abstract
Membrane-type matrix metalloproteinases (MT-MMPs) are a sub-family of zinc-dependent endopeptidases involved in the degradation of the extracellular matrix. Although MT-MMPs have been mainly characterized in tumor biology, they also play a relevant role during pregnancy. Placental MT-MMPs are required for cytotrophoblast migration and invasion of the uterine wall and in the remodeling of the spiral arteries. They are involved in the fusion of cytotrophoblasts to form the syncytiotrophoblast as well as in angiogenesis. All these processes are crucial for establishing and maintaining a successful pregnancy and, thus, MT-MMP activity has to be tightly regulated in time and space. Indeed, a de-regulation of MT-MMP expression has been linked with pregnancy complications such as preeclampsia (PE), fetal growth restriction (FGR), gestational diabetes mellitus (GDM) and was also found in maternal obesity. Here we review what is currently known about MT-MMPs in the placenta, with a focus on their general features, their localization and their involvement in pregnancy disorders.
KEYWORDS: extracellular matrix; fetal growth restriction, MT-MMP; preeclampsia; trophoblast
Introduction
The extracellular matrix (ECM) is a plastic matrix giving structure and grounding for the 3 dimensional organization of tissues. ECM is involved in multiple aspects of cell function including cell proliferation, differentiation, adhesion, migration and invasion.1 Its degradation and remodeling is central for structural and developmental changes. Thus, the establishment of pregnancy as well as embryonic and fetal development requires ECM degradation to allow implantation, placental development, angiogenesis and parturition.2 Vice versa, ECM composition modulates these processes. ECM degradation is tightly regulated since imbalances lead to pregnancy complications.3
Several proteases are involved in ECM degradation during pregnancy. These include serine proteases, cathepsins and matrix metalloproteinases (MMPs).4 MMPs are a family of 24 zinc dependent endopeptidases capable of degrading virtually all ECM components. They have been classified into 5 groups: collagenases, gelatinases, stromelysins, membrane-type MMPs (MT-MMPs) and other MMPs.5,6 This review will focus on MT-MMPs, a subgroup of 6 membrane anchored MMPs: MT1-MMP (MMP14), MT2-MMP (MMP15), MT3-MMP (MMP16), MT4-MMP (MMP17), MT5-MMP (MMP24) and MT6-MMP (MMP25).
Because of their key role in ECM degradation, various MMPs have been studied in depth regarding their function in pregnancy, but the majority of this work has focused on MMP2 and MMP9.7-9 In contrast to secreted MMPs, MT-MMPs are membrane anchored and thus, allow a directed and spatially regulated mode of action. MT-MMPs have been shown to be required for tumor proliferation, invasion and angiogenesis,10 processes that are also taking place in pregnancy, although tightly regulated. However, despite their function in ECM degradation, little is known about the role of MT-MMPs during pregnancy. This review summarizes the function of placental MT-MMPs, focusing on their localization, regulation and their involvement in the pathophysiology of pregnancy.
MT-MMPs: General features
MT-MMP structure
All members of the MMP family share a similar structure differing mainly in their domain organization. These domains include a pro-domain, a catalytic domain, a hinge region and a hemopexin domain.11
MT-MMPs, which are inserted in the membrane, can be further classified into 2 groups: i) MT-MMPs that are anchored by a transmembrane domain followed by a cytoplasmic domain (MT1-, MT2-, MT3- and MT5-MMP), and ii) MT-MMPs that are anchored by a glycosylphosphatidylinositol (GPI)-anchor and which lack a cytoplasmic domain (MT4- and MT6-MMP).12 Figure 1 shows the domain assembly of MT-MMPs.
Figure 1.
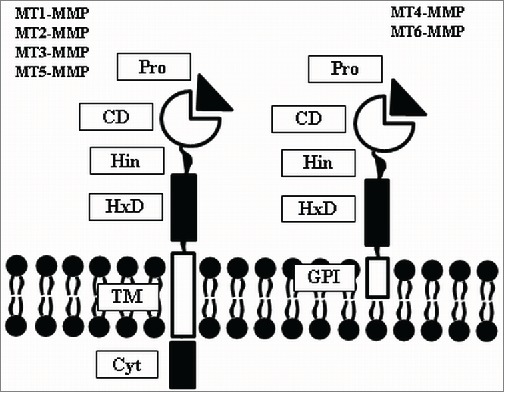
MT-MMP structure. MT-MMPs are anchored to the membrane either via transmembrane domain (MT1-, MT2-, MT3- and MT5-MMP) or via GPI-anchor (MT4- and MT6-MMP). Cyt: cytoplasmic domain; TM: transmembrane domain; GPI: glycophosphatidylinositol; HxD: hemopexin domain; Hin: hinge region; CD: catalytic domain; Pro: pro-domain.
MT-MMPs can function both as monomers or homodimers.13,14 Interestingly, homodimerization has been shown to play an important role in the regulation of MT-MMP activity, e.g. in MMP-2 activation mediated by MT1- and MT2-MMP (section 2.3.).
Although ECM degradation by the catalytic domain is the primary function of MMPs, other functions have been attributed to the rest of the MT-MMP domains. For instance, the hemopexin domain is involved in protein-protein interactions, allowing MT1-MMP oligomerization.15 The cytoplasmic domain can interact with other intracellular proteins, transducing information from the extracellular environment.16 Furthermore, it is thought to regulate MT-MMP intracellular trafficking, degradation and surface distribution.10 The GPI-anchor in MT4- and MT6-MMP is involved in signal transduction as well as in lipid raft localization, and confers their sensitivity to phospholipases.17
MT-MMP activation
MMPs are secreted as inactive zymogens with the protease inhibiting pro-domain located at the C-terminus. The pro-domain contains a cysteine residue that interacts with a zinc ion in the catalytic domain. Disruption of this interaction is required for MMP activation. This occurs in a 2-step process. First, the pro-domain is cleaved in a sequence-specific manner. Then, the intermediate MMP product as well as other proteases can completely remove the pro-domain, resulting in a fully-activated MMP.18 Additionally, MMP activation can also result from a conformational change of the pro-domain, or by activation by reactive oxygen species, which interact with the free cysteine in the pro-domain.19
In contrast to the general activation process of MMPs, MT-MMPs and MMP11, 21 and 28 contain a recognition sequence for Golgi-associated pro-protein convertases, i.e. RXRXKR, with R=Arg, K=Lys and X=non basic amino acid, and are thus activated in the Golgi network prior to secretion.19-21 MT-MMP activation might also occur in 2 steps, since MT1-MMP activation requires a first cleavage in the pro-domain prior to the second cleavage mediated by pro-protein convertases.22 Once in the plasma membrane, active-MT1-MMP can undergo autocatalysis generating an inactive cleavage product, which regulates the pool of active-MT1-MMP present on the cell surface by inhibiting active-MT1-MMP endocytosis.23,24 The membrane anchored MT1-MMP can also be cleaved and shed. Non-autocatalytical shedding results in a soluble active MT1-MMP.25 This shedding of an active MT1-MMP species produces a soluble enzyme and allows MT1-MMP action beyond the surface of the MT1-MMP producing cells.26
MT-MMP activity
MT-MMPs accept a wide array of substrates (Table 1). They degrade various ECM components, including those found in the uterine wall and the spiral arteries, such as fibronectin, vitronectin, collagen IV and laminin.27 Furthermore, MT-MMPs can cleave pro-forms of cytokines including TGF-ß and TNF-α, resulting in their activation.28-30 In addition to their regulation of the immune response, these cytokines also modulate some placental functions. TGF-ß has been shown to inhibit trophoblast invasion.31 TNF-α was able to induce MT1- and MT2-MMP expression in the human first trimester trophoblast cell line ACH-3P and in placental explants from the first trimester.32 However, several studies have also shown that TNF-α limits trophoblast invasion by increased secretion of plasminogen activator inhibitor (PAI1). PAI1 is the principal inhibitor of plasminogen activator (tPA) and urokinase (uPA), both activators of fibrinolysis.33,34 This mechanism would reduce ECM degradation independent of the MMP/TIMP system.
