Abstract
The aim of this study was to investigate the causes of elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in children. We analyzed the medical records for children aged 3 months to 18 years who presented to the hospital with ALT >45 IU/L and/or AST >50 IU/L, between 2012 and 2014, for various reasons, including those not related to liver disease. In total, 281 children met the study criteria. This group comprised of 125 (44.5%) females and 156 (55.5%) males. At the presentation, the most common patient complaint was fatigue (53.4%), while 15.7% of the patients reported no symptoms. The most common findings on the physical examination were jaundice and hepatomegaly. In 15% of the cases, the findings were normal. According to the diagnosis, the most common cause of the elevated transaminases were infections (34%), with hepatitis A virus (HAV) infection as the leading cause (18.9%). Drug-induced liver injury (DILI) was the cause in 18.1% of the cases and non-alcoholic fatty liver disease (NAFLD) in 11.1%. The highest transaminase levels were associated with HAV infection, while DILI and NAFLD caused only slightly elevated transaminases. Overall, our results show that the elevated transaminases in children are most often caused by infections, DILI, and NAFLD. In a majority of cases, elevated ALT and AST indicate liver disease, however, they could also be associated with conditions other than liver damage. Additionally, the elevated enzymes can be detected in completely healthy individuals.
KEY WORDS: Children, DILI, hepatitis, liver, myopathy, NAFLD, transaminases, ALT, AST
INTRODUCTION
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are normally found in the plasma at low levels [1-3]. When tests show increased levels of these enzymes, further analyzes must be performed in order to make a differential diagnosis. Although the elevated transaminases indicate liver damage in a majority of cases, other conditions can also cause this increase [4-7].
Several causes can lead to increased levels of the transaminases including infectious, metabolic, toxic, inflammatory, infiltrative, and traumatic causes. When serum AST levels are higher than ALT in the absence of signs and symptoms of liver disease, this should lead to a consideration of occult muscle disease as a probable source [8].
Several studies report that the prevalence of the elevated transaminases in children varies according to the region, food, sanitation, the period of year and the quality of healthcare. In underdeveloped countries, infections are the leading cause associated with the elevated liver enzymes, with hepatitis A virus (HAV) as the most common cause of the infections. On the contrary, non-alcoholic fatty liver disease (NAFLD) associated with obesity is the most common cause in developed countries [9-18].
Although the prevalence and causes of the elevated transaminases have been extensively studied in adults [19], there is no sufficient information regarding the prevalence and causes in children. In the pediatric population, the prevalence of elevated transaminases was reported to be 3.5-12.4% [2,20,21]. NAFLD and viral infections were determined as the most common causes of hypertransaminasemia [5,20-22].
To date, only one study was investigating the elevated transaminases in children age 0-15 years in Turkey. That study included only hospitalized children already receiving a specific treatment [23].
In contrast, we wanted to investigate demographic characteristics and diagnoses of children aged 3 months-18 years who presented to our hospital with elevated ALT and AST values, regardless of the condition.
MATERIALS AND METHODS
The transaminase levels of the outpatients and patients were recorded from the hospital records system (MEDULA). Local Clinical Research Ethics Committee approved the study and informed consent was obtained from all the subjects or parents.
We included patients aged between 3 months and 18 years, visiting our hospital as outpatients or hospitalized at any of the departments, between December 2012 and December 2014. The values of AST >50 IU/L and ALT >45 IU/L were considered as elevated in accordance with the literature [24], and the elevated enzyme levels had to be detected at least twice in the last month.
Patients below the age of 3 months and older than 18 years, as well as those with only one elevated result or with incomplete medical records were excluded from the study.
Data on demographic characteristics, complaints, physical examination, biochemical, serological, and molecular/genetic testing, ultrasonography (US) findings, liver biopsy results as well as the clinical course were recorded for all the patients.
Obesity-related NAFLD was diagnosed only if the child was obese or overweight. Body mass index (BMI) above the 95th and 85th percentile for age was used for defining obesity and overweight respectively. In addition, the observation of bright liver on ultrasound scanning was used to make the diagnosis in the absence of all other known causes of liver disease, in accordance with the literature [25].
Autoimmune hepatitis (AIH) was investigated according to the criteria established by the International Autoimmune Hepatitis Group [26].
The diagnosis of Epstein-Barr virus (EBV) infections was made based on the increase of serum ALT levels and early positive immunoglobulin M antibody to EBV viral capsid antigen (anti-EBV VCA IgM).
