Abstract
Acute administration of a monoclonal antibody (mAb) raised against the CD11d subunit of the leukocyte CD11d/CD18 integrin after spinal cord injury (SCI) in the rat greatly improves neurological outcomes. This has been chiefly attributed to the reduced infiltration of neutrophils into the injured spinal cord in treated rats. More recently, treating spinal cord-injured mice with a Ly-6G neutrophil-depleting antibody was demonstrated to impair neurological recovery. These disparate results could be due to different mechanisms of action utilized by the two antibodies, or due to differences in the inflammatory responses between mouse and rat that are triggered by SCI. To address whether the anti-CD11d treatment would be effective in mice, a CD11d mAb (205C) or a control mAb (1B7) was administered intravenously at 2, 24, and 48 h after an 8-g clip compression injury at the fourth thoracic spinal segment. The anti-CD11d treatment reduced neutrophil infiltration into the injured mouse spinal cord and was associated with increased white matter sparing and reductions in myeloperoxidase (MPO) activity, reactive oxygen species, lipid peroxidation, and scar formation. These improvements in the injured spinal cord microenvironment were accompanied by increased serotonin (5-HT) immunoreactivity below the level of the lesion and improved locomotor recovery. Our results with the 205C CD11d mAb treatment complement previous work using this anti-integrin treatment in a rat model of SCI.
Keywords: anti-CD11d, anti-inflammatory, neutrophils, spinal cord injury
Introduction
Mechanical injury to the spinal cord is followed by a process of secondary injury that is attributed to a robust inflammatory reaction (Bartholdi and Schwab, 1995; Blight, 1992; Hausmann, 2003; Lee et al., 2000; Popovich et al., 2002; Taoka and Okajima, 1998). The CD11/CD18 integrins, a family of membrane-bound glycoproteins, are found on the surface of leukocytes and bind to rat and human vascular cell adhesion molecule-1 (VCAM-1; Grayson et al., 1999; Van der Vieren et al., 1999), and human intercellular cell adhesion molecule-3 (ICAM-3; Van der Vieren et al., 1995). The expression of VCAM-1 and ICAM-3 is upregulated on damaged endothelial cells, allowing leukocytes to bind and migrate into the damaged tissue after injury (Schnell et al., 1999). A monoclonal antibody (mAb) 217L directed against the CD11d subunit of the CD11/CD18 integrin substantially decreased the number of neutrophils and macrophages in the cord after SCI in the rat (Mabon et al., 2000; Saville et al., 2004). This treatment resulted in decreased secondary damage (Bao et al., 2004a, 2004b, 2005), and significantly improved locomotor and autonomic recovery and decreased neuropathic pain (Gris et al., 2004; Oatway et al., 2005). These results are reinforced by several other studies that found that reducing neutrophils was neuroprotective (Bao et al., 2004a; Gorio et al., 2007; Hamada et al., 1996; Nguyen et al., 2007; Sroga et al., 2003; Taoka et al., 1997; Tonai et al., 2001).
In apparent contradiction to these CD11d mAb studies was a recent report in the spinal cord-injured mouse, that depleting neutrophils after SCI using the Ly-6G (RB6-8C5) mAb impeded functional recovery (Stirling et al., 2009). One possible explanation for these contradictory results is that decreasing intraspinal neutrophils in the rat after SCI might not work as effectively in the mouse due to differences in the onset and duration of neutrophil infiltration (Donnelly and Popovich, 2008; Kigerl et al., 2006). An alternative explanation is that the CD11d and Ly-6G mAb target leukocytes in different ways (depletion versus blocking migration), bringing about different results after SCI.
As a step toward resolving the discrepancy in results between the CD11d and the Ly-6G mAb-treated spinal cord-injured animals, we sought to evaluate the anti-CD11d treatment in spinal cord-injured mice. Mice were injured at the fourth thoracic spinal segment using an 8-g clip compression injury, and a CD11d mAb was administered intravenously at 2, 24, and 48 h post-injury. We herein report that the anti-CD11d treatment in the spinal cord-injured mouse decreases neutrophil infiltration by approximately 61% at 72 h post-injury, and results in reduced secondary damage and improved functional recovery. Thus despite differences in the neuroinflammatory response between the rat and mouse after SCI, the anti-CD11d treatment improves neurological outcomes in both species. Our results confirm the therapeutic effectiveness of reducing the early infiltration of leukocytes into the injured spinal cord, and suggest that the use of a non-depleting antibody has benefits not realized when a depleting antibody is used to target similar leukocyte populations.
Methods
Spinal cord injury
All procedures for this study were conducted in accordance with the University of Western Ontario Animal Care Committee Guidelines, adhering to the Canadian Guide to the Care and Use of Experimental Animals. Female C57BL/6 mice (Charles River) weighing 18–22 g were premedicated with atroprine (0.05 mg/kg SC; Sigma-Aldrich, St. Louis, MO). After 10 min, the mice were anesthetized by induction with 4% halothane and maintained with 1.5% halothane. The T4 spinal cord segment was exposed by dorsal laminectomy and injured, without disrupting the dura, by a 60-sec 8.3-g clip compression (Jacob et al., 2003; Joshi and Fehlings, 2002). The skin was closed and the injured mice were allowed to recover under a heat lamp. The mice were administered 0.5 mL of saline to replace loss of blood, buprenorphrine (0.01 mg/kg, and Baytril 25 mg/kg SC; Bayer, Toronto, Ontario, Canada) after surgery, and twice daily for the next 3 days. The bladders were manually emptied twice daily for the duration of the study.
Antibody treatment
The mice were administered a CD11d mAb (205C) or a control mAb (1B7, a dinitrophrenyl mAb) at 1.0 mg/kg IV, via tail vein injection at 2, 24, and 48 h after injury. This dose was optimized in spinal cord-injured rats and found to improve functional recovery (Gris et al., 2004; Mabon et al., 2000; Saville et al., 2004). A third group of spinal cord-injured mice was administered saline to control for any effects of IgG administration. The CD11d and 1B7 mAb were generously provided by Eli Lilly & Co. To test for any effects of the antibody treatments on circulating leukocytes we collected blood samples from the saphenous vein before injury and then again at 24 h after antibody treatments. White blood cell (WBC) counts were performed using a hemocytometer after RBC lysis. The mean WBC count before injury was 4.65×106±9.9×105 cells per mL and 6.2×106±2.5×105 cells per mL for the 1B7 and CD11d mAb-treated mice, respectively. The mean WBC count after injury was 3.2×106±4.6×105 cells per mL and 5.7×106±2.5×105 cells per mL for the 1B7 and CD11d mAb-treated mice, respectively. There were no significant differences in WBC counts between groups before or after injury (p<0.05 by two-way analysis of variance [ANOVA]).
Biochemical assays
At 24 h, 72 h, 1 week, or 2 weeks post-injury mice were anesthetized and perfused with cold 0.9% NaCl. A 0.5-cm segment of spinal cord centered on the lesion site was removed for analysis. The tissue was homogenized and assayed for markers of inflammation and oxidative damage. Neutrophil, and to a lesser extent macrophage, presence and activity was measured by myeloperoxidase (MPO) activity. The levels of reactive oxygen species (ROS) were measured by the oxidation of 2′-7′-dichlorofluorescin diacetate (DCFH-DA) to the fluorescent compound 2′-7′-dichlorofluorescein (DCF). The formation of the oxidized fluorescent derivative DCF was monitored at an excitation wavelength of 488 nm and an emission wavelength of 525 nm using a fluorescence spectrophotometer as previously described (Bao et al., 2005). The formation of ROS was quantified using a DCF standard curve, and results were expressed as fold changes compared to homogenates from uninjured spinal cords. Free radical-induced lipid peroxidation and enzymatic lipid peroxidation, a byproduct of arachidonic acid metabolism, was detected by a thiobarbituric acid reactive substances (TBARS) assay that measures the relative levels of malondialdehyde (MDA) and other aldehydes, as previously described (Ohkawa et al., 1979; Bao et al., 2004b).
