Abstract
Objective:
Pulmonary rehabilitation (PR) improves exercise capacity in most but not all COPD patients. The factors associated with treatment success and the role of chest wall mechanics remain unclear. We investigated the impact of PR on exercise performance in COPD with severe hyperinflation.
Methods:
We evaluated 22 COPD patients (age, 66 ± 7 years; FEV1 = 37.1 ± 11.8% of predicted) who underwent eight weeks of aerobic exercise and strength training. Before and after PR, each patient also performed a six-minute walk test and an incremental cycle ergometer test. During the latter, we measured chest wall volumes (total and compartmental, by optoelectronic plethysmography) and determined maximal workloads.
Results:
We observed significant differences between the pre- and post-PR means for six-minute walk distance (305 ± 78 vs. 330 ± 96 m, p < 0.001) and maximal workload (33 ± 21 vs. 39 ± 20 W; p = 0.02). At equivalent workload settings, PR led to lower oxygen consumption, carbon dioxide production (VCO2), and minute ventilation. The inspiratory (operating) rib cage volume decreased significantly after PR. There were 6 patients in whom PR did not increase the maximal workload. After PR, those patients showed no significant decrease in VCO2 during exercise, had higher end-expiratory chest wall volumes with a more rapid shallow breathing pattern, and continued to experience symptomatic leg fatigue.
Conclusions:
In severe COPD, PR appears to improve oxygen consumption and reduce VCO2, with a commensurate decrease in respiratory drive, changes reflected in the operating chest wall volumes. Patients with severe post-exercise hyperinflation and leg fatigue might be unable to improve their maximal performance despite completing a PR program.
Keywords: Pulmonary disease, chronic obstructive/rehabilitation; Exercise therapy; Respiratory therapy
Abstract
Objetivo:
A reabilitação pulmonar (RP) melhora a capacidade de exercício na maioria (mas não todos) dos pacientes com DPOC. Os fatores associados ao sucesso do tratamento e o papel da mecânica da parede torácica na determinação desse sucesso ainda não é claro. Investigamos o impacto da RP no desempenho ao exercício em pacientes com DPOC e hiperinsuflação grave.
Métodos:
Foram avaliados 22 pacientes com DPOC (idade, 66 ± 7 anos; VEF1 = 37,1 ± 11,8% do previsto) submetidos a oito semanas de exercícios aeróbicos e treino de força. Antes e depois da RP, cada paciente também realizou um teste de caminhada de seis minutos e um teste de exercício incremental em uma bicicleta ergométrica. Durante esse último, os volumes da parede torácica (total e compartimental por pletismografia optoeletrônica) e a carga de trabalho máxima foram determinados.
Resultados:
Diferenças significativas foram observadas entre as médias pré e pós-RP da distância percorrida no teste de caminhada de seis minutos (305 ± 78 vs. 330 ± 96 m; p < 0,001) e da carga máxima (33 ± 21 vs. 39 ± 20 W; p = 0,02). Sob parâmetros de carga de trabalho equivalente, a RP levou a valores menores de consumo de oxigênio, produção de dióxido de carbono (VCO2) e ventilação minuto. O volume inspiratório (operacional) da caixa torácica diminuiu significativamente após a RP. Em 6 pacientes, a RP não aumentou a carga máxima. Após a RP, esses pacientes não apresentaram uma diminuição significativa na VCO2 durante o exercício, tiveram maiores volumes expiratórios finais da parede torácica com padrão respiratório mais rápido e superficial e continuaram a apresentar fadiga sintomática nas pernas.
Conclusões:
Na DPOC grave, a RP parece melhorar o consumo de oxigênio e reduzir VCO2, com uma diminuição proporcional no drive respiratório, mudanças essas que são refletidas nos volumes operacionais da parede torácica. Pacientes com hiperinsuflação grave pós-exercício e fadiga nas pernas podem ser incapazes de melhorar seu desempenho máximo apesar de completarem um programa de RP.
INTRODUCTION
Pulmonary rehabilitation (PR) is one of the most effective interventions in the management of COPD and produces significant improvements in exercise performance, with a reduction in breathlessness,( 1 - 3 ) in patients with varying degrees of disease severity.( 4 ) However, not all patients benefit from PR programs.( 5 ) The reasons for this are complex; some patients decline to enroll in such programs, and some others initially recruited drop out, often due to factors related to expectation, smoking status, or perceived disability.( 6 ) Relatively little is known about why some patients who complete the planned course of a PR program fail to improve.( 7 )
Most studies of PR programs have shown that, although rehabilitation has no effect on lung function, it can reduce carbon dioxide production (VCO2) and increase the lactate threshold.( 6 , 8 ) It has been reported that lung and chest wall volumes decrease during exercise.( 1 , 8 , 9 ) It remains unclear whether that is an effect of a reduced ventilatory demand for a given amount of work or whether rehabilitation changes the way in which COPD patients who have chronic hyperinflation breathe. The effect may be most relevant when resting hyperinflation is more severe. However, there are relatively few data about the impact of PR in hyperinflated patients with extremely poor initial exercise tolerance. We hypothesized that the main effect of PR in such patients would be to decrease the metabolic stimulus for ventilation during exercise and that any changes in the inspiratory (operating) chest wall volume would be secondary. In addition, we anticipated that the patients in whom there was no increase in CO2 production during exercise (lack of such an increase being a marker of an impaired muscle performance) would not benefit from PR. Therefore, we aimed to investigate the impact that a general PR program has on exercise performance in COPD patients with severe hyperinflation and, consequently, worse baseline exercise performance than that of the COPD patients who have typically been studied.
