Abstract
The systemic inflammatory response syndrome (SIRS) follows spinal cord injury (SCI) and causes damage to the lungs, kidney, and liver due to an influx of inflammatory cells from the circulation. After SCI in rats, the SIRS develops within 12 h and is sustained for at least 3 days. We have previously shown that blockade of CD11d/ CD18 integrin reduces inflammation-driven secondary damage to the spinal cord. This treatment reduces the SIRS after SCI. In another study we found that blockade of α4β1 integrin limited secondary cord damage more effectively than blockade of CD11d/CD18. Therefore we considered it important to assess the effects of anti-α4β1 treatment on the SIRS in the lung, kidney, and liver after SCI. An anti-α4 antibody was given IV at 2 h after SCI at the fourth thoracic segment and the effects on the organs were evaluated at 24 h post-injury. The migration of neutrophils into the lungs and liver was markedly reduced and all three organs contained fewer macrophages. In the lungs and liver, the activation of the oxidative enzymes myeloperoxidase (MPO), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and gp91phox, the production of free radicals, lipid peroxidation, and cell death were substantially and similarly reduced. Treatment effects were less robust in the kidney. Overall, the efficacy of the anti-α4β1 treatment did not differ greatly from that of the anti-CD11d antibody, although details of the results differed. The SIRS after SCI impedes recovery, and attenuation of the SIRS with an anti-integrin treatment is an important, clinically-relevant finding.
Keywords: anti-integrin treatment, kidney, liver, lung, organ damage, spinal injury, systemic inflammation
Introduction
The systemic inflammatory response (SIRS) contributes to organ dysfunction after SCI, as it does after brain injury and other traumatic insults (Acosta et al., 1998; Baskaran et al., 2000; Bhatia et al., 2005; Gabay and Kushner, 1999; Ott et al., 1994). In SCI patients, the lung and kidney are major targets of the SCI-induced SIRS (Catz et al., 2002; De-Vivo et al., 1999; O’Connor, 2005; Pickett et al., 2006). The SIRS after central nervous system (CNS) trauma begins with an influx of leukocytes into the liver, secondary to hepatic chemokine expression that occurs within 2 h of injury (Campbell et al., 2003,2005; Perry et al., 2003; Wilcockson et al., 2002). The liver then produces acute phase proteins as well as chemokines that are released into the circulation (Campbell et al., 2005; Wilcockson et al., 2002), initiating a widespread inflammatory response.
In animal models the SIRS following traumatic injury to the brain and spinal cord causes damage to organs such as the lungs, kidney, and liver, due to an influx of inflammatory cells from the circulation (Bao et al., 2011b; Campbell et al., 2003,2005; Gris et al., 2008; Perry et al., 2003; Wilcockson et al., 2002). In a rat model of compression SCI, an inflammatory response in these organs is readily apparent by 12 h after the injury and is sustained for at least 3 days (Bao et al., 2011b; Gris et al., 2008). Indeed, our previous studies showed that SCI causes a leukocytosis with a prominent neutrophilia, activates circulating neutrophils, increases their longevity, and promotes their production of oxygen free radicals (Gris et al., 2008). These neutrophils invade the lung, kidney, and liver from 2 to 24 h after SCI (Bao et al., 2011b; Gris et al., 2008). In addition, resident and invading hematogenous macrophages in the organs are activated. This inflammatory condition within the organs is accompanied by increased activity and expression of oxidative enzymes and proteinases, lipid peroxidation, protein nitration, and cell death. The influx of leukocytes into the organs occurs in part by adhesion molecule-mediated diapedesis entailing binding of leukocyte integrins, such as the α4β1 integrin and the β2 integrin CD11d/ CD18, to their counter-receptors on the endothelial wall (Davenpeck et al., 1998; Kubes et al., 1995; Nandi et al., 2004; Van der Vieren et al., 1999). Our recent study of this process (Bao et al., 2011b) demonstrated that intravenous delivery of a monoclonal antibody to the CD11d subunit of the CD11d/ CD18 integrin at 2 h after SCI at the fourth thoracic segment (T4), caused a significant reduction in the inflammatory response within the lung, kidney, and liver at 12 h post-injury, also limiting the oxidative damage to the lung and kidney. At 12 h, the inflammatory response within the liver was less than in the lung or kidney, and the effect of the anti-CD11d treatment was correspondingly less than in the lung and kidney.
The purpose of the current study was to assess the impact on the post-SCI SIRS of an antibody against the α4β1 integrin. In our previous studies of the intraspinal inflammatory response after SCI, intravenous treatment with an antibody against the α4 subunit of the α4β1 integrin limited intraspinal inflammation and its secondary consequences, and improved neurological outcomes (Fleming et al., 2008). This treatment led to greater tissue sparing in the cord than a similar treatment with the anti-CD11d antibody (Gris et al., 2004). The α4β1 integrin is expressed by rat and human neutrophils, monocytes/macrophages, and lymphocytes, and mediates their extravasation via binding to vascular cell adhesion molecule-1 (VCAM-1) on endothelial cells (Davenpeck et al., 1998; Kubes et al., 1995). This integrin also binds to fibronectin in the extracellular matrix, mediating migration within tissues. The α4β1 integrin participates in leukocyte tethering and rolling, as well as in firm adhesion during the extravasation process, rendering it an excellent target for therapeutic intervention. Antibody binding to α4β1 obstructs its binding to its counter-receptors, and also causes it to internalize within the leukocyte; both mechanisms interfere with its contribution to diapedesis (Fleming et al., 2010; Leone et al., 2003). Indeed, in our laboratory incubation of neutrophils with the anti-α4 antibody blocked their migration across an endothelial layer in transwell assays (Fleming, 2008). Because of the excellent anti-inflammatory and tissue-sparing effects of the anti-α4 antibody in the animal studies, we tested its efficacy on the SIRS at 24 h after SCI at T4 in the rat. The study was done at 24 h rather than 12 h after SCI to optimize detection of liver responses. Some measures of oxidative activity within the liver, such as myeloperoxidase (MPO) activity, are greater at 24 than at 12 h after SCI (Gris, 2007).
Methods
Spinal cord injury and antibody treatment
All protocols for these experiments were done in accordance with the policies established by the Canadian Council on Animal Care. Twenty-eight female Wistar rats (Charles River, St. Constant, Quebec, Canada) weighing 200–220 g were used in this study, and 17 rats received a moderate clip compression SCI as described previously (Bao et al., 2004; Weaver et al., 2001). At 2 h post-injury, the rats were administered either the anti-α4 monoclonal antibody (mAb) (n = 9, clone TA-2, mouse anti-rat α4 immunoglobulin [Ig]G1, 2.5 mg/kg via the tail vein, a gift of BiogenIdec, Cambridge, MA, purchased from Seikagaku America Inc., East Falmouth, MA), or an isotype-matched irrelevant control mAb (n = 8, clone 1E6, mouse anti-human LFA-3 IgG1, BiogenIdec, 2.5 mg/kg), or vehicle (saline). One set of 9 SCI and 6 uninjured rats was used for biochemical assays, and a second set of 8 SCI rats and 5 uninjured rats was used for immunocytochemistry. Control SCI rats were those administered the 1E6 mAb or saline, and uninjured rats were untreated.
Tissue preparation for Western blotting analysis and morphological examination
For morphological examination, uninjured animals (n = 5) and SCI animals (n = 8, 4 control and 4 treated) at 24 h after injury were anesthetized and perfused transcardially with saline, followed by 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.2–7.4. The lungs were perfused separately via the pulmonary artery. The lung (left), liver (right lobe), and kidney (left) were removed, and post-fixed for 24 h at 4°C, then cryoprotected in increasing concentrations of sucrose. A piece of each organ approximately 0.5 cm thick was sampled from the center of the lobe of lung or liver and from the center of the kidney (oriented from hilus to apex) and sectioned into 25-μm sections and placed in buffer for immunohistochemical staining.
