ABSTRACT
The establishment of a functional placenta is pivotal for normal fetal development and the maintenance of pregnancy. In the course of early placentation, trophoblast precursors differentiate into highly invasive trophoblast subtypes. These cells, referred to as extravillous trophoblasts (EVTs), penetrate the maternal uterus reaching as far as the inner third of the myometrium. One of the most fundamental functions of EVTs is the transformation of spiral arteries to establish the uteroplacental blood circulation assuring an adequate nutrient and gas supply to the developing fetus. To achieve this, specialized EVT subpopulations interact with maternal immune cells, provoke elastolysis in the arterial wall and replace the endothelial cells lining the spiral arteries to induce intraluminal vascular remodeling. These and other trophoblast-mediated processes are tightly controlled by paracrine signals from the maternal decidua and furthermore underlie an intrinsic cell-type specific program. Various severe pregnancy complications such as preeclampsia or intrauterine growth retardation are associated with abnormal EVT function, shallow invasion, and decreased blood flow to the placenta. Hence a better understanding of human trophoblast invasion seems mandatory to improve therapeutic intervention. This approach, however, requires a profound knowledge of the human placenta, its various trophoblast subtypes and in particular a better understanding of the regulatory network that controls the invasive phenotype of EVTs.
Keywords: decidua, EVT, invasion, placenta, preeclampsia, trophoblast
Introduction
The objective of this article is to review and discuss our current understanding of regulatory mechanisms that guide and control human trophoblast invasion. Consequently, processes underlying placental formation and those involved in the function of non-invasive trophoblast subpopulations including villous cytotrophoblasts (vCTBs) and syncytiotrophoblasts (STs) will only be discussed in brief. Accordingly, we will firstly provide a short overview on placental development in humans and then focus on discussing formation and function of different human invasive trophoblast subtypes and the regulatory network that controls these cells.
The first cell differentiation event in mammalian development occurs during the transition from the morula to the blastocyst and leads to the establishment of 2 distinct cell lineages: the trophectoderm (TE) and the inner cell mass (ICM). While the latter population gives rise to the embryo, the TE is the origin of trophoblasts, which make up the epithelial compartment of the placenta. According to its basic function, which is to control fetal maternal exchange, the placenta is the prerequisite for the survival of the mammalian embryo. Remarkably, placental structure and diversity of trophoblast subtypes differs greatly among all mammals. Most rodents and primates, including humans, develop a so-called hemochorial placenta, being defined by direct contact between maternal blood and trophoblasts (Fig. 1A). Another feature of hemochorial placentas is their highly invasive placentation reaching deep into the pregnant uterus, referred to as decidua. In humans decidualization is induced by rising progesterone and cyclic AMP levels during the secretory phase of the menstrual cycle and is characterized by extensive remodeling events in the endometrial compartment. These changes include the transformation of spindle-shaped endometrial fibroblasts to large polygonal decidual stromal cells (DSCs) and the secretion of high amounts of insulin-like growth factor 1 and prolactin.1 Vasculogenesis and angiogenesis occur during the proliferative and secretory phase inducing vessel elongation and coiling as well as growth of spiral arteries, respectively.2 Another hallmark of decidualization is the expansion of a unique immune cell population, so-called uterine natural killer (uNK) cells (see below).3-5 Whether uNK cells derive from peripheral tissue sources.6 or from tissue-resident progenitors.7 is still a matter of debate. Human trophoblasts invade the decidua as deep as the inner third of the myometrium and therefore exhibit the most pronounced infiltration of the fetal maternal interface among all eutherian mammals.8 Deep placentation during human pregnancy most likely owes to the exceptionally rapid growth of the fetal brain demanding intensive support with nutrients.9 Trophoblast invasion of the decidua is necessary to establish pivotal changes in the fetal-maternal environment such as anchorage of the placenta to the decidua, remodeling of the vasculature (see below) as well as cross-talk to maternal decidual stromal cells and lymphocytes, including uNK cells.10,11 Failures in placentation and function of trophoblasts during early stages of gestation are associated with numerous pregnancy diseases such as miscarriage, preeclampsia (PE) and intrauterine growth restriction (IUGR).12,13 Indeed, uterine vascularization is already impaired in the first trimester of pregnancy in women who later on develop PE.14-16 PE and IUGR affect approximately 5-8% of pregnancies and may cause severe conditions during pregnancy, including hypertension and proteinuria in the mother as well as decreased fetal growth and may even lead to maternal or fetal death.17,18
In humans, trophoblast-mediated invasion of the decidua is accomplished by a specialized subtype referred to as the extravillous trophoblast (EVT). EVTs arise from a single epithelial cell layer consisting of so-called vCTBs. These cells reside at the basement membrane of placental villi, which separates trophoblasts from the underlying mesenchymal villous core. vCTBs provide a population of progenitor cells that are able to differentiate into 2 distinct entities, the ST and EVT lineage. STs form a continuous multinucleated syncytium that is in direct contact with maternal blood within the intervillous space. These terminally differentiated trophoblasts secrete numerous pregnancy-maintaining hormones including chorionic gonadotropin (hCG), human placental lactogen (hPL), estrogen and progesterone into the maternal circulation.19
Invasive Trophoblast Subtypes and Their Function
