ABSTRACT
Maternal gestational diabetes (GDM) is associated with hyperglycaemia and hyperinsulinemia in the fetal circulation which consequently may induce endothelial dysfunction in the feto-placental vasculature. In fact, feto-placental vasculature reveals various morphological changes in response to GDM. The cell adhesion molecules (CAMs) ICAM-1, VCAM-1 and E-selectin promote attachment and trans-endothelial migration of leukocytes, and are up regulated in inflammation and endothelial dysfunction. Thus, we hypothesized that the GDM environment upregulates ICAM-1, VCAM-1 and E-selectin in the feto-placental endothelium. We isolated primary feto-placental endothelial cells (fpEC) after normal (n=18) and GDM pregnancy (n=11) and analyzed mRNA (RT-qPCR) and protein expression (Immunoblot) of ICAM-1, VCAM-1 and E-selectin. While other CAMs were unchanged on mRNA and protein levels, ICAM-1 protein was decreased by GDM. Further analysis revealed also a decrease in the release of soluble ICAM-1 (sICAM-1), whose levels correlated negatively with maternal BMI. We conclude that this reduction of ICAM-1 protein species is the result of post-translational regulation, since ICAM-1 mRNA expression was unchanged. In fact, miRNAs targeting ICAM-1 were upregulated in GDM fpEC. Immunohistochemistry showed weaker ICAM-1 staining in the placental endothelium after GDM pregnancies, and demonstrated ICAM-1 binding partners CD11a and CD18 expressed on leukocytes in fetal circulation and on placental tissue macrophages. This study identified reduction of ICAM-1 protein in fpEC in GDM pregnancy, which was regulated post-transcriptionally. Low ICAM-1 protein production may represent a protective, placenta-specific mechanism to avoid leukocyte transmigration into the placenta in response to GDM.
KEYWORDS: endothelial dysfunction, E-selectin, feto-placental endothelium, GDM, ICAM-1, VCAM-1
Introduction
The endothelium, a layer of endothelial cells lining the blood vessels, is a major active site of blood vessel function. Endothelial functions include the formation of the endothelial barrier, leukocyte trafficking, regulation of vascular tone, blood coagulation, nutrient uptake and neovascularization.1,2 Endothelial dysfunction is the inability of endothelial cells to perform their physiological functions which is paralleled by a change of endothelial phenotype.3 Endothelial dysfunction contributes to vascular disease4,5 and is a long term complication of diabetes and metabolic disorders resulting from hyperglycemia, oxidative stress and a pro-inflammatory environment.6–8
Gestational diabetes mellitus (GDM) is a maternal glucose intolerance that is clinically diagnosed in the second trimester of gestation. GDM induces metabolic derangements in the maternal and in the fetal circulation.8 Thus, endothelial cells of the fetal circulation are exposed to hyperglycaemia, hyperinsulinemia, hyperleptinemia and often also to hypoxia9 which may induce dysfunction of the fetal endothelium.
The endothelium of the fetus cannot be studied in humans, but limited reports investigating the feto-placental endothelium in GDM indeed identified histological changes such as placental hypervascularisation10 and altered expression of junctional proteins11 and MMP14.12 These findings indicate an altered feto-placental endothelial function, but a clear demonstration has been pending.
Classical markers for the phenotypic changes of the functional endothelium are over-expression of endothelial cell adhesion molecules ICAM-1 (intercellular cell adhesion molecule-1), VCAM-1 (vascular cell adhesion molecule-1) and E-selectin.13,14 ICAM-1, VCAM-1 and E-selectin expression on endothelial cells greatly increases upon cytokine stimulation and their main function is to bind ligands present on leukocytes to promote leukocyte attachment and trans-endothelial migration.15 These CAMs can be cleaved and shed from the cell surface, releasing soluble forms sICAM-1, sVCAM-1 and sE-selectin. The biological significance of circulating CAMs may be manifold including the competitive inhibition of their binding receptors located on leukocytes, the reduction of endothelial binding sites as well as signaling functions.16
The soluble forms of ICAM-1, VCAM-1 and E-selectin are increasingly released from endothelial cells under inflammatory conditions and endothelial cell stimulation. Circulating CAMs serve as biomarkers for endothelial dysfunction since the cellular expression of CAMs is difficult to assess clinically.14 However, the placenta as a highly vascularized fetal organ can be obtained after pregnancy to investigate endothelial cell adhesion molecules directly in the feto-placental endothelium and in isolated primary feto-placental endothelial cells (fpEC).
We hypothesized that the diabetic environment of GDM alters the expression and release of CAMs in fpEC. To this end, we compared ICAM-1, VCAM-1 and E-selectin gene and protein expression in isolated primary fpEC after normal and GDM pregnancy. Results were confirmed in situ by immunohistochemistry.
Materials and Methods
Ethics statement
The present study was approved by the Ethical Committee of the Medical University of Graz. Informed consent of all patients was obtained. Patients' characteristics (Table 1) were collected on an anonymous basis after coding the patients' names.
Table 1.