Table 1.
Major substrates of MT-MMP (adapted from 18 with additional data from10,12, 16,17, 36,50. *Contradictory data showing that MT4-MMP and MT6-MMP are able/not able to activate pro-MMP2 17.
| MT-MMPs | ECM substrates | Non-ECM substrates |
|---|---|---|
| MT1-MMP (MMP14) | Aggrecan, collagen I, II, II, dermatan sulfate proteoglycan, entactin, fibrin, fibrillin, fibrinogen, fibronectin, gelatin, laminin-1, -2/4, -5, lumican, nidogen, perlecan, tenascin, vitronectin | α2-Macroglobulin, α1-PI, αvβ3 integrin, β-glycan δ-like 1, ADAM9, ApoE, CD44, CXCL-12, death-receptor 6, EMPRIN, factor XII, ICAM-1, IL-8, KiSS-1, LRP1, MCP-3, myelin-inhibitory protein, progranulin, pro-MMP2, pro-MMP8, pro-MMP13, pro-TGF-β, pro-TNF-α, RANKL, syndecan, tissue transglutaminase |
| MT2-MMP (MMP15) | Aggrecan, collagen I, IV, entactin, gelatin, fibrin, fibronectin, laminin-1, nidogen, perlecan, tenascin | pro-MMP2, pro-MMP13, pro-TNF-α, tissue transglutaminase |
| MT3-MMP (MMP16) | Aggrecan, collagen III, fibronectin, gelatin, laminin-1, vitronectin | α2-Macroglobulin, α1-PtdIns, casein, pro-MMP2, pro-MMP13, syndecan, tissue transglutaminase |
| MT4-MMP (MMP17) | Fibrin, fibrinogen, gelatin | ADAMTS4, KiSS-1, pro-TNF-α, pro-MMP2* |
| MT5-MMP (MMP24) | Chondroitin sulfate proteoglycan, dermatan sulfate proteoglycan, gelatin, fibronectin, plasminogen | pro-MMP2, pro-MMP13, tissue transglutaminase |
| MT6-MMP (MMP25) | Chondroitin sulfate proteoglycan, collagen IV, dermatan sulfate proteoglycan, fibrin, fibrinogen, fibronectin, gelatin, laminin | α1-PI, CCL2, CCL7, CCL13, CCL15, CCL23, CXCL2, CXCL5, CXCL12, pro-MMP2*, pro-MMP9, urokinase plasminogen activator receptor, vimentin |
Besides ECM degradation and cytokine activation, MT-MMPs can also inactivate cytokines,35 activate other MMPs such as MMP2, 8, 9 and 1330,36 as well as other proteases such as ADAM9 (a disintegrin and metalloproteinase 9)12 and ADAMTS4 (ADAM with thrombospondin motifs 4).37
Pro-MMP2 activation by MT1-MMP has been thoroughly studied, revealing a complex mechanism where TIMP2 (tissue inhibitor of MMPs 2), a natural MMP inhibitor, is also required. Once located on the membrane, MT1-MMP forms a homodimer. One MT1-MMP of this dimer binds TIMP2, which will subsequently bind pro-MMP2.23 Then, the other MT1-MMP molecule of the dimer can cleave pro-MMP2 and produce active MMP2. This mechanism is dependent on TIMP2 concentration and occurs only at basal TIMP2 levels. High TIMP2 levels inhibit both, MMP2 and MT1-MMP.38 Interestingly, MT2-MMP can activate pro-MMP2 without interaction with TIMP2. This mechanism also involves an MT2-MMP dimer, of which one MT2-MMP molecule binds pro-MMP2 directly, followed by activation by the other MT2-MMP molecule.39 Contradictory data regarding MMP2 activation have been reported for the other 3 MT-MMP members.17
MT-MMP regulation
It has been hypothesized that the different members of the MMP family originate from gene duplication, as they are widely spread across several chromosomes.21 However, promoter architecture highly differs among distinct MMPs, which allows discriminative regulation at the level of gene expression.
Based on basic promoter conformation, i.e., the distribution of the cis-elements along the promoter, MMPs are classified into 3 different groups: i) MMPs containing a TATA box at -30 bp and a proximal binding site for the transcription factor activator protein-1 (AP-1); ii) MMPs containing a TATA box but no proximal AP-1 binding site; and iii) MMPs lacking both, TATA box and AP-1 binding site.40,41
MT-MMPs belong to the third group, i.e., they lack a TATA-box and an AP-1 binding site, with the exception of MT2-MMP, which contains a TATA box. Thus, lack of common MMP regulatory elements in the promoter regions of MT-MMPs might allow their regulation different from other MMPs. Moreover, the basic promoter conformation represents only one determining aspect of the regulation of MMP expression. The majority of the regulatory elements in MMP promoters are non-canonical, which might also explain the finely tuned regulation with a different set of MMPs being activated in various cell types and at different developmental stages.
Finally, MMP expression is also regulated by cytokines, hormones and growth factors, adding a new level of complexity. Several interleukins such as IL-1, IL-6 and IL-15 have been shown to promote trophoblast invasion, whereas IL-10 inhibits this process.4 The role of placental hormones, e.g. human chorionic gonadotropin, in this regard remains still contradictory.42 We previously reported that insulin-like growth factors (IGFs) as well as insulin increase the expression of MT1-MMP in isolated first trimester trophoblast.43 Whether this entails invasion promotion remains to be studied.
Inhibition of MT-MMP activity could be considered as the last level of regulation of ECM degradation. Plasma proteins such as α2-macroglobulin are thought to be broad-range inhibitors of endopeptidases, including MMPs. However, α2-macroglobulin action has been mainly found in tissue fluids. Thus, its relevance for MT-MMP inhibition remains unclear.44
By contrast, tissue inhibitors of MMPs (TIMPs) are proteins specifically targeting MMPs. Four members of the TIMP family have been described, which bind to the catalytic domain of MMPs in a 1:1 molar ratio. The zinc ion in the MMP catalytic domain is then chelated, which results in MMP inhibition.45 TIMPs are broad-range MMP inhibitors affecting MMPs in general, but with different preference and efficiency. For instance, TIMP1 is a weak inhibitor of MT1-, MT2-, MT3- and MT5-MMP.46 Furthermore, different TIMP members can fulfill different functions: As mentioned above, low levels of TIMP2 are even required for MMP2 activation by MT1-MMP. TIMP4 also interacts with MT1-MMP and MMP2, but this interaction results in inhibition of both MMPs.47,48 Thus, the balance between the different TIMP members can also fine tune MMP activity.