EVT formation occurs at sites where placental villi attach to the decidua. These types of villi are named anchoring villi and form so-called cell columns. These consist of highly proliferative proximal trophoblasts, which differentiate into non-dividing trophoblasts at the distal end of the cell column (Fig. 1B). This differential switch is characterized by the upregulation of EVT-specific marker genes including HLA-G,20 integrin α (ITGA) 1 and -5,21 T-cell factor 4 (TCF4).22 as well as a disintegrin and metalloproteinase domain (ADAM) 12.23,24 Upon contacting the decidua, distal cell column trophoblasts start to either infiltrate the decidua as interstitial cytotrophoblasts (iCTBs) or colonize spiral arteries, referred to as endovascular cytotrophoblasts (eCTBs).25 Remarkably, during the first weeks of gestation eCTBs plug spiral arteries, and later on in pregnancy they transform these vessels into larger conduits in order to deliver low-pressure, high blood flow to the growing fetus.11 Trophoblast-mediated occlusion of the arterial openings into the intervillous space is believed to prevent precocious onset of the maternal-placental circulation, which possibly results in oxidative damage of the fetal-placental unit due to the generation of reactive oxygen species.26,27 Indeed, disorganized early onset of blood flow and incomplete plugging of the maternal vessels are features of miscarried pregnancies.28,29 As mentioned above, by the end of the first trimester of pregnancy (10th – 12th week) trophoblast plugs disappear from the spiral arteries and eCTBs replace the vascular endothelium to induce intraluminal remodeling of the spiral arteries.11 This event facilitates a vascular connection between mother and fetus and marks the transition from a histotrophic toward hemotrophic nutrition of the conceptus. Moreover, it is generally believed that EVTs do not invade uterine veins since ephrin B1 expressed on trophoblasts and EPHB4, a receptor associated with venous identity, generate a repulsive signal.30 However, it appears that this hypothesis certainly deserves further attention since some data exist to suggest interaction between uterine veins and trophoblasts in humans and macaques.31,32 iCTB invade the decidua as deep as the first third of the myometrium, where these cells undergo a final differentiation step into multinucleated trophoblasts giant cells.8 It is believed that their formation likely provides a mechanism which prevents deeper penetration into the uterine wall. In addition, these cells produce pregnancy-specific hormones such as hPL and hCG.33 It is still a matter of debate whether giant cells are formed by cellular fusion, aggregation or failed cytokinesis. Since iCTBs tend to form trophoblastic clusters at distal parts of the decidua it may very well be that giant cells are indeed formed by trophoblast aggregation as recently suggested.33 iCTBs also engage in the remodeling process of spiral arteries provoking the transformation of narrow vessels with relatively high resistance into highly dilated, low-resistance conduits. Transformation of the decidual and myometrial spiral arteries involves the interplay of the placenta-derived iCTB as well as maternal uNK cells and macrophages.34 Recent data suggest that macrophages and uNK cells initiate remodeling of spiral arteries in order to disrupt the tightly packed vascular layer of smooth muscle cells and extracellular matrix (ECM).35 In particular uNKs cells were found to secrete an array of factors including angiotensin 1 and 2, vascular growth factor c and metalloproteinases (MMP) promoting ECM degradation and vSMC disorganization.36 In a second wave, iCTBs cause further disruption for instance by the secretion of MMP-12,37 a functional elastolytic protease or by the induction of apoptosis in endothelial as well as smooth muscle cells.38-40 However, whether macrophages and/or uNK are imperative for spiral artery remodeling in humans is currently not known. Previous work in mice shows that depletion of uNK cells results in impaired vascular remodeling rather than loss of pregnancy or failure in implantation.41 The absence of uNK cells in rats delays but does not prevent conversion of spiral arteries. The authors of this study concluded that uNK cells induce angiogenesis and disruption of the arterial smooth muscle layer, thereby controlling uterine oxygen tension and invasive trophoblast differentiation.42 Different to macrophages and uNK cells, iCTBs reside in the arterial wall hence referred to as intramural CTBs (imCTB). Whether imCTBs can breach the vascular basal membrane, convert into eCTBs and replace endothelial cells is not known. The observation that vascular colonization by eCTBs also takes place at sites where no decidual EVTs are detectable speaks against a contribution of imCTBs.11 On the other hand, in vitro studies demonstrate that trophoblasts are capable of perfusing into the lumen of unmodified spiral arteries, obtained from uterine biopsies.43 Another important feature of iCTBs is their ability to interact with immune cells of the fetal-maternal interface. While non-invasive trophoblast subtypes do not express any pattern of human leukocyte antigen (HLA) class I or II molecules, iCTBs show cell surface expression of HLA-E, HLA-G and HLA-C.44 The latter was recently shown to interact with KIR receptors expressed by uNK cells, demonstrating that allo-recognition does occur in the fetal-maternal interface. This interaction is suggested to be critical for reproductive success, since mismatch of HLA-C/KIR haplotypes confers a significantly higher risk for preeclampsia and recurrent abortions.45 Finally, trophoblasts have also been noticed to invade endometrial glands.46 In general, endometrial glands are supposed to play an important role during early pregnancy but represents hitherto a neglected topic in the field of human reproduction. In other eutherian mammals, for instance in the sheep or horse, endometrial gland-derived proteins, lipids and carbohydrates are well characterized as important sources for the histotrophic nutrition of the embryo.47 Indeed, disruption of endometrial gland development in sheep results in faulty implantation and embryonic development, and finally loss of pregnancy.48 A similar important role for endometrial-derived factors during early human pregnancy is likely since endometrial glands have been shown to discharge into the intervillous space.49 Interestingly, it has been recognized that the endometrial secretions during early pregnancy lack sialic acid endcaps. As a result, endometrial gland-derived factors can only be active in the fetal-maternal interface as any of these factors will immediately be removed from the maternal circulation by asiaglycoprotein receptors in the liver.50 At least 2 trophoblast subtypes, the pre-syncytitrophoblast and the iCTBs, may likely coordinate erosion of endometrial glands, which results in the release of glandular secretions into the intervillous space. During early placental development the chorionic sac is surrounded by syncytiotrophoblasts and as this cell layer expands, it erodes the epithelium of adjacent uterine glands.51 Interestingly, iCTB were also found to actively invade the glandular epithelium and occasionally replace glandular epithelial cells. iCTB-mediated invasion of EGs may therefore represent an alternative mechanisms, which may support histotrophic nutrition of the embryo prior to the onset of maternal blood flow. Besides provision of nutrients to the embryo, endometrial gland function may also contribute to placental development and implantation by the secretion of specific growth factors including epidermal growth factor (EGF) and endocrine gland-derived vascular endothelial growth factor.51,52
Insights Into the Control of Trophoblast Invasion
In this chapter we shall discuss observational evidences that have been created to unravel the impact of cell-intrinsic regulation of trophoblast invasion and networks that are established between fetal trophoblasts and decidual tissue resident cells. Signaling pathways and transcription factors that lie downstream of this regulatory network have been reviewed extensively53-55 and thus will not be discussed.
