ABSTRACT
Trophoblast migration and invasion through the decidua and maternal uterine spiral arteries are crucial events in placentation. During this process, invasive trophoblast replace vascular endothelial cells as the uterine arteries are remodeled to form more permissive vessels that facilitate adequate blood flow to the growing fetus. Placentation failures resulting from either extensive or shallow trophoblastic invasion can cause pregnancy complications such as preeclampsia, intrauterine growth restriction, placenta creta, gestational trophoblastic disease and even maternal or fetal death. Consequently, the use of experimental animal models such as rats and mice has led to great progress in recent years with regards to the identification of mechanisms and factors that control trophoblast migration kinetics. This review aims to perform a comparative analysis of placentation and the mechanisms and factors that coordinate intrauterine trophoblast migration in humans, rats and mice under physiological and pathological conditions.
KEYWORDS: decidua, factors, human, mice, placentation, pathology, rat, trophoblast migration
Introduction
The migration of trophoblasts at the maternal-fetal interface is a key process in implantation and placentation and is therefore essential for successful pregnancy outcomes in women and rodents.1,2 Placentation failures resulting from either extensive or shallow trophoblastic invasion can cause pregnancy complications such as preeclampsia, intrauterine growth restriction, placenta creta, prematurity, gestational trophoblastic disease and even maternal or fetal death.3-6 Consequently, some reports have attempted to evaluate the molecular mechanisms controlling trophoblastic invasion and migration under physiological conditions,7-9 and under pathological conditions, e.g., preeclampsia, intrauterine growth restriction, gestational diabetes and maternal hypothyroidism.6,10-12,164
The trophoblasts that form the placenta originate from the embryonic trophectoderm and are the first cell lineage in mammalian development.13 In this moment, after its differentiation, whether the trophoblast is apposed to uterine epithelium, the endothelium of maternal vessels, or directly to maternal blood, placentas are classified as, respectively, epitheliochorial, endotheliochorial or hemochorial. In this last type of placenta, observed in humans, rats, mice, guinea pigs, armadillos, rabbits and apes, specialized populations of trophoblasts are able to leave the placenta and move toward the decidua to directly contact maternal blood.13,14 During this process, trophoblast stem cells proliferate and can differentiate into various trophoblast lineages. Between them, interstitial and endovascular trophoblasts exhibit migratory and invasive properties and have the capacity to recognize, modify and stimulate the behavior of other cell types at the maternal-fetal interface. This cellular communication is precisely controlled by maternal factors and factors released and/or expressed by trophoblastic cells themselves such as integrins, E-cadherin, proteases, cytokines, interleukins and growth factors. That allow the trophoblast cell to degrade extracellular matrix (ECM) proteins such as collagen IV, laminin, vitronectin and fibronectin to promote cell migration, while the decidua expresses a variety of inhibitory proteins that controls trophoblastic cell invasion.14 Consequently, invasive trophoblasts replace vascular endothelial cells as the uterine arteries are remodeled to form more permissive vessels that facilitate adequate blood flow to the growing fetus.15
Changes or inadequate responses within the regulatory pathways that control trophoblast invasion and migration compromise placental development and can negatively affect maternal and fetal health, as well as postnatal development.3,5,6,12 During the 1970s, Brosens et al.10,11 observed that failures in human trophoblast invasion and the absence of adequate vascular remodeling of the utero-placental arteries in the placental bed were associated with intrauterine growth restriction and/or preeclampsia. Since then, intrauterine trophoblast migration and invasion has been a major focus of placentation research. As there are some morphological and functional similarities among species that have hemochorial placenta, rat and mice animal models have been useful in the study of many aspects of human placentation.16,17 This review aims to perform a comparative analysis of placentation and the mechanisms and factors involved in the cellular interactions that coordinate intrauterine trophoblast migration in humans, rats and mice under physiological and pathological conditions.
Placental organization
Hemochorial placental development is characterized by close contact between maternal and fetal tissues and occurs in humans and rodents such as the rat and mouse. During this process, trophoblast stem cells originate from the embryonic trophectoderm and can differentiate into various trophoblast lineages. One of the key activities of differentiated trophoblast cells is remodeling uterine spiral arteries. Vascular remodeling transforms tightly coiled uterine spiral arteries into dilated vessels that are no longer under maternal control. Restructuring maternal vasculature is essential for the optimal delivery of nutrients to the fetus.1-3,16,17,26 However, despite the hemochorial placentation and especially trophoblast-directed vascular remodeling in humans, rats and mice are highly similar, there are differences in structure, placental development and some types of trophoblast cells between the human and rodent placentas.
Human
One of the initial processes in human pregnancy is characterized by the adhesion of the blastocyst to the uterine decidua. This apposition is the first step in implantation and occurs approximately 6 to 7 d following conception (Figure 1). At this stage, the endometrium has already been decidualized once; in contrast with mice, human decidualization is not dependent on blastocyst implantation and instead begins on day 14 of the menstrual cycle due to the effects of progesterone.46
Figure 1.
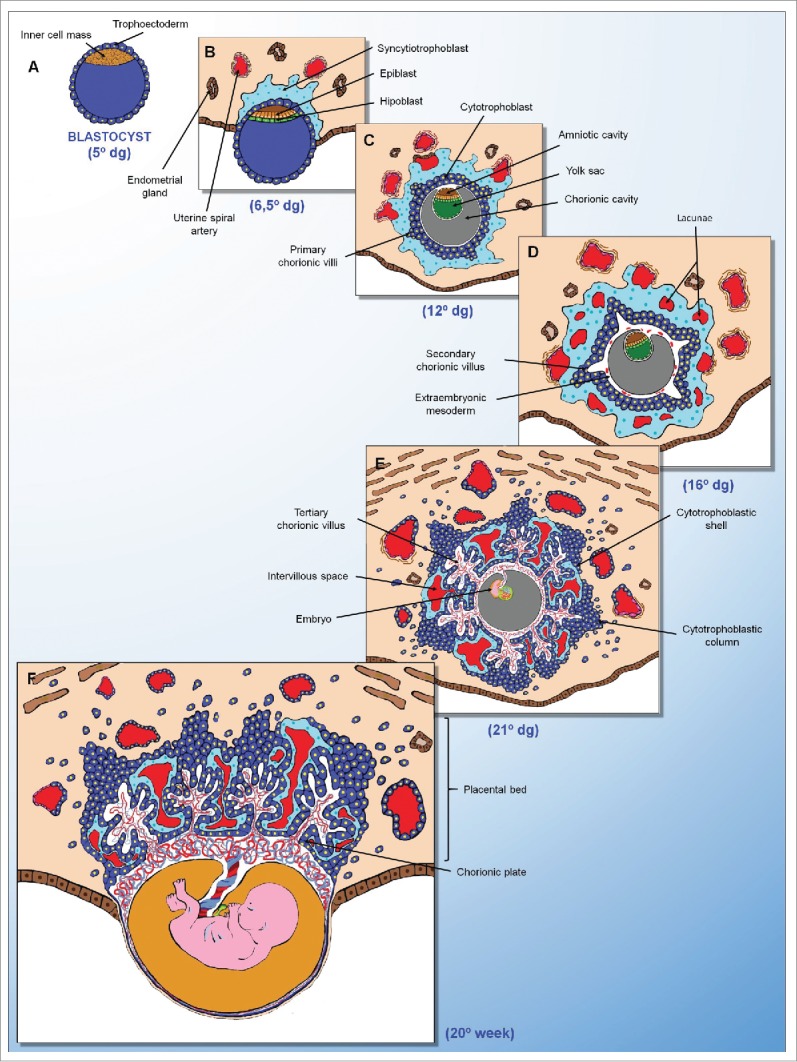
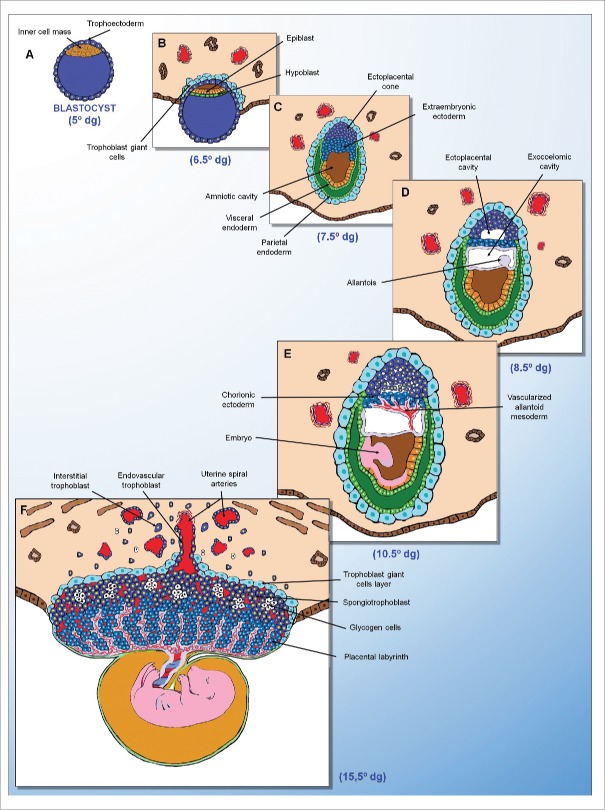
Human placental development. (A) Blastocyst (5° dg). (B) Implantation phase (6.5° dg). Trophectoderm differentiation and syncytiotrophoblast infiltration of the endometrium. (C) Post-implantation phase (12° dg). Syncytiotrophoblast-mediated erosion of the maternal blood vessels and endometrial glands, and formation of the primary chorionic villi from cytotrophoblasts. (D) Post-implantation phase (16° dg). Differentiation of the extraembryonic mesoderm and formation of the secondary chorionic villi. (E) Pre-placental phase (21° dg). Formation of the tertiary chorionic villi and cytotrophoblastic shell. (F) Definitive placenta (20° weeks). Evidence of the placental bed formed by the chorionic villi, decidualized endometrium and adjacent myometrium. dg = days of gestation.
The outer layer of the blastocyst is formed by trophectoderm. Close contact between the fetal trophoblasts and maternal uterine tissue leads to the formation of the maternal-fetal junctional zone. The junctional zone will form the basal plate, which is the maternal surface of the placenta, and the fetal surface is called the chorionic plate.47,48
After the formation of the junctional zone, implantation continues through trophoblast proliferation and differentiation in cytotrophoblast (innermost) and syncytiotrophoblast (outermost).13,15,16,21 The syncytiotrophoblast expands and erodes maternal capillaries and endometrial glands in the decidua to release blood and the contents of the glands inside the lacunae. The cytotrophoblast sequentially proliferates to form the primary, secondary and tertiary villi (villous trophoblast). The trophoblasts will then form columns, proliferate and extend beyond the syncytiotrophoblast, joining one another and forming the cytotrophoblastic shell. By the eighth week of pregnancy, the continuity of the cytotrophoblastic shell is lost and trophoblasts at the top of the anchoring villi will give rise to the extravillous trophoblast (EVT) that will migrate through the basal plate, decidualized endometrium and underlying myometrium to form the placental bed.47,48
The extravillous trophoblast, corresponding to all of the trophoblasts residing outside of placental villi, acquires the capacity to degrade extracellular matrix proteins to permit these cells to mobilize and invade the decidua (interstitial trophoblast) and the wall of maternal uterine spiral arteries (endovascular trophoblast). Endovascular trophoblasts invade the lumen of the spiral arteries and incorporate into the arterial wall (intramural trophoblast). The endothelium, the muscular layer and the elastic material of these arteries are destroyed and replaced with an extracellular matrix called the fibrinoid and by the EVT cells themselves. This process, known as trophoblastic arterial remodeling transforms the uterine spiral arteries into easily distensible, limp, thin-walled vessels that allow continuous and adequate blood supply in the placenta and thereby ensure the gestational success.6,7,16,50-52
The density of extravillous trophoblast and the depth of invasion of the utero-placental arteries are the most prominent in the central placental bed, and both the density and depth of invasion decrease with proximity to the placental margins. The control of this intrauterine migration allows a peak of trophoblastic invasion around the 12th week of pregnancy that is shortly followed by a decline. The space control restricts the depth of endovascular and interstitial trophoblast invasion to the decidua and proximal third of the myometrium.53,54 Deregulation of these processes results in a wide variety of pregnancy complications such as intrauterine growth restriction, preeclampsia, placenta creta, gestational trophoblastic disease and abortion.3,55-57 While preeclampsia and intrauterine growth restriction are characterized by a failure of trophoblastic invasion, placenta creta and gestational trophoblastic disease are characterized by excessive trophoblastic invasion by either “normal” trophoblasts, or by trophoblastic tumor or pre-tumor cells, respectively.58,59
The villous trophoblast, which gives rise to extravillous trophoblast, covers the connective tissue shaft, extending from the chorionic plate until the basal plate and is where the greater diameter fetal vessels are located. The chorionic villi originate from these fibrous septa (also called villous septa). The space between the villous septa is called the intervillous space and is filled with maternal blood from the uterine arteries. The chorionic villi are formed by a central axis of connective tissue that contains a vast fetal capillary network surrounded by a layer of cytotrophoblasts and the multinucleate syncytiotrophoblast.6,49,50
The syncytiotrophoblast is more abundant and forms the outer surface of the villi, making direct contact with maternal blood and is formed from the fusion of cytotrophoblasts. The syncytiotrophoblast undergoes a constant and controlled process of renewal and apoptosis, and its primary functions are nutrient absorption, waste product removal, and synthesis of hormones such as hCG. To facilitate the transport of nutrients to the fetus and the removal of waste and harmful toxins from fetal circulation, a wide variety of transporters are expressed in the syncytiotrophoblast, including ion channels, aquaporins and ATP-binding cassette transporters.6,49,50
The cytotrophoblasts are located below the syncytiotrophoblast and are mononuclear and globular. The fetal blood makes exchanges with the maternal blood circulating in the intervillous space and bathing the villi. As the pregnancy progresses, the number of blood vessels in the chorionic villi increases while the amount of connective tissue decreases, facilitating the exchange of metabolites between the mother and the fetus.6,49,50 In humans, the final structure of the placenta is discoid and becomes apparent at approximately 21 d of gestation.16,49
Rodent
Following implantation, particularly in rats and mice, the embryo is completely covered by various trophoblast cell types with distinct spatial locations and gene expression patterns. In contrast with humans, the extraembryonic ectoderm, particularly the polar trophectoderm, differentiates into chorionic ectoderm and the ectoplacental cone that will later give rise to the placental labyrinth and junctional zone, respectively (Figure 2).60,61 Mossman62 suggested that the ectoplacental cone in rats and mice is analogous to cytotrophoblastic shell formed in the human placenta; however, in rodents, the ectoplacental cone proliferates in an orderly manner to form the trophospongium, while the trophoblasts of the human cytotrophoblastic shell proliferate in a disorderly manner through the basal plate, decidua and underlying myometrium.16
Figure 2.
