Abstract
Biomaterials and biotechnology are increasing becoming an important area in modern medicine. The main aim in this area is the development of materials, which are biocompatible to normal tissue. Tissue-implant interactions with molecular, biological and cellular characteristics at the implant-tissue interface are important for the use and development of implants. Implantation may cause an inflammatory and immune response in tissue, foreign body reaction, systemic toxicity and imminent infection. Tissue-implant interactions determine the implant life-period. The aims of the study are to consider the biological response to implants. Biomaterials and host reactions to implants and their mechanisms are also briefly discussed.
KEY WORDS: Host versus graft disease, biomaterial, wound healing, transplant, tissue
INTRODUCTION
The medical field of biomaterials and bioengineering is nowadays becoming increasingly important. Although activity in this field is not new, the knowledge of molecular and cell biology has enabled significant progress, especially in the past 20 years. At first, more than 50 years ago, well-known synthetic materials were used. Later, through analyzing and exploring new forms of biomaterials, various implants for medical use were designed [1-3]. Materials, which are essentially well known in the technological field, had initially not been developed as biomaterials. These were the first used for tissue defects and reconstructions. Bone implants, for example, were made from stainless steel and other alloys, or from high-density polyethylene. Methacrylate polymers were used as bone cement in dental medicine, whilst polyethylene tetra phthalate fibers were used for the production of grafts for blood vessel reconstructions (Figure 1). During this pioneering period, cellulose membranes were also used as filter meshes in hemodialysis. At the start, the science of biomaterials was focused on in vitro studies, imitating living tissue [4-8]. Experiments in vivo followed. These enabled a deeper understanding of biological tissue response to implants and hence further studies about the use of alternative components within biological implants. Most of the materials used were synthetic and intended for the manufacture of permanent implants. These were proposed to replace the function and structure of damaged or diseased tissue [9-11].
FIGURE 1.
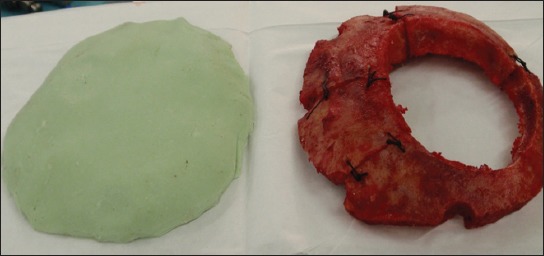
An example of biomaterial implementation for reconstruction of cranial bone defects. After cranial surgery, artificial flaps made of methylmetacrylate are being used for covering of bone defects. Such artificial flap (on the left) was made according to the original and will replace the damaged original bone flap (right).
The main objective in the science of biomaterials is the ongoing research and development of materials that specifically react with the biological environment for which were developed for. This includes the so-called tissue regeneration approach; biomaterials are supposed to act as a temporary scaffold or cell anchorage for three-dimensional tissue structures to be then colonized by specific cell types [4,9,12]. Their main purpose is to allow improved tissue regeneration. Over time, some biomaterials are degradable within the tissues that they were implanted in, whilst others are permanent. A new and specific function of biomaterials is their use in molecular transfer into target tissues for the treatment of diseases. An example of this is the transfer of encapsulated genes into the cells of the diseased tissues [13-17].
TISSUE AND BIOMATERIALS
The molecular biological interactions between the implant and the tissue are significant in the application and use of biomaterials as well as their interaction with tissue. For example, the study of biomaterials for vascular applications includes factors such as interactions between the blood and the implant, the factors influencing the response of blood components to the implant as well as the evaluation of these phenomena. Specific reactions of platelets, red blood cells and leukocytes to the artificial material, activation of the complement, coagulation cascade, adsorption of proteins and the fibrinolytic activity are important factors within the organism in terms of the interactions between blood and implants or grafts [7,8,18,19]. On the other hand, the reaction of blood components to the implant could be affected by the composition of the biomaterial, the presence of antithrombotic agents, active ingredients of drugs acting on an organism, the use of biomaterial and the type of injury or defect responsible for the implant used [20-23].
The aim of the study of biomaterials is to understand the biological response of tissue and organism to artificial implants. In recent years, this has enabled a great progress in the development of artificial materials. Exploring the impact of biomaterials on the human body and vice versa begins with in vitro studies. These studies are particularly important in the development of the biomaterial for its specific use. The tests performed under in vivo conditions follow. These may continue to clinical research and ultimately lead to the general use of the implant [7,21,22,24].
TISSUE REACTIONS TO BIOMATERIALS
A surgical implantation of a biomaterial into the body triggers an organism-inflammatory reaction with the associated healing of the damaged tissue. Depending upon the composition of the implanted material, the surface of the implant, the mechanism of fatigue and chemical decomposition there are a number of other reactions possible. These can be local as well as being systemic. These include immune response, foreign body reaction with the isolation of the implant with a vascular connective tissue, possible infection and impact on the lifespan of the implant [21,23].
Usually, implants are well-integrated into the surrounding tissue and may be serviceable for a long period of time. Depending on the use, they may improve the quality of life and prolong survival [21,25,26]. Since implants are made of artificial materials that are foreign to living tissues, they may trigger a reaction involving surrounding tissue. These reactions may be so pronounced that they may ultimately lead to tissue damage and failure of the implant, as well as to death of the organism. The interactions between the implant and the tissue are various (Figure 2). They can be divided into two groups: (1) The effect of the implant on the tissue, and (2) the influence of the organism on the implant. The first group includes: (I) The local effects with interactions between blood and implant, infection, influence on the normal course of treatment, toxicity and carcinogenesis, as well as, (II) the systemic effect of implant particle embolization, hypersensitivity reactions and the increased amount of chemical compounds within the implant in the body. The second group includes the interaction of the organism to the implant: (I) The biological effects of enzymatic degradation, calcification and absorption of the artificial material and (II) the physical and mechanical effect of the material such as fatigue, abrasion, corrosion, and dissolution [2,7,21,27-29].
FIGURE 2.
