ABSTRACT
Preeclampsia (PE) and intrauterine growth retardation (IUGR) are rare but severe pregnancy complications that are associated with placental insufficiency often resulting in premature birth. The clinical pathologies are related to gross placental pathologies and trophoblastic deficiencies that might derive from inflammatory processes and oxidative stress injury. The mesenchymal core of placental villi has been identified as a possible niche for trophoblast progenitor cells that are called upon to replenish the injured syncytiotrophoblast layer. These progenitor cells are known to express trophoblast stem cell (CDX2) and pluripotency (SOX2, NANOG and OCT4A) markers, however only little data is available characterizing the expression of these transcription factors beyond the blastocyst stage. We aimed to describe the expression of these factors in healthy 1st and 3rd trimester placentae as well as PE, IUGR and combined PE+IUGR placentae. We analyzed 8 respective samples derived from 1st trimester (elective abortions), and 3rd trimester (healthy controls, PE, IUGR and combined PE+IUGR). We accomplished immunoperoxidase staining to detect the stem cell markers: CDX2 (trophectoderm), SOX2, NANOG and OCT4A (embryonal). Immunoreative scoring was used for objective analyses of staining patterns. All markers display clearly elevated signals in 1st trimester villous samples as compared to healthy 3rd trimester counterparts. Especially CDX2 and NANOG were specific to the cytotrophoblast layer and the mesenchymal core. Specific and differential expression patterns were visible in the villous/extravillous compartment of each placenta-associated pregnancy complication (PE: pan elevated expression; IUGR elevated SOX2 in basal plate; combined PE+IUGR pan loss of expression). Reduction of stem cell transcription factor expression in term placentae indicates temporal regulation, and probably a specific function which is yet to be elucidated. The differential expression patterns within placentae complicated with placenta-associated pregnancy complications indicate that PE, IUGR and combined PE+IUGR are separate entities. It is unclear whether the alterations are the cause or the effect of the clinical pathology.
KEYWORDS: IUGR, placenta, pluripotency markers, preeclampsia, trophoblast stem cell markers
Introduction
Preeclampsia is a potentially lethal syndrome that affects 6–8 % of all pregnancies and accounts for approximately 15% of all maternal deaths worldwide.1 Although much debate exists on defining the exact pathomechanism, it is generally accepted that the placenta is the main culprit in triggering this disease, because only removal of the placenta amounts to causal therapy.
PE is often thought of as a multi-step disease with the first trimester setting the premises for the onset of the disease in the latter 2 trimesters (reviewed in ref. 2). One commonly accepted hypothesis puts forward that shallow or defective extravillous trophoblast invasion into the decidua during the first trimester, and thus insufficient transformation of deep maternal spiral arteries into low-resistant conduits, leads to suboptimal flow of maternal blood into the intervillous space, thus negatively altering the intervillous environment, which results in syncytial stress (in the syncytiotrophoblast) during the remaining 2 trimesters, which in turn triggers several mechanisms that are thought to cause PE symptoms and/or intrauterine growth retardation (IUGR).2-5
This hypothesis has been challenged recently by Huppertz et al. who recognizes that many PE-associated changes are found before placentation is completed, thus indicating that problematic early trophoblast differentiation processes are instead associated with these complications.6,7 According to this newer train of thought, faulty differentiation into extravillous trophoblast results in shallow invasion that is explanatory more for IUGR, while PE is associated more with problematic syncytiotrophoblast differentiation. When both PE and IUGR coincide, then the trauma might have occurred during an even earlier stage of trophoblast differentiation, during which both the syncytio-, as well as the extravillous trophoblast populations derived from the same precursor.6
In any case, several kinds of stress have been described to prevail in the villous compartment of the placenta during PE: oxidative stress,3 inflammation7 and endothelial dysfunction.8 Local stress during PE takes its toll on the syncytiotrophoblast, leading to a higher rate of cellular turnover that must be replenished by the layer of villous cytotrophoblast just below the syncytial layer.9-11 Villous cytotrophoblast cells are often considered a trophoblast stem cell, because this cell is able to differentiate into any other trophoblast population, but probably derive from a further progenitor for which the mesenchymal compartment of villi and chorion is the proposed niche, at least during the first trimester of pregnancy, but possibly in late gestation as well.12 Aberrant function of villous cytotrophoblast and chorionic mesenchymal stroma (or stem-like cells derived from human chorionic mesenchym, as termed by some groups) has been implicated in the development of PE characteristics.13-15
A commonly utilized marker of trophoblast stem/progenitor cells, CDX2, is also one that is thought to specify toward a trophoblast fate. These cells16 and villous mesenchymal stem/stroma cells have been described as expressing so-called pluripotency markers such as OCT4A, SOX2 and NANOG.13,17-19 More descriptive literature concerning the expression of all of these transcription factors (both trophoblast, as well as mesenchymal) is mainly restricted to the preimplantation phase and/or the murine model. Furthermore, to our knowledge, the expression profile of any of these markers has not been investigated in placenta-associated pregnancy pathologies such as PE and IUGR.
