Introduction
Middle-Ear (ME) pressure-regulation (MEPR) is a homeostatic mechanism serving to maintain the ME-ambient pressure-gradient within the narrow, zero-centered, range that maximizes hearing and preserves ME health1. Because the ME is a temperature stable, relatively non-collapsible, biological gas pocket, its pressure is determined primarily by the concentration of contained gases. Under physiologic conditions, the N2 pressure in the ME at ambient total pressure exceeds that of the local blood by about 600 daPa, a pressure-gradient that drives the diffusive, transmucosal (transMEM), net ME to blood exchange of N22,3 at a rate dependent on certain physiochemical constants for N2 and ME tissues, the existing ME-blood N2 pressure-gradient and the volume-rate of ME mucosal blood-perfusion1. Importantly, hearing acuity progressively deteriorates with decreasing ME pressure and at a critical ME gauge pressure of approximately -250 daPa, ME mucosal inflammation, vascular disruption and the accumulation of capillary fluids within the normally gas-filled ME occur4-7. Usually this pathology is prevented by periodic, muscle-assisted Eustachian tube (ET) openings which establish a gas-phase communication between the ME and nasopharynx8. During tubal openings, existing ME-nasopharyngeal total pressure-gradients are decreased by gradient-driven gas flow between the ME and nasopharynx1.
From these considerations, ME pressure is flow regulated such that, over a time-interval specific for each ear, the N2 volume lost from the ME to the local blood is balanced by an equivalent volume of mixed gases supplied to the ME gas during all ET openings that occur within the interval1. MEPR efficiency is inversely related to the length of the time-interval required for these opposing processes to achieve balanced gas volume transfers, with very short intervals characterizing ears that continuously maintain a near-0 daPa ME-ambient pressure-gradient and infinitely long intervals characterizing ears with persistent mucosal inflammation/effusion. Consequently, the efficiency of MEPR can be increased by effecting an increased frequency of ET openings and/or an increased ET conductance defined as the volume gas transfer per driving-pressure per ET opening, or, conversely, by effecting a decreased transMEM N2 exchange-rate.
Past experiments showed that the standardized rate for transMEM exchange of N2 and other inert gases such as N2O, the transMEM N2 conductance, is perfusion-limited and, thus, directly proportional to the rate of ME mucosal blood-perfusion9-11. Consequently, it is expected that, at a fixed ET opening frequency and conductance, MEPR efficiency can be modulated by interventions that affect mucosal blood-perfusion, either upgraded by decreasing perfusion or downgraded by increasing perfusion. This possibility was explored in a recent study that reported a decrease in the transMEM conductance of the inert gas, N2O, following systemic administration of the vasoconstrictor, pseudoephedrine HCL, to adults12.
Studies in monkeys reported that topical exposure of the nasal mucosa to certain inflammatory mediators such as histamine and PGD2 affected ME pressure, mucosal blood flow rate and transMEM N2O conductance13,14. This broad response of the continuous nasal-ET-ME mucosa to a localized regional stimulation is predicted by the “unified airway” model which links diffuse inflammation over the continuous nasal, sinus, ME and tracheobronchial tree mucosa in response to an organ-specific insult15,16. It was hypothesized that treatment of the nasal mucosa with a topical vasoconstrictor would decrease the blood-perfusion rate for the ME mucosa thus effecting a decreased transMEM inert gas conductance for the ME.
Here, that hypothesis is tested by measuring the transMEM inert gas conductance for adult humans17-19 within the context of a randomized, double-blind, cross-over design with measurements done after topical treatments of the nasal mucosa with saline (placebo) and oxymetazoline (Afrin) at a standard clinical dose.
Materials and Methods
Study Population
The protocol was approved by the IRB at the University of Pittsburgh. Healthy adults, recruited by advertisement signed an informed-consent for study participation. Consented subjects provided medical and surgical histories and a comprehensive history for ME diseases, comorbidities and predisposing conditions. They had an Ear Nose and Throat (ENT) examination with bilateral pneumatic otoscopy and tympanometry (Titan, Eden Prairie, MN) and women had a urine pregnancy test. Pregnant women and persons with a past adverse reaction to oxymetazoline or gas-mixtures containing N2O, or a history of chronic illness, having taken prescription medication within the previous month (except birth control) or oral/nasal decongestants within the 2 weeks before a test session, or had extant unilateral or bilateral otitis media or low tympanic membrane compliance or symptoms/signs of extant nasopharyngeal disease were excluded.