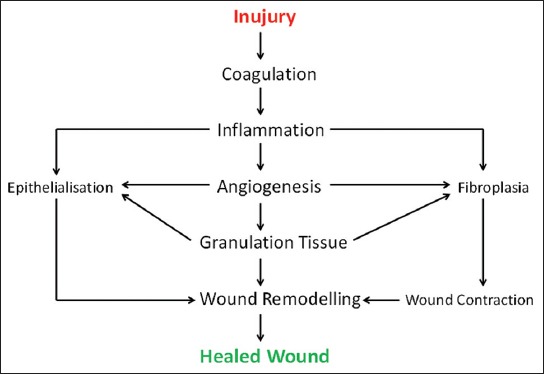
Scheme of wound healing. Implantation of biomaterial causes tissue injury and activates the mechanisms for tissue repair. Varieties of coordinated processes begin at the moment of wounding and persist until the reparation is completed.
Inflammatory response and tissue healing
The implantation of a biomaterial into the tissue by surgery, injection or insertion causes tissue damage and tissue response, leading to wound healing (Figure 3). This begins at the moment of injury and involves both resident and migratory cell populations, extracellular matrix, and the action of soluble mediators. The mechanisms underlying the processes involve: (I) Inflammatory mediators and growth factors; (II) cell-cell and cell-extracellular matrix interactions that govern cell proliferation, migration and differentiation; (III) events in epithelialization, fibroplasia and angiogenesis; (IV) wound contraction, and (V) remodeling. They are initiated at the time of physical injury and continue throughout the repair process [30-33]. The time taken for a wound to heal can be diverse, and some wounds may take up to a year or more to heal in their entirety (Figure 4). Despite the fact that in all tissues the processes of repair begin immediately after an injury occurs and that all wounds go through similar phases of healing, some specialized tissue types such as those within the liver, skeletal tissue and eye have a distinctive way of regeneration and repair and hence follow separate paths. In addition, there are differences among tissues in the time required to complete regeneration [34,35]. A completely healed wound is defined as one that has lead to normal anatomical structure, function and appearance of the tissue within a reasonable period of time. In contrast, some wounds do not heal in a timely and orderly manner. Multiple systemic and local factors can slow the course of wound healing by causing disturbances in the finely balanced repair processes involved. This results in chronic, non-healing wounds, which are difficult to treat even with the use of biomaterials and biotechnology [30,36].
FIGURE 3.
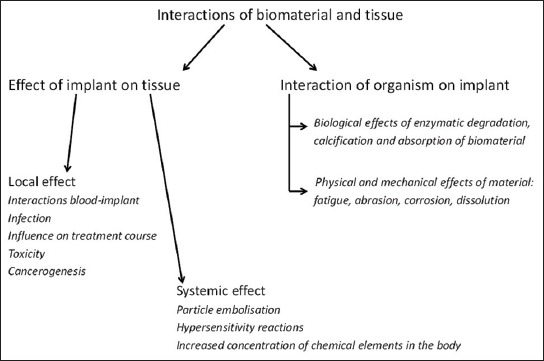
A schematic representation of various interactions between biomaterial and tissue.
FIGURE 4.
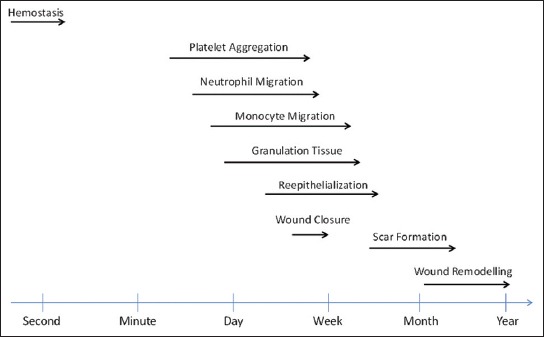
Flow diagram with events in wound healing.
Healing is a complex process involving the interaction between diverse immunological and biological systems. These activities do not occur in a haphazard manner. They occur as a cascade of carefully and precisely regulated steps and events that correlate with the appearance of various cell types during distinct stages of healing (Figure 5) [35,37-39]. The processes of reconstruction of damaged tissue after the implantation of biomaterial may be classified into four phases: (I) Coagulation and hemostasis, which begins immediately after the injury, (II) the inflammatory period (III), the proliferative period, beginning after a few days and represents the main phase of healing, and (IV) scarring [40-43].
FIGURE 5.
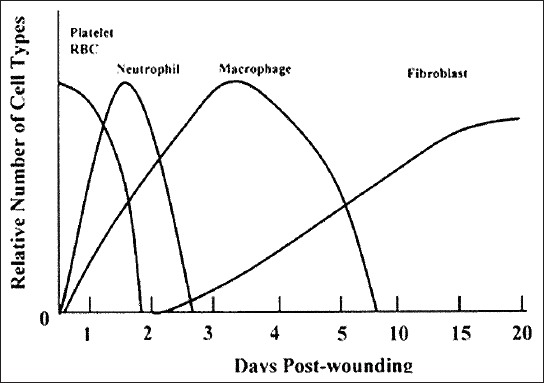
A graphical representation of time scale-emergence of different cell types in the wound and their relative numbers during the healing process.
Coagulation and hemostasis
This period begins in the wound immediately after the injury. The main objective is the prevention of hemorrhage. The second objective is a long-term one: A blood clot that forms will represent a basis for cell invasion in the later stages of healing [32,44]. A dynamic balance between endothelial cells, coagulation factors, platelets and fibrinolytic reactions regulates hemostasis and determines the amount of fibrin deposited in the wound and at the implant, thereby affecting subsequent reparative processes [33].
Adverse factors within the healing process can result in microvascular injury and extravasation of blood into the wound [33,45]. Damaged blood vessels rapidly contract due to the neuronal reflex mechanism through the contraction of smooth muscle cells in the vessel wall. Reflex vasoconstriction may temporarily reduce or even stop the bleeding. After a few minutes, the vascular tone diminishes due to hypoxia and acidemia in the vessel wall, which causes a passive relaxation with the bleeding subsequently starting again. If the insoluble fibrin plug did not form during this time, the vascular mechanisms would have been ineffective in the long-term [32,45,46].
Inflammatory period
Coagulation and hemostasis are followed by the humoral and cellular inflammatory response. The aim of this is the formation of an immune response against the foreign material and potential microorganisms present within the biomaterial, thereby forming an immune barrier around the foreign elements. The inflammatory response to the implant includes a component of acute and chronic inflammation and foreign body reaction with the formation of granulation tissue. Surface characteristics of the biomaterial, devices or implants, tissue regeneration potential, properties of the body and the level of damage during the implantation may all determine the course and the extent of the immune reaction [47,48]. The inflammatory period includes the early and the late inflammatory phases. It first begins in the late stages of the coagulation phase and has numerous functions. It activates the complement cascade and molecular events that eventually lead to the infiltration of the wound with neutrophils, the main task of these being the prevention of infection [48-52]. The late inflammatory phase begins 48-72 hours after the injury. The most important cells here are the macrophages, which continue the late inflammatory process by means of phagocytosis. Macrophages are attracted into the wound by various chemoattractant substances and are important for this late phase inflammatory response. They act as the main regulatory cells, activating keratinocytes, fibroblasts, and endothelial cells [52-55]. The last cells that come into the wound in the late inflammatory period are lymphocytes. They are attracted to the site of injury after 72 hours [48,53].