Subject characteristics.
| BMI before pregnancy (kg/m2) | BMI before birth (kg/m2) | Gestational age (weeks) | Placental weight (g) | Fetal sex ratio | Fetal ponderal index (kg/m3) | |
|---|---|---|---|---|---|---|
| Control (n=18 ) | 24.7±6 .1 | 30.9±7 .3 | 39±4 | 638±226 | 0.52 | 26.7±2 .7 |
| GDM (n=11 ) | 27.9±7 .8 | 33.1±7 .3 | 38±4 ** | 700±194 | 0.55 | 25.9±2 .6 |
p<0 .01; Fetal sex ratio=number of male/number of female fetuses
GDM screening
In our institution, a 75g oral glucose tolerance test (oGTT) is routinely performed between gestational weeks 24 and 28. Blood glucose is measured in venous plasma. Women are diagnosed for GDM if one measurement is above the defined maximum levels of normal glucose tolerance (fasting: <5.1mM; 1h glucose level <10.0mM; 2h glucose level <8.5mM). In case of one pathological value women receive nutritional counseling and are instructed in blood glucose self-monitoring. If blood glucose levels cannot be maintained in the normal range (fasting <95mg/dl and 1h after meals <140mg/dl) insulin therapy is initiated.17
Isolation and culture of human feto-placental arterial endothelial cells
Primary human fpEC were isolated from third-trimester human placentas after healthy pregnancies (control fpEC) and after pregnancies complicated by GDM (GDM fpEC) as described.18 Briefly, arterial chorionic blood vessels were resected, washed with HBSS (Gibco) and cells were isolated by perfusion of the arteries with prewarmed HBSS containing 0.1U/ml collagenase, 0.8U/ml dispase (Roche), and antibiotics (Gibco) for 7min. The perfusate was centrifuged (5min at 200 × g), resuspended in endothelial basal medium (EBM; Clonetics; Lonza) supplemented with the EGM-MV BulletKit (Clonetics; Lonza) containing gentamicin/amphotericin, hydrocortisone, human epidermal growth factor (EGF), bovine brain extract, and 5% fetal calf serum (FCS), and plated on culture plates precoated with 1% gelatin. This medium was also used for cell expansion. All cell preparations were subjected to immunocytochemical characterization for identity, purity, and functionality.18 For analysis of TNFa effect in ICAM-1 protein levels, fpEC (50,000/well) were seeded in a 24 well plate and cultured for 48h. Then, TNFa (Reliatech, Wolfenbüttel, Germany) was added to a final concentration of 0; 0.5; 5 and 50µg/ml for 24h after which protein was isolated. For all other experiments fpEC were seeded at a density of 11,000 cells per cm2 and cultured for 48h at 37°C and 21% oxygen. Cells were used up to passage 7.
Quantitative reverse transcription PCR (RT-qPCR)
Total RNA was isolated using the RNeasy mini Kit (Qiagen, Hilden, Germany). The quality and integrity of the RNA was determined by the ratio of spectrophotometric absorbance 260nm/280nm measured with the Scandrop 250 (Analytick Jena AG, Germany). The cDNA was synthesized from 50 ng total RNA according to the manufacturer's instructions (SuperScript II Reverse Transcriptase protocol from Invitrogen, USA). 3ng/µl of cDNA were used on a total reaction volume of 10µl in the ABI Prism 5,700 Sequence Detection System. RT-qPCR for ICAM-1, VCAM-1 and E-selectin (SELE) was performed using TaqMan assays: Hs00164932_m1, Hs01003372_m1 and Hs00174057_m1, respectively (Applied Biosystems, CA, USA). Mean expression of the housekeeping gene HPRT1 hypoxanthine-guanine phosphoribosyltransferase (HPRT1; Hs02800695_m1; Applied Biosystems, CA, USA) was not influenced by GDM and hence, was used to normalize gene expression with 2−ΔΔct method.19
Flow cytometry
Isolated fpEC were grown in 48 well plates for 48h (Greiner Bio-One GmbH, Germany) and detached with detachment buffer (25mM HEPES, 10mM EDTA in PBS) for 10min, collected and stained with antibodies against ICAM-1 (PE-Cy5 mouse anti-human CD54; BD Pharmingen, Vienna, Austria), VCAM-1 (FITC mouse anti-human CD106; eBioscience Inc, San Diego, CA) and E-selectin (PE mouse anti-human CD62E; BD Pharmigen, Vienna, Austria). Antibody dilutions (1:40) were prepared in Antibody diluent (DAKO, Glostrup, Denmark). Incubation was performed at 4°C in dark for 30min. Cells were washed with PBS, fixed and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany).
Immunoblot
Total protein was extracted with RIPA buffer containing proteinase inhibitors (Complete Protease Inhibitor Cocktail Tablets, Roche). Obtained cell lysates (10µg of total intracellular protein per lane) were applied to a gradient 4–20% SDS-PAGE, and electrophoretically transferred to 0.2µm nitrocellulose membranes (Trans-Blot Turbo Mini Nitrocellulose Transfer Membrane, BioRad) using the Trans-Blot Turbo Transfer System (BioRad). For detection of cell adhesion molecules following antibodies were used: anti-ICAM-1 rabbit monoclonal (ab109361, dilution 1:2000, Abcam), anti-VCAM-1 rabbit polyclonal (#12367S, dilution 1:1000, Cell Signaling) and anti-E-selectin mouse monoclonal (CBL180, dilution 1:1000, Millipore). Secondary antibody was HRP-conjugated anti-mouse or -rabbit antibody, respectively (HAF008; R&D Systems, UK). Signals were detected using the SuperSignal West Pico (34080, Pierce, Thermo Scientific). Thus, normalization was performed to total protein level after staining the membranes with Ponceau S. Signal intensity was calculated by DigiDoc 1000 software.
Immunohistochemistry
Placental tissue samples from pregnancies complicated by GDM (n=4) and healthy controls (n=4) were fixed and paraffin embedded using the HOPE (Hepes Glutamic Acid Buffer Mediated Organic Solvent Protection Effect) fixation technique (Innovative Diagnostik Systeme, Hamburg, Germany), as previously described.20 Placental tissue sections (5μm) were mounted on Superfrost Plus slides (Menzel/Thermo Fisher Scientific), deparaffinized in xylene for 10 min. and rehydrated in a graded series of ethanol.20 Sections were immunostained using the UltraVision Large Volume Detection System HRP Polymer Kit (Thermo Fisher Scientific) according to the manufacturer's protocol. Briefly, endogenous peroxidase was blocked using the hydrogen peroxidase block for 10min. Three washing steps with Tris-buffered saline (TBS) were followed by background blocking using Ultra Vision Protein Block for 5min. Rabbit monoclonal anti-ICAM1 antibody (ab109361, Abcam) was diluted 1:250 in Antibody Diluent (Dako) and incubated on slides for 45min at RT. Slides were washed and detection achieved by incubation with the anti-mouse/rabbit UltraVision HRP-labeled polymer system (15min) and 3-amino-9-ethylcarbacole (AEC, Thermo Scientific), according to the manufacturer's instructions. Nuclei were stained with hemalaun and slides were mounted with aqueous mounting agent Aquatex (Merk Millipore). For negative controls, slides were incubated with Polyclonal rabbit IgG (Negative Control for Rabbit IgG Ab-1, Thermo Scientific) at a concentration of 2µg/ml, which did not show any staining.