Protocol
This was a randomized, double-blind, placebo-controlled, cross-over study requiring that participants complete paired N2O breathing sessions at a minimum 1-week interval. Metered pump nasal spray bottles delivering 0.1ml/spray (Health Care Logistics Inc., Circleville, OH) were prepared by a pharmacist and identified only by the study and visit numbers to blind both investigators and subjects to delivered treatment. At presentation, the subject provided an interval history for medication use, ME and upper respiratory diseases and had a brief ENT examination. Then, the subject was seated comfortably in an examination chair and three sprays of either oxymetazoline hydrochloride 0.05% (0.05mg/spray – Major Pharmaceuticals, Livonia, MI) or 0.9% NaCl were delivered through each nostril at 1-minute intervals. The subject was fitted with the finger probe of a pulse oximeter (Massimo RDS1), the cuff of an automated blood-pressure monitor (Critikon Dynamap 1846SX) and a Rudolph Nasal & Mouth Breathing Silicone Face Mask (Model 8900, Kansas, USA) with a two-way non-rebreathing valve and surface EMG electrodes were placed on the skin over the submental muscles to detect swallowing-related muscle activity. The chair was reclined to about 30 degrees to the horizontal. Three gas sources could be placed “on-line” to the mask, room air, 50%N2O:50%O2 and 100%O2.
Figure 1 shows the procedure timing for a “standard” session. The session was begun after dosing and consisted of a 20-minute acclimation period (Period 1, room-air breathing), a 20-minute experimental period (Period 2, 50%O2:50% N2O breathing) and a 10-minute recovery period (Period 3, 100% O2 breathing). During Period 2, subjects were asked to refrain from swallowing. Throughout, the EMG was continuously recorded, bilateral ME pressures were recorded by tympanometry at 1-minute intervals, blood O2 saturation, heart-rate and blood-pressure were recorded every 5 minutes. At the end of Period 3, the subject was observed for 20 minutes, given a brief physical examination and dismissed if recovered from the N2O breathing. At least 1-week later, the experiment was repeated using the crossover nasal spray.
Figure 1.
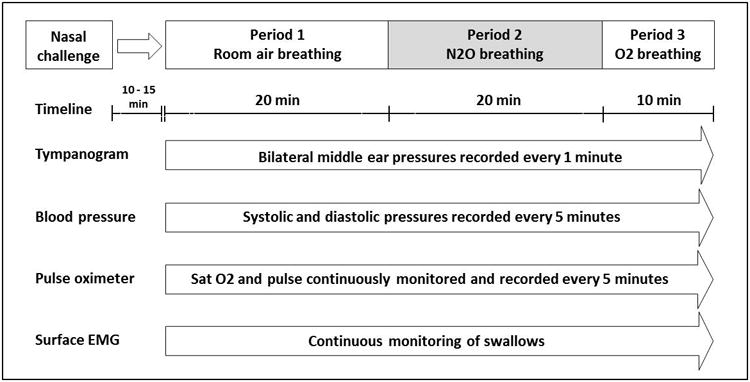
Overview detailing the activity timing for a typical session.
Data Analysis
For each session, the average blood O2 saturation, heart-rate and systolic and diastolic blood-pressures were calculated for Periods 1 and 2 and used as summary variables in the analysis. The EMG was examined and times with patterns suggestive of a swallow identified. For each minute interval that included swallowing, the possibility that the swallows were accompanied by functional ET openings was evaluated by calculating the Fractional Gradient Equilibrated (FGE) which is a measure of ET opening efficiency and defined as the difference between the measured pre-swallow and post-swallow ME pressures divided by the pre-swallow ME pressure ranging from 0, no gas transfer, to 1, complete gradient equilibration20 . ME pressure-time functions at 1-minute resolution were constructed for Period 2 and examined to identify the longest linear segment uninterrupted by pressure change identified as a functional ET opening. The slope of the identified linear segment was calculated by linear regression and used in the analysis as a measure of the standardized transMEM N2O exchange-rate free of any confounding effects of ET openings. The effects of Treatment (Oxymetazoline, placebo), Test Session (1, 2) and Period (room air, N2O) on the vital-signs measures were evaluated using repeated-measures ANOVAs operating on the blood O2 saturation, heart-rate and the systolic and diastolic blood-pressure data. Similarly, the effects of Treatment (oxymetazoline, placebo), Test Session (1, 2), Ear (right, left) and ME Disease History (positive, negative) on the ME pressure-time slope were evaluated for significance using a repeated-measures ANOVA. Period 2 active ET opening efficiency was defined as the average FGE for all swallows occurring at me gauge pressures ≥20 daPa. The NCSS 2007 statistical package, Kaysville, Utah, was used for statistical analyses and the format average ± standard deviation is used consistently.
