Abstract
Through Pavlovian conditioning, reward-associated neutral stimuli can acquire incentive salience and motivate complex behaviors. In smokers, cigarette-associated cues may induce cravings and trigger smoking. Understanding the brain mechanisms underlying conditioned responses to cigarette-associated relative to other inherently pleasant stimuli might contribute to the development of more effective smoking cessation treatments that emphasize the rehabilitation of reward circuitry. Here we measured brain responses to geometric patterns (the conditioned stimuli, CSs) predicting cigarette-related, intrinsically pleasant and neutral images (the unconditioned stimuli, USs) using event-related potentials (ERPs) in 29 never-smokers, 20 nicotine-deprived smokers, and 19 non-deprived smokers. Results showed that during US presentation, cigarette-related and pleasant images prompted higher cortical positivity than neutral images over centro-parietal sensors between 400 and 800 ms post-US onset (late positive potential, LPP). The LPP evoked by pleasant images was significantly larger than the LPP evoked by cigarette images. During CS presentation, ERPs evoked by geometric patterns predicting pleasant and cigarette-related images had significantly larger amplitude than ERPs evoked by CSs predicting neutral images. These effects were maximal over right parietal sites between 220 and 240 ms post-CS onset and over occipital and frontal sites between 308 and 344 ms post-CS onset. Smoking status did not modulate these effects. Our results show that stimuli with no intrinsic reward value (e.g., geometric patterns) may acquire rewarding properties through repeated pairings with established reward cues (i.e., cigarette-related, intrinsically pleasant).
Keywords: Event related potentials, ERP, Emotion, Nicotine dependence, Smoking, LPP, Higher-order conditioning
1. Introduction
For smokers, cigarette-related cues are salient stimuli that can induce cravings and trigger smoking behavior (Shiffman et al., 2007; Stewart, 2008). Animal models of drug addiction have demonstrated that drug-related cues acquire incentive salience via Pavlovian conditioning (Flagel et al., 2008; Kruzich et al., 2001; Uslaner et al., 2006). Through Pavlovian conditioning, an organism learns the relationship existing between two events, for example a light being turned on (the conditioned stimulus, CS) and the subsequent delivery of a primary reward, such as food or a drug infusion (the unconditioned stimulus, US; Rescorla, 1988). Once an organism learns the CS–US relationship, CSs can become incentive stimuli and, with their presence, trigger motivational states and addictive behaviors (Meyer et al., 2012; Mucha et al., 1998; Robinson and Berridge, 2001; Winkler et al., 2011).
Human studies assessing peripheral and central nervous system responses to emotional and cigarette-related stimuli have shown that smokers process cigarette-related cues similarly to inherently pleasant stimuli: both categories of stimuli inhibit startle and corrugator electro-myographic responses (Dempsey et al., 2007; Geier et al., 2000), increase activation in brain regions supporting attentional (e.g., the extended visual system) (Dunning et al., 2011; Littel et al., 2012; Minnix et al., 2013; Robinson et al., 2014) and reward processes (e.g., medial prefrontal cortex, striatum) (Engelmann et al., 2012; Sweitzer et al., 2014; Versace et al., 2011a; Wilson et al., 2014). These findings are consistent with the hypothesis that, by being repeatedly associated with nicotine delivery, cigarette-related stimuli acquire enough incentive salience to trigger cravings and may prompt smoking relapse among those trying to quit (e.g., Ferguson and Shiffman, 2009).
Recent studies, however, have reported that never-smokers also show enhanced brain responses to cigarette-related compared with neutral stimuli (Littel and Franken, 2012; McDonough and Warren, 2001; Minnix et al., 2013; Oliver and Drobes, 2012; Robinson et al., 2014). Such findings are puzzling because, unlike smokers, never-smokers should not have experienced the associative learning processes between nicotine and cigarette-related cues that turn them into motivationally relevant stimuli. Because never-smokers tend to respond to cigarette-related cues less than smokers, these authors speculated that for never-smokers, reactivity to cigarette cues is not due to their motivational relevance per se, but rather might be attributed to higher-order evaluative processes (Robinson et al., 2014), to specific perceptual characteristic of cigarette-related stimuli (Minnix et al., 2013; Robinson et al., 2014), to the familiarity that also never-smokers might have for cigarette-related stimuli (Oliver and Drobes, 2012), or to an overall more negative attitude toward smoking cues by never-smokers (Robinson et al., 2014).
Apart from directly recording electrocortical reactivity to cigarette-related cues, another approach to evaluate the level of motivational relevance attributed to cigarette-related cues is to use them in a second-order conditioning procedure. In second-order conditioning, a conditioned stimulus acquires associative strength and elicits a conditioned response by being paired not with a primary reward (e.g., nicotine), but with another conditioned stimulus (called a secondary reinforcer) that holds motivational relevance by having been previously associated with the primary reward (e.g., a cigarette-related cue). Animal models have shown that secondary reinforcers can be effective in maintaining complex behavioral chains even when primary rewards are not consistently delivered (Gewirtz and Davis, 2000; Grabus et al., 2005). These results suggest that cigarette-related stimuli should be effective secondary reinforcers in a second-order conditioning paradigm only to the extent to which they hold motivational relevance. Recently, Littel and Franken (2012) examined electrophysiological responses to geometric figures preceding either cigarette-related or neutral images in smokers and never-smokers. The authors reported that even though the geometric figures predicting cigarette cues were never directly paired with nicotine delivery, they elicited significantly larger positive ERPs than geometric figures predicting neutral cues. These effects were observed in both early (250–280 ms) and late (280–500 ms) time windows. Given that modulation of the ERP amplitude within these time windows is considered an index of enhanced motivated attention (Cuthbert et al., 2000; Hajcak et al., 2010; Lang et al., 1997; Lang and Bradley, 2010; Olofsson et al., 2008; Schupp et al., 2000), the findings by Littel and Franken (2012) support the idea that neutral stimuli can acquire relevance simply by being paired with cigarette-related cues.
A second-order conditioning paradigm might also contribute to clarifying the role that nicotine plays in influencing the level of incentive salience that smokers attribute to non-nicotine-related natural rewards (e.g., food, sex). Neurobiological models of drug addiction emphasize that drug use alters the brain mechanisms involved in the processing of rewards, and suggest that drug-induced amelioration of reward deficits might contribute to addictive disorders and substance abuse (Goldstein and Volkow, 2002; Koob and LeMoal, 2008; Koob and Volkow, 2010; Volkow et al., 2010). Mounting evidence shows that low hedonic capacity (i.e., diminished reactivity to pleasurable stimuli; (Huys et al., 2013) might contribute to both nicotine addiction vulnerability and smoking maintenance (Audrain-McGovern et al., 2012; Rubinstein et al., 2011; Versace et al., 2012). Individuals with low hedonic capacity might have more difficulty quitting smoking because they rely on nicotine to increase the salience of otherwise “bland” natural rewards (de Wit and Phan, 2010). This hypothesis is in line with the dual-reinforcement model of nicotine addiction: nicotine is not only a primary reinforcer, but also acts as a reinforcement enhancer, magnifying the incentive value of stimuli accompanying nicotine delivery (Caggiula et al., 2009). Accordingly, by amplifying the incentive value of natural rewards, nicotine might help individuals with low hedonic capacity normalize their hypo-responsive reward system. Hence, we hypothesized that nicotine deprivation should reduce the capacity of pleasant stimuli to act as secondary reinforcers.