METHODS
Patients and procedures
This was an exploratory observational study involving a convenience sample of 22 patients with moderate to very severe COPD( 10 ) and a residual volume > 150% predicted. All were current or former smokers, with a post-bronchodilator FEV1/FVC ratio < 0.7 and FEV1 < 70% of the predicted value. No patient had had an exacerbation in the last six weeks. All were treated with inhaled corticosteroids and long-acting bronchodilators, as well as short-acting rescue therapy when necessary. The exclusion criteria were having been diagnosed with asthma, requiring supplemental oxygen at rest, and having any concurrent illness that could limit exercise performance (including heart failure and neuromuscular disorders). The study was approved by the Research Ethics Committee of Aintree University Hospital, in Liverpool, England, and all participating patients gave written informed consent.
All measurements were performed before and after eight weeks of PR. Spirometry and lung volume measurements were performed using a plethysmograph (1085D; Medical Graphics Corporation, St. Paul, MN, USA), in accordance with the American Thoracic Society/European Respiratory Society recommendations.( 11 , 12 ) Maximal voluntary ventilation (MVV) was determined indirectly as the product of FEV1 × 37.5.( 13 ) All variables are expressed as a percentage of the predicted value for age.( 14 , 15 )
Each patient performed two six-minute walk tests in accordance with the American Thoracic Society recommendations.( 16 ) The six-minute walk distance (6MWD) was measured and compared with that obtained for an age-matched reference population evaluated in a study conducted in the city of Liverpool, United Kingdom.( 17 ) Peripheral oxygen saturation and heart rate were recorded with a pulse oximeter (PULSOX 3i; Konica Minolta, Ramsey, NJ, USA). During the test, subjects were asked to rate their breathlessness and leg fatigue, on a modified Borg scale,( 18 ) once every minute.
Optoelectronic plethysmography (OEP) was applied (see below), after which patients were seated on a cycle ergometer (Corival; Lode, Groningen, the Netherlands) and asked to execute three slow vital capacity maneuvers, followed by 2 min of quiet breathing, to establish baseline values for the chest wall volumes. Subjects then undertook an incremental exercise test on a cycle ergometer, pedaling without a load for 2 min, then with incremental load increases of 5 watts/min until exhaustion. Subjects were breathing through a mouthpiece with a nose clip, and breath-by-breath ventilatory variables were derived from the flow signal detected with a pneumotachograph (preVent; Medical Graphics Corporation). Oxygen consumption (VO2) and VCO2 were measured with a paramagnetic sensor and an infrared carbon dioxide analyzer, respectively, as part of an exercise testing system (CardioO2 system; Medical Graphics Corporation). During the exercise, subjects were asked to rate their breathlessness and leg fatigue, on a modified Borg scale,( 18 ) once every minute. The results were compared with those obtained for an age-matched population.( 19 ) The flow signal was synchronized with that of the motion analyzer used for OEP and transferred to a personal computer for subsequent analysis. Peripheral oxygen saturation was measured by pulse oximetry (Biox 3700e; Ohmeda, Louisville, CO, USA). Heart rate was determined on the basis of the R-R interval from a 4-lead electrocardiogram.
The kinematic data of the chest wall were analyzed with an OEP system (BTS Bioengineering, Milan, Italy). A complete description of the process has previously been published.( 20 , 21 ) In brief, the volume displacements of the two compartments of the chest wall were measured through the use of 89 retroreflective markers placed on the trunk at established anatomical reference points. ( 20 ) Three-dimensional coordinates of the markers were calculated with stereophotogrammetry and then linked with a mesh of triangles representing the surface of the trunk. The volume of the trunk enclosed by the defined surfaces was obtained through Gauss' theorem.
PR program
The PR program consisted of two supervised 60-min sessions and one unsupervised 60-min session of exercise per week, over a period of eight weeks. Patients performed aerobic upper and lower limb exercise, which included peripheral muscle strengthening and whole body endurance exercises delivered by a combination of cycle ergometry and corridor walking exercise. The patients trained to a symptom-limited intensity equivalent to a level of 3 to 4 on the modified Borg scale and were allowed 1-2 min of rest between each exercise. Patients were encouraged to increase the time spent on each exercise at each session. An individually tailored home exercise program was provided for the unsupervised session. After each supervised exercise session, there was also an education session focusing on aspects of behavior and lifestyle. This regime has previously been shown to improve exercise capacity in patients with a wide range of COPD severity.( 22 ) The attendance at each supervised session was documented and patients used a home diary to confirm adherence with the unsupervised portion of the program.
Data analysis
The chest wall was modeled in two compartments: rib cage and abdomen. The total volume displaced by the chest wall was calculated as the sum of the volumes swept by the two individual compartments. The boundaries between those two compartments were represented by the lower costal margin. The total lung capacity (TLC) was plotted to indicate the operating constraints on the chest wall volumes during cycling and was obtained as the sum of inspiratory capacity and the chest wall volume at the end of expiration.