Results
Thirty-two subjects were screened, 4 were disqualified for not meeting the inclusion/exclusion criteria. Of the 28 enrolled subjects, 4 completed the first session only and were lost to follow-up. Also, 1 subject panicked at the onset of N2O breathing and discontinued Session 2, 1 subject laughed/cried and 2 subjects repeatedly swallowed throughout both sessions making their ME pressure-time functions uninterpretable. The remaining 20 subjects (average age=30.7±7.6 years; 9 Males; 16 white, 3 black and 1 other race; 10 with and 10 without a history for OM) completed the 2 test-sessions.
The average values for each of the 4 vital signs over Periods 1 and 2 of both sessions were analyzed by repeated measures ANOVA to determine the significance of differences among Subjects and between Sessions, Breathing Periods and Treatments. Those analyses documented the expected differences among subjects in the systolic and diastolic blood-pressures and pulse (all P<0.01), the expected difference (P=0.03) in blood O2 saturation between Period 1, while breathing room air (96.7±0.6%), and Period 2 while breathing the 50%O2:50% N2O mixture (99.9±0.3%), and between-Treatment differences in the systolic (Oxymetazoline=109±8, placebo=107±8 mmHg; P<0.01) and diastolic (Oxymetazoline=72±7, placebo=69±7 mmHg; P<0.01)) blood-pressures.
Figure 2 shows the ME pressure-time functions for the left and right ears of Subject 6408 recorded at the placebo and active treatment sessions. All 4 functions consisted of a sequential series of parallel, positive-sloped linear segments separated by rapid, low-magnitude, “toward 0” shifts indicative of functional ET openings. The positive-sloped linear segments for each function were isolated and the slope for the longest segment was calculated to yield a measure of the transMEM N2O exchange-rate. There was no evidence that treatment affected displacement magnitude as evidenced by the similar FGE values during the active, 0.14±0.11, and placebo, 0.12±0.08, treatment sessions, P>0.35. In this example and typical for the other experiments, the functions for the 2 ears had similar slopes with left-right correlation coefficients of 0.80 and 0.50 for the active and placebo sessions, respectively.
Figure 2.
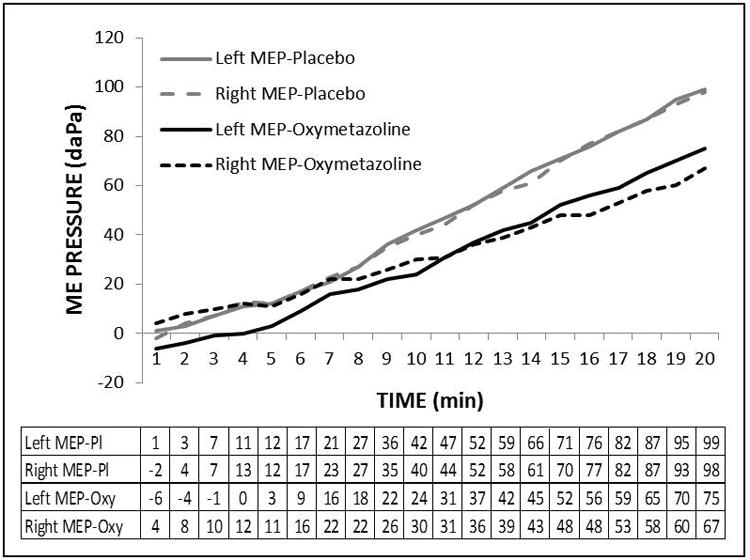
Example ME pressure-time functions for the left (solid lines) and right (broken lines) ears of Subject 6408 recorded during the placebo (gray line) and active (black line) treatment sessions and the tabulated source data for time (min.) and ME pressure (daPa). Note that the lines for the two ears at both sessions were parallel indicating similar slopes and that slopes of the lines for the active treatment were more shallow than those for the placebo treatment. Also, note that each line consisted of a series of roughly parallel, linear segments of positive slope interrupted by discrete displacements in the direction of 0 daPa pressure indicative of a pressure-regulating ET opening. The slope used in the analysis was extracted from the longest linear segment for each function by linear regression.