In this study, we recruited never-smokers and smokers under nicotine deprived or sated conditions and measured event-related potentials (ERPs) to geometric patterns (i.e., the conditioned stimuli, CS) preceding the presentation of cigarette-related, intrinsically pleasant (i.e., erotic), or neutral stimuli (i.e., the secondary reinforcers, US). ERPs are a particularly useful measure to explore cognitive processes and attentional biases in addiction, as the amplitude of the ERP provides information regarding the extent of attentional and motivational engagement in the presence of visual stimuli. Both pleasant and unpleasant stimuli lead to higher activity in the primary and secondary visual cortical areas and this activation can be reliably detected over posterior regions of the scalp (Keil et al., 2002; Lang et al., 1997; Liu et al., 2012; Schupp et al., 2000).
Both animal and human studies have shown that the presence of conditioned stimuli increases electrophysiological activity in the visual sensory areas (Ihssen et al., 2007; Shuler and Bear, 2006). Hence, we expected to observe increased cortical reactivity over occipital and parietal regions of the scalp to CSs paired with cigarette-related images in smokers, relative to never-smokers. Further, we expected both smokers and never-smokers to show increased cortical reactivity to CSs paired with inherently pleasant (erotic) images, relative to neutral images. To the extent that nicotine acts as a reinforcement enhancer, we hypothesized higher responses to CSs paired with erotic images in nicotine sated smokers versus nicotine deprived smokers.
2. Methods
2.1. Participants
Participants were recruited from the Houston metropolitan area using flyers, magazine, and newspaper advertisements. The inclusion/exclusion criteria are listed in Table 1. Never-smoking participants were recruited using the same methods and were subject to the same inclusion and exclusion criteria as the smokers, except that they must have smoked less than 100 cigarettes in their lifetime and produce a baseline expired CO less than 4 parts per million (ppm). The “less than 100 lifetime cigarettes” threshold was chosen because it is the oldest (Bondy et al., 2009) and most frequently used criterion for distinguishing never-smokers from ever smokers (CDC, 2011; Gilpin et al., 2004). The number of cigarettes that never-smokers reported smoking ranged from 0 to 10 (mean: 0.9, SD: 2.09).
Table 1.
Inclusion and exclusion criteria for smokers and non-smokers.
| Inclusion criteria |
| Aged 18–50 years |
| Fluent in English |
| Have a working telephone |
| Smokers |
| Smoke ≥5 cigarettes per day |
| Baseline expired carbon monoxide (CO) level >6 parts per million (ppm) |
| Non-smokers |
| Smoked <100 cigarettes in their lifetime |
| Baseline expired CO level <4 ppm |
| Exclusion criteria |
| Current psychiatric disorder (within 6 months, as established by self-report) |
| Current substance abuse (with the exception of smoking) |
| Current participation in a formal smoking-cessation activity |
| Current use of non-cigarette tobacco products (e.g., pipe tobacco, cigars, snuff, chewing tobacco, and hookah) |
| Currently pregnant or breast-feeding |
| Use of psychotropic medication or illicit drug use (within 30 days) |
| History of seizures or a seizure disorder |
| A head injury with a loss of consciousness |
| Visual or auditory problems |
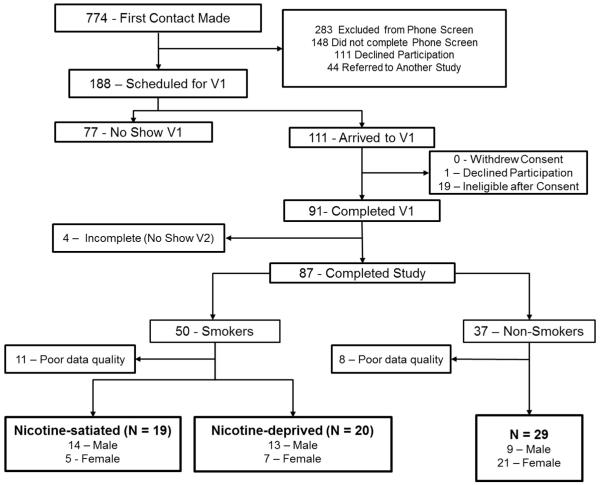
Fig. 1 explains the recruitment process for this study. Due to poor recording quality, largely attributed to excessive movement and eye blink artifacts, nineteen participants were excluded from further analysis. Laboratory data from 19 nicotine-satiated (SMO), 20 nicotine-deprived (DEP), and 29 never-smoking (NEV) participants were included in the final analyses, yielding a total of 68 participants. All participants received monetary compensation for their time and for parking/travel, totaling $60.
Fig. 1.
Diagram of the recruitment process. The final sample is highlighted in bold.
2.2. Procedure
The procedures were approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. All participants were initially screened in a 30 min telephone interview to establish initial eligibility for the study. Eligible participants were then invited to attend an in-person screening visit (V1), where a trained member of the staff explained the study procedures to the potential participants and collected written, informed consent. Biochemical verification of smoking status was assessed by measuring expired carbon monoxide (CO) levels, and patients completed questionnaires regarding demographic, medical, mood, and smoking history (see Table 2). Smokers were then randomized to either the nicotine-satiated or the nicotine-deprived group. Smokers in the nicotine-satiated group were permitted to smoke regularly until the second visit (V2); smokers in the nicotine-deprived group were instructed to observe a 24 hour period of abstinence leading up to the V2 appointment time, which was scheduled between 24 h and 7 days after V1. Participants were informed that smoking abstinence would be verified by a CO test at V2. Upon arrival at V2, all participants provided a CO sample to verify smoking status (successful abstinence was defined by a CO level < 6 ppm) and then completed a battery of questionnaires aimed at assessing current mood state and, for smokers, withdrawal symptoms. After questionnaire completion, smokers in the nicotine-satiated group were asked to smoke one cigarette; smokers in the nicotine-deprived group remained abstinent until after study completion. Participants then completed the EEG recording session (see Section 2.4). Since previous studies have shown that knowledge about the opportunity to smoke a cigarette at the end of the session can alter brain reactivity to cigarette related cues (Wilson et al., 2005), prior to the EEG recording, all smokers were told that they would have the opportunity to smoke a cigarette at the conclusion of the session (i.e., after the SAM rating procedure, see Section 2.4).
Table 2.
Participant demographics and baseline characteristics.
| Non-smokers |
Deprived smokers |
Non-deprived smokers |
|
|---|---|---|---|
| n = 30 | n = 21 | n = 22 | |
| Visit 1 | |||
| Gender | %(N) | %(N) | %(N) |
| Male | 30(9)* | 66(14) | 63(14) |
| Race | |||
| African American, non-Hispanic | 56.6(17) | 54.5(12) | 57.1(12) |
| White, non-Hispanic | 16.6(5) | 31.8(7) | 33.3(7) |
| Other | 26.6(6) | 13.8(3) | 9.5(2) |
| Mean (SE) | Mean (SE) | Mean (SE) | |
| Age | 33.0 (1.7)* | 41.9 (2.0) | 45.9 (2.0) |
| Cigarettes/day | – | 14.8 (1.8) | 18.7 (1.8) |
| FTNDa | – | 4.8 (0.4) | 5.7 (0.4) |
| Visit 2 | |||
| PANAS positive affectb | 37.5 (1.6) | 29.9 (1.9)* | 37.5 (1.9) |
| PANAS negative affectb | 13.2 (1.0) | 16.3 (1.2) | 15.1 (1.2) |
| CES-Dc | 7.9 (1.6) | 9.9 (1.9) | 8.7 (1.9) |
| QSUd | – | 106.0 (13.4)** | 65.1 (13.4) |
Fagerström test for nicotine dependence.