As pointed out above, establishment of the invasive EVT lineage is preceded by the formation of placental cell columns, which proliferate at their proximal ends and, upon expansion, trophoblasts differentiate, acquire an invasive phenotype and invade the decidua. Due to trophoblast-mediated plugging of the uterine vasculature (see above), early placental development occurs in a hypoxic environment, coinciding with a peak in trophoblast proliferation and placental growth.56 This has led to the assumption that hypoxia could be the main trigger of early trophoblast growth and by the end of the first trimester of pregnancy when blood flow is established, normoxia may trigger trophoblast invasion. Indeed, first trimester placental explant cultures cultivated under low oxygen conditions show elevated proliferative activity in trophoblasts.57,58 Moreover, it has further been suggested that low oxygen tension-induced proliferation suppresses EVT differentiation and therefore is a negative regulator of trophoblast invasion. The underlying mechanisms, in support of this model, involve hypoxia-inducible factor 1 α-induced expression of tumor growth factor β 3 (TGFβ3), an inhibitor of trophoblast motility in vitro.59 However, there are various observations and experimental data that speak against this hypothesis. Firstly, this model would suggest that overactivation of the cell cycle in trophoblasts inhibits differentiation and as a consequence invasion. On the contrary, experimental data suggest that trophoblast proliferation is a prerequisite for differentiation. For instance, EGF a potent inducer of trophoblast proliferation also enhances EVT formation and invasion,60 although EVTs are not responsive to EGF as these cells do not express its exclusive receptor EGFR.61 This suggests that expansion of proliferative trophoblast subtypes will also positively affect the conversion into non-dividing, differentiated EVTs. In support of these findings, hyperproliferative complete hydatidiform mole placentas also show accelerated trophoblast differentiation and invasion.22 Finally, experimental hypoxia in the rat was reported to enhance trophoblast invasion and vascular remodeling.62 In conclusion, although it is likely that hypoxia is a driver of trophoblast proliferation, existing data to support the notion that normoxia is a trigger of trophoblast invasion and suppressor of EVT differentiation remain controversial.
Various secreted molecules, such as growth factors, cytokines, and chemokines, which have been extensively reviewed,53,54 are thought to regulate EVT function in an auto- and/or paracrine manner. However, it is unclear to which extent this regulatory network is dependent on an intrinsic EVT-specific program or on signals originating from maternal cells in the decidua. There are various observations that support the notion that the EVTs ability to invade foreign tissue underlies an intrinsic program or at least does not depend on decidua-specific signals. Firstly, isolated trophoblasts, usually containing undifferentiated cytotrophoblasts as well as EVTs, spontaneously invade artificial matrices including fibronectin and Matrigel.22,63 Along these lines, it has been shown that at least a subset of these cells undergo differentiation toward the EVT lineage in vitro.64 Primary trophoblast cultures induce expression of EVT-specific gene signatures when cultivated on extracellular matrices in vitro as demonstrated by the induction of certain integrin signatures, HLA-G or ADAM-12 and downregulation of genes that are specifically expressed by non-invasive trophoblast subtypes such as epidermal growth factor receptor and ITGA6.23,63,64 Besides the proposed intrinsic, pro-invasive program in trophoblasts it may very well be that extracellular matrices used in these assays also affect trophoblast behavior. Interestingly, recent data suggest that isolated HLA-G+ trophoblasts undergo a further differentiation step when cultivated on fibronectin.65 Since fibronectin is expressed and secreted by DSC it may be that decidual contact further enhances differentiation into an invasive phenotype. Interestingly, in contrast to dermal stromal cells, DSCs express serine/arginine-rich protein serine/arginine-rich splicing factor 1 (SRSF1), which leads to more efficient fibronectin bundle deposition into the ECM. The same authors confirm that SRSF1-dependent splicing of fibronectin facilitates trophoblast invasion.66 However, fibronectin is also strongly produced by the EVT itself pointing toward auto –and paracrine mechanisms facilitating fibronectin-driven trophoblast differentiation and/or invasion.67 As pointed out above, isolated trophoblasts are also able to penetrate unmodified arteries from myometrial biopsies.43 In addition, EVT differentiation is unaffected in ectopic pregnancies such as tubal pregnancy, despite the absence of a decidua.68 Lastly, transplanted human villous placental explants invade tissue and vasculature of immuno-deficient SCID mice lacking supportive paracrine pregnancy-specific signals.38