We devised an a priori definition of post-PR improvement in exercise performance: an increase in the peak workload achieved during the incremental exercise test. We also conducted a post-hoc comparison in which post-PR improvement was defined as a clinically significant increase in the 6MWD.( 16 ) To evaluate the physiological responses to PR at equivalent workloads, we compared metabolic, ventilatory, and symptomatic variables, as well as the operating chest wall volumes, during conditions of quiet breathing, unloaded pedaling, and equivalent workload (50% and maximum), using the shortest cycling test workload as a reference value. Thus, for patients who achieved a higher workload after PR than before ("improvers"), the selected workload was obtained from the pre-PR incremental exercise test, whereas for those who did not ("non-improvers"), the selected workload was obtained from the post-PR test.
Statistical analysis
Results are presented as mean ± SD or as median and ranges for symptom scores and 6MWD. Values at peak exercise were compared by paired t-test and the Wilcoxon test for parametric and non-parametric distribution, respectively. Pre- and post-PR time courses on incremental exercise test were compared at equivalent workload settings with two-way repeated-measures analysis of variance. The level of statistical significance was set at p < 0.05. The estimation of the sample size was impaired because there is lack of studies involving OEP and PR. To our knowledge, there have been only two studies of OEP and PR in COPD,( 1 , 23 ) both of which investigated the effects of PR on operating volumes. Our sample was larger than those evaluated in either of those two studies.
RESULTS
Of the 22 patients evaluated, 15 were male. According to the Global Initiative for Chronic Obstructive Lung Disease staging system,( 16 ) all of the patients had moderate to severe COPD (stage II = 5; stage III = 12; and stage IV = 5), with substantial resting hyperinflation. There were no dropouts. As can be seen in Table 1, the overall pre-PR exercise performance was markedly impaired, with a mean peak workload < 25% predicted. Before PR, the mean peak VO2 showed a modest relationship with FEV1 as a percentage of the predicted value (r = 0.48, p = 0.02). Table 1 also shows that cycle exercise was limited by a combination of dyspnea and leg fatigue, a high mean pre-PR peak minute ventilation (VE)/MVV ratio (88.2 ± 20.1%) suggesting that ventilatory limitation was an important reason for exercise cessation. In addition, the mean pre-PR 6MWD was well below the predicted value (28.7 ± 6.8% of predicted).
Table 1. Demographic characteristics at baseline, together with BMI, spirometry parameters, and lung volumes, as well as exercise performance values (for an incremental exercise test on a cycle ergometer and for the six-minute walk test), before and after pulmonary rehabilitation, in patients with COPD (n = 22).a .
| Variable | Pre-PR | Post-PR | p |
|---|---|---|---|
| Age, years | 65.9 ± 7.1 | ||
| Male gender, n (%) | 15 (68.2) | ||
| BMI, kg/m2 | 24.4 ± 5.9 | 24.7 ± 5.7 | 0.21 |
| FEV1 | |||
| L | 1.00 ± 0.26 | 1.01 ± 0.33 | 0.77 |
| % of the predicted value | 37.1 ± 11.8 | 2.53 ± 0.79 | 0.72 |
| FVC | |||
| L | 2.49 ± 0.68 | 2.53 ± 0.79 | 0.67 |
| % of the predicted value | 66.2 ± 13.5 | 67.3 ± 15.6 | 0.71 |
| FEV1/FVC ratio, % | 42.5 ± 11.8 | 41.5 ± 10.0 | 0.39 |
| IC | |||
| L | 1.74 ± 0.54 | 1.79 ± 0.51 | 0.45 |
| % of the predicted value | 64.2 ± 16.0 | 66.2 ± 16.4 | 0.32 |
| FRC | |||
| L | 6.29 ± 2.10 | 6.10 ± 1.64 | 0.55 |
| % of the predicted value | 189.2 ± 46.3 | 184.2 ± 38.1 | 0.57 |
| TLC | |||
| L | 8.07 ± 2.28 | 7.84 ± 1.75 | 0.49 |
| % of the predicted value | 128.9 ± 23.4 | 125.7 ± 17.7 | 0.51 |
| RV | |||
| L | 5.35 ± 1.91 | 5.18 ± 1.45 | 0.62 |
| % of the predicted value | 239.8 ± 69.4 | 233.9 ± 59.1 | 0.68 |
| RV/TLC ratio | 0.65 ± 0.07 | 0.65 ± 0.07 | 0.92 |
| Peak incremental exercise values | |||
| Workload | |||
| W | 33 ± 21 | 39 ± 20 | 0.02 |
| % of the predicted value | 23.3 ± 13.7 | 29.0 ± 13.2 | 0.01 |
| VO2 | |||
| L/min | 0.74 ± 0.18 | 0.72 ± 0.20 | 0.43 |
| % of the predicted value | 44.7 ± 16.6 | 42.6 ± 14.4 | 0.23 |
| VCO2, L/min | 0.77 ± 0.22 | 0.73 ± 0.25 | 0.15 |
| VE, L/min | 32.8 ± 7.2 | 31.1 ± 9.4 | 0.07 |
| VE/MVV ratio, % | 88.2 ± 20.1 | 83.2 ± 23.5 | 0.04 |
| VT, L | 1.03 ± 0.31 | 1.00 ± 0.35 | 0.34 |
| RR, breaths/min | 31 ± 6 | 30 ± 5 | 0.7 |
| TI, s | 0.81 ± 0.19 | 0.81 ± 0.18 | 0.9 |
| TE, s | 1.25 ± 0.24 | 1.26 ± 0.28 | 0.9 |
| TI/Ttot ratio, % | 39.2 ± 4.6 | 39.2 ± 5.2 | 0.9 |
| SpO2, % | 92 ± 2 | 94 ± 8 | 0.3 |
| Post-6MWT values | |||
| 6MWD | |||
| m, median (range) | 305 (170-425) | 330 (230-490) | 0.001 |
| % of the predicted value | 28.7 ± 6.8 | 33.3 ± 8.0 | 0.001 |
| Borg dyspnea score | 3 (1.3) | 3 (4.6) | 0.8 |
| Borg leg fatigue score | 2 (3.0) | 1 (4.1) | 0.7 |
| SpO2, % | 92 ± 2 | 92 ± 2 | 0.003 |
PR: pulmonary rehabilitation; IC: inspiratory capacity; FRC: functional residual capacity; TLC: total lung capacity; RV: residual volume; VO2: oxygen consumption; VCO2: carbon dioxide production; VE: minute ventilation; MVV: maximal voluntary ventilation; VT: tidal volume; RR: respiratory rate; TI: inspiratory time, TE: expiratory time; Ttot: total respiratory time; 6MWT: six-minute walk test; and 6MWD: six-minute walk distance. aValues expressed as mean ± SD, except where otherwise indicated.