Placental MT-MMPs and their relevance during pregnancy
Table 2 summarizes the cellular and tissue distribution of MT-MMPs in human placenta and decidua in the course of pregnancy.
Table 2.
MT-MMP localization in human placenta and decidua.
| MT-MMPs | Cell type/Tissue | Trimester | References |
|---|---|---|---|
| MT1-MMP (MMP14) | Feto-placental endothelial cells | 1st | 59 |
| Syncytiotrophoblast (ST) | 1st | 58,59 | |
| Villous cytotrophoblasts (VTs) | 1st | 43,52,58,59,92 | |
| Extravillous cytotrophoblasts (EVTs) | 1st | 53-56,58-60 | |
| Cell columns | 1st | 57,60 | |
| Perivascular EVTs | 1st | 57,58 | |
| Decidua | 1st | 60,64,65,91 | |
| Decidua | 2nd | 64 | |
| Feto-placental endothelial cells | 3rd | 59,62 | |
| ST | 3rd | 59,62,66 | |
| VTs | 3rd | 59,62 | |
| Decidua | 3rd | 64 | |
| Amniochorion | 3rd | 69 | |
| MT2-MMP (MMP15) | VTs | 1st | 43 |
| EVTs | 1st | 53,54,60,61 | |
| Decidua | 1st | 64,65,91 | |
| Decidua | 2nd | 64 | |
| ST | 3rd | 3,67 | |
| Decidua | 3rd | 64 | |
| Amniochorion | 3rd | 70 | |
| MT3-MMP (MMP16) | Decidua | 1st | 64,65,91 |
| Decidua | 2nd | 64 | |
| ST | 3rd | 3,68 | |
| Decidua | 3rd | 64 | |
| MT4-MMP (MMP17) | Decidua | 1st | 64 |
| Decidua | 2nd | 64 | |
| Feto-placental endothelial cells | 3rd | 68 | |
| Decidua | 3rd | 64 | |
| Amniochorion | 3rd | 70 | |
| MT5-MMP (MMP24) | Decidua | 1st | 64,65,91 |
| Decidua | 2nd | 64 | |
| ST | 3rd | 93 | |
| Decidua | 3rd | 64 | |
| Amniochorion | 3rd | 70 | |
| MT6-MMP (MMP25) | ST | 3rd | 68 |
| Amniochorion | 3rd | 70 |
MT-MMPs during the first trimester of pregnancy
The placenta is a fast developing and growing villous organ. Specialized placental cells, the cytotrophoblasts, undertake various processes essential for placental function and pregnancy success. Three different cytotrophoblast subpopulations can be distinguished: the villous cytotrophoblasts (VTs), which can fuse and form the syncytiotrophoblast (ST), a syncytium that represents the classical placental barrier, transporting maternal nutrients to the fetal circulation and producing pregnancy hormones. Finally, the extravillous cytotrophoblasts (EVTs), which migrate from tips of some specialized placental villi, invade the maternal decidua and anchor the placenta in the uterus. This process predominantly takes place in the first trimester of pregnancy.4 Some EVTs also reach the uterine spiral arteries and remodel them into wide, low resistance vessels, allowing an adequate blood supply to the fetus.49 All these processes, i.e. trophoblast fusion, invasion and the remodeling of the spiral arteries, require ECM degradation, pointing out once more the relevance of MMPs in pregnancy. However, due to their involvement in the process of trophoblast invasion, MMP expression has been mainly characterized in first trimester trophoblasts.
Because of their membrane anchor, MT-MMPs can be located to specific membrane regions, where they play an active role in pericellular proteolysis,50 a feature required in the spatially directed process of trophoblast invasion. Indeed, in first trimester trophoblasts MT1-MMP is localized in invadopodia, a structure at the cellular edge of ECM degradation and invasion progression.51 MT1-MMP expression in cytotrophoblasts is maintained through the first trimester of pregnancy.52 This suggests that, together with its role as pro-MMP2 activator, MT1-MMP may be involved in trophoblast invasion. In fact, MT1-MMP expression has been observed in EVTs53-56 and in perivascular EVTs, i.e. EVTs migrating into the spiral arteries.57,58 Blocking of MT1-MMP with neutralizing antibodies reduced migration of a first trimester trophoblast cell line.59
Among the other members of the MT-MMP family, only MT2-MMP is expressed in isolated first trimester trophoblasts at levels similar to MT1-MMP.43,60 MT2-MMP is predominantly expressed in EVTs, with weaker expression in VTs.54,61 Thus, a role of MT2-MMP in trophoblast invasion is also likely.
However, besides trophoblast invasion, also other functions of placental development may involve MT-MMPs in the first trimester of pregnancy. MT1-MMP is produced in feto-placental endothelial cells forming the first placental vessels.62 In fact, MT1-MMP is a key player in matrix degradation during angiogenesis,63 and blocking of MT1-MMP reduces the network formation potential of human feto-placental endothelial cells isolated from full term placentas.62 This suggests a role of MT1-MMP in neovascularisation or vascular development of the early placenta.
In addition to its location in EVT and endothelial cells with both cell types performing directed migration during trophoblast invasion and angiogenesis, respectively, MT1-MMP is also expressed in VTs and ST.58,59 Here, MT1-MMP has been observed to promote VT proliferation as well as their fusion to ST in vitro.59
All MT-MMP members, except MT6-MMP, are expressed in first trimester decidua. MT1- and MT4-MMP are expressed in EVTs reaching the decidua, but also in decidual fibroblasts and uterine natural killer cells, 2 cell types controlling the depth of trophoblast invasion. MT2-MMP is predominantly produced in cytotrophoblasts, whereas MT3- and MT5-MMP are mainly located in decidual stromal cells.64 When MT1-, MT2-, MT3- and MT5-MMP expression was studied in the decidual secretory endometrium, in decidua parietalis and in decidua basalis, all 4 MT-MMPs were detected in all parts of the decidua, as well as in the syncytiotrophoblast and EVTs reaching the decidua basalis.65
Placental MT-MMPs during the second trimester of pregnancy
For obvious reasons, sampling during the second trimester of pregnancy is mainly based on non-invasive techniques such as serum screening and ultrasound. Therefore, in this period placental MT-MMPs remain uncharacterized. All MT-MMP members except MT6-MMP were detected in second trimester decidua.64
Placental MT-MMPs during the third trimester of pregnancy
All MT-MMP members are present in the third trimester placenta.3,66-68 This is especially surprising for MT6-MMP, which is not expressed in the placenta during the first trimester of pregnancy. In the third trimester, MT1-MMP is expressed in several placental cell types including the syncytiotrophoblast, the underlining VTs and the endothelium of the feto-placental vessels.59,62,66 As mentioned above, in vitro experiments suggest a role of MT1-MMP in proliferation and fusion of VTs as well as in feto-placental angiogenesis.59,62 MT2-, MT3-, MT5- and MT6-MMP have been localized in the syncytiotrophoblast,67,68 with MT2-, MT3- and MT5-MMP being also present in VTs.3 By contrast, MT4-MMP expression was predominantly observed in fetal vessels.68
Similar to their expression in the first and the second trimester of pregnancy, all MT-MMP members except for MT6-MMP are expressed in the decidua at term.64 MT-MMPs have also been characterized in fetal membranes after delivery. With the only exception of MT3-MMP, all MT-MMPs are expressed in the amniochorion.69,70
Changes in placental MT-MMP expression during pregnancy
Another aspect regarding placental MT-MMPs that has been insufficiently documented is the dynamic in their expression during the course of pregnancy. In a microarray study71 several genes involved in different biological processes such as cell proliferation, differentiation and angiogenesis were differentially regulated in first and third trimester human placenta, with most genes involved in these processes, including MT1-MMP but not MT2-MMP, being higher expressed in first trimester placenta.