The proliferative phase
When the action of harmful factor stops, hemostasis is achieved and the immune response is under way. The events in the acute wounds move toward the phases of tissue reconstruction [30,39]. The proliferative phase begins on the 3rd day after wounding and lasts for around 2 weeks. The main characteristic of the proliferative phase is the migration of fibroblasts and the deposition of the newly generated extracellular matrix in the wound, acting as a substitute for the temporary network of fibrin and fibronectin [52-54].
The migration of fibroblasts
The injury acts as a stimulus for fibroblast and myofibroblast proliferation in the vicinity of the wound. Fibroblasts first appear in the wound on the 3rd day after the injury [56,57]. After arriving there, they proliferate vigorously [58,59]. At the end of the 1st week, a large amount of extracellular matrix has already been deposited. This further promotes cell migration and is important for the process of reparation. Now, the fibroblasts change their phenotype into myofibroblasts. Wound contraction now occurs due to the contraction of pseudopodia. This is an important event in the reparative process that approximates the wound edges [59,60].
The synthesis of collagen
Collagens are important components in all stages of healing. Synthesized by fibroblasts, they allow the strength and integrity of the tissue [51]. The collagens play a key role in the proliferative phase of inflammation and in the period of wound remodeling and thus act as a basis for the formation of extracellular matrix in the wound [49,61,62].
Angiogenesis and the formation of granulation tissue
The formation and transformation of new blood vessels occur simultaneously with other steps of the reparative process. In addition to attracting neutrophils and macrophages, growth factors that are secreted in the period of hemostasis also promote angiogenesis [63,64]. Macrophages secrete a number of angiogenic substances and potentiate the proliferation of new endothelial cells. The capillary sprouts from the edges of the wound and grow into the blood clot that was formed in the earlier stages of the healing process (Figure 6). After a few days, a microvascular network forms, composed of a number of new capillaries. Together with collagen, fibrinogen, fibronectin and hyaluronic acid, the macrophages, fibroblasts and vascularized stroma compose the acute granulation tissue, which replaces the temporary fibrin network in the wound gap. With the accumulation of collagen, the density of blood vessels decreases and the granulation tissue matures, leading to the formation of scar tissue [65,66].
FIGURE 6.
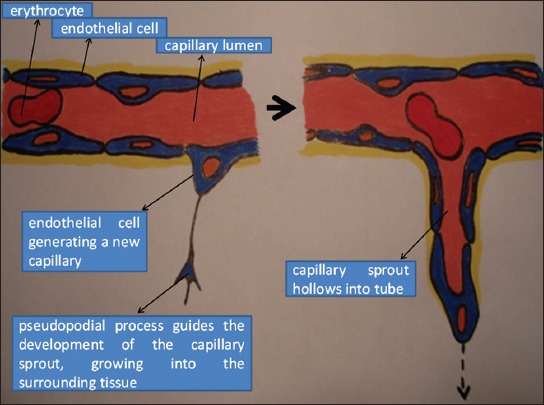
A schematic representation of angiogenesis. New capillary sprouts form from the wall of a pre-existing vessel by migration and proliferation of endothelial cells.
Epithelialization
The migration of epithelial cells takes place from the wound edges. It begins a few hours after wounding. One cell layer is formed first covering the defect. It is accompanied by increased mitotic activity along the wound edges. When the growing and proliferating cells from the wound edges meet, the migration activity stops and the basal membrane starts to form [53,56,57,63].
The formation of scars and phase of wound transformation
This stage of wound transformation is the last phase of wound healing. New epithelium forms and scar tissue is finally rearranged. The synthesis of the extracellular matrix starts simultaneously in the proliferative phase and the stage of wound transformation with the formation of granulation tissue. However, both last for up to 1 or 2 years, sometimes even longer [58,59]. The tensile strength of the wound increases proportionally with the formation and deposition of collagen [67].
The initial accumulation of collagen in the wound is disorganized. Final organization of collagen fibers is achieved in the last stages of wound transformation due to the contraction of the wound. The connective tissue deep in the wound contracts due to the interaction of fibroblasts with the extracellular matrix and approximates the wound edges. The number of fibroblasts and macrophages decrease with time as does the growth of capillaries. The blood flow is gradually reduced and metabolic activity of the scar decreases. The final result is the formation of a mature scar with high tensile strength [67-69].
Foreign body reaction
Foreign body reaction to the implanted biomaterials involves three stages: (I) The incipient period, (II) the phase of progression and (III) the phase of resolution. The main cells during the foreign body reaction are macrophages and giant cells. They are gathered on the surface of the foreign body, surrounded with the granulation tissue, which is composed of fibroblasts, collagen deposits and young capillaries [27]. Macrophages play a central role in the tissue response against the implant. The composition of the implant, on the other hand, also has influence on macrophages, granulation tissue formation and its composition. The flat and smooth surfaces of the implant are surrounded by macrophages in one or two layers, and here fibrosis is the major component of granulation tissue. Implants with a rough surface, such as vascular prostheses, are covered with a layer of macrophages and giant cells with a different amount of granulation tissue. Such a layer may surround the implant throughout its lifetime and may isolate it from the local tissue [21]. The final stage of tissue healing at the implant site is reparation, i.e. the proliferation of connective tissue cells with the formation of the fibrous capsule, isolating the implant or regeneration where damaged tissue is replaced with parenchymal cells as they were before the injury. Usually, both processes are expressed to various extents. After tissue damage, the changes in the growth and differentiation of cells with hypertrophy, hyperplasia, atrophy or metaplasia may occur. At the site of the implant, the tissue may become atrophic due to decreased blood flow or load. Since the implant acts as a foreign body within the tissue, it may inhibit the normal healing process. Therefore, restitution is a rare phenomenon. Local and systemic factors may all affect the final outcome with sufficient blood flow, exposure to infection, concomitant illnesses, medications and health status of the host all potentially playing a role [70,71].
