abstract
The objective of this study was to investigate whether human placental multipotent mesenchymal stromal cell (hPMSC)-derived Slit2 and endothelial cell Roundabout (Robo) receptors are involved in placental angiogenesis. The hPMSC-conditioned medium and human umbilical vein endothelial cells were studied for Slit2 and Robo receptor expression by immunoassay and RT-PCR. The effect of the conditioned medium of hPMSCs with or without Slit2 depletion on endothelial cells was investigated by in vitro angiogenesis using growth factor-reduced Matrigel. hPMSCs express Slit2 and both Robo1 and Robo4 are present in human umbilical vein endothelial cells. Human umbilical vein endothelial cells do not express Robo2 and Robo3. The hPMSC-conditioned medium and Slit2 recombinant protein significantly inhibit the endothelial cell migration, but not by the hPMSC-conditioned medium with Slit2 depletion. The hPMSC-conditioned medium and Slit2 significantly enhance endothelial tube formation with increased cumulated tube length, polygonal network number and vessel branching point number compared to endothelial cells alone. The tube formation is inhibited by the depletion of Slit2 from the conditioned medium, or following the expression of Robo1, Robo4, and both receptor knockdown using small interfering RNA. Furthermore, co-immunoprecipitation reveals Slit2 binds to Robo1 and Robo4. Robo1 interacts and forms a heterodimeric complex with Robo4. These results suggest the implication of both Robo receptors with Slit2 signaling, which is involved in endothelial cell angiogenesis. Slit2 in the conditioned medium of hPMSCs has functional effect on endothelial cells and may play a role in placental angiogenesis.
KEYWORDS: angiogenesis, endothelial cells, placental multipotent mesenchymal stromal cells, Robo, Slit2
Introduction
Slit proteins were initially described in the developing central nervous system of Drosophila as axonal repellents, which regulated the migration of neurons and axons by binding to cognate Roundabout (Robo)receptors.1 Slit has 3 isoforms (Slit1–3) and Robo has 4 (Robo1–4). Robo2 and Robo3 are abundantly expressed in the nervous system but undetectable in the vascular system.1,2 Slit–Robo signaling functions in a variety of developmental processes, such as kidney development,3 chemoattractants of vascular endothelial cells,4 leukocytes and cancer cell migration.5,6 Therefore, it is insightful to investigate the roles of Slit–Robo signaling in the placenta and their mechanisms.
Endothelial cells have been found to express Robo1 and Robo4, suggesting the involvement of Slit2–Robo signaling in vascular development.7 Robo4 is expressed specifically in vascular endothelial cells.2 Previous studies on endothelial migration induced by Slit–Robo signaling however are inconsistent. Some reports suggest that Slit2 promotes angiogenesis in human umbilical vein endothelial cells (HUVECs) through Robo1,7,8 and others show that Slit2 inhibits migration of HUVECs through Robo4.2,9 Slit2 was shown to inhibit VEGF-induced microvascular endothelial cell migration, tube formation and endothelial cell permeability in a Robo4-dependent manner.10 Additionally, Robo1 was proposed to form a heterodimer with Robo4,11 and siRNA knockdown of Robo1 or Robo4 reduced the inhibitory effect of Slit on HUVEC migration and permeability.12 Thus, Robo1 and Robo4 have been suggested to co-express in endothelial cells,9 and regulate Slit2 responses differentially through signaling cascades.13,14
Placental angiogenesis starts from day 32 of gestation, but development of the vascular tree continues to term.15 Angiogenesis is a complex process that involves extracellular matrix alteration, endothelial cell proliferation, differentiation and migration, and vessel stabilization by pericytes and mural cells.16 We previously isolated human placental multipotent mesenchymal stromal cells (hPMSCs) from placentas.17,18 These cells were found to distribute in villous stroma, but their role in the placental villous microenvironment remains unknown. Multipotent mesenchymal stromal cells are known to produce various soluble growth factors and cytokines.19-21 We previously observed that hPMSCs expressed IL-6, IL-8 and HGF, which are involved in endothelial cell protection from oxidative stress and trophoblast migration.22,23 In the present study, we also observed that hPMSCs express Slit2. However, the role of Slit2 in the placental villous microenvironment has not been explored. Thus, we hypothesize that hPMSCs express Slit2, which may modulate endothelial cells in placental angiogenesis via Robo1/4 receptors.
Results
Differential expression of Slit2, Robo1 and Robo4 in HUVECs and hPMSCs
The mRNA encoding Robo1–4 of HUVECs was studied, and mRNAs of Robo1 and Robo4 were found in HUVECs (Fig. 1A). Slit2 mRNA was also observed in hPMSCs, while Slit3 mRNA expressed significantly less than Slit2 (Fig. 1C). Protein expression of Robo1 and Robo4 in HUVECs and that of Slit2 in hPMSCs were further confirmed by Western blot (Figs. 1B and D).
Figure 1.