CD11 and CD18 staining was performed on standard formalin fixed paraffin embedded (FFPE) term placenta sections (5µm). Standard deparaffinization procedure was followed by boiling slides in Epitope Retrieval Solution pH9.0 (Novocostra, Leica) for 7min at 120°C in a decloaking chamber (Biocare Medical). Sections were stained as described above using monoclonal anti-CD11 antibody (clone EP1345Y, Millipore, 1:50) and polyclonal anti-CD18 (NBP1–88128, Novusbio, 1:10). Images were acquired using a Zeiss Axiophot microscope equipped with an AxioCamHRc digital camera.
Enzyme-linked immunosorbent assay (ELISA)
Supernatants of control fpEC and GDM fpEC were collected after 48h culture at 37°C and 21% oxygen. Cord blood serum (CBS) was obtained from umbilical vein and arteries of term human placentas after healthy pregnancies or pregnancies complicated with GDM and diluted before the assay (1:20). ELISA was performed according to the manufacturer´s instructions (DCD540, R&D Systems, UK). Optical density was determined at 450nm using a Spectrophotometer (Spectromax). Obtained data were normalized to total protein concentration determined using BCA kit (23227 Pierce, Thermo Scientific).
Methylation of the ICAM-1 gene
1µg of DNA isolated from 9 control fpEC and 5 GDM fpEC was bisulphite converted using the MethylEasyTM bisulphite modification kit (Human Genetic Signatures, Sydney, Australia), according to the manufacturer's instructions. Conversion efficiency was assessed by bisulphite-specific PCR. Hybridization of bisulfite-treated samples to Illumina Infinium Human Methylation450 (HM450) Beadchips was performed at the Australian Genome Research Facility (AGRF), Melbourne. Raw data (IDAT files) were exported from GenomeStudio (Illumina, San Diego, CA). The Bioconductor package minfi was used to read the data into R, carry out quality control, preprocessing and normalization using the subset-quantile within array normalization (SWAN) method.21 Additional quality control was carried out using the Bioconductor packages arrayQualityMetrics and limma. The limma package was used to fit a linear model to compare control and GDM fpEC.
M-values were calculated after removing probes on the sex chromosomes to eliminate any potential gender bias and any poor performing probes, defined as those with a detection p-value cut-off >0 .05 for all samples. β-values were derived from intensities as defined by the ratio of methylated to unmethylated probes given by β =M / (U+M+100) and were used as a measure of effect size.
Statistical analysis
Data were analyzed using SigmaPlot software Version 12.5 (SigmaStat). Data are expressed as mean ± SD. Student's t test was applied after testing for normal distribution (Kolmogorov-Smirnov test). p-values below 0.05 were considered statistically significant.
Results
Cell adhesion molecules in fpEC from normal and GDM pregnancies
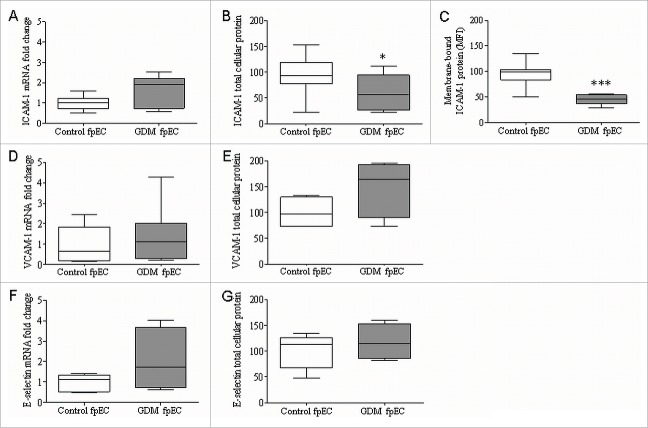
In order to determine if fpEC exposed to diabetic environment of GDM will show signs of pro-inflammatory phenotype, we determined mRNA and protein levels of ICAM-1, VCAM-1 and E-selectin in fpEC isolated after GDM complicated pregnancy (GDM fpEC) and compared them to fpEC after normal pregnancy (control fpEC). No difference in mRNA levels of ICAM-1 (Fig. 1A), VCAM-1 (Fig. 1D) or E-selectin (Fig. 1F) was found by RT-qPCT in control vs GDM fpEC. Total cellular ICAM-1 was significantly reduced by 31% in GDM (p=0.03) (Fig. 1B), while no significant differences were found in cellular protein levels of VCAM-1 (Fig. 1E) and E-selectin (Fig. 1G) in GDM fpEC vs control. Flow cytometric analysis revealed 53% lower levels of membrane bound ICAM-1 in GDM fpEC compared to control fpEC (p<0.001) (Fig. 1C), while membrane bound VCAM-1 and membrane bound E-selectin were below detection limit of flow cytometry (data not shown). Treatment of fpEC with TNFa in vitro revealed a dose dependent increase in ICAM-1 protein, demonstrating that these cells are capable to classically respond to pro-inflammatory signals (Fig. S1).
Figure 1.