There were no significant post-PR changes in spirometry parameters or lung volumes at rest (Table 1). The mean values for peak ventilation, tidal volume, and respiratory rate were similar before and after PR, as were the intensity of symptoms and the 6MWD (Table 1).
Analyzing the equivalent workload conditions, based on the highest load achieved in the pre-PR incremental exercise test, we found that VO2 and VCO2 were both lower after PR, with a corresponding significant drop in VE, as shown in Figure S1 of the supplementary file (available online at http://www.jornaldepneumologia.com.br/detalhe_anexo.asp?id=45). This post-PR reduction in ventilation was due to decreases in tidal volume (−2.7 ± 20.3%) and respiratory rate (−5.9 ± 16.3%). There was no significant difference between the pre- and post-PR values for the VE/VCO2 slope (31.8 ± 4.1 vs. 33.3 ± 3.8, p > 0.05). Breathlessness and leg fatigue during cycling were also similar before and after PR (p > 0.05 for both).
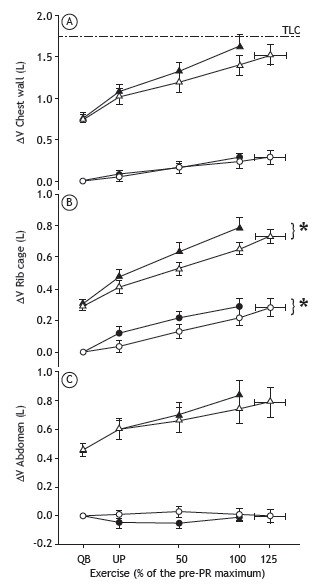
The end-expiratory chest wall volume (EECWV) increased during exercise. At peak exercise, the end-inspiratory chest wall volume (EICWV) approached TLC. The PR program had no effect of the behavior on the chest wall volumes or on the timing of the increase in the EECWV (Figure 1A). Given the lower ventilation after exercise at any given workload, the tidal volume was lower and this is in line with the decrease in EICWV in the isovolumic comparisons. However, the regional distribution of tidal volume did change after rehabilitation, despite a significant reduction in the volume of the rib cage compartment and a non-significant increase in abdominal volume (Figures 1B and 1C).
Figure 1. Changes, before and after pulmonary rehabilitation (PR), in the inspiratory (operating) volume of the chest wall (A), rib cage (B), and abdomen (C) during incremental exercise at an equivalent workload, defined as the percentage of the maximum workload achieved during the pre-PR incremental exercise test. White symbols: before PR; black symbols: after PR; ∆V: volume change from resting state; TLC (---): total lung capacity, measured before PR; triangles: end-inspiratory volumes; circles: end-expiratory volumes; QB: quiet breathing; and UP: unloaded pedaling. *p < 0.05 vs. pre-PR value.
In 16 patients (72.7%), the workload was higher after PR than before (46 ± 17 vs. 36 ± 22 W, p < 0.05). The gender distribution was comparable between the two groups, females accounting for 5 (31.2%) of the 16 patients in the improver group and for 2 (33.3%) of the 6 patients in the non-improver group. Although all of the patients attended the same number of training sessions, as well as having similar anthropometric and spirometric characteristics before PR, the post-PR lung volumes (functional residual capacity and TLC) tended to be higher in the non-improvers (Table 2). There were no significant differences between the pre- and post-PR lung function, within or between subgroups (p > 0.05 for both). The differences in exercise performance between the two subgroups were reflected in the 6MWD (Table 3). However the post-PR change in the response to the incremental exercise test was not very predictive of that, several of the patients in the non-improver group showing considerable increases in their 6MWD. In comparison with the improvers, the non-improvers showed lower metabolic and ventilatory responses to incremental exercise before PR (p < 0.05) and did not show an increase in their whole body VO2 during exercise, as can be seen in Figure S2 of the supplementary file (available online at http://www.jornaldepneumologia.com.br/detalhe_anexo.asp?id=45). In addition, the non-improvers reported greater leg fatigue at maximal exercise in the pre-PR incremental exercise test than did the improvers (p < 0.05).
