ABSTRACT
Proper placental development and function is crucial for a healthy pregnancy, and there has been substantial research to identify markers of placental dysfunction for the early detection of pregnancy complications. Low first-trimester levels of a disintegrin and metalloproteinase 12 (ADAM12) and pregnancy-associated plasma protein-A (PAPP-A) have been consistently associated with the subsequent development of preeclampsia and fetal growth restriction. These molecules are both metalloproteinases secreted by the placenta that cleave insulin-like growth factor binding proteins (IGFBPs), although ADAM12 also has numerous other substrates. Recent work has identified ADAM12, and particularly its shorter variant, ADAM12S, as a regulator of the migration and invasion of trophoblasts into the lining of the uterus, a critical step in normal placental development. While the mechanisms underlying this regulation are not yet clear, they may involve the liberation of heparin-binding EGF-like growth factor (HB-EGF) and/or IGFs from IGFBPs. In contrast, there has been relatively little functional work examining PAPP-A or the IGFBP substrates of ADAM12 and PAPP-A. Understanding the functions of these markers and the mechanisms underlying their association with disease could improve screening strategies and enable the development of new therapeutic interventions.
Keywords: A disintegrin and metalloproteinase 12, ADAM12, fetal growth restriction, invasion, pregnancy-associated plasma protein-A, PAPP-A, preeclampsia, trophoblast
Placental Development and Adverse Pregnancy Outcomes
Abnormal placental development and function threaten the health and wellbeing of both the fetus and the mother, causing conditions such as fetal growth restriction and preeclampsia. These complications affect 5–7% of pregnancies and are leading causes of perinatal and maternal mortality.1 Both of these conditions are thought to be caused, at least in part, by deficiencies in processes critical to placental development, such as the migration and invasion of trophoblast cells into the maternal lining of the uterus (decidua) and the uterine wall (myometrium). Following the implantation of the blastocyst, cytotrophoblast cells proliferate and form columns at sites where the placental villi, the site of maternal-fetal nutrient exchange, come into contact with the maternal uterine stroma. Cytotrophoblasts at the distal portions of cell columns detach, migrate and invade into maternal tissue; invasive trophoblasts are collectively referred to as extravillous trophoblasts (EVTs). EVTs invade the maternal tissue via both interstitial and endovascular routes. Interstitial EVTs, together with uterine natural killer cells, break down the smooth muscle surrounding maternal spiral arteries while endovascular EVTs replace the endothelium of the maternal blood vessels. This spiral artery remodeling extends into the myometrium, and the removal of the smooth muscle increases vessel diameter and precludes vasoconstriction, thereby ensuring adequate blood flow required for oxygen and nutrient delivery to the placenta.2 (Fig. 1)
Figure 1.
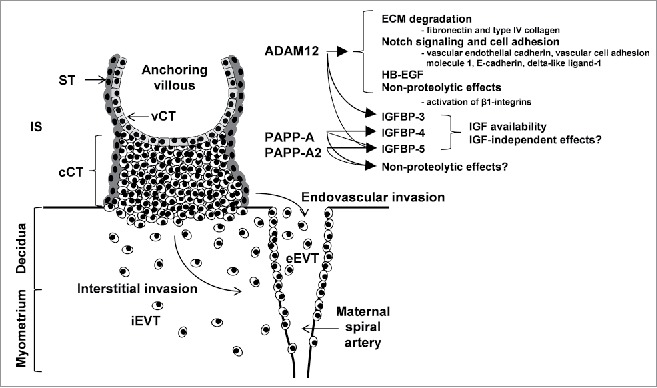
Trophoblast invasion and the roles of ADAM12, PAPP-A and PAPP-A2, which are expressed in the syncytiotrophoblast, extravillous trophoblasts and, in the case of ADAM12 and PAPP-A, decidual cells. cCT = columnar cytotrophoblast; eEVT = endovascular extravillous trophoblast; iEVT = interstitial extravillous trophoblast; IS = intervillous space; ST = syncytiotrophoblast; vCT = villous cytotrophoblast.