The diagnosis of suspected drug-induced liver injury (DILI) was made as described by Tajiri et al. [27]. DILI was classified into three types: hepatocellular, cholestatic, and mixed according to the American College of Gastroenterology Clinical Guideline [28].
The diagnosis of NAFLD was made based on the evidence of hepatic steatosis by US findings, and also based on the evidence that there were no causes of hepatic fat accumulation such as significant alcohol consumption, the use of steatogenic medication, or hereditary disorders. Ultrasonography findings scored in this study included hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring defined by Hamaguchi et al. [29]. Scores ranged from 0 to 6 points and NAFLD was defined if US score was ≥1.
The patients were subdivided into 4 groups according to the age, ALT, and AST values. The age groups were 3 months-2 years, 3-5 years, 6-12 years, and 13-18 years. The ALT values for grouping were 46-100 IU/L, 101-500 IU/L, 501-1.000 IU/L, and >1.000 IU/L. The AST values for grouping were 50-100 IU/L, 101-500 IU/L, 501-1.000 IU/L, and >1.000 IU/L. The diagnoses were compared according to the groups.
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Science for Windows (SPSS) version 15.0 (SPSS Inc. Chicago, IL, USA, 2006) software. Measurable variables were stated as mean±standard deviation (SD) or standard error (SE). For comparing groups defined by numbers the Chi-square test was used. The Mann Whitney U-test was used to compare the mean values of the measurements of two independent groups. p<0.05 was considered statistically significant.
RESULTS
From a total of 41,155 children aged 3 months-18 years, who presented at the hospital within the 2-year period, the transaminase tests were requested for 10,915 children. Among these, the elevated enzyme levels were detected in 786 cases (7.2%). In this group 505 cases did not met the study criteria and were excluded from the study. A total of 281 cases met all the study criteria and were evaluated. These comprised of 125 (44.5%) females and 156 (55.5%) males with a mean age of 7.01±5.24 years (range, 3 months-18 years). According to the gender, no statistically significant difference was determined in the mean age (p=0.061) nor in the age groups (p=0.174).
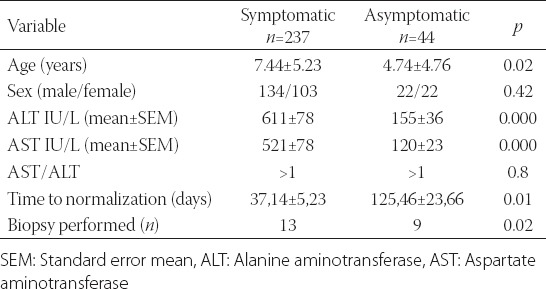
The most common complaint on the presentation was fatigue, with 150 patients (53.4%) reporting this symptom. In 45 cases, the elevated transaminase levels were determined using preoperative testing before surgery (e.g. tonsillectomy) which was performed outside the pediatric clinic. Complaints of the patients are presented in Table 1. Two hundred thirty-seven patients (84.3%) had at least 1 complaint on the presentation. Forty-four of them (15.7%) were asymptomatic. A comparison between the two groups is presented in Table 2.
TABLE 1.
Complaints of the patients
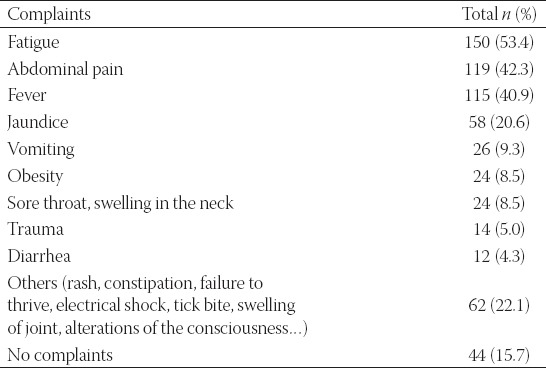
TABLE 2.
Differences between symptomatic and asymptomatic patients with respect to age, sex, mean ALT and AST values, and follow up
The most common findings on the physical examination were jaundice and hepatomegaly, both determined in 64 patients (22.8%). Splenomegaly was observed in 30 (10.7%) and tonsillitis and upper respiratory tract symptoms in 12 patients (4.3%). In 42 patients (15%), the physical examination was completely normal (Table 3).
TABLE 3.