Expression of markers for endothelial dysfunction in control and GDM-exposed feto-placental endothelial cells. RT-qPCR showed no significant difference in mRNA expression of ICAM-1 (A), VCAM-1 (D) or E-selectin (F) between control vs GDM derived fpEC (n control/GDM= 7/7). Immunoblotting for total cellular ICAM-1 revealed significant reduction of ICAM-1 levels in GDM fpEC (B) (n control/GDM=18/11). No difference between control and GDM fpEC were seen in total cellular protein VCAM-1 (E) (n control/GDM=5/5) or E-selectin (G) (n control/GDM=5/5). Detection of ICAM-1 by flow cytometry demonstrated decreased levels of membrane bound ICAM-1 in fpEC from GDM placenta (C) (n control/GDM=8/8). Membrane bound VCAM-1 and membrane bound E-selectin were below detection of flow cytometry (data not shown). mRNA levels are shown as fold change in GDM fpEC. Total cellular protein levels are shown normalized to PonceauS staining. *p<0.05, ***p<0.001.
ICAM-1 protein in normal and GDM placenta
Since ICAM-1 was the only molecule that showed significant difference between control and GDM fpEC, we further evaluated ICAM-1 expression in the human placenta. Immunohistochemical analysis of human third trimester placenta revealed strongest ICAM-1 staining in the feto-placental endothelium and a very weak staining in placental stromal cells, presumably macrophages (Hofbauer cells). Comparison of the intensity of ICAM-1 staining in feto-placental endothelium of control and GDM placenta by visual inspection revealed reduced endothelial ICAM-1 levels in GDM placenta which paralleled reduced ICAM-1 expression levels revealed in isolated fpEC (Fig. 2).
Figure 2.
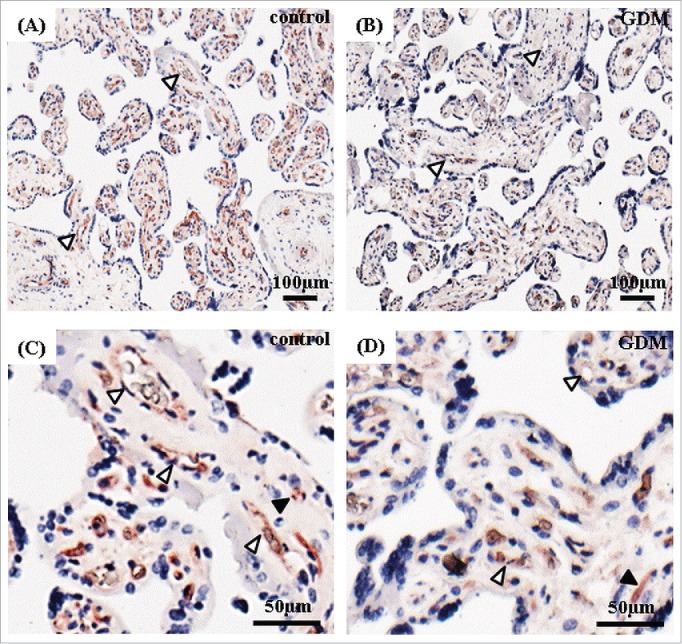
Immunolocalization of ICAM-1 in human term placenta after normal and GDM pregnancy. Placental tissue after normal (A, C) and GDM pregnancies (B, D) stained for ICAM-1 (shown in red). Nuclei are stained with DAPI (blue). Feto-placental endothelial cells (white arrowheads) showed a positive staining that was reduced in GDM placenta. Placental stromal cells (black arrowheads) were stained less intensive. Representative pictures of 4 control and 4 GDM placentas are shown.
Soluble ICAM-1 levels in fpEC culture supernatants and in cord blood serum after GDM pregnancy
To investigate if increased shedding of ICAM-1 accounts for unexpected lower levels of its cellular and membrane bound forms in GDM, sICAM-1 was quantified by ELISA in culture supernatants of control and GDM fpEC. In comparison to control, GDM fpEC secreted 57% less sICAM-1 during 48h of culture (Fig. 3A). These results show that GDM reduces total, cellular and secreted ICAM-1 levels. In fpEC isolated after normal pregnancy sICAM release into the culture medium negatively correlated with maternal BMI before pregnancy (Fig. 3C). In GDM derived cells, the correlation showed the same trend, but sICAM-1 levels were lower than ICAM-1 released by control fpEC as indicated by a shift of the regression. sICAM-1 levels measured in CBS of control and GDM pregnancies were unchanged (Fig. 3B).
Figure 3.
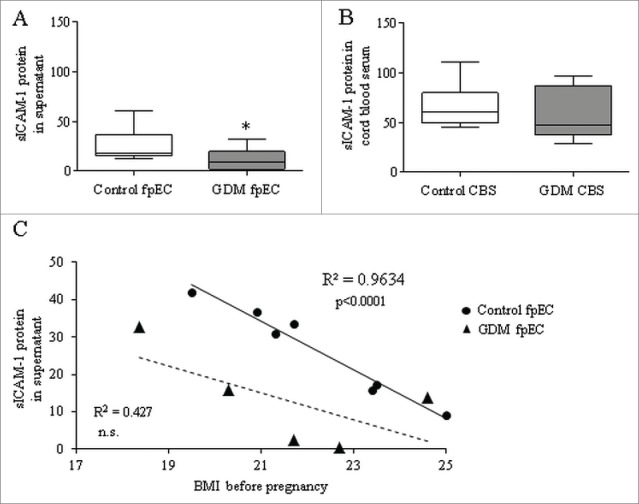
sICAM-1 in culture supernatant of control and GDM feto-placental endothelial cells and in cord blood serum. Levels of sICAM-1 quantified by ELISA showed significant reduction in supernatant of GDM vs control fpEC (A) (n control/GDM=11/6). In cord blood serum (CBS) collected after healthy (control CBS) and GDM pregnancies (GDM CBS) sICAM-1 levels were similar (B) (n control/GDM=12/17). Correlation of sICAM-1 levels released in the supernatant of fpEC isolated after normal pregnancies (circles) and GDM pregnancy (triangles) with maternal BMI before pregnancy. The linear regression line is given as continuous line for the controls and as dached line for the GDM derived fpEC. (C) *p<0.05.