Taking into consideration that trophoblast progenitor cells, possibly derived from the mesenchymal compartment of the chorionic villi, would be called on to compensate the stressed syncytium or extravillous compartment in some way, we aimed here to characterize the expression of all above-mentioned progenitor cell markers during the course of a healthy pregnancy and compare that to said expression profiles during placenta-associated pregnancy pathologies, namely PE or IUGR or both.
Material and methods
Patient material
All samples of placental tissues were obtained from the Department of Obstetrics of the University Hospital Jena or from the Department of Obstetrics and Gynecology of the Ludwig Maximillian University in Munich after informed consent and after respective local Ethics Committee approval.
Women donated their placentae following prospective inclusion to the study subsequent to either receiving an elective abortion during the 1. trimester (5.-12. gestational weeks [GW], n = 8) in an otherwise healthy pregnancy, delivering at term after a healthy pregnancy (>36. GW; 39.2 GW, n = 8), after a pregnancy complicated by third trimester PE with IUGR (>30. GW; 30.9 GW, n = 8; average birth weight 1109 g) or IUGR alone (> 30. GW; 31.6 GW, n = 8; average birth weight 1410 g) or PE alone (>30. GW; 35.0 GW, n = 9; average birth weight 2138 g).
Diagnosis of preeclampsia (PE) was based on the guidelines as recommended by the German Society of Obstetrics and Gynecology (DGGG): hypertension, as measured by systolic blood pressure ≥140 mmHg and / or diastolic blood pressure ≥90 mmHg measured twice at an interval between 6 hours and 1 week, or singular measurement ≥150 / 100 mmHg; and proteinuria as measured by a urine dipstick test ≥1+ or ≥300 mg total protein in 24-hour urine collection. Intrauterine growth retardation (IUGR) was diagnosed when birth weight <10th percentile according to Voigt in a pregnancy in which gestational age and normal fetal morphology was confirmed in the first trimester and in which placental insufficiency was confirmed via elevated pulsatility index (PI) in the uterine arteries (>90th centile of reference population).
Exclusion criteria for all patients were the existence of potentially confounding factors such as multiple pregnancy, chromosomal and syndromal disorders, congenital infections, e.g. TORCH, amnion infection syndrome, fetal alcohol syndrome and/or maternal substance abuse (including nicotine), maternal diabetes mellitus or gestational diabetes, and inborn or acquired disorders leading to growth restriction in childhood.
All control term patients gave birth to infants that were considered appropriate-for-gestational age. All control patients of the first trimester group presented at 5th – 12th GW with vital pregnancies without any obvious fetal malformations as identified via transvaginal sonography and with the wish for an elective abortion due to sociopsycho- or economic reasons and not due to maternal health issues. Only third trimester pregnancies of both pregnancy pathology groups were included into the study.
Immunohistochemistry
In order to decipher the localization, expression pattern and expression intensity alterations of the target proteins during the course of normal pregnancy and in pregnancies complicated by either PE or IUGR, we employed immunohistochemistry as previously described.20
Briefly, all tissues were fixed in 4% buffered formalin for 20–24 hours, embedded in paraffin, then cut into 5µm sections and mounted on SuperFrost/Plus slides (Menzel, Germany). The samples were then deparaffinized and rehydrated. To inhibit endogenous peroxidise activity, placenta tissues were incubated in methanol/H2O2 for 30 min and washed for 5 min in phosphate-buffered saline (PBS, pH 7.4). Antigenetic retrieval was accomplished with citrate buffer. To reduce non-specific background staining, all samples were incubated with human serum (Typ AB, PAA, # C11-062) as recommended by Honig et al.21 and as confirmed for study relevant antibodies by Weber et al.22 as well as goat serum at room temperature for 20 min (Vector Laboratories). Tissues were incubated with primary antibodies (Table 1) for 1 hour at room temperature. Antibodies were diluted in DAKO Antibody Diluent with Background Reducing Components (DAKO, #S3022). In a next step they were incubated with the biotinylated secondary antibody (Vector Laboratories) again for 30 min at room temperature. For a listing of all antibodies, please refer to Table 1. Following incubation with the secondary antibody, an incubation period with ABC-Complex (avidin-biotinylated peroxidise; Vector Laboratories) for 30 min at room temperature was completed. Between each step, all samples were washed profusely with PBS. The peroxidase reaction was achieved with DAB (diaminobenzidine/H2O2; 1mg/ml; Vector Laboratories) and, after 5 minutes, discontinued with water. Haematoxylin staining was followed by mounting the cover slide with Histofluid.
Table 1.