For biochemical and Western blotting analyses, uninjured rats (n = 6) and the rats at 24 h post-injury (n = 9, 4 control SCI and 5 treated) were perfused with cold 0.9% NaCl, first transcardially and then via the pulmonary artery. The lung (left), liver (right lobe), and kidney (left) were removed and stored at −80°C until later homogenization for various analyses. Tissue samples were taken from the approximate center of the lobe of lung or liver, and from the center of the kidney. Each piece of the organ was then divided into three parts for different biochemical analyses. All homogenization steps were done with a glass homogenizer on ice. For Western blotting, lung (350 mg) or liver (800 mg) or kidney (600 mg) samples were homogenized and centrifuged, and the supernatant was used for Western blot analysis as described previously (Bao et al., 2004). For measurement of MPO activity, different samples from the organs (lung, 130 mg; liver, 220 mg; kidney, 320 mg) were homogenized with the inclusion of hexadecyltrimethylammonium bromide (to extract the MPO from the neutrophil granules), and centrifuged and the supernatant was used for MPO assay as described previously (Bao et al., 2004). For measurement of thiobarbituric acid reactive substance (TBARS) and free radicals, the tissue samples (lung, 170 mg; liver, 250 mg; kidney, 280 mg) were homogenized and centrifuged, and the supernatant used for TBARS and 2′-7′-dichlorofluorescin diacetate (DCFH-DA) assays as described previously (Bao et al., 2004,2005). The protein concentrations of the samples were determined using the modified Bradford method (Bio-Rad Protein assay kit II; Bio-Rad, Hercules, CA) with bovine serum albumin as standard.
Assessing infiltration of phagocytic leukocytes
Infiltration of phagocytic leukocytes was detected by an activity assay for the oxidative enzyme MPO, by immunohistochemical staining of tissue sections and by Western blotting. For the MPO assay, 10 μL of the tissue homogenates (lung, liver, and kidney) was incubated in a 96-well plate in 100 μL of K-PBS and 100 μL of o-dianosinisidine (12.5 mg per 10 mL distilled water and 9 μL of 30% H2O2). The reaction was stopped by the addition of 100 μL of 1% NaH3 into each well. The plate was scanned using a 96-well plate reader (Multiskan Ascent; Thermo Fisher Scientific, Waltham, MA) at a wavelength of 450 nm. For every plate, one standard curve in triplicate was performed using MPO from human leukocytes (Sigma-Aldrich, St. Louis, MO) (Bao et al., 2004). MPO activity is expressed in units/mg protein.
For immunohistochemical staining, randomly selected sections from the pools of lung, liver, and kidney sections (generated as described above) were processed free-floating for staining as described previously (Weaver et al., 2001). A rabbit anti-rat neutrophil polyclonal antibody diluted 1:20,000 (Anthony et al., 1998; a gift of Dr. Daniel Anthony, Oxford University, Oxford, U.K.) was used to identify neutrophil infiltration. The anti-neutrophil antibody binds to a 56-kDa protein in rat neutrophils. An anti-ED-1 antibody (1:500; Serotec, Raleigh, NC) was used to identify phagocytic macrophages in the tissues. The sections were next incubated overnight with biotinylated donkey anti-rabbit antibody (1:500 dilution; Jackson ImmunoResearch, West Grove, PA). The immunoreactivity was visualized using diaminobenzidine (DAB; Sigma-Aldrich) as a chromogen. The slides were viewed using an Olympus microscope (BX50; Olympus America Inc., Center Valley, PA), and photomicrographs were acquired using a digital camera (Retiga; Quantitative Imaging Corporation, Burnaby, B.C., Canada) and analyzed with Image Pro software version 5.1 (Media Cybernetics, Silver Spring, MD). The sections were examined only qualitatively to affirm that inflammatory cells were present in the organs. Quantitative analyses of neutrophil influx or macrophage activation were done by Western blotting as described below.
ED-1 protein levels in the lung, liver, and kidney were quantified by Western blot analysis. We used the anti-neutrophil antibody in the Western blots as a quantitative estimate of the neutrophil influx. Proteins derived from tissue homogenates were loaded onto 7% or 10% polyacrylamide gels and separated by SDS-PAGE using a Bio-Rad Mini-Protean 3 apparatus, and transferred to polyvinylidene di-fluoride (PVDF) membranes (0.45 μm pore size; Millipore, Mississauga, Ontario, Canada). The membranes were first blocked with 5% non-fat powdered milk, and then incubated with the ED-1 antibody or the anti-neutrophil antibody, followed by incubation with horseradish peroxidase (HP)-conjugated donkey anti-mouse secondary antibody. The signal was developed using an enhanced chemiluminescence (ECL plus) detection system (Amersham, Oakville, Ontario, Canada). Band intensity was measured using Lab Works software (UVP, Upland, CA). Densitometric values were normalized for protein loading using β-actin (antibody from Sigma-Aldrich) as a loading control and for local background. Molecular weights of the proteins detected were determined using known molecular weight protein standards (BioRad Prestained Precision Protein Standards). Western blots with the anti-neutrophil antibody yielded a clear band at 56 kDa.
Assessing oxidative enzymes
Oxidative enzymes in lung were detected and quantified by Western blot analysis with an antibody raised against the catalytic subunit (gp91phox) of the nicotinamide adenine di-nucleotide phosphate (NADPH) oxidase (mouse anti-rat gp91phox, 1:500; Upstate Biotechnology, Lake Placid, NY) using methods described previously (Bao et al., 2004). Expression levels of two additional oxidative enzymes, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), were also assayed by Western blot analysis with polyclonal rabbit anti-iNOS (Oxford Biomedical Research, Oxford, MI), and polyclonal rabbit anti-COX-2 (Cayman Chemical, Ann Arbor, MI) antibodies using the methods described above (Bao et al., 2004).
Assessing free radical production (DCFH-DA assay)
To assess free radical production in lung, liver, and kidney, we used DCFH-DA as a probe for free radical detection, and the resulting DCF formation was monitored by the fluorescence intensity (Bao et al., 2005). DCFH-DA is hydrolyzed to DCFH by esterase cleavage of the diacetate group. This compound is oxidized by reactive oxygen species to form the fluorescent compound, 2′-7′-dichlorofluorescein (DCF). For ex vivo detection of free radicals, an aliquot of the lung or kidney homogenate sample (25 μL) from the same animals used for MDA assay was incubated with 0.1 mM DCFH-DA at 37°C for 30 min. The formation of the oxidized fluorescent derivative DCF was monitored at an excitation wavelength of 485 nm and an emission wavelength of 527 nm using a fluorescence spectrophotometer as described previously (Bao et al., 2005). Background fluorescence was corrected by the inclusion of parallel blanks. The formation of ROS was quantified using a DCF standard curve, and results were expressed as nmol DCF/mg protein.
Assessing lipid peroxidation and cell death
A TBARS assay was used to detect malondialdehyde (MDA) and other aldehyde products of lipid peroxidation, as described previously (Bao et al., 2004). MDA is also produced as a by-product of enzymatic lipid peroxidation during the arachidonic acid cascade. The TBARS estimate of lipid peroxidation was quantified in the homogenates of the lung, liver, and kidney. A standard curve was established using MDA bis(dimethyl acetal) (Sigma-Aldrich), and lipid peroxidation was expressed as μmol of TBARS/g tissue. Lipid peroxidation in lung was also detected by the presence of 4-hydroxynonenal (HNE)-bound proteins by Western blots, using a mouse anti-HNE monoclonal antibody (1:5000; Alpha Diagnostic International, San Antonio, TX) and 10% polyacrylamide gels. Cell death in the lung was quantified by Western blotting for caspase-3 (anti-caspase-3; Upstate Biotechnology).
Statistical analyses
Mean values are expressed ± standard error (SE). The results were subjected to parametric statistical analysis using one-way analysis of variance (ANOVA; Snedecor and Cochran, 1989). This analysis included data from the uninjured rats and from rats with injury at T4. Differences between means were determined by the post-hoc Student Neuman-Keuls test. Significance was set at p < 0.05. The power of the tests performed always exceeded 0.80.