However, there are various evidences in support of a pivotal decidua-specific role in the regulation of trophoblast invasion. For instance, decidua formation is specific to haemochorial placentation and does not occur in non-invasive epithelia chorial placentas.10 In support of this observation, comparative studies in primates show a positive correlation between the degree of decidualization and depth of trophoblast invasion.69 Along these lines, it has been suggested that the decidua plays an important role in the provision of restraining signals, which restrict the invasive EVT in spatiotemporal manner. Lessons from placental pathologies, which display no or defective decidua formation, such as placenta creta or ectopic pregnancies support this theory as these disorders are associated with excessive trophoblast invasion.70 Examples for such anti-invasive cues include DSC-mediated generation of collagen XVIII-derived endostatin or secretion of TGF-β3, both of which are potent inhibitors of trophoblast motility in vitro.63,71 Interestingly, both factors were found to be elevated in preeclamptic patients.72,73 On the other hand, by critically reviewing existing literature it becomes obvious that decidual cells, including uNK cells, macrophages and DSCs not only provide anti-invasive signals but also a myriad of factors that support EVT motility.53 For instance, uNK cells, in contrast to peripheral NK populations, were identified as potent secretors of an array of chemokines, cytokines and growth factors, which induced trophoblast motility both in vivo and in vitro.74 It therefore seems apparent that decidual cells may rather represent a “gatekeeper” function allowing trophoblasts to access maternal tissues and blood supply by achieving an accurate balance between under- and over-invasion. Although the causes of PE remain poorly understood und thus are under intense investigation, impaired trophoblast differentiation, survival and in particular invasion have been attributed to this severe pregnancy-related pathology. In support of this concept, both iCTBs and imCTBs from PE patients were found to be reduced in number,75-78 showed signs of increased apoptosis75 and were more susceptible to cell death when cultivated in vitro.79 It the light of this assumption it is interesting to note, that specific non-favorable phenotypic alterations in trophoblasts isolated from preeclamptic placentas change to control values when cultivated in vitro.80 The authors concluded that the adverse trophoblast phenotype noticed in preeclamptic patients is at least partly induced by the uterine environment, implicating that the pathological phenotype of trophoblasts noticed in PE could be reversed by therapeutically targeting the uterine environment.
In the light of these data, it would be highly interesting to study potential phenotypic alterations in decidual cell populations obtained from complicated pregnancies and, if present, to test their influence on EVT function.
Conclusion
The study of human trophoblast subtype-specific function and their control is challenging since there are various limitations imposed by ethical considerations and the high diversity in placenta development among animal species. Although our knowledge, in particular about early events during trophoblast invasion, remains scarce recent advances and increasing interest in the field promise progress in the near future. In this context, some interesting questions yet to be answered include: • Can imCTBs breach the basal membrane of spiral arteries and thus contributing to intramural remodeling of these vessels? • Do iCTBs and/or imCTBs invade and remodel decidual veins? • Do endometrial glands contribute to early placental development and is iCTB-mediated invasion and replacement of the glandular epithelium of functional relevance? • To which extent does proper EVT function depend on paracrine signals from the decidua? • Are complicated pregnancies with impaired EVT function also associated with adverse decidual cell phenotypes? In summary, further research is necessary to receive a clear picture of EVT subtype-specific functions and in understanding the dynamics in the interplay between trophoblasts and decidual cells (Fig. 1).
Figure 1.
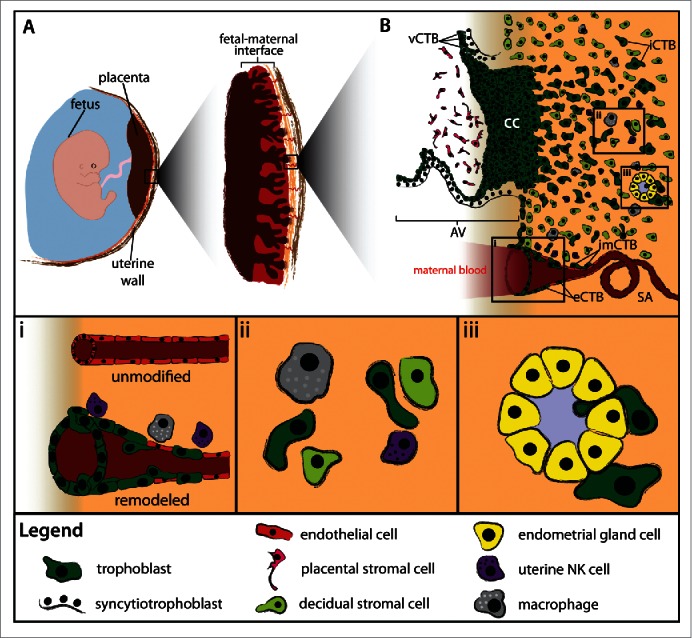
Human trophoblast invasion. (A) The placenta connects the fetus to the uterine wall and establishes a vascular connection between mother and child. The placenta is structured as villous tree and is in direct contact with maternal blood and, thus referred to as hemochorial. The site where the placenta comes in direct contact with the maternal decidua is called the fetal-maternal interface. (B) During early pregnancy, vCTBs fuse to form multinucleated STs, which surround the placental villus. STs transport nutrients and gases from the maternal to the fetal circulation and represent the major endocrine unit of the placenta by secreting hormones such as chorionic gonadotropin, placental growth hormone or placental lactogen. AV form cell columns that attach to the maternal decidua and give rise to the EVT lineage. Invasive EVTs can be divided into iCTBs, which invade the decidual stroma and become terminally differentiated multinucleated GCs, or eCTBs. (i) The latter colonise the lumen of uterine spiral arteries and together with iCTBs, macrophages and uNK cells convert these vessels into larger conduits to guarantee adequate blood flow to the growing fetus. (ii) Various decidual cell types such as macrophages, uNK cells and decidual stromal cells interact with the interstitial cytotrophoblasts in order to control iCTB invasion in an in a spatio-temporal manner. (iii) In addition, iCTBs invade and replace the epithelium of endometrial glands. AV, anchoring villus; CC, cell column; EVT, extravillous cytotrophoblast; eCTB, endovascular cytotrophoblast; iCTB, interstitial cytotrophoblast; SA, spiral artery; ST, syncytiotrophoblast; uNK, uterine natural killer cell.