Antibodies and kits.
| Antibody | Clone | Isotype | Concentration | Source |
|---|---|---|---|---|
| CDX2 | (Order#:3977) | Polyclonal Rabbit | 1: 200 | Cell Signaling |
| SOX2 | D6D9 (Order#:3579) | Monoclonal Rabbit | 1: 100 | Cell Signaling |
| NANOG | -(Order#:3580) | Polyclonal Rabbit | 1: 400 | Cell Signaling |
| OCT4A | C52G3 (Order#:2890) | Monoclonal Rabbit | 1: 300 | Cell Signaling |
| Isotype control | Clone DA1E (Order#:3900) | Monoclonal Rabbit | 1: 100 | Cell Signaling |
| ABC Elite-Kit (rabbit IgG) | PK-6101 | Vector Laboratories (Lörrach, Germany) |
A negative control was prepared by replacing the primary antibody with a DAKO control antibody with the same isotype compared to the primary antibody in diluent. Please refer to Table 1 for isotype control.
All samples were analyzed with a Zeiss microscope and statistical analyses were performed with the immunoreactive score (IRS) system.
The IR score was calculated by multiplication of optical staining intensity (grading of staining intensity: 0-none, 1- weak, 2-moderate, 3-strong) and percentage of positive cells stained (grading for percentage of stained cells: 0- none, 1- under 10% of cells, 2- up to 50%, 3- up to 80%, 4- up to 100%). Samples were studied by 3 blinded, independent scorers, who were not given any prior information of diagnosis or staining target. The IRS score is a fair indicator for the intensity of protein expression in a certain tissue.
Only nuclear staining of SOX2, OCT4A, NANOG and CDX2 was considered for the IRS score. One full-thickness walnut-sized sample per placenta was taken from an area at the midpoint between umbilical cord and placental rim. In all, 10 areas per sample were considered for IRS analysis.
Statistical analysis
The SPSS/PC software package, version 17 (SPSS, Munich, Germany) was used for collection, processing and statistical analysis of all data. Statistical analysis was performed using the nonparametrical Wilcoxon-Mann-Whitney test for comparison of the IRS score distribution. P < 0.05 values were considered statistically significant.
Results
CDX2, NANOG, SOX2 and OCT4A staining in the villous compartment is clearly elevated in the first trimester as compared to the healthy third trimester.
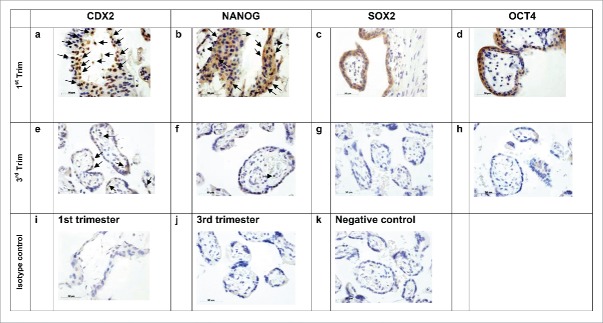
The villous compartment of first trimester placentas stained positively for all markers investigated in this study, while staining in the same compartment was significantly lower for the same markers in third trimester samples. Some nuclei of the syncytiotrophoblast layers of the first trimester placenta stained positive for CDX2 and NANOG, however these proteins showed an altogether more mosaic type pattern within the villous and in several areas it seemed that especially the cytotrophoblast layer expressed CDX2 and NANOG (please refer to Fig. 1, panels a and b, arrows pointing downward). It was surprising to find that according to villous morphology positive signals for OCT4A and SOX2 were visible especially in the nuclei of the syncytiotrophoblast layer (versus the cytotrophoblast) in first trimester placentas (Fig. 1, panels c and d).
Figure 1.
IHC 1st vs. 3rd Trimester, villous sections. Diagonal arrows (↘) indicate marker staining within the cytotrophoblast layer of the villous. Horizontal arrows (←, →) indicate marker staining in cells within the mesenchymal core of the villous. All investigated markers are expressed within the villous of 1st trimester placentas (a-d). Especially CDX2 (a) and NANOG (b) appear to be expressed in the cytotrophoblast and villous mesenchymal core cell populations, while OCT4A (c) and SOX2 (d) appear to be expressed mainly in the cytoplasm of the syncytiotrophobast. Expression of all markers is markedly decreased (CDX2 and NANOG in e and f respectively) or lost (OCT4A and SOX2 in g and h respectively) in the villous of healthy placentas. Scale bars indicate 50 µm.
Nuclei within the mesenchymal core generally stained positive for CDX2 and NANOG in first trimester placentae, while the other pluripotency markers, OCT4A and SOX2, were usually negative (Fig. 1, panels a-d, horizontal arrows). For the sake of simplicity, we did not segregate IRS scores of villous trophoblast and mesenchymal core, especially since all signals were either significantly reduced (CDX2, NANOG; statistics in Fig. 4, panels A and B) or completely eliminated (SOX2, OCT4A, statistics in Fig. 3, panels C and D) in the third trimester villous compartment, both among the villous trophoblast, as well as within the mesenchymal core (Fig. 1, panels e-h).