Results
Anti-α4 treatment reduces lung neutrophils and macrophages after SCI
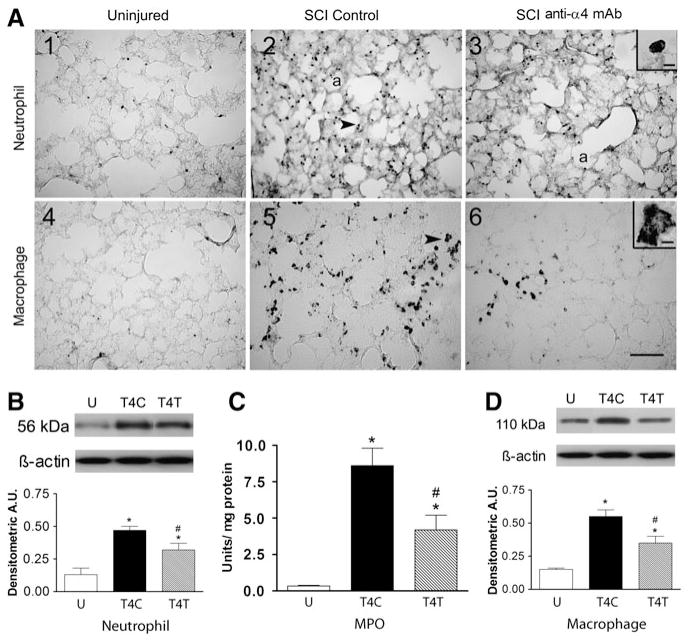
SCI at T4 caused an influx of neutrophils into the lung at 24 h after injury (Fig. 1A1–3). The inset in A3 shows the lobed nucleus within a cell that is typical of neutrophils. In the uninjured rat, the few neutrophils (identified by the anti-neutrophil antibody) found in the lung (Fig. 1A1) were predominantly located within blood vessels. In contrast, after T4 injury in SCI control rats, an infiltrate of neutrophils appeared within the alveoli and extravascular tissue (Fig. 1A2). After the anti-α4 treatment, fewer neutrophils were detected within the lung (Fig. 1A3). Quantification of the effects of the anti-α4 treatment on the neutrophil infiltrate at T4 was done using Western blotting. The anti-neutrophil antibody detected the neutrophil protein at a molecular weight of 56 kDa. After the T4 SCI, the 56-kDa neutrophil protein expression changed significantly (Fig. 1B; ANOVA, F2,19 = 13.70, p = 0.002), increasing by 3.7-fold compared to the values in the uninjured rats (p = 0.002). The anti-α4 treatment reduced this increase significantly, by ~30% (to a 2.5-fold change; p = 0.045). MPO activity in lung homogenates (assessed mostly as an estimate of neutrophil activity, and to a lesser degree of macrophage activity) changed significantly with SCI (Fig. 1C; ANOVA, F2,12 = 25.46, p < 0.001), increasing by about 25-fold in the lungs of T4 control (T4C) SCI rats (p < 0.001). This increase was significantly attenuated in anti-α4 treated (T4T) rats (p = 0.003).
FIG. 1.
The anti-α4 treatment decreases neutrophils and macrophages in the lung at 24 h after spinal cord injury (SCI). (A) Photomicrographs of lung sections immunostained by an anti-neutrophil antibody (panels 1–3), and by an ED-1 antibody to detect macrophages (panels 4–6) from an uninjured rat, a T4 SCI control rat, and a T4 SCI rat treated with the anti-α4 monoclonal antibody (SCI anti-α4 mAb). The insets in A3 and A6 show high-power detail of stained cells (a, alveolus). The arrows in A2 and A5 point to a neutrophil and a macrophage, respectively (scale bar = 100 μm in A6 applies to A1–A6; scale bar = 10 μm in insets). (B) Neutrophil protein, identified by Western blotting in lung homogenates from uninjured and SCI rats (n = 4 for all groups) expressed in arbitrary units (A.U.; U, uninjured rats; T4C, T4 control SCI rats; T4T, T4 SCI rats treated with the anti-α4 mAb). A representative autoradiogram of a Western blot showing relative protein expression, compared to loading controls (β-actin), is shown above the bar graph. (C) Myeloperoxidase (MPO) activity in lung homogenates from uninjured rats (n = 6), T4 SCI control rats (n = 4), and T4 SCI rats treated with the anti-α4 mAb (n = 5). (D) Macrophage protein (ED-1) expression (Western blotting) in lung homogenates from uninjured and SCI rats (n = 4/group). In this and all figures values are means ± standard error (*significantly different from uninjured; #significantly different from SCI control; p ≤ 0.05 by Student Neuman-Keuls test for all comparisons).
The normal uninjured lung contains a population of resident ED-1-immunoreactive macrophages within the tissue parenchyma surrounding the alveoli (Fig. 1A4). At 24 h after T4 SCI in control rats, the density of this macrophage population appeared increased (Fig. 1A5). These cells, although larger, were similar in morphology and location to those in the uninjured lungs. An example of a large irregular macrophage is shown in the Figure 1A6 inset. After anti-α4 treatment, the macrophages appeared to be less prevalent in the lungs of the SCI rats than in the control SCI rats (Fig. 1A6). Expression of ED-1 in lung homogenates changed significantly after T4 SCI (Fig. 1D; ANOVA, F2,9 = 20.72, p < 0.001), increasing by 3.6-fold in control SCI rats compared to uninjured rats (p < 0.001). The anti-CD11d treatment reduced this change significantly, by ~40% (p = 0.011).
Anti-α4 treatment reduces expression of oxidative enzymes and the concentration of free radicals in the lung after SCI
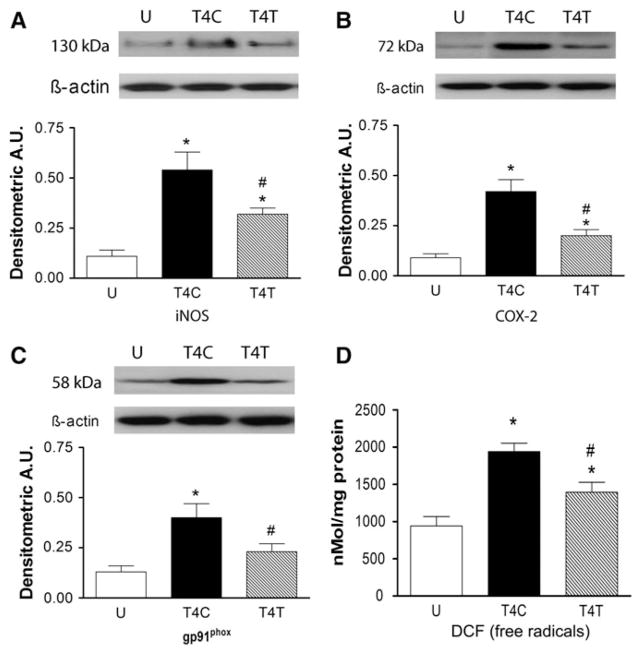
In addition to the MPO results described above, other oxidative enzymes associated with injury to the spinal cord were evaluated in the lung homogenates using Western blotting. Expression of iNOS changed significantly in lung homogenates after T4 SCI (Fig. 2A; ANOVA, F2,9 = 13.41, p = 0.002), increasing by fivefold (p = 0.002). The anti-α4 treatment reduced this change significantly, by ~40% to a threefold increase (p = 0.027). COX-2 expression in the lung homogenates also changed after SCI (Fig. 2B; ANOVA, F2,9 = 20.56, p < 0.001), increasing by fivefold after T4 SCI (p < 0.001). This increase was significantly (~50%) smaller (2.3-fold greater than uninjured) after anti-α4 treatment (p = 0.003). The pattern of changes in expression of the enzyme gp91phox (the NADPH oxidase catalytic subunit) followed that of iNOS and COX-2. Expression of gp91phox was significantly changed after SCI (Fig. 2C; ANOVA, F2,9 = 9.21, p = 0.007), increasing by threefold in the lung homogenates after T4 SCI (p = 0.006). Treatment with the anti-α4 mAb reduced this increase by almost half (p = 0.024), to levels no different from the uninjured values. A quantitative DCF assay for free radicals in the lung homogenates revealed significant changes in DCF after SCI (Fig. 2D; ANOVA, F2,12 = 14.47, p < 0.001). In the control SCI rats, a twofold increase in DCF occurred (p < 0.001), and anti-α4 treatment reduced this response by ~30% (p = 0.016).
