fibronectin synthesis by high glucose level mediated proliferation of mouse embryonic stem cells:Involvement of ANG II and TGF-beta1. J Cell Physiol. 2010;223(2):397–407. doi: 10.1002/jcp.22048. http://dx.doi.org/10.1002/jcp.22048 . [DOI] [PubMed] [Google Scholar]
- [18].Ng J. TGF-beta signals regulate axonal development through distinct Smad-independent mechanisms. Development. 2008;135:4025–35. doi: 10.1242/dev.028209. http://dx.doi.org/10.1242/dev.028209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sun J, Zhou W, Sha B, Yang Y. Ischemia induced neural stem cell proliferation and differentiation in neonatal rat involved vascular endothelial growth factor and transforming growth factor-beta pathways. Brain Dev. 2010;32:191–200. doi: 10.1016/j.braindev.2009.01.004. http://dx.doi.org/10.1016/j.braindev.2009.01.004 . [DOI] [PubMed] [Google Scholar]
- [20].Shih SC, Ju M, Liu N, Mo JR, Ney JJ, Smith LE. Transforming growth factor beta1 induction of vascular endothelial growth factor receptor 1:Mechanism of pericyte-induced vascular survival in vivo. Proc Natl Acad Sci U S A. 2003;100(26):15859–64. doi: 10.1073/pnas.2136855100. http://dx.doi.org/10.1073/pnas.2136855100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40(6):1133–45. doi: 10.1016/s0896-6273(03)00766-9. http://dx.doi.org/10.1016/S0896-6273(03)00766-9 . [DOI] [PubMed] [Google Scholar]