There have been enormous efforts to identify markers of placental dysfunction that can predict the development of complications such as preeclampsia and fetal growth restriction early in pregnancy, before symptoms develop. Such predictive tools would enable earlier detection, closer monitoring and potentially preventative treatment. Most of this work has focused on associations between levels of specific proteins in the maternal circulation in the first trimester and the subsequent development of complications.3,4 More recently, other potential markers in the maternal circulation have also been examined, including microRNAs and cell free fetal DNA. Currently, there is no established first trimester screening for complications arising from placental dysfunction because the predictive value of known markers is not sufficiently high, perhaps due to the heterogeneous nature of these conditions.1,5 For example, recent work examining placental gene expression at delivery has identified subclasses of preeclampsia at a molecular level.6
Individual first trimester markers have poor specificity and sensitivity for the prediction of subsequent complications, whereas combinations of circulating markers and other measures of placental performance are more promising.4 Understanding the functions of potential markers and the mechanisms underlying associations between markers and disease would enable markers to be combined in an informed way to distinguish between subtypes of disease and to improve predictive value.5 In addition to allowing earlier identification of at-risk pregnancies, understanding the underlying mechanisms could also enable the development of new therapeutic interventions.
Proteins in the first trimester maternal circulation that have been associated with preeclampsia and/or fetal growth restriction most frequently include activin A, A disintegrin and metalloproteinase 12 (ADAM12), free β human chorionic gonadotrophin, inhibin-A, placental growth factor, placental protein 13, pregnancy-associated plasma protein-A (PAPP-A), soluble endoglin, and soluble fms-like tyrosine kinase 1.3,4,7 Some of these molecules likely influence the migration and invasion of extravillous trophoblasts, potentially playing a causal role in placental pathology. However, while the regulation of extravillous trophoblast invasion is an area of intense research, some proteins showing consistent associations between first trimester levels and subsequent development of disease have received little attention. This commentary will focus on 2 related proteins, describing recent advances in our understanding of the functions of ADAM12 in extravillous trophoblast migration and invasion, and arguing that the functions and regulation of PAPP-A deserve similar attention.
Diverse Proteases Coordinate Invasive Trophoblast Biology
Extravillous trophoblasts share many molecular signatures with invasive tumor cells, and not surprisingly, utilize many of the same processes that tumor cells use to invade and “de-differentiate” from well-organized epithelial structures into senescent migratory mesenchymal-like cells.2 Establishment of a microenvironment conducive for initiating and controlling extravillous trophoblast differentiation is complex and necessitates interactions with multiple cell subtypes within the maternal-fetal interface. For instance, invasive trophoblasts interact with uterine decidualized stroma and uterine glandular epithelia, multiple immune cell subtypes of both innate (e.g., uterine natural killer) and adaptive lineage, and smooth muscle and endothelial cells of the uterine vasculature.2 Trophoblast-derived proteases play central roles in the remodelling and degradation of the uterine extracellular matrix (ECM) that are required for cell invasion. To this end, both matrix metalloproteinases (MMPs) and plasmins are major protease families expressed by invasive trophoblasts that, similar to invasive tumor cells, direct trophoblast invasion via direct degradation of uterine ECM.8 The importance of MMP activity in placentation has been shown recently in mice where MMP9 deficiency leads to impaired trophoblast invasion, aberrant placental development, and the acquisition of clinical features resembling preeclampsia.9
In contrast to proteases that directly degrade ECM components, groups of specialized proteases modulate diverse cellular processes through proteolytic cleavage of substrates leading to activation or inhibition of pathways involved in cell growth and invasion. Notably, protease-directed cleavage of membrane bound, soluble and ECM-tethered substrates affects multiple cellular pathways, including growth factor/receptor availability, cell-cell adhesion competency, and cytokine network activation. ADAM12 and PAPP-A, 2 related metzincin proteases highly expressed in the developing placenta, function primarily in controlling growth factor ligand accessibility and activity. Although ADAM12s role in controlling tumor proliferation, survival and invasion is known,10 the functions and mechanisms of ADAM12 and PAPP-A in directing trophoblast differentiation into invasive cell subsets required for healthy placental function are not well described.