FIG. 2.
The anti-α4 treatment decreases expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and gp91phox, and production of free radicals in the lung at 24 h after spinal cord injury (SCI). (A) iNOS expression was examined in uninjured rats and SCI rats (n = 4 for all groups; U, uninjured rats; T4C, T4 control SCI rats; T4T, T4 SCI rats treated with the anti-α4 monoclonal antibody [mAb]; A.U., arbitrary units). (B and C) COX-2 and gp91phox expression was also evaluated in these rats (n = 4 for all groups). (D) The concentration of 2′-7′-dichlorofluorescein (DCF) was assayed as a free radical marker in lung homogenates from the uninjured (n = 6), T4C (n = 4), and T4T (n = 5) rats (*significantly different from uninjured; #significantly different from SCI controls, p ≤ 0.05 by Student Neuman-Keuls test).
Anti-α4 treatment reduces lipid peroxidation and cell death in lung after SCI
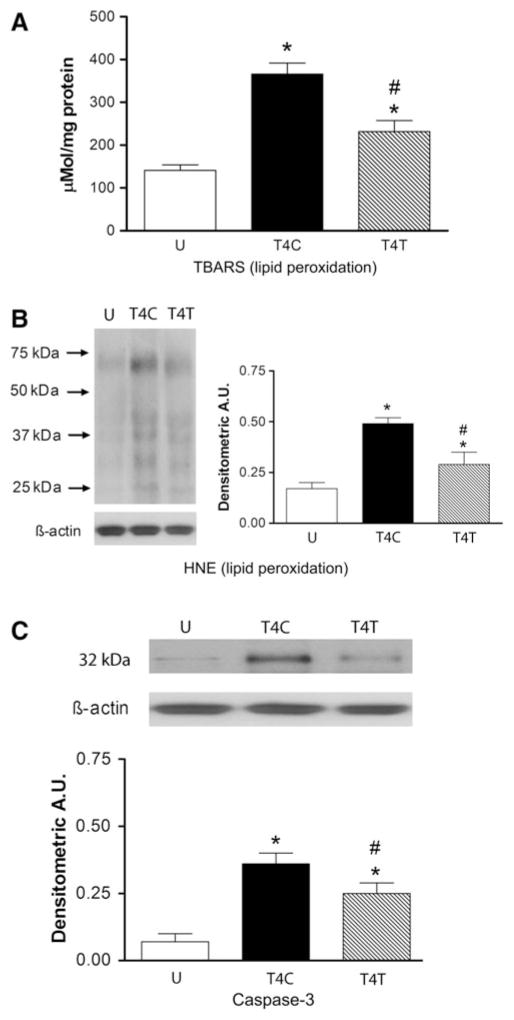
Lung damage was evaluated by examining lipid peroxidation of cell membranes and expression of the pro-apoptotic enzyme caspase-3. Lipid peroxidation was first estimated by assaying relative levels of aldehyde products, including MDA, using the TBARS assay. Lipid peroxidation was significantly altered by SCI (Fig. 3A; ANOVA, F2,12 = 26.94, p < 0.001), increasing by 2.6-fold after T4 SCI (p < 0.001). Anti-α4 treatment reduced this increase significantly, by ~40% (p = 0.001), to a 1.6-fold change. Western blotting for the presence of HNE also revealed changes in lipid peroxidation after SCI (Fig. 3B; ANOVA, F2,9 = 17.44, p < 0.001). After T4 SCI, lung HNE increased significantly, by threefold (p < 0.001). This increase was reduced by anti-α4 treatment, by ~50% to a 1.7-fold change (p = 0.006). Caspase-3 was examined as a marker of apoptotic cell death in the lungs. Quantification of caspase-3 expression by Western blotting revealed very limited expression of this enzyme in lungs of uninjured rats, but significant changes in expression after SCI (Fig. 3C; ANOVA, F2,9 = 17.49, p < 0.001). After T4 SCI, caspase-3 levels in the lung increased significantly, by ~5-fold (p < 0.001), and anti-α4 treatment reduced this by ~30%, to a 3.4-fold increase (p = 0.046).
FIG. 3.
The anti-α4 treatment decreases lipid peroxidation and cell death in the lung at 24 h after spinal cord injury (SCI). (A) Lipid peroxidation was assessed by the thiobarbituric acid reactive substance (TBARS) assay for aldehydes, including malondialdehyde in lung homogenates from uninjured (n = 6), T4C (n = 4), and T4T (n = 5) rats, and also by Western blotting for 4-hydroxynonenol (HNE)-bound proteins (B) in most of these rats (n = 4 per group). Western blot illustrates an example of expression of HNE-bound proteins with different molecular weights. The bar graphs display the sums of areas of all bands. (C) Caspase-3 expression was also evaluated by Western blotting in the homogenates from these rats (n = 4 for all groups; *significantly different from uninjured; #significantly different from T4 SCI controls; p ≤ 0.05 by Student Neuman-Keuls test; U, uninjured rats; T4C, T4 control SCI rats; T4T, T4 SCI rats treated with the anti-α4 monoclonal antibody [mAb]; A.U., arbitrary units).
Anti-α4 treatment reduces the influx of inflammatory cells into the kidney after SCI
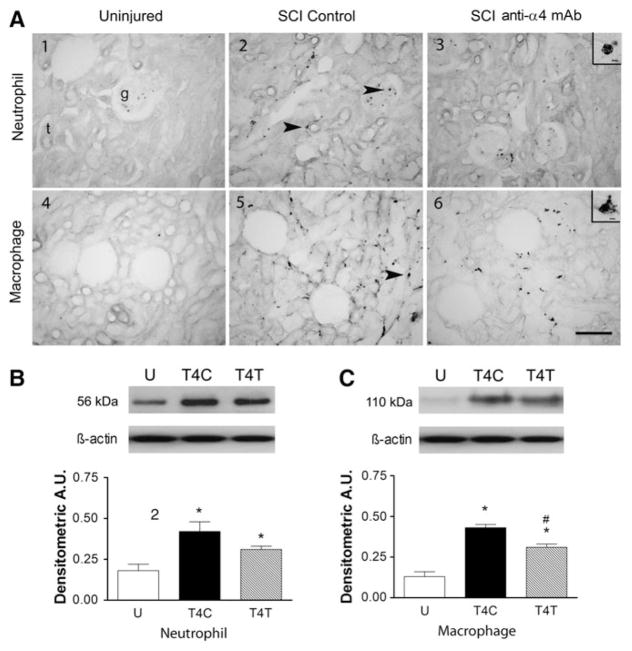
Cells with morphology typical of neutrophils were readily identified in kidney sections from uninjured and SCI rats (see inset in Fig. 4A3). In uninjured rats, neutrophils were found predominantly within blood vessels of the glomeruli, and not within the kidney parenchyma (Fig. 4A1). After T4 SCI, neutrophils were also found within the parenchyma, often adjacent to tubules (see arrow in Fig. 4A2). In rats after anti-α4 treatment, the density of neutrophils within the glomeruli and adjacent to tubules was reduced but remained more prevalent than in kidneys of uninjured rats (Fig. 4A3). Quantification of the neutrophil influx into the kidney by Western blotting also revealed that the 56-kDa neutrophil protein changed significantly (Fig. 4B; ANOVA, F2,9 = 8.11, p = 0.010), increasing by 2.3-fold compared to values in the uninjured rats (p < 0.008). After the anti-α4 treatment the 1.7-fold increase was not significantly different from that in control SCI rats (p = 0.110), but was marginally different from the values in the uninjured rats (p = 0.052). Variability in the MPO assay caused low power in the statistical analysis of the results. Despite a doubling of MPO activity after SCI, and values after anti-α4 treatment that were almost identical to those in uninjured rats, no significant differences among the groups were detected.