Implant triggered carcinogenesis
Metaplasia of cells can sometimes lead to the carcinogenesis [72]. Foreign body reaction is the basis for an inflammatory reaction with cell proliferation, which would otherwise facilitate incorporation of the implant into the tissue. Prolonged inflammation may accelerate the formation of tumor cells and tumor progression. This is mainly due to the release of reactive oxygen radicals from inflammatory cells, which represent one of the strongest genotoxic mediators and partly due to the promotion of permanent cell proliferation. Vivid proliferation in the inflammatory period may result in the formation of pre-neoplastic cells at the implant site, which is delineated from their surroundings by a fibrous capsule. These calls are thus partly isolated from the organism and its anticancer control. They may divide, grow and finally lose all control mechanisms of proliferation, which is followed by an uncontrolled growth of sarcoma cells [21,72].
Physical characteristics and chemical properties of the foreign bodies have a greater impact on carcinogenesis. Solid implants with a large surface area are potentially the most carcinogenic. This is less pronounced with implants having blunt edges, perforated surface or with fine particles. Chemical induction of tumor formation is a result of the chemical composition of the implanted material [21,70,72].
The immune response
The main pillar of the body defense system is the immune response. It is primarily oriented against microbes. It may be also activated against non-infectious foreign substances. The main task of the immune system is to identify and distinguish foreign molecules from its own. Immunity can be distinguished between innate and acquired immunity, which can be active or passive. The immune response against biomaterials includes the activation of: (I) Humoral and (II) cell components [21,73].
The humoral components form antibodies and are the basis and the complement system. When an organism first comes into contact with the antigen, the specific antibody is raised in the serum after a few days or weeks. The time taken depends on the characteristics, mode of application and dose of the antigen. Immunoglobulin M (IgM) antibodies form first, followed by the IgG, IgA or both. Upon re-encounter with an identical antigen, the antibody response is faster owing to the memory cells sensitized to a specific antigen. The amount of IgM antibodies is the same as during the primary response, the amount of IgG, however, is greater and lasts longer [21,73]. The task of complement is to initiate a localized inflammatory reaction to a foreign body [73-79].
The interaction between the implant, blood and coagulation process
Tissue damage at the implantation side causes damage to blood vessels and activates the homeostatic mechanisms that prevent bleeding. The contact of the foreign material with blood also triggers a similar reaction. In both cases, there is interaction between the implant, vascular surfaces, platelets and coagulation factors. This leads to the formation of a blood clot. The control mechanisms ensure that the process is limited solely to the site of injury leaving the flow of blood in the vicinity undisturbed. Disturbances in this control mechanism may result in progressive blood clotting and the spread of thrombus away from the site of its formation, i.e. on the vessel with the wall replaced with artificial material, resulting in the vessel obstruction [21,32,33,43,44].
The artificial surfaces and damaged blood vessel walls trigger the adhesion of platelets, followed by platelet activation, secretion and aggregation. Platelets adhere to fibrin, which has been formed from fibrinogen. In blood plasma, under the influence of thrombin, blood clot forms. This then further shrinks and approximates the edges of the damaged vessel [33,45-47].
Systemic influence of biomaterials on the organism
Systemic influence of biomaterials on the organism may form due to a number of reasons, such as exaggerated inflammatory reaction, the formation of vasoactive products and the activation of the immune system or due to the direct toxic effects of the implant constituents that are released from it, damaging the target organs. Biomaterials are typically made up of many components, each of which can affect the tissue. Not only is the chemical composition important, but also the shape, size and quantity of particles emitted from the implanted material. Before general clinical use, it is important to test biomaterials on cell cultures and study their cytotoxic effects. Such testing is probably the reason for the relatively low incidence of toxic effects of biomaterials that are used in clinical practice [21,80-83].
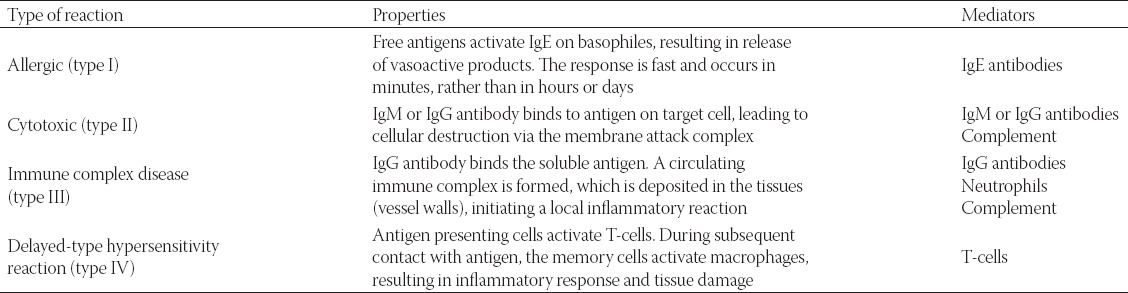
Hypersensitivity reactions concerning biomaterials are mainly described in connection with the fitting of prosthetic implants containing a number of components, including metal, polymers and ceramics such as artificial joints but also in relation to the silicone and collagen structures especially those not of human origin. Collagen especially is a potent antigen. All four types of hypersensitivity reactions are possible (Table 1). Type IV reactions occur most commonly, with Type I and II reactions also occasionally occurring. Type III reactions occur with systems used for the application of active substances into the body that slowly release its components into the tissue [21,12].
TABLE 1.
Summary of hypersensitivity reactions and their properties
Due to the increasing use of biomaterials in modern medicine, graft versus host disease presents one of the most frequent and serious complications in transplantation practice. Over the previous decades, many new therapeutic approaches to early diagnosis, prevention and treatment of graft versus host disease have been implemented. Even so, rejection of the transplant is regarded as one of the leading causes of late mortality. A better understanding of its pathology is aiding the development of biomarkers for the severity of acute graft versus host disease and hence appropriate treatment response. Although corticosteroids are the mainstay of treatment, various modes of disease management are being used in practice such as new immunosuppressive drugs and, in particular, cellular treatments with extracorporeal photopheresis and mesenchymal stem cells [12,84]. In the future, the treatment of graft versus host disease will likely involve multiple modalities, such as enhancement of suppressor cytokines and cellular subsets, modulation of immunologic checkpoints, graft manipulation, modulation of the microbiota and other donor-based prophylaxis strategies [84-88].