ICAM-1 binding partners
ICAM-1 interacts with membrane-bound β 2 integrin receptors CD11a/CD18 (LFA-1) and CD11b/CD18 (Mac-1).16,22 Hence, in order to identify those cells representing potential ICAM-1 mediated binding partners for feto-placental endothelium, we stained placental sections with antibodies against CD11a and CD18. Immunohistochemistry revealed fetal blood cells (white arrowheads) as main ICAM-1 binding partners as they showed positive staining for both CD11 (Fig. 4A-B) and CD18 (Fig. 4C-D). Also placental macrophages (Hofbauer cells) (black arrowheads) expressed CD11 (Fig. 4C).
Figure 4.
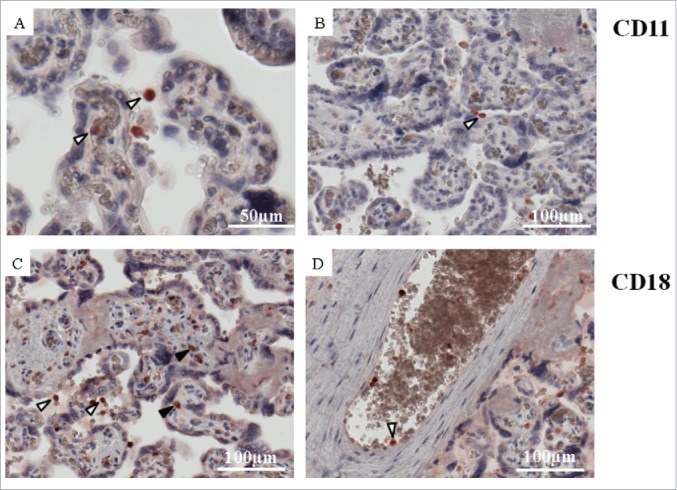
Expression of ICAM-1 binding partners CD11a and CD18 subunits of LFA-1. Placental tissue after normal pregnancies stained for CD11a (A, B) and CD18 (C, D) in red. Nuclei were stained with DAPI (blue). Materal and fetal blood cells (white arrowheads) showed positive staining for both CD11a (A, B) and CD18 (C, D). Placental macrophages (Hofbauer cells) (black arrowheads) expressed only CD11a (C). Representative pictures of 4 control placentas are shown.
Discussion
We hypothesized that the GDM environment induces alterations in the expression of cell adhesion molecules ICAM-1, VCAM-1 and E-selectin in the feto-placental endothelium, which are classical markers for endothelial dysfunction. The key findings were: 1) ICAM-1 protein was decreased in GDM exposed fpEC, while there were no changes in the other CAMs, and 2) only ICAM-1 protein, but not mRNA was reduced in GDM suggesting it to be a result of post-translational regulation.
ICAM-1 is constitutively expressed on endothelial cells and cells of the immune system. It stabilizes cell-cell interactions and facilitates leukocyte endothelial transmigration. Under pro-inflammatory conditions, ICAM-1 expression is rapidly increased by several cytokines i.e. TNFa, IL-1b and IFNg.23 ICAM-1 binds to its ligands LFA-1 (CD11b/CD18) or Mac-1 (CD11a/CD18) that are expressed on leukocytes and monocytes, enabling them to bind to endothelial cells and transmigrate into tissues. Furthermore, proteolytic cleavage of the extracellular portion of ICAM-1 produces a soluble form, sICAM-1. This shedding of sICAM-1 from the cell membrane is regarded as a protective mechanism to prevent from excessive leukocyte and monocyte attachment. Moreover, shedded sICAM-1 adds to this protective effect by blocking LFA-1 and Mac-1, respectively.13
Since maternal GDM is a pro-inflammatory state and causes hyperglycaemia, hyperinsulinemia and altered levels of cytokines in the fetal circulation, we hypothesized that the altered GDM metabolic milieu may cause endothelial dysfunction and induce upregulation of either the cell surface bound ICAM-1, or released sICAM-1. Indeed, increased levels of sICAM-1 in cord blood were observed in other pro-inflammatory conditions in pregnancy, i.e. in placental vascular disease24 and in maternal Type 1 diabetes25 indicating that the feto-placental vasculature is capable of the classical ICAM-1 upregulation upon cytokine stimulation. Cytokine dependent upregulation of ICAM-1 protein in fpEC was also confirmed by us in vitro using TNFa as classical pro-inflammatory mediator. However, the derangements of GDM did not elevate ICAM-1 expression in the feto-placental vasculature. By contrast, total cellular, membrane-bound and soluble forms of ICAM-1 protein were reduced.
Our finding of reduced ICAM-1 in feto-placental endothelium and in cultured fpEC is in line with a previous study by Kurt et al.26 who observed decreased ICAM-1 immunohistochemically in the endothelial compartment of GDM placentas. The analysis of primary fpEC after normal and GDM pregnancy using distinct methods enabled us to determine mRNA levels and discriminate between membrane bound and sICAM-1 protein. Unaltered mRNA excluded transcriptional regulation of ICAM-1 in GDM. Indeed, global DNA methylation analysis revealed that GDM does not alter DNA methylation profile of ICAM-1 gene in fpEC (Figure S2). The discrepancy between mRNA and protein suggests a repressive post-transcriptional mechanism to reduce ICAM-1 protein. MicroRNAs represent such mechanism, and several microRNAs were shown to down regulate ICAM-1 protein synthesis.27–30 Microarray analysis comparing whole genome expression between fpEC isolated after normal vs GDM pregnancy (Cvitic at al., unpublished) revealed mir221 and mir222, 2 negative regulators of ICAM-1,28,31 significantly upregulated in GDM (Table 2), indicating that GDM-associated overexpression of mir221 and 222 may cause reduced ICAM-1 levels in the feto-placental endothelium in GDM.