FIG. 4.
The anti-α4 treatment decreases neutrophils and macrophages in the kidney at 24 h after spinal cord injury (SCI). (A) Photomicrographs of kidney sections from uninjured and T4 SCI rats, immunostained by the anti-neutrophil antibody (A1–A3), and by the ED-1 antibody (A4–A6). Insets in A2 and A6 show high-power detail of cells with morphology typical of neutrophils and macrophages, respectively (g, glomerulus; t, tubule). Arrows in A2 point to neutrophils in the glomerulus and near a tubule. Arrow in A5 points to a macrophage near a tubule (scale bar = 100 μm in A6 also applies to A1–A6; scale bar = 10 μm in insets). (B) Neutrophil protein expression was examined by Western blotting in uninjured and T4 SCI rats (n = 4 for all groups). (C) Macrophage protein (ED-1) expression was also detected by Western blotting in these rats (n = 4 per group; *significantly different from uninjured; #significantly different from T4 SCI controls; U, uninjured rats; T4C, T4 control SCI rats; T4T, T4 SCI rats treated with the anti-α4 monoclonal antibody [mAb]; A.U., arbitrary units).
Tissue sections of the normal uninjured kidney rarely contained ED-1-immunoreactive macrophages (Fig. 4A4). A cell with typical macrophage morphology is shown in the inset of Figure 4A6. After T4 injury in control SCI rats, the density of this macrophage population appeared increased, particularly adjacent to the tubules (see arrow in Fig. 4A5). After anti-α4 treatment, the macrophage presence in the kidneys appeared to be reduced compared to that in the control SCI rats (Fig. 4A6). Western blotting showed that expression of the macrophage marker ED-1 in kidney homogenates changed significantly after SCI (Fig. 4C; ANOVA, F2,9 = 40.21, p < 0.001). ED-1 expression increased by 3.4-fold within the kidneys of control SCI rats (p < 0.001). The anti-α4 treatment reduced this change significantly, by 29% (p = 0.006).
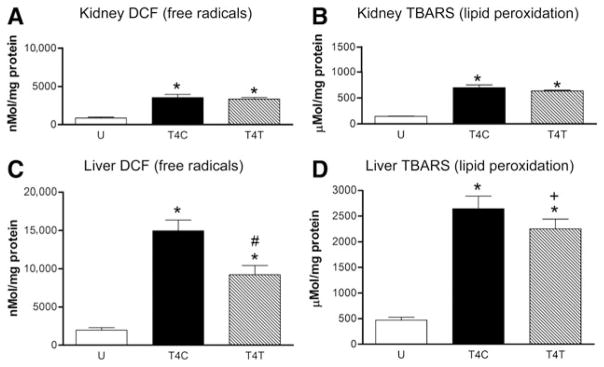
Oxidative activity within the kidney was estimated by the DCF assay for free radicals (Fig. 5A). Low levels of DCF were present in the kidneys of uninjured rats, and SCI significantly altered the concentrations of this free radical detector (ANOVA, F2,12 = 38.38, p < 0.001). DCF concentrations increased by fourfold in the kidneys of control SCI rats (p < 0.001), and anti-α4 treatment did not alter this response. Lipid peroxidation within the kidney, estimated by the TBARS assay for aldehydes, was significantly altered by the T4 SCI (Fig. 5B; ANOVA, F2,12 = 146.73, p < 0.001), increasing by 4.7-fold after injury (p < 0.001) compared to that seen in uninjured rats. Anti-α4 treatment had no significant effect on this increase in lipid peroxidation.
FIG. 5.
The anti-α4 treatment decreases the production of free radicals and lipid peroxidation in the kidney and liver at 24 h after spinal cord injury (SCI). (A and C) The concentration of 2′-7′-dichlorofluorescein (DCF) was assayed as a free radical marker in the kidney (A) and liver (C) homogenates from the uninjured (n = 6), T4C (n = 4), and T4T (n = 5) rats. (B and D) Lipid peroxidation was assessed by the thiobarbituric acid reactive substance (TBARS) assay for aldehydes, including malondialdehyde, in lung homogenates from the same groups of rats (group numbers the same as those in A and C; *significantly different from uninjured; #significantly different from T4 SCI controls; + tended to differ from T4 controls; p = 0.063; U, uninjured rats; T4C, T4 control SCI rats; T4T, T4 SCI rats treated with the anti-α4 monoclonal antibody [mAb]).
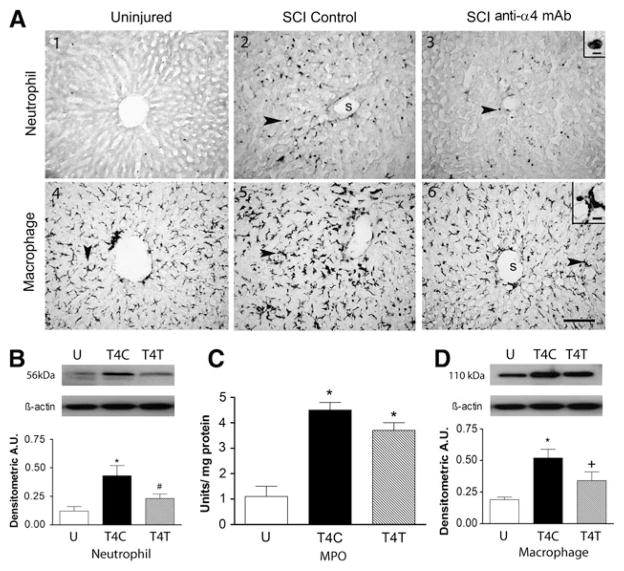
Anti-α4 treatment reduces neutrophil and macrophage activation in the liver after SCI
The liver is also infiltrated by neutrophils after SCI. In uninjured rats only a few neutrophils were found within the tissue parenchyma of liver sections, and a few neutrophils were seen within the vascular sinusoids (Fig. 6A1). After T4 injury, many neutrophils were distributed throughout the liver, among the hepatocytes and adjacent to the sinusoids (see arrow in Fig. 6A2). The morphology of the cell in the inset of Figure 6A3 is typical of a neutrophil. Liver sections from anti-α4-treated rats after SCI had visibly fewer neutrophils than the control SCI rats, but appeared to have more of these cells than uninjured rats (Fig. 6A3). In the liver homogenates, quantification of the 56-kDa neutrophil protein by Western blotting revealed significant changes after T4 SCI (Fig. 6B; ANOVA, F2,9 = 7.45, p = 0.012), with 3.7-fold increases in the livers of control SCI rats (p = 0.010), and a 45% reduction of this increase in rats with anti-α4 treatment (p = 0.045), to values no different from uninjured rats. MPO activity in the liver homogenates was significantly changed after T4 SCI (Fig. 6C; ANOVA, F2,12 = 33.33, p < 0.001), increasing by 4.2-fold (p < 0.001). However, the ~20% reduction in this increase after anti-α4 treatment was insufficient to yield a significant difference between the control and treated SCI rats.
FIG. 6.