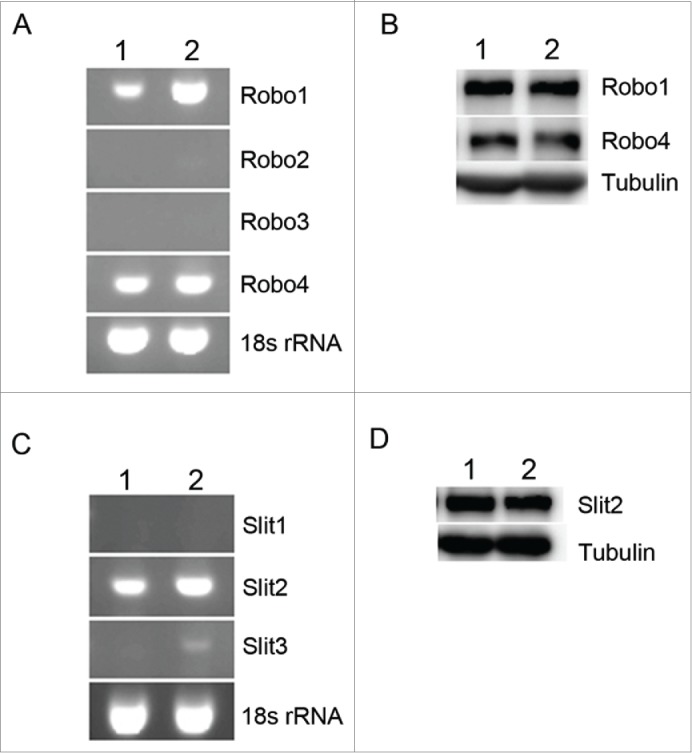
Determination of the levels of Slit2, Roundabout (Robo)1 and Robo4 in human umbilical vein endothelial cells (HUVECs) and human placental multipotent mesenchymal stromal cells (hPMSCs). The mRNA and protein levels of Slit2, Robo1 and Robo4 were examined 24 hours after cell plating by RT-PCR (A, C) and Western blotting (B, D). The mRNA (A) and protein (B) expression of Robo1 and Robo4 expressions were detected in HUVECs. Both mRNA (C) and protein (D) levels of Slit2 were observed in hPMSCs. 1, 2: different strains of cells in HUVECs (A, B) or hPMSCs (C, D).
Effect of Slit2 and hPMSC-conditioned medium on HUVEC migration
Analyzing the conditioned medium of hPMSCs by Western blot and ELISA revealed that hPMSCs expressed Slit2. The Slit2 level in the hPMSC-conditioned medium significantly decreased after Slit2 depletion (Fig. 2A). When the HUVECs were exposed to Slit2 recombinant protein or the hPMSC-conditioned medium, decreased migration of HUVECs compared to HUVECs in the EGM control medium was observed. The inhibition effect of the hPMSC-conditioned medium on HUVEC migration was abrogated when Slit2 in hPMSC-conditioned medium was depleted by a blocking antibody (p < 0.01; Fig. 2B).
Figure 2.
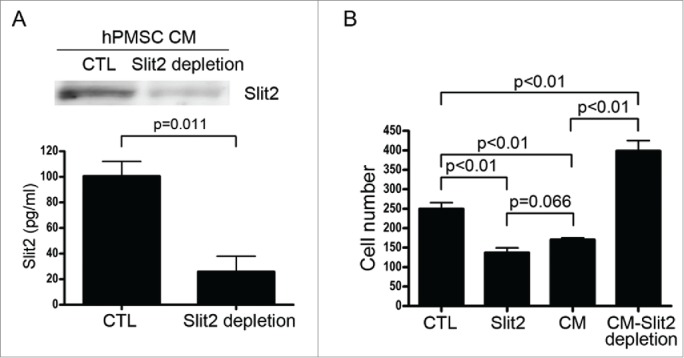
Slit2 was expressed by human placental multipotent mesenchymal stromal cells (hPMSCs) and the Slit2 effect on HUVEC migration. (A) The Slit2 levels in the hPMSC-conditioned medium before and after Slit2 depletion by immunoprecipitation was assessed by Western blot (upper panel) and enzyme-linked immunosorbent assay (lower panel). CTL: hPMSC-conditioned medium without Slit2 depletion. Data are mean ± SD of 3 independent experiments. (B) The HUVEC migration ability was significantly reduced by EGM with 2% FBS (CTL) containing 1 μg/ml Slit2 (Slit2), or CTL mixed with a hPMSC-conditioned medium containing 2% FBS (1:1 ratio; CM), but the inhibition was abrogated by Slit2 depletion from the conditioned medium (CTL mixed with hPMSC-conditioned medium with 2% FBS after Slit2 depletion, 1:1 ratio; CM-Slit2 depletion). CTL: EGM with 2% FBS. Data are mean ± SD of 3 independent experiments.
Endothelial cell-hPMSC interactions mediated by Slit2–Robo1/4 in vitro
To investigate the functional correlation of the endothelial cell–hPMSC interaction in vascularization, we investigated the effects of Slit2 and hPMSC-conditioned medium on formation of tube structures and endothelial cell-network stabilization formed by HUVECs on basement membrane-like growth factor-reduced Matrigel. HUVECs cultured in the EGM control medium were used as a control, and they were able to form characteristic tube structures (Fig. 3A). Culture medium containing either Slit2 recombinant protein or the hPMSC-conditioned medium significantly enhanced HUVEC elongation and formation of interconnecting cell networks. The tube structure was significantly inhibited in HUVECs cultured in medium containing hPMSC-conditioned medium with Slit2 depletion. These findings indicated that Slit2 significantly increased the ability of HUVECs to form tube structures (Figs. 3B–D). Quantification of the tube structures by measuring the cumulative tube length, polygonal network number and vessel branching point number was shown in Figures 3E–G. Thus, Slit2 expressed by hPMSCs supported tube formation of HUVECs in Matrigel assays.
Figure 3.