In another microarray study we have previously analyzed MT-MMP gene expression in cytotrophoblasts from first trimester placenta (FT) and showed that only MT1- and MT2-MMP could be detected in FT.43 Here we present novel RT-PCR data comparing MT-MMP expression between cytotrophoblasts isolated from first (FT) and third trimester placenta (TT) by tissue digestion, Percoll gradient centrifugation and immunopurification72,73 (Fig. 2). Of all MT-MMPs, only MT1-MMP and MT2-MMP were expressed in both FT and TT, with MT2-MMP expression being higher in FT (Fig. 2). This expression change underlines the functional difference of FT and TT, since in contrast to FT, TT cannot fulfill endovascular remodeling both in vivo and in vitro.74 Interestingly, we were not able to detect MT3- and MT5-MMP in TT, despite its previously reported presence3 (Table 2). This may in part be due to differences between mRNA and protein, or due to differences in the trophoblast subpopulations investigated. While freshly isolated trophoblasts mainly resemble cytotrophoblast cells, Zhu et al. investigated whole placental tissue and identified the syncytiotrophoblast as major MT3- and MT5-MMP producing site.
Figure 2.

RT-PCR analysis of MT-MMP expression in primary trophoblasts isolated from first trimester placenta (FT) vs. third trimester placenta (TT). For the housekeeping gene RPL30 24 cycles were used, for all MT-MMP 27 cycles.
Placental MT-MMPs in pregnancy complications
It is well established that severe pregnancy complications associated with shallow or impaired trophoblast invasion and spiral artery remodeling, such as preeclampsia (PE) and fetal growth restriction (FGR), have their origin in early pregnancy.49 However, PE and FGR clinically manifest only during the second trimester.75 Therefore, the relationship of MMPs with PE and FGR has been mainly analyzed in third trimester placentas from preterm and term pregnancies.
Investigating chorionic villous biopsies provides the possibility to study placental tissue in the period of potential functional failure. Huisman et al.76 found no differences in MMP2 and MMP9 activity in first trimester placenta from pregnancy subsequently developing PE or FGR, but the study was limited by the small sample size of the groups.
Also from the studies investigating placenta from third trimester, the majority of analyses focused on MMP2 and MMP9.77-79 Few publications have studied the role of MT-MMPs in FGR. We have previously shown a decrease in MT1-MMP mRNA and protein levels in FGR placentas when compared to gestational age matched healthy controls.59 This change in FGR may be limited to MT1-MMP, because MT2-, MT3- and MT5-MMP are unaltered in FGR.3 However, more data are required to draw firm conclusions.
In PE placental MT6-MMP is down-regulated,68 whereas MT2-MMP67,80 and MT4-MMP68 are up-regulated. Since MT4-MMP is involved in angiogenesis, its up-regulation might be a compensatory mechanism to overcome poor blood supply to the fetus with placental hypervascularisation.68 The role of MT-MMPs in the pathology of PE may involve their function in cleavage and thus activation or inactivation of circulating substrates. For instance, soluble endoglin (sEng), a soluble form of the TGF-β receptor, is increased in PE.81 Both sEng and MT1-MMP have been localized in the ST of third trimester placenta. Indeed, although its expression is not upregulated in pre-eclamptic placentas, MT1-MMP has been reported to cleave endoglin.66
Thus, contrasting the concept that poor placentation may relate to lower MMP expression, some MT-MMP members are up-regulated in PE and FGR during the third trimester of pregnancy. This might represent a compensatory mechanism to overcome poor placentation due to MT-MMP down-regulation in the first trimester of pregnancy.82
Diabetes in pregnancy is associated with both an increased risk for PE83 and FGR, suggesting placental invasion failure, as well as excessive fetal fat accretion and fetal overgrowth. Furthermore, the placenta in diabetes is characterized by hypervascularization.84 Accordingly, MT1-MMP imbalances have been described in diabetes. MT1-MMP is increased in first trimester placentas of Type 1 diabetic women, and upregulation of trophoblast MT1-MMP by insulin, IGF-1, IGF-2 and TNF-α may underlie this observation.43 Gestational diabetes mellitus (GDM) is a glucose intolerance with first clinical manifestation in the second trimester. We showed an up-regulation of MT1-MMP protein levels in third trimester placenta as a result of GDM in lean women. The upregulation of MT1-MMP by insulin and IGF-2 in endothelial cells accounts for the increase.62 In contrast, MT1-MMP is downregulated in GDM placentas of obese women.85 This suggests a complex interplay between diabetes-associated and obesity-associated factors to ultimately determine placental MT1-MMP levels in the third trimester of pregnancy.
Indeed, maternal obesity is associated with an increased risk of preterm delivery, PE and fetal death86 and might also play a role in the regulation of placental MT-MMPs. In a rat model mimicking maternal obesity, trophoblast invasion and blood vessel remodeling is altered.87 Leptin, a classical hormone upregulated in obesity and involved in placental lipid metabolism,88 can also modulate placental ECM molecule expression89 and up-regulates MT1-MMP expression in human EVTs.90 However, more studies are required to identify the obesity-related factors regulating MT-MMPs.
MT-MMP expression is also altered in pregnancy disorders leading to miscarriage. Decidual MT2- and MT5-MMP expression is increased in first trimester decidua of spontaneous abortions,91 whereas absence of MT4-MMP in amniochorion has been described in preterm premature rupture of the membranes (pPROM) when compared with term labor placenta.70
Conclusion
In the present review we have summarized current knowledge about MT-MMPs with a focus on the human placenta. Their location, functions and involvement in pregnancy complications highlight the crucial role of MT-MMPs for a successful pregnancy. However, it is obvious that MT-MMPs are understudied and more research is required to completely understand their role in placental implantation and trophoblast invasion. Additional studies addressing MT-MMP activity and function in PE and FGR could help in finding diagnostic markers for these pregnancy complications.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors contributions
AM reviewed the literature and wrote the first draft. UH modified the first draft and critically discussed and reviewed the manuscript. NG, UL, GD and MD critically discussed and reviewed the manuscript.
Funding
The work was supported by funds of the Oesterreichische Nationalbank (Anniversary Fund, project number: 14796), by the Doctorate program MOLIN (FWF, W1241) and the Medical University of Graz.