The anti-α4 treatment decreases neutrophils and macrophages in the liver at 24 h after spinal cord injury (SCI). (A) Photomicrographs of liver sections from uninjured and T4 SCI rats, immunostained by the anti-neutrophil antibody (A1–A3), and by the ED-1 antibody (A4–A6). Insets in A3 and A6 show high-power detail of stained cells (s, liver sinusoid). Arrows point to neutrophils (A2 and A3) and macrophages (A5 and A6; scale bar = 100 μm in A6 also applies to A1–A6; scale bar = 10 μm in insets of A3 and A6). (B) Neutrophil protein expression was revealed by Western blotting in uninjured and T4 SCI rats (n = 4 for all groups). (C) Myeloperoxidase (MPO) activity was assayed in liver homogenates of uninjured (n = 6), T4C rats (n = 4), and T4T rats (n = 5). (D) Macrophage protein (ED-1) expression was also examined by Western blotting in these rats (n = 4 for all groups; *significantly different from uninjured; #significantly different from T4 SCI control; + tended to differ from T4 controls; p = 0.065; U, uninjured rats; T4C, T4 control SCI rats; T4T, T4 SCI rats treated with the anti-α4 monoclonal antibody [mAb]; A.U., arbitrary units).
Staining for macrophages by ED-1 after T4 SCI revealed these cells within the livers of uninjured, control SCI, and anti-α4-treated rats (Fig. 6A4–6). The large irregular cell in the inset of Figure 6A6 appears to be a tissue macrophage, and these macrophages likely were Kupffer cells. The relative abundance of these cells was similar among all groups of rats, although they seemed to be larger in the control SCI rats. Indeed, Western blotting for the ED-1 in liver homogenates showed significant changes in expression after SCI (Fig. 6D; ANOVA, F2,9 = 7.58, p = 0.012), with 2.7-fold increases in the livers of control SCI rats (p = 0.009). After the anti-α4 treatment, the 1.8-fold increase tended to differ from the values in the control SCI rats (p = 0.065).
Free radicals and lipid peroxidation within the liver were estimated with the DCF and TBARS assays, respectively, in tissue homogenates from the uninjured and T4 SCI rats. Changes in liver DCF concentrations were detected after SCI (Fig. 5C; ANOVA, F2,12 = 44.57, p < 0.001), with 7.5-fold increases occurring (p < 0.001). The anti-α4 treatment reduced this change, by 40% to a 4.6-fold increase (p = 0.002). Changes in lipid peroxidation were found in liver homogenates after SCI (Fig. 5D; ANOVA, F2,12 = 85.96, p < 0.001), with significant 5.6-fold increases in the TBARS measure of aldehydes in SCI rats (p < 0.001). Treatment with the anti-α4 mAb caused a 15% reduction in lipid peroxidation, reaching a level that nearly attained a statistically significant difference from the value in control SCI rats (p = 0.064).
Discussion
The compression injury at T4 evoked a vigorous SIRS, as we have described previously (Bao et al., 2011b; Gris et al., 2008), and this response was clearly attenuated by blocking the leukocyte integrin α4β1. The migration of neutrophils into the lungs and liver was markedly reduced and all three organs contained fewer macrophages. The macrophages probably came from vascular monocyte transmigration and from activation of tissue macrophages. In the lungs and liver, the oxidative sequelae that typically follow this influx were similarly reduced. Effects of the treatment were less robust in the kidney.
In the lungs, the anti-α4 treatment attenuated the increase in the expression of the oxidative enzymes iNOS, COX-2, and gp91phox, with parallel decreases in reactive oxygen species, lipid peroxidation, and cell death. These effects are highly relevant, as respiratory failure is a leading cause of morbidity and mortality in the acute phase of human SCI (Catz et al., 2002; DeVivo et al., 1999; O’Connor, 2005; Pickett et al., 2006). Lung dysfunction is considered to be very difficult to treat in SCI patients (Bhatia et al., 2005; Kyono and Coates, 2002), and averting the early inflammatory response within the lungs would be an important step toward eliminating this serious secondary consequence of SCI. We have previously shown that this anti-α4 treatment is highly effective in reducing the influx of neutrophils and monocytes/macrophages into the injured spinal cord, improving tissue preservation and neurological outcomes (Fleming et al., 2008). The present study shows further utility of this treatment in protection of the lungs from the SIRS.
In previous studies we have employed an anti-β2 integrin (CD11d/CD18) and the anti-α4 integrin (α4β1) strategies to reduce the influx of leukocytes into the injured spinal cord (Fleming et al., 2008; Gris et al., 2004; Oatway et al., 2005; Saville et al., 2004). Both entailed intravenous delivery of a monoclonal antibody within the first 72 h after SCI. Blockade of either integrin limited inflammation and secondary injury within the injured cord and improved neurological outcomes. In comparing the two treatments a key outcome was greater tissue sparing in the spinal cord after the anti-α4 treatment. That background prompted us to query whether this treatment would also lead to better organ protection from SIRS. Our findings did not support this hypothesis with respect to effects on the lung. Both antibodies had clear anti-inflammatory and tissue-protecting effects in the lung. The difference in time of treatment delivery between the anti-CD11d antibody used in our previous study (12 h) (Bao et al., 2011b) and the anti-α4 integrin antibody used in the present study (24 h) appeared to have no impact on the outcome. Generally, the influx of neutrophils and density of macrophages within the organs was reduced similarly by both antibodies, with reduction of oxidative activity and tissue damage in the lung.
The kidney is also an important target of the SIRS (Catz et al., 2002; DeVivo et al., 1999; O’Connor, 2005; Pickett et al., 2006), as renal failure is a serious component of the multi-organ failure that can occur after SCI. The kidneys were in-filtrated by neutrophils and contained activated macrophages at 24 h after the SCI. Within the organ, this was accompanied by a large increase in the concentration of free radicals and robust lipid peroxidation. Although the anti-α4 treatment reduced the density of macrophages within the kidney, the reduction in neutrophils was not statistically significant, and the treatment did not alter the concentration of free radicals or degree of lipid peroxidation. The modest, non-significant reduction in neutrophils in the kidney caused by the anti-α4 treatment correlated with the absence of change in this very high oxidative activity, despite the significant reduction in macrophages. The small sample size in this study may have limited detection of the change in neutrophils in the kidney, but the lack of change in oxidative activity was clear.
These findings in the kidney contrast with the significant reduction of neutrophils, macrophages, free radicals, and lipid peroxidation observed at 12 h post-injury after anti-CD11d integrin treatment in our previous study (Bao et al., 2011b). However, the increases in the two measures of oxidative activity were much smaller at 12 h after T4 SCI than those measured at 24 h in the current study. We conclude that the number of cells remaining after treatment in the current study was sufficient to support the ongoing free radical production and lipid peroxidation, and/or that factors other than intrarenal inflammatory cells were generating the oxidative activity and membrane damage. As our study did not include any measures of renal blood flow, oxygenation, or possible ischemia, we are unable to speculate about alternative causes of the sustained responses. Changes in each of these intrarenal measures could augment the production of free radicals and tissue damage (Paller et al., 1984).
The liver sustained a robust infiltration of neutrophils and increased density of macrophages by 24 h after the T4 SCI. This response was accompanied by a substantial increase in MPO activity, free radical production, and lipid peroxidation. The neutrophil influx and increase in macrophages were much greater at 24 h than 12 h after injury. At 24 h a sharp increase in free radical production and lipid peroxidation occurred in the liver, whereas such responses were not yet present in the liver in our study at 12 h (Bao et al., 2011b). The anti-α4 treatment significantly reduced the inflammatory response and free radical production; the reduction in lipid peroxidation due to this treatment was very close to statistical significance. The anti-α4 treatment had markedly greater effects on the liver during the SIRS than our previously studied anti-CD11d treatment (Bao et al., 2011b). The greater efficacy in the liver of the anti-α4 antibody might relate to the later time of analysis (24 h versus 12 h post-injury), with greater ongoing oxidative activity and tissue damage at that time providing a better treatment target.