CONCLUSIONS
Graft versus host disease is an auto- and allo-immune disorder, exhibiting a variable clinical course. It can manifest in either acute or chronic form, affecting multiple organs and tissues and causing serious complications in clinical practice, both during transplantation and implementation of biocompatible materials.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Rossomando EF. Prosthodontics and implants:From xenodontics to biodontics. Compend Contin Educ Dent. 2007;28(8):418–20. [PubMed] [Google Scholar]
- [2].Binyamin G, Shafi BM, Mery CM. Biomaterials:A primer for surgeons. Semin Pediatr Surg. 2006;15(4):276–83. doi: 10.1053/j.sempedsurg.2006.07.007. http://dx.doi.org/10.1053/j.sempedsurg.2006.07.007 . [DOI] [PubMed] [Google Scholar]
- [3].Latour RA., Jr Future materials for foot surgery. Clin Podiatr Med Surg. 1995;12(3):519–44. [PubMed] [Google Scholar]
- [4].Navarro M, Michiardi A, Castaño O, Planell JA. Biomaterials in orthopaedics. J R Soc Interface. 2008;5(27):1137–58. doi: 10.1098/rsif.2008.0151. http://dx.doi.org/10.1098/rsif.2008.0151 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zim S. Skeletal volume enhancement:Implants and osteotomies. Curr Opin Otolaryngol Head Neck Surg. 2004;12(4):349–56. doi: 10.1097/01.moo.0000130576.04818.55. http://dx.doi.org/10.1097/01.moo.0000130576.04818.55 . [DOI] [PubMed] [Google Scholar]
- [6].Sepulveda P, Jones JR, Hench LL. Bioactive sol-gel foams for tissue repair. J Biomed Mater Res. 2002;59(2):340–8. doi: 10.1002/jbm.1250. http://dx.doi.org/10.1002/jbm.1250 . [DOI] [PubMed] [Google Scholar]
- [7].Courtney JM, Irvine L, Jones C, Mosa SM, Robertson LM, Srivastava S. Biomaterials in medicine - A bioengineering perspective. Int J Artif Organs. 1993;16(3):164–71. [PubMed] [Google Scholar]
- [8].Warren SM, Sylvester K, Chen CM, Hedrick MH, Longaker MT. New directions in bioabsorbable technology. Orthopedics. 2002;25(10 Suppl):s1201–10. doi: 10.3928/0147-7447-20021002-12. http://dx.doi.org/10.3171/spi.2002.97.4.0481 . [DOI] [PubMed] [Google Scholar]
- [9].Wilkinson CD. Making structures for cell engineering. Eur Cell Mater. 2004;22(8):21–6. doi: 10.22203/ecm.v008a03. [DOI] [PubMed] [Google Scholar]
- [10].Ashammakhi N, Ndreu A, Yang Y, Ylikauppila H, Nikkola L, Hasirci V. Tissue engineering:A new take-off using nanofiber-based scaffolds. J Craniofac Surg. 2007;18(1):3–17. doi: 10.1097/01.scs.0000236444.05345.53. http://dx.doi.org/10.1097/01.scs.0000236444.05345.53 . [DOI] [PubMed] [Google Scholar]
- [11].Yang J, Yamato M, Kohno C, Nishimoto A, Sekine H, Fukai F, et al. Cell sheet engineering:Recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26(33):6415–22. doi: 10.1016/j.biomaterials.2005.04.061. http://dx.doi.org/10.1016/j.biomaterials.2005.04.061 . [DOI] [PubMed] [Google Scholar]
- [12].Sigler M, Paul T, Grabitz RG. Biocompatibility screening in cardiovascular implants. Z Kardiol. 2005;94(6):383–91. doi: 10.1007/s00392-005-0231-4. http://dx.doi.org/10.1007/s00392-005-0231-4 . [DOI] [PubMed] [Google Scholar]
- [13].Greisler HP, Petsikas D, Lam TM, Patel N, Ellinger J, Cabusao E, et al. Kinetics of cell proliferation as a function of vascular graft material. J Biomed Mater Res. 1993;27(7):955–61. doi: 10.1002/jbm.820270715. http://dx.doi.org/10.1002/jbm.820270715 . [DOI] [PubMed] [Google Scholar]
- [14].Padera RF, Colton CK. Time course of membrane microarchitecture-driven neovascularization. Biomaterials. 1996;17(3):277–84. doi: 10.1016/0142-9612(96)85565-7. http://dx.doi.org/10.1016/0142-9612(96)85565-7 . [DOI] [PubMed] [Google Scholar]
- [15].Kirkpatrick CJ, Krump-Konvalinkova V, Unger RE, Bittinger F, Otto M, Peters K. Tissue response and biomaterial integration:The efficacy of in vitro methods. Biomol Eng. 2002;19(2-6):211–7. doi: 10.1016/s1389-0344(02)00019-9. http://dx.doi.org/10.1016/S1389-0344(02)00019-9 . [DOI] [PubMed] [Google Scholar]
- [16].Tiwari A, Salacinski H, Seifalian AM, Hamilton G. New prostheses for use in bypass grafts with special emphasis on polyurethanes. Cardiovasc Surg. 2002;10(3):191–7. doi: 10.1016/s0967-2109(02)00004-2. http://dx.doi.org/10.1016/S0967-2109(02)00004-2 . [DOI] [PubMed] [Google Scholar]
- [17].Greisler HP, Cabusao EB, Lam TM, Murchan PM, Ellinger J, Kim DU. Kinetics of collagen deposition within bioresorbable and nonresorbable vascular prostheses. ASAIO Trans. 1991;37(3):M472–5. [PubMed] [Google Scholar]
- [18].Greisler HP, Joyce KA, Kim DU, Pham SM, Berceli SA, Borovetz HS. Spatial and temporal changes in compliance following implantation of bioresorbable vascular grafts. J Biomed Mater Res. 1992;26(11):1449–61. doi: 10.1002/jbm.820261105. http://dx.doi.org/10.1002/jbm.820261105 . [DOI] [PubMed] [Google Scholar]
- [19].Shin H. Fabrication methods of an engineered microenvironment for analysis of cell-biomaterial interactions. Biomaterials. 2007;28(2):126–33. doi: 10.1016/j.biomaterials.2006.08.007. http://dx.doi.org/10.1016/j.biomaterials.2006.08.007 . [DOI] [PubMed] [Google Scholar]
- [20].Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29(12):1517–24. doi: 10.1002/jbm.820291208. http://dx.doi.org/10.1002/jbm.820291208 . [DOI] [PubMed] [Google Scholar]