ADAM12
A disintegrin and metalloproteinase (ADAM) proteins are multidomain molecules structurally defined by an N-terminal signal sequence, a prodomain, a metalloproteinase domain, a disintegrin domain containing a cysteine-rich region, an epidermal growth factor-like domain, a transmembrane domain and a cytoplasmic tail.11 Predictably, ADAMs affect multiple processes fundamental in promoting cell invasion, migration, proliferation and survival. ADAM12, expressed as 2 alternatively spliced gene variants, a long transmembrane isoform (ADAM12L) and a short secreted variant (ADAM12S), is restrictedly expressed in regenerating and developing tissues, tissues linked to chronic disease states, and in specialized cells, defined in part by their abilities to fuse to form multinuclear structures (e.g., bone, muscle and placenta). For example, gene-targeting strategies in mice indicate ADAM12 as a key regulator in myogenesis12 and adipogenesis,13 while in vitro studies have assigned roles for ADAM12 in promoting cell fusion in osteoclasts14 and trophoblasts.15 ADAM12 also positively associates with, and in some cases promotes, the progression of chronic disease states including multiple subtypes of cancer,16-18 fibrosis,19 and cardiac hypertrophy.20 The secreted variant, ADAM12S, strongly links with cancer progression where serum levels are elevated in patients with highly-invasive metastatic breast cancer, while ADAM12S over-expression promotes breast cancer cell invasion.21 In these diverse systems, little is known about the regulatory mechanisms controlling ADAM12 activity, however studies have indicated that Notch signaling can either promote17,22 or restrain23 ADAM12 expression.
Multiple ADAM12 substrates have been described, including insulin-like growth factor binding proteins 3 and 5 (IGFBP-3 and -5), heparin-binding EGF-like growth factor (HB-EGF) and certain ECM components such as fibronectin and type IV collagen.10 Moreover, ADAM12 has recently been shown to cleave several cell adhesion molecules and components of the Notch signaling pathway, including vascular endothelial cadherin, vascular cell adhesion molecule 1, E-cadherin and delta-like ligand-1 (DLL1)15,16,24 (Fig. 1). Taken together, proteolytic processing of diverse soluble and membrane-anchored substrates position ADAM12 as a candidate protease important in regulating healthy placenta development by controlling cell migration and invasion signaling networks essential for placental establishment in early pregnancy.
ADAM12 spatially localizes to multiple trophoblast populations within placental villi, and importantly localizes to trophoblasts in distal anchoring columns as well as to invasive matrix-degrading extravillous trophoblasts.25,26 Consistent with the notion that ADAM12 plays a role in healthy placenta development, a functional role for ADAM12 in promoting trophoblast invasion and migration has been recently shown.25,26 Although the mechanism(s) central to ADAM12-directed trophoblast invasion are not fully elucidated, Biadasiewicz et al did show that ADAM12S promotes spreading via a β1-integrin dependent effect.26 While the secreted variant, ADAM12S, may be the dominant isoform in promoting trophoblast invasion, a definitive role for ADAM12L has yet to be shown. Studies examining ectopic expression of ADAM12L in tumor cells and activated fibroblasts highlight a role for ADAM12L in controlling matrix metalloproteinase-14 activation and localization to actin-rich structures.27,28 Consistent with these observations, ectopic expression of ADAM12L in a trophoblast model promoted cell invasion.26 However, it is important to note that in these examples ADAM12L was expressed as a truncated variant lacking its cytoplasmic tail, thereby complicating the interpretation of the role of wild-type ADAM12L in promoting invasion. Nonetheless, a functional role for ADAM12L in regulating cell motility is still conceivable, in part due to ADAM12L's proline-rich sequences within its cytoplasmic tail that facilitate Src homology domain 3 interaction with c-Src and integrin/actin-rich filaments at the leading edge of migratory cells. Components of ADAM12's extracellular domain may also affect cell migration through proteolytic-independent mechanisms, as both its disintegrin and cysteine-rich regions interact with and activate pro-migratory β1-integrins expressed on the cell surface.29,30
Proteolytic events directed by ADAM12 that may play central roles in promoting trophoblast invasion involve cleavage of membrane-bound pro-HB-EGF, or cleavage of IGFBP-3 and -5; proteolysis of either protein results in liberation of HB-EGF or IGF-I/II and interaction with and activation of their cognate receptors, leading to the activation of invasion pathways. Soluble HB-EGF promotes trophoblast invasion in vitro,31 and IGF signaling also plays an important role in trophoblast biology (described below). ADAM12 may also regulate trophoblast motility via effects on ectodomain cleavage of the Notch receptor ligand, DLL1, resulting in subsequent Notch inactivation.24 Lastly, paracrine effects of ADAM12 in regulating growth factor signalling and invasion in trophoblasts is also possible as ADAM12 is expressed by decidual cells (activated uterine stroma).32
PAPP-A