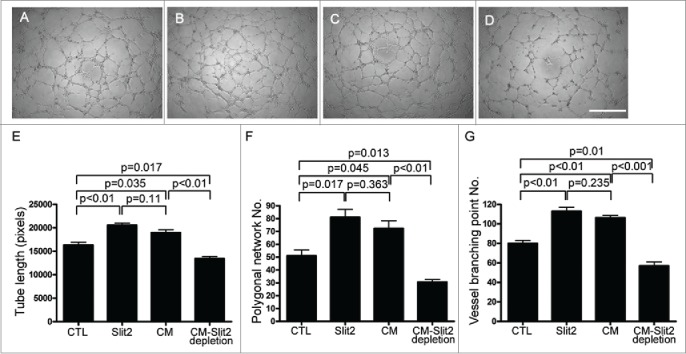
In vitro angiogenesis: The effect of Slit2 on the formation of tube structures by human umbilical vein endothelial cells (HUVECs). Slit2 increased the angiogenesis in HUVECs. HUVECs (1 × 104 cells/well) were plated on growth factor-reduced Matrigel and cultured in 50 μl of (A) EGM with 2% FBS (CTL), (B) CTL containing 1 μg/ml Slit2 (Slit2), (C) CTL mixed with hPMSC conditioned medium with 2% FBS (1:1 ratio; CM) or (D) CTL mixed with hPMSC-conditioned medium with 2% FBS after Slit2 depletion (1:1 ratio; CM-Slit2 depletion). HUVECs formed characteristic tube structures. Cell elongation and interconnecting cell networks were observed. Slit2 significantly increased the ability of HUVECs to form tube structures. Representative data of 5 different experiments are shown. Quantification of the tube structures by measuring (E) the cumulative tube length, (F) the polygonal network number and (G) vessel branching point number formed by HUVECs in different culture conditions. A significant inhibition of tube structure formation was observed when Slit2 was depleted from the hPMSC conditioned medium. Error bar: SD. Scale bar: 100 μm.
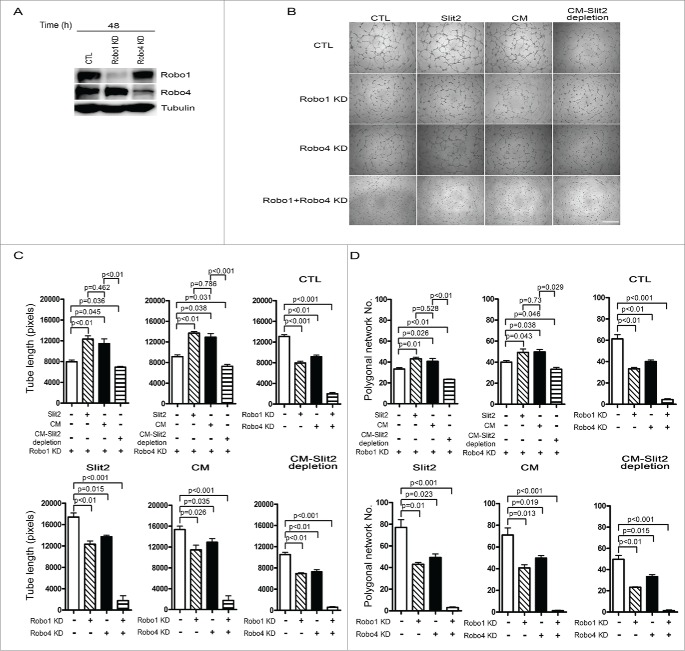
To examine the role of Robo1 and Robo4 in angiogenesis, Robo1 and Robo4 genes were transiently knocked down in HUVECs using siRNAs. The expression of Robo1 or Robo4 was significantly reduced in Robo1- or Robo4-silenced endothelial cells, respectively. The expression of Robo4 in endothelial cells with Robo1 knockdown was not affected compared to that with non-silencing control siRNA transfection. Similarly, the expression of Robo1 did not alter in endothelial cells with Robo4 knockdown (Fig. 4A). In vitro angiogenesis assays using growth factor-reduced Matrigel, Robo1 knockdown HUVECs showed an impaired ability to form a tube network, as fewer branches were formed. Similarly, tube formation was significantly inhibited in Robo4 knockdown HUVECs compared to the controls (Fig. 4B). The tube formation was significantly increased in Robo1- or Robo4-silenced HUVECs cultured in the medium containing Slit2 recombinant protein or hPMSC-conditioned medium, but was significantly reduced in cells cultured in the medium containing an Slit2-depleted hPMSC-conditioned medium (Fig. 4B). Quantification of the tube structures by measuring the cumulative tube length, the polygonal network number (Figs. 4C and 4D) and vessel branching point number was shown (Fig. S1). However, even though the knockdown efficiencies of Robo4 and Robo1 varied slightly, we did find an additive effects due to knockdown of both Robo1 and Robo4 on suppressing tube formation (Figs. 4A, 4C and 4D). There was nearly no tube formation in the HUVECs with or without medium containing Slit2 recombinant protein or hPMSC-conditioned medium. (Figs. 4B–D, Fig. S1). Based on these observations, we could not conclude which Robo receptor played a more important role in angiogenesis.
Figure 4.
Robo1 and Robo4 receptors were involved in angiogenesis in human umbilical vein endothelial cells (HUVECs). Robo1, Robo4, and both receptor knockdown (KD) significantly inhibited angiogenesis. (A) The Robo1- (Robo1 KD) and Robo4-silenced (Robo4 KD) in HUVECs were shown by Western blot. (B) In vitro angiogenesis was evaluated 4 hours after Robo1, Robo4 and both receptor knockdown in HUVECs. The cells were plated on growth factor-reduced Matrigel, treated with EGM with 2% FBS (CTL), CTL containing 1 μg/ml Slit2 (Slit2), CTL mixed with hPMSC-conditioned medium with 2% FBS (1:1 ratio; CM) or CTL mixed with hPMSC-conditioned medium with 2% FBS after Slit2 depletion (1:1 ratio; CM-Slit2 depletion). Quantitative analysis showed that the cumulative tube length (C), polygonal network number (D) in various culture conditions were increased significantly with 1 μg/ml Slit2 and CM treatment, and the enhanced effects were blocked significantly after Robo1, Robo4 and both receptor knockdown. HUVECs in CTL were transfected with non-silencing control siRNA. Error bar: SD. Scale bar: 100 μm.