Table 2. Comparison between COPD patients who improved after pulmonary rehabilitation and those who did not, in terms of baseline age, BMI, spirometry parameters, and lung volumes.a,b .
| Variable at baseline | Post-PR improvers | Post-PR non-improvers |
|---|---|---|
| (n = 16) | (n = 6) | |
| Age, years | 65.5 ± 6.8 | 67.1 ± 8.4 |
| BMI, kg/m2 | 25.1 ± 6.4 | 22.5 ± 4.5 |
| FEV1 | ||
| L | 1.01 ± 0.29 | 1.00 ± 0.19 |
| % of the predicted value | 37.4 ± 12.2 | 36.5 ± 10.1 |
| FVC | ||
| L | 2.40 ± 0.62 | 2.73 ± 0.84 |
| % of the predicted value | 64.3 ± 12.6 | 71.5 ± 15.7 |
| FEV1/FVC ratio, % | 44.1 ± 12.9 | 38.3 ± 7.6 |
| IC | ||
| L | 1.75 ± 0.42 | 1.61 ± 0.47 |
| % of the predicted value | 63.4 ± 12.3 | 56 ± 10.7 |
| FRC | ||
| L | 5.67 ± 2.48 | 6.68 ± 1.02 |
| % of the predicted value | 174.3 ± 54.1 | 204.2 ± 13.0 |
| TLC | ||
| L | 7.42 ± 2.66 | 8.29 ± 1.39 |
| % of the predicted value | 122.1 ± 26.0 | 135.2 ± 10.7 |
| RV | ||
| L | 4.93 ± 2.19 | 5.48 ± 0.82 |
| % of the predicted value | 228.0 ± 82.6 | 242.0 ± 23.7 |
| RV/TLC ratio, % | 64.8 ± 7.3 | 66.7 ± 9.1 |
PR: pulmonary rehabilitation; IC: inspiratory capacity; FRC: functional residual capacity; TLC: total lung capacity; and RV: residual volume. aValues expressed as mean ± SD. bThere were no statistical differences between the two subgroups for any of these variables.
Table 3. Comparison between COPD patients who improved after pulmonary rehabilitation and those who did not, in terms of pre- and post-pulmonary rehabilitation exercise performance on an incremental exercise test and the six-minute walk test.a .
| Variable | Post- PR improvers | Post- PR non-improvers | ||
|---|---|---|---|---|
| (n = 16) | (n = 6) | |||
| Pre-PR | Post-PR | Pre-PR | Post-PR | |
| Peak incremental exercise values | ||||
| Workload | ||||
| W | 36 ± 22 | 46 ± 17* | 27 ± 18 | 21 ± 13† |
| % of the predicted value | 24.8 ± 14.3 | 34.2 ± 10.3* | 19.1 ± 12 | 15.1 ± 9.6 |
| VO2, L/min | 0.76 ± 0.18 | 0.77 ± 0.21 | 0.70 ± 0.19 | 0.60 ± 0.15 |
| VCO2, L/min | 0.81 ± 0.23 | 0.80 ± 0.25 | 0.69 ± 0.20 | 0.53 ± 0.12† |
| VE, L/min | 33.7 ± 8.1 | 33.7 ± 9.7 | 30.4 ± 3.6 | 24.3 ± 4.1* |
| VE/MVV ratio, % | 87.9 ± 18.6 | 87.0 ± 21.2 | 89.1 ± 25.7 | 72.8 ± 28.3* |
| VT, L | 1.08 ± 0.34 | 1.09 ± 0.37 | 0.92 ± 0.20 | 0.76 ± 0.14† |
| RR, breaths/min | 32 ± 6 | 30 ± 6 | 30 ± 3 | 30 ± 4 |
| TI, s | 0.79 ± 0.19 | 0.81 ± 0.18 | 0.86 ± 0.18 | 0.83 ± 0.21 |
| TE, s | 1.24 ± 0.27 | 1.27 ± 0.31 | 1.25 ± 0.15 | 1.22 ± 0.19 |
| TI/Ttot ratio | 0.39 ± 0.04 | 0.39 ± 0.05 | 0.39 ± 0.05 | 0.39 ± 0.06 |
| SpO2, % | 93 ± 2 | 94 ± 2 | 92 ± 2 | 92 ± 1 |
| Post-6MWT values | ||||
| 6MWD | ||||
| m, median (range) | 310 (170-425) | 338 (230-490)* | 285 (190-340) | 290 (230-466) |
| % of the predicted value | 29.8 ± 7.2 | 34.5 ± 7.7* | 25.7 ± 5.0 | 30.2 ± 8.7 |
| Borg dyspnea score, median (range) | 3 (0-5) | 3 (0.5-5) | 3.5 (2-5) | 3 (2-9) |
| Borg leg fatigue score, median (range) | 2 (0-5) | 1 (0-5) | 1.75 (0-4) | 1.5 (0-7) |
| SpO2, % | 92 ± 3 | 90 ± 4* | 92 ± 1 | 88 ± 2 |
PR: pulmonary rehabilitation; VO2: oxygen consumption; VCO2: carbon dioxide production; VE: minute ventilation; MVV: maximal voluntary ventilation; VT: tidal volume; RR: respiratory rate; TI: inspiratory time, TE: expiratory time; Ttot: total respiratory time; 6MWT: six-minute walk test; and 6MWD: six-minute walk distance. aValues expressed as mean ± SD, except where otherwise indicated. *p < 0.05 vs. pre-PR value. †p < 0.05 vs. post-PR improvers.