Findings of the physical examination

The mean ALT values in all the cases were determined as 540±67 IU/L (range, 44–9.176 IU/L), and the mean AST values as 474±69 IU/L (range, 9–11.507 IU/L). No statistically significant difference was determined between the age groups with respect to the mean ALT and AST values (p=0.37, p=0.99).
When the patients were grouped according to the diagnosis, the leading cause were infections, occurring in 96 cases (34%). These infections were associated with HAV (18.5%), hepatitis B virus (HBV) (1.1%), EBV (5%), Cytomegalovirus (CMV), Parvovirus, and brucellosis (1.1%). The second most common cause of the elevated transaminases was suspected DILI, detected in 51 (18.9%) cases. Types of liver injury were hepatocellular in 35 patients, cholestatic in 11 patients, and mixed in 5 patients. Other diagnoses were NAFLD in 31 (11.1%) patients, multi-trauma in 14 (5%), muscular dystrophy in 9 (3.2%), total parenteral nutrition related in 9 (3.2%), sepsis in 6 (2.1%), AIH in 6 (2.1%), metabolic diseases in 5 (1.8%), cholesistitis with cholelithiasis in 5 (1.8%), non-drug-related toxic hepatitis in 3 (1.1%), hypoxia in 2 (0.7%), rhabdomyolysis in 2 (0.7%), mushroom poisoning in 2 (0.7%), malnutrition in 2 (0.7%), and other diagnosis in 13 (4.5%) patients. Idiopathic cases with unknown etiology were determined in 18 patients (6.4%). Causes that are not associated with liver disease were determined in 4% of the patients.
In asymptomatic patients, the most frequent causes of the elevated transaminases were DILI (31.8%), idiopatic (18.2%), and myopathy (11.4%). However, HAV (21.5%), DILI (16.5%) and NAFLD (11.4%) were the most frequent causes in symptomatic patients.
When the evaluation of diagnosis was made according to the age groups, the most common causes in the infant group (aged 3 months-2 years) were drugs and idiopathic causes. In 15 (19.2%) patients, the elevated transaminase levels were caused by DILI, in 15 patients (19.2%) the underlying reason could not be determined, and in 14 (17.9%) the cause was related to infections other than viral hepatitis. In the preschool-age group (3-5 years), HAV infection-related elevated transaminase levels were determined in 8 (16.6%) patients, DILI in 7 (14.5%) and EBV in 7 (14.5%). In school-age children (6-12 years) HAV infection was the primary cause as determined in 32 (30%) patients, DILI was detected in 20 (18.6%), and NAFLD in 16 (14.9%). In the adolescent-age group (13-18 years), the leading cause of the elevated transaminase levels was NAFLD, determined in 13 (27%) patients, followed by HAV infection detected in 12 (25%), and DILI in 9 cases (18.7%).
In the group with slightly elevated levels of ALT (46-100 IU/L), DILI was determined as the cause in 33 (28.7%) patients and NAFLD in 19 (16.5%). In the group with moderately elevated levels of ALT (101-500 IU/L), DILI was again the leading cause, determined in 18 (17.1%) patients. In the group with highly elevated levels of ALT (501-1.000 IU/L) HAV infection was diagnosed in 13 patients (65%). Finally, in the group with very high levels of ALT (>1.000 IU/L) HAV infection was also the leading cause, determined in 33 (82.5%) patients.
In the group with slightly elevated levels of AST (50-100 IU/L), DILI was determined as the cause in 33 (28.7%) patients, and NAFLD in 11 (10%) patients. In the group with moderately elevated levels of AST (101-500 IU/L), DILI was again the leading cause, determined in 16 (16.5%) patients. In the group with highly elevated levels of AST (501-1.000 IU/L) HAV infection was diagnosed in 12 patients (57.1%). In the group with very high levels of AST (>1.000 IU/L) HAV infection was also the leading cause, determined in 27 (79.4%) patients.