Positive and Negative Affect Scale.
Center for Epidemiologic Studies Depression Scale.
Questionnaire of Smoking Urges.
Denotes a significant difference from both groups, p < 0.004.
Denotes a significant difference from a single group, p < 0.03.
2.3. Self-report measures
In smokers, nicotine dependence was measured using the Fagerström Test for Nicotine Dependence (FTND), a 6-item questionnaire that assesses various components of smoking behavior such as daily intake and time to first cigarette after waking (Fagerström, 1982). The Questionnaire on Smoking Urges — Brief Version (QSU; Cox et al., 2001) was used to measure both desire and intent to smoke and anticipation of relief from negative affect and desire to smoke. In all participants, affect was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D), a 20-item self-report measure developed to assess depressive symptoms in community (non-clinical) populations (Ross and Mirowsky, 1984), as well as the Positive and Negative Affect Scale (PANAS; Watson et al., 1988), comprised of two 10-item mood scales, Positive Affect (PA) and Negative Affect (NA), rated on a scale of 1–5.
2.4. Stimuli and experimental paradigm
During the conditioning phase, participants viewed a series of geometric patterns and picture stimuli presented with a PC computer using E-Prime software (version 2.0.8.74; PST Inc., Pittsburgh, PA) on a 17-in LCD monitor. Picture stimuli consisted of 96 intrinsically pleasant (ERO; erotic couples), 96 cigarette-related (CIG; people smoking, cigarettes, ashtrays), and 96 neutral (NEU; neutral people, household objects) pictures selected in part from the International Affective Picture System (Lang et al., 2005) and other picture collections previously used in our laboratory (Minnix et al., 2013; Robinson et al., 2014), and served as the US. One of three geometric patterns was paired with each of the picture categories, and served as the CS. Picture presentation was pseudo-randomized such that no more than two pictures of the same category were presented consecutively, and CS–US pairings were counterbalanced across participants.
Each trial began with a variable inter-trial interval between 1069 and 2269 ms, during which a white fixation cross was presented in the center of the screen. Subsequently, one of the three geometric patterns was presented in either the upper left or upper right corner of the screen for 469 ms. Following CS presentation, the corresponding ERO, CIG, or NEU US was added to the center of the screen for a duration of 469 ms, and subtended a horizontal visual angle of 7°. See Fig. 2 for a schematic representation of the trial sequence.
Fig. 2.
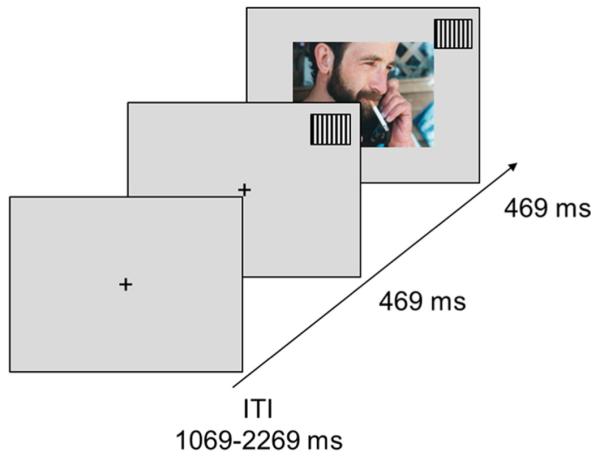
Schematic representation of the experimental paradigm.
The experiment was divided into 12 blocks of 26 trials. Within each block, a total of 8 ERO, 8 CIG, and 8 NEU pictures were paired with the corresponding CS cue. CS cues were presented in either the upper right or upper left corner, and were equally distributed (4 right, 4 left) for each picture category. Two checkerboard targets, one with a right CS cue and one with a left CS cue, were inserted into each block to ensure participants were attending to the screen. Participants were instructed to respond with a speeded button press as soon as the checkerboard target appeared on the screen. The CS cues preceding the checkerboard targets were counterbalanced, such that no one geometric pattern predicted the appearance of the checkerboard target. Thus, within a block, 13 CS cues were presented on the right and 13 CS cues were presented on the left. In total there were 312 trials: 96 erotic, 96 cigarette-related, 96 neutral and 24 checkerboard (target) trials. After each block, participants were given a brief 30 second break. The entire picture presentation lasted approximately 20 min.
Following the experimental session, participants were asked about their awareness of the different geometric patterns and of any contingency between the images and the geometric patterns. Participants were also asked to rate a subset of the images presented during the experiment using a computerized version of the Self-Assessment Manikin (SAM; Lang, 1980) on the dimensions of affective valence and arousal. For consistency with other studies from our laboratory (e.g., J. D. Robinson et al., 2014), an equal number of unpleasant (e.g., mutilation, attack) images were included in the SAM rating procedure. At the conclusion of the experimental session and after all rating procedures and debriefing, smokers were permitted to smoke a cigarette.
2.5. EEG data reduction and analyses
EEG was recorded using a 129-channel Geodesic Sensor System (Geodesic EEG System 250; Electrical Geodesics, Inc., Eugene, OR). After data collection, a 60-Hz low-pass filter was applied off-line. Data were visually inspected, and channels contaminated by artifacts for more than 50% of the recording were interpolated with use of spherical splines. On average, approximately 2% of the channels met this criterion and were interpolated. Eye blinks were then corrected using a spatial filtering method implemented in BESA version 5.1.8.10 (MEGIS Software GmbH, Gräfelfing, Germany), and the EEG data were then transformed to the average reference. The Brain Vision Analyzer (Brain Products GmbH, Munich, Germany) software program was used to extract single epochs from the continuous EEG signal. For the CSs, the epochs started 100 ms before the onset of the geometric pattern and ended 600 ms later; for the USs the epoch started 100 ms before the onset of the images and ended 900 ms later. Using the segmented data, artifacts affecting sensors within specific trials were identified. Artifacts were defined by the following criteria: EEG amplitude above 100 or below −100 μV; absolute voltage difference between any two data points within the segment larger than 100 μV; voltage difference between two contiguous data points above 25 μV and less than 0.5 μV variation for more than 100 ms. A segment was excluded from the subsequent averages if more than 10% of the sensors within the segment were contaminated by artifacts. Overall, fewer than 5% of the segments were excluded.
2.5.1. Unconditioned stimuli (pictures, US)
As a first step and manipulation check, we sought to confirm that the presentation of emotionally arousing (including cigarette-related) images resulted in increased LPP amplitude, a well-established neural signature of emotional relevance typically peaking between 400 and 700 ms post-stimulus onset (e.g., Cuthbert et al., 2000; Keil et al., 2002; Schupp et al., 2000). To be consistent with previous work from our laboratory (Minnix et al., 2013; Robinson et al., 2014; Versace et al., 2012), voltages from 10 centro-parietal electrodes (EGI HydroCel Geodesic Sensor Net 128 sensors: 7, 31, 37, 54, 55, 79, 80, 87, 106, 129) were averaged from 400 to 800 ms for ERO, CIG, and NEU US conditions, for each participant.