Like ADAM12S, PAPP-A is a secreted metalloproteinase that is expressed by both invasive trophoblasts and decidual stromal cells, although the known substrates of PAPP-A are much more limited: IGFBP-4 and -533 (Fig. 1). IGFBPs regulate the availability of IGF-I and IGF-II, which play important roles in placental development, promoting trophoblast proliferation, extravillous trophoblast migration, hormone secretion, glucose and amino acid uptake, and reducing apoptosis.34 For example, deletion of a placental specific transcript of the Igf2 gene in mice reduces placental nutrient transport, as well as placental and fetal growth.33 The IGFs exert their effects on trophoblast invasion and migration through activation of the ERK1/2, PI3K-Akt and FAK-Rho-ROCK pathways,35 acting through both the type-I and -II IGF receptors.33 Given the importance of IGF signaling in placental function, it is tempting to speculate that the association between low ADAM12 and PAPP-A levels in the first trimester and the increased risk of subsequent complications reflects causation, whereby reduced PAPP-A levels lead to increased IGFBP levels, and therefore reduced IGF availability and impaired placental development and function. However, in addition to sequestering the IGFs, IGFBPs may also increase their half-life, concentrate them in particular regions and/or potentiate their effects.33 Furthermore, IGFBPs have been found to have IGF-independent effects.33,36 Therefore, PAPP-A is not necessarily expected to simply reduce levels of intact IGFBP-4 and -5 and increase IGF-mediated migration and invasion. Furthermore, it is not clear to what extent PAPP-A would affect IGF availability given that it does not proteolyse IGFBP-1, the most abundant IGFBP in decidual cells.33 Moreover, it has recently been proposed that PAPP-A may also have non-proteolytic functions,37 although these have not been studied in the placenta. The mechanisms underlying associations between low PAPP-A and ADAM12 levels and adverse pregnancy complications may therefore be much more complex than simple changes in IGF availability.
Remarkably, despite the consistent associations between pregnancy complications and circulating PAPP-A, the mechanistic function of PAPP-A in the placenta remains unstudied. At a clinical level, there is evidence of associations between low PAPP-A levels and ultrasound measures of placental function and morphology.38 Apart from understanding its function, identifying factors that affect its regulation and cause it to be downregulated in complicated pregnancies would help to distinguish between subtypes of disease. The regulation of PAPP-A expression in the placenta appears complex. Peroxisome proliferator-activated receptor-γ (PPARγ), which limits trophoblast invasion, inhibits the expression and secretion of PAPP-A in primary cultures of extravillous (i.e., invasive) trophoblasts, but not in trophoblasts of villous origin.39 The inhibition of PAPP-A by PPARγ would be expected to reduce IGFBP proteolysis and therefore reduce IGF availability, thereby limiting invasion. Similarly, IGF-II decreases PAPP-A expression in endometrial stromal cells, but not trophoblasts.40 These observations are consistent with a negative feedback loop whereby IGF-II reduces PAPP-A expression in the decidua, thereby reducing IGFBP proteolysis and reducing IGF availability, potentially representing a mechanism by which the decidua controls invasion. In contrast, progesterone increases PAPP-A expression in endometrial stromal cells 40 and trophoblastic cells, increasing the proliferation and adhesion of the latter to uterine epithelial cells.41
The function of the IGFBP substrates of ADAM12 and PAPP-A in the placenta have also received little study. In cell line models of extravillous trophoblasts, IGFBP-4 and -5 have inhibitory effects on IGF-stimulated migration.42,43 IGFBP-3 reduces IGF-stimulated proliferation of cytotrophoblasts in placental explants, and also reduces proliferation through IGF-independent mechanisms.36 First trimester levels of an IGFBP-5 protease paralogous to PAPP-A, PAPP-A2, are elevated in pregnancies that subsequently develop preeclampsia,44 perhaps in response to hypoxia.45,46 Consistent associations between IGFBP proteases and pregnancy complications emphasize the need to understand the functions of the IGFBPs in migration and invasion, and suggest that the IGFBPs themselves are worthy of investigation as candidate markers.47
Conclusion
This commentary has focused on ADAM12 and PAPP-A, although there are undoubtedly other proteolytic mechanisms driving trophoblast differentiation along the invasive pathway. Despite consistent associations between first trimester levels and subsequent development of disease, there has been relatively little study of the roles of these proteins and those of their IGFBP substrates in migration and invasion, their regulation, and the mechanisms underlying their associations with disease. ADAM12 and PAPP-A may serve as markers of healthy trophoblasts, and so a better understanding of these issues will help to distinguish the different etiologies of preeclampsia, and to combine markers based on function to develop screening strategies with improved predictive ability to allow earlier intervention. In particular, understanding the factors influencing the secretion of these molecules in various trophoblast populations may allow screening strategies to distinguish impairment in different types of trophoblasts.1 Currently, there are no cures for conditions resulting from placental dysfunction such as preeclampsia and fetal growth restriction other than inducing delivery, which has long-lasting effects on offspring health in the case of early-onset complications. However, in the future it may be possible to identify placentae with impaired function early in pregnancy and to intervene pharmacologically.