The Table reports the results for the repeated measures ANOVA evaluating the significance of the differences in the ME pressure-time slopes among subjects and between ears, sessions, OM histories and treatments. There, significant differences among subjects and between Treatments (placebo slope=5.70±3.58, Oxymetazoline slope=4.64±2.38 daPa/min) were documented. The effect on slope is depicted visually for individual ears in Figure 3 as a scatterplot of the paired slopes recorded during oxymetazoline treatment as a function of the respective slopes recorded during the saline treatment. Those paired slopes for the individual ears were linearly related by the regression equation; Oxymetazoline slope = 0.49 × Placebo slope + 1.87 (r2=0.54). Note that a majority of the data points lay below the line of identity for the placebo treatment documenting lesser ME pressure-time slopes for the oxymetazoline treatment.
Table.
ANOVA results for the ME Pressure-Time Slopes listing the sources of variation and, for each source, the associated degrees of freedom (df), sum of squares (SSQ), mean square (MSQ), F-Ratio and probability level (P-Value).
| Source | df | SSQ | MSQ | F-Ratio | P-Value |
|---|---|---|---|---|---|
| Individual | 17 | 481.2 | 28.31 | 6.95 | <0.01* |
| Ear | 1 | 0.2 | 0.21 | 0.01 | 0.93 |
| Session | 1 | 0.4 | 0.35 | 0.09 | 0.77 |
| History of ME Disease | 1 | 1.9 | 1.93 | 0.07 | 0.80 |
| Treatment | 1 | 22.6 | 22.64 | 5.56 | 0.02* |
| Error | 58 | 236.1 | 4.07 | ||
| Total (Adjusted) | 79 | 742.2 | |||
| Total | 80 |
Significant at alpha ≤ 0.05
Figure 3.
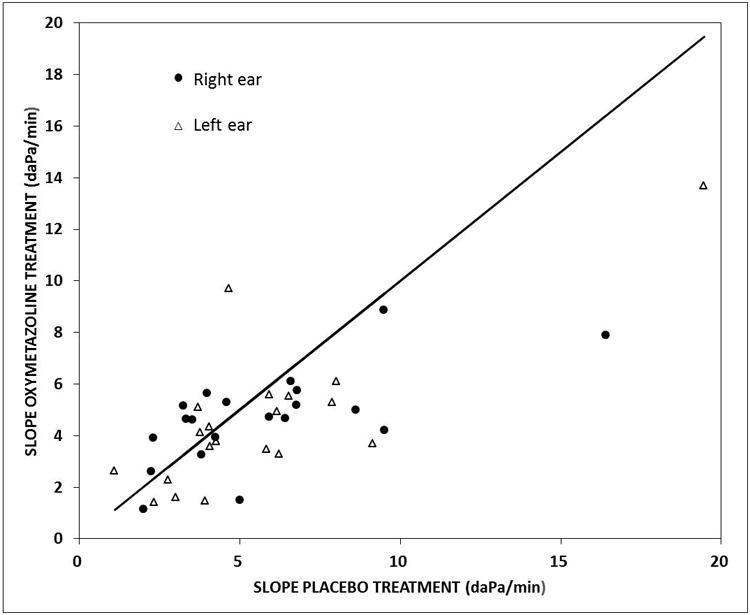
Scatterplot of the ME Pressure-Time slopes recorded after oxymetazoline treatment as a function of the paired slopes recorded after the placebo treatment for Right (solid circles) and Left (open triangles) ears. The solid line is the line of identity for the placebo slopes.
Because the oxymetazoline treatment affected both the systemic blood-pressure and the ME pressure-time slope, it is possible that the latter was mediated by the former. Coupled mediation requires that the proposed mediator, blood-pressure, and response, ME pressure-time slope, share information quantifiable as the square of the correlation coefficient. To explore this possibility, the Pearson product-moment correlation coefficient between the placebo-active difference for each blood-pressure parameter and that difference for the ME pressure-time slope was calculated. Correlation coefficients between the active-placebo difference in blood-pressure and ME pressure-time slope were 0.08 (t-value=0.52, P=0.61) for the systolic and 0.16 (t-value=0.97, P=0.34) for the diastolic blood-pressure, thus rejecting directional mediation.