Rat placental development. (A) Blastocyst (5° dg). (B) Implantation phase (6.5° dg). Trophectoderm differentiation and primary trophoblast giant cell infiltration of the endometrium. (C) Post-implantation phase (7.5° dg). Trophectoderm differentiation with the formation of giant cells around the embryo and ectoplacental cone. (D) Post-implantation phase (8.5° dg). Allantois formation from the posterior epiblast. (E) Pre-placental phase (10.5° dg). Placental labyrinth formation from the fusion of the allantoid mesoderm with the chorionic ectoderm. (F) Definite placenta (15° dg). Evidence of the trophoblast giant cell layer, spongiotrophoblast and placental labyrinth. dg = days of gestation.
The definitive placenta of mice and rats is discoid and becomes established at approximately the 11th and 12th days of gestation, respectively, ie, in the middle of gestation. It has 3 anatomically and physiologically distinct regions (Figure 3): the placental labyrinth located more internally on the fetal-placental unit; the junctional zone, which is composed of the spongiotrophoblast and glycogen cells and is formed from the original wall of the blastocyst and remains unicellular; and the trophoblast giant cell layer formed by the penetration of trophoblasts into the endometrium.18,63
Figure 3.
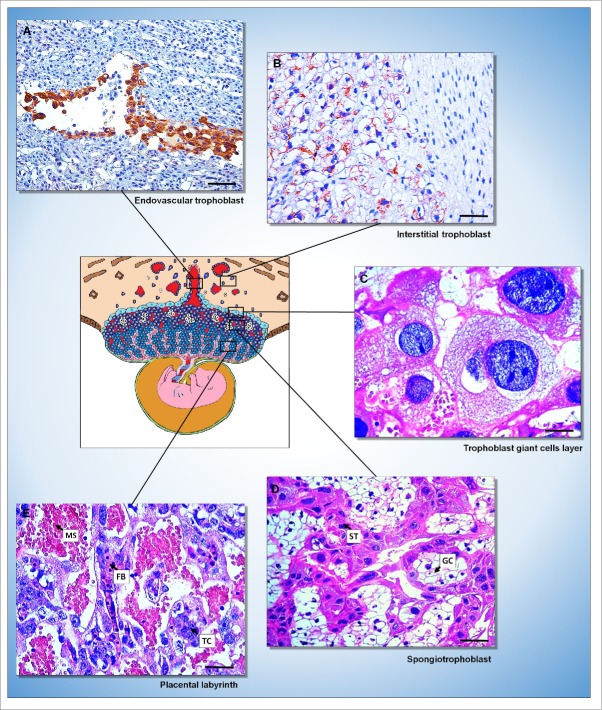
Rat placenta. (A and B) Immunohistochemical evidence of the endovascular (A) and interstitial (B) trophoblast in the decidua stained with AE1/AE3 cytokeratin antibody. [Streptavidin-biotin-peroxidase method, Harris' hematoxylin counterstain, bar = 150 µm (A); 64 µm (B)]. (C-E) Histological evidence of the 3 layers of the placenta [trophoblast giant cells layer (C); spongiotrophoblast (D) formed by spongiotrophoblasts (ST) and glycogen cells (GC); placental labyrinth (E) formed by fetal blood vessels (FB), trophoblast cells (TC) and maternal sinusoids (MS)]. [Hematoxylin and eosin, bar = 64 µm].
The trophoblast giant cell layer (Figure 3C) is located more externally on the fetal-placental unit and are the first cells that mediate fetal implantation and uterine invasion. These are known as secondary trophoblast giant cells and are derived from the polar trophoectoderm, whereas the primary trophoblast giant cells do not contribute to the definitive placenta and are derived from the mural trophoectoderm.18 The trophoblast giant cells arises from of trophoblasts that leave the cell cycle, stop dividing, and become polypoid through endoreplication, undergoing cycles of DNA replication without mitosis. They are one of the major endocrine cells of the placenta because produce many cytokines and hormones that regulate the flow of maternal blood to the implantation site, ovarian progesterone synthesis and lactogenesis.67,68 Most trophoblast giant cells migrate to the decidua; however, a subtype of trophoblastic giant cells that invades the spiral arteries that bring the maternal blood to the implantation site was identified in rat placenta.69 Based on this evidence, the trophoblastic giant cells are also known to participate in the uterine vascular remodeling, at least in rats.68
The junctional zone, the middle layer of the placenta, is bordered by trophoblast giant cell layer and, more externally, by mesometrial decidua. This region is also referred to as the “trophospongium,” “spongiotrophoblast” and “spongy region.” Four trophoblast lineages differentiate from the progenitor cells present in the junctional zone: secondary trophoblastic giant cells, spongiotrophoblasts, glycogen cells, and invasive trophoblasts. In response to close contact with the inner cell mass, all of these cells undergo fibroblast growth factor 4 (FGF4)-mediated proliferation.18,67,69,70 The spongiotrophoblasts are ovoid and mononuclear, while the glycogen cells, which appear in the placenta from the 12th day of gestation, possess more condensed nuclei and have glycogen granules in their cytoplasm.68,71
The spongiotrophoblast (Figure 3D) acts as an endocrine glandular compartment that maintains corpus luteum secretion of progesterone and performs other functions. The spongiotrophoblast, and secondarily, the syncytiotrophoblast, produce luteotropic and lactogenic hormones during pregnancy.8,13 Another supposed spongiotrophoblast function is the limiting of fetal endothelium growth in the maternal placenta by secreting anti-angiogenic factors such as soluble fms-like tyrosine kinase-1 (sFlt-1) and proliferin-related protein (rPlf).69,72 In the event of changes in placental development, qualitative changes occur in the balance between spongiotrophoblasts and glycogen cells.70,73
Glycogen cells are trophoblasts of an uncertain origin that arise in the spongiotrophoblast. They are believed to originate from spongiotrophoblasts that express a specific gene, Tpbp (4311).69 Alternatively, there is evidence that glycogen cells may be distinct from the spongiotrophoblast once they express protocadherin 12 (PCDH12), a specific marker of a cell group that is distinct from that which gives rise to spongiotrophoblasts in the ectoplacental cone.74
Glycogen cells are also similar to the human extravillous trophoblast in that they invade the uterine spiral arteries that carry blood to the implantation site and remodel the arterial muscle wall. This process results in increased blood flow, carrying oxygen and nutrients to the implantation site. In rats, glycogen cells invade the maternal decidua and can interact with the uterine natural killer cells (uNKs), stimulating them to modify the uterine spiral arteries.69 Once in the decidua, the glycogen cells undergo a cytolytic process, releasing their intracellular components, glycogen and hormones into the interstitial space. These components are likely to be important as energy sources and in hormonal control of pregnancy and/or fetal development.74
Invasive trophoblasts, which originate from the junctional zone, also participate in uterine vascular remodeling along with the glycogen cells and uNKs, being that the exit of these cells from the placenta and their movements into the uterine stroma and/or myometrium is temporally and spatially well defined. These cells surround and penetrate the uterine spiral arteries and exhibit a retrograde migration similar to that of the human extravillous trophoblast.16 The invasive endovascular trophoblasts (Figure 3A) replace the endothelium while the interstitial trophoblasts are localized between the blood vessels.8,9 Prior to 13.5 d of gestation, trophoblast invasion in rats and mice is limited to the endovascular environment and is confined to the mesometrial decidua. Following 14.5 d of gestation, endovascular and interstitial trophoblast (Figure 3B) in rats can be identified in the metrial gland.8,32 Research has demonstrated that arteries present at the maternal-fetal interface begin to dilate when they are near interstitial trophoblasts that are expressing nitric oxide synthase (NOS), endothelial nitric oxide synthase (eNOS) and possibly inducible nitric oxide synthase (iNOS).66 Furthermore, Cartwright et al.75 demonstrated that in vitro cell motility and trophoblast invasion is highly dependent upon trophoblast-produced NOS. The precise coordination of the entire uterine vascular remodeling process is essential to ensure proper delivery of nutrients to the fetus and prevent inadequate fetal exposure to the deleterious effects of reactive oxygen species (ROS).76
Based on these observations, the interstitial and endovascular trophoblast invasion and vascular changes in rats and mice are very similar to those processes in humans.7,53 In contrast, the incorporation of human invasive trophoblasts into the blood vessel wall is associated with a greater deposition of PAS-positive fibrinoid matrix than in rats. The incorporation of intramural trophoblasts in humans is also associated with the elimination of vascular smooth muscle and elastic fibers as observed in rats and mice, followed by a process of re-endothelialization.66 One difference between these species is that interstitial invasion in humans precedes endovascular invasion, which can be related to the fact that in humans the interstitial trophoblast also plays a role in early vascular remodeling.53 In contrast, rat and mice endovascular invasion precedes interstitial invasion.9 Similarly to humans, rat trophoblasts deeply invade the decidua, extending to the mesometrial triangle. In mice, on the contrary, interstitial trophoblastic invasion does not extend into the myometrium and endovascular invasion is very limited and confined to the mesometrial decidua. Moreover, mice invasive trophoblasts follow a perivascular pathway and are not intraluminal as in rats. Therefore, the rat may represent a more promising animal model in relation to mouse for the study of experimental conditions limiting trophoblast invasion.69,77
The placental labyrinth (Figure 3E), which is the largest portion of the placental disk, originates from the interaction of the allantois with the chorionic ectoderm, syncytium formation by trophoblasts and establishment of maternal-fetal barrier.64 The chorionic ectoderm overlying the exocoelomic cavity and the allantois originates from the posterior primitive trunk of the epiblast within the exocoelomic cavity. The allantois develops toward the ectoplacental cone and fuses with the chorionic ectoderm at 8.5 and 9.5 d of gestation in mice and rats, respectively; this period coincides with the initiation of the heartbeat and circulation within the embryo.65 It is known that chorioallantoic fusion is dependent on the cell adhesion molecule VCAM1, which is expressed on the allantois, and its ligand α4-integrin, which is expressed by the chorionic mesothelium. However, not all VCAM1- or α4-integrin- deficient mice fail in chorioallantoic fusion, suggesting that other redundant adhesion mechanisms are involved.64
During the chorioallantoic fusion, the trophoblast begins to differentiate into 3 layers, 2 syncytiotrophoblast layers in contact with the fetal endothelium, and one cytotrophoblast layer in contact with maternal blood. This type of placental development is known as haemotrichorial placentation, different from human placenta which is haemomonochorial.16,18 Syncytiotrophoblast is formed by the fusion of trophoblast cells expressing Gcm1 gene and its differentiation does not begin until chorioallantoic fusion in the endometrium. The placental labyrinth is also comprised of stem cells capable of differentiating into trophoblast giant cells. However, that giant cells possess a restricted capacity for hormone production.8 The placental labyrinth is the main area of maternal-fetal exchange, since syncytial trophoblast cells mediate the transfer of nutrients and wastes between maternal and fetal compartments. Maternal and fetal blood flows in a countercurrent manner within the labyrinth to maximize nutrient transport.8,64 This region corresponds to the human placental villi that are surrounded by intervillous space through which maternal blood flows.66
Trophoblast differentiation
Early in mammalian development, the conceptus differentiates into an inner cell mass and an outer sphere of cells, the trophectoderm. During this process, to form the hemochorial placenta of humans, rats and mice, stem cells present in the trophectoderm infiltrates the endometrial epithelium and differentiates into a variety of cell subtypes, each with specific functions.18,19 The trophectoderm does not contribute to the formation of any fetal tissues but does contribute to the formation of the placenta and other embryonic annexes. The proliferation and differentiation of trophectoderm cells makes it the largest component of the placenta.19 The tissues of the embryo are all derived from the inner cell mass as are the amnion and major components of the yolk sac and allantois.18,19
The molecular events involved in the differentiation of the trophectoderm are not fully known.20 In rats and mice, critical transcription factors such as Eomes, Cdx2 and Mash2 control the development of various trophoblast cell subtypes21; however, it is unclear what factors dictate invasive trophoblast differentiation in humans.22 For example, although transcription factors that are important during invasive trophoblast giant cell differentiation, a subtype of trophoblast cell, have been described in mice, the expression and distribution of these factors in human invasive trophoblasts do not necessarily suggest the same roles.23 These discrepancies may be explained not only by differences in observed placental morphology and trophoblast cell subtypes in human and rodent placenta but also by the difficulty of examining human invasive trophoblasts during various gestational periods. As an example, homozygous mutant embryos from Hand1-null mutant mice by gene targeting arrested by embryonic day 7.5 of gestation with defects in trophoblast giant cell differentiation, i.e, mice Hand-1 promotes trophoblast stem cell differentiation into invasive giant cells during early pregnancy.24 In human, on the contrary, Hand-1 expression can only be detected in blastocysts and during in vitro trophectoderm differentiation.25
Regardless, some transcription factors, at least in rats and mice, are known to have critical roles in promoting and directing the initial differentiation of trophoblasts. The generation of mouse trophoblast stem cell lines from early-stage mouse embryos has been useful for studying this process as well as the mechanisms that promote self-renewal of the trophoblast stem cell population. Trophoblast stem cells (TSCs) can be derived and maintained in vitro in the presence of medium conditioned by mouse embryonic fibroblasts (MEF) supplemented with fibroblast growth factor 4 (FGF4). Without MEF-conditioned medium and FGF4 occurs the differentiation of trophoblast stem cells into various cell types of the placenta including spongiotrophoblast, syncytiotrophoblast and giant cells. MEF-conditioned medium provides TGFβ and/or Activin, that enable proliferation and maintenance of the undifferentiated state as inhibition of the TGFβ pathway results in TSCs differentiation.156,157 However, attempts to use an FGF4-based strategy to derive human trophoblast stem cells have failed, which suggests that others factors are also required for self-renewal of human trophoblast stem cells.158
The first transcription factors to be expressed, Eomes and Cdx-2, influence trophoblast stem cell differentiation during the preimplantation period and are up-regulated in response to FGF4 treatment of mouse trophoblast stem cells.20 Others transcription factors such as Tead4, Elf5, GATA3, Tcfap2c, Esrrb and Sox2 are also critical for specification and/or maintenance of the multipotent state of mouse trophoblast stem cells, since knockdown mouse embryos to these genes do not express or have reduced expression of trophectoderm specific genes, including Eomes and Cdx2, and died at pre-implantation stages.159,160
During the implantation and post-implantation periods, sequential expression of transcription factors Hand1, AP-2y, ETs-2, Mash2, Gcm1, Ascl2, GATA2 and FosL1 induce the differentiation of various trophoblast subpopulations. Gcm1-null mouse, for example, has defects in the formation of syncytiotrophoblasts of the labyrinth layer. However, Gcm1 alone is not sufficient to promote syncytiotrophoblasts formation in vitro from mouse trophoblast stem cell, although expression of an antisense Gcm1 transcript blocks syncytiotrophoblasts differentiation. Hand1, on the contrary, is important for mouse trophoblast giant cells formation, while Mash2 is required for the production of the precursor population that gives rise to the spongiotrophoblast layer.20,27-29 Human trophoblast progenitors also express Mash2, which is downregulated in vitro as the cells differentiate along the invasive pathway.158
Research in both humans and rodents has demonstrated that low oxygen tension directs trophoblast stem cell differentiation toward a phenotype that is associated with the junctional zone,30,31 i.e., an invasive phenotype. This developmental process depends upon hypoxia-dependent signaling pathways, and the invasive trophoblast lineage in rats depends upon the hypoxia stimulus primarily between 8.5 and 9.5 d of gestation.30,32 Similar to rats, in vitro exposure of human cytotrophoblastic cells to low oxygen tension also stimulates their differentiation along the extravillous trophoblast phenotype, which has invasive potential.33
Additionally, multiple homeobox genes controlling trophoblast differentiation potential such as HB24 and DLX4 have been identified in invasive trophoblasts from first-trimester human placenta.34,35 Jia et al.36 showed that Cdx2 promotes HTR-8/SVneo extravillous trophoblast cell invasiveness and migration to stimulate MMP9 expression and reduce metallopeptidase inhibitor 1 (TIMP1) expression. Unlike Cdx2, HTR-8/SVneo extravillous trophoblast cells transfected with Cdx1 reduces in vitro invasion and migration by reducing MMP9 expression and increasing TIMP1 expression.37
Another homeobox gene, the signal transducer and activator of transcription 3, also known as STAT3, was identified in JEG-3 human trophoblasts and in first trimester trophoblasts, but not in late gestation trophoblasts, suggesting a role in trophoblast invasion.38 Placental leptin, which is known to inhibit trophoblastic invasion, increases STAT3 expression and activity in JEG-3 trophoblasts and human cytotrophoblasts.39
Activation of another transcription regulatory factor, peroxisome proliferator-activated receptor gamma (PPAR-γ), also reduces the in vitro invasion of human cytotrophoblasts and HIPEC 65 trophoblastic cells, while its inhibition through of antagonists increases the invasiveness of these cells, suggesting an inhibitory role for this factor in trophoblast motility.40 Interestingly, PPAR-γ activation affects extravillous trophoblast expression and secretion of human chorionic gonadotropin (hCG), suggesting that the negative effects of PPAR-y on trophoblast invasion could be mediated by reductions in the levels of hCG during pregnancy.41
Yu et al.42 also demonstrated that Notch-1 expression in the JEG3 human trophoblast lineage is important for trophoblast migration and invasion such that for these cells, the in vitro inhibition of this factor through RNA interference-mediated knockdown results in the reduced expression of MMPs 2 and 9, reduced NF-κB signaling and increased expression of E-cadherin, a marker for the epithelial-mesenchymal transition.43 Notch-1 expression is reduced in placentas with preeclampsia.44 Similar to the role of Notch-1 in human trophoblasts, inactivation of Notch-2 in human trophoblast lineages also affects intrauterine trophoblastic migration, reducing trophoblastic invasion of the maternal uterine spiral arteries.45
Intrauterine trophoblast migration
Trophoblast cells invade the decidua and possess a variety of functions such as communication with maternal immune cells, hormone and cytokine production, substitution of endothelial cells of maternal arterioles and angiogenesis. However, the regulation of these features depends upon plentiful extra- and intracellular signals.