Figure 4.
Statistical evaluation of IRS results. Statistical evaluation of IRS results recapitulates the observations described in Figures 1–3. Expression of CDX2, NANOG, SOX2 and OCT4A is visible in the villous section of first trimester placentas (first lanes of A-D). Expression of CDX2 and NANOG are significantly diminished in the villous section of healthy third trimester placentas (second lanes of A and B, p* respectively), while OCT4A and SOX2 signals are lost (second lanes of C and D, p* respectively). No significant changes as compared to healthy third trimester controls are visible in the villous of placentas derived from intra-uterine growth retardation complicated pregnancies (fourth lanes of A-D), while CDX2 and NANOG expression are lost in preeclampsia-altered villous placentas (third lanes in A and B, p** respectively), and thus significantly altered as compared to healthy controls.Only SOX2 expression is altered in any of the investigated extravillous placental sections. In this case, SOX2 expression is significantly elevated in the extravillous of intrauterine growth retardation-derived placentas as compared to healthy third trimester controls (G, p*).
Figure 3.
IHC of healthy vs. PE- and IUGR-derived placental extravillous sections. No positive staining for any of the investigated markers is apparent in the extravillous of healthy placentas (i-l) or preeclampsia-altered placentas (a-d). Horizontal arrows (→) indicate marker staining (SOX2) in cells within the extravillous section of placentas altered by intrauterine growth retardation (g). Further identification of SOX2 positive cells was not possible; SOX2 expression was erratic and often not visible again in the serial section (data not shown). Scale bars indicate 50 µm.
Specific and differential expression patterns of CDX2, NANOG, SOX2 and OCT4A is visible in the villous compartment of all placenta-associated pregnancy complications vs. healthy controls.
Following the hypothesis that PE pregnancy is associated with oxidative stress and inflammation that leads to overly high syncytiotrophoblast turnover, we suspected that any form of syncytiotrophoblast progenitor (such as the cytotrophoblast or trophoblast progenitor cells of the villous compartment) must be called on to replenish the synctiotrophoblast layer. Indeed, in comparison to healthy controls, we were able to confirm that CDX2, SOX2 and OCT4 (but not NANOG) expression was significantly elevated in PE cases (Fig. 2, panels i-l vs. Fig. 1, panels e-h).
Figure 2.
IHC of PE+IUGR versus PE vs. IUGR in 3rd trimester placental villous sections. Diagonal arrows (↘) indicate marker staining within the cytotrophoblast layer of the villous. Horizontal arrows (←, →) indicate marker staining in cells within the mesenchymal core of the villous. None of the investigated markers are visible in any part of the combined preeclampsia + intrauterine growth retardation (PE+IUGR) -altered placental villous (a-d). Staining of CDX2 (e) and NANOG (f) are weak in the villous (both cytotrophoblast and mesenchymal core cells) of placentas derived from pregnancies complicated by intrauterine growth retardation, while OCT4A (g) and SOX2 (h) are not detecable. This staining pattern is similar to that seen in the villous placenta of healthy third trimester pregnancies. In preeclampsia villous, all investigated markers are detectable in the mesenchymal core and often in the villous cytotrophoblast (i-l). This staining pattern is more similar to that seen in the villous placenta of healthy first trimester pregnancies. Scale bars indicate 50 µm.
Interestingly, the signals for CDX2 and NANOG, which were diminished within the healthy third trimester villous (Fig. 1, panels e and f) had completely disappeared within the villous of PE+IUGR placentae (Fig. 2, panels a and b). In contrast, pure IUGR cases did not differ significantly in their marker expression pattern from healthy controls controls (Fig. 2E and Fig. 2F vs. Fig. 1E Fig. 1F).
The panels A-D in Figure 4 reiterates these descriptive assertions as the statistical evaluation of blinded IRS points. The p-value < 0.05 reveals significance for comparisons of both CDX2 and NANOG signals in healthy 3rd trimester villous vs. PE+IUGR villous, during which the signal is lost. The same panels in Figure 4 also demonstrate that both CDX2 and NANOG signals tend to be less visible in IUGR villous as compared to healthy third trimester villous, but this does not reach statistical significance. It is of note that all investigated factors are elevated in the villous compartment of pure PE placentae in comparison to PE+IUGR placentae. When comparing pure PE with pure IUGR, it becomes apparent that CDX2 and SOX2 expression is diminished in IUGR placentae.
The expression of SOX2 is elevated in the basal plate of placentae complicated by IUGR versus control.