Abbreviations
- ADAM12
a disintegrin and metalloproteinase 12
- DSC
decidual stromal cell
- ECM
extracellular matrix
- eCTB
endothelial cytotrophoblast
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EVT
extravillous trophoblast
- hCG
human chorionic gonadotropin
- HLA
human leukocyte antigen
- hPL
human placental lactogen
- ICM
inner cell mass
- iCTB
interstitial cytotrophoblast
- imCTB
intramural cytotrophoblast
- ITGA
integrin α
- IUGR
intrauterine growth restriction
- MMP
matrixmetalloproteinase
- PE
preeclampsia
- SRSF1
serine/arginine-rich protein serine/arginine-rich splicing factor 1
- ST
syncytiotrophoblast
- TCF4
t-cell factor 4
- TE
trophectoderm
- TGFβ3
tumor growth factor β3
- uNK
uterine natural killer cell
- vCTB
villous cytotrophoblast.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the Austrian Science Fund (grant P-25187-B13 to J.P.) and the Herzfelder´sche Familienstiftung (grant 00685).
References
- 1.Daly DC, Maslar IA, Riddick DH. Prolactin production during in vitro decidualization of proliferative endometrium. Am J Obstet Gynecol 1983; 145:672-8; PMID:6829654 [DOI] [PubMed] [Google Scholar]
- 2.Maas JW, Groothuis PG, Dunselman GA, de Goeij AF, Struyker Boudier HA, Evers JL. Endometrial angiogenesis throughout the human menstrual cycle. Hum Reprod 2001; 16:1557-61; PMID:11473943; http://dx.doi.org/ 10.1093/humrep/16.8.1557 [DOI] [PubMed] [Google Scholar]
- 3.Starkey PM, Clover LM, Rees MC. Variation during the menstrual cycle of immune cell populations in human endometrium. Eur J Obstet Gynecol Reprod Biol 1991; 39:203-7; PMID:1709605; http://dx.doi.org/ 10.1016/0028-2243(91)90058-S [DOI] [PubMed] [Google Scholar]
- 4.Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod 1991; 6:791-8; PMID:1757516 [DOI] [PubMed] [Google Scholar]
- 5.King A, Loke YW. On the nature and function of human uterine granular lymphocytes. Immunol Today 1991; 12:432-5; PMID:1786078; http://dx.doi.org/ 10.1016/0167-5699(91)90014-K [DOI] [PubMed] [Google Scholar]
- 6.Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD, Koopman LA, Strominger JL. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc Natl Acad Sci USA 2007; 104:3378-83; PMID:17360654; http://dx.doi.org/ 10.1073/pnas.0611098104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vacca P, Vitale C, Montaldo E, Conte R, Cantoni C, Fulcheri E, Darretta V, Moretta L, Mingari MC. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci U S A 2011; 108:2402-7; PMID:21248224; http://dx.doi.org/ 10.1073/pnas.1016257108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980; 1:3-19; PMID:7443635; http://dx.doi.org/ 10.1016/S0143-4004(80)80012-9 [DOI] [PubMed] [Google Scholar]
- 9.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol 2013; 13:133-44; PMID:23334245; http://dx.doi.org/ 10.1038/nri3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol 2006; 6:584-94; PMID:16868549; http://dx.doi.org/ 10.1038/nri1897 [DOI] [PubMed] [Google Scholar]
- 11.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006; 27:939-58; PMID:16490251; http://dx.doi.org/ 10.1016/j.placenta.2005.12.006 [DOI] [PubMed] [Google Scholar]
- 12.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1972; 1:177–91; PMID:4669123 [PubMed] [Google Scholar]
- 13.Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. British J Obstet Gynaecol 1977; 84:656-63; PMID:911717; http://dx.doi.org/ 10.1111/j.1471-0528.1977.tb12676.x [DOI] [PubMed] [Google Scholar]
- 14.Sibiude J, Guibourdenche J, Dionne MD, Le Ray C, Anselem O, Serreau R, Goffinet F, Tsatsaris V. Placental growth factor for the prediction of adverse outcomes in patients with suspected preeclampsia or intrauterine growth restriction. PloS One 2012; 7:e50208; PMID:23209675; http://dx.doi.org/ 10.1371/journal.pone.0050208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11-13 weeks. Prenat Diagn 2011; 31:66-74; PMID:21210481; http://dx.doi.org/ 10.1002/pd.2660 [DOI] [PubMed] [Google Scholar]
- 16.Gebb J, Dar P. Colour Doppler ultrasound of spiral artery blood flow in the prediction of pre-eclampsia and intrauterine growth restriction. Best Pract Res Clin Obstet Gynaecol 2011; 25:355-66; PMID:21377937; http://dx.doi.org/ 10.1016/j.bpobgyn.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 17.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005; 308:1592-4; PMID:15947178; http://dx.doi.org/ 10.1126/science.1111726 [DOI] [PubMed] [Google Scholar]
- 18.Shennan AH, Redman C, Cooper C, Milne F. Are most maternal deaths from pre-eclampsia avoidable? Lancet 2012; 379:1686-7; PMID:22177535; http://dx.doi.org/ 10.1016/S0140-6736(11)60785-X [DOI] [PubMed] [Google Scholar]
- 19.Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocrine Rev 2006; 27:141-69; PMID:16434511; http://dx.doi.org/ 10.1210/er.2005-0011 [DOI] [PubMed] [Google Scholar]
- 20.Ellis SA, Palmer MS, McMichael AJ. Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA Class I molecule. J Immunol 1990; 144:731-5 [PubMed] [Google Scholar]