Heterodimerization of Robo1 and Robo4
The Robo receptors were known to form homo- and heterodimers, which were important for their functions.11 Slit2 could modulate the expression of Robo1 and Robo4 receptor in endothelial cells (Fig. 5A). We further demonstrated that the expression levels of Robo1 and Robo4 receptors in endothelial cells decreased with increasing doses of Slit2 from 0.5 to 2 μg/ml (Fig. 5A). To study heterodimerization, endogenous Robo1 was immunoprecipitated from HUVEC lysates with or without Slit2 treatment. Robo1 and Robo4 Western blots showed that endogenous Robo1 co-immunoprecipitated with Robo4, suggesting that these proteins interacted in HUVECs. Furthermore, immunoprecipitates followed by Slit2 immunblot also revealed the interaction between Slit2 and Robo receptors (Fig. 5B).
Figure 5.
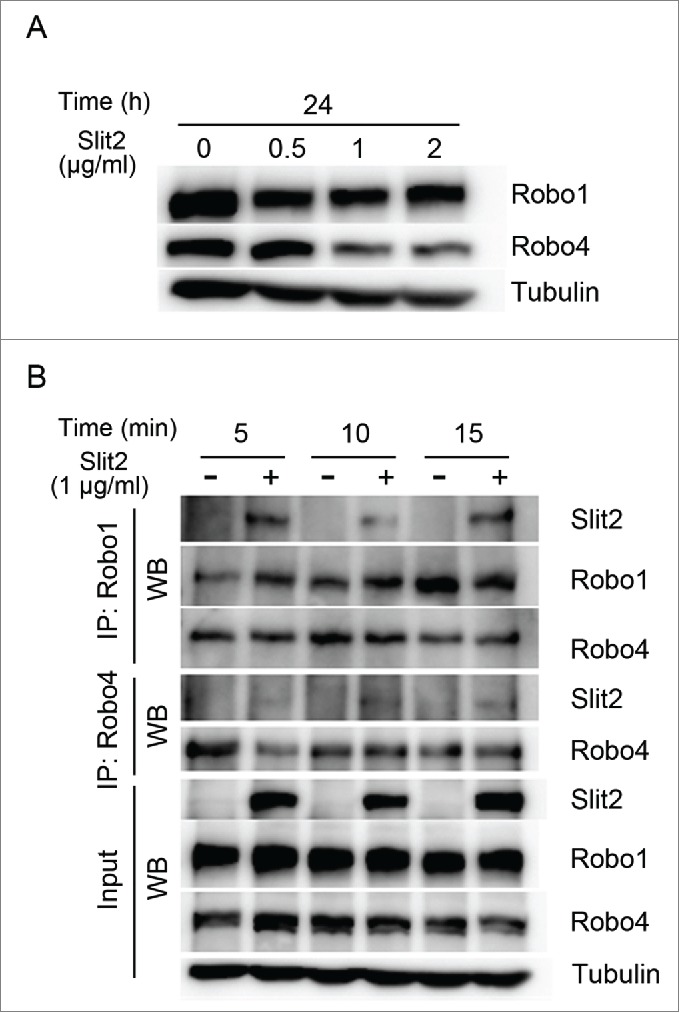
Slit2 modulates the expression and interaction of Robo1 and Robo4 receptors in human umbilical vein endothelial cells (HUVECs). (A) Robo1 and Robo4 were expressed in HUVECs. The Robo1 and Robo4 levels decreased as Slit2 treatment concentration increased as shown in Western blot. (B) The interaction between Robo1 and Robo4 with or without 1 μg/mL Slit2 for 5 to 15 minutes was examined by co-immunoprecipitation (IP) in HUVEC lysate. The HUVEC Robo1 was immunoprecipitated from HUVEC lysate and probed by an antibody against Robo1, Robo4 or Slit2. Western blot (WB) revealed that endogenous Robo1 co-immunoprecipitates with Robo4. Slit2 interacted with the Robo1/4 receptor complex. The lower panel showed the input of Slit2, Robo1, Robo4 and α-tubulin.
Discussion
This study revealed that the Slit2 secreted by hPMSCs was an important modulator for Robo1/Robo4 mediated endothelial cell migration and angiogenesis in vitro. Robo2 and Robo3 mRNA was not detected in endothelial cells, whereas Robo1 and Robo4 mRNA exhibited relatively high levels of expression. Robo1 and Robo4 are the cognate receptors of endothelial cells for Slit2. Slit2 binds to the heterodimer receptor formed by Robo1 and Robo4. Co-culture of hPMSCs with endothelium on a growth factor-reduced Matrigel, the Slit2 or an hPMSC-conditioned medium containing Slit2 could enhance endothelial cells forming more tube networks than endothelial cells alone. Tube formation was significantly reduced in endothelial cells cultured with the Slit2-depleted hPMSC-conditioned medium or in endothelial cells transfected with siRNA for Robo1, Robo4 or both. These observations indicated that hPMSC-derived Slit2 was involved in tube formation. Robo1 cooperated with Robo4 to modulate signaling pathways downstream of Slit2 and mediate angiogenesis in vitro.