The liver is known to be a key organ in the instigation of the SIRS after CNS injury, as it produces and releases chemokines and acute phase proteins soon after brain or spinal cord injury (Campbell et al., 2003,2005; Perry et al., 2003; Wilcockson et al., 2002). This release into the circulation permits the attraction of leukocytes into other highly perfused organs such as the lungs and kidneys, creating the potential for intra-organ inflammation, damage, and ultimately failure. A recent study using intravital microscopy (Hundt et al., 2011) demonstrated early extravasation of leukocytes into the liver of rats after SCI, and associated this influx with hepatocyte cell death that increased with time after injury and severity of injury. This was a study of the very acute period after SCI (90 min), but it demonstrated the potential for liver damage after cord injury. At 12 h after T4 SCI in rats, staining of alkaline phosphatase in the liver is spread throughout the entire organ, in contrast to the staining limited to endothelial cells found in uninjured animals (Gris, 2007). The presence of this enzyme is routinely used in the clinical setting to evaluate liver injury (Dufour et al., 2000; Rochling, 2001), and its occurrence in the rat liver at 12 h after SCI foretells the cell damage that we found at 24 h. Moreover, in a large proportion of patients with SCI, serum transaminases (markers of liver damage) have been shown to be increased for several months after injury (Bloom and Freed, 1989). Liver dysfunction after traumatic injuries is known to worsen the clinical prognosis (Harbrecht et al., 2001). Therefore the significant reduction in liver neutrophils and macrophages, with parallel decreases in oxidative stress and lipid peroxidation caused by the anti-α4 integrin treatment, has potentially important clinical relevance.
We compared the oxidative activity and lipid membrane damage in the three organs at 24 h after SCI, as they seemed to follow a pattern of increasing intensity from lung to kidney to liver. The basal level of free radicals in the liver of uninjured rats was > 2 times greater than that in the lung and kidney (p = 0.004 versus lung; p = 0.006 versus kidney), and the lipid peroxidation (TBARS) in the liver was > 3 times that in the lung and kidney (p < 0.001 for both comparisons). The increases caused by T4 SCI were greater in the liver as well (p < 0.001 for both comparisons). In order of relative magnitude, these responses to SCI were smallest in the lung (2- to 2.6-fold), greater in the kidney (4- to 4.7-fold), and greatest in the liver (5.6- to 7.5-fold).
The SIRS response of the rat has features similar to that of humans. In humans, SIRS is characterized by either increases or decreases in body temperature, increased heart rate, increased respiratory rate and leukocytosis or leukopenia (Bone et al., 1992). For several days after T4 SCI, rats are hypothermic, requiring assistance with body temperature maintenance, and have rapid, shallow breathing (Weaver, unpublished observations). Within 24 h of this SCI, their heart rates increase by 40–70 beats/min, and this increase reaches 100 beats/min by 3 days after the injury (Fleming et al., 2008; Mayorov et al., 2001). Finally, as described above, rats have a leukocytosis within 12–24 h of T4 SCI (Gris et al., 2008). These changes after SCI in the rat may be partially attributed to the acute loss of descending neural control systems, but they occur after moderate as well as more severe injuries. Although the clinical aspects of the SIRS in rats have not been examined systematically, and the physiological impact on organ function has not yet been studied, the rat model of this important problem appears to be sound.
The anti-α4β1 treatment examined in this study and the anti-CD11d/CD18 treatment that we investigated previously (Bao et al., 2011b) may yield somewhat different outcomes, as the integrins themselves differ in several ways. First, the α4β1 integrin mediates rolling and tethering as well as firm adhesion (Davenpeck et al., 1998; Kubes et al., 1995), whereas the CD11d/CD18 is only known to mediate firm adhesion (Grayson et al., 1998; Van der Vieren et al., 1995,1999). Accordingly the anti-α4β1 treatment might block a larger portion of the diapedesis process. Next, expression of the two different integrins on human neutrophils and monocytes follows a generally similar time course after SCI, but expression of α4β1 on neutrophils increases sooner than CD11d/CD18 (12 h versus 48 h) (Bao et al., 2011a). Furthermore, binding of α4β1 to a ligand increases its avidity. Such changes in the binding properties of the CD11d/CD18 integrin have not been reported. Earlier increases in surface expression and increased avidity might make α4β1 a more important target for blockade after SCI. Nonetheless, the very early involvement of the α4β1 integrin in diapedesis may make it a less attractive integrin for clinical blockade therapy than the CD11d/CD18, as the neuroprotective effects of blocking the α4β1 integrin are reduced when treatment is delayed to 6 h after SCI (Fleming et al., 2009). This finding demonstrates that blockade of the earliest involvement of the α4β1 integrin is essential. In contrast CD11d/CD18 blockade commencing at 6 h after SCI still yields excellent neuroprotective effects (Ditor et al., 2006).
In conclusion, blockade of the α4β1 integrin after SCI markedly reduces the impact of the SIRS on the lungs and liver. The α4β1 and CD11d/CD18 integrins are each unique targets for treatment, due to their distinct intrinsic properties. An ideal therapeutic strategy may be a combination treatment that blocks both integrins in the first hours and days after SCI. The utility of the anti-integrin neuroprotective treatments in attenuating the SIRS is an important and clinically relevant new finding.
Acknowledgments
This research was supported by a grant from the Canadian Institutes of Health Research. The authors thank BiogenIdec for donating the anti-α4 mAb and isotype-matched control mAb used in this study. We appreciate the critical review of the manuscript by Dr. Canio Polosa.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- Acosta JA, Yang JC, Winchell RJ, Simons RK, Fortlage DA, Hollingsworth-Fridlund P, Hoyt DB. Lethal injuries and time to death in a level I trauma center. J Am Coll Surg. 1998;186:528–533. doi: 10.1016/s1072-7515(98)00082-9. [DOI] [PubMed] [Google Scholar]
- Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, Walker K. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr Biol. 1998;8:923–926. doi: 10.1016/s0960-9822(07)00373-9. [DOI] [PubMed] [Google Scholar]
- Bao F, Bailey CS, Gurr KR, Bailey SI, Rosas-Arellano MP, Brown A, Dekaban GA, Weaver LC. Human spinal cord injury causes specific increases in surface expression of beta integrins on leukocytes. J Neurotrauma. 2011a;28:269–280. doi: 10.1089/neu.2010.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao F, Brown A, Dekaban GA, Omana V, Weaver LC. CD11d integrin blockade reduces the systemic inflammatory response syndrome after spinal cord injury. Exp Neurol. 2011b;231:272–283. doi: 10.1016/j.expneurol.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 2004;88:1335–1344. doi: 10.1046/j.1471-4159.2003.02240.x. [DOI] [PubMed] [Google Scholar]
- Bao F, Dekaban GA, Weaver LC. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. J Neurochem. 2005;94:1361–1373. doi: 10.1111/j.1471-4159.2005.03280.x. [DOI] [PubMed] [Google Scholar]
- Baskaran H, Yarmush ML, Berthiaume F. Dynamics of tissue neutrophil sequestration after cutaneous burns in rats. J Surg Res. 2000;93:88–96. doi: 10.1006/jsre.2000.5955. [DOI] [PubMed] [Google Scholar]