Histology
At 72 h, 2 weeks, and 42 weeks after SCI, mice were anesthetized and perfused with cold 0.9% NaCl, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) as previously described (Brown et al., 2004, 2007). A 1-cm segment of the spinal cord centered on the lesion site was dissected, post-fixed for 2 h, and cryoprotected in 20% sucrose in PBS (pH 7.4) overnight. Tine spinal cord segment was embedded in tissue embedding medium, frozen, and stored at −80°C. Serial cross-sections of the spinal cord were cut at 16 μm using a cryostat, thaw-mounted on Superfrost™ glass slides (Fisherbrand; Fisher Scientific, Ottawa, Ontario, Canada), and stored at −20°C until processing for histological analysis. Every 10th section was collected on the same slide so that each slide had 10 sections, with each section separated by 160 μm. All histological staining and immunohistochemistry were carried out on sections separated by 160 μm, spanning from 5.0 mm rostral to 5.0 mm caudal to the lesion site. Histochemical staining with solochrome and trichrome was used to assess white matter sparing and collagen deposition after SCI, respectively (Xu et al., 2010).
Immunohistochemistry
The commercial sources and concentrations of the primary and secondary antibodies used in the immunohistochemical analyses are summarized in Table 1. For Ly-6G, Mac-1, and MPO immunohistochemistry, the sections were washed 3×10 min in TBS. Endogenous peroxidases were blocked by submerging sections in 3% hydrogen peroxide for 10 min, and washed 3×10 min in TBS. The sections were treated with 5% goat serum and 0.1% Triton-X-100 in TBS for at least 2 h at room temperature. Primary antibodies were left on tissue sections overnight at 4°C in a humidified chamber. The sections were incubated with biotinylated secondary antibodies in TBS for 1 h at room temperature, then washed 3×10 min in TBS and incubated in avidin-peroxidase conjugate (Elite Kit; Vector Laboratories, Burlingame, CA) for 45 min at room temperature. The signal was visualized using a peroxidase-diaminobenzidine (DAB; Zymed, Carlsbad, CA) reaction.
Table 1.
List of Antibodies Used in Immunohistochemistry
| Primary antibody | Antigen | Secondary antibody | Time point analyzed |
|---|---|---|---|
| Biotinylated mouse anti-Ly-6G (1:800; eBiosciences, San Diego, CA) | Ly-6G | ExtrAvidin®-Peroxidase (1:1000; Sigma-Aldrich, St. Louis, MO), followed by diaminobenzidine (DAB; Zymed, Carlsbad, CA) | 3, 14, 42 days post-injury |
| Rat mac-1 (CD11b, 1:600; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) | CD11b | Biotinylated goat anti-rat IgG (1:200; Jackson ImmunoResearch, West Grove, PA), followed by avidin-peroxidase conjugate (Vector Laboratories, Burlingame, CA), and diaminobenzidine (DAB; Zymed) | 3, 14, 42 days post-injury |
| Rabbit anti-human myeloperoxidase (1:500; Dako, Glostrup, Denmark) | MPO | Biotinylated donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch, West Grove, PA), followed by Avidin-peroxidase conjugate (Vector Laboratories) and diaminobenzine (DAB, Zymed) | 3, 14 days post-injury |
| Rabbit anti-5-HT (1:400; ImmunoStar, Hudson, WI) | Goat anti-rabbit IgG (AF488, 1:300; Invitrogen) | 42 days post-injury |
For serotonin (5-HT) immunostaining, sections were washed 3×10 minutes in TBS and then treated with 10% goat serum, 2% bovine serum albumin, and 0.3% Triton-X-100 in TBS for 2 h at room temperature. The sections were incubated in primary antibody (Table 1) in 3% goat serum, 2% bovine serum albumin, and 0.3% Triton X-100 in TBS overnight at 4°C in a humidified chamber. The sections were washed 3×10 min in TBS, incubated with the appropriate secondary antibodies (Table 1) with 3% goat serum, and 0.3% Triton-X-100 in TBS for 1 h at room temperature. The slides were washed 3×10 min in TBS and cover-slips were secured to the slides using Vectashield HardSet Mounting Media containing the fluorescent nuclear stain 4,6-diamino-2-phenylindole (DAPI; both from Vector Laboratories). The fluorescently-labeled sections were examined using an Olympus epifluorescence microscope (BX51). Control slides were processed in the same manner with the exception that blocking solution replaced the primary antibody.
Quantification of Ly-6G, MPO, and 5-HT expression
Every 10th transverse serial section from 5.0 mm rostral to 5.0 mm caudal to the lesion site was analyzed by immunohistochemistry for the number of Ly-6G- and MPO-positive cells at 3 and 14 days post-injury. The number of Ly-6G- and MPO-positive cells was counted using a light microscope. The area of 5-HT immunoreactivity (area per area of interest) was measured in the intermediolateral columns and in the ventral horns in sections obtained ~1920 μm caudal to the injury using Image Pro Plus Software.
Quantification of macrophages/microglia, white matter sparing, and collagen
Sections were immunostained with a Mac-1 antibody to identify macrophages/microglia. Sections were stained with solochrome cyanine to identify myelin, or with Mason’s trichrome to identify collagen (Xu et al., 2010). Images of every 10th section (160 μm apart) were digitally captured and the area demonstrating signal (per area of interest) was measured using Image Pro Plus Software. Because of the frequent distortion of the cord by scar and the compression at and near the lesion, white matter (per area) of sections from relatively intact cord at 3 mm rostral to the lesion epicenter in the same animal was also quantified and used to normalize the white matter areas closer to the lesion.
Locomotor function
The spinal cord-injured mice were assessed for locomotor outcomes using the Basso Mouse Scale (BMS; Basso et al., 2006). Locomotor function of the animals was assessed by two observers blinded to the experimental treatment of each mouse. Mice were scored in an open field-environment for 4 min. Scores ranged from 0 (complete paralysis) to 9 (normal mobility). Animals that scored higher than 0.5 on the day after surgery were excluded from the study. Individual scores from each hindlimb were averaged. The saline and 1B7 mAb-treated controls scored an average of 0.5 on the BMS scale, which is comparable to the degree of locomotor recovery seen in other studies using the 8-g clip compression injury in C57BL/6 mice (Chu et al., 2007).
Statistical analysis
Mean values are expressed ± standard error (SE). Statistical analyses were performed using GB-STAT software. Comparisons of the number of Ly-6G+ and MPO+ cells in different groups and assay results were made using Student’s t-tests. Data for locomotor recovery, myelin sparing, and Mac-1-positive cell density were analyzed by a two-way repeated measures ANOVA, as these measures change with time/distance and treatment. Differences between means were determined by a post-hoc Fisher least squares protected t-test. Changes in trichrome staining (scar formation) were analyzed by a one-way ANOVA, as these changes do not change with distance from the lesion. Differences between means were determined with a Newman-Keuls post-hoc test. In all cases comparisons of means were only performed if the results from the one-way or two-way ANOVA were significant. To evaluate whether the area of 5-HT immunoreactivity caudal to the lesion was greater in CD11d-treated mice, a one-tailed t-test was used. The correlation between the BMS scores and area of 5-HT immunoreactivity 1920 μm caudal to the injury was calculated using a simple linear regression analysis. Differences were considered statistically significant if p<0.05.
Results
Effect of the anti-CD11d treatment on leukocyte infiltration into the injured spinal cord 3 days post-injury
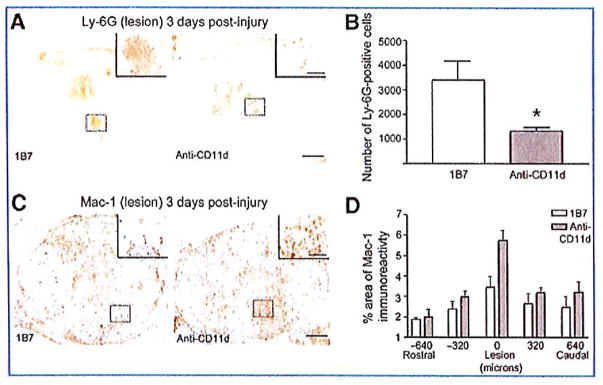
We assessed the effect of the anti-CD11d treatment on leukocyte infiltration into the injured mouse spinal cord. Three days post-injury the presence of neutrophils was assessed using the marker Ly-6G. Ly-6G immunostaining revealed that Ly-6G-expressing cells were found only at the lesion site, a distance that approximately spanned a length ±0.48 mm from the epicenter. At 72 h post-injury the number of Ly-6G+ cells at the lesion site in the CD11d mAb-treated group was significantly less than the number of Ly-6G+ cells at the lesion site in the 1B7 mAb-treated group (1328±147 and 3398±781, respectively; Fig. 1A and B; 6 mice/group, p<0.05 by Student’s t-test).