Invasive trophoblasts
Placenta trophoblast invasion into the uterine tissue and maternal blood vessels is an essential process during rodent and human gestation and fetal development. Due to their remarkable plasticity, invasive trophoblasts fulfill numerous functions such as the anchoring of the placenta in maternal tissue, secreting hormones, modulating decidual angiogenesis/lymphangiogenesis and remodeling maternal uterine spiral arteries. This latter function is critical to increase blood flow to the placenta and thus ensure adequate transfer of nutrients and oxygen to the developing fetus.30,78
Failures in uterine and placental vascular remodeling are associated with important obstetric and neonatal complications such as preeclampsia, intrauterine growth restriction, early miscarriage, premature birth and even maternal or fetal death.1,2,6 Consequently, basic research in this specific area has sought to identify the molecular mechanisms controlling trophoblast invasion under physiological and pathological conditions.
An increasing number of growth factors, angiogenic factors, cytokines and proteases that control trophoblastic cell migration kinetics have been identified, primarily through the use of first-trimester and full-term human chorionic villus explants and/or human trophoblast cell lines such as HIPEC 65, JEG3, BeWo and HTR8/SVneo.40,78,79 However, the significance of most of these factors during in vivo placentation in humans, rats and mice is unknown.
Much of the in vitro control of trophoblastic invasion is exerted by growth factors and cytokines through activation of the phosphatidylinositol-3-kinase/serine-threonine (PI3K/Akt) intracellular signaling pathway, which regulates the trophoblast invasive phenotype in rats and humans.78 Disruption of PI3K or Akt inhibits the expression of genes associated with the invasive phenotype and trophoblast cell invasion through an extracellular matrix. These actions are mediated, at least in part, through promoting the nuclear accumulation of FosLl protein. FosL1 transcription factor is important in the regulation of the invasive trophoblast cell phenotype in humans and rats, but does not have the same function in mice. Knockdown of FosL1 in human trophoblast cells in vitro and in rat models disrupted the trophoblast migration and invasion and the expression of a subset of genes associated with the invasive-vascular remodeling trophoblast phenotype, including the MMPs.26,27 In FosL1 null mouse, on the contrary, mutation in the Fosl1 gene disrupts vascularization of the labyrinth zone with prenatal lethality.165
However, most of the PI3K/Akt signaling activators are secreted not only by the trophoblast itself but also by the decidua, uNKs cells and uterine macrophages, suggesting that a complex network of various cell types, mediators and signaling pathways regulates trophoblast invasiveness.2,78
During implantation and placentation in rodents, intrauterine trophoblast invasion is a prominent feature. Rat and mouse invasive trophoblast cells have been characterized by their polyploidy, epithelial nature, accumulation of glycogen and expression of a unique subset of prolactin family cytokines. The first trophoblast cells penetrating into the uterine mesometrial compartment take an endovascular route and the depth of their invasion is generally limited to the mesometrial decidua compartment. In the rat, endovascular trophoblast cells can be identified at midgestation and expand into blood vessels situated in the uterine decidua and subsequently into the myometrium as gestation advances. During this process occurs an intraluminal invasion pathway, in contrast to the suggested invasion pathway in decidual spiral arteries of the mouse that is perivascularly followed by replacement of the endothelium from the outside.8,9,13,161-163
A second wave of trophoblast cell invasion begins after gestation day 13.5 and includes both interstitial and endovascular trophoblast cells. Invading interstitial trophoblast cells similarly penetrate the uterine decidua and in rats invade into the myometrium; however, their movements are restricted to the last third of gestation. It is not clear whether this invasion involves cell migration or proliferation-mediated extension toward the junctional zone, but it is believed that the invasion involves cell migration because there are no mitotic cells in the junctional zone at and after 14° day of gestation. Besides, invasive cells, unlike spongiotrophoblasts, are the only trophoblasts after 13° day of gestation expressing a gene coding for urokinase type plasminogen activator (uPA), a secreted proteolytic enzyme thought to be involved in cell migration.8,9,13,161-163
It is known that the trophoblast invasion in rats and mice involves degradation and remodeling of uterine extracellular matrix (ECM), partly regulated by MMPs and their tissue inhibitors (TIMPS). MMP-9 and MMP-14 have been reported to be highly expressed in the invading cells, and embryo-derived MMP-9 and uterus-derived TIMP-3 appear to be key regulators of ECM degradation by mouse trophoblast. However, the factors and regulatory mechanisms that controls the propulsion of endovascular and interstitial trophoblast cells along the decidua and myometrium of rats and mice have not been elucidated as in vitro research using human trophoblast cells. Some factors such as differential expression of adhesion molecules, colony stimulating factor-I, epidermal growth factor, hepatocyte growth factor, and insulin-like growth factor-I have been implicated as stimulators of human trophoblast cell invasion.8,9,13,161-163 Whether these and others factors also participate in the regulation of trophoblast cell invasion in the rat or mouse remains to be elucidated.
During implantation in humans, cytotrophoblasts leave the top of the anchoring villi and become extravillous trophoblasts (Figure 4). Initially, the extravillous trophoblasts form cell columns and then invade and migrate toward the decidua. The extravillous trophoblasts at the top of the villi express α6β4 integrin, while extravillous trophoblasts from the column are positive for α5β1 integrin and the extravillous trophoblasts that invade the decidua express α1β1 integrin. All of these observations show that at least in humans, trophoblast differentiation toward an invasive phenotype depends on a change in the expression of integrins on the cell surface.80,81
Figure 4.
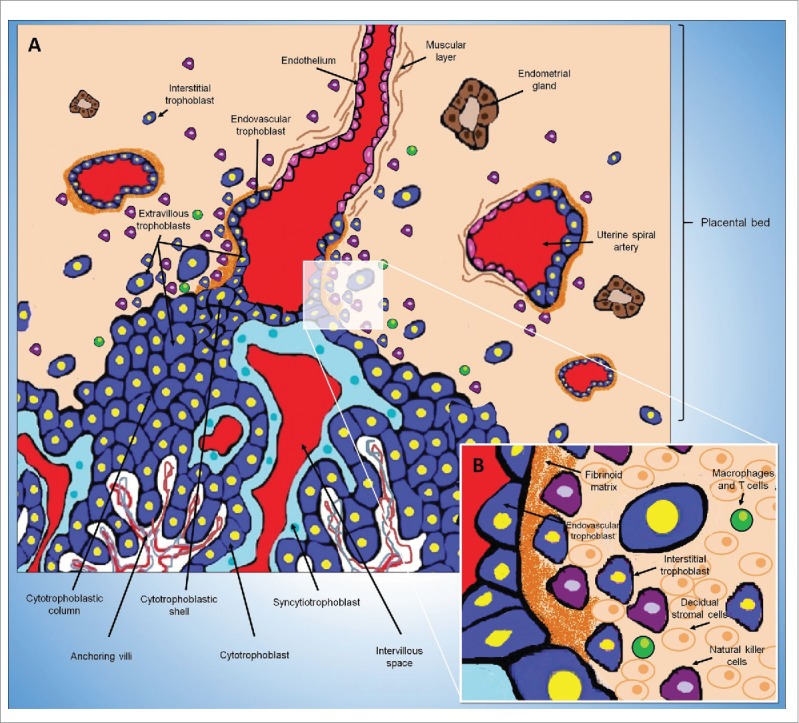
Intrauterine trophoblast invasion in humans. (A) The placental villi are covered by villous trophoblast cells (an inner cytotrophoblast layer covered by syncytiotrophoblast). O core of villus contains fetal blood vessels, fibroblasts and fetal macrophages. Maternal blood in the intervillous space reaches the placenta through the uterine spiral arteries. Cytotrophoblasts leave the top of the anchoring villi and become extravillous trophoblasts. Initially, the extravillous trophoblasts form cell columns and then invade and migrate toward the decidua. The extravillous trophoblast residing outside of placental villi acquires the capacity to degrade extracellular matrix proteins to invade the decidua (interstitial trophoblast) and the wall of maternal uterine spiral arteries (endovascular trophoblast). Endovascular trophoblasts invade the lumen of the spiral arteries and incorporate into the arterial wall. The endothelium, the muscular layer and the elastic material of these arteries are destroyed and replaced with an extracellular matrix called the fibrinoid and by the EVT cells themselves. (B) Representation of the placental bed showing interstitial trophoblast cells between decidual stromal cells, uterine natural killer cells and others maternal leukocytes (macrophages and T cells).
Adhesion molecules
Change in the integrin profile during human placental development is involved in the epithelial-mesenchymal transition exhibited by invasive trophoblasts.43 The α1β1 integrin promotes an interaction with different collagens and laminins, such as laminin 2, which is abundantly expressed in the decidua.82 Wang et al.83,84 showed that RNA interference-mediated silencing of laminin receptor 1 (LR1) suppresses human trophoblast-like cell (JEG3) migration and invasion by reducing the expression of MMPs 2 and 9, and increasing TIMP expression. LR1 also contributes to hypoxia-induced trophoblastic migration via MMP9 expression.85 The placentas of women with preeclampsia exhibit reduced LR1 expression in cytotrophoblasts and syncytiotrophoblasts.86 Hypoxia also activates an epithelial-mesenchymal-like transformation in vitro in rat trophoblast stem cells that is associated with a decrease in the expression of E-cadherin and increases in the expression of MMPs 9 and 12 and movement through an extracellular matrix. These responses are dependent on activation of the HIF signaling pathway.2,17
Zhou et al.50,87 suggest that failures in trophoblastic invasion of the spiral arteries result from a defect in the epithelial-mesenchymal transition and in the expression profile of vascular adhesion molecules. During normal pregnancy, extravillous trophoblast downregulate the expression of adhesion receptors that are characteristic of their epithelial origin, and upregulate the expression of adhesion receptors that are expressed by vascular cells. E-cadherin expression is reduced, while expression of VE-cadherin, vascular cell adhesion molecule 1 (VECAM-1), Platelet endothelial cell adhesion molecule 1 (PECAM-1) and α4 integrins increase. Endovascular trophoblasts continue to express these receptors and begin to express αVβ3 integrins, similarly to activated endothelial cells. The extravillous trophoblasts of patients with preeclampsia fail to express most of these endothelial markers, suggesting that trophoblast expression of a vascular phenotype is necessary for endovascular invasion success.50,87 In contrast, other studies yielded conflicting results, showing no extravillous trophoblast expression of PECAM-1, or that expression of PECAM-1 and E-cadherin did not vary between normal pregnancies and those with preeclampsia and IUGR.88-90
Integrin glycosylation at the maternal-fetal interface is also of great importance in the control of trophoblastic migration and invasion during pregnancy as it mediates the interactions between trophoblasts and the extracellular matrix.91 Liao et al.92 demonstrated in vitro that β-1,4-galactosyltransferase III (B4GALT3)-transfected trophoblast cell have reduced invasion, increasing their adhesion to laminin and trophoblast degradation of β1 integrin. As B4GALT3 expression in humans primarily occurs during the last trimester of pregnancy in the extravillous trophoblast, it is believed to be a critical regulator of trophoblast invasion in late pregnancy.92 Similar roles have been suggested for mucin 1 and 15 (MUC1/MUC15), as their expression in human placenta increases during pregnancy and in vitro studies have demonstrated that MUC1 and MUC15-transfected trophoblast-like cells (JAR and JEG-3 cells) have reduced invasion and migration through reduced MMP9 expression, increased TIMP1 expression, and/or inhibition of β1 integrin activity.93-95 Shyu et al.93 showed that MUC1 expression is increased in human placentas with preeclampsia.