2.5.2. Second-order conditioned stimuli (geometric patterns, CS)
To investigate the spatio-temporal dynamics of the cortical activity evoked by conditioned stimuli, we used a multi-step approach that aimed at reducing the risk of inflating the Type I error rate when no a-priori time window or spatial region of interest could be established. First, we conducted a repeated measures Analysis of Variance (ANOVA) at each electrode and each time point using predicted stimulus content (ERO, CIG, NEU). Then we identified the time windows in which the predicted stimulus content main effect was significant at an uncorrected threshold of p < 0.01 in at least 10 electrodes for at least 16 ms. This allowed us to identify two distinct segments of time in which cortical activity was differently modulated by the CSs: a segment extending from 220 to 240 ms post-CS onset and another from 308 to 344 ms post-CS onset. The 220–240 ms time window included 13 right parietal electrodes: 77, 78, 79, 83, 84, 85, 86, 90, 91, 92, 95, 96, and 97. The 308–344 time window included two spatially distinct group of electrodes, one in the occipital region (electrodes: 50, 57, 59, 64, 65, 69, 73, 74, 81, 82, 83, 84, 90, 91, 95), and one in the frontal region (electrodes: 5, 6, 7, 11, 12, 13, 20, 30, 55, 105, 112, 113, 118, 129). To test whether smoking status modulated ERPs to CSs, we computed the mean voltage evoked by each CS within each region of interest and we used these values to test the significance of the interaction group (NEV, SMO, DEP) by stimulus content (ERO, CIG, NEU).
2.6. Statistical analyses
2.6.1. Unconditioned stimuli (pictures, US)
The LPP served as the primary unit of analysis for the US. Mean LPP amplitude between 400 and 800 ms, which was computed for each group (NEV, SMO, DEP) and each stimulus content (ERO, CIG, NEU) using a repeated measures ANOVA using Statistica (v12.0.1133.6; StatSoft, Inc., Tulsa, OK, USA). Where appropriate, significant effects were further evaluated using Bonferroni error corrected pairwise comparisons.
2.6.2. Second-order conditioned stimuli (geometric patterns; CS)
Electrocortical amplitude modulations in response to the CS were analyzed separately for each of the previously defined spatial (parietal, occipital, frontal) ROI (see Section 2.5.2). ANOVAs were computed separately for each time window, first testing for a main effect of stimulus content (ERO, CIG, NEU). An ANOVA was then computed to test for a group by stimulus content interaction, using group (NEV, SMO, DEP) as a between subjects factor and stimulus content (ERO, CIG, NEU) as within subjects factor.
To ensure that the rate at which the CS–US contingency was established did not differ by group, we conducted an ANOVA using block (1, 2, 3, 4) and stimulus content (ERO, CIG, NEU) as within subjects factors and group (NEV, SMO, DEP) as a between subjects factor. To test specifically for differences in ERP amplitude modulation of CS predicting emotional, relative to neutral, stimuli, follow-up analyses were conducted using planned comparisons. ANOVAs were conducted separately for each spatial (parietal, occipital, frontal) ROI.
2.6.3. Participant characteristics and SAM ratings
To compare participant characteristics across sessions and by group, questionnaire and demographic data were analyzed using an ANOVA with session (V1, V2) as within subjects factor and group (NEV, SMO, DEP) as a between subjects factor.
Self-reported hedonic valence and emotional arousal of pictorial stimuli (US) were analyzed separately using ANOVA with group (NEV, SMO, DEP) as a between subjects factor and stimulus content (PLE, CIG, NEU, UNP) as within subjects factor. Post hoc analyses were conducted using Bonferroni univariate error corrected pairwise comparisons.
3. Results
3.1. Participant characteristics
Participant characteristics for both smokers and never-smokers are presented in Table 2. The never-smokers did not differ from smokers on baseline measures of depressive symptomology (as measured by the CES-D) or affect (as measured by the PANAS), but had a significantly different racial composition (p = 0.04) and were, on average, 10.9 years younger (p < 0.0001) than the smoking sample. Although the racial composition differed among never-smokers and current smokers, correlation analyses testing the relationship between observed LPP amplitude modulation (by condition) and participant group were not affected by race (all p > 0.25). To evaluate whether the age difference influenced self-reported measures, we repeated the analyses using age as covariate. The results showed that age did not affect any measures of smoking behavior, nicotine dependence, depressive symptomology, or affect. Within the smoking sample, nicotine-deprived smokers had a significantly lower Positive Affect score on the PANAS (p = 0.004) and a significantly higher score on the QSU (p = 0.04), suggesting that, compared to nicotine-satiated smokers, nicotine-deprived smokers were currently experiencing less pleasure and a greater urge to smoke at the second laboratory session.
To assess whether group differences in self-report are related to the psychophysiological response, normalized values for erotica and cigarette-related US were calculated by deviating the LPP amplitude for erotica and cigarette-related stimuli from neutral using a simple subtraction method (e.g., erotica minus neutral, cigarette-related minus neutral). These values were used to test separately 1) the relationship between the PANAS positive affect scale with LPP amplitude to erotic stimuli (ERO–NEU) in both satiated and deprived smokers, and 2) to test the relationship between the QSU with LPP amplitude to cigarette-related stimuli (CIG–NEU) in both satiated and deprived smokers.
For deprived smokers, there was a weak, negative relationship between the PANAS positive affect scale and the normalized LPP amplitude to erotic stimuli (r(18) = −0.0078, p = 0.974), and a weak, positive relationship between the QSU and the normalized LPP amplitude to cigarette-related stimuli (r(18) = 0.320, p = 0.168). Although non-significant, these correlations are consistent with the self-report data where deprived smokers report significantly lower PANAS positive affect scores than never- and satiated smokers, and likewise report a greater urge to smoke than do satiated smokers.
For satiated smokers, there was a weak, positive relationship between the PANAS positive affect scale and the normalized LPP amplitude to erotic stimuli (r(17) = 0.187, p = 0.44) and a weak, negative relationship between the QSU and the normalized LPP amplitude to cigarette-related stimuli (r(17) = −0.324, p = 0.175). Although non-significant, these correlations are also consistent with the self-report data where satiated smokers report significantly higher PANAS positive affect scores than deprived smokers, and do not report a greater urge to smoke than deprived smokers.
3.2. Unconditioned stimuli (pictures; US)
A main effect of US (F(2, 130) = 151.63, p < 0.0001) was observed over centro-parietal sensors between 400 and 800 ms (Fig. 3). Post-hoc comparisons revealed an effect specific to emotionally arousing USs: the LPP of both ERO (p < 0.0001) and CIG (p = 0.005) pictures prompted higher cortical positivity than NEU over centro-parietal sensors. The LPP evoked by ERO was significantly larger than the LPP evoked by CIG images (p < 0.001). Smoking status did not modulate this effect (Stimulus Content × Group, F(4, 130) = 0.24, p = 0.92).
Fig. 3.