Abbreviations
- ADAM12
A disintegrin and metalloproteinase 12
- DLL1
Delta-like ligand-1
- ECM
Extracellular matrix
- EVT
Extravillous trophoblast
- HB-EGF
Heparin-binding epidermal growth factor-like
- IGFBP
Insulin-like growth factor binding protein
- MMP
Matrix metalloproteinase
- PAPP-A
Pregnancy-associated plasma protein-A
- PPARγ
Peroxisome proliferator-activated receptor-γ.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Huppertz B. Placental origins of preeclampsia - challenging the current hypothesis. Hypertension 2008; 51:970-5; PMID:18259009; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.107.107607 [DOI] [PubMed] [Google Scholar]
- 2.Pijnenborg R, Vercruysse L, Brosens I. Deep placentation. Best Pract Res Clin Obstet Gynaecol 2011; 25:273-85; PMID:21212025; http://dx.doi.org/ 10.1016/j.bpobgyn.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 3.Allen RE, Rogozinska E, Cleverly K, Aquilina J, Thangaratinam S. Abnormal blood biomarkers in early pregnancy are associated with preeclampsia: A meta-analysis. Eur J Obstet Gynecol Reprod Biol 2014; 182:194-201; PMID:25305662; http://dx.doi.org/ 10.1016/j.ejogrb.2014.09.027 [DOI] [PubMed] [Google Scholar]
- 4.Kane SC, Costa FdS, Brennecke S. First trimester biomarkers in the prediction of later pregnancy complications. Biomed Res Int 2014; 2014:807196; PMID:24800250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halscott TL, Ramsey PS, Reddy UM. First trimester screening cannot predict adverse outcomes yet. Prenat Diagn 2014; 34:668-76; PMID:24855016 [DOI] [PubMed] [Google Scholar]
- 6.Leavey K, Bainbridge SA, Cox BJ. Large scale aggregate microarray analysis reveals three distinct molecular subclasses of human preeclampsia. Plos One 2015; 10:UNSP e0116508; PMID:25679511; http://dx.doi.org/ 10.1371/journal.pone.0116508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christians JK, Gruslin A. Altered levels of insulin-like growth factor binding protein proteases in preeclampsia and intrauterine growth restriction. Prenat Diag 2010; 30:815-20; PMID:20658698; http://dx.doi.org/ 10.1002/pd.2583 [DOI] [PubMed] [Google Scholar]
- 8.Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta 2006; 27:783-93; PMID:16249026; http://dx.doi.org/ 10.1016/j.placenta.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 9.Plaks V, Rinkenberger J, Dai J, Flannery M, Sund M, Kanasaki K, Ni W, Kalluri R, Werb Z. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc Natl Acad Sci U S A 2013; 110:11109-14; PMID:23776237; http://dx.doi.org/ 10.1073/pnas.1309561110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kveiborg M, Albrechtsen R, Couchman JR, Wewer UA. Cellular roles of ADAM12 in health and disease. Int J Biochem Cell Biol 2008; 40:1685-702; PMID:18342566; http://dx.doi.org/ 10.1016/j.biocel.2008.01.025 [DOI] [PubMed] [Google Scholar]
- 11.Reiss K, Saftig P. The “A disintegrin and metalloprotease” (ADAM) family of sheddases: Physiological and cellular functions. Semin Cell Dev Biol 2009; 20:126-37; PMID:19049889; http://dx.doi.org/ 10.1016/j.semcdb.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 12.Kurisaki T, Masuda A, Sudo K, Sakagami J, Higashiyama S, Matsuda Y, Nagabukuro A, Tsuji A, Nabeshima Y, Asano M, et al.. Phenotypic analysis of meltrin alpha (ADAM12)-deficient mice: Involvement of meltrin α in adipogenesis and myogenesis. Mol Cell Biol 2003; 23:55-61; PMID:12482960; http://dx.doi.org/ 10.1128/MCB.23.1.55-61.