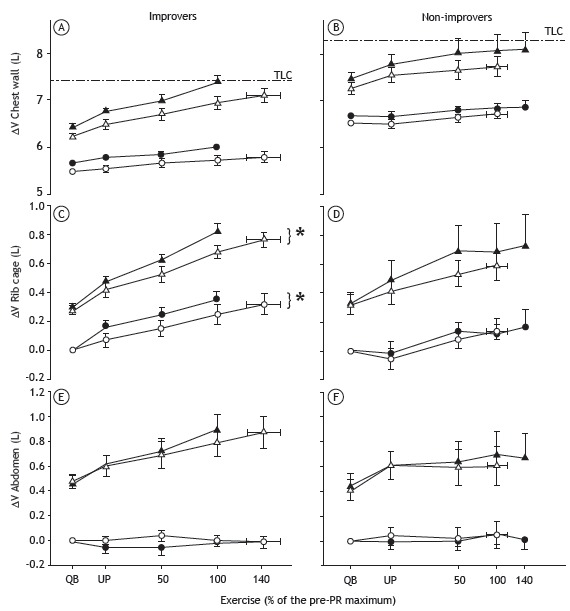
Although the change in end-exercise total chest wall volume under an equivalent workload was no different after PR in the improver group or in the non-improver group (Figures 2A and 2B, respectively), a significant post-PR decrease in rib cage operating volume was seen in the former and not in the latter (Figures 2C and 2D, respectively). In addition, the end-expiratory volume of the abdominal compartment during submaximal exercise was higher in the improvers than in the non-improvers (Figures 2E and 2F, respectively), although the difference did not reach statistical significance. We saw the same changes in end-expiratory lung volume when the response to rehabilitation was categorized by the ability to increase walking distance by 30 m or more, a recently proposed minimum clinically important difference.( 16 ) The ventilatory pattern also differed between the two subgroups, the improvers showing a post-PR change to a less rapid and less shallow breathing pattern at an equivalent VE.
Figure 2. Comparison between COPD patients who improved after pulmonary rehabilitation (PR) and those who did not, in terms of pre- and post-PR changes in the inspiratory (operating) volume of the chest wall (A and B), rib cage (C and D), and abdomen (E and F) during incremental exercise at an equivalent workload, defined as the percentage of the maximum workload achieved during the pre-PR incremental exercise test. White symbols: before PR; black symbols: after PR; ∆V: volume change from resting state; TLC (---): total lung capacity, measured before PR; triangles: end-inspiratory volumes; circles: end-expiratory volumes; QB: quiet breathing; and UP: unloaded pedaling. *p < 0.05 vs. pre-PR value.
DISCUSSION
Most patients with COPD report reduced exercise tolerance and show varying degrees of dynamic hyperinflation during exercise.( 24 , 25 ) That change has been observed even in patients with mild COPD.( 26 ) After PR, the rate of rise of end-expiratory lung volume during exercise appears to decrease, at least in some patients,( 2 , 8 ) whereas it has also been shown that chest wall volumes were reduced after a PR program in which the respiratory rate at an equivalent workload fell.( 1 ) Our data for COPD patients with even more marked resting hyperinflation suggest that this is not always the case, even when an equivalent degree of improvement in workload is achieved. In addition, some of our subjects continued to be limited despite PR, possibly as a consequence of hyperinflation and the accompanying impairment of the extrapulmonary peripheral muscles.
Our patients are similar to those with severe COPD evaluated in other studies, save for their more marked degree of resting hyperinflation, with a mean functional residual capacity of 6.29 L, compared with the 5.56 L reported in the literature,( 1 ) which contributed to their relatively poor exercise performance. Although we followed an incremental exercise test protocol similar to that described by Georgiadou et al.,( 1 ) pre-PR workloads were higher in the subjects evaluated by those authors than in our subjects. Our patients exercised with a high fraction of their MVV at peak exercise, and their end-inspiratory lung volume reached their predicted TLC. After PR, there was a small yet statistically significant increase in peak workload similar to that reported in other studies employing incremental exercise tests,( 1 , 8 ) and that was reflected in a similar improvement in the 6MWD. For a given workload, VO2 and VCO2 both decreased after PR in our patients, suggesting that a true training effect had occurred. However, because the VE/VCO2 slope was not changed after PR, it is likely that the lower ventilation seen at the equivalent workload was a consequence mainly of the lower metabolic stimulus (reduced VCO2).
Like other authors, we observed no relationship between the timing of the change in chest wall volume and the response to PR. In a recent study, we observed that changes in the EECWV during exercise are associated with the presence of paradoxical movement of the lower rib cage at rest.( 27 ) The data obtained in the present study suggest that although this might be undesirable in terms of energy expenditure, it does not influence the ability to achieve a training effect, at least in hyperinflated patients. Although the total EECWV was unaffected by PR, there was a post-PR decrease in the rib cage volumes, with less recruitment of the abdominal compartment during exercise. The end-expiratory abdominal volume remained constant and there was a proportionate decrease in the volume contained within the rib cage compartment at any given workload. Although the EECWV was unaffected by PR in either subgroup, this new behavior in the rib cage and abdominal compartments after PR, resulting in a more physiological pattern and less distortion of the total chest wall, was seen only in the improvers. However, in the study conducted by Georgiadou et al.,( 1 ) who evaluated patients in Athens, Greece, rib cage and abdominal volumes at equivalent workloads both decreased after PR as a result of a change in the breathing pattern and an increase in expiratory time. These discrepancies might reflect differences in the resting lung volumes, our hyperinflated subjects tending to show a reduction in tidal volume rather than in the respiratory rate. Another possible explanation is that the exercise regimes employed may have been different, particularly because some of the patients evaluated by Georgiadou et al.( 1 ) underwent an interval training regime rather than a general physical exercise program.