Growth factors
Numerous growth factors have been identified as acting at the maternal-fetal interface to stimulate human trophoblast cell proliferation, adhesion and/or invasion. Among these factors are epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), placental growth factor (PIGF), colony stimulating factor 1 (CSF-1) and insulin-like growth factors 1 and 2 (IGF1 and IGF2).96-98 In contrast, addition in culture medium of inhibitory proteins such as transforming growth factor β (TGFβ) family members, interferon gamma (INFγ), endostatin, tumor necrosis factor α (TNFα) and fetuin-A limit human trophoblastic lineage migration and invasion in vitro.99-102
Cytokines
Cytokines, e.g., CX3CL1, CCL14, CCL4, CXCL16 and CCL21 are also capable of stimulating human trophoblast migration and invasion,103,104 unlike CXCL6 and CXCL14 cytokines that inhibit human trophoblast cell migration and invasion in vitro by inhibiting gelatinase activity. Human CXCL14 expression occurs primarily during early pregnancy and predominantly in villous cytotrophoblast, in contrast with CXCL6, which exhibits increased expression during pregnancy, particularly in the extravillous trophoblast.105,106
Interleukins
Interleukins, such as IL1, IL6, IL8, and IL11, that are secreted by trophoblastic or decidual cells also promote increased human trophoblast migration and invasion, with increased expression of MMPs 2 to 9 and/or integrins α1, α5, α6 and β1.107-109 The leukemia inhibitory factor (LIF) is another key cytokine in development and embryo implantation, and increases the expression of protein O-fucosyltransferase 1 (poFUT1) in human trophoblasts in vitro, promoting their migration and invasion at the maternal-fetal interface by activating the PI3K/Akt signaling pathway and stimulating MMP2 expression.110
It is notable that the importance of the growth factors and interleukins may vary during pregnancy depending upon the patterns of temporal expression, abundance of soluble inhibitors and receptor expression.78
Placental hormones
The hormones produced by the placenta itself also influence trophoblast migration and vascular remodeling at the maternal-fetal interface. Estradiol modulates the release in vitro of macrophage migration inhibitory factor (MIF) in first-trimester chorionic villous explants in a dose-dependent manner.112 Krivokuca et al.113 showed that MIF stimulates the in vitro migration and invasion of extravillous trophoblasts concomitant with increased levels of MMPs 2 and 9, and α1 integrin. Additionally, estrogen regulates the expression of placental leptin in a dose-dependent manner.114,115 Placental leptin also increases the expression of MMPs 2 and 9 and trophoblastic migration in vitro.114,115
Progesterone is another hormone produced by the placenta and is one of the factors that controls trophoblastic cell invasiveness by reducing trophoblast secretion of gelatinases, although this effect is dependent upon the gestational period.116 The progesterone-induced blocking factor (PIBF), identified in both humans and mice, was originally described as a progesterone-induced molecule that mediates the effects of progesterone during pregnancy. The distribution of PIBF within the human decidua during the first trimester of pregnancy coincides with sites of trophoblastic invasion, with strong PIBF immunostaining in the extravillous trophoblast.117 Furthermore, PIBF has been shown to reduce the expression of placental leptin and its receptor, revealing another way in which progesterone affects trophoblastic invasion.118
Prolactin and placental growth hormone (pGH) also stimulate in vitro migration of human trophoblasts by increasing expression of the α1 and α5 integrins and galectin-1.119,120 Galectin 1, expressed by the trophoblast column during cytotrophoblast differentiation to form the extravillous trophoblast, also stimulates in vitro trophoblastic migration.121 pGH is believed to potentially act as an autocrine stimulator of trophoblastic invasion, particularly during the early stages of cytotrophoblast differentiation into extravillous trophoblast.119
Others factors
Recently, human and rodent kisspeptins were implicated not only in the inhibition of tumor metastasis but also in the inhibition of trophoblastic invasion via the G protein-coupled receptor Kiss-1R. In human placenta, the expression levels of kisspeptin and its receptor are highest during the first trimester of pregnancy, while for rats, they are most highly expressed at 12.5° days of pregnancy. This period coincides with the peak of trophoblastic invasion and with when regulation of trophoblastic invasion is critical. In rats, as in humans, the levels of Kiss-1 and its receptor decline during placental maturation, reaching low and/or undetectable levels at the end of gestation.48 Human kisspeptins are primarily produced by the syncytiotrophoblast, while its receptor is expressed in the syncytiotrophoblast, cytotrophoblast and extravillous trophoblast, indicating paracrine regulation of trophoblastic invasion by the syncytiotrophoblast. In rat placenta, which is structurally different from human placenta, both Kiss-1 and its receptor are predominantly expressed by the invasive trophoblast giant cells, suggesting autocrine control over the invasion of these cells.48 Research has shown that kisspeptins reduce trophoblastic migration by reducing MMP expression, and increasing TIMP expression and invasive trophoblast adhesion through type 1 collagen in the decidual stroma.48,111
Recent research has also shown that microRNAs (miRNAs) can influence trophoblastic invasion and migration. miRNAs are a class of endogenous small RNAs that act as post-transcriptional negative regulators of gene expression, and increased expression of certain miRNAs (miR-29b, miR-16, miR-222 and miR-519d-3p) in the plasma and/or placenta has been observed in women with preeclampsia.122,123 Recently, Ding et al.122 demonstrated that in vitro overexpression of miR-519d-3p significantly inhibit trophoblast cell migration and invasion, whereas transfection of a miR-519d-3p inhibitor enhance trophoblast cell migration and invasion. Besides, miR-519d-3p overexpression reduce MMP-2 mRNA and protein expression, while MMP-2 knockdown using a siRNA attenuates the increased trophoblast migration and invasion promoted by the miR-519d-3p inhibitor. miR-519d-3p is exclusively expressed in placenta and regulates fetal-maternal signaling.122
Interestingly, in vitro work by Liu et al.110 also demonstrated that Gadd45α, a downstream target of p53, negatively regulates trophoblastic migration and invasion in early human pregnancy by reducing the expression of MMPs 2 and 9 and increasing the expression of TIMP1 and 2. Additionally, Gadd45α expression is elevated in preeclamptic placenta.110 Gadd45α is an important factor in cell cycle regulation, apoptosis, signal transduction and genome stability, and its expression inhibits tumor cell invasion and metastasis by maintaining cell adhesion and reducing MMP expression.124
Interactions between invasive trophoblasts, extracellular matrix and uterine natural killer cells (uNK cells) during uterine vascular remodeling
During gestation in humans and rats, the endovascular and interstitial trophoblast invasion is restricted to the decidua and the initial third of the myometrium, as the extent of trophoblast invasion is precisely controlled by maternal factors and factors released by trophoblastic cells themselves.96,97 Tantbirojn et al.3 showed that in humans, deep trophoblastic invasion occurs in the absence of decidua resulting from either scarce uterine tissue or ectopic pregnancy, revealing the importance of decidua-released factors in the control of trophoblast invasion.
In both humans and rodents, the invasive trophoblast is equipped with various proteases systems that allow it to degrade extracellular matrix (ECM) proteins such as collagen IV, laminin, vitronectin and fibronectin to promote cell migration. Acting in opposition, the decidua expresses a variety of inhibitory proteins that restrict trophoblastic cell invasion. Invasive trophoblasts express various members of the MMP family, such as cathepsins and urokinase plasminogen activator (uPA),125 while decidual cells produce TIMPs and plasminogen activator inhibitor (PAI).78 Extravillous trophoblasts also express CCN1 and CCN3, which are extracellular matrix proteins that are involved in cell migration, and their levels are decreased during conditions of preeclampsia; unglycosylated CCN3 protein increases the invasion and migration potential of JEG3 human trophoblast cells in vitro.126,127 Both CCN1 and CCN3 also promote the migration of human trophoblasts under hypoxic conditions in vitro, and their expression levels are upregulated by hypoxia-inducible factor 1-α (HIF1α) and TGFβ3.126,127
uNK cells are the principal maternal immune system cells that are present in the decidualized endometrium before and during placentation in humans, rats and mice. These cells are members of the lymphoid lineage, are similar to T and B lymphocytes, and can be divided into several subpopulations. Uterine NK cells are phenotypically and functionally distinct from those present in the systemic circulation.17,128 Their exact role in pregnancy is unknown, but they are believed to be involved in the regulation of placentation; however, research has shown that they function with trophoblasts to coordinate uterine spiral artery remodeling.128 Mice lacking uNK cells (TgE26 mice) are fertile, but exhibit inappropriate uterine vascular remodeling, poor decidualization and low fetal weight, highlighting the importance of uNK cells in placentation.129 These cells function by secreting a variety of cytokines and growth factors such as VEGF, INFγ, nitric oxide (NO), and interleukin 15 (IL-15), etc.1,32 In humans, rats and mice, NK cells primarily participate in uterine vascular remodeling during the early stages of pregnancy as uNK cells are absent from the maternal-fetal interface during late pregnancy.1,17
Research using human placenta has suggested that extravillous trophoblasts are primarily responsible for the vascular remodeling of the uterine spiral arteries53; however, new evidence indicates that the early vascular remodeling process is primarily performed by uNK cells, meaning that the extravillous trophoblasts have a secondary role. The vascular remodeling of the maternal-fetal interface in humans and mice begins before intrauterine trophoblastic migration, when the decidual uNK cell population has already been established.69,130
In vitro experiments co-culturing human trophoblasts with uNK cells have shown that uNK cells inhibit invasive trophoblast migration to reduce cytotrophoblastic expression of MMP2 and 9, and PAI-1. These effects are believe to be mediated at least in part by INFγ; however, uNK cells affect neither extravillous trophoblast cell proliferation and apoptosis nor the formation of trophoblastic villi.131 uNK cells also produce other soluble molecules, e.g., TGFβ1, TGFβ2, IL-10, TIMP-1 and TNFα, which also limit human trophoblast cell migration and invasion in vitro.1,2,132,133 However, other factors that are also produced by uNK cells promote the migration of extravillous trophoblast, e.g., IL-8 and interferon gamma-induced protein 10 (IP-10).134
Recently, Hu et al.135 showed that uNK cell-mediated inhibition of human trophoblast cell migration in vitro is mediated by DNA hypermethylation, causing a reduction in the expression of claudin-4 (CLDN4) and fucosyltransferase IV (FUTIV), which are important for trophoblastic migration. DNA methylation is an important epigenetic modification in programmed gene regulation during cell differentiation and adaptation to environmental conditions, and has been associated with changes in gene expression. Research has shown that DNA methylation is involved in pregnancy complications such as preeclampsia.136,137
Trophoblast migration, placental stress and diseases associated with failure of intrauterine trophoblast migration
Blood flow at the maternal-fetal interface increases during pregnancy, and abnormal pregnancies can be predicted by changes in the flow as measured by Doppler. Normally, the utero-placental arteries are invaded by the endovascular trophoblast and remodeled into blood vessels that are dilated, inelastic and lack maternal vasomotor control. When intrauterine trophoblastic migration is deficient, vascular remodeling is impaired, and this impairment has been implicated as a cause of IUGR and preeclampsia.6,138-140
Failures in extravillous trophoblast invasion lead to the absence of arterial remodeling, resulting in vessels that permit highly pressured pulses to reach the delicate structures of the chorionic villi and intermittent blood flow in the placental site. During pregnancy, this condition leads to a hypoxic environment and/or ischemia reoxygenation, and the continual production of reactive oxygen species (ROS) and depletion of intracellular ATP concentrations. Consequently, inflammatory mediators are released into maternal circulation and cause preeclampsia syndrome that is characterized by hypertension and proteinuria.140 Other clinical signs and changes may also be observed during preeclampsia, including oliguria, epigastric pain, thrombocytopenia, hyperuricemia, elevated liver enzymes and cyanosis.141 Preeclampsia is a major cause of morbidity and mortality in pregnant women and neonates, affecting between 2 and 8% of pregnancies worldwide. Most cases begin late in the third trimester of pregnancy, but approximately 10% of cases start before 34 weeks of gestation; in these cases, which are also known as early onset preeclampsia, the disease is more severe and is often associated with IUGR.142 Notably, even when IUGR is not accompanied by pre-eclampsia, failure of intrauterine vascular remodeling has also been observed; in these cases, interstitial trophoblastic invasion is increased, unlike that observed in preeclampsia.143
The placental stress associated with clinical changes observed in preeclampsia results from oxidative stress,139 nitrosative stress144 and as described more recently, endoplasmic reticulum (ER) stress at the maternal-fetal interface.138,145 During this process, ischemia-reperfusion injury and hypoxia favor the occurrence of ER stress through changes in calcium homeostasis and increased ROS production via the mitochondrial pathway or proteolytic cleavage of xanthine dehydrogenase to form xanthine oxidase. Furthermore, the protein folding that occurs during ER stress generates ROS, and ER stress stimulates some inflammatory pathways.138,140
In preeclampsia, the NF-κB inflammatory pathway is activated and levels of plasma inflammatory cytokines such as TNFα and IL-6 increase.140 Experimental evidence using Eif2s1(tm1RjK) mutants mice has shown that preeclampsia ER stress-induced apoptosis occurs particularly in the syncytiotrophoblast and decidua.146 This process causes the release of placental microparticles and cell debris into the maternal bloodstream and activates several inflammatory pathways that trigger the inflammatory response and systemic endothelial dysfunction, the main pathophysiological events of preeclampsia.140 Explants of late gestation human placenta under conditions of in vitro hypoxia-reoxygenation also exhibit increased secretion of pro-inflammatory cytokines such as TNFα and IL-1β in addition to increased levels of anti-angiogenic factors such as sFlt-1.147,148
Certain factors and metabolic disorders such as obesity, malnutrition, smoking, uterine infection and diabetes may also predispose mothers to preeclampsia. With regards to diabetes, although no research has shown changes in uterine vascular remodeling in response to hyperglycemia, maternal hyperinsulinemia in rats decreases the number and depth of endovascular invasive trophoblast cells.164 Han et al.149 recently showed that excess glucose reduces the in vitro migratory capacity of extravillous trophoblasts and promotes trophoblast synthesis of inflammatory cytokines such as IL-1β, IL-6, IL-8 and G-CSF. This pro-inflammatory status and the failure of trophoblastic migration in vitro suggests that diabetic hyperglycemia can result in the changes in uterine vascular remodeling that are observed in preeclampsia.149 Interestingly, obesity in humans also favors a pro-inflammatory environment at the maternal-fetal interface, increasing the macrophage population and TNFα expression. This environment has been suggested to compromise trophoblastic migration and consequently affect uterine vascular remodeling.150 Hayes et al.151 demonstrated that obese rats have reduced trophoblast migration during late pregnancy, as well as failure of intrauterine vascular remodeling, corroborating the hypothesis of Challier et al.150
Recent studies in rats have also shown that other metabolic disorders, such as thyroid dysfunction, can affect intrauterine trophoblastic migration, and an environment of placental stress at the maternal-fetal interface is suspected in these animals.12,152
Silva et al.152 observed that the placentas from hypothyroid rats have increased populations of glycogen cells in the spongiotrophoblast and of trophoblastic giant cells, suggesting a failure in the migration kinetics of these cells toward the decidua. In vitro work by Silva et al.153 showed that trophoblasts of rats treated with triiodothyronine exhibit increased Tpbp and Prl3b1 gene expression, which are only expressed in the spongiotrophoblast and invasive trophoblast, respectively, during the differentiation of trophoblasts from the ectoplacental cone.67 In 2014,12 the same group of researchers demonstrated that hypothyroid rats exhibited reduced endovascular and interstitial trophoblast invasion that could be associated with low placental and fetal weight. These animals also exhibited reduced iNOS expression during pregnancy,12 and Cartwright et al.75 demonstrated that in vitro, trophoblastic migration and invasion are heavily dependent on trophoblast-produced NOS.