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafuste P, Sonnet C, Chazaud B, Dreyfus PA, Gherardi RK, Wewer UM, Authier FJ. ADAM12 and alpha(9)beta(1) integrin are instrumental in human myogenic cell differentiation. Mol Biol Cell 2005; 16:861-70; PMID:15574885; http://dx.doi.org/ 10.1091/mbc.E04-03-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe E, Mocharla H, Yamate T, Taguchi Y, Manolagas SC. Meltrin-α, a fusion protein involved in multinucleated giant cell and osteoclast formation. Calcif Tissue Int 1999; 64:508-15; PMID:10341023; http://dx.doi.org/ 10.1007/s002239900641 [DOI] [PubMed] [Google Scholar]
- 15.Aghababaei M, Hogg K, Perdu S, Robinson WP, Beristain AG. ADAM12-directed ectodomain shedding of E-cadherin potentiates trophoblast fusion. Cell Death Differ 2015; 22:1970-1984; PMID:25909890; http://dx.doi.org/ 10.1038/cdd.2015.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frohlich C, Klitgaard M, Noer JB, Kotzsch A, Nehammer C, Kronqvist P, Berthelsen J, Blobel C, Kveiborg M, Albrechtsen R, et al.. ADAM12 is expressed in the tumour vasculature and mediates ectodomain shedding of several membrane-anchored endothelial proteins. Biochem J 2013; 452:97-109; PMID:23458101; http://dx.doi.org/ 10.1042/BJ20121558 [DOI] [PubMed] [Google Scholar]
- 17.Li H, Solomon E, Muggy SD, Sun D, Zolkiewska A. Metalloprotease-disintegrin ADAM12 expression is regulated by notch signaling via MicroRNA-29. J Biol Chem 2011; 286:21500-10; PMID:21518768; http://dx.doi.org/ 10.1074/jbc.M110.207951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimura T, Dagher A, Sachdev M, Ebi M, Yamada T, Yamada T, Joh T, Moses MA. Urinary ADAM12 and MMP-9/NGAL complex detect the presence of gastric cancer. Cancer Prev Res 2015; 8:240-8; PMID:25591790; http://dx.doi.org/ 10.1158/1940-6207.CAPR-14-0229 [DOI] [PubMed] [Google Scholar]
- 19.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med 2012; 18:1262; PMID:22842476; http://dx.doi.org/ 10.1038/nm.2848 [DOI] [PubMed] [Google Scholar]
- 20.Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, Ohmoto H, Node K, Yoshino K, Ishiguro H, et al.. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: Metalloproteinase inhibitors as a new therapy. Nat Med 2002; 8:35-40; PMID:11786904; http://dx.doi.org/ 10.1038/nm0102-35 [DOI] [PubMed] [Google Scholar]
- 21.Roy R, Rodig S, Bielenberg D, Zurakowski D, Moses MA. ADAM12 transmembrane and secreted isoforms promote breast tumor growth A distinct role for ADAM12-S protein in tumor metastasis. J Biol Chem 2011; 286:20758-68; PMID:21493715; http://dx.doi.org/ 10.1074/jbc.M110.216036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz B, Yuen A, Iizuka S, Higashiyama S, Courtneidge SA. Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia. J Cell Biol 2013; 201:279-92; PMID:23589494; http://dx.doi.org/ 10.1083/jcb.201209151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velicky P, Haider S, Otti GR, Fiala C, Pollheimer J, Knoefler M. Notch-dependent RBPJ kappa inhibits proliferation of human cytotrophoblasts and their differentiation into extravillous trophoblasts. Mol Hum Reprod 2014; 20:756-66; PMID:24850908; http://dx.doi.org/ 10.1093/molehr/gau038 [DOI] [PubMed] [Google Scholar]
- 24.Dyczynska E, Sun D, Yi H, Sehara-Fujisawa A, Blobel CP, Zolkiewska A. Proteolytic processing of delta-like 1 by ADAM proteases. J Biol Chem 2007; 282:436-44; PMID:17107962; http://dx.doi.org/ 10.1074/jbc.M605451200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aghababaei M, Perdu S, Irvine K, Beristain AG. A disintegrin and metalloproteinase 12 (ADAM12) localizes to invasive trophoblast, promotes cell invasion and directs column outgrowth in early placental development. Mol Hum Reprod 2014; 20:235-49; PMID:24243624; http://dx.doi.org/ 10.1093/molehr/gat084 [DOI] [PubMed] [Google Scholar]