Mesenchymal stem cells have been reported to cooperate with endothelial cells to enhance and stabilize vascular network formation24,25 through secreting angiogenic growth factors with or without the need of a direct cell to cell contact.26,27 We previously showed that hPMSCs have a phenotype similar to that of bone marrow mesenchymal stem cells.18 Here, we also observed that the hPMSC-conditioned medium could stabilize endothelial cell vascularization with increasing cumulative tube length, polygonal network number and vessel branching point number. Analysis of hPMSC-secreted proteins revealed Slit2 mediated these responses, extending our knowledge of vascular endothelial growth factor, bFGF, and hepatocyte growth factor secreted by stem cells associated with angiogenesis.19,28,29
Our data have shown that Slit2-Robo receptor signaling was involved in angiogenesis.7,9 Slit2 released from tumor cells has been found to affect Robo1-dependent tumor angiogenesis.7 By binding to Robo4, Slit2 can stabilize the vasculature and inhibit VEGF-induced endothelial cell migration and permeability in vitro, and prevent pathologic angiogenesis in mouse retina.10 Vessel maturation during angiogenesis requires recruitment of mural cells and development of surrounding matrix to stabilize endothelial cells and inhibit endothelial cell migration.16 We thus suggested that Slit2 could modulate placenta angiogenesis and maintain vessel integrity via Slit2-Robo1/Robo4 interaction. We observed that hPMSCs from placental villous stroma expressed Slit2, which inhibited endothelial cell migration to stabilize tube formation, as demonstrated by the effects of Slit2 recombinant protein or the hPMSC-conditioned medium on cell elongation and tube network formation. Additionally, pericytes and vascular smooth muscle cells constitutively expressed Slit2 and/or Robo1 and Robo4,10,11,30 raising the possibility of Slit2-based autocrine and paracrine influence on vascularization. These findings supported the hypothesis that hPMSC-derived Slit2 might contribute to placenta angiogenesis in a similar communication network involved mural cells and endothelial cells. Nonetheless, there were contradictory results showing that binding of Slit2 to Robo1/4 receptor induced actin cytoskeleton movements, filopodia formations, and led to endothelial cell migration.11
Our results revealed Slit2 modulation of Robo1 and Robo4 receptor expression in endothelial cells. The expression levels of Robo1 and Robo4 receptors decreased as Slit2 concentrations were increased from 0.5 to 2 μg/ml. This finding could be explained by the observation that microRNA-218 encoded intronically in Slit2 gene and shared the same transcript as Slit2, targeted the untranslated region of the Robo receptor and inhibited its protein translation.31 HUVECs treated by Slit2 significantly increased the intracellular levels of Slit2 and microRNA-218, revealing a positive regulation of Slit2 expression.31 The microRNA-218 negatively regulated the expression of Robo1 and Robo2.31 This suggested potentially there was a reciprocal modulation between Slit2 and microRNA-218 on Robo expression. Conceivably, increasing Slit2 protein expression drove synthesis of microRNA-218, which could then suppress the expression of Robo receptor and Slit2-Robo signaling. A study on zebrafish heart tube formation also observed Slit2 stimulation suppressed Robo1 and Robo2 expression and inhibited endocardial migration.32. In our co-immunoprecipitation experiments, the interaction between Slit2 and Robo1/Robo4 was not altered. Thus, the optimal levels of Slit2 and Robo expression involved in angiogenesis will need further investigation. Additionally, Robo4 can be co-immunoprecipitated with Robo1 in cultured endothelial cells with or without Slit2 stimulation. In agreement with previous reports,11,13 this finding suggests that Robo1–Robo4 heterodimerization responds to Slit2 binding, leading to Robo1 and/or Robo4 downstream signaling and cellular responses.13,14
A variety of signaling factors are known to orchestrate placenta angiogenesis, including VEGF, PDGF and Ang/Tie 2.33-35 Here we found that Slit2–Robo signaling might also play an important role. Slit2 is expressed by hPMSCs and therefore may act in a paracrine fashion through interacting with Robo receptors expressed by endothelial cells. The present data extend our understanding of hPMSC functions in villous development, particularly in placenta vascularization.
Materials and methods
hPMSC isolation and culture
Placental tissue was obtained after informed consent of the women, and all experiments were approved by the Institutional Review Board of Mackay Memorial Hospital, Taipei. hPMSCs were isolated from clinically normal human term placentas (37 to 40 weeks of gestation) collected after cesarean section as previously described.17,18 Briefly, about 100 g of tissue from central placental cotyledons was minced. The tissue was trypsinized (0.25% trypsin-EDTA solution; Invitrogen, Carlsbad, CA) and treated with 10 U/ml DNAse I (Sigma-Aldrich, St. Louis, MO) in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Grand Island, NY, USA) at 37°C for 5 min 3 times, and finally filtered through a cell strainer (BD Biosciences, San Diego, CA). The supernatants were centrifuged and the mononuclear cells in the supernatants were recovered by Percoll density gradient fractionation (1.073 g/ml, Sigma-Aldrich). The cell cultures were maintained in DMEM (Gibco-BRL) with 10% FBS (Hyclone, Logan, UT) at 37°C. The hPMSCs which were initially isolated from tissue and plated on culture dish were defined as passage 0. The cells were used for experiments at passage 4 to 5.
The cell phenotype of the hPMSCs was characterized by a panel of phycoerythrin- or fluorescein isothiocyanate-conjugated antibodies using standard fluorescence-activated cell sorting analysis and CellQuest software as previously described.17,18
The hPMSCs isolated from term placentas were found to express CD13, CD29, CD44, CD49b, CD54, CD73, CD90, CD105, CD166 and SSEA4, independent of gestational age. The cells are negative for CD14, CD34, CD45, HLA-DR, but are multipotent with the ability to differentiate into osteocytes, adipocytes and endothelial cells (not shown).18,23,36
HUVECs collected as previously described18 were cultivated using an endothelial cell growth medium (EGM) kit with SupplementPack (PromoCell GmbH, Heidelberg, Germany). The cells were used for experiments at passage 4 to 6.
Preparation of the conditioned medium
hPMSCs were grown in DMEM (Gibco-BRL) with 10% FBS (Hyclone) until 80% confluent. The culture medium was removed and cell layers were washed and incubated with endothelial cell basal medium (PromoCell) with 2% FBS (Hyclone) for 2 days. Conditioned medium was collected, centrifuged at 2300 × g for 5 min, passed through a 0.22 μm filter, and stored at −80°C for use in subsequent experiments.