FIG. 1.
The anti-CD11d treatment reduces neutrophil infiltration into the cord at 3 days post-injury. Shown are representative photomicrographs of sections from the lesion epicenters of 1B7 and CD11d monoclonal antibody (mAb)-treated mice 3 days after spinal cord injury (SCI), immunostained with a Ly-6G antibody (A) or a Mac-1 antibody (C). Staining for neutrophils (Ly-6G) was decreased in mice treated with the CD11d mAb compared to mice treated with the control mAb (1B7). (B) The number of Ly-6G-positive cells at the lesion epicenter in CD11d mAb-treated mice (gray bars) was significantly reduced compared to control 1B7-treated mice (open bars). (D) The mean percent area of Mac-1 immunoreactivity at the lesion epicenter in CD11d mAb-treated mice (gray bars) tended to be increased compared to control 1B7-treated mice (open bars; scale bars in A and C = 100 μm, in high-power inset = 50 μm). In B and D values are means ± standard error (*p<0.05 in B significantly different from controls by two-tailed Student’s t-test, 6 animals/group; p=0.093 in D by two-way analysis of variance, 5 animals/group).
The effect of the anti-CD11d treatment on monocyte/microglia/macrophage populations in the spinal lesion was assessed by quantifying Mac-1 (CD11b) immunoreactivity. At 72 h post-injury, Mac-1-expressing cells were present both rostral and caudal to the lesion. At this time point the majority of Mac-1+ cells observed at the lesion site had large cell bodies with limited processes. The area of Mac-1+ cells was not significantly different between the anti-CD11d- and 1B7-treated groups (Fig. 1C and D; 5 mice/group, p=0.093 by two-way ANOVA).
Anti-CD11d treatment reduces neutrophil and macrophage infiltration into the injured spinal cord at 2 and 6 weeks post-injury
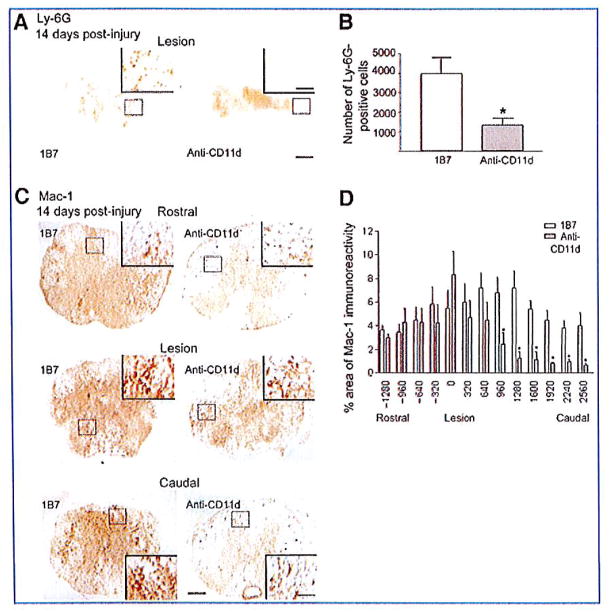
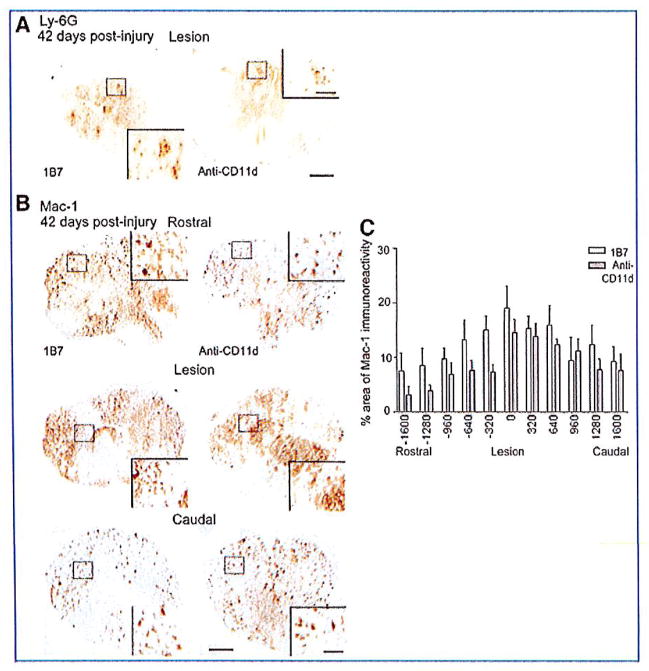
In the mouse model of SCI, peak numbers of neutrophils and macrophages are found between 3 and 14 days post-injury and persist chronically (Sroga et al., 2003). We therefore examined the influence of CD11d mAb on leukocyte infiltration at 2 and 6 weeks post-injury. At 14 days post-injury, Ly-6G+ cells were restricted to the region of tissue damage and within regions of tissue fibrosis. The number of Ly-6G+ cells in the injured spinal cords of mice treated with the CD11d mAb was significantly less than the number in 1B7 mAb-treated controls (1341±346 versus 3697±833, respectively, 6 mice/group, p<0.05 by Student’s t-test; Fig. 2A and B). At the lesion epicenters Mac-1-expressing cells were large, foamy, round, and mostly found in uninjured tissue outside the fibrous scar. Rostral to the lesion, the majority of Mac-1+ cells were stratified, with small cell bodies bearing multiple processes (Fig. 2C). The areas of Mac-1 immunoreactivity at the lesion epicenters, or rostral to the epicenters, did not differ significantly between the CD11d and 1B7 mAb-treated control mice. Caudal to the lesion sites the area of Mac-1 immunoreactivity in the CD11d mAb-treated spinal cords was significantly less than that in the control mice (p=0.025 by two-way ANOVA and p<0.05 by Fisher protected t-test, 6 mice/group; Fig. 2D). At 42 days post-injury there was no significant difference in the number of Ly-6G+ cells or the area of Mac-1+ immunoreactivity in CD11d mAb-treated mice compared to 1B7 mAb-treated controls (5 animals/1B7 group and 6 animals/anti-CD11d group, two-way ANOVA; Fig. 3).
FIG. 2.
CD11d monoclonal antibody (mAb) treatment reduces neutrophil and macrophage/microglia numbers in the injured mouse spinal cord 14 days post-injury. (A and C) Shown are representative photomicrographs of sections from the lesion epicenters of 1B7 and CD11d mAb-treated mice 14 days after spinal cord injury (SCI), immunostained with a Ly-6G antibody. (B) CD11d mAb treatment (gray bar) significantly reduced the number of neutrophils (Ly-6G+ cells) compared to controls (1B7, open bar). (C) Representative photomicrographs of sections at the epicenter or 960 μm caudal or rostral to the epicenter from anti-CD11d or control 1B7 mAb-treated mice stained with a Mac-1 antibody. Mac-1-expressing cells were large, foamy, and round at the lesion epicenters in both CD11d and 1B7 mAb-treated mice, and caudal to the epicenter in 1B7 mAb-treated mice. Rostral to the lesions in both CD11d and 1B7 mAb-treated mice, and caudal to the lesion epicenter in CD11d mAb-treated mice, the majority of Mac-1+ cells were stratified, with small cell bodies bearing multiple processes. (D) CD11d mAb (gray bars) treatment significantly reduced the percent area of Mac-1-immunoreactivity caudal to the lesion compared to controls (1B7, open bars; scale bars in A and C = 100 μm, 50 μm in high-power insets). In B and D values are means ± standard error (in B *p<0.05 significantly different from controls by two-tailed Student’s t-test, 6 mice/group; in D *p=0.025 by two-way analysis of variance and p<0.05 by Fisher protected t-test, 6 mice/group).
FIG. 3.