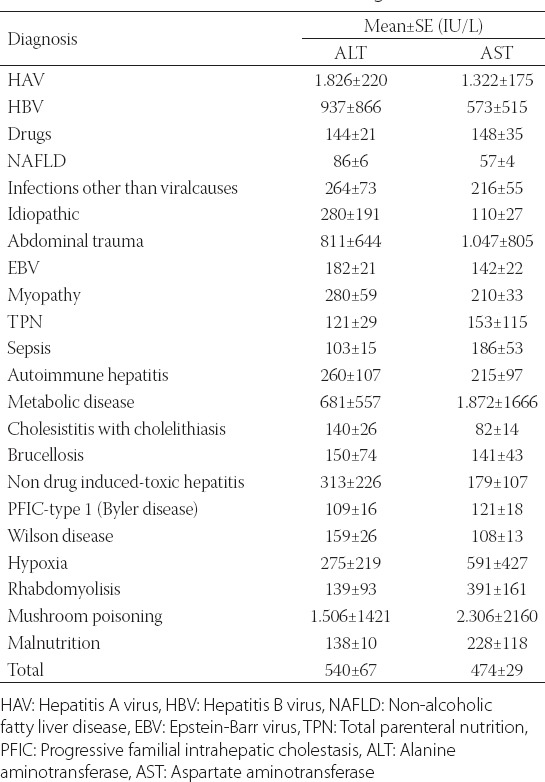
When the mean ALT and AST values were evaluated according to diagnosis, the highest levels of the enzymes were detected in the viral hepatitis group and the lowest levels in the DILI group (Table 4).
TABLE 4.
Mean ALT and AST values according to the causes
Liver biopsies were performed on 22 (7.8%) patients. In 19 (86%) of them, the histopathological result contributed to diagnosis. Based on the biopsy results, hepatosteatosis, AIH, and chronic hepatitis were each determined in 4 patients. In 2 cases, the results of biopsy were evaluated as normal.
Serological testing was performed in 196 patients (70%), among these, in 83 cases (42%) diagnosis was made based on the test results. Using this method, HAV was most commonly diagnosed, with 51 cases showing a HAV infection. Following were EBV, diagnosed in 18 patients, CMV or herpes simplex virus (HSV) in 6, HBV in 3, brucellosis in 3, Fasciola hepatica in 1, and Parvovirus B19 in 1 case.
The US examination was carried out in 156 (55%) cases, and contributed to making a diagnosis in 51 patients (32%). Among these, hepatosteatosis was diagnosed in 27 cases, gallstones in 9, liver or spleen contusion and/or laceration in 7, a heterogeneous appearance in 3, a cyst or mass in 3, Fasciola hepatica in 1, and hemangioma in 1 case.
Genetic-molecular testing was requested in 8 patients. In 1 case (Wilson disease), the diagnosis was made based on these results.
Magnetic resonance imaging or computed tomography was applied to 8 (3%) patients who had a severe abdominal trauma, providing a diagnosis in 6 cases.
During the follow-up period, 2 patients were transferred to another center for liver transplantation, because of fungal poisoning in the first and progressive familial intrahepatic cholestasis in the second case.
The mean normalization times of the transaminases were 12.4±2.5, 27.21±3.4, 29.4±8.49, and 95.66±27.24 days in the EBV, DILI, HAV, and idiopathic groups, respectively. The normalization time was significantly shorter in the EBV and DILI group compared to the other groups. Among 31 patients with NAFLD there were 5 patients with normal weight, 4 overweight, and 22 obese patients. In seven of them, the transaminase levels returned to normal within 235.71±53.75 days due to weight loss.
Throughout the study period, 7 patients died. These include: 3 cases of malignancy (2 brain tumors, 1 Ewing sarcoma), 2 cases of sepsis (1 with cystic fibrosis), 1 case of pulmonary aspiration, and 1 case of metabolic disease (organic acidemia).
DISCUSSION
In a community-based study in the USA, the prevalence of elevated ALT was determined as 7.4% in Caucasians, 11.5% in Mexican Americans, and 6% in African Americans [20]. Regarding other community-based studies, rates of elevated ALT were determined in 6.5% of the Korean adolescents [2], as well as in 3.8% of the Mexican girls and 9.8% of the Mexican boys [21]. To our knowledge, no similar community-based studies were conducted in Turkey nor in other countries. Even though the current study was hospital-based, the elevated levels of the transaminases were determined in children at the rate of 7.2%.
Complaints accompanying the elevated transaminases can show many variations. In addition, the elevated enzymes can be detected incidentally in completely asymptomatic cases [6,23]. In the current study, the most common complaint on the presentation was fatigue (53.4%). In 15.7% of the cases no complaints were reported, and the diagnosis was made completely by coincidence. As expected, the most common physical examination findings were hepatomegaly and jaundice (22.8%). In 2% of the cases, the Gowers’ sign was positive. For 15% of the patients, the physical findings were normal.