- Bhatia RK, Pallister I, Dent C, Jones SA, Topley N. Enhanced neutrophil migratory activity following major blunt trauma. Injury. 2005;36:956–962. doi: 10.1016/j.injury.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Bloom KK, Freed MM. Liver enzyme abnormalities in spinal cord injury. J Am Paraplegia Soc. 1989;12:11–13. doi: 10.1080/01952307.1989.11735800. [DOI] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Campbell SJ, Hughes PM, Iredale JP, Wilcockson DC, Waters S, Docagne F, Perry VH, Anthony DC. CINC-1 is an acute-phase protein induced by focal brain injury causing leukocyte mobilization and liver injury. FASEB J. 2003;17:1168–1170. doi: 10.1096/fj.02-0757fje. [DOI] [PubMed] [Google Scholar]
- Campbell SJ, Perry VH, Pitossi FJ, Butchart AG, Chertoff M, Waters S, Dempster R, Anthony DC. Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am J Pathol. 2005;166:1487–1497. doi: 10.1016/S0002-9440(10)62365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz A, Thaleisnik M, Fishel B, Ronen J, Spasser R, Fredman B, Shabtay E, Gepstein R. Survival following spinal cord injury in Israel. Spinal Cord. 2002;40:595–598. doi: 10.1038/sj.sc.3101391. [DOI] [PubMed] [Google Scholar]
- Davenpeck KL, Sterbinsky SA, Bochner BS. Rat neutrophils express alpha4 and beta1 integrins and bind to vascular cell adhesion molecule-1 (VCAM-1) and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) Blood. 1998;91:2341–2346. [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Ditor DS, Bao F, Chen Y, Dekaban GA, Weaver LC. A therapeutic time window for anti-CD 11d monoclonal antibody treatment yielding reduced secondary tissue damage and enhanced behavioral recovery following severe spinal cord injury. J Neurosurg Spine. 2006;5:343–352. doi: 10.3171/spi.2006.5.4.343. [DOI] [PubMed] [Google Scholar]
- Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I Performance characteristics of laboratory tests. Clin Chem. 2000;46:2027–2049. [PubMed] [Google Scholar]
- Fleming JC. Inflammation in injured human spinal cords: the foundation for an anti-integrin treatment for cord injury. University of Western Ontario; London, Ontario: 2008. [Google Scholar]
- Fleming JC, Bao F, Cepinskas G, Weaver LC. Anti-alpha4beta1 integrin antibody induces receptor internalization and does not impair the function of circulating neutrophilic leukocytes. Inflamm Res. 2010;59:647–657. doi: 10.1007/s00011-010-0177-5. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Bao F, Chen Y, Hamilton EF, Gonzalez-Lara LE, Foster PJ, Weaver LC. Timing and duration of anti-alpha4beta1 integrin treatment after spinal cord injury: effect on therapeutic efficacy. J Neurosurg Spine. 2009;11:575–587. doi: 10.3171/2009.6.SPINE08915. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Bao F, Chen Y, Hamilton EF, Relton JK, Weaver LC. Alpha4beta1 integrin blockade after spinal cord injury decreases damage and improves neurological function. Exp Neurol. 2008;214:147–159. doi: 10.1016/j.expneurol.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Grayson MH, Van der Vieren M, Sterbinsky SA, Michael Gallatin W, Hoffman PA, Staunton DE, Bochner BS. Alphadbeta2 integrin is expressed on human eosinophils and functions as an alternative ligand for vascular cell adhesion molecule 1 (VCAM-1) J Exp Med. 1998;188:2187–2191. doi: 10.1084/jem.188.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gris D. Systemic inflammation after spinal cord injury. University of Western Ontario; London, Ontario: 2007. [Google Scholar]
- Gris D, Hamilton EF, Weaver LC. The systemic inflammatory response after spinal cord injury damages lungs and kidneys. Exp Neurol. 2008;211:259–270. doi: 10.1016/j.expneurol.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbrecht BG, Doyle HR, Clancy KD, Townsend RN, Billiar TR, Peitzman AB. The impact of liver dysfunction on outcome in patients with multiple injuries. Am Surg. 2001;67:122–126. [PubMed] [Google Scholar]
- Hundt H, Fleming JC, Phillips JT, Lawendy A, Gurr KR, Bailey SI, Sanders D, Bihari R, Gray D, Parry N, Bailey CS, Badhwar A. Assessment of hepatic inflammation after spinal cord injury using intravital microscopy. Injury. 2011;42:691–696. doi: 10.1016/j.injury.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Kubes P, Niu XF, Smith CW, Kehrli ME, Jr, Reinhardt PH, Woodman RC. A novel beta 1-dependent adhesion pathway on neutrophils: a mechanism invoked by dihydrocytochalasin B or endothelial transmigration. FASEB J. 1995;9:1103–1111. [PubMed] [Google Scholar]
- Kyono W, Coates TD. A practical approach to neutrophil disorders. Pediatr Clin North Am. 2002;49:929–971. viii. doi: 10.1016/s0031-3955(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Leone DR, Giza K, Gill A, Dolinski BM, Yang W, Perper S, Scott DM, Lee WC, Cornebise M, Wortham K, Nickerson-Nutter C, Chen LL, LePage D, Spell JC, Whalley ET, Petter RC, Adams SP, Lobb RR, Pepinsky RB. An assessment of the mechanistic differences between two integrin alpha 4 beta 1 inhibitors, the monoclonal antibody TA-2 and the small molecule BIO5192, in rat experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther. 2003;305:1150–1162. doi: 10.1124/jpet.102.047332. [DOI] [PubMed] [Google Scholar]
- Mayorov DN, Adams MA, Krassioukov AV. Telemetric blood pressure monitoring in conscious rats before and after compression injury of spinal cord. J Neurotrauma. 2001;18:727–736. doi: 10.1089/089771501750357663. [DOI] [PubMed] [Google Scholar]
- Nandi A, Estess P, Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest; CD44 association with VLA-4 in T cell extravasation. Immunity. 2004;20:455–465. doi: 10.1016/s1074-7613(04)00077-9. [DOI] [PubMed] [Google Scholar]
- O’Connor PJ. Survival after spinal cord injury in Australia. Arch Phys Med Rehabil. 2005;86:37–47. [PubMed] [Google Scholar]
- Oatway MA, Chen Y, Bruce JC, Dekaban GA, Weaver LC. Anti-CD11d integrin antibody treatment restores normal serotonergic projections to the dorsal, intermediate, and ventral horns of the injured spinal cord. J Neurosci. 2005;25:637–647. doi: 10.1523/JNEUROSCI.3960-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott L, McClain CJ, Gillespie M, Young B. Cytokines and metabolic dysfunction after severe head injury. J Neurotrauma. 1994;11:447–472. doi: 10.1089/neu.1994.11.447. [DOI] [PubMed] [Google Scholar]
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Pickett GE, Campos-Benitez M, Keller JL, Duggal N. Epidemiology of traumatic spinal cord injury in Canada. Spine (Phila Pa 1976) 2006;31:799–805. doi: 10.1097/01.brs.0000207258.80129.03. [DOI] [PubMed] [Google Scholar]
- Rochling FA. Evaluation of abnormal liver tests. Clin Cornerstone. 2001;3:1–12. doi: 10.1016/s1098-3597(01)90074-2. [DOI] [PubMed] [Google Scholar]
- Saville LR, Pospisil CH, Mawhinney LA, Bao F, Simedrea FC, Peters AA, O’Connell PJ, Weaver LC, Dekaban GA. A monoclonal antibody to CD11d reduces the inflammatory infiltrate into the injured spinal cord: a potential neuroprotective treatment. J Neuroimmunol. 2004;156:42–57. doi: 10.1016/j.jneuroim.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Iowa State University Press; Ames: 1989. [Google Scholar]
- Van der Vieren M, Crowe DT, Hoekstra D, Vazeux R, Hoffman PA, Grayson MH, Bochner BS, Gallatin WM, Staunton DE. The leukocyte integrin alpha D beta 2 binds VCAM-1: evidence for a binding interface between I domain and VCAM-1. J Immunol. 1999;163:1984–1990. [PubMed] [Google Scholar]
- Van der Vieren M, Le Trong H, Wood CL, Moore PF, St John T, Staunton DE, Gallatin WM. A novel leukointegrin, alpha d beta 2, binds preferentially to ICAM-3. Immunity. 1995;3:683–690. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Weaver LC, Verghese P, Bruce JC, Fehlings MG, Krenz NR, Marsh DR. Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J Neurotrauma. 2001;18:1107–1119. doi: 10.1089/08977150152693782. [DOI] [PubMed] [Google Scholar]
- Wilcockson DC, Campbell SJ, Anthony DC, Perry VH. The systemic and local acute phase response following acute brain injury. J Cereb Blood Flow Metab. 2002;22:318–326. doi: 10.1097/00004647-200203000-00009. [DOI] [PubMed] [Google Scholar]