To remove Slit2 from the conditioned medium, the medium was incubated with 2.4 μg/ml of anti-Slit2 polyclonal antibody (GeneTex, Hsinchu, Taiwan) for 4 to 6 h at 4°C with constant rotation. Then, 100 μl of protein G-agarose beads (50% slurry; Thermo Scientific, Rockford, Illinois, USA) were added and incubated overnight at 4°C with rotation. Conditioned medium containing agarose beads was centrifuged at 800 × g for 3 min and the supernatant was collected and used immediately. To verify that Slit2 had been removed from the conditioned medium, an aliquot of the conditioned medium stripped of Slit2 through immunoprecipitation was tested for Slit2 concentration by an enzyme-linked immunosorbent assay.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was used to assess the Slit2 concentration in the hPMSC supernatant as per the manufacturer′s instructions (MyBioSource, San Diego, California, USA).
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA (1 μg) of isolated HUVECs and hPMSCs was extracted using the TRIzol reagent (Life Technologies, Carlsbad, CA) according to the manufacturer's protocol, and cDNA was synthesized using oligodeoxythymidine (Promega Corporation, Madison, WI) and a Superscript II reverse transcriptase (Invitrogen). The cDNA was amplified via PCR for 35 cycles at 95°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec. 18s rRNA was used as an internal control. PCR products were checked by 2% agarose gel (Amresco, Solon, OH) electrophoresis. The primers used were shown in Table 1.
Table 1.
The primer sequences used for RT-PCR and siRNA.
| RT-PCR | ||
|---|---|---|
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
| Slit1 | TGGCCTTCCCTGACTTCAGGTGTG | GTTCCTTGTAGCCAGTCTTCACCC |
| Slit2 | CAGATCAAAAGCAAGAAATTCCGTTG | GAACATCTTATGCTGCACATTTTCC |
| Slit3 | CCTCTGTCAGCATGAGGCCAAGTGC | CGTGGCCCTGGTACAGCTCCAGTG |
| Robo1 | CAGCCATGCATCTGGTAGCAGC | CACTATCTGCTCCTTGAAATTCATT |
| Robo2 | GATCAGATTGTTGCTCAAGGTCG | GTAAATCCCTCCTTTAACCAGC |
| Robo3 | GGGAAGCTGATGATGTCACATAC | TCCTTCTGCCAGAAGATGGCAG |
| Robo4 | GCAGCAGCAGCCTCAGCAGTCG | TCTGCAGGGGCCAGAGACAAGC |
| 18s rRNA | TAGAGCTAATACATGCCGACGG | GGGCCTCGAAAGAGTCCTGTATT |
| siRNA | ||
| Gene | SMART pool (5′-3′) | |
| Robo1 | GAAUCAGACUGGUUAGUUU | |
| GCAGGUACUUGGAGGAUAU | ||
| GAGGGCAGCUAAUGCAUAU | ||
| GGAUGUAUUUGCAACAAGA | ||
| Robo4 | CCUCAGAGUUCACGGACAU | |
| GGGCCAAGACUACGAGUUC | ||
| GGGAGGAUCAAGACAGCGU | ||
| UAGCUUUGGUUUCGGUCUA | ||
Western blot
Total protein (30 μg) was separated by 8% sodium dodecyl sulfate-polyacrylamide gel and transferred on to an Immobilon polyvinylidene difluoride membrane (Amersham Biosciences, Piscataway, NJ). We conducted immunoblot analysis using antibodies against Slit2 (1:1000; GeneTex), Robo1 (1:1000; GeneTex), Robo4 (1:1000; Santa Cruz Biotechnology, Dallas, TX), α-Tubulin (1:5000; Millipore, Temecula, CA) overnight at 4°C, and followed by incubation with the appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The proteins were visualized by chemiluminescence detection kit (Millipore).
Transwell migration
HUVECs (1.0 × 104 cells/well) were added to the upper chamber of a Transwell® device (Costar) and allowed to migrate for 24 h in EGM (PromoCell) with 2% FBS containing either Slit2 (1 μg/ml, Peprotech, Rocky Hill, NJ), EGM (PromoCell) mixed with hPMSC conditioned medium with or without Slit2 depletion (1:1 ratio) in the lower chamber. Trans-membrane migrated cells were stained by DAPI (Sigma-Aldrich) and quantified under microscopy (magnification 50; Axiovert 200; Carl Zeiss MicroImaging) equipped with Image-Pro Plus software (Media Cybernetics).
Transfection with Robo1 siRNAs or Robo4 siRNAs
The HUVECs (5 × 105) were seeded in a 6-cm dish with EGM (PromoCell GmbH) containing 2% FBS (Hyclone) for overnight. The HUVECs were then transfected with the siRobo1 (SMART pool; Dharmacon, Lafayette, CO), siRobo4 (SMART pool; Dharmacon), or both (Table 1) and non-silencing control siRNA (Invitrogen) using a jetPRIME (Polyplus, New York, NY) transfection reagent. After 48 h of transfection, the levels of Robo1 and Robo4 were confirmed by Western blotting analysis.
Co-immunoprecipitation assay
To demonstrate the homo- or heterodimerization of Robo1 and Robo4, HUVECs treated with or without 1 μg/ml Slit2 recombinant protein (Peprotech) for 5 to 15 min were extracted in 1× CHAPS lysis buffer (20 mM Tris, 136.8 mM NaCl pH7.5, 1% CHAPS) and centrifuged at 10,000 × g for 15 min at 4°C to obtain cell lysates. Cell lysates (150 μg) were precleared with 40 μl protein G beads (Thermo Fisher Scientific, Rockford, IL) for 30 min at 4°C and subsequently centrifuged at 4,000 × g for 5 min at 4°C to discard protein G beads (Thermo Fisher Scientific). The cell lysates were incubated with an anti-Robo1 or anti-Robo4 (R&D) antibody overnight at 4°C, followed by an addition of 40 μl protein G beads (Thermo Fisher Scientific) for 3 h at 4°C. Protein G beads (Thermo Fisher Scientific) were collected by centrifugation at 3,000 × g for 5 min at 4°C, and the immunoprecipitates were washed twice with 1× CHAPS lysis buffer and once with PBS. The protein complexes were subjected to immunoblot with specific primary anti-Slit2 (GeneTex), Robo1 (GeneTex), and Robo4 (Abcam, Cambridge, UK) antibodies, and subsequently hybridized with the respective secondary antibody.