- 21.Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest 1992; 89:210-22; PMID:1370295; http://dx.doi.org/ 10.1172/JCI115565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollheimer J, Loregger T, Sonderegger S, Saleh L, Bauer S, Bilban M, Czerwenka K, Husslein P, Knöfler M. Activation of the canonical wingless/T-cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol 2006; 168:1134-47; PMID:16565489; http://dx.doi.org/ 10.2353/ajpath.2006.050686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biadasiewicz K, Fock V, Dekan S, Proestling K, Velicky P, Haider S, Knöfler M, Fröhlich C, Pollheimer J. Extravillous trophoblast-associated ADAM12 exerts pro-invasive properties, including induction of integrin β 1-mediated cellular spreading. Biol Reprod 2014; 90:101; PMID:24695627; http://dx.doi.org/ 10.1095/biolreprod.113.115279 [DOI] [PubMed] [Google Scholar]
- 24.Aghababaei M, Perdu S, Irvine K, Beristain AG. A disintegrin and metalloproteinase 12 (ADAM12) localizes to invasive trophoblast, promotes cell invasion and directs column outgrowth in early placental development. Mol Hum Reprod 2014; 20:235-49; PMID:24243624; http://dx.doi.org/ 10.1093/molehr/gat084 [DOI] [PubMed] [Google Scholar]
- 25.Hamilton WJ, Boyd JD. Development of the human placenta in the first three months of gestation. J Anat 1960; 94:297-328; PMID:14399291 [PMC free article] [PubMed] [Google Scholar]
- 26.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat 2009; 215:27-35; PMID:19175804; http://dx.doi.org/ 10.1111/j.1469-7580.2008.00978.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton GJ, Hempstock J, Jauniaux E. Oxygen, early embryonic metabolism and free radical-mediated embryopathies. Reprod Biomed Online 2003; 6:84-96; PMID:12626148; http://dx.doi.org/ 10.1016/S1472-6483(10)62060-3 [DOI] [PubMed] [Google Scholar]
- 28.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 2000; 157:2111-22; PMID:11106583; http://dx.doi.org/ 10.1016/S0002-9440(10)64849-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khong TY, Liddell HS, Robertson WB. Defective haemochorial placentation as a cause of miscarriage: a preliminary study. British J Obstet Gynaecol 1987; 94:649-55; PMID:3620413; http://dx.doi.org/ 10.1111/j.1471-0528.1987.tb03169.x [DOI] [PubMed] [Google Scholar]
- 30.Red-Horse K, Kapidzic M, Zhou Y, Feng KT, Singh H, Fisher SJ. EPHB4 regulates chemokine-evoked trophoblast responses: a mechanism for incorporating the human placenta into the maternal circulation. Development 2005; 132:4097-106; PMID:16107476; http://dx.doi.org/ 10.1242/dev.01971 [DOI] [PubMed] [Google Scholar]
- 31.Blankenship TN, Enders AC, King BF. Trophoblastic invasion and modification of uterine veins during placental development in macaques. Cell Tissue Res 1993; 274:135-44; PMID:7694799; http://dx.doi.org/ 10.1007/BF00327994 [DOI] [PubMed] [Google Scholar]
- 32.Craven CM, Zhao L, Ward K. Lateral placental growth occurs by trophoblast cell invasion of decidual veins. Placenta 2000; 21:160-9; PMID:10736238; http://dx.doi.org/ 10.1053/plac.1999.0449 [DOI] [PubMed] [Google Scholar]
- 33.Al-Lamki RS, Skepper JN, Burton GJ. Are human placental bed giant cells merely aggregates of small mononuclear trophoblast cells? An ultrastructural and immunocytochemical study. Hum Reprod 1999; 14:496-504; PMID:10100001; http://dx.doi.org/ 10.1093/humrep/14.2.496 [DOI] [PubMed] [Google Scholar]
- 34.Harris LK. IFPA Gabor Than Award lecture: Transformation of the spiral arteries in human pregnancy: key events in the remodelling timeline. Placenta 2011; 32 Suppl 2:S154-8; PMID:21167598; http://dx.doi.org/ 10.1016/j.placenta.2010.11.018 [DOI] [PubMed] [Google Scholar]
- 35.Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J pathol 2009; 174:1959-71; PMID:19349361; http://dx.doi.org/ 10.2353/ajpath.2009.080995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD, Baker PN, Robson SC, Bulmer JN. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J 2012; 26:4876-85; PMID:22919072; http://dx.doi.org/ 10.1096/fj.12-210310 [DOI] [PubMed] [Google Scholar]
- 37.Harris LK, Smith SD, Keogh RJ, Jones RL, Baker PN, Knöfler M, Cartwright JE, Whitley GS, Aplin JD. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am J Pathol 2010; 177:2103-15; PMID:20802175; http://dx.doi.org/ 10.2353/ajpath.2010.100182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Red-Horse K, Rivera J, Schanz A, Zhou Y, Winn V, Kapidzic M, Maltepe E, Okazaki K, Kochman R, Vo KC, et al.. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J Clin Invest 2006; 116:2643-52; PMID:16998586; http://dx.doi.org/ 10.1172/JCI27306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keogh RJ, Harris LK, Freeman A, Baker PN, Aplin JD, Whitley GS, Cartwright JE. Fetal-derived trophoblast use the apoptotic cytokine tumor necrosis factor-α-related apoptosis-inducing ligand to induce smooth muscle cell death. Circ Res 2007; 100:834-41; PMID:17322170; http://dx.doi.org/ 10.1161/01.RES.0000261352.81736.37 [DOI] [PubMed] [Google Scholar]