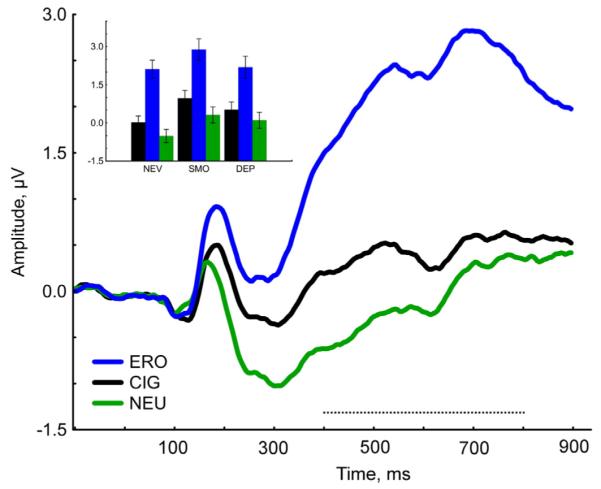
Event-related potentials to erotica, cigarette-related and neutral images (US) averaged across all participant groups. The waveforms represent grand-averages from 10 centro-parietal electrodes (sensors: 7, 31, 37, 54, 55, 79, 80, 87, 129). The black dashed line (400–800 ms) indicates the time window used for the analysis of the late positive potential. Inset: Mean amplitude for erotica, cigarette-related and neutral US averaged between 400 and 800 ms, separately for never-smokers (NEV), nicotine-satiated smokers (SMO) and 24-hr nicotine-deprived smokers (DEP).
3.3. Second-order conditioned stimuli (geometric patterns; CS)
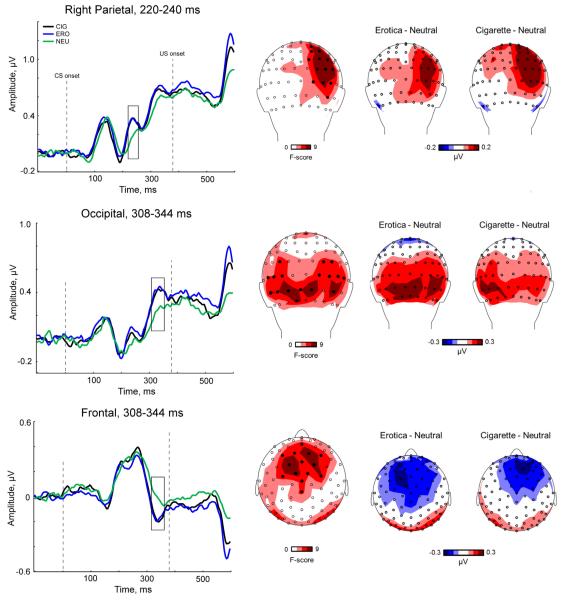
Over right parietal sensors, we observed a significant main effect of stimulus content (F(2, 130) = 14.423, p < 0.0001) between 220 and 240 ms, indicating that CSs preceding emotionally arousing pictures evoked larger regional mean amplitudes compared to CSs preceding neutral, across all participants (Fig. 4, top row). Both ERO (p < 0.0001) and CIG (p < 0.0001) CSs prompted significantly larger ERPs than the amplitude elicited by NEU CSs. Smoking status did not modulate effects observed over right parietal sensors (F(4, 130) = 2.04, p = 0.09), although deprived smokers did show a trend in larger ERPs to CSs elicited by ERO, relative to CIG (p = 0.07).
Fig. 4.
Top row, left: Event-related potentials to the geometric patterns (CS) preceding erotica, cigarette-related and neutral pictures averaged across all participant groups. The waveforms represent grand-averages from a cluster of parietal electrodes during the time region of interest, 220–240 ms, denoted by a black box. Top row, center: Topographical distribution of F-scores between 220–240ms. The black filled circles denote 1) the electrodes used to calculate the grand-averaged waveforms and 2) electrodes with an F-score > 4.75 (p < 0.01). Top row, right: Difference topographies of the mean amplitude for erotica minus neutral (left) and cigarette minus neutral (right) conditions, averaged across all participants between 220–240 ms. Grand-averaged waveforms, topographical distribution of F-scores, and difference topographies are shown for the occipital and frontal electrode clusters during the time region of interest (308–344 ms) on the middle and top rows, respectively. Please note the different scale for difference topographies.
A main effect of stimulus content was also observed over both occipital (F(2, 130) = 12.73, p < 0.0001; Fig. 4, middle row) and frontal (F(2, 130) = 10.42, p < 0.0001; Fig. 4, bottom row) sites between 308 and 344 ms. Post-hoc comparisons revealed that the ERP amplitude elicited by CSs preceding ERO (p < 0.0001) and CIG (p < 0.0002) images over occipital sensors was significantly greater than CSs preceding NEU images. The same pattern of effects were observed for CSs preceding ERO (p < 0.0001) and CIG (p = 0.0008) images with respect to frontal sites. Smoking status did not modulate these effects (Occipital: Stimulus Content × Group, F(4, 130) = 0.74, p = 0.56; Frontal: Stimulus Content × Group, F(4, 130) = 0.24, p = 0.91).
With near complete coverage of the volume conductor and average reference, the overall signal of the evoked potentials must sum to zero; thus, the positive occipital potentials must have a negative frontal counterpart (e.g., Peyk et al., 2009; Schupp et al., 2004). As shown in Fig. 4, the overall topography showed an ERP component with positive polarity over occipital sensors and negative polarity over frontal sites. We observed the hypothesized differences in ERP amplitude modulation over occipital areas as a function of stimulus content, and thus attribute the observed frontal negativity to the volume conductor.
There was no significant block by stimulus content interaction for any of the spatial regions of interest (all p > 0.21). However, in right parietal, occipital and frontal electrode sites, CS predicting emotional US elicited significantly greater ERP amplitudes than did CS predicting neutral US, beginning in Block 1 (see Table 3). These results were not modulated by smoking status (all p > 0.84). These findings suggest that for all groups, the CS–US contingency was effectively established in Block 1 and was maintained throughout the duration of the experiment.
Table 3.
Results of block analysis.
| Right parietal | Occipital | Frontal | |
|---|---|---|---|
| Block × condition × group | F(12, 336) = 0.59, p = .85 | F(12, 336) = 1.14, p = .32 | F(12, 336) = 0.98, p = .47 |
| Block × condition | F(6, 348) = 1.39, p = .22 | F(6, 348) = 0.97, p = .44 | F(6, 348) = 0.90, p = .50 |
| Emotional vs NEU, Block 1 | 0.001 | 0.002 | 0.001 |
| Emotional vs NEU, Block 2 | 0.003 | 0.050 | 0.003 |
| Emotional vs NEU, Block 3 | 0.003 | 0.0001 | 0.006 |
| Emotional vs NEU, Block 4 | 0.137 | 0.032 | 0.002 |
Bold values indicate a significant difference in ERP amplitude elicited by CS predicting emotional stimuli, relative to neutral at the p < 0.05 level.