In vitro angiogenesis
The μ-slide angiogenesis (ibidi GmbH, München, Germany) was coated with 10 μl per well of growth factor-reduced Matrigel (BD Biosciences). Slides were incubated at 37°C for 30 min. The HUVECs were plated at a number of 1 × 104 cells/well. HUVECs were cultured in 50 μl of EGM/2% FBS, EGM/2% FBS with 1 μg/ml Slit2 (Peprotech), EGM/2% FBS mixed with hPMSCs conditioned medium (1:1 ratio) or EGM/2% FBS mixed with hPMSCs conditioned medium after Slit2 depletion (1:1 ratio) at 37°C with 5% CO2 for 4 h and monitored by a microscope (Zeiss). For studying the role of Robo receptors in angiogenesis, HUVECs were transfected by Robo1 siRNA, Robo4 siRNA (Dharmacon), both siRNA or non-silencing siRNA control (Invitrogen) and incubated as described. Tube formation was quantified by counting the sum of the tube length or the tube formation structures by measuring the polygonal network and vessel branching point number in each well. The total length of the tubes was calculated as the sum of the length of the individual branches including several tube-like structures merged together or branched.18 Results are represented as total tube length (pixels) or a number for 6 random photographic fields per experimental condition (magnification 50; Axiovert 200; Carl Zeiss MicroImaging).
Statistics
The data are presented as means ± standard deviation. At least 3 independent experiments were carried out for each experiment. Differences were assessed using the Student′s t test. A p value of <0.05 is considered significant.
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest.
Funding
This work was supported by a grant from Mackay Memorial Hospital (MMH-E 103001) and Taiwan's Ministry of Science and Technology (MOST-104-2314-B-195- 010-MY3 to C.-P Chen).
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
References
- 1.Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Ann Rev Cell Dev Biol 2006; 22:651-75; PMID:17029581; http://dx.doi.org/ 10.1146/annurev.cellbio.21.090704.151234 [DOI] [PubMed] [Google Scholar]
- 2.Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol 2003; 261:251-67; PMID:12941633; http://dx.doi.org/ 10.1016/S0012-1606(03)00258-6 [DOI] [PubMed] [Google Scholar]
- 3.Grieshammer U, Le M, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell 2004; 6:709-17; PMID:15130495; http://dx.doi.org/ 10.1016/S1534-5807(04)00108-X [DOI] [PubMed] [Google Scholar]
- 4.Wang LJ, Zhao Y, Han B, Ma YG, Zhang J, Yang DM, Mao JW, Tang FT, Li WD, Yang Y, et al.. Targeting Slit-Roundabout signaling inhibits tumor angiogenesis in chemical-induced squamous cell carcinogenesis. Cancer Sci 2008; 99:510-7; PMID:18201275; http://dx.doi.org/ 10.1111/j.1349-7006.2007.00721.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu JY, Feng L, Park HT, Havlioglu N, Wen L, Tang H, Bacon KB, Jiang Z, Zhang X, Rao Y. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 2001; 410:948-52; PMID:11309622; http://dx.doi.org/ 10.1038/35073616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuasa-Kawada J, Kinoshita-Kawada M, Rao Y, Wu JY. Deubiquitinating enzyme USP33/VDU1 is required for Slit signaling in inhibiting breast cancer cell migration. Proc Natl Acad Sci U S A 2009; 106:14530-5; PMID:19706539; http://dx.doi.org/ 10.1073/pnas.0801262106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang B, Xiao Y, Ding BB, Zhang N, Yuan X, Gui L, Qian KX, Duan S, Chen Z, Rao Y, et al.. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 2003; 4:19-29; PMID:12892710; http://dx.doi.org/ 10.1016/S1535-6108(03)00164-8 [DOI] [PubMed] [Google Scholar]
- 8.Howitt JA, Clout NJ, Hohenester E. Binding site for Robo receptors revealed by dissection of the leucine-rich repeat region of Slit. EMBO J 2004; 23:4406-12; PMID:15496984; http://dx.doi.org/ 10.1038/sj.emboj.7600446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seth P, Lin Y, Hanai J, Shivalingappa V, Duyao MP, Sukhatme VP. Magic roundabout, a tumor endothelial marker: expression and signaling. Biochem Biophys Res Commun 2005; 332:533-41; PMID:15894287; http://dx.doi.org/ 10.1016/j.bbrc.2005.03.250 [DOI] [PubMed] [Google Scholar]
- 10.Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F, Mukouyama YS, et al.. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med 2008; 14:448-53; PMID:18345009; http://dx.doi.org/ 10.1038/nm1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheldon H, Andre M, Legg JA, Heal P, Herbert JM, Sainson R, Sharma AS, Kitajewski JK, Heath VL, Bicknell R. Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors. FASEB J 2009; 23:513-22; PMID:18948384; http://dx.doi.org/ 10.1096/fj.07-098269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, et al.. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med 2010; 2:23ra19; PMID:20375003; http://dx.doi.org/ 10.1126/scitranslmed.3000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur S, Samant GV, Pramanik K, Loscombe PW, Pendrak ML, Roberts DD, Ramchandran R. Silencing of directional migration in roundabout4 knockdown endothelial cells. BMC Cell Biol 2008; 9:61; PMID:18980679; http://dx.doi.org/ 10.1186/1471-2121-9-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huminiecki L, Gorn M, Suchting S, Poulsom R, Bicknell R. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics 2002; 79:547-52; PMID:11944987; http://dx.doi.org/ 10.1006/geno.2002.6745 [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta 2004; 25:114-26; PMID:14972444; http://dx.doi.org/ 10.1016/j.placenta.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 16.Ribatti D, Crivellato E. “Sprouting angiogenesis”, a reappraisal. Dev Biol 2012; 372:157-65; PMID:23031691; http://dx.doi.org/ 10.1016/j.ydbio.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 17.Chen CP, Liu SH, Huang JP, Aplin JD, Wu YH, Chen PC, Hu CS, Ko CC, Lee MY, Chen CY. Engraftment potential of human placenta-derived mesenchymal stem cells after in utero transplantation in rats. Hum Reprod 2009; 24:154-65; PMID:18845668; http://dx.doi.org/ 10.1093/humrep/den356 [DOI] [PubMed] [Google Scholar]