In the present study, there was a high level of adherence to treatment. Nevertheless, some patients failed to improve in terms of their response to the incremental exercise test or their 6MWD. In general, the non-improvers exercised to a lower peak workload before PR and tended to have more severe resting hyperinflation than did the improvers, although the small numbers of patients in each of the subgroups precluded any inferences regarding statistical significance. More striking was the pre-PR inability of the non-improvers to increase their VCO2 during exercise. Previous studies have suggested that there are differences among patients in terms of the ability of peripheral muscles to increase their VO2 during exercise,( 19 ) which could explain why some COPD patients are limited by peripheral muscle fatigue rather than by ventilatory factors.( 28 - 30 ) Although we did not collect data related to peripheral muscle strength, the fact that the non-improvers reported significantly higher pre-PR levels of muscle fatigue than did the improvers is consistent with such a mechanism. The changes in regional operating lung volumes were confined to the patients who improved their exercise performance, suggesting that such changes were secondary to the reduced overall metabolic drive to breathing at any given workload. After PR, the improvers had a relatively slower and deeper breathing pattern at any VE, which could explain why these patients were able to exercise for longer without further increasing their reported levels of breathlessness. However, whether the change in rib cage volume was a result of a reduction in the activation of the muscles acting on that compartment or a consequence of a reduced central respiratory drive cannot be answered on the basis of our findings in the present study. In contrast, the non-improvers reported higher degrees of muscle fatigue before PR, which were still present at lower absolute workloads after PR. The fact that the non-improvers showed no improvement in VO2 and no decrease in VCO2 could explain why they also showed no post-PR changes in VE or regional chest wall volumes.
Our study has certain limitations. We used a relatively arbitrary threshold to define improvers and non-improvers in terms of the response to the incremental exercise test, given that there is no established minimum clinically important difference for that test. However, our findings were unchanged when we separated patients according to the ability to achieve a clinically important improvement in the 6MWD after PR. In addition, the protocol involved incremental rather than constant-load exercise, which could have decreased its sensitivity to detect post-PR improvements in exercise capacity. Nevertheless, the use of incremental exercise allowed us to analyze variables at different exercise intensities over the course of the test. Furthermore, we did not specifically identify peripheral muscle weakness, although that would be a plausible explanation for the differences we observed. Future studies of COPD patients who do not improve after PR, however they are defined, should include objective measurements of peripheral muscle fatigue. The small number of patients who did not improve after our PR is encouraging given our selection of individuals with significant hyperinflation. Finally, we did not measure ventilatory muscle strength, which could have had some effect on the operating volumes during exercise.
In summary, we have shown that in COPD patients with resting hyperinflation the major effect that PR has on exercise capacity is that of improving VO2 and reducing VCO2, with a commensurate decrease in respiratory drive. The changes in operating lung volumes reflect this reduction in respiratory drive for a given workload. When severe hyperinflation is present, the ability to reduce operating lung volumes with a reduction in respiratory drive is more limited, although subtle changes in the distribution of volume between the rib cage and abdominal compartments could be a useful way to delay the onset of limiting symptoms. Such changes occurred only in patients in whom an objective training effect could be demonstrated. It is encouraging to see that a majority of patients with severe COPD can improve after completing a conventional non-specific exercise program. However, certain patients (those with significant resting hyperinflation) might require a different approach to rehabilitation and those in whom breathlessness or leg fatigue is a dominant symptom at low workloads might require specific peripheral muscle training.
Footnotes
Financial support: None.
Study carried out in the Disciplina de Pneumologia, Instituto do Coração, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil.