- 40.Harris LK, Keogh RJ, Wareing M, Baker PN, Cartwright JE, Aplin JD, Whitley GS. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism. Am J Pathol 2006; 169:1863-74; PMID:17071607; http://dx.doi.org/ 10.2353/ajpath.2006.060265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod 1997; 56:169-79; PMID:9002646; http://dx.doi.org/ 10.1095/biolreprod56.1.169 [DOI] [PubMed] [Google Scholar]
- 42.Chakraborty D, Rumi MA, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci U S A 2011; 108:16295-300; PMID:21900602; http://dx.doi.org/ 10.1073/pnas.1109478108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cartwright JE, Kenny LC, Dash PR, Crocker IP, Aplin JD, Baker PN, Whitley GS. Trophoblast invasion of spiral arteries: a novel in vitro model. Placenta 2002; 23:232-5; PMID:11945091; http://dx.doi.org/ 10.1053/plac.2001.0760 [DOI] [PubMed] [Google Scholar]
- 44.Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunol 2009; 127:26-39; PMID:19368562; http://dx.doi.org/ 10.1111/j.1365-2567.2008.03019.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L, et al.. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest 2010; 120:4102-10; PMID:20972337; http://dx.doi.org/ 10.1172/JCI43998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moser G, Gauster M, Orendi K, Glasner A, Theuerkauf R, Huppertz B. Endoglandular trophoblast, an alternative route of trophoblast invasion? Analysis with novel confrontation co-culture models. Hum Reprod 2010; 25:1127-36; PMID:20176592; http://dx.doi.org/ 10.1093/humrep/deq035 [DOI] [PubMed] [Google Scholar]
- 47.Spencer TE, Bazer FW. Uterine and placental factors regulating conceptus growth in domestic animals. J Animal Sci 2004; 82 E-Suppl:E4-13; PMID:15471813 [DOI] [PubMed] [Google Scholar]
- 48.Gray CA, Taylor KM, Ramsey WS, Hill JR, Bazer FW, Bartol FF, Spencer TE. Endometrial glands are required for preimplantation conceptus elongation and survival. Biol Reprod 2001; 64:1608-13; PMID:11369585; http://dx.doi.org/ 10.1095/biolreprod64.6.1608 [DOI] [PubMed] [Google Scholar]
- 49.Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metabolism 2002; 87:2954-9; PMID:12050279; http://dx.doi.org/ 10.1210/jcem.87.6.8563 [DOI] [PubMed] [Google Scholar]
- 50.Pepys MB, Rademacher TW, Amatayakul-Chantler S, Williams P, Noble GE, Hutchinson WL, Hawkins PN, Nelson SR, Gallimore JR, Herbert J, et al.. Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure. Proc Natl Acad Sci USA 1994; 91:5602-6; PMID:8202534; http://dx.doi.org/ 10.1073/pnas.91.12.5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burton GJ, Jauniaux E, Charnock-Jones DS. Human early placental development: potential roles of the endometrial glands. Placenta 2007; 28 Suppl A:S64-9; PMID:17349689; http://dx.doi.org/ 10.1016/j.placenta.2007.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hempstock J, Cindrova-Davies T, Jauniaux E, Burton GJ. Endometrial glands as a source of nutrients, growth factors and cytokines during the first trimester of human pregnancy: a morphological and immunohistochemical study. Reprod Biol Endocrinol 2004; 2:58; http://dx.doi.org/ 10.1186/1477-7827-2-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knöfler M, Pollheimer J. IFPA Award in Placentology lecture: molecular regulation of human trophoblast invasion. Placenta 2012; 33 Suppl:S55-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pollheimer J, Knöfler M. Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta 2005; 26 Suppl A:S21-30; PMID:15837062; http://dx.doi.org/ 10.1016/j.placenta.2004.11.013 [DOI] [PubMed] [Google Scholar]
- 55.Knöfler M, Pollheimer J. Human placental trophoblast invasion and differentiation: a particular focus on Wnt signaling. Front Genet 2013; 4:190; PMID:24133501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol 1999; 181:718-24; PMID:10486489; http://dx.doi.org/ 10.1016/S0002-9378(99)70518-1 [DOI] [PubMed] [Google Scholar]
- 57.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science 1997; 277:1669-72; PMID:9287221; http://dx.doi.org/ 10.1126/science.277.5332.1669 [DOI] [PubMed] [Google Scholar]
- 58.Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta 2000; 21 Suppl A:S25-30; PMID:10831118; http://dx.doi.org/ 10.1053/plac.1999.0522 [DOI] [PubMed] [Google Scholar]
- 59.Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest 2000; 105:577-87; PMID:10712429; http://dx.doi.org/ 10.1172/JCI8316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright JK, Dunk CE, Amsalem H, Maxwell C, Keating S, Lye SJ. HER1 signaling mediates extravillous trophoblast differentiation in humans. Biol Reprod 2010; 83:1036-45; PMID:20739666; http://dx.doi.org/ 10.1095/biolreprod.109.083246 [DOI] [PubMed] [Google Scholar]
- 61.Fock V, Plessl K, Fuchs R, Dekan S, Milla SK, Haider S, Fiala C, Knöfler M, Pollheimer J. Trophoblast subtype-specific EGFR/ERBB4 expression correlates with cell cycle progression and hyperplasia in complete hydatidiform moles. Hum Reprod 2015; 30:789-99; PMID:25740878; http://dx.doi.org/ 10.1093/humrep/dev027 [DOI] [PubMed] [Google Scholar]