3.4. SAM ratings
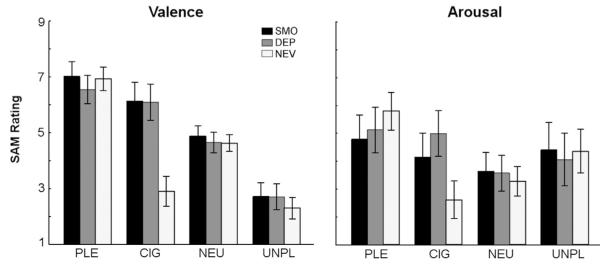
Significant interactions of smoking status and stimulus content (US) emerged for the SAM valence (Group × Content, F(6, 192) = 17.9, p < 0.0001) and arousal (Group × Content, F(6, 192) = 5.6, p < 0.0001) ratings. Post-hoc comparisons indicated that nicotine-satiated and nicotine-deprived smokers rated CIG images as being more pleasant than both NEU (SMO: p = 0.029; DEP: p = 0.0017) and UNP (both p < 0.0001) images, but did not differ significantly in terms of pleasure ratings from PLE (both p < 0.86) images. For never-smokers, PLE images were rated as being more pleasant than NEU (p < 0.0001), whereas CIG and UNP images were both rated as more unpleasant than NEU (both p < 0.0001) images. Ratings did not differ between CIG and UNP images (p > 1).
With respect to arousal, nicotine-deprived smokers rated CIG as significantly more arousing than never-smokers (p < 0.001), whereas nicotine-satiated smokers and never-smokers did not differ in their arousal ratings for CIG (p = 0.3). Never-smokers rated PLE (p < 0.0001) and UNP (p < 0.02) as being significantly more arousing than CIG. See Fig. 5 for smokers' and never-smokers' mean valence and arousal scores.
Fig. 5.
Self-assessment manikin (SAM) ratings on the dimensions of valence (left) and arousal (right), for all participants. Error bars represent the standard error of the mean. PLE = pleasant; CIG = cigarette-related; NEU = neutral; UNPL = unpleasant picture type.
4. Discussion
We used a second-order conditioning paradigm to investigate the level of motivational relevance smokers and never-smokers attribute to cigarette-related cues and non-cigarette-related pleasant stimuli. Conditioned brain responses were obtained by pairing three geometric patterns (CS) with cigarette-related, erotic, and neutral images (US).
With respect to the US, our results replicate previous findings that emotionally arousing stimuli (including drug-related stimuli) elicit greater LPP amplitude relative to neutral stimuli (Dunning et al., 2011; Littel et al., 2012; Minnix et al., 2013; Robinson et al., 2014; Versace et al., 2011b). These findings suggest that the presence of cigarette-related cues engaged cortical processes similar to those involved in the processing of intrinsically motivating stimuli (Minnix et al., 2013; Versace et al., 2011a). Interestingly, LPP amplitude modulation to cigarette-related stimuli was not specific to smokers: we observed enhanced LPP amplitude to cigarette-related images in both smokers and never-smokers. Other studies have reported enhanced brain responses toward cigarette-related cues in never-smokers, albeit less pronounced than those observed in smokers (Minnix et al., 2013; Littel et al., 2012; Robinson et al., 2014; McDonough and Warren, 2001). As a recent meta-analysis summarizing electrophysiological responses to substance-related cues shows (Littel et al., 2012), this finding is not limited to cigarette-related stimuli: although substance users show larger responses than non-users, both groups react to drug-related stimuli more than to neutral ones.
One characteristic of stimuli with motivational relevance is that they can be used as reinforcers in second-order conditioning procedures (Gewirtz and Davis, 2000; Littel et al., 2012). We expected conditioning effects to emerge only for CS–US pairings for which the US was motivationally relevant. In line with this hypothesis, we observed enhanced cortical reactivity to CSs paired with inherently pleasant (i.e., erotic) images over parietal, occipital, and frontal electrode sites in both smokers and never-smokers. Over the same spatial regions, we observed enhanced cortical reactivity to CSs paired with cigarette-related stimuli. However, similar to the LPP findings, enhanced cortical reactivity to cues predicting cigarette-related stimuli was not specific to smokers. These results are in line with those of Littel and Franken (2012), who observed an overall conditioning effect for geometric patterns associated with cigarette-related stimuli across all participants in their study.
Although our findings suggest that cigarette-related cues are relevant stimuli for both smokers and never-smokers, the neurobiological underpinnings of this effect might be different in the two groups: ERPs to pleasant and unpleasant stimuli are comparable (e.g., Bradley et al., 2012; Schupp et al., 2004). With ERPs alone we are unable to discriminate a pleasant from an unpleasant stimulus, and thus cannot determine whether never-smokers react to cigarette-related images because they are interpreted as an unpleasant, versus a pleasant, stimulus. However, the self-report ratings of the stimuli used in this study suggest that cigarette-related stimuli might be relevant to never-smokers because they are unpleasant to them (Fig. 5). Previous exposure to smoking (i.e., second-hand smoke) may have contributed to the unpleasant rating of cigarette-related stimuli and might make them both unpleasant and motivationally significant for never-smokers. Oliver and Drobes (2012) suggest that exposure to cigarette-related cues might be sufficient to bias attention in never smokers. On the other hand, there is also the possibility that never-smokers might have been exposed to cigarette smoke in pleasant contexts, such as at a bar or a party. As a result, cigarette-related cues might be relevant to them because they are linked to positive contextual cues in a longer chain of conditioning.
Similarly to other studies that investigated the effects of deprivation on electrocortical reactivity evoked by drug-related cues (Littel et al., 2012) we did not observe satiation-specific conditioning effects among our smoking sample. However, self-reported ratings of valence and arousal revealed that nicotine-deprived smokers rated the cigarette-related images as more arousing than nicotine-satiated smokers. This finding is in line with the results from the PANAS and the QSU, in which nicotine-deprived smokers reported experiencing less pleasure and a greater urge to smoke, respectively, relative to nicotine-satiated smokers. Further, all smokers rated the cigarette-related images as being as pleasant as naturally rewarding stimuli and more pleasant than did never-smokers.
Because chronic drug use might lead to the attribution of excessive motivational salience toward drugs at the expense of natural rewards (Goldstein and Volkow, 2002; Koob and Volkow, 2010; Volkow et al., 2010), it has been hypothesized that reduced hedonic capacity that addicts might experience while attempting to quit might contribute to relapse (Volkow et al., 2010). Clinical observations seem to support this hypothesis, but empirical studies have reported contradictory results (Fiorino and Phillips, 1999; Goldstein et al., 2010; Grigson and Twining, 2002; Wyvell and Berridge, 2001). In contrast to the SAM ratings, but in line with our previous findings (Robinson et al., 2014), we did not find electrophysiological evidence that would suggest that inherently pleasant stimuli hold less relevance for smokers relative to never-smokers. One important consideration, however, is the possibility that group analyses might mask individual differences in hedonic capacity (i.e., the individual's ability to extract pleasure from natural rewards). Recently, our laboratory reported that only a subset of smokers are characterized by an attenuated LPP response to intrinsically pleasant stimuli, relative to cigarette-related stimuli (Versace et al., 2012, 2011a) and that these differences are associated to reduced blood oxygen-level dependent (BOLD) responses in reward-sensitive areas such as the striatum (Versace et al., 2014). Although the size of our smoking sample did not permit such analyses as those reported in Versace et al. (2012), it will be important for future studies to consider whether differences in hedonic capacity influence second-order conditioning processes.