Hypothyroid rats also exhibit reduced expression of MMPs 2 and 9 and placental leptin, in contrast with hyperthyroid rats that exhibit increased trophoblast MMP2 expression.12 These results corroborate the work of Oki et al.154 who observed that thyroid hormone deficiency in vitro reduced human extravillous trophoblast expression of MMPs 2 and 3, fetal fibronectin and integrins. Expression of MMPs 2 and 9 is also stimulated by placental leptin,114 corroborating the findings of Silva et al.12
Hyperthyroid rats exhibit reduced endovascular trophoblast invasion during late gestation,12 and this is believed to be related to the premature birth presented by these animals. For adequate delivery and afterbirth, invasive trophoblasts die and are removed.2,17 Contrary to that observed in hyperthyroid rats,12 increased trophoblastic invasion in late pregnancy, accompanied by failure to remove these cells from the decidua, is a major cause of retained placenta, dystocia and postpartum hemorrhage in women and domestic animal species, and can be fatal.3,55
Another example of excessive trophoblastic invasion occurs in placenta creta (accreta, increta and percreta), wherein the anchoring villi and extravillous trophoblasts penetrate beyond the initial third of the myometrium, reaching the final third of the myometrium, the perimetrium or even extra-uterine tissues and organs. Depending upon the extent of invasion, the placenta is considered to be accreta, increta or percreta, with placenta percreta being the most severe. In all these situations, failure of decidua formation is also observed, such that the placental villi and extravillous trophoblasts more easily reach the myometrium. Interestingly, although trophoblasts are more invasive in these situations, incomplete vascular remodeling of maternal uterine spiral arteries still occurs in placenta cretas. All of these placental abnormalities may result in heavy bleeding during pregnancy and following childbirth, as well as hinder the release of the placenta following the birth of the fetus.3,58
Unlike placenta creta, in which excessive trophoblastic invasion of ‘normal’ trophoblasts occurs, gestational trophoblastic disease (GTD) exhibits an excess of trophoblastic invasion by tumor cells or pre-tumor cells derived from the placental villi, presenting 5 major clinical and pathological forms: hydatiform mole (complete or partial), invasive mole, choriocarcinoma, placental site trophoblastic tumor and epithelioid trophoblastic tumor. As observed in placenta creta, severe bleeding can occur in all GTD cases, in addition to the possibility of fatality if untreated. Despite its great invasive and sometimes even metastatic potential, little is known about the regulation of GTD development and growth.4,59
Regarding recurrent spontaneous abortion, another gestational condition that can occur at the beginning and end of pregnancy, failures in the uterine vascular remodeling have also been observed in humans, with an increase in the population of uNK cells and reduction of intramural and endovascular trophoblast populations.155 The causes underlying these changes and the consequent abortion are still unknown; therefore, more research is needed in this area.
Conclusions
Trophoblast migration and invasion through the decidua and maternal uterine spiral arteries are crucial events in placentation. This review shows that despite the differences in structure and some types of trophoblasts between the human and rodent placentas, some aspects and functions are highly similar, especially when considering intrauterine trophoblastic migration. Consequently, the use of experimental animal models such as rats and mice has led to great progress in recent years with regards to the identification of mechanisms and factors that control trophoblast migration kinetics. Some of this progress can also be attributed to the use of in vitro models of immortalized trophoblasts or placental explants in monoculture or co-culture system that are either bi or tri-dimensional, in vivo and in vitro gene transfection of trophoblast cells, and the use of genetically modified rats and mice. These tools have facilitated a better understanding of trophoblastic migration under physiological conditions and especially during some pathologies, such as preeclampsia and intrauterine growth restriction. In this context, the current perspectives in reproductive medicine allow the development of in vitro experimental techniques that can more accurately simulate the placental environment to promote a better understanding of all of the biological events occurring at the maternal-fetal interface during pregnancy, especially those concerning the human placenta, as there are limitations on in vivo research. Additionally, the rat should receive greater use an experimental model to determine whether the in vivo roles of various studied factors correspond to their functions in trophoblast culture. The greater our understanding of placentation, the more disease prevention and maintenance of maternal-fetal and postnatal health will be improved.
Abbreviations
- MMP-9
metalloproteinase 9
- TIMP1
metallopeptidase inhibitor 1
- STAT3
signal transducer and activator of transcription 3
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- hCG
human chorionic gonadotropin
- EVT
extravillous trophoblast
- FGF4
fibroblast growth factor 4
- sFlt-1
soluble fms-like tyrosine kinase-1
- rPlf
proliferin-related protein
- PCDH12
protocadherin 12
- uNKs
uterine natural killer cells
- NOS
nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- PI3K/Akt
phosphatidylinositol-3-kinase/serine-threonine
- VECAM-1
vascular cell adhesion molecule 1
- PECAM-1
platelet endothelial cell adhesion molecule 1
- IUGR
intrauterine growth restriction
- IL
interleukin
- B4GALT3
β-1,4-galactosyltransferase III
- EGF
epidermal growth factor
- VEGF
vascular endothelial growth factor
- PDGF
platelet-derived growth factor
- PIGF
placental growth factor
- CSF-1
colony stimulating factor 1
- IGF1/ IGF2
insulin-like growth factors 1/2
- TGFβ
transforming growth factor β
- INFγ
interferon gamma
- TNFα
tumor necrosis factor α
- LIF
leukemia inhibitory factor
- MIF
macrophage migration inhibitory factor
- PIBF
progesterone-induced blocking factor, pGH, placental growth hormone
- uPA
urokinase plasminogen activator
- PAI
plasminogen activator inhibitor
- HIF1α
hypoxia-inducible factor 1-α
- IP-10
interferon gamma-induced protein 10
- ROS
reactive oxygen species
- GTD
gestational trophoblastic disease
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from Postgraduate Program in Animal Science from Universidade Federal de Minas Gerais (UFMG).
References
- 1.Hammer A. Immunological regulation of trophoblast invasion. J Reprod Immunol 2011; 90:21-8; PMID:21641660; http://dx.doi.org/ 10.1016/j.jri.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 2.Soares MJ, Chakraborty D, Kubota K, Renaud SJ, Rumi MA. Adaptive mechanisms controlling uterine spiral artery remodeling during the establishment of pregnancy. Int J Dev Biol 2014; 58:247-59; PMID:25023691; http://dx.doi.org/ 10.1387/ijdb.140083ms [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tantbirojn P, Crum CP, Parast MM. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta 2008; 29:639-45; PMID:18514815; http://dx.doi.org/ 10.1016/j.placenta.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 4.Bolze PA, Attia J, Massardier J, Seckl MJ, Massuger L, van Trommel N, Niemann I, Hajri T, Schott AM, Golfier F, et al.. Formalised consensus of the European Organisation for Treatment of Trophoblastic Diseases on management of gestational trophoblastic diseases. Eur J Cancer 2015; 51(13):1725-31; PMID:26092638 [DOI] [PubMed] [Google Scholar]
- 5.Smith JM, Currie S, Cannon T, Armbruster D, Perri J. Are national policies and programs for prevention and management of postpartum hemorrhage and preeclampsia adequate? A key informant survey in 37 countries. Glob Health Sci Pract 2014; 2:275-84; PMID:25276587; http://dx.doi.org/ 10.9745/GHSP-D-14-00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 2003; 69:1-7; PMID:12620937; http://dx.doi.org/ 10.1095/biolreprod.102.014977 [DOI] [PubMed] [Google Scholar]
- 7.Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 1983; 4:397-413; PMID:6634666; http://dx.doi.org/ 10.1016/S0143-4004(83)80043-5 [DOI] [PubMed] [Google Scholar]
- 8.Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol 2003; 260:176-90; PMID:12885563; http://dx.doi.org/ 10.1016/S0012-1606(03)00210-0 [DOI] [PubMed] [Google Scholar]
- 9.Caluwaerts S, Vercruysse L, Luyten C, Pijnenborg R. Endovascular trophoblast invasion and associated structural changes in uterine spiral arteries of the pregnant rat. Placenta 2005; 26:574-84; PMID:15993707; http://dx.doi.org/ 10.1016/j.placenta.2004.09.007 [DOI] [PubMed] [Google Scholar]
- 10.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1972; 1:177-91; PMID:4669123 [PubMed] [Google Scholar]
- 11.Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol 1977; 84:656-63; PMID:911717; http://dx.doi.org/ 10.1111/j.1471-0528.1977.tb12676.x [DOI] [PubMed] [Google Scholar]
- 12.Silva JF, Ocarino NM, Serakides R. Maternal thyroid dysfunction affects placental profile of inflammatory mediators and the intrauterine trophoblast migration kinetics. Reproduction 2014; 147:803-16; PMID:24534949; http://dx.doi.org/ 10.1530/REP-13-0374 [DOI] [PubMed] [Google Scholar]
- 13.Malassine A, Frendo JL, Evain-Brion D. A comparison of placental development and endocrine functions between the human and mouse model. Hum Reprod Update 2003; 9:531-9; PMID:14714590; http://dx.doi.org/ 10.1093/humupd/dmg043 [DOI] [PubMed] [Google Scholar]
- 14.Rosario GX, Ain R, Konno T, Soares MJ. Intrauterine fate of invasive trophoblast cells. Placenta 2009; 30:457-63; PMID:19344949; http://dx.doi.org/ 10.1016/j.placenta.2009.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lunghi L, Ferretti ME, Medici S, Biondi C, Vesce F. Control of human trophoblast function. Reprod Biol Endocrinol 2007; 5:6; PMID:17288592; http://dx.doi.org/ 10.1186/1477-7827-5-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pijnenborg R, Robertson WB, Brosens I, Dixon G. Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta 1981; 2:71-91; PMID:7010344; http://dx.doi.org/ 10.1016/S0143-4004(81)80042-2 [DOI] [PubMed] [Google Scholar]
- 17.Soares MJ, Chakraborty D, Karim Rumi MA, Konno T, Renaud SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta 2012; 33:233-43; PMID:22284666; http://dx.doi.org/ 10.1016/j.placenta.2011.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross JC. Genetic insights into trophoblast differentiation and placental morphogenesis. Semin Cell Dev Biol 2000; 11:105-13; PMID:10873707; http://dx.doi.org/ 10.1006/scdb.2000.0156 [DOI] [PubMed] [Google Scholar]
- 19.Armant DR. Blastocysts don't go it alone. Extrinsic signals fine-tune the intrinsic developmental program of trophoblast cells. Dev Biol 2005; 280:260-80; PMID:15882572; http://dx.doi.org/ 10.1016/j.ydbio.2005.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imakawa K, Chang KT, Christenson RK. Pre-implantation conceptus and maternal uterine communications: molecular events leading to successful implantation. J Reprod Dev 2004; 50:155-69; PMID:15118242; http://dx.doi.org/ 10.1262/jrd.50.155 [DOI] [PubMed] [Google Scholar]
- 21.Cross JC, Baczyk D, Dobric N, Hemberger M, Hughes M, Simmons DG, Yamamoto H, Kingdom JC. Genes, development and evolution of the placenta. Placenta 2003; 24:123-30; PMID:12596737; http://dx.doi.org/ 10.1053/plac.2002.0887 [DOI] [PubMed] [Google Scholar]
- 22.Loregger T, Pollheimer J, Knofler M. Regulatory transcription factors controlling function and differentiation of human trophoblast–a review. Placenta 2003; 24(Suppl A):S104-10; PMID:12842421; http://dx.doi.org/ 10.1053/plac.2002.0929 [DOI] [PubMed] [Google Scholar]
- 23.Meinhardt G, Husslein P, Knofler M. Tissue-specific and ubiquitous basic helix-loop-helix transcription factors in human placental trophoblasts. Placenta 2005; 26:527-39; PMID:15993702; http://dx.doi.org/ 10.1016/j.placenta.2004.09.005 [DOI] [PubMed] [Google Scholar]
- 24.Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet 1998; 18:271-5; PMID:9500551; http://dx.doi.org/ 10.1038/ng0398-271 [DOI] [PubMed] [Google Scholar]