References
- 1.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor Perspect Biol 2011; 3:a005058; PMID:21917992; http://dx.doi.org/ 10.1101/cshperspect.a005058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forbes K, Westwood M. Maternal growth factor regulation of human placental development and fetal growth. J Endocrinol 2010; 207(1):1-16; PMID:20817666; http://dx.doi.org/ 10.1677/JOE-10-0174 [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Zhong M, Pang Z, Yu Y. Dysregulated expression of matrix metalloproteinases and their inhibitors may participate in the pathogenesis of pre-eclampsia and fetal growth restriction. Early Hum Dev 2014; 90(10):657-64; PMID:25194834; http://dx.doi.org/ 10.1016/j.earlhumdev.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 4.Bischof P, Meisser A, Campana A. Biochemistry and molecular biology of trophoblast invasion. Ann N Y Acad Sci 2001; 943:157-62; PMID:11594536; http://dx.doi.org/ 10.1111/j.1749-6632.2001.tb03799.x [DOI] [PubMed] [Google Scholar]
- 5.Seiki M. Membrane-type matrix metalloproteinases. APMIS 1999; 107(1):137-43; PMID:10190290; http://dx.doi.org/ 10.1111/j.1699-0463.1999.tb01536.x [DOI] [PubMed] [Google Scholar]
- 6.Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta 2006; 27(8):783-93; PMID:16249026; http://dx.doi.org/ 10.1016/j.placenta.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 7.Isaka K, Usuda S, Ito H, Sagawa Y, Nakamura H, Nishi H, Suzuki Y, Li YF, Takayama M. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta 2003; 24(1):53-64; PMID:12495660; http://dx.doi.org/ 10.1053/plac.2002.0867 [DOI] [PubMed] [Google Scholar]
- 8.Seval Y, Akkoyunlu G, Demir R, Asar M. Distribution patterns of matrix metalloproteinase (MMP)-2 and -9 and their inhibitors (TIMP-1 and TIMP-2) in the human decidua during early pregnancy. Acta Histochem 2004; 106(5):353-62; PMID:15530550; http://dx.doi.org/ 10.1016/j.acthis.2004.07.005 [DOI] [PubMed] [Google Scholar]
- 9.Huppertz B, Kertschanska S, Demir AY, Frank HG, Kaufmann P. Immunohistochemistry of matrix metalloproteinases (MMP), their substrates, and their inhibitors (TIMP) during trophoblast invasion in the human placenta. Cell Tissue Res 1998; 291(1):133-48; PMID:9394051; http://dx.doi.org/ 10.1007/s004410050987 [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Barrantes S, Bernardo M, Toth M, Fridman R. Regulation of membrane type-matrix metalloproteinases. Semin Cancer Biol 2002; 12(2):131-8; PMID:12027585; http://dx.doi.org/ 10.1006/scbi.2001.0421 [DOI] [PubMed] [Google Scholar]
- 11.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006; 69(3):562-73; PMID:16405877; http://dx.doi.org/ 10.1016/j.cardiores.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 12.Itoh Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol 2015; 44-46:207-23; PMID:25794647; http://dx.doi.org/ 10.1016/j.matbio.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 13.Sela-Passwell N, Rosenblum G, Shoham T, Sagi I. Structural and functional bases for allosteric control of MMP activities: can it pave the path for selective inhibition? Biochim Biophys Acta 2010; 1803(1):29-38; PMID:19406173; http://dx.doi.org/ 10.1016/j.bbamcr.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 14.Sohail A, Marco M, Zhao H, Shi Q, Merriman S, Mobashery S, Fridman R. Characterization of the dimerization interface of membrane type 4 (MT4)-matrix metalloproteinase. J Biol Chem 2011; 286(38):33178-89; PMID:21828052; http://dx.doi.org/ 10.1074/jbc.M111.253369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehti K, Lohi J, Juntunen MM, Pei D, Keski-Oja J. Oligomerization through hemopexin and cytoplasmic domains regulates the activity and turnover of membrane-type 1 matrix metalloproteinase. J Biol Chem 2002; 277(10):8440-8; PMID:11779859; http://dx.doi.org/ 10.1074/jbc.M109128200 [DOI] [PubMed] [Google Scholar]
- 16.Marco M, Fortin C, Fulop T. Membrane-type matrix metalloproteinases: key mediators of leukocyte function. J Leukoc Biol 2013; 94(2):237-46; PMID:23695309; http://dx.doi.org/ 10.1189/jlb.0612267 [DOI] [PubMed] [Google Scholar]
- 17.Sohail A, Sun Q, Zhao H, Bernardo MM, Cho JA, Fridman R. MT4-(MMP17) and MT6-MMP (MMP25), A unique set of membrane-anchored matrix metalloproteinases: properties and expression in cancer. Cancer Metastasis Rev 2008; 27(2):289-302; PMID:18286233; http://dx.doi.org/ 10.1007/s10555-008-9129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003; 92(8):827-39; PMID:12730128; http://dx.doi.org/ 10.1161/01.RES.0000070112.80711.3D [DOI] [PubMed] [Google Scholar]
- 19.Löffek S, Schilling O, Franzke CW. Series “matrix metalloproteinases in lung health and disease:” Biological role of matrix metalloproteinases: a critical balance. Eur Respir J 2011; 38(1):191-208; PMID:21177845; http://dx.doi.org/ 10.1183/09031936.00146510 [DOI] [PubMed] [Google Scholar]
- 20.Yana I, Weiss SJ. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol Biol Cell 2000; 11(7):2387-401; PMID:10888676; http://dx.doi.org/ 10.1091/mbc.11.7.2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanjul-Fernandez M, Folgueras AR, Cabrera S, Lopez-Otin C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim Biophys Acta 2010; 1803(1):3-19; PMID:19631700; http://dx.doi.org/ 10.1016/j.bbamcr.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 22.Golubkov VS, Chekanov AV, Shiryaev SA, Aleshin AE, Ratnikov BI, Gawlik K, Radichev I, Motamedchaboki K, Smith JW, Strongin AY. Proteolysis of the membrane type-1 matrix metalloproteinase prodomain: implications for a two-step proteolytic processing and activation. J Biol Chem 2007; 282(50):36283-91; PMID:17938169; http://dx.doi.org/ 10.1074/jbc.M706290200 [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Barrantes S, Toth M, Bernardo MM, Yurkova M, Gervasi DC, Raz Y, Sang QA, Fridman R. Binding of active (57 kDa) membrane type 1-matrix metalloproteinase (MT1-MMP) to tissue inhibitor of metalloproteinase (TIMP)-2 regulates MT1-MMP processing and pro-MMP-2 activation. J Biol Chem 2000; 275(16):12080-9; PMID:10766841; http://dx.doi.org/ 10.1074/jbc.275.16.12080 [DOI] [PubMed] [Google Scholar]