Macrophage/microglia and neutrophils are seen in the injured cord at 42 days post-injury. (A) Representative photomicrographs of sections from the lesion epicenters of 1B7 and CD11d monoclonal antibody (mAb)-treated mice 42 days after spinal cord injury (SCI), immunostained with a Ly-6G antibody. There was no significant difference in the number of Ly-6G+ cells in CD11d mAb and 1B7 mAb-treated mice 42 days after SCI. (B) Representative photomicrographs of sections at the epicenter or 960 μm caudal or rostral to the epicenter from 1B7 and CD11d mAb-treated mice 42 days after SCI, immunostained with a Mac-1 antibody. Mac-1+ cells were generally stratified in appearance caudal and rostral to the lesion site. At the lesion site, Mac-1 cells were more round in appearance. (C) There was no significant difference in Mac-1 areas of immunoreactivity between 1B7 mAb-treated mice (open bars) compared to anti-CD11d-treated mice (gray bars; 6 mice/1B7 group and 6 mice/anti-CD11d group, two-way analysis of variance; scale bars in A and B = 100 μm and 50 μm for high-power insets).
The anti-CD11d treatment reduces markers of secondary damage
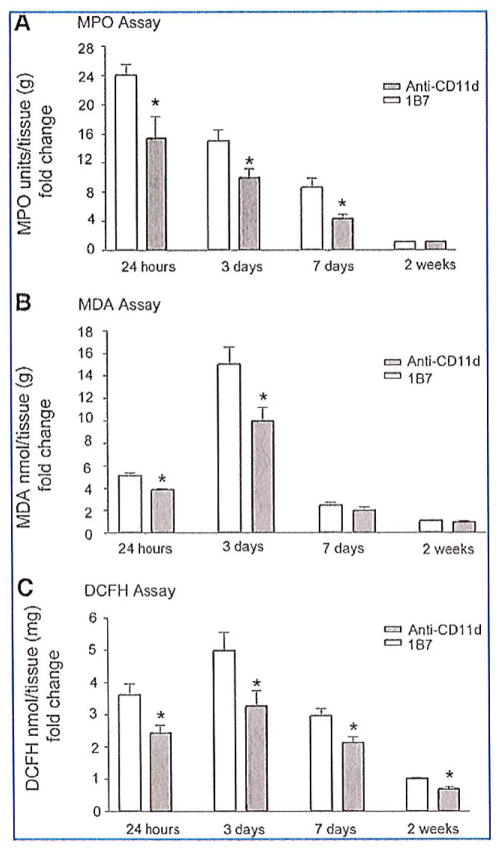
Leukocytes are major producers of hydrogen peroxide, superoxide anion, and free radicals in injured tissue (Braughler and Hall, 1992; Clark, 1990; Smith, 1994; Tidball, 2005). Myeloperoxidase expressed in neutrophils and macrophages catalyzes a reaction between hydrogen peroxide and chloride that yields hypochlorite, a strong oxidizing agent (Panasenko et al., 1995; Weiss, 1989). These ROS in the spinal lesion react with polyunsaturated fatty acids and result in lipid peroxidation and membrane damage. We predicted that the significant decrease of intraspinal neutrophils at 3 days post-injury in CD11d mAb-treated mice would lead to reductions in oxidative damage in their spinal cords. We performed biochemical assays to measure MPO activity, ROS, and lipid peroxidation levels. MPO activity peaked at 24 h after SCI, increasing 24-fold compared to uninjured controls. Treatment with the CD11d mAb significantly reduced MPO activity at 1, 3, and 7 days post-injury compared to 1B7 mAb-treated controls (p<0.05 by Student’s t-test; Fig. 4A).
FIG. 4.
CD11d monoclonal antibody (mAb) treatment decreases inflammation and oxidative damage in the injured spinal cord. (A) Myeloperoxidase (MPO) activity was measured in cord homogenates. CD11d mAb (gray bars) treatment significantly reduced MPO activity at 24 h, 3 days, and 7 days post-injury compared to controls (1B7, open bars). (B) Malondialdehyde (MDA), a measure of lipid peroxidation, was significantly reduced in cord homogenates by the anti-CD11d treatment (gray bars) compared to control treatment (1B7, open bars) at 24 h and 3 days post-injury. (C) 2′-7′-Dichlorofluorescin diacetate (DCFH-DA) oxidation, a measure of reactive oxygen species in injured cord, was significantly reduced by CD11d mAb treatment (gray bars) compared to control treatment (1B7, open bars). Values are means ± standard error (*p<0.05 significantly different from controls by Student’s t-test; n=4 at 24 h; n=6 at 3 days; n=4 at 7 days; n=6 at 2 weeks).
An indication of lipid peroxidation that could be due to free radical tissue damage and/or to activation of the arachidonic acid cascade was evaluated by a TBARS assay that measures the relative levels of MDA in tissue homogenates. Treatment with the CD11d mAb significantly reduced MDA levels, by 40% at 24 h and by 33% at 72 h post-injury compared to 1B7 mAb-treated controls. MDA activity in the spinal cord of CD11d and 1B7 mAb-treated mice returned to uninjured levels by 2 weeks post-injury (p<0.05 by Student’s t-test; Fig. 4B). We used the conversion of DCFH-DA to the oxidized fluorescent product DCF to assess the amount of ROS in the injured spinal cords. DCFH-DA was added to tissue homogenates and the formation of DCF was monitored by fluorescence. Concentrations of DCFH in spinal cord homogenates peaked 3 days post-injury. DCF levels in the spinal cord homogenates from CD11d mAb-treated mice were decreased by approximately 30% compared to DCF levels in 1B7 mAb-treated control homogenates at all time points (p<0.05 by Student’s t-test; Fig. 4C).
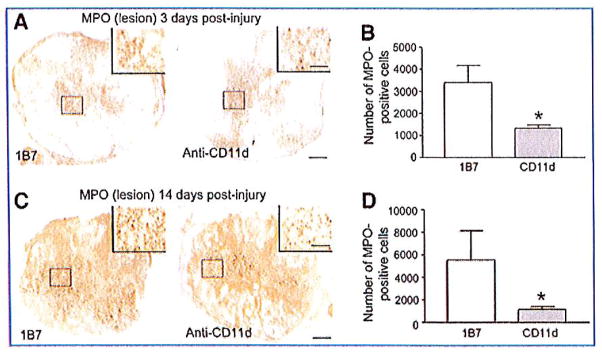
We were surprised to find no detectable levels of MPO activity at 14 days post-injury, as we detected neutrophils with immunohistochemistry using Ly-6G antibody at this same time point (Fig. 4A). To clarify this apparent discrepancy, we performed immunohistochemistry using an MPO antibody. Whereas the MPO activities measured at 2 weeks post-injury in tissue homogenates from the CD11d and 1B7-mAb-treated groups were very low, and not significantly different from one another, the MPO immunohistochemistry demonstrated a statistically significant reduction in MPO-expressing cells in CD11d mAb-treated mice compared to 1B7 mAb-treated controls (Fig. 5), as was also demonstrated using Ly-6G immunostaining (Fig. 2).
FIG. 5.
Myeloperoxidase (MPO) immunoreactivity in anti-CD11d and 1B7 monoclonal antibody (mAb)-treated mice. MPO-expressing cells were identified by immunostaining at 3 days post-injury (A) and 14 days post-injury (B) at the lesion epicenter. There was a significant decrease in MPO-expressing cells at 3 days post-injury and at 14 days post-injury in CD11d mAb-treated mice compared to 1B7 controls (scale bars = 100 μm and 50 μm for high-power inset). Values are means±standard error; *p<0.05 significantly different from controls by Student’s t-test, 4 animals/group).
Anti-CD11d treatment improves locomotor recovery in spinal cord-injured mice
The 8.3-g clip compression injury was severe and in all mice caused flaccid paralysis with little or no hindlimb movement during the first week post-injury (Fig. 6). CD11d mAb-treated mice began to improve at 3 days post-injury, and received significantly better scores by 14 days post-injury compared to 1B7 mAb-treated or saline control mice. At 42 days post-injury the CD11d mAb-treated group had an average score of 3.8±1.1 SEM. A score of 4 indicates occasional stepping and a score of 3 indicates plantar placement. Mice treated with the control mAb, 1B7, achieved an average score of 0.3±0.2 SEM. A score of 0 reflects no movement and a score of 1 indicates slight movement in one ankle (n=6 mice/1B7 group, n=4 mice/saline controls, and n=7 mice/anti-CD11d group; p<0.0001 by two-way ANOVA, and p<0.05 by Fisher’s protected t-test; Fig. 6).