- 25.Knofler M, Meinhardt G, Bauer S, Loregger T, Vasicek R, Bloor DJ, Kimber SJ, Husslein P. Human Hand1 basic helix-loop-helix (bHLH) protein: extra-embryonic expression pattern, interaction partners and identification of its transcriptional repressor domains. Biochem J 2002; 361:641-51; PMID:11802795; http://dx.doi.org/ 10.1042/bj3610641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kent LN, Rumi MA, Kubota K, Lee DS, Soares MJ. FOSL1 is integral to establishing the maternal-fetal interface. Mol Cell Biol 2011; 31:4801-13; PMID:21947281; http://dx.doi.org/ 10.1128/MCB.05780-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renaud SJ, Kubota K, Rumi MA, Soares MJ. The FOS transcription factor family differentially controls trophoblast migration and invasion. J Biol Chem 2014; 289:5025-39; PMID:24379408; http://dx.doi.org/ 10.1074/jbc.M113.523746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes M, Dobric N, Scott IC, Su L, Starovic M, St-Pierre B, Egan SE, Kingdom JC, Cross JC. The Hand1, Stra13 and Gcm1 transcription factors override FGF signaling to promote terminal differentiation of trophoblast stem cells. Dev Biol 2004; 271:26-37; PMID:15196947; http://dx.doi.org/ 10.1016/j.ydbio.2004.03.029 [DOI] [PubMed] [Google Scholar]
- 29.Ray S, Dutta D, Rumi MA, Kent LN, Soares MJ, Paul S. Context-dependent function of regulatory elements and a switch in chromatin occupancy between GATA3 and GATA2 regulate Gata2 transcription during trophoblast differentiation. J Biol Chem 2009; 284:4978-88; PMID:19106099; http://dx.doi.org/ 10.1074/jbc.M807329200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakraborty D, Rumi MA, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci U S A 2011; 108:16295-300; PMID:21900602; http://dx.doi.org/ 10.1073/pnas.1109478108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev 2000; 14:3191-203; PMID:11124810; http://dx.doi.org/ 10.1101/gad.853700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol 2008; 314:362-75; PMID:18199431; http://dx.doi.org/ 10.1016/j.ydbio.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robins JC, Heizer A, Hardiman A, Hubert M, Handwerger S. Oxygen tension directs the differentiation pathway of human cytotrophoblast cells. Placenta 2007; 28:1141-6; PMID:17706280; http://dx.doi.org/ 10.1016/j.placenta.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 34.Quinn LM, Latham SE, Kalionis B. Homeobox gene HB24, a regulator of haematopoiesis, is a candidate for regulating differentiation of the extra-embryonic trophoblast cell lineage. Reprod Fertil Dev 1997; 9:617-23; PMID:9551666; http://dx.doi.org/ 10.1071/R97025 [DOI] [PubMed] [Google Scholar]
- 35.Quinn LM, Latham SE, Kalionis B. A distal-less class homeobox gene, DLX4, is a candidate for regulating epithelial-mesenchymal cell interactions in the human placenta. Placenta 1998; 19:87-93; PMID:9481790; http://dx.doi.org/ 10.1016/S0143-4004(98)90103-5 [DOI] [PubMed] [Google Scholar]
- 36.Jia RZ, Ding GC, Gu CM, Huang T, Rui C, Wang YX, Lu Q. CDX2 enhances HTR-8/SVneo trophoblast cell invasion by altering the expression of matrix metalloproteinases. Cell Physiol Biochem 2014; 34:628-36; PMID:25171776; http://dx.doi.org/ 10.1159/000363028 [DOI] [PubMed] [Google Scholar]
- 37.Jia RZ, Rui C, Li JY, Cui XW, Wang X. CDX1 restricts the invasion of HTR-8/SVneo trophoblast cells by inhibiting MMP-9 expression. Placenta 2014; 35:450-4; PMID:24836459; http://dx.doi.org/ 10.1016/j.placenta.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 38.Corvinus FM, Fitzgerald JS, Friedrich K, Markert UR. Evidence for a correlation between trophoblast invasiveness and STAT3 activity. Am J Reprod Immunol 2003; 50:316-21; PMID:14672334; http://dx.doi.org/ 10.1034/j.1600-0897.2003.00099.x [DOI] [PubMed] [Google Scholar]
- 39.Poehlmann TG, Fitzgerald JS, Meissner A, Wengenmayer T, Schleussner E, Friedrich K, Markert UR. Trophoblast invasion: tuning through LIF, signalling via Stat3. Placenta 2005; 26(Suppl A):S37-41; PMID:15837065; http://dx.doi.org/ 10.1016/j.placenta.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 40.Tarrade A, Schoonjans K, Pavan L, Auwerx J, Rochette-Egly C, Evain-Brion D, Fournier T. PPARgamma/RXRalpha heterodimers control human trophoblast invasion. J Clin Endocrinol Metab 2001; 86:5017-24; PMID:11600579 [DOI] [PubMed] [Google Scholar]
- 41.Handschuh K, Guibourdenche J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T. Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-gamma. Endocrinology 2007; 148:5011-9; PMID:17628005; http://dx.doi.org/ 10.1210/en.2007-0286 [DOI] [PubMed] [Google Scholar]
- 42.Yu Y, Wang L, Tang W, Zhang D, Shang T. RNA interference-mediated knockdown of Notch-1 inhibits migration and invasion, down-regulates matrix metalloproteinases and suppresses NF-kappaB signaling pathway in trophoblast cells. Acta Histochem 2014; 116:911-9; PMID:24681113; http://dx.doi.org/ 10.1016/j.acthis.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 43.Tripathi V, Popescu NC, Zimonjic DB. DLC1 induces expression of E-cadherin in prostate cancer cells through Rho pathway and suppresses invasion. Oncogene 2014; 33:724-33; PMID:23376848; http://dx.doi.org/ 10.1038/onc.2013.7 [DOI] [PubMed] [Google Scholar]
- 44.Cobellis L, Mastrogiacomo A, Federico E, Schettino MT, De Falco M, Manente L, Coppola G, Torella M, Colacurci N, De Luca A. Distribution of Notch protein members in normal and preeclampsia-complicated placentas. Cell Tissue Res 2007; 330:527-34; PMID:17955263; http://dx.doi.org/ 10.1007/s00441-007-0511-6 [DOI] [PubMed] [Google Scholar]
- 45.Haider S, Meinhardt G, Velicky P, Otti GR, Whitley G, Fiala C, Pollheimer J, Knofler M. Notch signaling plays a critical role in motility and differentiation of human first-trimester cytotrophoblasts. Endocrinology 2014; 155:263-74; PMID:24189144; http://dx.doi.org/ 10.1210/en.2013-1455 [DOI] [PubMed] [Google Scholar]
- 46.Zhang P, Tang M, Zhong T, Lin Y, Zong T, Zhong C, Zhang B, Ren M, Kuang H. Expression and function of kisspeptin during mouse decidualization. PLoS One 2014; 9:e97647; PMID:24830702; http://dx.doi.org/ 10.1371/journal.pone.0097647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hertig AT. On the development of the amnion and exoccelomic membrane in the previllous human ovum. Yale J Biol Med 1945; 18:107-15; PMID:21007544 [PMC free article] [PubMed] [Google Scholar]
- 48.Hiden U, Bilban M, Knofler M, Desoye G. Kisspeptins and the placenta: regulation of trophoblast invasion. Rev Endocr Metab Disord 2007; 8:31-9; PMID:17351756; http://dx.doi.org/ 10.1007/s11154-007-9030-8 [DOI] [PubMed] [Google Scholar]
- 49.Hamilton WJ, Grimes DH. Growth relationship between the foetus and the placenta. Proc R Soc Med 1970; 63:496-8; PMID:5460385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest 1997; 99:2139-51; PMID:9151786; http://dx.doi.org/ 10.1172/JCI119387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Yuge A, Rajah AM, Unek G, Rinaudo PF, Maltepe E. LIMK1 regulates human trophoblast invasion/differentiation and is down-regulated in preeclampsia. Am J Pathol 2014; 184:3321-31; PMID:25307528; http://dx.doi.org/ 10.1016/j.ajpath.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pollheimer J, Knofler M. Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta 2005; 26(Suppl A):S21-30; PMID:15837062; http://dx.doi.org/ 10.1016/j.placenta.2004.11.013 [DOI] [PubMed] [Google Scholar]
- 53.Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol 1967; 93:569-79; PMID:6054057; http://dx.doi.org/ 10.1002/path.1700930218 [DOI] [PubMed] [Google Scholar]
- 54.Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980; 1:3-19; PMID:7443635; http://dx.doi.org/ 10.1016/S0143-4004(80)80012-9 [DOI] [PubMed] [Google Scholar]
- 55.Ophir E, Singer-Jordan J, Odeh M, Hirch Y, Maksimovsky O, Shaider O, Yvry S, Solt I, Bornstein J. Abnormal placental invasion–a novel approach to treatment case report and review. Obstet Gynecol Surv 2009; 64:811-22; PMID:19939295; http://dx.doi.org/ 10.1097/OGX.0b013e3181c46913 [DOI] [PubMed] [Google Scholar]
- 56.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005; 308:1592-4; PMID:15947178; http://dx.doi.org/ 10.1126/science.1111726 [DOI] [PubMed] [Google Scholar]
- 57.Shih IM, Kurman RJ. New concepts in trophoblastic growth and differentiation with practical application for the diagnosis of gestational trophoblastic disease. Verh Dtsch Ges Pathol 1997; 81:266-72; PMID:9474880 [PubMed] [Google Scholar]
- 58.Clausen C, Lonn L, Langhoff-Roos J. Management of placenta percreta: a review of published cases. Acta Obstet Gynecol Scand 2014; 93:138-43; PMID:24266548; http://dx.doi.org/ 10.1111/aogs.12295 [DOI] [PubMed] [Google Scholar]
- 59.Hubener C, Bidlingmaier M, Wu Z, Diebold J, Delius M, Friese K, Strasburger CJ, Hasbargen U. Human placental growth hormone: a potential new biomarker in gestational trophoblastic disease. Gynecol Oncol 2015; 136:264-8; PMID:25448485; http://dx.doi.org/ 10.1016/j.ygyno.2014.11.077 [DOI] [PubMed] [Google Scholar]
- 60.Ain R, Konno T, Canham LN, Soares MJ. Phenotypic analysis of the rat placenta. Methods Mol Med 2006; 121:295-313; PMID:16251750 [DOI] [PubMed] [Google Scholar]
- 61.Gardner RL. Analysis of determination and differentiation in the early mammalian embryo using intra- and interspecific chimeras. Symp Soc Dev Biol 1975:207-36; PMID:1098201 [DOI] [PubMed] [Google Scholar]
- 62.Mossman HW. Comparative morphogenesis of the fetal membranes and accessory uterine structures. Contrib Embryol 1937; 26:133-247; PMID:2034592 [DOI] [PubMed] [Google Scholar]
- 63.Hemberger M, Cross JC. Genes governing placental development. Trends Endocrinol Metab 2001; 12:162-8; PMID:11295572; http://dx.doi.org/ 10.1016/S1043-2760(01)00375-7 [DOI] [PubMed] [Google Scholar]
- 64.Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005; 20:180-93; PMID:15888575; http://dx.doi.org/ 10.1152/physiol.00001.2005 [DOI] [PubMed] [Google Scholar]
- 65.Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell 2005; 8:365-75; PMID:15737932; http://dx.doi.org/ 10.1016/j.devcel.2004.12.016 [DOI] [PubMed] [Google Scholar]
- 66.Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta 2004; 25:114-26; PMID:14972444; http://dx.doi.org/ 10.1016/j.placenta.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 67.Cross JC. How to make a placenta: mechanisms of trophoblast cell differentiation in mice–a review. Placenta 2005; 26(Suppl A):S3-9; PMID:15837063; http://dx.doi.org/ 10.1016/j.placenta.2005.01.015 [DOI] [PubMed] [Google Scholar]
- 68.Cross JC, Hemberger M, Lu Y, Nozaki T, Whiteley K, Masutani M, Adamson SL. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol 2002; 187:207-12; PMID:11988329; http://dx.doi.org/ 10.1016/S0303-7207(01)00703-1 [DOI] [PubMed] [Google Scholar]
- 69.Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol 2002; 250:358-73; PMID:12376109; http://dx.doi.org/ 10.1006/dbio.2002.0773 [DOI] [PubMed] [Google Scholar]
- 70.Coan PM, Conroy N, Burton GJ, Ferguson-Smith AC. Origin and characteristics of glycogen cells in the developing murine placenta. Dev Dyn 2006; 235:3280-94; PMID:17039549; http://dx.doi.org/ 10.1002/dvdy.20981 [DOI] [PubMed] [Google Scholar]
- 71.Burdon C, Mann C, Cindrova-Davies T, Ferguson-Smith AC, Burton GJ. Oxidative stress and the induction of cyclooxygenase enzymes and apoptosis in the murine placenta. Placenta 2007; 28:724-33; PMID:17222904; http://dx.doi.org/ 10.1016/j.placenta.2006.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem 1999; 274:25130-5; PMID:10455194; http://dx.doi.org/ 10.1074/jbc.274.35.25130 [DOI] [PubMed] [Google Scholar]
- 73.Milstone DS, Redline RW, O'Donnell PE, Davis VM, Stavrakis G. E-selectin expression and function in a unique placental trophoblast population at the fetal-maternal interface: regulation by a trophoblast-restricted transcriptional mechanism conserved between humans and mice. Dev Dyn 2000; 219:63-76; PMID:10974672; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 74.Bouillot S, Rampon C, Tillet E, Huber P. Tracing the glycogen cells with protocadherin 12 during mouse placenta development. Placenta 2006; 27:882-8; PMID:16269175; http://dx.doi.org/ 10.1016/j.placenta.2005.09.009 [DOI] [PubMed] [Google Scholar]
- 75.Cartwright JE, Holden DP, Whitley GS. Hepatocyte growth factor regulates human trophoblast motility and invasion: a role for nitric oxide. Br J Pharmacol 1999; 128:181-9; PMID:10498850; http://dx.doi.org/ 10.1038/sj.bjp.0702757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat 2009; 215:27-35; PMID:19175804; http://dx.doi.org/ 10.1111/j.1469-7580.2008.00978.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hemberger M, Nozaki T, Masutani M, Cross JC. Differential expression of angiogenic and vasodilatory factors by invasive trophoblast giant cells depending on depth of invasion. Dev Dyn 2003; 227:185-91; PMID:12761846; http://dx.doi.org/ 10.1002/dvdy.10291 [DOI] [PubMed] [Google Scholar]