- 24.Cho JA, Osenkowski P, Zhao H, Kim S, Toth M, Cole K, Aboukameel A, Saliganan A, Schuger L, Bonfil RD, et al.. The inactive 44-kDa processed form of membrane type 1 matrix metalloproteinase (MT1-MMP) enhances proteolytic activity via regulation of endocytosis of active MT1-MMP. J Biol Chem 2008; 283(25):17391-405; PMID:18413312; http://dx.doi.org/ 10.1074/jbc.M708943200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toth M, Hernandez-Barrantes S, Osenkowski P, Bernardo MM, Gervasi DC, Shimura Y, Meroueh O, Kotra LP, Gálvez BG, Arroyo AG, et al.. Complex pattern of membrane type 1 matrix metalloproteinase shedding. Regulation by autocatalytic cells surface inactivation of active enzyme. J Biol Chem 2002; 277(29):26340-50; http://dx.doi.org 10.1074/jbc.M200655200 [DOI] [PubMed] [Google Scholar]
- 26.Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP). J Cell Physiol 2004; 200(1):2-10; PMID:15137052; http://dx.doi.org/ 10.1002/jcp.20064 [DOI] [PubMed] [Google Scholar]
- 27.Kemp B, Kertschanska S, Kadyrov M, Rath W, Kaufmann P, Huppertz B. Invasive depth of extravillous trophoblast correlates with cellular phenotype: a comparison of intra- and extrauterine implantation sites. Histochem Cell Biol 2002; 117(5):401-14; PMID:12029487; http://dx.doi.org/ 10.1007/s00418-002-0396-0 [DOI] [PubMed] [Google Scholar]
- 28.English WR, Puente XS, Freije JM, Knauper V, Amour A, Merryweather A, Lopez-Otin C, Murphy G. Membrane type 4 matrix metalloproteinase (MMP17) has tumor necrosis factor-α convertase activity but does not activate pro-MMP2. J Biol Chem 2000; 275(19):14046-55; PMID:10799478; http://dx.doi.org/ 10.1074/jbc.275.19.14046 [DOI] [PubMed] [Google Scholar]
- 29.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, et al.. Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature 1994; 370(6490):555-7; PMID:8052310; http://dx.doi.org/ 10.1038/370555a0 [DOI] [PubMed] [Google Scholar]
- 30.Sounni NE, Paye A, Host L, Noel A. MT-MMPS as Regulators of Vessel Stability Associated with Angiogenesis. Front Pharmacol 2011; 2:111; PMID:21687519; http://dx.doi.org/ 10.3389/fphar.2011.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng JC, Chang HM, Leung PC. Transforming growth factor-beta1 inhibits trophoblast cell invasion by inducing Snail-mediated downregulation of vascular endothelial-cadherin protein. J Biol Chem 2013; 288(46):33181-92; PMID:24106276; http://dx.doi.org/ 10.1074/jbc.M113.488866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiden U, Wadsack C, Prutsch N, Gauster M, Weiss U, Frank HG, Schmitz U, Fast-Hirsch C, Hengstschläger M, Pötgens A, et al.. The first trimester human trophoblast cell line ACH-3P: a novel tool to study autocrine/paracrine regulatory loops of human trophoblast subpopulations–TNF-α stimulates MMP15 expression. BMC Dev Biol 2007; 7:137; PMID:18093301; http://dx.doi.org/ 10.1186/1471-213X-7-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M. Tumor necrosis factor-α inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab 2004; 89(2):812-22; PMID:14764800; http://dx.doi.org/ 10.1210/jc.2003-031351 [DOI] [PubMed] [Google Scholar]
- 34.Renaud SJ, Postovit LM, Macdonald-Goodfellow SK, McDonald GT, Caldwell JD, Graham CH. Activated macrophages inhibit human cytotrophoblast invasiveness in vitro. Biol Reprod 2005; 73(2):237-43; PMID:15800179; http://dx.doi.org/ 10.1095/biolreprod.104.038000 [DOI] [PubMed] [Google Scholar]
- 35.Tam EM, Morrison CJ, Wu YI, Stack MS, Overall CM. Membrane protease proteomics: Isotope-coded affinity tag MS identification of undescribed MT1-matrix metalloproteinase substrates. Proc Natl Acad Sci U S A 2004; 101(18):6917-22; PMID:15118097; http://dx.doi.org/ 10.1073/pnas.0305862101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folgueras AR, Pendas AM, Sanchez LM, Lopez-Otin C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol 2004; 48(5-6):411-24; PMID:15349816; http://dx.doi.org/ 10.1387/ijdb.041811af [DOI] [PubMed] [Google Scholar]
- 37.Gao G, Plaas A, Thompson VP, Jin S, Zuo F, Sandy JD. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J Biol Chem 2004; 279(11):10042-51; PMID:14701864; http://dx.doi.org/ 10.1074/jbc.M312100200 [DOI] [PubMed] [Google Scholar]
- 38.Lu KV, Jong KA, Rajasekaran AK, Cloughesy TF, Mischel PS. Upregulation of tissue inhibitor of metalloproteinases (TIMP)-2 promotes matrix metalloproteinase (MMP)-2 activation and cell invasion in a human glioblastoma cell line. Lab Invest 2004; 84(1):8-20; PMID:14631378; http://dx.doi.org/ 10.1038/labinvest.3700003 [DOI] [PubMed] [Google Scholar]
- 39.Morrison CJ, Butler GS, Bigg HF, Roberts CR, Soloway PD, Overall CM. Cellular activation of MMP-2 (gelatinase A) by MT2-MMP occurs via a TIMP-2-independent pathway. J Biol Chem 2001; 276(50):47402-10; PMID:11584019; http://dx.doi.org/ 10.1074/jbc.M108643200 [DOI] [PubMed] [Google Scholar]
- 40.Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol 2008; 40(6-7):1362-78; PMID:18258475; http://dx.doi.org/ 10.1016/j.biocel.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 41.Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol 2007; 211(1):19-26; PMID:17167774; http://dx.doi.org/ 10.1002/jcp.20948 [DOI] [PubMed] [Google Scholar]
- 42.Bischof P, Meisser A, Campana A. Paracrine and autocrine regulators of trophoblast invasion–a review. Placenta 2000; 21 Suppl A:S55-60; PMID:10831123; http://dx.doi.org/ 10.1053/plac.2000.0521 [DOI] [PubMed] [Google Scholar]
- 43.Hiden U, Glitzner E, Ivanisevic M, Djelmis J, Wadsack C, Lang U, Desoye G. MT1-MMP expression in first-trimester placental tissue is upregulated in type 1 diabetes as a result of elevated insulin and tumor necrosis factor-α levels. Diabetes 2008; 57(1):150-7; PMID:17928399; http://dx.doi.org/ 10.2337/db07-0903 [DOI] [PubMed] [Google Scholar]
- 44.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 2002; 115(Pt 19):3719-27; PMID:12235282; http://dx.doi.org/ 10.1242/jcs.00063 [DOI] [PubMed] [Google Scholar]
- 45.Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol 2011; 12(11):233; PMID:22078297; http://dx.doi.org/ 10.1186/gb-2011-12-11-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 2015;44-46:247-54; PMID:25805621; http://dx.doi.org/ 10.1016/j.matbio.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 47.Hernandez-Barrantes S, Shimura Y, Soloway PD, Sang QA, Fridman R. Differential roles of TIMP-4 and TIMP-2 in pro-MMP-2 activation by MT1-MMP. Biochem Biophys Res Commun 2001; 281(1):126-30; PMID:11178970; http://dx.doi.org/ 10.1006/bbrc.2001.4323 [DOI] [PubMed] [Google Scholar]