In underdeveloped countries, the leading cause of the elevated transaminases is associated with HAV [11], while in developed countries the leading cause is NAFLD associated with obesity [20,21]. In the current study, the primary (34%) etiology were infections (18.9% cases with HAV) followed by DILI in 18.1%, and NAFLD in 11.1% patients.
The study conducted in Italy, showed that among 425 children with the elevated transaminases, infections, primarily EBV infections, were diagnosed in 31% of the cases, liver disease associated with obesity in 18%, and genetic diseases in 12% of the patients. However, major hepatotropic viruses were excluded from the analysis [5]. Thus, our results of HAV and NAFLD as the most common causes of the elevated transaminases, indicate that our country is between developed and underdeveloped countries.
Another study from Turkey [23] showed that the rate for infectious causes was 34.4%, and among these, 8% were HAV-related causes. These results are very similar to those obtained in this study. Higher HAV-related rate obtained in the current study could be related to different inclusion criteria. For example, Çeltik et al. [23] only included hospitalized patients and the upper age limit was 15 years. In addition, infants below the age of 3 months were not included.
In the current study, the causes could not be determined in 18 (6.4%) patients. In all these idiopathic cases, the transaminase levels spontaneously recovered within a mean of 95 days. In the study by Çeltik et al. [23] the idiopathic rate was found to be 27.1% and was reported to recover within 1 year. Iorio et al. reported idiopathic hypertransaminasemia in 31.7% of the cases. In 84% of these patients, the enzymes returned to the normal levels within 6 months [5]. In both studies, the rates of idiopathic cases were higher than those reported in the current study. This could be due to the younger age group in the first and the exclusion of major viral causes in the second study.
In this study, causes not related to liver disease were determined in 4% patients. These included: 9 cases of myopathy, 1 electric shock, and 1 case of rhabdomyolysis following vaccination. Similar to these results, muscular dystrophy was reported at 3% by Iorio et al. [5] and at 2% by Nobili et al. [22].
The transaminases can also be elevated due to rotavirus, which is the most common cause of gastroenteritis [14]. The results of this study show that the rotavirus infection was diagnosed in 2 patients. This should be consider when treating rotavirus-gastroenteritis, in order to avoid unnecessary tests.
Obesity is a significant cause of elevated ALT [15,21]. The results from this study show that NAFLD was determined in 31 cases, among these, 84% were associated with being overweight or obese. It is therefore important to monitor ALT in young obese patients, in order to prevent liver diseases which may develop in the future.
According to the literature, the aminotransferase levels in patients with NAFLD are approximately 10 times below 300 IU/L [4]. In the current study, the mean ALT levels in the patients with NAFLD were 86 IU/L and thus consistent with the data from the literature.
When the mean ALT and AST values were evaluated according to the diagnosis, the highest levels were detected in the viral hepatitis group and the lowest levels were associated with drug toxicity. These findings were consistent with the literature [4].
However, this study demonstrates several limitations and these include: 1) The absence of a specific upper normal limit for the transaminases in our society, as well as, all over the world 2) Retrospective study 3) All diagnostic tools were not performed in all the patients.
The causes of the elevated transaminases can vary even in the same geographic region due to changes in environmental conditions, sanitation, and vaccinations [11]. Since the nationwide HBV vaccination program was started in Turkey in 1998, hepatitis B virus has been rarely detected in children. In contrast, a high rate of HAV has been observed. However, the national vaccination program for HAV was initiated in 2014, thus a dramatic reduction in this disease is expected over time.
In this study, the second most common cause was related to medications, followed by NAFLD. Considering that obesity among children is increasing in this country, NAFLD could become the greatest risk in Turkey in the future, similarly as in developed countries.