Discussion
Maintaining the ME-ambient pressure-gradient at near 0 daPa, i.e. efficient MEPR, is prerequisite both to normal ME function in hearing and to preventing certain ME pathologies8. Most past studies of MEPR focused on the pressure-regulating role played by active ET openings with the contribution to MEPR of transMEM gas exchange only explored in more recent modeling1 and animal experiments18,19. The latter studies show that the standardized rate of transMEM N2 exchange prescribes the minimum gas volume that needs to be transferred to the ME during ET openings for efficient MEPR, or more simply, the transMEM N2 exchange-rate defines the demand placed upon the ET for ME volume gas supply. By consequence, MEPR efficiency can be improved by increasing the ME gas supply (e.g. increased frequency of ET openings, increased volume gas transfer per opening) or, under most conditions, by decreasing the ME gas demand (e.g. by decreasing the transfer rate of N2 from ME to local blood).
Pharmacological modulation of the ME gas demand to improve MEPR is a relatively new concept. One recently published human experiment showed that pharmacologically induced mucosal vasoconstriction decreased the ME gas demand and, specifically, that oral administration of a single standard dose of pseudoephedrine HCL, a drug with well characterized vasoconstrictive effects, decreased the basal transMEM N2O exchange-rate by approximately 33%12. That effect was attributed to a decreased ME mucosal blood perfusion rate consequent to the decreased ME mucosal blood volume under conditions of drug-induced vasoconstriction. Noting that the standardized transMEM exchange-rate for inert gases such as N2O and N2 is perfusion-limited, that rate scales directly to the respective tissue solubilities of the gases (relative solubility in water based tissues for N2/N2O ≈ 1:33), the existing transMEM partial-pressure gradient and the mucosal blood perfusion rate, decreasing the mucosal blood perfusion rate effects a decrease in the transMEM exchange-rate10,17. Using similar methods, the present study documented a similar effect on the transMEM N2O exchange-rate of topically applying the vasoconstrictor, oxymetazoline, to the nasal mucosa. Specifically, topical treatment effected an approximate 19% decrease in the basal transMEM N2O exchange-rate for the ME.
The exact mechanism coupling the observed ME response to a nasal treatment is not known. For example, in this experiment, increased systemic blood-pressure was noted following the active treatment, an effect possibly explained by drug absorption into the systemic circulation with the implied possibility of widespread re-distribution and distal actions or as a feedback response to the regional increases in vascular resistance. Some mechanistic insight is gained from the correlation analysis which showed that the blood-pressure and ME responses were independent and by simple calculations documenting extremely dilute blood oxymetazoline concentrations even if the locally applied dose was completely absorbed. More reasonably, the ME effects of topical intranasal drug delivery represents a broad response of the continuous nasal-ET-ME mucosa to a localized stimulus, an effect presumed by those who recommend topical nasal decongestants to improve or prevent situational ET dysfunction21-23. That mechanism is also supported by empirical data for monkey experiments documenting changes in certain measures of ME mucosal blood perfusion after exposing the nasal mucosa to inflammatory substances13,14 and is predicted by the “Unified Airway” theory15,16.
Irrespective of mechanism, the results for this and the previous study demonstrate the feasibility of pharmacologically modulating the transMEM N2 exchange-rate, an intervention with potential clinical applications. This is illustrated in Figure 4 which shows ME gauge pressure-time trajectories predicted by a mathematical model of MEPR1 whose parameters were calibrated to their known/measured values while varying the ET opening efficiency expressed by the FGE (a) or the mucosal blood perfusion rate expressed as the inverse of the time to complete replacement of one mucosal blood volume (b). The horizontal line at -250 daPa represents the critical ME gauge pressure necessary for the development of mucosal inflammation and the accumulation of ME effusion6,7.
Figure 4.