FIG. 6.
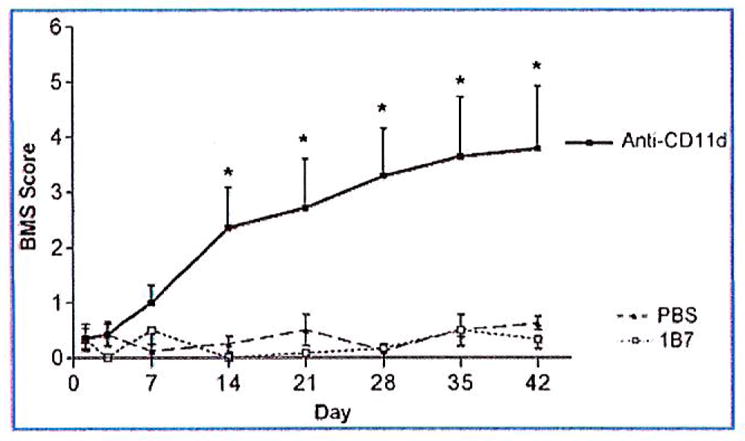
Locomotor recovery after a spinal cord compression injury. Locomotor function was assessed using the Basso Mouse Scale (BMS). CD11d monoclonal antibody (mAb; black boxes) treatment improved recovery beginning at 14 days post-injury compared to 1B7 controls (open boxes) or saline controls (black triangles). Values are means ± standard error; *significantly different from controls, p< 0.0001 by two-way analysis of variance, and p<0.05 by Fisher’s protected t-test; 6 mice/1B7 group, 4 mice/saline group, 7 mice/anti-CD11d group).
Anti-CD11d treatment increased myelin sparing and reduced scar formation
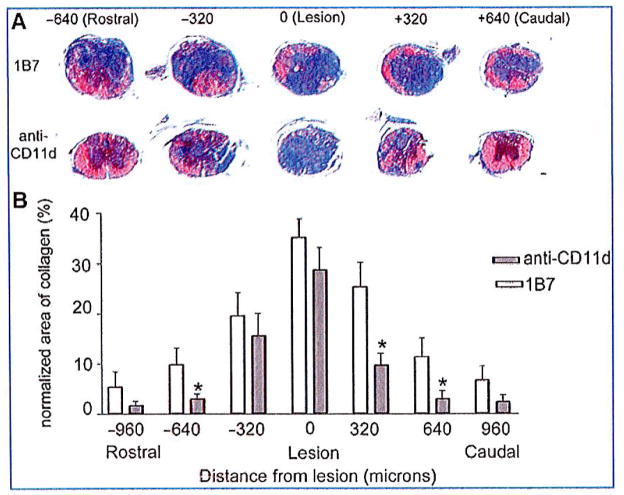
After locomotor evaluation at 42 days post-injury, the mice were euthanized by cardiac perfusion and their spinal cords processed for histology. Myelin sparing was assessed by measuring the area of blue staining in solochrome-stained sections, and the extent of scarring was assessed by the area of blue staining in Mason’s trichrome-stained sections. Improved functional recovery correlated with an increase in myelin sparing and reduced collagenous scar tissue. Solochrome staining revealed a near-total absence of myelin at all lesion epicenters, with only a small amount of myelin staining evident in the pial rim. Myelin sparing at the lesion epicenters, where the least amount of myelin was observed, did not differ between CD11d and 1B7-mAb treated mice. A significant increase in myelin sparing was observed beginning at 0.32 mm rostral and caudal to the lesion epicenter in CD11d mAb-treated mice compared to 1B7 mAb-treated controls (5 mice/1B7 group and 6 mice/anti-CD11d group; p = 0.008 by two-way ANOVA, and p< 0.05 by Fisher’s protected t-test; Fig. 7A and B). Reduction in the collagenous scar was also associated with the CD11d mAb treatment. The scar was most pronounced at the lesion epicenters in all spinal cord-injured mice. The scar was significantly reduced in the spinal cords of CD11d mAb-treated mice compared to 1B7 mAb-treated controls, beginning at 0.64 mm rostral and 0.32 mm caudal to the lesion epicenter (Fig. 8).
FIG. 7.
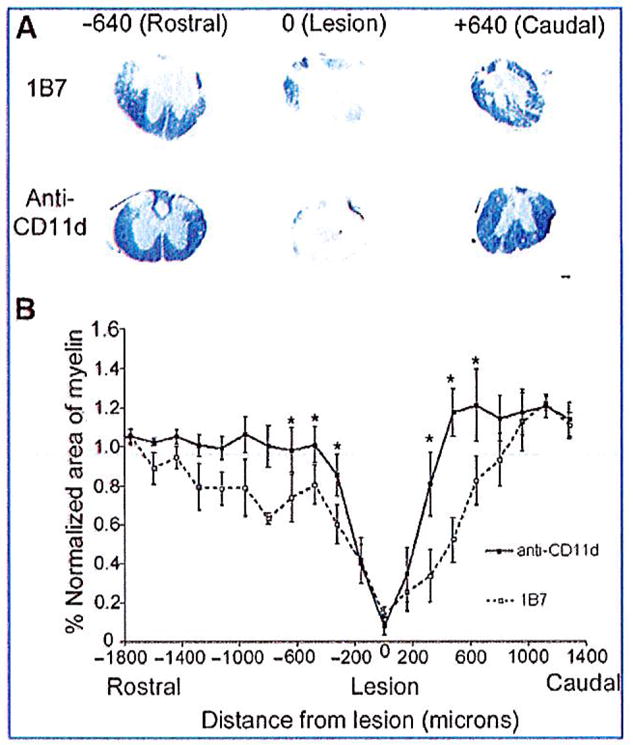
CD11d monoclonal antibody (mAb) treatment increases myelin sparing at 42 days after spinal cord injury (SCI). (A) Photomicrographs of solochrome-stained spinal cord sections from 1B7 and CD11d mAb-treated mice rostral (− 640 μm), caudal (+ 640 μm), and at the lesion site (0 μm). Very little myelin (indicated by blue staining) was present at the lesion epicenters. However, myelin increased rostral and caudal to the lesion in CD11d mAb-treated mice. (B) Solochrome staining of consecutive transverse sections from anti-CD11d and 1B7 mAb-treated mice was used to plot the amount of myelin from 1400 μm rostral to 1400 μm caudal to the lesion epicenters. The area of myelin in each section was normalized to the area of myelin in a section with normal morphology from the same cord far from the injury site. CD11d mAb treatment (solid line) increased the amount of myelin beginning at 360 μm rostral and caudal to the lesion compared to control treatment (1B7, dashed line; scale bar in A = 100 μm). Values are means ± standard error (*significantly different from controls, p= 0.008 by two-way analysis of variance, and p<0.05 by Fisher’s protected t-test; 5 mice/1B7 group and 6 mice/anti-CD11d group).
FIG. 8.
CD11d monoclonal antibody (mAb) treatment reduces the collagenous scar 42 days after spinal cord injury. (A) Trichrome staining was used to delineate the presence of collagen (blue color). Representative photomicrographs demonstrate large amounts of collagen at the injury site in CD11d and 1B7 mAb-treated mice. Rostral and caudal to their lesion epicenters, the CD11d mAb-treated mice had reduced levels of blue-stained collagen compared to the 1B7 control section, and almost no collagen was present in sections 640 μm rostral and 640 μm caudal to their lesion epicenters. (B) The area of collagen was reduced with CD11d mAb treatment (gray bars), rostral to the lesion site (−640 μm) and caudal to the lesion site (+320 μm), compared to controls (1B7, open bars; scale bar in A = 100 μm). (B) Values are means ± standard error (*significantly different from controls, p<0.0001 by one-way analysis of variance, and p<0.05 by Student Newman-Keuls test; 5 mice/1B7 group, 6 mice/anti-CD11d group).