- [21].Anderson JM, Gristina AG, Hanson SR, Harker LA, Johnson RJ, Meritt K, et al. Host reactions to biomaterials and their evaluation. In: Rattner BD, Hoffman AS, editors. Biomaterials Science. San Diego: Academic Press; 1996. pp. 165–214. http://dx.doi.org/10.1016/B978-0-08-050014-0.50009-2 . [Google Scholar]
- [22].Greisler HP, Dennis JW, Endean ED, Ellinger J, Buttle KF, Kim DU. Derivation of neointima in vascular grafts. Circulation. 1988;78:I6–12. [PubMed] [Google Scholar]
- [23].Tardito E, Biondo B, Caputo V, Freddi G, Grosso E, Mantero S, et al. Biodegradation of dacron vascular prostheses. Physico-chemical, histological, morphometric and ultra-structural study. Minerva Cardioangiol. 1993;41(3):59–80. [PubMed] [Google Scholar]
- [24].Lindenauer SM, Stanley JC, Zelenock GB, Cronenwett JL, Whitehouse WM, Jr, Erlandson EE. Aorto-iliac reconstruction with Dacron double velour. J Cardiovasc Surg (Torino) 1984;25(1):36–42. [PubMed] [Google Scholar]
- [25].Esposito M, Grusovin MG, Worthington HV. Interventions for replacing missing teeth:Antibiotics at dental implant placement to prevent complications. Cochrane Database Syst Rev. 2013;7:CD004152. doi: 10.1002/14651858.CD004152.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Orenstein SB, Saberski ER, Klueh U, Kreutzer DL, Novitsky YW. Effects of mast cell modulation on early host response to implanted synthetic meshes. Hernia. 2010;14(5):511–6. doi: 10.1007/s10029-010-0680-1. http://dx.doi.org/10.1007/s10029-010-0680-1 . [DOI] [PubMed] [Google Scholar]
- [27].Anderson JM. Inflammatory response to implants. Asaio Trans. 1988;34(2):101–7. doi: 10.1097/00002480-198804000-00005. http://dx.doi.org/10.1097/00002480-198804000-00005 . [DOI] [PubMed] [Google Scholar]
- [28].Albrektsson T. Hard tissue implant interface. Aust Dent J. 2008;53(1):34–8. doi: 10.1111/j.1834-7819.2008.00039.x. http://dx.doi.org/10.1111/j.1834-7819.2008.00039.x . [DOI] [PubMed] [Google Scholar]
- [29].Christensen L. Normal and pathologic tissue reactions to soft tissue gel fillers. Dermatol Surg. 2007;33(Suppl 2):S168–75. doi: 10.1111/j.1524-4725.2007.33357.x. http://dx.doi.org/10.1097/00042728-200712001-00009 , http://dx.doi.org/10.1111/j.1524-4725.2007.33357.x . [DOI] [PubMed] [Google Scholar]
- [30].Robson MC, Steed DL, Franz MG. Wound healing:Biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38(2):72–140. doi: 10.1067/msg.2001.111167. http://dx.doi.org/10.1067/msg.2001.111167 . [DOI] [PubMed] [Google Scholar]
- [31].Labler L, Mica L, Härter L, Trentz O, Keel M. Influence of V.A.C.-therapy on cytokines and growth factors in traumatic wounds. Zentralbl Chir. 2006;131(Suppl 1):S62–7. doi: 10.1055/s-2006-921511. http://dx.doi.org/10.1055/s-2006-921511 . [DOI] [PubMed] [Google Scholar]
- [32].Broughton G, 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(Suppl 7):12S–34. doi: 10.1097/01.prs.0000225430.42531.c2. http://dx.doi.org/10.1097/01.prs.0000225430.42531.c2 . [DOI] [PubMed] [Google Scholar]
- [33].Lawrence WT. Physiology of the acute wound. Clin Plast Surg. 1998;25(3):321–40. [PubMed] [Google Scholar]
- [34].Richardson M. Acute wounds:An overview of the physiological healing process. Nurs Times. 2004;100:50–3. [PubMed] [Google Scholar]
- [35].Okan D, Woo K, Ayello EA, Sibbald G. The role of moisture balance in wound healing. Adv Skin Wound Care. 2007;20(1):39–53. doi: 10.1097/00129334-200701000-00013. http://dx.doi.org/10.1097/00129334-200701000-00013 , http://dx.doi.org/10.1097/00129334-200701000-00014 . [DOI] [PubMed] [Google Scholar]
- [36].Strecker-McGraw MK, Jones TR, Baer DG. Soft tissue wounds and principles of healing. Emerg Med Clin North Am. 2007;25(1):1–22. doi: 10.1016/j.emc.2006.12.002. http://dx.doi.org/10.1016/j.emc.2006.12.002 . [DOI] [PubMed] [Google Scholar]
- [37].Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K. Clinical approach to wounds:Débridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg. 2006;117(7 Suppl):72S–109. doi: 10.1097/01.prs.0000225470.42514.8f. http://dx.doi.org/10.1097/01.prs.0000225470.42514.8f . [DOI] [PubMed] [Google Scholar]
- [38].Broughton G, Janis JE, Attinger CE. Wound healing:An overview. Plast Reconstr Surg. 2006;117(Suppl 7):1–32. doi: 10.1097/01.prs.0000222562.60260.f9. http://dx.doi.org/10.1097/01.prs.0000222562.60260.f9 , http://dx.doi.org/10.1097/01.prs.0000205564.61816.11 . [DOI] [PubMed] [Google Scholar]
- [39].Rice J. Secondary intention wound healing - Pathphysiology and management. Collegian. 2000;7(3):40–1. doi: 10.1016/s1322-7696(08)60377-7. http://dx.doi.org/10.1016/S1322-7696(08)60377-7 . [DOI] [PubMed] [Google Scholar]
- [40].Vanwijck R. Surgical biology of wound healing. Bull Mem Acad R Med Belg. 2001;156(3-4):175–84. [PubMed] [Google Scholar]
- [41].Degreef HJ. How to heal a wound fast. Dermatol Clin. 1998;16(2):365–75. doi: 10.1016/s0733-8635(05)70019-x. http://dx.doi.org/10.1016/S0733-8635(05)70019-X . [DOI] [PubMed] [Google Scholar]