- 78.Knofler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol 2010; 54:269-80; PMID:19876833; http://dx.doi.org/ 10.1387/ijdb.082769mk [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pavan L, Tarrade A, Hermouet A, Delouis C, Titeux M, Vidaud M, Therond P, Evain-Brion D, Fournier T. Human invasive trophoblasts transformed with simian virus 40 provide a new tool to study the role of PPARgamma in cell invasion process. Carcinogenesis 2003; 24:1325-36; PMID:12807721; http://dx.doi.org/ 10.1093/carcin/bgg074 [DOI] [PubMed] [Google Scholar]
- 80.Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest 1992; 89:210-22; PMID:1370295; http://dx.doi.org/ 10.1172/JCI115565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aplin JD. Expression of integrin alpha 6 beta 4 in human trophoblast and its loss from extravillous cells. Placenta 1993; 14:203-15; PMID:7685095; http://dx.doi.org/ 10.1016/S0143-4004(05)80261-9 [DOI] [PubMed] [Google Scholar]
- 82.Church HJ, Vicovac LM, Williams JD, Hey NA, Aplin JD. Laminins 2 and 4 are expressed by human decidual cells. Lab Invest 1996; 74:21-32; PMID:8569185 [PubMed] [Google Scholar]
- 83.Wang L, Yu Y, Guan H, Liu T, Qiao C. 67-kDa Laminin receptor contributes to hypoxia-induced migration and invasion of trophoblast-like cells by mediating matrix metalloproteinase-9. Clin Exp Pharmacol Physiol 2015; 42:549-58; PMID:25800042; http://dx.doi.org/ 10.1111/1440-1681.12389 [DOI] [PubMed] [Google Scholar]
- 84.Wang L, Zhang D, Yu Y, Guan H, Qiao C, Shang T. RNA interference-mediated silencing of laminin receptor 1 (LR1) suppresses migration and invasion and down-regulates matrix metalloproteinase (MMP)-2 and MMP-9 in trophoblast cells: implication in the pathogenesis of preeclampsia. J Mol Histol 2013; 44:661-8; PMID:23729238; http://dx.doi.org/ 10.1007/s10735-013-9515-6 [DOI] [PubMed] [Google Scholar]
- 85.Formisano P, Ragno P, Pesapane A, Alfano D, Alberobello AT, Rea VE, Giusto R, Rossi FW, Beguinot F, Rossi G, et al.. PED/PEA-15 interacts with the 67 kD laminin receptor and regulates cell adhesion, migration, proliferation and apoptosis. J Cell Mol Med 2012; 16:1435-46; PMID:21895963; http://dx.doi.org/ 10.1111/j.1582-4934.2011.01411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurdoglu M, Kurdoglu Z, Ozen S, Kucukaydin Z, Bulut G, Erten R, Kamaci M. Expression of laminin receptor 1 in human placentas from normal and preeclamptic pregnancies and its relationship with the severity of preeclampsia. J Perinat Med 2011; 39:411-6; PMID:21391874; http://dx.doi.org/ 10.1515/jpm.2011.024 [DOI] [PubMed] [Google Scholar]
- 87.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest 1997; 99:2152-64; PMID:9151787; http://dx.doi.org/ 10.1172/JCI119388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Divers MJ, Bulmer JN, Miller D, Lilford RJ. Beta 1 integrins in third trimester human placentae: no differential expression in pathological pregnancy. Placenta 1995; 16:245-60; PMID:7543673; http://dx.doi.org/ 10.1016/0143-4004(95)90112-4 [DOI] [PubMed] [Google Scholar]
- 89.Floridon C, Nielsen O, Holund B, Sunde L, Westergaard JG, Thomsen SG, Teisner B. Localization of E-cadherin in villous, extravillous and vascular trophoblasts during intrauterine, ectopic and molar pregnancy. Mol Hum Reprod 2000; 6:943-50; PMID:11006324; http://dx.doi.org/ 10.1093/molehr/6.10.943 [DOI] [PubMed] [Google Scholar]
- 90.Lyall F, Bulmer JN, Duffie E, Cousins F, Theriault A, Robson SC. Human trophoblast invasion and spiral artery transformation: the role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol 2001; 158:1713-21; PMID:11337369; http://dx.doi.org/ 10.1016/S0002-9440(10)64127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janik ME, Litynska A, Vereecken P. Cell migration-the role of integrin glycosylation. Biochim Biophys Acta 2010; 1800:545-55; PMID:20332015; http://dx.doi.org/ 10.1016/j.bbagen.2010.03.013 [DOI] [PubMed] [Google Scholar]
- 92.Liao WC, Liu CH, Chen CH, Hsu WM, Liao YY, Chang HM, Lan CT, Huang MC, Shyu MK. beta-1,4-Galactosyltransferase III suppresses extravillous trophoblast invasion through modifying beta1-integrin glycosylation. Placenta 2015; 36:357-64; PMID:25659296; http://dx.doi.org/ 10.1016/j.placenta.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 93.Shyu MK, Chen CW, Lin NY, Liao WC, Chen CH, Lin CJ, Huang HC, Lee JJ, Huang MJ, Tseng GF, et al.. MUC1 expression is elevated in severe preeclamptic placentas and suppresses trophoblast cell invasion via beta1-integrin signaling. J Clin Endocrinol Metab 2011; 96:3759-67; PMID:21917866; http://dx.doi.org/ 10.1210/jc.2011-1368 [DOI] [PubMed] [Google Scholar]
- 94.Shyu MK, Lin MC, Liu CH, Fu YR, Shih JC, Lee CN, Chen HY, Huang J, Huang MC, Hsieh FJ. MUC1 expression is increased during human placental development and suppresses trophoblast-like cell invasion in vitro. Biol Reprod 2008; 79:233-9; PMID:18417712; http://dx.doi.org/ 10.1095/biolreprod.108.067629 [DOI] [PubMed] [Google Scholar]
- 95.Shyu MK, Lin MC, Shih JC, Lee CN, Huang J, Liao CH, Huang IF, Chen HY, Huang MC, Hsieh FJ. Mucin 15 is expressed in human placenta and suppresses invasion of trophoblast-like cells in vitro. Hum Reprod 2007; 22:2723-32; PMID:17720698; http://dx.doi.org/ 10.1093/humrep/dem249 [DOI] [PubMed] [Google Scholar]
- 96.Bischof P, Meisser A, Campana A. Paracrine and autocrine regulators of trophoblast invasion–a review. Placenta 2000; 21(Suppl A):S55-60; PMID:10831123; http://dx.doi.org/ 10.1053/plac.2000.0521 [DOI] [PubMed] [Google Scholar]
- 97.Lala PK, Hamilton GS. Growth factors, proteases and protease inhibitors in the maternal-fetal dialogue. Placenta 1996; 17:545-55; PMID:8916202; http://dx.doi.org/ 10.1016/S0143-4004(96)80071-3 [DOI] [PubMed] [Google Scholar]
- 98.Mayama R, Izawa T, Sakai K, Suciu N, Iwashita M. Improvement of insulin sensitivity promotes extravillous trophoblast cell migration stimulated by insulin-like growth factor-I. Endocr J 2013; 60:359-68; PMID:23197113; http://dx.doi.org/ 10.1507/endocrj.EJ12-0241 [DOI] [PubMed] [Google Scholar]
- 99.Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab 2004; 89:812-22; PMID:14764800; http://dx.doi.org/ 10.1210/jc.2003-031351 [DOI] [PubMed] [Google Scholar]
- 100.Gomez LM, Anton L, Srinivas SK, Elovitz MA, Parry S. Effects of increased fetuin-A in human trophoblast cells and associated pregnancy outcomes. Am J Obstet Gynecol 2012; 207:484.e1-8; PMID:23108067 [DOI] [PubMed] [Google Scholar]
- 101.Lala PK, Graham CH. Mechanisms of trophoblast invasiveness and their control: the role of proteases and protease inhibitors. Cancer Metastasis Rev 1990; 9:369-79; PMID:2097085; http://dx.doi.org/ 10.1007/BF00049525 [DOI] [PubMed] [Google Scholar]
- 102.Lash GE, Otun HA, Innes BA, Kirkley M, De Oliveira L, Searle RF, Robson SC, Bulmer JN. Interferon-gamma inhibits extravillous trophoblast cell invasion by a mechanism that involves both changes in apoptosis and protease levels. FASEB J 2006; 20:2512-8; PMID:17142800; http://dx.doi.org/ 10.1096/fj.06-6616com [DOI] [PubMed] [Google Scholar]
- 103.Huang Y, Zhu XY, Du MR, Wu X, Wang MY, Li DJ. Chemokine CXCL16, a scavenger receptor, induces proliferation and invasion of first-trimester human trophoblast cells in an autocrine manner. Hum Reprod 2006; 21:1083-91; PMID:16431903; http://dx.doi.org/ 10.1093/humrep/dei436 [DOI] [PubMed] [Google Scholar]
- 104.Red-Horse K, Kapidzic M, Zhou Y, Feng KT, Singh H, Fisher SJ. EPHB4 regulates chemokine-evoked trophoblast responses: a mechanism for incorporating the human placenta into the maternal circulation. Development 2005; 132:4097-106; PMID:16107476; http://dx.doi.org/ 10.1242/dev.01971 [DOI] [PubMed] [Google Scholar]
- 105.Kuang H, Chen Q, Zhang Y, Zhang L, Peng H, Ning L, Cao Y, Duan E. The cytokine gene CXCL14 restricts human trophoblast cell invasion by suppressing gelatinase activity. Endocrinology 2009; 150:5596-605; PMID:19833716; http://dx.doi.org/ 10.1210/en.2009-0570 [DOI] [PubMed] [Google Scholar]
- 106.Zhang H, Hou L, Li CM, Zhang WY. The chemokine CXCL6 restricts human trophoblast cell migration and invasion by suppressing MMP-2 activity in the first trimester. Hum Reprod 2013; 28:2350-62; PMID:23814098; http://dx.doi.org/ 10.1093/humrep/det258 [DOI] [PubMed] [Google Scholar]
- 107.Jovanovic M, Stefanoska I, Radojcic L, Vicovac L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1. Reproduction 2010; 139:789-98; PMID:20133364; http://dx.doi.org/ 10.1530/REP-09-0341 [DOI] [PubMed] [Google Scholar]
- 108.Jovanovic M, Vicovac L. Interleukin-6 stimulates cell migration, invasion and integrin expression in HTR-8/SVneo cell line. Placenta 2009; 30:320-8; PMID:19251319; http://dx.doi.org/ 10.1016/j.placenta.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 109.Librach CL, Feigenbaum SL, Bass KE, Cui TY, Verastas N, Sadovsky Y, Quigley JP, French DL, Fisher SJ. Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J Biol Chem 1994; 269:17125-31; PMID:8006017 [PubMed] [Google Scholar]
- 110.Liu S, Wang J, Qin HM, Yan XM, Yang XS, Liu C, Yan Q. LIF upregulates poFUT1 expression and promotes trophoblast cell migration and invasion at the fetal-maternal interface. Cell Death Dis 2014; 5:e1396; PMID:25165882; http://dx.doi.org/ 10.1038/cddis.2014.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taylor J, Pampillo M, Bhattacharya M, Babwah AV. Kisspeptin/KISS1R signaling potentiates extravillous trophoblast adhesion to type-I collagen in a PKC- and ERK1/2-dependent manner. Mol Reprod Dev 2014; 81:42-54; PMID:24273038; http://dx.doi.org/ 10.1002/mrd.22279 [DOI] [PubMed] [Google Scholar]
- 112.Ietta F, Bechi N, Romagnoli R, Bhattacharjee J, Realacci M, Di Vito M, Ferretti C, Paulesu L. 17{beta}-Estradiol modulates the macrophage migration inhibitory factor secretory pathway by regulating ABCA1 expression in human first-trimester placenta. Am J Physiol Endocrinol Metab 2010; 298:E411-8; PMID:20173014; http://dx.doi.org/ 10.1152/ajpendo.00522.2009 [DOI] [PubMed] [Google Scholar]
- 113.Jovanovic Krivokuca M, Stefanoska I, Abu Rabi T, Al-Abed Y, Stosic-Grujicic S, Vicovac L. Pharmacological inhibition of MIF interferes with trophoblast cell migration and invasiveness. Placenta 2015; 36:150-9; PMID:25530499; http://dx.doi.org/ 10.1016/j.placenta.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 114.Gambino YP, Maymo JL, Perez Perez A, Calvo JC, Sanchez-Margalet V, Varone CL. Elsevier Trophoblast Research Award lecture: Molecular mechanisms underlying estrogen functions in trophoblastic cells–focus on leptin expression. Placenta 2012; 33(Suppl):S63-70; PMID:22197627; http://dx.doi.org/ 10.1016/j.placenta.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 115.Gambino YP, Maymo JL, Perez-Perez A, Duenas JL, Sanchez-Margalet V, Calvo JC, Varone CL. 17Beta-estradiol enhances leptin expression in human placental cells through genomic and nongenomic actions. Biol Reprod 2010; 83:42-51; PMID:20237333; http://dx.doi.org/ 10.1095/biolreprod.110.083535 [DOI] [PubMed] [Google Scholar]
- 116.Shimonovitz S, Hurwitz A, Hochner-Celnikier D, Dushnik M, Anteby E, Yagel S. Expression of gelatinase B by trophoblast cells: down-regulation by progesterone. Am J Obstet Gynecol 1998; 178:457-61; PMID:9539508; http://dx.doi.org/ 10.1016/S0002-9378(98)70420-X [DOI] [PubMed] [Google Scholar]
- 117.Anderle C, Hammer A, Polgar B, Hartmann M, Wintersteiger R, Blaschitz A, Dohr G, Desoye G, Szekeres-Bartho J, Sedlmayr P. Human trophoblast cells express the immunomodulator progesterone-induced blocking factor. J Reprod Immunol 2008; 79:26-36; PMID:18817979; http://dx.doi.org/ 10.1016/j.jri.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 118.Miko E, Halasz M, Jericevic-Mulac B, Wicherek L, Arck P, Arato G, Skret Magierlo J, Rukavina D, Szekeres-Bartho J. Progesterone-induced blocking factor (PIBF) and trophoblast invasiveness. J Reprod Immunol 2011; 90:50-7; PMID:21632119; http://dx.doi.org/ 10.1016/j.jri.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 119.Lacroix MC, Guibourdenche J, Fournier T, Laurendeau I, Igout A, Goffin V, Pantel J, Tsatsaris V, Evain-Brion D. Stimulation of human trophoblast invasion by placental growth hormone. Endocrinology 2005; 146:2434-44; PMID:15718272; http://dx.doi.org/ 10.1210/en.2004-1550 [DOI] [PubMed] [Google Scholar]