The CD11d mAb increased serotonergic input and axon growth at and caudal to the lesion epicenters
Descending serotonergic (5-HT-positive) axons provide excitatory input to motor neurons, and the loss of these inputs has been correlated with locomotor dysfunction (Saruhashi et al., 1996). Serotonergic axons were examined in the injured spinal cords at 42 days post-injury. 5-HT-positive fibers could easily be identified at the lesion epicenters in both anti-CD11d- and 1B7-treated mice and appeared disorganized (Fig. 9). Whereas 5-HT-positive axons were largely absent caudal to the lesion site in all 1B7 mAb-treated control mice examined (n = 5), the CD11d mAb-treated mice (n = 6) demonstrated many 5-HT-positive axons caudal to the lesion that were localized to the intermediolateral columns and ventral horns (Fig. 9). Measuring the area of 5-HT-immunoreactivity per area of interest at 1920 μm caudal to the lesion epicenter using Image Pro Plus Software revealed a statistically significant increase in 5-HT immunoreactivity in the CD11d mAb-treated mice compared to 1B7 mAb-treated controls (p<0.05 by one-tailed t-test). Linear regression analysis showed a significant correlation between BMS scores 6 weeks post-injury and areas of 5-HT immunoreactivity 1920 μm caudal to the lesion epicenter (r2 = 0.89). These results suggest that the anti-CD11d treatment may help preserve serotonergic pathways.
FIG. 9.
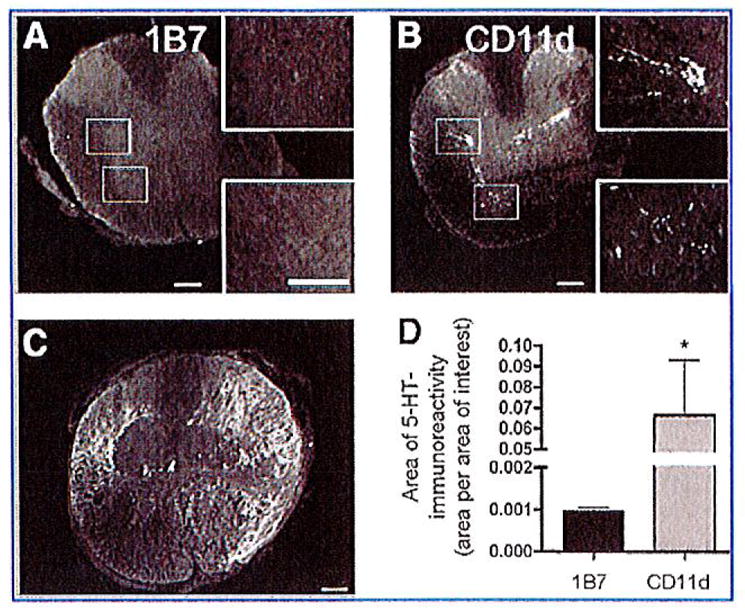
CD11d monoclonal antibody (mAb) treatment increases serotonin (5-HT) immunoreactivity caudal to the lesion. Immunohistochemistry was used to detect 5-HT in the spinal cord 42 days post-injury. Representative photomicrographs of sections stained for 5-HT immunoreactivity 1920 μm caudal to the lesion epicenter from 1B7- (A) or CD11d-treated (B) mice. (A) Almost no observable 5-HT staining was detected caudal to the lesion in 1B7-treated mice (5 mice/group). (B) Serotonin immunoreactivity was observed caudal to the lesion in the intermediolateral cell column and ventral horn in the injured spinal cords from animals treated with the CD11d mAb (6 mice/group). (C) Representative photomicrograph of a section from the lesion epicenter of a CD11d-treated mouse stained for 5-HT immunoreactivity. The appearance of the 5-HT immunoreactivity in the lesion epicenters of 1B7 mAb-treated controls was no different from the appearance of the 5-HT immunoreactivity seen in the CD11d mAb-treated mice (scale bars = 100 μm). (D) Measuring the area of 5-HT-immunoreactivity (area/area of interest) at 1920 μm caudal to the lesion epicenter in CD11d- and 1B7-treated spinal cord-injured mice revealed a significant increase in 5-HT immunoreactivity in the CD11d mAb-treated group.
Discussion
The anti-CD11d treatment was given acutely at 2, 24, and 48 h post-injury. Within the first 48 h after SCI in mice, neutrophils infiltrate the spinal cord in high numbers, while monocytes/macrophages have only begun to enter the injured cord. Immunohistochemistry using both Ly-6G and MPO antibodies demonstrate that the CD11d mAb reduced the number of neutrophils in the injured mouse spinal cord by approximately threefold at 3 days post-injury. In confirmation of these results we also observed reduced MPO activity in tissue homogenates from CD11d mAb-treated mice compared to tissue homogenates from 1B7 mAb-treated mice at 1, 3, and 7 days after injury. Despite reductions in MPO immunostaining at 2 weeks post-injury we did not detect any reduction in MPO activity in tissue homogenates from CD11d mAb-treated rats at 2 weeks after injury compared to 1B7 mAb-treated controls. This is likely due to reduced MPO enzyme activity in neutrophils and macrophages in the lesion site as inflammation enters a more chronic state.
The anti-CD11d treatment also reduced macrophage infiltration of the injured cord. We observed a reduction in Mac-1 immunostaining at 2 weeks post-injury. Neutrophil infiltration into injured tissue typically signals the onset of the inflammatory phase of wound healing, including the recruitment of monocytes to the injury site (Soehnlein et al., 2008). Furthermore, depletion of neutrophils impairs monocyte recruitment (Florido et al., 1997; Shiohara et al., 2004; Soehnlein et al., 2008). Thus the macrophage reduction observed in CD11d mAb-treated mice may be a response to the decrease in neutrophil activation and/or recruitment within their injured cords, rather than resulting from a direct blockade of macrophage infiltration.
To investigate the possibility that spared supraspinal inputs may contribute to the locomotor recovery seen in CD11d mAb-treated mice, we stained sections for 5-HT immunoreactivity, as supraspinal serotonergic inputs to the ventral horn are critical to motor function after SCI (Saruhashi et al., 1996). Sparing as little as 5–10% of the fibers at the lesion site is sufficient to produce basic locomotion in spinal cord-injured rats (Basso et al., 2006). The strong 5-HT immunoreactivity caudal to the lesion epicenter in the CD11d mAb-treated mice contrasts with the near-absence of 5-HT staining in all the 1B7 mAb-treated controls. The strong correlation (r2 = 0.89) between BMS scores at 6 weeks post-injury and the area of 5-HT immunoreactivity caudal to the lesion suggests that the increased BMS scores in CD11d mAb-treated mice are likely due to increased sparing of supraspinal serotonergic inputs.
We found that in mice, reduced intraspinal neutrophil numbers due to the anti-CD11d treatment led to improved neuroprotection and improved functional recovery. However, these results conflict with a recent mouse SCI study that found reduced functional recovery after acutely depleting neutrophils with a Ly-6G mAb (RB6-8C5) treatment (Stirling et al., 2009). There are several possible explanations for these conflicting results. The first is that the Ly-6G antibody may not be specific for neutrophils. The anti-Ly-6G antibody can also bind to Ly-6C, that in addition to neutrophils (Fleming et al., 1993), is also expressed on dendritic cells (Tvinnereim et al., 2002), lymphocytes (Han and Cutler, 1997), and Gr-1 + monocyte subsets (Daley et al., 2008). Thus the Ly-6G mAb may have depleted more than just neutrophils.
The second possible explanation for the differences seen between the Ly-6G and CD11d mAb treatment results in cord-injured mice is that the two antibodies reduce intraspinal neutrophil numbers via different mechanisms. If, as suggested by Stirling and associates, the anti-Ly-6G antibody not only depletes circulating but also the bone marrow neutrophil reserves (Stirling et al., 2009), the effects will be long-lasting, requiring time for the bone marrow to replenish neutrophil progenitor populations. In contrast, the CD11d mAb does not deplete CD11d+ cells, but rather just blocks the CD11d interaction with its receptor VCAM-I on endothelial cells, resulting in direct effects that are likely short-lived. By virtue of the fact that the Ly-6G antibody depletes bone marrow reserves and circulating neutrophils, this treatment may have had a more profound and protracted effect on neutrophil numbers in the injured cord, and in the circulation. This possibility is difficult to evaluate, as the Ly-6G antibody study did not evaluate neutrophil numbers in the injured spinal cord at times later than 48 h post-injury, nor were the effects of the neutrophil depletion on subsequent monocyte-derived macrophage recruitment that peaks at 7–14 days post-SCI assessed. It is likely that a moderate presence of neutrophils is necessary for wound healing. The benefit of the acute CD11d mAb treatment is that it blocks the early proinflammatory infiltration of leukocytes into the cord without depleting their number or progenitors, so that these cells may infiltrate at a later time point when the environment may promote their wound-healing and repair functions.