Table 2.
Expression of ICAM-1 regulating microRNAs in fpEC after normal (n=10) vs. GDM (n=10) pregnancies as determined by microarray analysis.
| Gene Symbol | RefSeq | p-value | Fold-change |
|---|---|---|---|
| MIR222 | NR_029636 | 0.0000 | 3.2 |
| MIR221 | NR_029635 | 0.0001 | 2.0 |
| MIR223 | NR_029637 | n.s. | NC |
| MIR296 | NR_029844 | n.s. | NC |
| MIR339 | NR_029898 | n.s. | NC |
n.s.: not significant; NC: no change
The negative correlation of sICAM secreted by primary fpEC with maternal BMI suggests pro-inflammatory and adiposity related factors to trigger this mechanism. In fact, a correlation of BMI with sICAM was demonstrated.32 However, the reduced sICAM-1 release of GDM-derived fpEC regardless of whether maternal BMI was high or low suggests that GDM can be regarded as an independent regulator of ICAM-1. The reduction of sICAM could—as a hypothesis—represent mechanism counteracting overflowing leukocyte attachment and transmigration. If so, this mechanism however, is likely to be placenta specific, since we found unchanged sICAM-1 levels in fetal cord blood after GDM pregnancy, which is in line with other studies.5,33 Thus, other fetal sources of sICAM-1 release counteract the reduced ICAM shedding from the feto-placental endothelium.
In order to identify cells interacting with ICAM-1 on the feto-placental endothelium, we stained placental tissue for the ICAM-1 binding partners CD11a and CD18. This identified fetal blood cells (CD11a and CD18) and placental macrophages (CD18) as potential target cells of ICAM-1 mediated cell-cell interaction.
Besides ICAM-1, we also measured mRNA and protein expression of other CAMs, VCAM-1 and E-selectin, and found no difference in fpEC that were exposed to GDM environment. These unchanged levels may indeed suggest absent of the classical endothelial dysfunction markers in GDM despite of altered feto-placental endothelial phenotype and function shown previously by us and others.6,7,34 Indeed, decreased ICAM-1 expression further indicates a change in endothelial function. Thus in opposite to maternal endothelium,6,7,34 and the decreased ICAM-1 protein expression observed here is a further indication that endothelial cell function is altered. Thus, in opposite to maternal endothelium,5,35 the feto-placental endothelium does not classically respond to the GDM environment with increased CAM expression. Table S2 summarizes studies on placenta, fetal and maternal expression of these markers, and also includes their levels in offspring and women after GDM pregnancy.
In summary, our data show that GDM does not induce upregulation of the classical endothelial dysfunction markers ICAM-1, VCAM-1 and E-selectin in feto-placental endothelium, and we suggest this to be a protective mechanism of GDM placenta.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
FD-P and SC performed experiments, analyzed data and wrote the manuscript. UH designed the study and wrote the manuscript. MG, VK and JL performed experiments, GD, AH, IL and RS designed the study and contributed to discussion. All authors revised and approved the manuscript.
Funding
The work was supported by the doctorate program MOLIN (FWF, W1241), Medical University of Graz and by the Oesterreichische Nationalbank (Anniversary Fund, project number: 14844).
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
References
- 1.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol 2015; 209:13-22; PMID:25869663; http://dx.doi.org/ 10.1083/jcb.201412052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannarile F, Valentini V, Mirabelli G, Alunno A, Terenzi R, Luccioli F, Gerli R, Bartoloni E. Cardiovascular disease in systemic sclerosis. Ann Transl Med 2015; 3:8,5839.2014.12.12; PMID:25705640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton SJ, Watts GF. Endothelial dysfunction in diabetes: Pathogenesis, significance, and treatment. Rev Diabet Stud 2013; 10:133-56; PMID:24380089; http://dx.doi.org/ 10.1900/RDS.2013.10.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vairappan B. Endothelial dysfunction in cirrhosis: Role of inflammation and oxidative stress. World J Hepatol 2015; 7:443-59; PMID:25848469; http://dx.doi.org/ 10.4254/wjh.v7.i3.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mordwinkin NM, Ouzounian JG, Yedigarova L, Montoro MN, Louie SG, Rodgers KE. Alteration of endothelial function markers in women with gestational diabetes and their fetuses. J Matern Fetal Neonatal Med 2013; 26:507-12; PMID:23046386; http://dx.doi.org/ 10.3109/14767058.2012.736564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leach L. Placental vascular dysfunction in diabetic pregnancies: Intimations of fetal cardiovascular disease? Microcirculation 2011; 18:263-9; PMID:21418381; http://dx.doi.org/ 10.1111/j.1549-8719.2011.00091.x [DOI] [PubMed] [Google Scholar]
- 7.Sobrevia L, Abarzua F, Nien JK, Salomon C, Westermeier F, Puebla C, Cifuentes F, Guzman-Gutierrez E, Leiva A, Casanello P. Review: Differential placental macrovascular and microvascular endothelial dysfunction in gestational diabetes. Placenta 2011; 32 Suppl 2:S159-64; http://dx.doi.org/ 10.1016/j.placenta.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 8.Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. the insulin and cytokine network. Diabetes Care 2007; 30 Suppl 2:S120-6; http://dx.doi.org/ 10.2337/dc07-s203 [DOI] [PubMed] [Google Scholar]