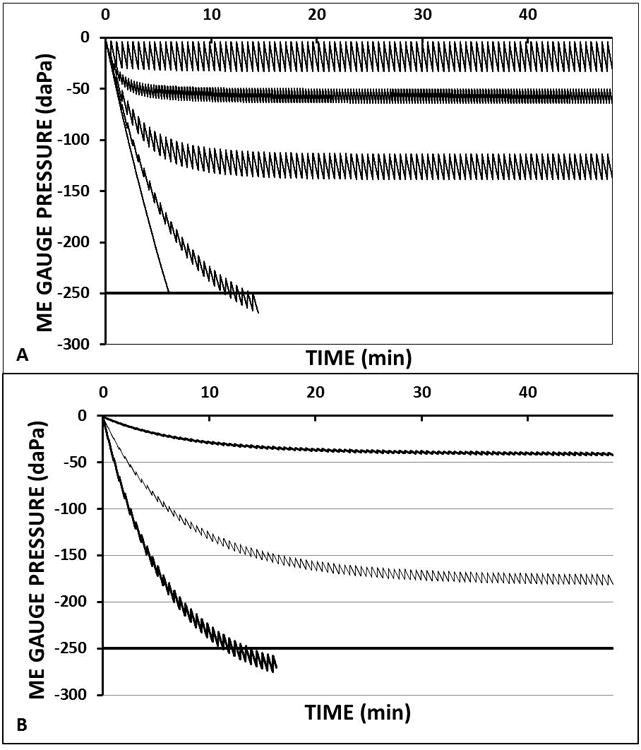
Time trajectories for the middle-ear gauge pressures predicted by a standard mathematical model of middle-ear pressure regulation1 while holding most parameters constant but varying (A) the ET opening efficiency measured as the fractional gradient (FGE=1.0, 0.5, 0.2, 0.07 and 0.0 from least to greatest exponential slopes) at a fixed ET opening frequency of 1/30 minutes and mucosal perfusion rate of 1 mucosal blood volume replaced per second (Q=1) and (B) varying mucosal perfusion represented by the number of mucosal blood volumes replaced in 1 second (Q=1, Q=0.5, Q=0.01 from greatest to least exponential slopes) at a fixed FGE of 0.07 and ET opening frequency of 1/30 minutes. Solid horizontal line at -250 daPa defines the critical middle-ear gauge pressure for the development of mucosal inflammation and effusion. For all simulations, fixed constants were taken from the literature for blood and for the tissue (water), middle ear volume was set to 5 ml and the mucosal surface area was calculated from the measured surface area/volume ratio for the tympanum and mastoid of 1626.
Figure 4a shows predicted ME gauge pressure trajectories at different FGEs. In general, those trajectories are characterized by low magnitude oscillations about a negative exponential trend line. For that function, the absolute value of the slope increased and of the asymptote decreased with decreasing FGE. Two trajectories, characterized by FGEs of 0.07 (extremely inefficient ET opening) and 0.0 (no ET openings), crossed the critical value for pathology. Note that the absolute difference between a trajectory's asymptote and a 0 ME gauge pressure scales directly to the predicted magnitude of hearing loss and that trajectories crossing the critical value predict the development of ME pathology and a conductive hearing loss on the order of 40 dB Hl. Figure 4b shows the effect of changing the ME mucosal blood perfusion rate on the pathological trajectory characterized by an FGE of 0.07. There, reducing the mucosal blood perfusion rate progressively decreased the absolute value of the slope and increased the asymptotic ME gauge pressure, effects with the expected consequences of preventing ME pathology and preserving more “normal” hearing.
This model shows that downgraded MEPR caused by low, but not 0, ET opening efficiency can be partly restored by an intervention that decreases the transMEM inert gas exchange-rate as for, example, treatment with vasoactive drugs12. While provocative, the introduction of any such treatment to improve MEPR with the goal of reducing the morbidity burden attributable to hearing loss and ME pathology in “at risk” populations defined by constitutively low ET opening efficiency is premature and overly ambitious. There, any effective treatment would need be long-term which is unacceptable given the expected side-effects and loss of activity for any biologically active drug as, for example, the risks for rhinitis medicamentosa and “rebound” vasodilation during long-term topical nasal vasoconstrictors24, and the unknown, but potentially significant, risks associated with continuous suppression of systemic or, preferably, local inert gas transMEM gas-exchange. More acceptable is the restriction of this “treatment” strategy to individuals evidencing a self-limited period of downgraded MEPR secondary to the compromised ET opening efficiency occurring during an upper respiratory tract infection25.
An alternative hypothesis suggested to explain the effect of oxymetazoline on the ME pressure-time slope involves a decreased mucosal vascular volume accompanied by a consequent increase in ME gas volume after oxymetazoline treatment. There, by Boyle's law, at identical blood to ME N2O transfer-rates, ME gas pressure will change more slowly for larger volume MEs (oxymetazoline treatment) when compared to smaller volume MEs (placebo treatment) irrespective of a drug effect on mucosal blood perfusion. However, a consideration of the two relevant volumes suggests that any reasonable change in vascular volume would have a negligible effect on ME gas volume. This hypothesis was tested using the mathematical model discussed above and calibrated to “normal” conditions. Simulations of the experiment showed that, for non-inflamed MEs, a complete collapse of the mucosal vascular volume would decrease the pressure-time slope by less than 1%. Moreover, even an unreasonably large increase in a typical ME volume (7 ml) of 1 ml would only decrease the ME pressure-time slope by 12% which is less than the 19% change measured in this study. Consequently, we reject the alternative hypothesis as an explanation of the observed effects.