References
- 1.Georgiadou O, Vogiatzis I, Stratakos G, Koutsoukou A, Golemati S, Aliverti A. Effects of rehabilitation on chest wall volume regulation during exercise in COPD patients. Eur Respir J. 2007;29(2):284–291. doi: 10.1183/09031936.00121006. [DOI] [PubMed] [Google Scholar]
- 2.Gigliotti F, Coli C, Bianchi R, Romagnoli I, Lanini B, Binazzi B. Exercise training improves exertional dyspnea in patients with COPD: evidence of the role of mechanical factors. Chest. 2003;123(6):1794–1802. doi: 10.1378/chest.123.6.1794. [DOI] [PubMed] [Google Scholar]
- 3.Vogiatzis I, Zakynthinos S. The physiological basis of rehabilitation in chronic heart and lung disease. J Appl Physiol (1985) 2013;115(1):16–21. doi: 10.1152/japplphysiol.00195.2013. [DOI] [PubMed] [Google Scholar]
- 4.Berry MJ, Rejeski WJ, Adair NE, Zaccaro D. Exercise rehabilitation and chronic obstructive pulmonary disease stage. Am J Respir Crit Care Med. 1999;160(4):1248–1253. doi: 10.1164/ajrccm.160.4.9901014. [DOI] [PubMed] [Google Scholar]
- 5.Troosters T, Gosselink R, Decramer M. Exercise training in COPD: how to distinguish responders from nonresponders. J Cardiopulm Rehabil. 2001;21(1):10–17. doi: 10.1097/00008483-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner CF, Wasserman K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis. 1991;143(1):9–18. doi: 10.1164/ajrccm/143.1.9. [DOI] [PubMed] [Google Scholar]
- 7.Vogiatzis I. Strategies of muscle training in very severe COPD patients. Eur Respir J. 2011;38(4):971–975. doi: 10.1183/09031936.00075011. [DOI] [PubMed] [Google Scholar]
- 8.Porszasz J, Emtner M, Goto S, Somfay A, Whipp BJ, Casaburi R. Exercise training decreases ventilatory requirements and exercise-induced hyperinflation at submaximal intensities in patients with COPD. Chest. 2005;128(4):2025–2034. doi: 10.1378/chest.128.4.2025. [DOI] [PubMed] [Google Scholar]
- 9.Puente-Maestu L, Abad YM, Pedraza F, Sánchez G, Stringer WW. A controlled trial of the effects of leg training on breathing pattern and dynamic hyperinflation in severe COPD. Lung. 2006;184(3):159–167. doi: 10.1007/s00408-005-2576-x. [DOI] [PubMed] [Google Scholar]
- 10.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 11.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 12.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 13. ERS Task Force, Palange P, Ward SA, Carlsen KH, Casaburi R, Gallagher CG, et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29(1):185–209. doi: 10.1183/09031936.00046906. [DOI] [PubMed] [Google Scholar]
- 14.Roca J, Burgos F, Sunyer J, Saez M, Chinn S, Antó JM. References values for forced spirometry. Group of the European Community Respiratory Health Survey. Eur Respir J. 1998;11(6):1354–1362. doi: 10.1183/09031936.98.11061354. [DOI] [PubMed] [Google Scholar]
- 15.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8(3):492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 16.Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons WJ, Fruchter N, Sloan S, Levy RD. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil. 2001;21(2):87–93. doi: 10.1097/00008483-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Koch B, Schäper C, Ittermann T, Spielhagen T, Dörr M, Völzke H. Reference values for cardiopulmonary exercise testing in healthy volunteers: the SHIP Study. Eur Respir J. 2009;33(2):389–397. doi: 10.1183/09031936.00074208. [DOI] [PubMed] [Google Scholar]
- 20.Cala SJ, Kenyon CM, Ferrigno G, Carnevali P, Aliverti A, Pedotti A. Chest wall and lung volume estimation by optical reflectance motion analysis. J Appl Physiol (1985) 1996;81(6):2680–2689. doi: 10.1152/jappl.1996.81.6.2680. [DOI] [PubMed] [Google Scholar]
- 21.Aliverti A, Stevenson N, Dellacà RL, Lo Mauro A, Pedotti A, Calverley PM. Regional chest wall volumes during exercise in chronic obstructive pulmonary disease. Thorax. 2004;59(3):210–216. doi: 10.1136/thorax.2003.011494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker PP, Burnett A, Flavahan PW, Calverley PM. Lower limb activity and its determinants in COPD. Thorax. 2008;63(8):683–689. doi: 10.1136/thx.2007.087130. [DOI] [PubMed] [Google Scholar]
- 23.Romagnoli I, Gigliotti F, Lanini B, Bruni GI, Coli C, Binazzi B. Chest wall kinematics and breathlessness during unsupported arm exercise in COPD patients. Respir Physiol Neurobiol. 2011;178(2):242–249. doi: 10.1016/j.resp.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 24.O'Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770–777. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 25.Calverley PM. Dynamic hyperinflation: is it worth measuring? Proc Am Thorac Soc. 2006;3(3):239–244. doi: 10.1513/pats.200508-084SF. [DOI] [PubMed] [Google Scholar]
- 26.Ofir D, Laveneziana P, Webb KA, Lam YM, O'Donnell DE. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(6):622–629. doi: 10.1164/rccm.200707-1064OC. [DOI] [PubMed] [Google Scholar]
- 27.Aliverti A, Quaranta M, Chakrabarti B, Albuquerque AL, Calverley PM. Paradoxical movement of the lower ribcage at rest and during exercise in COPD patients. Eur Respir J. 2009;33(1):49–60. doi: 10.1183/09031936.00141607. [DOI] [PubMed] [Google Scholar]
- 28.Saey D, Debigare R, LeBlanc P, Mador MJ, Cote CH, Jobin J. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(4):425–430. doi: 10.1164/rccm.200208-856OC. [DOI] [PubMed] [Google Scholar]
- 29.Man WD, Soliman MG, Gearing J, Radford SG, Rafferty GF, Gray BJ. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(5):562–567. doi: 10.1164/rccm.200302-162OC. [DOI] [PubMed] [Google Scholar]
- 30.Aliverti A, Macklem PT. The major limitation to exercise performance in COPD is inadequate energy supply to the respiratory and locomotor muscles. J Appl Physiol (1985) 2008;105(2):749–751. doi: 10.1152/japplphysiol.90336.2008. [DOI] [PubMed] [Google Scholar]