- 48.Bigg HF, Morrison CJ, Butler GS, Bogoyevitch MA, Wang Z, Soloway PD, Overall CM. Tissue inhibitor of metalloproteinases-4 inhibits but does not support the activation of gelatinase A via efficient inhibition of membrane type 1-matrix metalloproteinase. Cancer Res 2001; 61(9):3610-8; PMID:11325829 [PubMed] [Google Scholar]
- 49.Whitley GS, Cartwright JE. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta 2010; 31(6):465-74; PMID:20359743; http://dx.doi.org/ 10.1016/j.placenta.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001; 17:463-516; PMID:11687497; http://dx.doi.org/ 10.1146/annurev.cellbio.17.1.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel A, Dash PR. Formation of atypical podosomes in extravillous trophoblasts regulates extracellular matrix degradation. Eur J Cell Biol 2012; 91(3):171-9; PMID:22284833; http://dx.doi.org/ 10.1016/j.ejcb.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu P, Wang YL, Zhu SJ, Luo SY, Piao YS, Zhuang LZ. Expression of matrix metalloproteinase-2, -9, and -14, tissue inhibitors of metalloproteinase-1, and matrix proteins in human placenta during the first trimester. Biol Reprod 2000; 62(4):988-94; PMID:10727268; http://dx.doi.org/ 10.1095/biolreprod62.4.988 [DOI] [PubMed] [Google Scholar]
- 53.Tarrade A, Goffin F, Munaut C, Lai-Kuen R, Tricottet V, Foidart JM, Vidaud M, Frankenne F, Evain-Brion D. Effect of matrigel on human extravillous trophoblasts differentiation: modulation of protease pattern gene expression. Biol Reprod 2002; 67(5):1628-37; PMID:12390897; http://dx.doi.org/ 10.1095/biolreprod.101.001925 [DOI] [PubMed] [Google Scholar]
- 54.Harris LK, Smith SD, Keogh RJ, Jones RL, Baker PN, Knofler M, Cartwright JE, Whitley GS, Aplin JD. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am J Pathol 2010; 177(4):2103-15; PMID:20802175; http://dx.doi.org/ 10.2353/ajpath.2010.100182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Onogi A, Naruse K, Sado T, Tsunemi T, Shigetomi H, Noguchi T, Yamada Y, Akasaki M, Oi H, Kobayashi H. Hypoxia inhibits invasion of extravillous trophoblast cells through reduction of matrix metalloproteinase (MMP)-2 activation in the early first trimester of human pregnancy. Placenta 2011; 32(9):665-70; PMID:21764444; http://dx.doi.org/ 10.1016/j.placenta.2011.06.023 [DOI] [PubMed] [Google Scholar]
- 56.Francis VA, Abera AB, Matjila M, Millar RP, Katz AA. Kisspeptin regulation of genes involved in cell invasion and angiogenesis in first trimester human trophoblast cells. PloS One 2014; 9(6):e99680; PMID:24923321; http://dx.doi.org/ 10.1371/journal.pone.0099680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurskainen T, Seiki M, Apte SS, Syrjakallio-Ylitalo M, Sorsa T, Oikarinen A, Autio-Harmainen H. Production of membrane-type matrix metalloproteinase-1 (MT-MMP-1) in early human placenta. A possible role in placental implantation? J Histochem Cytochem 1998; 46(2):221-9; PMID:9446829; http://dx.doi.org/ 10.1177/002215549804600211 [DOI] [PubMed] [Google Scholar]
- 58.Bai SX, Wang YL, Qin L, Xiao ZJ, Herva R, Piao YS. Dynamic expression of matrix metalloproteinases (MMP-2, -9 and -14) and the tissue inhibitors of MMPs (TIMP-1, -2 and -3) at the implantation site during tubal pregnancy. Reproduction 2005; 129(1):103-13; PMID:15615902; http://dx.doi.org/ 10.1530/rep.1.00283 [DOI] [PubMed] [Google Scholar]
- 59.Hiden U, Ghaffari-Tabrizi N, Gauster M, Tam-Amersdorfer C, Cetin I, Dieber-Rotheneder M, Lang U, Desoye G. Membrane-type matrix metalloproteinase 1 regulates trophoblast functions and is reduced in fetal growth restriction. Am J Pathol 2013; 182(5):1563-71; PMID:23470162; http://dx.doi.org/ 10.1016/j.ajpath.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 60.Bjorn SF, Hastrup N, Larsen JF, Lund LR, Pyke C. Messenger RNA for membrane-type 2 matrix metalloproteinase, MT2-MMP, is expressed in human placenta of first trimester. Placenta 2000; 21(2-3):170-6; PMID:10736239; http://dx.doi.org/ 10.1053/plac.1999.0447 [DOI] [PubMed] [Google Scholar]
- 61.Pollheimer J, Fock V, Knofler M. Review: the ADAM metalloproteinases - novel regulators of trophoblast invasion? Placenta 2014; 35 Suppl:S57-63; PMID:24231445; http://dx.doi.org/ 10.1016/j.placenta.2013.10.012 [DOI] [PubMed] [Google Scholar]
- 62.Hiden U, Lassance L, Tabrizi NG, Miedl H, Tam-Amersdorfer C, Cetin I, Lang U, Desoye G. Fetal insulin and IGF-II contribute to gestational diabetes mellitus (GDM)-associated up-regulation of membrane-type matrix metalloproteinase 1 (MT1-MMP) in the human feto-placental endothelium. J Clin Endocrinol Metab 2012; 97(10):3613-21; PMID:22893718; http://dx.doi.org/ 10.1210/jc.2012-1212 [DOI] [PubMed] [Google Scholar]
- 63.Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol 2004; 167(4):757-67; PMID:15545316; http://dx.doi.org/ 10.1083/jcb.200405001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anacker J, Segerer SE, Hagemann C, Feix S, Kapp M, Bausch R, Kämmerer U. Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases. Mol Hum Reprod 2011; 17(10):637-52; PMID:21565864; http://dx.doi.org/ 10.1093/molehr/gar033 [DOI] [PubMed] [Google Scholar]
- 65.Plaisier M, Koolwijk P, Willems F, Helmerhorst FM, van Hinsbergh VW. Pericellular-acting proteases in human first trimester decidua. Mol Hum Reprod 2008; 14(1):41-51; PMID:18175789; http://dx.doi.org/ 10.1093/molehr/gam085 [DOI] [PubMed] [Google Scholar]
- 66.Kaitu'u-Lino TJ, Palmer KR, Whitehead CL, Williams E, Lappas M, Tong S. MMP-14 is expressed in preeclamptic placentas and mediates release of soluble endoglin. Am J Pathol 2012; 180(3):888-94; PMID:22296769; http://dx.doi.org/ 10.1016/j.ajpath.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 67.Kaitu'u-Lino TJ, Palmer K, Tuohey L, Ye L, Tong S. MMP-15 is upregulated in preeclampsia, but does not cleave endoglin to produce soluble endoglin. PloS one 2012; 7(6):e39864; PMID:22768148; http://dx.doi.org/ 10.1371/journal.pone.0039864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaitu'u-Lino TJ, Tuohey L, Ye L, Palmer K, Skubisz M, Tong S. MT-MMPs in pre-eclamptic placenta: relationship to soluble endoglin production. Placenta 2013; 34(2):168-73; PMID:23261267; http://dx.doi.org/ 10.1016/j.placenta.2012.11.034 [DOI] [PubMed] [Google Scholar]
- 69.Fortunato SJ, Menon R, Lombardi SJ. Expression of a progelatinase activator (MT1-MMP) in human fetal membranes. Am J Reprod Immunol 1998; 39(5):316-22; http://dx.doi.org/ 10.1111/j.1600-0897.1998.tb00524.x [DOI] [PubMed] [Google Scholar]