Another limitation of our study is the significant age difference that we observed between smokers and never-smokers. When we statistically controlled for this difference, the results did not change, however the presence of this difference highlights the challenges that researchers interested in comparing smokers and never-smokers face when repeated measures designs, like in this case, cannot be adopted. Future studies should carefully consider the inclusion criteria to obtain groups more closely matched as far as demographic variables are concerned.
Future studies may also benefit from a within-subjects design. In the present study, we found a trend toward larger ERPs to CSs elicited by erotic, relative to cigarette-related images, in nicotine-deprived smokers. However, the reduction in power resulting from a between-subjects design may have diluted any group-specific effects.
In sum, we observed associative learning for second-order emotionally arousing stimuli in both smokers and never-smokers. All participants, irrespective of smoking status, showed increased electrocortical reactivity for cigarette-related CS and US conditions. Although additional research is warranted, these findings show that through Pavlovian conditioning, stimuli with no intrinsic reward value may acquire motivational relevance, capture attentional resources, and act as secondary reinforcers, contributing to the maintenance of complex behaviors like smoking.
Acknowledgments
The authors thank Kristin Cortese, Jennifer Ng, Aurelija Slapin, and Kimberly Claiborne for help with data collection.
Funding
This work was supported by a Global Research Awards for Nicotine Dependence (GRAND) (WS1895436) grant, an independent competitive grant program supported by Pfizer, to Francesco Versace; and by MD Anderson's Cancer Center Support Grant (CA016672). Menton M. Deweese was supported in part, by a cancer prevention educational award (R25T CA057730, Dr. Shine Chang, Ph.D., Principal Investigator), and by the MD Anderson's Cancer Center Support Grant (CA016672, Ron DePinho, M.D., Principal Investigator) funded by the National Cancer Institute. None of the institutions providing funds for this study had any role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Declaration of interests
FV received grants from the National Institute on Drug Abuse. JDR received grants from the National Institutes of Health, Duncan Family Institute for Cancer Prevention and Risk Assessment, and Cancer Prevention and Research Institute of Texas. PMC served on the scientific advisory board of Pfizer Pharmaceuticals and has received grant support from Pfizer and the National Institute on Drug Abuse.
References
- Audrain-McGovern J, Rodriguez D, Leventhal AM, Cuevas J, Rodgers K, Sass J. Where is the pleasure in that? Low hedonic capacity predicts smoking onset and escalation. Nicotine Tob. Res. 2012;14:1187–1196. doi: 10.1093/ntr/nts017. http://dx.doi.org/10.1093/ntr/nts017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy SJ, Victor JC, Diemert LM. Origin and use of the 100 cigarette criterion in tobacco surveys. Tob. Control. 2009;18:317–323. doi: 10.1136/tc.2008.027276. http://dx.doi.org/10.1136/tc.2008.027276. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Keil A, Lang PJ. Orienting and emotional perception: facilitation, attenuation, and interference. Front. Psychol. 2012;3:493. doi: 10.3389/fpsyg.2012.00493. http://dx.doi.org/10.3389/fpsyg.2012.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. In: Bevins RA, Caggiula AR, editors. The Motivational Impact of Nicotine and Its Role in Tobacco Use, Nebraska Symposium on Motivation 55. Springer-Verlag; New York: 2009. pp. 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Vital signs: current cigarette smoking among adults aged >18 years — United States, 2005–2010. MMWR Morb Mortal Wkly Rep, Morbidity and Mortality Weekly Report. 2011;60:1207–1212. [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res. 2001;3:7–16. doi: 10.1080/14622200020032051. http://dx.doi.org/10.1080/14622200020032051_. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol. Psychol. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. http://dx.doi.org/10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Dempsey JP, Cohen LM, Hobson VL, Randall PK. Appetitive nature of drug cues re-confirmed with physiological measures and the potential role of stage of change. Psychopharmacology. 2007;194:253–260. doi: 10.1007/s00213-007-0839-3. http://dx.doi.org/10.1007/s00213-007-0839-3. [DOI] [PubMed] [Google Scholar]
- de Wit H, Phan L. Positive reinforcement theories of drug use. In: Kassel JD, editor. Substance Abuse and Emotion. The American Psychological Association; Washington DC: 2010. pp. 43–60. [Google Scholar]
- Dunning JP, Parvaz MA, Hajcak G, Maloney T, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users — an ERP study. Eur. J. Neurosci. 2011;33:1716–1723. doi: 10.1111/j.1460-9568.2011.07663.x. http://dx.doi.org/10.1111/j.1460-9568.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. NeuroImage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. http://dx.doi.org/10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström KO. A comparison of psychological and pharmacological treatment in smoking cessation. J. Behav. Med. 1982;5:343–351. doi: 10.1007/BF00846161. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J. Subst. Abus. Treat. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. http://dx.doi.org/10.1016/j.jsat.2008.06.005_. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior in male rats following d-amphetamine-induced behavioral sensitization. Psychopharmacology. 1999;142:200–208. doi: 10.1007/s002130050880. http://dx.doi.org/10.1007/s002130050880. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav. Brain Res. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. http://dx.doi.org/10.1016/j.bbr.2007.07.022_. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology. 2000;150:283–291. doi: 10.1007/s002130000404. http://dx.doi.org/10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. Using Pavlovian higher-order conditioning paradigms to investigate the neural substrates of emotional learning and memory. Learn. Mem. 2000;7:257–266. doi: 10.1101/lm.35200. [DOI] [PubMed] [Google Scholar]
- Gilpin EA, Lee L, Pierce JP. Changes in population attitudes about where smoking should not be allowed: California versus the rest of the USA. Tob. Control. 2004;13:38–44. doi: 10.1136/tc.2003.004739. http://dx.doi.org/10.1136/tc.2003.004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. http://dx.doi.org/10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Moeller SJ, Telang F, Jayne M, Wong C, Wang GJ, Fowler JS, Volkow ND. Liking and wanting of drug and non-drug rewards in active cocaine users: the STRAP-R questionnaire. J. Psychopharmacol. 2010;24:257–266. doi: 10.1177/0269881108096982. http://dx.doi.org/10.1177/0269881108096982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Batman AM, Tyndale RF, Sellers E, Damaj MI. Nicotine physical dependence and tolerance in the mouse following chronic oral administration. Psychopharmacology. 2005;178:183–192. doi: 10.1007/s00213-004-2007-3. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav. Neurosci. 2002;116:321–333. http://dx.doi.org/10.1037/0735-7044.116.2.321. [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Dev. Neuropsychol. 2010;35:129–155. doi: 10.1080/87565640903526504. http://dx.doi.org/10.1080/87565640903526504 (919237754 [pii]) [DOI] [PubMed] [Google Scholar]