Nuclei of cells within the basal plate generally remained signal negative for all investigated nuclear transcription factors regardless of cell population or pregnancy pathology (Fig. 3, panels a-l). SOX2 nucleic staining appeared distinctly positive in isolated cells within the extravillous, basal plate section of pure IUGR cases and statistical evaluation of IRS results revealed a significantly higher SOX2 score in pure IUGR placentae as compared to PE+IUGR placentae (p = 0.036 in Fig. 4, panel g).
We had aimed to identify whether SOX2 staining was within the extravillous trophoblast by employing CK7 as a trophoblast marker in immunofluorescence experiments.
However, due to the fact that the SOX2 signals within the extravillous compartment were elevated only in isolated cells of IUGR complicated placentae, it was difficult to pinpoint these specific cells for IF staining in serial sections (data not shown) so that currently, we are unable to verify whether the few cells that stained positive for SOX2 in PE extravillous cells were actually trophoblast.
It is also of note that we were unable to process the basal plate of pure PE cases due to technical difficulties that had resulted in the loss of tissue.
Discussion
Our data demonstrates the expression of CDX2 and NANOG especially in the cytotrophoblast, and OCT4A and SOX2 in the syncytiotrophoblast of the villous compartment of first trimester placentas, while these markers are reduced (CDX2 and NANOG) or nearly deleted (SOX2 and OCT4) at the end of gestation. In the same compartment of placentas complicated by PE and IUGR, the CDX2 and NANOG signal is completely lost and thus significantly altered in comparison to healthy third trimester placentae. In pure PE cases, especially SOX2 and OCT4 staining within the mesenchymal core of chorionic villi is elevated. The SOX2 signal was significantly elevated in the basal plate of pure IUGR-complicated placentae vs. their healthy counterparts.
Ergo, In summary, we demonstrate that there is a unique staining pattern of trophoblast progenitor cell – and pluripotency markers at different timepoints of the healthy placenta and within each observed pathology associated with the placenta (PE, IUGR and PE+IUGR).
This suggests that the investigated markers hold a trimester-dependent function, the deregulation of which would lead to separate, although clinically similar, pathologies.
Before we discuss the above-mentioned denotations, we first extend our attention to several points pertaining the reliability of our results.
Although other studies have described the expression of these markers in the first trimester placenta and during the course of pregnancy, our study is the first in which this was accomplished comprehensively and with visual verification of protein localization. Two groups demonstrated CDX2 expression in the first trimester cytotrophoblast, which was lost in late gestation.12,16 Some demonstrated similar findings for cytotrophoblastic NANOG expression.17,23 Others found cells derived from the chorion (with various terminology: mesenchymal stem cells, mesenchymal stromal cells, chorionic stem cells) that expressed some or all of the pluripotency-associated markers, OCT4A, SOX2 and NANOG.13,18,19 One group demontrated that first trimester placentae expressed higher levels of pluripotency-associated markers than those of third trimester placentae19. The cumulative results of these studies reassure us that our data concerning healthy pregnancies is reliable, suggesting also that our staining procedure is sound.
The observation of pluripotency markers (OCT4A and SOX2) in the cytoplasm of a terminally differentiated cell (syncytiotrophoblast) was unexpected. This was surprising for 2 reasons: (1) because pluripotency markers usually disappear after differentiation and (2) because these transcription factors are thought to be active exclusively in the nucleus. The possibility of false positive staining must be taken seriously, however, we have taken all recommended precautions to minimize this known phenomenon, e.g., by using a monoclonal antibody specific to the N-terminus of OCT-4A,24 2 separate negative controls (omission of secondary antibody, isotype control), and further experiment specific controls (internal controls, use of both conventional, as well as human serum blocking procedures22), making it unlikely that our signals are false positive. Further analyses, e.g. RT-PCR and Western blot might verify our results, albeit these experiments would then disregard cell population. Our samples were stored in formalin rendering them improper for these procedures, but the literature interestingly demonstrates cytoplasmic staining of some of these markers in other cell populations25-28 and in non-human trophoblastic cells.29,30 In our study, cytoplasmic staining of transcription factors was seen only in the terminally differentiated STB, suggesting that functional differences of these markers correlate to their position within the cell. We suspect that OCT4 and SOX2 expression within STB cytoplasm does not reflect pluripotent capacity, but rather is a remnant of the CTB daughter cell life cycle after fusion into the STB, meaning that OCT4/SOX2 expression has become redundant in the STB.
Our final point concerning reliability is the questionablity of comparing placental material with gestational age disparity. Albeit still stemming from the same trimester, the gestational age descripancy in the case groups versus the control group is up to 9 weeks. The manifestation of severe PE + IUGR is rare in late gestation as they are usually terminated in the second trimester (before the 30th GW) before becoming life-threatening to mother and child. Finding an appropriate control group for 2nd trimester placentae is difficult as extreme prematurity is potentially confounding in itself. Our compromise included all cases in the third trimester thus lowering the gestational age discrepancy, while maintaining clear diagnostic criteria. However, since the case placentas derive from similar GWs, but display staining distinction between subgroups, it seems unlikely that the observed differences are due to gestational age alone.