- 26.Biadasiewicz K, Fock V, Dekan S, Proestling K, Velicky P, Haider S, Knofler M, Frohlich C, Pollheimer J. Extravillous trophoblast-associated ADAM12 exerts pro-invasive properties, including induction of integrin beta 1-mediated cellular spreading. Biol Reprod 2014; 90:101; PMID:24695627; http://dx.doi.org/ 10.1095/biolreprod.113.115279 [DOI] [PubMed] [Google Scholar]
- 27.Albrechtsen R, Kveiborg M, Stautz D, Vikesa J, Noer JB, Kotzsh A, Nielsen FC, Wewer UM, Frohlich C. ADAM12 redistributes and activates MMP-14, resulting in gelatin degradation, reduced apoptosis and increased tumor growth. J Cell Sci 2013; 126:4707-20; PMID:24006261; http://dx.doi.org/ 10.1242/jcs.129510 [DOI] [PubMed] [Google Scholar]
- 28.Albrechtsen R, Stautz D, Sanjay A, Kveiborg M, Wewer UM. Extracellular engagement of ADAM12 induces clusters of invadopodia with localized ectodomain shedding activity. Exp Cell Res 2011; 317:195-209; PMID:20951132; http://dx.doi.org/ 10.1016/j.yexcr.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi N, Sundberg C, Kveiborg M, Moghadaszadeh B, Asmar M, Dietrich N, Thodeti CK, Nielsen FC, Moller P, Mercurio AM, et al.. ADAM12 induces actin cytoskeleton and extracellular matrix reorganization during early adipocyte differentiation by regulating beta 1 integrin function. J Cell Sci 2003; 116:3893-904; PMID:12915587; http://dx.doi.org/ 10.1242/jcs.00699 [DOI] [PubMed] [Google Scholar]
- 30.Zhao ZF, Gruszczynska-Biegala J, Cheuvront T, Yi HQ, von der Mark H, von der Mark K, Kaufman SJ, Zolkiewska A. Interaction of the disintegrin and cysteine-rich domains of ADAM12 with integrin alpha 7 beta 1. Exp Cell Res 2004; 298:28-37; PMID:15242759; http://dx.doi.org/ 10.1016/j.yexcr.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 31.Leach RE, Kilburn B, Wang J, Liu ZT, Romero R, Armant DR. Heparin-binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol 2004; 266:223-37; PMID:14738873; http://dx.doi.org/ 10.1016/j.ydbio.2003.09.026 [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Guo W, Chen Q, Fan X, Zhang Y, Duan E. Adam12 plays a role during uterine decidualization in mice. Cell Tissue Res 2009; 338:413-21; PMID:19841944; http://dx.doi.org/ 10.1007/s00441-009-0884-9 [DOI] [PubMed] [Google Scholar]
- 33.Nayak NR, Giudice LC. Comparative biology of the IGF system in endometrium, decidua, and placenta, and clinical implications for foetal growth and implantation disorders. Placenta 2003; 24:281-96; PMID:14626217; http://dx.doi.org/ 10.1053/plac.2002.0906 [DOI] [PubMed] [Google Scholar]
- 34.Sferruzzi-Perri AN, Owens JA, Pringle KG, Roberts CT. The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. J Physiol 2011; 589:7-20; http://dx.doi.org/ 10.1113/jphysiol.2010.198622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knofler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol 2010; 54:269-80; PMID:19876833; http://dx.doi.org/ 10.1387/ijdb.082769mk [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forbes K, Souquet B, Garside R, Aplin JD, Westwood M. Transforming growth factor-beta (TGF beta) receptors I/II differentially regulate TGF beta 1 and IGF-binding protein-3 mitogenic effects in the human placenta. Endocrinology 2010; 151:1723-31; PMID:20172969; http://dx.doi.org/ 10.1210/en.2009-0896 [DOI] [PubMed] [Google Scholar]