In summary, topical, nasal administration of the vasoconstrictive drug, oxymetazoline, decreased the transMEM N2O exchange-rate for the ME, an effect similar to that following oral administration of Pseudoephedrine. Because the transMEM exchange of inert-gases is perfusion-limited, this effect is expected to also characterized the transMEM N2 exchange-rate, the primary determinant of the ET opening efficiency required for normal hearing. These and other drugs with similar effects on mucosal blood perfusion could find use in preventing the consequences of short-duration, situational ET dysfunction.
Acknowledgments
This study was supported in part by a grant from the National Institutes of Health, P50 DC007667. The authors thank Dr. J. Douglas Swarts for his input into the study design and data interpretation, Dr. Ellen Mandel for preparing the necessary IRB submissions and related correspondences, and Mr. James T. Seroky, Ms. Julianne Banks and Ms. Jenna El-Wagaa for assisting with subject recruitment and testing.
Funding: NIH Grant DC007667
Footnotes
Financial Disclosure Information: The authors have no financial disclosures.
Conflict of Interest Statement: None of the listed authors have any real or apparent conflicts of interest with respect to the material presented in this manuscript.
References
- 1.Doyle W, Rosowski J, Merchant S, editors. Middle ear pressure regulation. The Hague, The Netherlands: Kugler Publications; 2000. The Function and Mechanics of Normal, Diseased and Reconstructed Middle Ears; pp. 3–21. [Google Scholar]
- 2.Hergils L, Magnuson B. Human middle ear, gas composition studied by mass spectrometry. Acta Otolaryngol. 1990 Jul-Aug;110(1-2):92–99. doi: 10.3109/00016489009122520. [DOI] [PubMed] [Google Scholar]
- 3.Hergils L, Magnuson B. Middle ear gas composition in pathologic conditions: mass spectrometry in otitis media with effusion and atelectasis. Ann Otol Rhinol Laryngol. 1997 Sep;106(9):743–745. doi: 10.1177/000348949710600905. [DOI] [PubMed] [Google Scholar]
- 4.Fria TJ, Saad MM, Doyle WJ, Cantekin EI. Auditory brain stem responses in rhesus monkey with otitis media with effusion. Otolaryngol Head Neck Surg. 1982 Nov-Dec;90(6):824–830. doi: 10.1177/019459988209000626. [DOI] [PubMed] [Google Scholar]
- 5.Casselbrant ML, Cantekin EI, Dirkmaat DC, Doyle WJ, Bluestone CD. Experimental paralysis of tensor veli palatini muscle. Acta Otolaryngol. 1988 Sep-Oct;106(3-4):178–185. doi: 10.3109/00016488809106423. [DOI] [PubMed] [Google Scholar]
- 6.Swarts JD, Alper CM, Seroky JT, Chan KH, Doyle WJ. In vivo observation with magnetic resonance imaging of middle ear effusion in response to experimental underpressures. Ann Otol Rhinol Laryngol. 1995 Jul;104(7):522–528. doi: 10.1177/000348949510400704. [DOI] [PubMed] [Google Scholar]
- 7.Alper CM, Tabari R, Seroky JT, Doyle WJ. Magnetic resonance imaging of the development of otitis media with effusion caused by functional obstruction of the eustachian tube. Ann Otol Rhinol Laryngol. 1997 May;106(5):422–431. doi: 10.1177/000348949710600511. [DOI] [PubMed] [Google Scholar]
- 8.Bluestone CD. Eustachian Tube Structure, Function, Role in Otitis Media. Hamilton, Ontario: BC Decker Inc; 2005. [Google Scholar]
- 9.Doyle WJ, Seroky JT. Middle ear gas exchange in rhesus monkeys. Ann Otol Rhinol Laryngol. 1994 Aug;103(8 Pt 1):636–645. doi: 10.1177/000348949410300811. [DOI] [PubMed] [Google Scholar]
- 10.Doyle WJ, Seroky JT, Alper CM. Gas exchange across the middle ear mucosa in monkeys. Estimation of exchange rate. Arch Otolaryngol Head Neck Surg. 1995 Aug;121(8):887–892. doi: 10.1001/archotol.1995.01890080055011. [DOI] [PubMed] [Google Scholar]