- [42].Panuncialman J, Falanga V. The science of wound bed preparation. Surg Clin North Am. 2009;89(3):611–26. doi: 10.1016/j.suc.2009.03.009. http://dx.doi.org/10.1016/j.suc.2009.03.009 . [DOI] [PubMed] [Google Scholar]
- [43].Szycher M, Lee SJ. Modern wound dressings:A systematic approach to wound healing. J Biomater Appl. 1992;7(2):142–213. doi: 10.1177/088532829200700204. http://dx.doi.org/10.1177/088532829200700204 . [DOI] [PubMed] [Google Scholar]
- [44].Pool JG. Normal hemostatic mechanisms:A review. Am J Med Technol. 1977;43(8):776–80. [PubMed] [Google Scholar]
- [45].Whitney JD. Overview:Acute and chronic wounds. Nurs Clin North Am. 2005;40(2):191–205. doi: 10.1016/j.cnur.2004.09.002. http://dx.doi.org/10.1016/j.cnur.2004.09.002 . [DOI] [PubMed] [Google Scholar]
- [46].Natarajan S, Williamson D, Stiltz AJ, Harding K. Advances in wound care and healing technology. Am J Clin Dermatol. 2000;1(5):269–75. doi: 10.2165/00128071-200001050-00002. http://dx.doi.org/10.2165/00128071-200001050-00002 . [DOI] [PubMed] [Google Scholar]
- [47].Jespersen J. Pathophysiology and clinical aspects of fibrinolysis and inhibition of coagulation. Experimental and clinical studies with special reference to women on oral contraceptives and selected groups of thrombosis prone patients. Dan Med Bull. 1988;35(1):1–33. [PubMed] [Google Scholar]
- [48].Hart J. Inflammation 1:Its role in the healing of acute wounds. J Wound Care. 2002;11(6):205–9. doi: 10.12968/jowc.2002.11.6.26411. http://dx.doi.org/10.12968/jowc.2002.11.6.26411 . [DOI] [PubMed] [Google Scholar]
- [49].Steed DL. Wound-healing trajectories. Surg Clin North Am. 2003;83(3):547–55. doi: 10.1016/S0039-6109(02)00208-6. vi-vii. [DOI] [PubMed] [Google Scholar]
- [50].Flanga M. The physiology of wound healing. J Wound Care. 2000;9(6):299–300. doi: 10.12968/jowc.2000.9.6.25994. http://dx.doi.org/10.12968/jowc.2000.9.6.25994 . [DOI] [PubMed] [Google Scholar]
- [51].Edwards SL. Tissue viability:Understanding the mechanisms of injury and repair. Nurs Stand. 2006;21(13):48–56. doi: 10.7748/ns2006.12.21.13.48.c6390. quiz 58. http://dx.doi.org/10.7748/ns2006.12.21.13.48.c6390 . [DOI] [PubMed] [Google Scholar]
- [52].Diegelmann RF, Evans MC. Wound healing:An overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. http://dx.doi.org/10.2741/1184 . [DOI] [PubMed] [Google Scholar]
- [53].Hunt TK, Hopf H, Hussain Z. Physiology of wound healing. Adv Skin Wound Care. 2000;13(2 Suppl):6–11. [PubMed] [Google Scholar]
- [54].Glat PM, Longaker MT. Wound healing. In: Aston SJ, Beasley RW, Thorne CH, editors. Grabb and Smith's Plastic Surgery. 5th ed. Philadelphia: Lippincott; 1997. pp. 3–12. [Google Scholar]
- [55].Sieggreen MY. Healing of physical wounds. Nurs Clin North Am. 1987;22(2):439–47. [PubMed] [Google Scholar]
- [56].Ennis WJ, Meneses P. Wound healing at the local level:the stunned wound. Ostomy Wound Manage. 2000;46(1A Suppl):39S–48S. quiz 49S-50. [PubMed] [Google Scholar]
- [57].Hess CT. Skin care basics. Adv Skin Wound Care. 2000;13:127–8. [PubMed] [Google Scholar]
- [58].Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77(3):509–28. doi: 10.1016/s0039-6109(05)70566-1. http://dx.doi.org/10.1016/S0039-6109(05)70566-1 . [DOI] [PubMed] [Google Scholar]
- [59].Ramasastry SS. Acute wounds. Clin Plast Surg. 2005;32(2):195–208. doi: 10.1016/j.cps.2004.12.001. http://dx.doi.org/10.1016/j.cps.2004.12.001 . [DOI] [PubMed] [Google Scholar]
- [60].Greenhalgh DG. The role of apoptosis in wound healing. Int J Biochem Cell Biol. 1998;30(9):1019–30. doi: 10.1016/s1357-2725(98)00058-2. http://dx.doi.org/10.1016/S1357-2725(98)00058-2 . [DOI] [PubMed] [Google Scholar]
- [61].Walmsley GG, Maan ZN, Wong VW, Duscher D, Hu MS, Zielins ER, et al. Scarless wound healing:Chasing the holy grail. Plast Reconstr Surg. 2015;135(3):907–17. doi: 10.1097/PRS.0000000000000972. http://dx.doi.org/10.1097/PRS.0000000000000972 . [DOI] [PubMed] [Google Scholar]
- [62].Yates CC, Hebda P, Wells A. Skin wound healing and scarring:fetal wounds and regenerative restitution. Birth Defects Res C Embryo Today. 2012;96(4):325–33. doi: 10.1002/bdrc.21024. http://dx.doi.org/10.1002/bdrc.21024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Baum CL, Arpey CJ. Normal cutaneous wound healing:Clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31(6):674–86. doi: 10.1111/j.1524-4725.2005.31612. http://dx.doi.org/10.1097/00042728-200506000-00011 . [DOI] [PubMed] [Google Scholar]
- [64].Servold SA. Growth factor impact on wound healing. Clin Podiatr Med Surg. 1991;8(4):937–53. [PubMed] [Google Scholar]
- [65].Moon JJ, Matsumoto M, Patel S, Lee L, Guan JL, Li S. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J Cell Physiol. 2005;203(1):166–76. doi: 10.1002/jcp.20220. http://dx.doi.org/10.1002/jcp.20220 . [DOI] [PubMed] [Google Scholar]
- [66].Gouverneur M, Berg B, Nieuwdorp M, Stroes E, Vink H. Vasculoprotective properties of the endothelial glycocalyx:Effects of fluid shear stress. J Intern Med. 2006;259(4):393–400. doi: 10.1111/j.1365-2796.2006.01625.x. http://dx.doi.org/10.1111/j.1365-2796.2006.01625.x . [DOI] [PubMed] [Google Scholar]