Now that we have adressed certain methodoligical arguments, we discuss the observed results.
As this is an observational study, we can only speculate as to why the first trimester trophoblast (including chorionic mesenchymal cells), seems to express pluripotency factors, or why this expression is lost at the end of gestation, however it is likely due to STB expansion. According to newer models of STB renewal, the STB layer is replenished by the underlying CTB layer, which in turn is probably replenished by pluripotency marker-expressing cells within the chorionic mesenchymal core, which can also replenish all other trophoblast populations, such as the invasive EVT, but also the STBs.12 For the sake of simplicity, we will term these cells “trophoblast progenitor cells” from here on. Syncytium expansion is at its highest during the first trimester of pregnancy, meaning that villous CTBs and trophoblast progenitor cells must constantly supply the syncytium with further STBs in order to accommodate the development of this layer. This is accomplished by fusion of an underlying villous CTB (or trophoblast progenitor cell) daughter cell with the syncytium.11 According to our observations, it is tempting to hypothesize that CDX2 and NANOG work in concert during villous CTB or trophoblast progenitor cell production of daughter cells. In the third trimester, syncytium expansion is arrested so villous CTBs need only to maintain the status quo, which explains the decrease or loss of trophoblast progenitor- and pluripotency marker signals in the villous compartment of third trimester placentae.
The observed elevation of the investigated markers in the villous of PE complicated pregnancies was expected, because, as reviewed in 11, the STB within a pure PE placenta is stressed therefore cellular turnover is elevated, resulting in higher villous CTB proliferation and STB differentiation. This train of thought is mirrored in our study, since most investigated markers were elevated during PE in comparison both to healthy counterparts and pure IUGR placentas. The observed loss of signals in villous samples of combined PE + IUGR was unique (not resembling pure IUGR nor pure PE) and unexpected.
This is especially counterintuitive if the common denominator of all 3 pathologies is thought to be defective extravillous trophoblast invasion, but not if all 3 situations are understood as separate entities with separate formation mechanisims. Recent literature indicates that although PE is often associated with IUGR and both pregnancy pathologies originate from the placenta, they are indeed 2 separate entities with separate origins. Although STBs and EVTs have a common up-stream precursor (trophoblast progenitor cells), they also probably differentiate from a separate set of intermediate precursor cells even late in pregnancy31, so, as Huppertz et al has recently put forward, PE characteristics might stem more from defective STB maturation processes, while insufficiency of the placenta to produce invasive extravillous trophoblast seems rather to lead to IUGR. Combined PE+IUGR possibly occurs when the common upstream precursor, the trophoblast progenitor cell, is defective. Recent publications suggest that functions such as cytokine or exosomal secretion by chorionic mesenchymal core cells, possibly trophoblast progenitor cells, is negatively altered in PE placentas and thus contributes to main PE characteristics, indicating that these cells react in a destructive manner to their environment.13-15 According to our data, it appears that the trophoblast progenitor cells overexpress several (trophoblast) progenitor cell markers during pure PE, while these are underexpressed during PE + IUGR. Ergo, our results are better explained by the PE/IUGR-hypotheses provided by Huppertz et al.
Functionally speaking, we suspect that lack of (trophoblast) progenitor cell marker expression the trophoblast progenitor cells of PE+IUGR indicates its inablity to react to the dire intervillous environment, which exists in all PE cases, a situation that might contribute to the further exacerbation of PE by triggering IUGR. Further studies are necessary to prove whether this train of thought has merit. We wish to stress here that older, in-house results demonstrate significantly lower expression of another molecule associated with pluripotency, STAT3 (Signal Transducer and Activator of Transcription 3), in PE+IUGR placentae.32
In this context, it is noteworthy that significant differences in SOX2 staining was visible in the (extravillous) basal plate compartment of pure IUGR complicated pregnancies. This observation is also congruent with the above theory by Huppertz et al, as this implies that some sort of progenitor cell action is taking place in an extravillous portion of IUGR complicated pregnancies.
Taken together, the results of our study indicate that PE, IUGR and combined PE+IUGR are 3 separate entities in which trophoblast differentiation processes are uniquely abberant, however we are unable to maintain whether this observation is the cause or the symptom of the pathology.
Conclusions
We conclude that trophobalst stem cell and pluripotency markers are expressed in specific cell populations of the early placenta and down-regulated later on in pregnancy. Out of the observation that placentas altered by PE+IUGR are not able to express some of these markers (CDX2, NANOG), while pure PE, pure IUGR and control placentas are, we speculate that these 3 entities have differing pathogeneses. Due to the fact that these marker expression differences are seen mainly in the villous cytotrophoblast and villous mesenchymal core cells, which are now considered trophoblast progenitor cells, we speculate that these cell populations are functionally perturbed in PE + IUGR placentas.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
We wish to acknowledge the IZKF Jena (Interdisciplinary Center for Clinical Research in Jena; project number J12), ProChance Jena and the DAAD (German Academic Exchange Service; project number Po-50735372).