- 18.Lee MY, Huang JP, Chen YY, Aplin JD, Wu YH, Chen CY, Chen PC, Chen CP. Angiogenesis in differentiated placental multipotent mesenchymal stromal cells is dependent on integrin alpha5beta1. PLoS One 2009; 4:e6913; PMID:19847290; http://dx.doi.org/ 10.1371/journal.pone.0006913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 2004; 94:678-85; PMID:14739163; http://dx.doi.org/ 10.1161/01.RES.0000118601.37875.AC [DOI] [PubMed] [Google Scholar]
- 20.Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 2007; 25:2363-70; PMID:17540857; http://dx.doi.org/ 10.1634/stemcells.2006-0686 [DOI] [PubMed] [Google Scholar]
- 21.Barbet R, Peiffer I, Hatzfeld A, Charbord P, Hatzfeld JA. Comparison of Gene Expression in Human Embryonic Stem Cells, hESC-Derived Mesenchymal Stem Cells and Human Mesenchymal Stem Cells. Stem Cells Int 2011; 2011:368192; PMID:21941565; http://dx.doi.org/ 10.4061/2011/368192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu SH, Huang JP, Lee RK, Huang MC, Wu YH, Chen CY, Chen CP. Paracrine factors from human placental multipotent mesenchymal stromal cells protect endothelium from oxidative injury via STAT3 and manganese superoxide dismutase activation. Biol Reprod 2010; 82:905-13; PMID:20107204; http://dx.doi.org/ 10.1095/biolreprod.109.081828 [DOI] [PubMed] [Google Scholar]
- 23.Chen CP, Huang JP, Chu TY, Aplin JD, Chen CY, Wu YH. Human placental multipotent mesenchymal stromal cells modulate trophoblast migration via Rap1 activation. Placenta 2013; 34:913-23; PMID:23896030; http://dx.doi.org/ 10.1016/j.placenta.2013.06.311 [DOI] [PubMed] [Google Scholar]
- 24.Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, Germanguz I, Amit M, Itskovitz-Eldor J. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation 2012; 125:87-99; PMID:22095829; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.111.048264 [DOI] [PubMed] [Google Scholar]
- 25.Kreutziger KL, Muskheli V, Johnson P, Braun K, Wight TN, Murry CE. Developing vasculature and stroma in engineered human myocardium. Tissue Eng Part A 2011; 17:1219-28; PMID:21187004; http://dx.doi.org/ 10.1089/ten.tea.2010.0557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, et al.. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005; 11:367-8; PMID:15812508; http://dx.doi.org/ 10.1038/nm0405-367 [DOI] [PubMed] [Google Scholar]
- 27.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004; 109:1292-8; PMID:14993122; http://dx.doi.org/ 10.1161/01.CIR.0000121425.42966.F1 [DOI] [PubMed] [Google Scholar]
- 28.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol 2007; 42:441-8; PMID:17187821; http://dx.doi.org/ 10.1016/j.yjmcc.2006.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR, Cohen IS, Doronin SV. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells 2007; 25:1761-8; PMID:17395769; http://dx.doi.org/ 10.1634/stemcells.2007-0022 [DOI] [PubMed] [Google Scholar]
- 30.Guijarro-Munoz I, Cuesta AM, Alvarez-Cienfuegos A, Geng JG, Alvarez-Vallina L, Sanz L. The axonal repellent Slit2 inhibits pericyte migration: potential implications in angiogenesis. Exp Cell Res 2012; 318:371-8; PMID:22198087; http://dx.doi.org/ 10.1016/j.yexcr.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 31.Zhao H, Anand AR, Ganju RK. Slit2-Robo4 pathway modulates lipopolysaccharide-induced endothelial inflammation and its expression is dysregulated during endotoxemia. J Immunol 2014; 192:385-93; PMID:24272999; http://dx.doi.org/ 10.4049/jimmunol.1302021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fish JE, Wythe JD, Xiao T, Bruneau BG, Stainier DY, Srivastava D, Woo S. A Slit/miR-218/Robo regulatory loop is required during heart tube formation in zebrafish. Development 2011; 138:1409-19; PMID:21385766; http://dx.doi.org/ 10.1242/dev.060046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charnock-Jones DS, Kaufmann P, Mayhew TM. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta 2004; 25:103-13; PMID:14972443; http://dx.doi.org/ 10.1016/j.placenta.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 34.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 2005; 97:512-23; PMID:16166562; http://dx.doi.org/ 10.1161/01.RES.0000182903.16652.d7 [DOI] [PubMed] [Google Scholar]
- 35.Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 2009; 29:630-8; PMID:19164813; http://dx.doi.org/ 10.1161/ATVBAHA.107.161521 [DOI] [PubMed] [Google Scholar]
- 36.Chen CP, Chen YY, Huang JP, Wu YH. The effect of conditioned medium derived from human placental multipotent mesenchymal stromal cells on neutrophils: possible implications for placental infection. Mol Hum Reprod 2014; 20:1117-25; PMID:25140001; http://dx.doi.org/ 10.1093/molehr/gau062 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.