- [67].Clark RA. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci. 1993;306(1):42–8. doi: 10.1097/00000441-199307000-00011. http://dx.doi.org/10.1097/00000441-199307000-00011 . [DOI] [PubMed] [Google Scholar]
- [68].Mulder GD, Vande Berg JS. Cellular senescence and matrix metalloproteinase activity in chronic wounds. Relevance to debridement and new technologies. J Am Podiatr Med Assoc. 2002;92(1):34–7. doi: 10.7547/87507315-92-1-34. http://dx.doi.org/10.7547/87507315-92-1-34 . [DOI] [PubMed] [Google Scholar]
- [69].Falanga V. Wound healing and chronic wounds. J Cutan Med Surg. 1998;3(Suppl 1):S1-1–5. [PubMed] [Google Scholar]
- [70].Luttikhuizen DT, Harmsen MC, Van Luyn MJ. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006;12(7):1955–70. doi: 10.1089/ten.2006.12.1955. http://dx.doi.org/10.1089/ten.2006.12.1955 . [DOI] [PubMed] [Google Scholar]
- [71].Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. http://dx.doi.org/10.1016/j.smim.2007.11.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Okada F. Beyond foreign-body-induced carcinogensis:Impact of reactive oxigen species derived from inflammatory cells in tumorigenic conversion and tumor progression. Int J Cancer. 2007;121(11):2364–72. doi: 10.1002/ijc.23125. http://dx.doi.org/10.1002/ijc.23125 . [DOI] [PubMed] [Google Scholar]
- [73].Lim HW. The complement system. Activation, modulation, and clinical relevance. Dermatol Clin. 1990;8(4):609–18. [PubMed] [Google Scholar]
- [74].Bohana-Kashtan O, Ziporen L, Donin N, Kraus S, Fishelson Z. Cell signals transduced by complement. Mol Immunol. 2004;41(6-7):583–97. doi: 10.1016/j.molimm.2004.04.007. http://dx.doi.org/10.1016/j.molimm.2004.04.007 . [DOI] [PubMed] [Google Scholar]
- [75].Podack ER, Tschopp J. Membrane attack by complement. Mol Immunol. 1984;21(7):589–603. doi: 10.1016/0161-5890(84)90044-0. http://dx.doi.org/10.1016/0161-5890(84)90044-0 . [DOI] [PubMed] [Google Scholar]
- [76].Müller-Eberhard HJ. The killer molecule of complement. J Invest Dermatol. 1985;85(1 Suppl):47s–52. doi: 10.1111/1523-1747.ep12275445. http://dx.doi.org/10.1111/1523-1747.ep12275445 . [DOI] [PubMed] [Google Scholar]
- [77].Morgan BP. Regulation of the complement membrane attack pathway. Crit Rev Immunol. 1999;19(3):173–98. http://dx.doi.org/10.1615/CritRevImmunol.v19.i3.10 . [PubMed] [Google Scholar]
- [78].Zhou H, Li Q, Zou P, You Y. Endothelial cells:A novel key player in immunoregulation in acute graft-versus-host disease? Med Hypotheses. 2009;72(5):567–9. doi: 10.1016/j.mehy.2008.12.028. http://dx.doi.org/10.1016/j.mehy.2008.12.028 . [DOI] [PubMed] [Google Scholar]
- [79].Biedermann BC. Vascular endothelium and graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21(2):129–38. doi: 10.1016/j.beha.2008.02.003. http://dx.doi.org/10.1016/j.beha.2008.02.003 . [DOI] [PubMed] [Google Scholar]
- [80].Patel ZS, Mikos AG. Angiogenesis with biomaterial-based drug- and cell-delivery systems. J Biomater Sci Polym Ed. 2004;15(6):701–26. doi: 10.1163/156856204774196117. http://dx.doi.org/10.1163/156856204774196117 . [DOI] [PubMed] [Google Scholar]
- [81].Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis:The potential for engineering bone. Eur Cell Mater. 2008;15:100–14. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- [82].Naderi H, Matin MM, Bahrami AR. Review paper:Critical issues in tissue engineering:Biomaterials, cell sources, angiogenesis, and drug delivery systems. J Biomater Appl. 2011;26(4):383–417. doi: 10.1177/0885328211408946. http://dx.doi.org/10.1177/0885328211408946 . [DOI] [PubMed] [Google Scholar]
- [83].Binzen E, Rickert D, Kelch S, Fuhrmann R. Angiogenesis around new AB-polymer networks after one week of implantation in mice. Clin Hemorheol Microcirc. 2003;28(3):183–8. [PubMed] [Google Scholar]
- [84].Dhir S, Slatter M, Skinner R. Recent advances in the management of graft-versus-host disease. Arch Dis Child. 2014;99(12):1150–7. doi: 10.1136/archdischild-2013-304832. http://dx.doi.org/10.1136/archdischild-2013-304832 . [DOI] [PubMed] [Google Scholar]
- [85].Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease:A bench-to-bedside update. Blood. 2014;124(3):363–73. doi: 10.1182/blood-2014-01-514786. http://dx.doi.org/10.1182/blood-2014-01-514786 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Jaglowski SM, Devine SM. Graft-versus-host disease:Why have we not made more progress? Curr Opin Hematol. 2014;21(2):141–7. doi: 10.1097/MOH.0000000000000026. http://dx.doi.org/10.1097/MOH.0000000000000026 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Guenther DA, Madsen JC. Advances in strategies for inducing central tolerance in organ allograft recipients. Pediatr Transplant. 2005;9(3):277–81. doi: 10.1111/j.1399-3046.2005.00308.x. http://dx.doi.org/10.1111/j.1399-3046.2005.00308.x . [DOI] [PubMed] [Google Scholar]
- [88].Adams DH, Afford SC. Effector mechanisms of nonsuppurative destructive cholangitis in graft-versus-host disease and allograft rejection. Semin Liver Dis. 2005;25(3):281–97. doi: 10.1055/s-2005-916320. http://dx.doi.org/10.1055/s-2005-916320 . [DOI] [PubMed] [Google Scholar]