On the other hand, another group using the same Ly-6G antibody (RB6-8C5) have shown that while this antibody depletes circulating neutrophils, it does not deplete neutrophil progenitors in the bone marrow, but on the contrary induces myeloid cell expansion and inhibits the suppressor activity of myeloid-derived suppressor cell populations (Ribechini et al., 2009). If the Ly-6G antibody does indeed induce myelopoiesis and inhibit suppressor cell activity, then its use after SCI would be predicted to lead to a delayed but more robust inflammatory response, increased secondary damage, and decreased neurological recovery. We suggest that it may be the effect on myelopoiesis and lack of suppressor cell activity and not the acute effect on circulating neutrophils that explains the poor recovery in Ly-6G antibody-treated spinal cord-inured mice.
A third possible explanation for the discrepancy seen in the results of the Ly-6G and CD11d mAb studies rests on the different types of injuries used. The Ly-6G antibody study utilized a moderate contusion injury using the Infinite Horizon Impactor (Precision Scientific, Lexington, KY), whereas the CD11d mAb study utilized a much more severe clip compression injury that includes a significant ischemia-reperfusion component. The difference in the severity of these two types of injuries is apparent when one considers that the control animals in the Ly-6G mAb study achieved average BMS scores of 5.1 by 4 weeks post-injury, compared to average BMS scores < 0.5 for control animals in the CD11d mAb study. The more severe inflammatory reactions triggered by severe injuries may promote the damaging effects of neutrophils over their reparative functions. Thus severe injuries may respond more favorably than less severe injuries to strategies that reduce neutrophil infiltration into the injured cord. A fourth and related possible explanation for the discrepancy in the results of the Ly-6G and CD11d mAb studies is that different strains of mice and different levels of SCI were used in each study. Studies have shown that different mouse strains respond differently to spinal cord injury with respect to locomotor recovery and inflammatory reactions (Basso et al., 2006; Kigerl et al., 2006; Ma et al., 2004). It is likely that CD-1 and C57BL/6 mice respond differently to SCI such that reducing neutrophils in the injured spinal cord is detrimental to CD-1 recovery, but beneficial to C57BL/6 recovery. Furthermore it has also been reported that the immune response triggered by SCI may be different for high thoracic SCI (as performed in our study) compared to lower thoracic SCI (as performed in the study by Stirling and associates), as higher levels of SCI (T3–T6) impair sympathetic innervation of lymphoid tissue (Lucin et al., 2007; Popovich and McTigue, 2009).
Our results strongly support the use of the anti-CD11d treatment as a viable option for SCI. Our results further point to the importance of characterizing the effect of anti-inflammatory treatments on all relevant cell populations and at acute and chronic time points post-injury. Finally anti-inflammatory treatments may need to be assayed in models with a variety of injury severities, as we predict that the greatest beneficial effects of anti-inflammatory strategies may rest in those instances in which inflammation plays the greatest role in the evolving secondary damage.
Acknowledgments
This work was supported in part a grant from the Canadian Institutes of Health Research (CIHR).
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bao F, Chen Y, Dekaban GA, Weaver LC. An anti-CD11d integrin antibody reduces cyclooxygenase-2 expression and protein and DNA oxidation after spinal cord injury in rats. J Neurochem. 2004;90:1194–1204. doi: 10.1111/j.1471-4159.2004.02580.x. [DOI] [PubMed] [Google Scholar]
- 2.Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 2004;88:1335–1344. doi: 10.1046/j.1471-4159.2003.02240.x. [DOI] [PubMed] [Google Scholar]
- 3.Bao F, Dekaban GA, Weaver LC. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. J Neurochem. 2005;94:1361–1373. doi: 10.1111/j.1471-4159.2005.03280.x. [DOI] [PubMed] [Google Scholar]
- 4.Bartholdi D, Schwab ME. Methylprednisolone inhibits early inflammatory processes but not ischemic cell death after experimental spinal cord lesion in the rat. Brain Res. 1995;672:177–186. doi: 10.1016/0006-8993(94)01410-j. [DOI] [PubMed] [Google Scholar]
- 5.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 6.Blight AR. Macrophages and inflammatory damage in spinal cord injury. J Neurotrauma. 1992; 9(Suppl 1):S83–S91. [PubMed] [Google Scholar]
- 7.Braughler JM, Hall ED. Involvement of lipid peroxidation in CNS injury. J Neurotrauma. 1992;9(Suppl 1):S1–S7. [PubMed] [Google Scholar]
- 8.Brown A, Ricci MJ, Weaver LC. NGF message and protein distribution in the injured rat spinal cord. Exp Neurol. 2004;188:115–127. doi: 10.1016/j.expneurol.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Brown A, Ricci MJ, Weaver LC. NGF mRNA is expressed in the dorsal root ganglia after spinal cord injury in the rat. Exper Neurol. 2007;205:283–286. doi: 10.1016/j.expneurol.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Chu GK, Yu W, Fehlings MG. The p75 neurotrophin receptor is essential for neuronal cell survival and improvement of functional recovery after spinal cord injury. Neuroscience. 2007;148:668–682. doi: 10.1016/j.neuroscience.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Clark RA. The human neutrophil respiratory burst oxidase. J Infect Dis. 1990;161:1140–1147. doi: 10.1093/infdis/161.6.1140. [DOI] [PubMed] [Google Scholar]
- 12.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukocyte Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exper Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 15.Florido M, Appelberg R, Orme IM, Cooper AM. Evidence for a reduced chemokine response in the lungs of beige mice infected with Mycobacterium avium. Immunology. 1997;90:600–606. doi: 10.1046/j.1365-2567.1997.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorio A, Madaschi L, Zadra G, Marfia G, Cavalieri B, Bertini R, Di Giulio AM. Reparixin, an inhibitor of CXCR2 function, attenuates inflammatory responses and promotes recovery of function after traumatic lesion to the spinal cord. J Pharm Exp Ther. 2007;322:973–981. doi: 10.1124/jpet.107.123679. [DOI] [PubMed] [Google Scholar]
- 17.Grayson MH, Van der Vieren M, Sterbinsky SA, Gallantin WM, Hoffman P, Staunton D, Bochner BS. alphadbeta2 integrin is a ligand for vascular cell adhesion molecule-1. Int Arch Allergy Immunol. 1999;118:263–264. doi: 10.1159/000024094. [DOI] [PubMed] [Google Scholar]
- 18.Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamada Y, Ikata T, Katoh S, Nakauchi K, Niwa M, Kawai Y, Fukuzawa K. Involvement of an intercellular adhesion molecule 1-dependent pathway in the pathogenesis of secondary changes after spinal cord injury in rats. J Neurochem. 1996;66:1525–1531. doi: 10.1046/j.1471-4159.1996.66041525.x. [DOI] [PubMed] [Google Scholar]
- 20.Han Y, Cutler JE. Assessment of a mouse model of neutropenia and the effect of an anti-candidiasis monoclonal antibody in these animals. J Infect Dis. 1997;175:1169–1175. doi: 10.1086/516455. [DOI] [PubMed] [Google Scholar]
- 21.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 22.Jacob JE, Gris P, Fehlings MG, Weaver LC, Brown A. Autonomic dysreflexia after spinal cord transection or compression in 129Sv, C57BL, and Wallerian degeneration slow mutant mice. Exp Neurol. 2003;183:136–146. doi: 10.1016/s0014-4886(03)00161-4. [DOI] [PubMed] [Google Scholar]
- 23.Joshi M, Fehlings MG. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 1. Clip design, behavioral outcomes, and histopathology. J Neurotrauma. 2002;19:175–190. doi: 10.1089/08977150252806947. [DOI] [PubMed] [Google Scholar]