- 37.Kjaer-Sorensen K, Engholm DH, Kamei H, Morch MG, Kristensen AO, Zhou J, Conover CA, Duan C, Oxvig C. Pregnancy-associated plasma protein A (PAPP-A) modulates the early developmental rate in zebrafish independently of its proteolytic activity. J Biol Chem 2013; 288:9982-92; PMID:23430244; http://dx.doi.org/ 10.1074/jbc.M112.426304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huynh L, Kingdom J, Akhtar S. Low pregnancy-associated plasma protein A level in the first trimester. Can Fam Physician 2014; 60:899-903; PMID:25316741 [PMC free article] [PubMed] [Google Scholar]
- 39.Handschuh K, Guibourdenche J, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T. Modulation of PAPP-A expression by PPAR gamma in human first trimester trophoblast. Placenta 2006; 27:S127-34; PMID:16388849; http://dx.doi.org/ 10.1016/j.placenta.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 40.Giudice LC, Conover CA, Bale L, Faessen GH, Ilg K, Sun I, Imani B, Suen LF, Irwin JC, Christiansen M, et al.. Identification and regulation of the IGFBP-4 protease and its physiological inhibitor in human trophoblasts and endometrial stroma: Evidence for paracrine regulation of IGF-II bioavailability in the placental bed during human implantation. J Clin Endocrinol Metab 2002; 87:2359-66; PMID:11994388; http://dx.doi.org/ 10.1210/jcem.87.5.8448 [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Liu S, Qin H, Zhao Y, Wang X, Yan Q. Pregnancy-associated plasma protein A up-regulated by progesterone promotes adhesion and proliferation of trophoblastic cells. Int J Clin Exp Pathol 2014; 7:1427-37; PMID:24817938 [PMC free article] [PubMed] [Google Scholar]
- 42.Lee BPL, Rushlow WJ, Chakraborty C, Lala PK. Differential gene expression in premalignant human trophoblast: Role of IGFBP-5. Int J Cancer 2001; 94:674-84; http://dx.doi.org/ 10.1002/ijc.1532 [DOI] [PubMed] [Google Scholar]
- 43.Crosley EJ, Dunk CE, Beristain AG, Christians JK. IGFBP-4 and -5 are expressed in first-trimester villi and differentially regulate the migration of HTR-8/SVneo cells. Reprod Biol Endocrinol 2014; 12:123; http://dx.doi.org/ 10.1186/1477-7827-12-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crosley EJ, Durland U, Seethram K, Macrae S, Gruslin A, Christians JK. First-trimester levels of pregnancy-associated plasma protein A2 (PAPP-A2) in the maternal circulation are elevated in pregnancies that subsequently develop preeclampsia. Reprod Sci 2014; 21:754-60; PMID:24336677; http://dx.doi.org/ 10.1177/1933719113512532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macintire K, Tuohey L, Ye L, Palmer K, Gantier M, Tong S, Kaitu'u-Lino TJ. PAPPA2 is increased in severe early onset pre-eclampsia and upregulated with hypoxia. Reprod Fertil Dev 2014; 26:351-7; PMID:23484525; http://dx.doi.org/ 10.1071/RD12384 [DOI] [PubMed] [Google Scholar]
- 46.Wagner PK, Otomo A, Christians JK. Regulation of pregnancy-associated plasma protein A2 (PAPPA2) in a human placental trophoblast cell line (BeWo). Reprod Biol Endocrinol 2011; 9:48; PMID:21496272; http://dx.doi.org/ 10.1186/1477-7827-9-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu Q, Bell M, Lu X, Yan X, Rodger M, Walker M, Wen S, Bainbridge S, Wang H, Gruslin A. Significance of IGFBP-4 in the development of fetal growth restriction. J Clin Endocrinol Metab 2012; 97:E1429-39; PMID:22689691; http://dx.doi.org/ 10.1210/jc.2011-2511 [DOI] [PubMed] [Google Scholar]