Finally, the elevated transaminases can also be detected in completely healthy individuals, and they do not always indicate liver disease. In addition, muscular diseases should not be overlooked in children presenting with the elevated transaminases.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, et al. SAFETY study:alanine aminotranferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010;138(4):1357–1364. doi: 10.1053/j.gastro.2009.12.052. http://dx.doi.org/10.1053/j.gastro.2009.12.052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Park SH, Park HY, Kang JW, Park J, Shin KJ. Aminotransferase upper reference limits and the prevalence of elevated aminotransferases in the Korean adolescent population. J Pediatr Gastroenterol Nutr. 2012;55(6):668–672. doi: 10.1097/MPG.0b013e3182660669. http://dx.doi.org/10.1097/MPG.0b013e3182660669 . [DOI] [PubMed] [Google Scholar]
- [3].Park HK, Hwang JS, Moon JS, Lee JA, Kim DH, Lim JS. Healthy range of serum alanine aminotransferase and its predictive power for cardiovascular risk in children and adolescents. J Pediatr Gastroenterol Nutr. 2013;56(6):686–691. doi: 10.1097/MPG.0b013e31828b4e67. http://dx.doi.org/10.1097/MPG.0b013e31828b4e67 . [DOI] [PubMed] [Google Scholar]
- [4].Giannini EG, Testa R, Savarino V. Liver enzyme alteration:a guide for clinicians. CMAJ. 2005;172(3):367–379. doi: 10.1503/cmaj.1040752. http://dx.doi.org/10.1503/cmaj.1040752 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Iorio R, Sepe A, Giannattasio A, Cirillo F, Vegnente A. Hypertransaminasemia in childhood as a marker of genetic liver disorders. J Gastroenterol. 2005;40(8):820–826. doi: 10.1007/s00535-005-1635-7. http://dx.doi.org/10.1007/s00535-005-1635-7 . [DOI] [PubMed] [Google Scholar]
- [6].Bugeac N, Pacht A, Mandel H, Iancu T, Tamir A, Srugo I, et al. The significance of isolated elevation of serum aminotransferases in infants and young children. Arch Dis Child. 2007;92(12):1109–1112. doi: 10.1136/adc.2007.121194. http://dx.doi.org/10.1136/adc.2007.121194 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367–1384. doi: 10.1053/gast.2002.36061. http://dx.doi.org/10.1053/gast.2002.36061 . [DOI] [PubMed] [Google Scholar]
- [8].Vajro P, Maddaluno S, Veropalumba C. Persistent hypertansaminasemia in asymptomatic children:a stepwise approach. World J Gastroenterol. 2013;19(18):2740–2751. doi: 10.3748/wjg.v19.i18.2740. http://dx.doi.org/10.3748/wjg.v19.i18.2740 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hennes HM, Smith DS, Schneider K, Hegenbarth MA, Duma MA, Jona JZ. Elevated liver transaminase levels in children with blunt abdominal trauma:a predictor of liver injury. Pediatrics. 1990;86(1):87–90. [PubMed] [Google Scholar]
- [10].Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes:a meta-analysis. Obes Surg. 2013;23(11):1815–1825. doi: 10.1007/s11695-013-0981-4. http://dx.doi.org/10.1007/s11695-013-0981-4 . [DOI] [PubMed] [Google Scholar]
- [11].Shen G, Zhang L, Zhang YL, Hu LP, Li MH, Lu Y, et al. Study on the etiology of acute hepatitis hospitalized patients in Beijing Ditan Hospital from 2002 to 2011 [Article in Chinese] Zhonghua Shi Yan He Lin Chuang Bing Du XueZaZhi. 2013;27(4):266–269. [PubMed] [Google Scholar]
- [12].Ellis RD, Dicko A, Sagara I, Kamate B, Guindo O, Niambele MB, et al. Short report:elevated levels of alanine aminotransferase and hepatitis A in the context of a pediatric malaria vaccine trial in a village in Mali. Am J Trop Med Hyg. 2008;79(6):980–982. [PMC free article] [PubMed] [Google Scholar]
- [13].Ameli M, Besharati S, Nemati K, Zamani F. Relationship between elevated liver enzyme with iron overload and viral hepatitis in thalassemia major patients in Northern Iran. Saudi Med J. 2008;29(11):1611–1615. [PubMed] [Google Scholar]
- [14].Akelma AZ, Kütükoğlu I, Köksal T, Çizmeci MN¸Kanburoğlu MK, Çatal F, et al. Serum transaminase elevation in children with rotavirus gastroenteritis:seven years' experience. Scand J Infect Dis. 2013;45(5):362–367. doi: 10.3109/00365548.2012.740573. http://dx.doi.org/10.3109/00365548.2012.740573 . [DOI] [PubMed] [Google Scholar]