- 62.Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol 2008; 314:362-75; PMID:18199431; http://dx.doi.org/ 10.1016/j.ydbio.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pollheimer J, Haslinger P, Fock V, Prast J, Saleh L, Biadasiewicz K, Jetne-Edelmann R, Haraldsen G, Haider S, Hirtenlehner-Ferber K, et al.. Endostatin suppresses IGF-II-mediated signaling and invasion of human extravillous trophoblasts. Endocrinology 2011; 152:4431-42; PMID:21933871; http://dx.doi.org/ 10.1210/en.2011-1196 [DOI] [PubMed] [Google Scholar]
- 64.Tarrade A, Lai Kuen R, Malassine A, Tricottet V, Blain P, Vidaud M, Evain-Brion D. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab Invest 2001; 81:1199-211; PMID:11555668; http://dx.doi.org/ 10.1038/labinvest.3780334 [DOI] [PubMed] [Google Scholar]
- 65.Tilburgs T, Crespo AC, van der Zwan A, Rybalov B, Raj T, Stranger B, Gardner L, Moffett A, Strominger JL. Human HLA-G+ extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes. Proc Natl Acad Sci USA 2015; 112:7219-24; PMID:26015573; http://dx.doi.org/ 10.1073/pnas.1507977112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez-Mejia IC, De Toledo M, Della Seta F, Fafet P, Rebouissou C, Deleuze V, Blanchard JM, Jorgensen C, Tazi J, Vignais ML. Tissue-specific and SRSF1-dependent splicing of fibronectin, a matrix protein that controls host cell invasion. Mol Biol Cell 2013; 24:3164-76; PMID:23966470; http://dx.doi.org/ 10.1091/mbc.E13-03-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aplin JD, Haigh T, Jones CJ, Church HJ, Vicovac L. Development of cytotrophoblast columns from explanted first-trimester human placental villi: role of fibronectin and integrin alpha5beta1. Biol Reprod 1999; 60:828-38; PMID:10084955; http://dx.doi.org/ 10.1095/biolreprod60.4.828 [DOI] [PubMed] [Google Scholar]
- 68.Goffin F, Munaut C, Malassine A, Evain-Brion D, Frankenne F, Fridman V, Dubois M, Uzan S, Merviel P, Foidart JM. Evidence of a limited contribution of feto-maternal interactions to trophoblast differentiation along the invasive pathway. Tissue Antigens 2003; 62:104-16; PMID:12889991; http://dx.doi.org/ 10.1034/j.1399-0039.2003.00085.x [DOI] [PubMed] [Google Scholar]
- 69.Ramsey EM, Houston ML, Harris JW. Interactions of the trophoblast and maternal tissues in three closely related primate species. Am J Obstet Gynecol 1976; 124:647-52; PMID:816200 [DOI] [PubMed] [Google Scholar]
- 70.Tantbirojn P, Crum CP, Parast MM. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta 2008; 29:639-45; PMID:18514815; http://dx.doi.org/ 10.1016/j.placenta.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 71.Graham CH, Lala PK. Mechanisms of placental invasion of the uterus and their control. Biochem Cell Biol 1992; 70:867-74; PMID:1297352; http://dx.doi.org/ 10.1139/o92-135 [DOI] [PubMed] [Google Scholar]
- 72.Hirtenlehner K, Pollheimer J, Lichtenberger C, Wolschek MF, Zeisler H, Husslein P, Knöfler M. Elevated serum concentrations of the angiogenesis inhibitor endostatin in preeclamptic women. J Soc Gynecol Investig 2003; 10:412-7; PMID:14519482; http://dx.doi.org/ 10.1016/S1071-5576(03)00142-4 [DOI] [PubMed] [Google Scholar]
- 73.Caniggia I, Grisaru-Gravnosky S, Kuliszewsky M, Post M, Lye SJ. Inhibition of TGF-β 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest 1999; 103:1641-50; PMID:10377170; http://dx.doi.org/ 10.1172/JCI6380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, et al.. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006; 12:1065-74; PMID:16892062; http://dx.doi.org/ 10.1038/nm1452 [DOI] [PubMed] [Google Scholar]
- 75.Genbacev O, DiFederico E, McMaster M, Fisher SJ. Invasive cytotrophoblast apoptosis in pre-eclampsia. Hum Reprod 1999; 14 Suppl 2:59-66; http://dx.doi.org/ 10.1093/humrep/14.suppl_2.59 [DOI] [PubMed] [Google Scholar]
- 76.Naicker T, Khedun SM, Moodley J, Pijnenborg R. Quantitative analysis of trophoblast invasion in preeclampsia. Acta Obstet Et Gynecol Scand 2003; 82:722-9; PMID:12848643; http://dx.doi.org/ 10.1034/j.1600-0412.2003.00220.x [DOI] [PubMed] [Google Scholar]
- 77.Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension 2013; 62:1046-54; PMID:24060885; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.113.01892 [DOI] [PubMed] [Google Scholar]
- 78.Kadyrov M, Kingdom JC, Huppertz B. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am J Obstet Gynecol 2006; 194:557-63; PMID:16458661; http://dx.doi.org/ 10.1016/j.ajog.2005.07.035 [DOI] [PubMed] [Google Scholar]
- 79.Whitley GS, Dash PR, Ayling LJ, Prefumo F, Thilaganathan B, Cartwright JE. Increased apoptosis in first trimester extravillous trophoblasts from pregnancies at higher risk of developing preeclampsia. Am J Pathol 2007; 170:1903-9; PMID:17525258; http://dx.doi.org/ 10.2353/ajpath.2007.070006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Y, Gormley MJ, Hunkapiller NM, Kapidzic M, Stolyarov Y, Feng V, Nishida M, Drake PM, Bianco K, Wang F, et al.. Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J ClinInvest 2013; 123:2862-72; PMID:23934129; http://dx.doi.org/ 10.1172/JCI66966 [DOI] [PMC free article] [PubMed] [Google Scholar]