References
- [1].Berzan E, Doyle R, Brown CM. Treatment of preeclampsia: current approach and future perspectives. Curr Hypertension Rep 2014; 16:473; PMID:25135649; http://dx.doi.org/ 10.1007/s11906-014-0473-5 [DOI] [PubMed] [Google Scholar]
- [2].Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005; 308:1592-4; PMID:15947178; http://dx.doi.org/ 10.1126/science.1111726 [DOI] [PubMed] [Google Scholar]
- [3].Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009; 30:473-82; PMID:19375795; http://dx.doi.org/ 10.1016/j.placenta.2009.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lyall F. Priming and remodelling of human placental bed spiral arteries during pregnancy–a review. Placenta 2005; 26 Suppl A:S31-6; PMID:15837064; http://dx.doi.org/ 10.1016/j.placenta.2005.02.010 [DOI] [PubMed] [Google Scholar]
- [5].Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension 2010; 56:1026-34; PMID:20956732; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.110.157743 [DOI] [PubMed] [Google Scholar]
- [6].Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension 2008; 51:970-5; PMID:18259009; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.107.107607 [DOI] [PubMed] [Google Scholar]
- [7].Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol 2010; 63:534-43; PMID:20331588; http://dx.doi.org/ 10.1111/j.1600-0897.2010.00831.x [DOI] [PubMed] [Google Scholar]
- [8].Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Ann Rev Pathol 2010; 5:173-92; PMID:20078220; http://dx.doi.org/ 10.1146/annurev-pathol-121808-102149 [DOI] [PubMed] [Google Scholar]
- [9].Redman CW, Tannetta DS, Dragovic RA, Gardiner C, Southcombe JH, Collett GP, Sargent IL. Review: does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 2012; 33 Suppl:S48-54; PMID:22217911; http://dx.doi.org/ 10.1016/j.placenta.2011.12.006 [DOI] [PubMed] [Google Scholar]
- [10].Carolina S, Pamela JR, Colleen CN. Exosomes in prostate cancer: putting together the pieces of a puzzle. Cancers 2013; 5:1522-44; PMID:24351670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mayhew TM. A stereological perspective on placental morphology in normal and complicated pregnancies. J Anatomy 2009; 215:77-90; PMID:19141109; http://dx.doi.org/ 10.1111/j.1469-7580.2008.00994.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Genbacev O, Lamb JD, Prakobphol A, Donne M, McMaster MT, Fisher SJ. Human trophoblast progenitors: where do they reside? Semin Reproduct Med 2013; 31:56-61; PMID:23329637; http://dx.doi.org/ 10.1055/s-0032-1331798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rolfo A, Giuffrida D, Nuzzo AM, Pierobon D, Cardaropoli S, Piccoli E, Giovarelli M, Todros T. Pro-inflammatory profile of preeclamptic placental mesenchymal stromal cells: new insights into the etiopathogenesis of preeclampsia. PloS One 2013; 8:e59403; PMID:23527185; http://dx.doi.org/ 10.1371/journal.pone.0059403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, Rice GE. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PloS One 2013; 8:e68451; PMID:23861904; http://dx.doi.org/ 10.1371/journal.pone.0068451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Newhouse SM, Davidge ST, Winkler-Lowen B, Demianczuk N, Guilbert LJ. In vitro differentiation of villous trophoblasts from pregnancies complicated by intrauterine growth restriction with and without pre-eclampsia. Placenta 2007; 28:999-1003; PMID:17559930; http://dx.doi.org/ 10.1016/j.placenta.2007.04.008 [DOI] [PubMed] [Google Scholar]
- [16].Hemberger M, Udayashankar R, Tesar P, Moore H, Burton GJ. ELF5-enforced transcriptional networks define an epigenetically regulated trophoblast stem cell compartment in the human placenta. Hum Mol Gen 2010; 19:2456-67; PMID:20354077; http://dx.doi.org/ 10.1093/hmg/ddq128 [DOI] [PubMed] [Google Scholar]