- 70.Fortunato SJ, Menon R. Screening of novel matrix metalloproteinases (MMPs) in human fetal membranes. J Assist Reprod Genet 2002; 19(10):483-6; PMID:12416653; http://dx.doi.org/ 10.1023/A:1020362519981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sitras V, Fenton C, Paulssen R, Vartun A, Acharya G. Differences in gene expression between first and third trimester human placenta: a microarray study. PloS One 2012; 7(3):e33294; PMID:22442682; http://dx.doi.org/ 10.1371/journal.pone.0033294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blaschitz A, Hutter H, Leitner V, Pilz S, Wintersteiger R, Dohr G, Sedlmayr P. Reaction patterns of monoclonal antibodies to HLA-G in human tissues and on cell lines: a comparative study. Hum Immunol 2000; 61(11):1074-85; PMID:11137210; http://dx.doi.org/ 10.1016/S0198-8859(00)00207-X [DOI] [PubMed] [Google Scholar]
- 73.Cervar M, Blaschitz A, Dohr G, Desoye G. Paracrine regulation of distinct trophoblast functions in vitro by placental macrophages. Cell Tissue Res 1999; 295(2):297-305; PMID:9931376; http://dx.doi.org/ 10.1007/s004410051236 [DOI] [PubMed] [Google Scholar]
- 74.Kalkunte S, Lai Z, Tewari N, Chichester C, Romero R, Padbury J, Sharma S. In vitro and in vivo evidence for lack of endovascular remodeling by third trimester trophoblasts. Placenta 2008; 29(10):871-8; PMID:18775564; http://dx.doi.org/ 10.1016/j.placenta.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Vivo A, Baviera G, Giordano D, Todarello G, Corrado F, D'Anna R. Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet Gynecol Scand 2008; 87(8):837-42; PMID:18607829; http://dx.doi.org/ 10.1080/00016340802253759 [DOI] [PubMed] [Google Scholar]
- 76.Huisman MA, Timmer A, Zeinstra M, Serlier EK, Hanemaaijer R, Goor H, Erwich JJ. Matrix-metalloproteinase activity in first trimester placental bed biopsies in further complicated and uncomplicated pregnancies. Placenta 2004; 25(4):253-8; PMID:15028416; http://dx.doi.org/ 10.1016/j.placenta.2003.10.006 [DOI] [PubMed] [Google Scholar]
- 77.Galewska Z, Romanowicz L, Jaworski S, Bankowski E. Gelatinase matrix metalloproteinase (MMP)-2 and MMP-9 of the umbilical cord blood in preeclampsia. Clin Chem Lab Med 2008; 46(4):517-22; PMID:18298353; http://dx.doi.org/ 10.1515/CCLM.2008.083 [DOI] [PubMed] [Google Scholar]
- 78.Shokry M, Omran OM, Hassan HI, Elsedfy GO, Hussein MR. Expression of matrix metalloproteinases 2 and 9 in human trophoblasts of normal and preeclamptic placentas: preliminary findings. Exp Mol Pathol 2009; 87(3):219-25; PMID:19716817; http://dx.doi.org/ 10.1016/j.yexmp.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 79.Myers JE, Merchant SJ, Macleod M, Mires GJ, Baker PN, Davidge ST. MMP-2 levels are elevated in the plasma of women who subsequently develop preeclampsia. Hypertens Pregnancy 2005; 24(2):103-15; PMID:16036395; http://dx.doi.org/ 10.1081/PRG-200059836 [DOI] [PubMed] [Google Scholar]
- 80.Pang ZJ, Xing FQ. Expression profile of trophoblast invasion-associated genes in the pre-eclamptic placenta. Br J Biomed Sci 2003; 60(2):97-101; PMID:12866918 [DOI] [PubMed] [Google Scholar]
- 81.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al.. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006; 12(6):642-9; PMID:16751767; http://dx.doi.org/ 10.1038/nm1429 [DOI] [PubMed] [Google Scholar]
- 82.Palei AC, Granger JP, Tanus-Santos JE. Matrix metalloproteinases as drug targets in preeclampsia. Curr Drug Targets 2013; 14(3):325-34; PMID:23316964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep 2015; 15(3):9; PMID:25644816; http://dx.doi.org/ 10.1007/s11892-015-0579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Setji TL, Brown AJ, Feinglos MN. Gestational Diabetes Mellitus. Clin Diab 2005; 23(1):17-24; http://dx.doi.org/ 10.2337/diaclin.23.1.17 [DOI] [Google Scholar]
- 85.Radaelli T, Varastehpour A, Catalano P, Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes 2003; 52(12):2951-8; PMID:14633856; http://dx.doi.org/ 10.2337/diabetes.52.12.2951 [DOI] [PubMed] [Google Scholar]
- 86.Lamminpaa R, Vehvilainen-Julkunen K, Gissler M, Selander T, Heinonen S. Pregnancy outcomes of overweight and obese women aged 35 years or older - A registry-based study in Finland. Obes Res Clin Pract 2015; PMID:26054598. [DOI] [PubMed] [Google Scholar]
- 87.Hayes EK, Tessier DR, Percival ME, Holloway AC, Petrik JJ, Gruslin A, Raha S. Trophoblast invasion and blood vessel remodeling are altered in a rat model of lifelong maternal obesity. Reprod Sci 2014; 21(5):648-57; PMID:24155067; http://dx.doi.org/ 10.1177/1933719113508815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.White V, Gonzalez E, Capobianco E, Pustovrh C, Martinez N, Higa R, Baier M, Jawerbaum A. Leptin modulates nitric oxide production and lipid metabolism in human placenta. Reprod Fertil Dev 2006; 18(4):425-32; PMID:16737635; http://dx.doi.org/ 10.1071/RD05105 [DOI] [PubMed] [Google Scholar]
- 89.Castellucci M, De Matteis R, Meisser A, Cancello R, Monsurro V, Islami D, Sarzani R, Marzioni D, Cinti S, Bischof P. Leptin modulates extracellular matrix molecules and metalloproteinases: possible implications for trophoblast invasion. Mol Hum Reprod 2000; 6(10):951-8; PMID:11006325; http://dx.doi.org/ 10.1093/molehr/6.10.951 [DOI] [PubMed] [Google Scholar]
- 90.Wang H, Cheng H, Shao Q, Dong Z, Xie Q, Zhao L, Wang Q, Kong B, Qu X. Leptin-promoted human extravillous trophoblast invasion is MMP14 dependent and requires the cross talk between Notch1 and PI3K/Akt signaling. Biol Reprod 2014; 90(4):78; PMID:24571988; http://dx.doi.org/ 10.1095/biolreprod.113.114876 [DOI] [PubMed] [Google Scholar]
- 91.Plaisier M, Dennert I, Rost E, Koolwijk P, van Hinsbergh VW, Helmerhorst FM. Decidual vascularization and the expression of angiogenic growth factors and proteases in first trimester spontaneous abortions. Hum Reprod 2009; 24(1):185-97; PMID:18854409; http://dx.doi.org/ 10.1093/humrep/den296 [DOI] [PubMed] [Google Scholar]
- 92.Xu P, Wang Y, Piao Y, Bai S, Xiao Z, Jia Y, Luo S, Zhuang L. Effects of matrix proteins on the expression of matrix metalloproteinase-2, -9, and -14 and tissue inhibitors of metalloproteinases in human cytotrophoblast cells during the first trimester. Biol Reprod 2001; 65(1):240-6; PMID:11420245; http://dx.doi.org/ 10.1095/biolreprod65.1.240 [DOI] [PubMed] [Google Scholar]