- 11.Doyle WJ, Alper CM, Seroky JT. Trans-mucosal inert gas exchange constants for the monkey middle ear. Auris Nasus Larynx. 1999 Jan;26(1):5–12. doi: 10.1016/s0385-8146(98)00060-1. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira MS, Alper CM, Martin BS, Cullen Doyle BM, Doyle WJ. Oral pseudoephedrine decreases the rate of transmucosal nitrous oxide exchange for the middle ear. Laryngoscope. 2015 Jul 7; doi: 10.1002/lary.25221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuksel S, Doyle WJ, Banks J, Seroky JT, Alper CM. Nasal prostaglandin challenge increases N2O exchange from blood to middle ear. Auris Nasus Larynx. 2005 Mar;32(1):29–32. doi: 10.1016/j.anl.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Seroky JT, Alper CM, Tabari R, Doyle WJ. Effects of intranasal challenge with histamine, bradykinin and prostaglandin on middle ear pressure and blood flow in cynomolgus monkeys. Acta Otolaryngol. 1995 Jan;115(1):83–87. doi: 10.3109/00016489509133352. [DOI] [PubMed] [Google Scholar]
- 15.Krouse JH. The unified airway--conceptual framework. Otolaryngol Clin North Am. 2008 Apr;41(2):257–266. v. doi: 10.1016/j.otc.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Marple BF. Allergic rhinitis and inflammatory airway disease: interactions within the unified airspace. Am J Rhinol Allergy. 2010 Jul-Aug;24(4):249–254. doi: 10.2500/ajra.2010.24.3499. [DOI] [PubMed] [Google Scholar]
- 17.Doyle WJ, Banks JM. Middle ear pressure change during controlled breathing with gas mixtures containing nitrous oxide. J Appl Physiol (1985) 2003 Jan;94(1):199–204. doi: 10.1152/japplphysiol.00634.2002. [DOI] [PubMed] [Google Scholar]
- 18.Doyle WJ. The mastoid as a functional rate-limiter of middle ear pressure change. Int J Pediatr Otorhinolaryngol. 2007 Mar;71(3):393–402. doi: 10.1016/j.ijporl.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alper CM, Kitsko DJ, Swarts JD, et al. Role of the mastoid in middle ear pressure regulation. Laryngoscope. 2011 Feb;121(2):404–408. doi: 10.1002/lary.21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyle WJ, Singla A, Banks J, El-Wagaa J, Swarts JD. Pressure chamber tests of eustachian tube function document lower efficiency in adults with colds when compared to without colds. Acta Otolaryngol. 2014 Jul;134(7):691–697. doi: 10.3109/00016489.2014.892213. [DOI] [PubMed] [Google Scholar]
- 21.Jensen JH, Leth N, Bonding P. Topical application of decongestant in dysfunction of the Eustachian tube: a randomized, double-blind, placebo-controlled trial. Clin Otolaryngol Allied Sci. 1990 Jun;15(3):197–201. doi: 10.1111/j.1365-2273.1990.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 22.Rashid M. Rationale and feasibility of intranasal delivery of drugs to the eustachian tube orifice. Curr Allergy Asthma Rep. 2012 Dec;12(6):541–546. doi: 10.1007/s11882-012-0310-3. [DOI] [PubMed] [Google Scholar]
- 23.Llewellyn A, Norman G, Harden M, et al. Interventions for adult Eustachian tube dysfunction: a systematic review. Health Technol Assess. 2014 Jul;18(46):1–180. v–vi. doi: 10.3310/hta18460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortuaire G, de Gabory L, Francois M, et al. Rebound congestion and rhinitis medicamentosa: nasal decongestants in clinical practice. Critical review of the literature by a medical panel. Eur Ann Otorhinolaryngol Head Neck Dis. 2013 Jun;130(3):137–144. doi: 10.1016/j.anorl.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Doyle WJ, Alper CM. Prevention of otitis media caused by viral upper respiratory tract infection: vaccines, antivirals, and other approaches. Curr Allergy Asthma Rep. 2003 Jul;3(4):326–334. doi: 10.1007/s11882-003-0093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swarts JD, Foley S, Alper CM, Doyle WJ. A cross-sectional study of the change in mastoid geometry with age in children without a history of otitis media. Laryngoscope. 2012 Mar;122(3):649–653. doi: 10.1002/lary.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]