- 24.Kigerl KA, McGaughy VM, Popovich PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol. 2006;494:578–594. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YL, Shin K, Bao P, Ghirnikar RS, Eng LF. Cytokine chemokine expression in contused rat spinal cord. Neurochem Int. 2000;36:417–425. doi: 10.1016/s0197-0186(99)00133-3. [DOI] [PubMed] [Google Scholar]
- 26.Lucin KM, Sanders VM, Jones TB, Malarkey WB, Popovich PG. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exper Neurol. 2007;207:75–84. doi: 10.1016/j.expneurol.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma M, Wei P, Wei T, Ransohoff RM, Jakeman LB. Enhanced axonal growth into a spinal cord contusion injury site in a strain of mouse (129X1/SvJ) with a diminished inflammatory response. J Comp Neurol. 2004;474:469–486. doi: 10.1002/cne.20149. [DOI] [PubMed] [Google Scholar]
- 28.Mabon PJ, Weaver LC, Dekaban GA. Inhibition of monocyte/macrophage migration to a spinal cord injury site by an antibody to the integrin alphaD: A potential new anti-inflammatory treatment. Exp Neurol. 2000;166:52–64. doi: 10.1006/exnr.2000.7488. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen HX, O’Barr TJ, Anderson AJ. Polymorphonuclear leukocytes promote neurotoxicity through release of matrix metalloproteinases reactive oxygen species, and TNF-alpha. J Neurochem. 2007;102:900–912. doi: 10.1111/j.1471-4159.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 30.Oatway MA, Chen Y, Bruce JC, Dekaban GA, Weaver LC. Anti-CD11d integrin antibody treatment restores normal serotonergic projections to the dorsal, intermediate, and ventral horns of the injured spinal cord. J Neurosci. 2005;25:637–647. doi: 10.1523/JNEUROSCI.3960-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 32.Panasenko OM, Evgina SA, Driomina ES, Sharov VS, Sergienko VI, Vladimirov YA. Hypochlorite induces lipid peroxidation in blood lipoproteins and phospholipid liposomes. Free Radic Biol Med. 1995;19:133–140. doi: 10.1016/0891-5849(94)00211-2. [DOI] [PubMed] [Google Scholar]
- 33.Popovich P, McTigue D. Damage control in the nervous system: beware the immune system in spinal cord injury. Nature Med. 2009;15:736–737. doi: 10.1038/nm0709-736. [DOI] [PubMed] [Google Scholar]
- 34.Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- 35.Ribechini E, Leenen PJ, Lutz MB. Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur J Immunol. 2009;39:3538–3551. doi: 10.1002/eji.200939530. [DOI] [PubMed] [Google Scholar]
- 36.Saruhashi Y, Young W, Perkins R. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp Neurol. 1996;139:203–213. doi: 10.1006/exnr.1996.0094. [DOI] [PubMed] [Google Scholar]
- 37.Saville LR, Pospisil CH, Mawhinney LA, Bao F, Simedrea FC, Peters AA, O’Connell PJ, Weaver LC, Dekaban GA. A monoclonal antibody to CD11d reduces the inflammatory infiltrate into the injured spinal cord: a potential neuroprotective treatment. J Neuroimmunol. 2004;156:42–57. doi: 10.1016/j.jneuroim.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Schnell L, Fearn S, Klassen H, Schwab ME, Perry VH. Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J Neurosci. 1999;11:3648–3658. doi: 10.1046/j.1460-9568.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- 39.Shiohara M, Gombart AF, Sekiguchi Y, Hidaka E, Ito S, Yamazaki T, Koeffler HP, Komiyama A. Phenotypic and functional alterations of peripheral blood monocytes in neutrophil-specific granule deficiency. J Leukocyte Biol. 2004;75:190–197. doi: 10.1189/jlb.0203063. [DOI] [PubMed] [Google Scholar]
- 40.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukocyte Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 41.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- 43.Stirling DP, Liu S, Kubes P, Yong VW. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J Neurosci. 2009;29:753–764. doi: 10.1523/JNEUROSCI.4918-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taoka Y, Okajima K. Spinal cord injury in the rat. Prog Neurobiol. 1998;56:341–358. doi: 10.1016/s0301-0082(98)00049-5. [DOI] [PubMed] [Google Scholar]
- 45.Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, Naruo M, Okabe H, Takatsuki K. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79:1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 46.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 47.Tonai T, Shiba K, Taketani Y, Ohmoto Y, Murata K, Muraguchi M, Ohsaki H, Takeda E, Nishisho T. A neutrophil elastase inhibitor (ONO-5046) reduces neurologic damage after spinal cord injury in rats. J Neurochem. 2001;78:1064–1072. doi: 10.1046/j.1471-4159.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- 48.Tvinnereim AR, Hamilton SE, Harty JT. CD8(+)-T-cell response to secreted and nonsecreted antigens delivered by recombinant Listeria monocytogenes during secondary infection. Infect Immun. 2002;70:153–162. doi: 10.1128/IAI.70.1.153-162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van der Vieren M, Crowe DT, Hoekstra D, Vazeux R, Hoffman PA, Grayson MH, Bochner BS, Gallatin WM, Staunton DE. The leukocyte integrin alpha D beta 2 binds VCAM-1: evidence for a binding interface between I domain and VCAM-1. J Immunol. 1999;163:1984–1990. [PubMed] [Google Scholar]
- 50.Van der Vieren M, Le Trong H, Wood CL, Moore PF, St John T, Staunton DE, Gallatin WM. A novel leukointegrin, alpha d beta 2, binds preferentially to ICAM-3. Immunity. 1995;3:683–690. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 51.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 52.Xu X, Geremia N, Bao F, Pniak A, Rossoni M, Brown A. Schwann cell co-culture improves the therapeutic effect of bone marrow stromal cells on recovery in spinal cord-injured mice. Cell Transplantation. 2010;20:1065–1086. doi: 10.3727/096368910X544906. [DOI] [PMC free article] [PubMed] [Google Scholar]
This article has been cited by
- 1.Zhang B, Gensel JC. Is neuroinflammation in the injured spinal cord different than in the brain? Examining intrinsic differences between the brain and spinal cord. Experimental Neurology. 2014;258:112–120. doi: 10.1016/j.expneurol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Bastien Dominic, Lacroix Steve. Cytokine pathways regulating glial and leukocyte function after spinal cord and peripheral nerve injury. Experimental Neurology. 2014;258:62–77. doi: 10.1016/j.expneurol.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Nardone Raffaele, Höller Yvonne, Thomschewski Aljoscha, Höller Peter, Lochner Piergiorgio, Golaszewski Stefan, Brigo Francesco, Trinka Eugen. Serotonergic transmission after spinal cord injury. Journal of Neural Transmission. 2014 doi: 10.1007/s00702-014-1241-z. [DOI] [PubMed] [Google Scholar]
- 4.John C. GenselSpinal Cord Injury. pp. 339–361. [Google Scholar]
- 5.Vaughn Chloe N, Iafrate Julia L, Henley Jessica B, Stevenson Edward K, Shlifer Igor G, Bucky Jones T. Cellular Neuroinflammation in a Lateral Forceps Compression Model of Spinal Cord Injury. The Anatomical Record. 2013;296(8):1229–1246. doi: 10.1002/ar.22730. [DOI] [PubMed] [Google Scholar]
- 6.Shultz Sandy R, Bao Feng, Weaver Lynne C, Cain Donald P, Arthur Brown. Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion. Journal of Neuroinflammation. 2013;10(1):26. doi: 10.1186/1742-2094-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stahel Philip F, VanderHeiden Todd, Finn Michael A. Management strategies for acute spinal cord injury. Current Opinion in Critical Care. 2012;18(6):651–660. doi: 10.1097/MCC.0b013e32835a0e54. [DOI] [PubMed] [Google Scholar]
- 8.Brown JM, Xia J, Zhuang B, Cho K-S, Rogers CI, Gama CI, Rawat M, Tully SE, Uetani N, Mason DE, Tremblay ML, Peters EC, Habuchi O, Chen DF, Hsieh-Wilson LC. A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proceedings of the National Academy of Sciences. 2012;109(13):4768–4773. doi: 10.1073/pnas.1121318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan Faith H, Anderson Aileen J, Taylor Stephen M, Woodruff Trent M, Marc J Ruitenberg. Complement activation in the injured central nervous system: another dual-edged sword? Journal of Neuroinflammation. 2012;9(1):137. doi: 10.1186/1742-2094-9-137. [DOI] [PMC free article] [PubMed] [Google Scholar]