- Huys QJ, Pizzagalli DA, Bogdan R, Dayan P. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol. Mood. Anxiety Disord. 2013;3:12. doi: 10.1186/2045-5380-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihssen N, Heim S, Keil A. The costs of emotional attention: affective processing inhibits subsequent lexico-semantic analysis. J. Cogn. Neurosci. 2007;19:1932–1949. doi: 10.1162/jocn.2007.19.12.1932. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. http://dx.doi.org/10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, Congleton KM, See RE. Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav. Neurosci. 2001;115:1086–1092. doi: 10.1037//0735-7044.115.5.1086. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: computer applications. In: Sidowski JB, Johnson JH, William TA, editors. Technology in Mental Health Care Delivery Systems. Ablex; Norwood, NJ: 1980. pp. 119–137. [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Psychology. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. http://dx.doi.org/10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system: technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention; Gainesville, FL: 2005. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: affect, activation, and action. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and Orienting: Sensory and Motivational Processes. Lawrence Erlbaum Associates; Hillsdale, N.J.: 1997. pp. 97–135. [Google Scholar]
- Littel M, Euser AS, Munafo MR, Franken IH. Electrophysiological indices of biased cognitive processing of substance-related cues: a meta-analysis. Neurosci. Biobehav. Rev. 2012;36:1803–1816. doi: 10.1016/j.neubiorev.2012.05.001. http://dx.doi.org/10.1016/j.neubiorev.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Littel M, Franken IH. Electrophysiological correlates of associative learning in smokers: a higher-order conditioning experiment. BMC Neurosci. 2012;13:8. doi: 10.1186/1471-2202-13-8. http://dx.doi.org/10.1186/1471-2202-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Huang H, McGinnis-Deweese M, Keil A, Ding M. Neural substrate of the late positive potential in emotional processing. J. Neurosci. 2012;32:14563–14572. doi: 10.1523/JNEUROSCI.3109-12.2012. http://dx.doi.org/10.1523/JNEUROSCI.3109–12.2012 ([pii] 32/42/14563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough BE, Warren CA. Effects of 12-h tobacco deprivation on event-related potentials elicited by visual smoking cues. Psychopharmacology. 2001;154:282–291. doi: 10.1007/s002130000647. http://dx.doi.org/10.1007/s002130000647. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS ONE. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnix JA, Versace F, Robinson JD, Lam CY, Engelmann JM, Cui Y, Brown VL, Cinciripini PM. The late positive potential (LPP) in response to varying types of emotional and cigarette stimuli in smokers: a content comparison. Int. J. Psychophysiol. 2013;89:18–25. doi: 10.1016/j.ijpsycho.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Pauli P, Angrilli A. Conditioned responses elicited by experimentally produced cues for smoking. Can. J. Physiol. Pharmacol. 1998;76:259–268. [PubMed] [Google Scholar]
- Oliver JA, Drobes DJ. Visual search and attentional bias for smoking cues: the role of familiarity. Exp. Clin. Psychopharmacol. 2012;20:489–496. doi: 10.1037/a0029519. http://dx.doi.org/10.1037/a0029519. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biol. Psychol. 2008;77:247–265. doi: 10.1016/j.biopsycho.2007.11.006. http://dx.doi.org/10.1016/j.biopsycho.2007.11.006 (S0301-0511(07)00191-3 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyk P, Schupp HT, Keil A, Elbert T, Junghofer M. Parallel processing of affective visual stimuli. Psychophysiology. 2009;46:200–208. doi: 10.1111/j.1469-8986.2008.00755.x. http://dx.doi.org/10.1111/j.1469-8986.2008.00755.x (PSYP755 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning. It's not what you think it is. Am. Psychoanal. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Versace F, Engelmann JM, Cui Y, Slapin A, Oum R, Cinciripini PM. The Motivational Salience of Cigarette-related Stimuli Among Former, Never, and Current Smokers. 2014. [DOI] [PMC free article] [PubMed]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J. Components of depressed mood in married men and women. The Center for Epidemiologic Studies' Depression Scale. Am. J. Epidemiol. 1984;119:997–1004. doi: 10.1093/oxfordjournals.aje.a113819. [DOI] [PubMed] [Google Scholar]
- Rubinstein ML, Luks TL, Dryden WY, Rait MA, Simpson GV. Adolescent smokers show decreased brain responses to pleasurable food images compared with nonsmokers. Nicotine Tob. Res. 2011;13:751–755. doi: 10.1093/ntr/ntr046. http://dx.doi.org/10.1093/ntr/ntr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. http://dx.doi.org/10.1111/1469-8986.3720257. [PubMed] [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Hickcox M, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug Alcohol Depend. 2007;91:159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler MG, Bear MF. Reward timing in the primary visual cortex. Science. 2006;311:1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- Stewart J. Review. Psychological and neural mechanisms of relapse. Philosophical transactions of the Royal Society of London. Series B. Biol. Sci. 2008;363:3147–3158. doi: 10.1098/rstb.2008.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Geier CF, Joel DL, McGurrin P, Denlinger RL, Forbes EE, Donny EC. Dissociated effects of anticipating smoking versus monetary reward in the caudate as a function of smoking abstinence. Biol. Psychiat. Alcohol. Smoking. 2014;76:681–688. doi: 10.1016/j.biopsych.2013.11.013. http://dx.doi.org/10.1016/j.biopsych.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav. Brain Res. 2006;169:320–324. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Jackson EF, Costa VD, Robinson JD, Lam CY, Minnix JA, Brown VL, Wetter DW, Cinciripini PM. Do brain responses to emotional images and cigarette cues differ? An fMRI study in smokers. Eur. J. Neurosci. 2011a;34:2054–2063. doi: 10.1111/j.1460-9568.2011.07915.x. http://dx.doi.org/10.1111/j.1460-9568.2011.07915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Robinson JD, Jackson EF, Green CE, Lam CY, Minnix JA, Karam-Hage M, Brown VL, Wetter DW, Cinciripini PM. Pre-quit fMRI responses to pleasant and cigarette cues predict cessation outcome. Nicotine Tob. Res. 2014;16:697–708. doi: 10.1093/ntr/ntt214. http://dx.doi.org/10.1111/j.1460-9568.2011.07915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Lam CY, Engelmann JM, Robinson JD, Minnix JA, Brown VL, Cinciripini PM. Beyond cue reactivity: blunted brain responses to pleasant stimuli predict long term smoking abstinence. Addict. Biol. 2012;17:991–1000. doi: 10.1111/j.1369-1600.2011.00372.x. http://dx.doi.org/10.1111/j.1369-1600.2011.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Minnix JA, Robinson JD, Lam CY, Brown VL, Cinciripini PM. Brain reactivity to emotional, neutral and cigarette-related stimuli in smokers. Addict. Biol. 2011b;16:296–307. doi: 10.1111/j.1369-1600.2010.00273.x. http://dx.doi.org/10.1111/j.1369-1600.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. BioEssays. 2010;32:748–755. doi: 10.1002/bies.201000042. http://dx.doi.org/10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS Scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. http://dx.doi.org/10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Delgado MR, McKee SA, Grigson PS, MacLean RR, Nichols TT, Henry SL. Weak ventral striatal responses to monetary outcomes predict an unwillingness to resist cigarette smoking. Cogn. Affect. Behav. Neurosci. 2014;14:1196–1207. doi: 10.3758/s13415-014-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob. Res. 2005;7:637–645. doi: 10.1080/14622200500185520. http://dx.doi.org/10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler MH, Weyers P, Mucha RF, Stippekohl B, Stark R, Pauli P. Conditioned cues for smoking elicit preparatory responses in healthy smokers. Psychopharmacology. 2011;213:781–789. doi: 10.1007/s00213-010-2033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: Increased cue-triggered “wanting” for sucrose reward. J. Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]