- [15].Hudson OD, Nunez M, Shaibi GQ. Ethnicity and elevated liver transaminases among newly diagnosed children with type 2 diabetes. BMC Pediatr. 2012;12:174. doi: 10.1186/1471-2431-12-174. http://dx.doi.org/10.1186/1471-2431-12-174 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].M'Koma AE, Longo WE. Postoperative liver enzyme abnormalities are related to staged restorative proctocolectomy. Int J Colorectal Dis. 2007;22(3):283–288. doi: 10.1007/s00384-006-0130-9. http://dx.doi.org/10.1007/s00384-006-0130-9 . [DOI] [PubMed] [Google Scholar]
- [17].González-Contreras J, Villalobos Gámez JL, Gómez-Sánchez AI, García-Almeida JM, Enguix Armanda A, Rius Diaz F, et al. Cholestasis induced by total parenteral nutrition:effects of the addition of Taurine (Tauramin®) on hepatic function parameters;possible synergistic action of structured lipids (SMOFlipid®) Nutr Hosp. 2012;27(6):1900–1907. doi: 10.3305/nh.2012.27.6.6047. http://doi:10.3305/nh.2012.27.6.6047 . [DOI] [PubMed] [Google Scholar]
- [18].Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, et al. Drug-Induced Liver Injury Network (DILIN). Hepatic histological findings in suspected drug-induced liver injury:systematic evaluation and clinical associations. Hepatology. 2014;59(2):661–670. doi: 10.1002/hep.26709. http://dx.doi.org/10.1002/hep.26709 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hyun HJ, Shim JJ, Kim JW, Lee JS, Lee CK, Jang JY, et al. The prevalence of elevated alanine transaminase and its possible causes in the general Korean population. J Clin Gastroenterol. 2014;48(6):534–539. doi: 10.1097/MCG.0b013e3182a474d3. doi:10.1097/MCG.0b013e3182a474d3. [DOI] [PubMed] [Google Scholar]
- [20].Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors:NHANES 1999-2004. Gastroenterology. 2007;133(6):1814–1820. doi: 10.1053/j.gastro.2007.08.077. http://dx.doi.org/10.1053/j.gastro.2007.08.077 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Purcell M, Flores YN, Zhang ZF, Denova-Gutiérrez E, Salmeron J. Prevalence and predictors of alanine aminotransferase elevation among normal weight, overweight and obese youth in Mexico. J Dig Dis. 2013;14(9):491–499. doi: 10.1111/1751-2980.12072. http://dx.doi.org/10.1111/1751-2980.12072 . [DOI] [PubMed] [Google Scholar]
- [22].Nobili V, Reale A, Alisi A, Morino G, Trenta I, Pisani M, et al. Elevated serum ALT in children presenting to the emergency unit:Relationship with NAFLD. Dig Liver Dis. 2009;41(10):749–752. doi: 10.1016/j.dld.2009.02.048. http://dx.doi.org/10.1016/j.dld.2009.02.048 . [DOI] [PubMed] [Google Scholar]
- [23].Çeltik C, Erbaş H, Kurşun ÖS, Bostancıoğlu M, Inan M, Öner N, et al. The Reasons of Elevated Serum Transaminases in Childhood [Article in Turkish] Turk J Biochem. 2008;33(4):175–181. [Google Scholar]
- [24].Gilbert-Barness E, Barness LA, Farrell PM. Clinical Use of Pediatric Diagnostic Tests. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins; 2003. [Google Scholar]
- [25].Fishbein MH, Miner M, Mogren C, Chalekson J. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr. 2003;36(1):54–61. doi: 10.1097/00005176-200301000-00012. http://dx.doi.org/10.1097/00005176-200301000-00012 . [DOI] [PubMed] [Google Scholar]
- [26].Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International Autoimmune Hepatitis Group Report:review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31(5):929–938. doi: 10.1016/s0168-8278(99)80297-9. http://dx.doi.org/10.1016/S0168-8278(99)80297-9 . [DOI] [PubMed] [Google Scholar]
- [27].Tajiri K, Shimizu Y. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J Gastroenterol. 2008;14(44):6774–6785. doi: 10.3748/wjg.14.6774. http://dx.doi.org/10.3748/wjg.14.6774 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chalasani NP, Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, et al. Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline:the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950–966. doi: 10.1038/ajg.2014.131. http://dx.doi.org/10.1038/ajg.2014.131 . [DOI] [PubMed] [Google Scholar]
- [29].Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102(12):2708–2715. doi: 10.1111/j.1572-0241.2007.01526.x. HTTP://DX.DOI.ORG/10.1111/J.1572-0241.2007.01526.X . [DOI] [PubMed] [Google Scholar]