- 9.Cvitic S, Desoye G, Hiden U. Glucose, insulin, and oxygen interplay in placental hypervascularisation in diabetes mellitus. Biomed Res Int 2014; 2014:145846; PMID:25258707; http://dx.doi.org/ 10.1155/2014/145846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jirkovska M, Kubinova L, Janacek J, Moravcova M, Krejci V, Karen P. Topological properties and spatial organization of villous capillaries in normal and diabetic placentas. J Vasc Res 2002; 39:268-78; PMID:12097825; http://dx.doi.org/ 10.1159/000063692 [DOI] [PubMed] [Google Scholar]
- 11.Babawale MO, Lovat S, Mayhew TM, Lammiman MJ, James DK, Leach L. Effects of gestational diabetes on junctional adhesion molecules in human term placental vasculature. Diabetologia 2000; 43:1185-96; PMID:11043866; http://dx.doi.org/ 10.1007/s001250051511 [DOI] [PubMed] [Google Scholar]
- 12.Hiden U, Lassance L, Tabrizi NG, Miedl H, Tam-Amersdorfer C, Cetin I, Lang U, Desoye G. Fetal insulin and IGF-II contribute to gestational diabetes mellitus (GDM)-associated up-regulation of membrane-type matrix metalloproteinase 1 (MT1-MMP) in the human feto-placental endothelium. 2012; 97:3613-21.; PMID:22893718 [DOI] [PubMed] [Google Scholar]
- 13.Zonneveld R, Martinelli R, Shapiro NI, Kuijpers TW, Plotz FB, Carman CV. Soluble adhesion molecules as markers for sepsis and the potential pathophysiological discrepancy in neonates, children and adults. Crit Care 2014; 18:204; http://dx.doi.org/ 10.1186/cc13733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: The role of endothelial dysfunction. Clin Sci (Lond) 2005; 109:143-59; PMID:16033329 [DOI] [PubMed] [Google Scholar]
- 15.Fotis L, Giannakopoulos D, Stamogiannou L, Xatzipsalti M. Intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 in children. do they play a role in the progression of atherosclerosis? Hormones (Athens) 2012; 11:140-6; PMID:22801559 [DOI] [PubMed] [Google Scholar]
- 16.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep 2009; 61:22-32; PMID:19307690; http://dx.doi.org/ 10.1016/S1734-1140(09)70004-0 [DOI] [PubMed] [Google Scholar]
- 17.Kautzky-Willer A, Bancher-Todesca D, Pollak A, Repa A, Lechleitner M, Weitgasser R. Gestational diabetes mellitus. Wien Klin Wochenschr 2012; 124 Suppl 2:58-65; http://dx.doi.org/ 10.1007/s00508-012-0265-3 [DOI] [PubMed] [Google Scholar]
- 18.Lang I, Schweizer A, Hiden U, Ghaffari-Tabrizi N, Hagendorfer G, Bilban M, Pabst MA, Korgun ET, Dohr G, Desoye G. Human fetal placental endothelial cells have a mature arterial and a juvenile venous phenotype with adipogenic and osteogenic differentiation potential. Differentiation 2008; 76:1031-43; PMID:18673379; http://dx.doi.org/ 10.1111/j.1432-0436.2008.00302.x [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001; 25:402-8 [DOI] [PubMed] [Google Scholar]
- 20.Blaschitz A, Gauster M, Dohr G. Application of cryo-compatible antibodies to human placenta paraffin sections. Histochem Cell Biol 2008; 130:595-9; PMID:18563432; http://dx.doi.org/ 10.1007/s00418-008-0458-z [DOI] [PubMed] [Google Scholar]
- 21.Maksimovic J, Gordon L, Oshlack A. SWAN: Subset-quantile within array normalization for illumina infinium HumanMethylation450 BeadChips. Genome Biol 2012; 13:R44,2012-13-6-r44; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest 1989; 83:2008-17; PMID:2566624; http://dx.doi.org/ 10.1172/JCI114111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anbarasan C, Bavanilatha M, Latchumanadhas K, Ajit Mullasari S. ICAM-1 molcular mechanism and genome wide SNP's association studies. Indian Heart J 2015; 67:282-7; PMID:26138191; http://dx.doi.org/ 10.1016/j.ihj.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Athayde N, Trudinger B. Endothelial cell expression of adhesion molecules is induced by fetal plasma from pregnancies with umbilical placental vascular disease. BJOG 2002; 109:770-7; PMID:12135213; http://dx.doi.org/ 10.1111/j.1471-0528.2002.01240.x [DOI] [PubMed] [Google Scholar]
- 25.Nelson SM, Sattar N, Freeman DJ, Walker JD, Lindsay RS. Inflammation and endothelial activation is evident at birth in offspring of mothers with type 1 diabetes. Diabetes 2007; 56:2697-704; http://dx.doi.org/ 10.2337/db07-0662 [DOI] [PubMed] [Google Scholar]
- 26.Kurt M, Zulfikaroglu E, Ucankus NL, Omeroglu S, Ozcan U. Expression of intercellular adhesion molecule-1 in umbilical and placental vascular tissue of gestational diabetic and normal pregnancies. Arch Gynecol Obstet 2010; 281:71-6; PMID:19343356; http://dx.doi.org/ 10.1007/s00404-009-1066-4 [DOI] [PubMed] [Google Scholar]