- [17].Genbacev O, Donne M, Kapidzic M, Gormley M, Lamb J, Gilmore J, Larocque N, Goldfien G, Zdravkovic T, McMaster MT, Fisher SJ. Establishment of human trophoblast progenitor cell lines from the chorion. Stem cells (Dayton, Ohio) 2011; 29:1427-36; PMID:21755573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Arakawa R, Aoki R, Arakawa M, Saito K. Human first-trimester chorionic villi have a myogenic potential. Cell Tissue Res 2012; 348:189-97; PMID:22370594; http://dx.doi.org/ 10.1007/s00441-012-1340-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jones GN, Moschidou D, Puga-Iglesias TI, Kuleszewicz K, Vanleene M, Shefelbine SJ, Bou-Gharios G, Fisk NM, David AL, De Coppi P, Guillot PV. Ontological differences in first compared to third trimester human fetal placental chorionic stem cells. PloS One 2012; 7:e43395; PMID:22962584; http://dx.doi.org/ 10.1371/journal.pone.0043395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jeschke U, Mayr D, Schiessl B, Mylonas I, Schulze S, Kuhn C, Friese K, Walzel H. Expression of galectin-1, -3 (gal-1, gal-3) and the Thomsen-Friedenreich (TF) antigen in normal, IUGR, preeclamptic and HELLP placentas. Placenta 2007; 28:1165-73; PMID:17664004; http://dx.doi.org/ 10.1016/j.placenta.2007.06.006 [DOI] [PubMed] [Google Scholar]
- [21].Honig A, Rieger L, Kapp M, Dietl J, Kammerer U. Immunohistochemistry in human placental tissue–pitfalls of antigen detection. J Histochem Cytochem: Off J Histochem Soc 2005; 53:1413-20; PMID:16009964; http://dx.doi.org/ 10.1369/jhc.5A6664.2005 [DOI] [PubMed] [Google Scholar]
- [22].Weber M, Knoefler I, Schleussner E, Markert UR, Fitzgerald JS. HTR8/SVneo cells display trophoblast progenitor cell-like characteristics indicative of self-renewal, repopulation activity, and expression of “stemness-” associated transcription factors. BioMed Res Int 2013; 2013:243649; PMID:23586024; http://dx.doi.org/ 10.1155/2013/243649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Siu MK, Wong ES, Chan HY, Ngan HY, Chan KY, Cheung AN. Overexpression of NANOG in gestational trophoblastic diseases: effect on apoptosis, cell invasion, and clinical outcome. Am J Pathol 2008; 173:1165-72; PMID:18772339; http://dx.doi.org/ 10.2353/ajpath.2008.080288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liedtke S, Stephan M, Kogler G. Oct4 expression revisited: potential pitfalls for data misinterpretation in stem cell research. Biol Chem 2008; 389:845-50; PMID:18627312; http://dx.doi.org/ 10.1515/BC.2008.098 [DOI] [PubMed] [Google Scholar]
- [25].Gu TT, Liu SY, Zheng PS. Cytoplasmic NANOG-positive stromal cells promote human cervical cancer progression. Am J Pathol 2012; 181:652-61; PMID:22683467; http://dx.doi.org/ 10.1016/j.ajpath.2012.04.008 [DOI] [PubMed] [Google Scholar]
- [26].Luo W, Li S, Peng B, Ye Y, Deng X, Yao K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PloS One 2013; 8:e56324; PMID:23424657; http://dx.doi.org/ 10.1371/journal.pone.0056324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kao CS, Warren S, Idrees MT. Merkel cell carcinoma exhibiting cytoplasmic OCT4 staining: a potential new diagnostic immunohistochemical marker. Am J Dermatopathol 2013; PMID:23291584 [DOI] [PubMed] [Google Scholar]
- [28.Alexander RE, Cheng L, Grignon DJ, Idrees M. Cytoplasmic staining of OCT4 is a highly sensitive marker of adrenal medullary-derived tissue. Am J Surg Pathol 2013; 37:727-33; PMID:23588367; http://dx.doi.org/ 10.1097/PAS.0b013e3182793dc2 [DOI] [PubMed] [Google Scholar]
- [29].Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 2003; 17:126-40; PMID:12514105; http://dx.doi.org/ 10.1101/gad.224503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Madeja ZE, Sosnowski J, Hryniewicz K, Warzych E, Pawlak P, Rozwadowska N, Plusa B, Lechniak D. Changes in sub-cellular localisation of trophoblast and inner cell mass specific transcription factors during bovine preimplantation development. BMC Dev Biol 2013; 13:32; PMID:23941255; http://dx.doi.org/ 10.1186/1471-213X-13-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].James JL, Stone PR, Chamley LW. The isolation and characterization of a population of extravillous trophoblast progenitors from first trimester human placenta. Hum Reproduct 2007; 22:2111-9; PMID:17580299; http://dx.doi.org/ 10.1093/humrep/dem144 [DOI] [PubMed] [Google Scholar]
- [32].Weber M, Kuhn C, Schulz S, Schiessl B, Schleussner E, Jeschke U, Markert UR, Fitzgerald JS. Expression of signal transducer and activator of transcription 3 (STAT3) and its activated forms is negatively altered in trophoblast and decidual stroma cells derived from preeclampsia placentae. Histopathology 2012; 60:657-62; PMID:22211353; http://dx.doi.org/ 10.1111/j.1365-2559.2011.04063.x [DOI] [PubMed] [Google Scholar]