- 120.Stefanoska I, Jovanovic Krivokuca M, Vasilijic S, Cujic D, Vicovac L. Prolactin stimulates cell migration and invasion by human trophoblast in vitro. Placenta 2013; 34:775-83; PMID:23849393; http://dx.doi.org/ 10.1016/j.placenta.2013.06.305 [DOI] [PubMed] [Google Scholar]
- 121.Kolundzic N, Bojic-Trbojevic Z, Kovacevic T, Stefanoska I, Kadoya T, Vicovac L. Galectin-1 is part of human trophoblast invasion machinery–a functional study in vitro. PLoS One 2011; 6:e28514; PMID:22174828; http://dx.doi.org/ 10.1371/journal.pone.0028514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ding J, Huang F, Wu G, Han T, Xu F, Weng D, Wu C, Zhang X, Yao Y, Zhu X. MiR-519d-3p suppresses invasion and migration of trophoblast cells via targeting MMP-2. PLoS One 2015; 10:e0120321; PMID:25803859; http://dx.doi.org/ 10.1371/journal.pone.0120321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li H, Ge Q, Guo L, Lu Z. Maternal plasma miRNAs expression in preeclamptic pregnancies. Biomed Res Int 2013; 2013:970265; PMID:24195082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hildesheim J, Bulavin DV, Anver MR, Alvord WG, Hollander MC, Vardanian L, Fornace AJ Jr. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res 2002; 62:7305-15; PMID:12499274 [PubMed] [Google Scholar]
- 125.Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta 2003; 24:575-87; PMID:12828917; http://dx.doi.org/ 10.1016/S0143-4004(03)00063-8 [DOI] [PubMed] [Google Scholar]
- 126.Wolf N, Yang W, Dunk CE, Gashaw I, Lye SJ, Ring T, Schmidt M, Winterhager E, Gellhaus A. Regulation of the matricellular proteins CYR61 (CCN1) and NOV (CCN3) by hypoxia-inducible factor-1{alpha} and transforming-growth factor-{beta}3 in the human trophoblast. Endocrinology 2010; 151:2835-45; PMID:20237132; http://dx.doi.org/ 10.1210/en.2009-1195 [DOI] [PubMed] [Google Scholar]
- 127.Yang W, Wagener J, Wolf N, Schmidt M, Kimmig R, Winterhager E, Gellhaus A. Impact of CCN3 (NOV) glycosylation on migration/invasion properties and cell growth of the choriocarcinoma cell line Jeg3. Hum Reprod 2011; 26:2850-60; PMID:21784733; http://dx.doi.org/ 10.1093/humrep/der239 [DOI] [PubMed] [Google Scholar]
- 128.Tessier DR, Yockell-Lelievre J, Gruslin A. Uterine Spiral Artery Remodeling: The Role of Uterine Natural Killer Cells and Extravillous Trophoblasts in Normal and High-Risk Human Pregnancies. Am J Reprod Immunol 2015; 74:1-11; PMID:25472023; http://dx.doi.org/ 10.1111/aji.12345 [DOI] [PubMed] [Google Scholar]
- 129.Colucci F, Boulenouar S, Kieckbusch J, Moffett A. How does variability of immune system genes affect placentation? Placenta 2011; 32:539-45; PMID:21665273; http://dx.doi.org/ 10.1016/j.placenta.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Charalambous F, Elia A, Georgiades P. Decidual spiral artery remodeling during early post-implantation period in mice: investigation of associations with decidual uNK cells and invasive trophoblast. Biochem Biophys Res Commun 2012; 417:847-52; PMID:22206676; http://dx.doi.org/ 10.1016/j.bbrc.2011.12.057 [DOI] [PubMed] [Google Scholar]
- 131.Hu Y, Dutz JP, MacCalman CD, Yong P, Tan R, von Dadelszen P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol 2006; 177:8522-30; PMID:17142750; http://dx.doi.org/ 10.4049/jimmunol.177.12.8522 [DOI] [PubMed] [Google Scholar]
- 132.Lash GE, Otun HA, Innes BA, Bulmer JN, Searle RF, Robson SC. Inhibition of trophoblast cell invasion by TGFB1, 2, and 3 is associated with a decrease in active proteases. Biol Reprod 2005; 73:374-81; PMID:15858216; http://dx.doi.org/ 10.1095/biolreprod.105.040337 [DOI] [PubMed] [Google Scholar]
- 133.Vigano P, Gaffuri B, Somigliana E, Infantino M, Vignali M, Di Blasio AM. Interleukin-10 is produced by human uterine natural killer cells but does not affect their production of interferon-gamma. Mol Hum Reprod 2001; 7:971-7; PMID:11574666; http://dx.doi.org/ 10.1093/molehr/7.10.971 [DOI] [PubMed] [Google Scholar]
- 134.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, et al.. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006; 12:1065-74; PMID:16892062; http://dx.doi.org/ 10.1038/nm1452 [DOI] [PubMed] [Google Scholar]
- 135.Hu Y, Blair JD, Yuen RK, Robinson WP, von Dadelszen P. Genome-wide DNA methylation identifies trophoblast invasion-related genes: Claudin-4 and Fucosyltransferase IV control mobility via altering matrix metalloproteinase activity. Mol Hum Reprod 2015; 21:452-65; PMID:25697377; http://dx.doi.org/ 10.1093/molehr/gav007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Blair JD, Yuen RK, Lim BK, McFadden DE, von Dadelszen P, Robinson WP. Widespread DNA hypomethylation at gene enhancer regions in placentas associated with early-onset pre-eclampsia. Mol Hum Reprod 2013; 19:697-708; PMID:23770704; http://dx.doi.org/ 10.1093/molehr/gat044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yuen RK, Penaherrera MS, von Dadelszen P, McFadden DE, Robinson WP. DNA methylation profiling of human placentas reveals promoter hypomethylation of multiple genes in early-onset preeclampsia. Eur J Hum Genet 2010; 18:1006-12; PMID:20442742; http://dx.doi.org/ 10.1038/ejhg.2010.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burton GJ, Yung HW. Endoplasmic reticulum stress in the pathogenesis of early-onset pre-eclampsia. Pregnancy Hypertens 2011; 1:72-8; PMID:22242213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hung TH, Burton GJ. Hypoxia and reoxygenation: a possible mechanism for placental oxidative stress in preeclampsia. Taiwan J Obstet Gynecol 2006; 45:189-200; PMID:17175463; http://dx.doi.org/ 10.1016/S1028-4559(09)60224-2 [DOI] [PubMed] [Google Scholar]
- 140.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta 2009; 30(Suppl A):S38-42; PMID:19138798; http://dx.doi.org/ 10.1016/j.placenta.2008.11.021 [DOI] [PubMed] [Google Scholar]
- 141.Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet 1993; 341:1447-51; PMID:8099148; http://dx.doi.org/ 10.1016/0140-6736(93)90889-O [DOI] [PubMed] [Google Scholar]
- 142.Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol 2003; 189:1173-7; PMID:14586374; http://dx.doi.org/ 10.1067/S0002-9378(03)00576-3 [DOI] [PubMed] [Google Scholar]
- 143.Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension 2013; 62:1046-54; PMID:24060885; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.113.01892 [DOI] [PubMed] [Google Scholar]
- 144.Myatt L, Rosenfield RB, Eis AL, Brockman DE, Greer I, Lyall F. Nitrotyrosine residues in placenta. Evidence of peroxynitrite formation and action. Hypertension 1996; 28:488-93; PMID:8794838; http://dx.doi.org/ 10.1161/01.HYP.28.3.488 [DOI] [PubMed] [Google Scholar]
- 145.Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta 2009; 30(Suppl A):S43-8; PMID:19081132; http://dx.doi.org/ 10.1016/j.placenta.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yung HW, Hemberger M, Watson ED, Senner CE, Jones CP, Kaufman RJ, Charnock-Jones DS, Burton GJ. Endoplasmic reticulum stress disrupts placental morphogenesis: implications for human intrauterine growth restriction. J Pathol 2012; 228:554-64; PMID:22733590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cindrova-Davies T, Spasic-Boskovic O, Jauniaux E, Charnock-Jones DS, Burton GJ. Nuclear factor-kappa B, p38, and stress-activated protein kinase mitogen-activated protein kinase signaling pathways regulate proinflammatory cytokines and apoptosis in human placental explants in response to oxidative stress: effects of antioxidant vitamins. Am J Pathol 2007; 170:1511-20; PMID:17456758; http://dx.doi.org/ 10.2353/ajpath.2007.061035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hung TH, Charnock-Jones DS, Skepper JN, Burton GJ. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol 2004; 164:1049-61; PMID:14982858; http://dx.doi.org/ 10.1016/S0002-9440(10)63192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Han CS, Herrin MA, Pitruzzello MC, Mulla MJ, Werner EF, Pettker CM, Flannery CA, Abrahams VM. Glucose and metformin modulate human first trimester trophoblast function: a model and potential therapy for diabetes-associated uteroplacental insufficiency. Am J Reprod Immunol 2015; 73:362-71; PMID:25394884; http://dx.doi.org/ 10.1111/aji.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, Hauguel-de Mouzon S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 2008; 29:274-81; PMID:18262644; http://dx.doi.org/ 10.1016/j.placenta.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hayes EK, Tessier DR, Percival ME, Holloway AC, Petrik JJ, Gruslin A, Raha S. Trophoblast invasion and blood vessel remodeling are altered in a rat model of lifelong maternal obesity. Reprod Sci 2014; 21:648-57; PMID:24155067; http://dx.doi.org/ 10.1177/1933719113508815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Silva JF, Vidigal PN, Galvao DD, Boeloni JN, Nunes PP, Ocarino NM, Nascimento EF, Serakides R. Fetal growth restriction in hypothyroidism is associated with changes in proliferative activity, apoptosis and vascularisation of the placenta. Reprod Fertil Dev 2012; 24:923-31; PMID:22935153; http://dx.doi.org/ 10.1071/RD11219 [DOI] [PubMed] [Google Scholar]
- 153.Silva JF, Ocarino NM, Serakides R. In vitro effects of triiodothyronine on gene expression in mouse trophoblast cells. Placenta 2015; 36:97-9; PMID:25468542; http://dx.doi.org/ 10.1016/j.placenta.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 154.Oki N, Matsuo H, Nakago S, Murakoshi H, Laoag-Fernandez JB, Maruo T. Effects of 3,5,3′-triiodothyronine on the invasive potential and the expression of integrins and matrix metalloproteinases in cultured early placental extravillous trophoblasts. J Clin Endocrinol Metab 2004; 89:5213-21; PMID:15472228; http://dx.doi.org/ 10.1210/jc.2004-0352 [DOI] [PubMed] [Google Scholar]
- 155.Ball E, Bulmer JN, Ayis S, Lyall F, Robson SC. Late sporadic miscarriage is associated with abnormalities in spiral artery transformation and trophoblast invasion. J Pathol 2006; 208:535-42; PMID:16402350; http://dx.doi.org/ 10.1002/path.1927 [DOI] [PubMed] [Google Scholar]
- 156.Erlebacher A, Price KA, Glimcher LH. Maintenance of mouse trophoblast stem cell proliferation by TGF-beta/activin. Dev Biol 2004; 275:158-69; PMID:15464579; http://dx.doi.org/ 10.1016/j.ydbio.2004.07.032 [DOI] [PubMed] [Google Scholar]
- 157.Tanaka S. Derivation and culture of mouse trophoblast stem cells in vitro. Methods Mol Biol 2006; 329:35-44; PMID:16845982 [DOI] [PubMed] [Google Scholar]
- 158.Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest 2004; 114:744-54; PMID:15372095; http://dx.doi.org/ 10.1172/JCI200422991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Latos PA, Hemberger M. Review: the transcriptional and signalling networks of mouse trophoblast stem cells. Placenta 2014; 35:S81-5; PMID:24220516; http://dx.doi.org/ 10.1016/j.placenta.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 160.Nishioka N, Yamamoto S, Kiyonari H, Sato H, Sawada A, Ota M, Nakao K, Sasaki H. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech Dev 2008; 125:270-83; PMID:18083014; http://dx.doi.org/ 10.1016/j.mod.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 161.Lei T, Hohn HP, Behr R, Denker HW. Influences of extracellular matrix and of conditioned media on differentiation and invasiveness of trophoblast stem cells. Placenta 2007; 28:14-21; PMID:16563500; http://dx.doi.org/ 10.1016/j.placenta.2006.01.020 [DOI] [PubMed] [Google Scholar]
- 162.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 2002; 23:3-19; PMID:11869088; http://dx.doi.org/ 10.1053/plac.2001.0738 [DOI] [PubMed] [Google Scholar]
- 163.Vercruysse L, Caluwaerts S, Luyten C, Pijnenborg R. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta. 2006; 27:22-33; PMID:16310034; http://dx.doi.org/ 10.1016/j.placenta.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 164.Skarzinski G, Khamaisi M, Bursztyn M, Mekler J, Lan D, Evdokimov P, Ariel I. Intrauterine growth restriction and shallower implantation site in rats with maternal hyperinsulinemia are associated with altered NOS expression. Placenta 2009; 30:898-906; PMID:19709742; http://dx.doi.org/ 10.1016/j.placenta.2009.07.014 [DOI] [PubMed] [Google Scholar]
- 165.Schreiber M, Wang ZQ, Jochum W, Fetka I, Elliott C, Wagner EF. Placental vascularisation requires the AP-1 component fra1. Development 2000; 127:4937-48; PMID:11044407 [DOI] [PubMed] [Google Scholar]