- 27.Ueda R, Kohanbash G, Sasaki K, Fujita M, Zhu X, Kastenhuber ER, McDonald HA, Potter DM, Hamilton RL, Lotze MT, et al.. Dicer-regulated microRNAs 222 and 339 promote resistance of cancer cells to cytotoxic T-lymphocytes by down-regulation of ICAM-1. Proc Natl Acad Sci U S A 2009; 106:10746-51; PMID:19520829; http://dx.doi.org/ 10.1073/pnas.0811817106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duan M, Yao H, Hu G, Chen X, Lund AK, Buch S. HIV tat induces expression of ICAM-1 in HUVECs: Implications for miR-221/-222 in HIV-associated cardiomyopathy. PLoS One 2013; 8:e60170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabet F, Vickers KC, Cuesta Torres LF, Wiese CB, Shoucri BM, Lambert G, Catherinet C, Prado-Lourenco L, Levin MG, Thacker S, et al.. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat Commun 2014; 5:3292; PMID:24576947; http://dx.doi.org/ 10.1038/ncomms4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Chen Q, Yan J, Wang Y, Zhu C, Chen C, Zhao X, Xu M, Sun Q, Deng R, et al.. MiRNA-296-3p-ICAM-1 axis promotes metastasis of prostate cancer by possible enhancing survival of natural killer cell-resistant circulating tumour cells. Cell Death Dis 2013; 4:e928; PMID:24263102; http://dx.doi.org/ 10.1038/cddis.2013.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu G, Gong AY, Liu J, Zhou R, Deng C, Chen XM. miR-221 suppresses ICAM-1 translation and regulates interferon-gamma-induced ICAM-1 expression in human cholangiocytes. Am J Physiol Gastrointest Liver Physiol 2010; 298:G542-50; PMID:20110463; http://dx.doi.org/ 10.1152/ajpgi.00490.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glowinska B, Urban M, Peczynska J, Florys B. Soluble adhesion molecules (sICAM-1, sVCAM-1) and selectins (sE selectin, sP selectin, sL selectin) levels in children and adolescents with obesity, hypertension, and diabetes. Metabolism 2005; 54:1020-6; http://dx.doi.org/ 10.1016/j.metabol.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 33.Cross JA, Temple RC, Hughes JC, Dozio NC, Brennan C, Stanley K, Murphy HR, Fowler D, Hughes DA, Sampson MJ. Cord blood telomere length, telomerase activity and inflammatory markers in pregnancies in women with diabetes or gestational diabetes. Diabet Med 2010; 27:1264-70; PMID:20950384; http://dx.doi.org/ 10.1111/j.1464-5491.2010.03099.x [DOI] [PubMed] [Google Scholar]
- 34.Hiden U, Lassance L, Tabrizi NG, Miedl H, Tam-Amersdorfer C, Cetin I, Lang U, Desoye G. Fetal insulin and IGF-II contribute to gestational diabetes mellitus (GDM)-associated up-regulation of membrane-type matrix metalloproteinase 1 (MT1-MMP) in the human feto-placental endothelium. J Clin Endocrinol Metab 2012; 97:3613-21; PMID:22893718; http://dx.doi.org/ 10.1210/jc.2012-1212 [DOI] [PubMed] [Google Scholar]
- 35.Krauss T, Emons G, Kuhn W, Augustin HG. Predictive value of routine circulating soluble endothelial cell adhesion molecule measurements during pregnancy. Clin Chem 2002; 48:1418-25; PMID:12194917 [PubMed] [Google Scholar]
- 36.Giri H, Chandel S, Dwarakanath LS, Sreekumar S, Dixit M. Increased endothelial inflammation, sTie-2 and arginase activity in umbilical cords obtained from gestational diabetic mothers. PLoS One 2013; 8:e84546; PMID:24376824; http://dx.doi.org/ 10.1371/journal.pone.0084546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Fulvio P, Pandolfi A, Formoso G, Di Silvestre S, Di Tomo P, Giardinelli A, De Marco A, Di Pietro N, Taraborrelli M, Sancilio S, et al.. Features of endothelial dysfunction in umbilical cord vessels of women with gestational diabetes. Nutr Metab Cardiovasc Dis 2014; 24:1337-45; http://dx.doi.org/ 10.1016/j.numecd.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 38.Sultan SA, Liu W, Peng Y, Roberts W, Whitelaw D, Graham AM. The role of maternal gestational diabetes in inducing fetal endothelial dysfunction. J Cell Physiol 2015; 230:2695-705; PMID:25808705; http://dx.doi.org/ 10.1002/jcp.24993 [DOI] [PubMed] [Google Scholar]
- 39.West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia 2011; 54:504-7; PMID:21153896; http://dx.doi.org/ 10.1007/s00125-010-2008-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manderson JG, Mullan B, Patterson CC, Hadden DR, Traub AI, McCance DR. Cardiovascular and metabolic abnormalities in the offspring of diabetic pregnancy. Diabetologia 2002; 45:991-6; PMID:12136397; http://dx.doi.org/ 10.1007/s00125-002-0865-y [DOI] [PubMed] [Google Scholar]
- 41.Gobl CS, Bozkurt L, Yarragudi R, Prikoszovich T, Tura A, Pacini G, Koppensteiner R, Kautzky-Willer A. Biomarkers of endothelial dysfunction in relation to impaired carbohydrate metabolism following pregnancy with gestational diabetes mellitus. Cardiovasc Diabetol 2014; 13:138,014-0138-3; http://dx.doi.org/ 10.1186/s12933-014-0138-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Telejko B, Zonenberg A, Kuzmicki M, Modzelewska A, Niedziolko-Bagniuk K, Ponurkiewicz A, Nikolajuk A, Gorska M. Circulating asymmetric dimethylarginine, endothelin-1 and cell adhesion molecules in women with gestational diabetes. Acta Diabetol 2009; 46:303-8; PMID:19139802; http://dx.doi.org/ 10.1007/s00592-008-0088-x [DOI] [PubMed] [Google Scholar]
- 43.Kautzky-Willer A, Fasching P, Jilma B, Waldhausl W, Wagner OF. Persistent elevation and metabolic dependence of circulating E-selectin after delivery in women with gestational diabetes mellitus. J Clin Endocrinol Metab 1997; 82:4117-21; PMID:9398724; http://dx.doi.org/ 10.1210/jcem.82.12.4419 [DOI] [PubMed] [Google Scholar]
- 44.Lawrence NJ, Kousta E, Penny A, Millauer B, Robinson S, Johnston DG, McCarthy MI. Elevation of soluble E-selectin levels following gestational diabetes is restricted to women with persistent abnormalities of glucose regulation. Clin Endocrinol (Oxf) 2002; 56:335-40; http://dx.doi.org/ 10.1046/j.1365-2265.2002.01473.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.