Abstract
Traumatic injury to the spinal cord triggers a systemic inflammatory response syndrome (SIRS), in which inflammatory cells from the circulation invade organs such as the liver, lung and kidney, leading to damage of these organs. Our previous study (Gris, et al, Exp. Neurol, 2008) demonstrated that spinal cord injury (SCI) activates circulating neutrophils that then invade the lung and kidney from 2 to 24 h after injury, increasing myeloperoxidase activity, cyclooxygenase-2 and matrix metalloproteinase-9 expression and lipid peroxidation in these organs. The present study was designed to ascertain whether a treatment that limits the influx of leukocytes into the injured spinal cord would also be effective in reducing the SIRS after SCI. This treatment is intravenous delivery of a monoclonal antibody (mAb) against the CD11d subunit of the CD11d/CD18 integrin expressed by neutrophils and monocytes. We delivered the anti-CD11d mAb at 2 h post moderate clip compression SCI at the 4th or 12th thoracic segments and assessed inflammation, oxidative activity and cellular damage within the lung, kidney and liver at 12 h post-injury. In some analyses we compared high and low thoracic injuries to evaluate the importance of injury level on the intensity of the SIRS. After T4 injury, treatment with the anti-integrin mAb reduced the presence of neutrophils and macrophages in the lung, with associated decreases in expression of NF-κB and oxidative enzymes and in the concentration of free radicals in this organ. The treatment also reduced lipid peroxidation, protein nitration and cell death in the lung. The anti-CD11d treatment also reduced the inflammatory cells within the kidney after T4 injury, as well as the free radical concentration and amount of lipid peroxidation. In the liver, the mAb treatment reduced the influx of neutrophils but most of the other measures examined were unaffected by SCI. The inflammatory responses within the lung and kidney were often greater after T4 than T12 injury. Clinical studies show that SIRS, with its associated organ failure, contributes significantly to the morbidity and mortality of SCI patients. This anti-integrin treatment may block the onset of SIRS after SCI.
Keywords: Spinal cord injury, Systemic inflammatory response, Organ damage, Anti-integrin treatment, Lung, Liver, Kidney
Traumatic injury to the brain and spinal cord triggers a systemic inflammatory response syndrome (SIRS), in which inflammatory cells from the circulation invade organs such as the liver, lung and kidney, leading to damage of these organs (Campbell et al., 2003, 2005; Gris et al., 2008; Perry et al., 2003; Wilcockson et al., 2002). In the spinal cord, these events occur in parallel with the well known secondary damage caused by the acute inflammatory response to the injury (Blight 1992; Fleming et al., 2006; Popovich et al., 1997; Saville et al., 2004; Taoka et al., 1997). Organ failure contributes significantly to the morbidity and mortality of SCI patients (Acosta et al., 1998); the lung and kidney are major targets of the SCI-induced SIRS (Catz et al., 2002; Devivo et al., 1999; O’Connor 2005; Pickett et al., 2006). Life-threatening lung dysfunction is extremely difficult to treat (Bhatia et al., 2005; Kyono and Coates 2002; Ryugo et al., 2006). The SIRS may be initiated by a cord injury-induced influx of leukocytes into the liver, secondary to hepatic chemokine expression that occurs within 2 h of the injury (Campbell et al., 2003, 2005; Perry et al., 2003; Wilcockson et al., 2002). The liver then produces acute phase proteins such as complement protein C3 and C-reactive protein as well as CXC and CC chemokines, CINC1 and CCL-2; these proteins and chemokines are then released into the circulation (Campbell et al., 2005; Wilcockson et al., 2002).
Such systemic inflammation likely contributes to organ dysfunction after SCI, as it does after brain injury and other traumatic insults (Acosta et al., 1998; Baskaran et al., 2000; Bhatia et al., 2005; Gabay and Kushner 1999; Ott et al., 1994). Indeed, our previous study (Gris et al., 2008) demonstrated that SCI activates circulating neutrophils, increases their longevity and promotes their production of oxygen free-radicals. These neutrophils invaded the lung and kidney from 2 to 24 h after SCI, with major effects noted at 12 h. In parallel with this invasion, myeloperoxidase (MPO) activity, cyclooxygenase-2 (COX-2) and matrix metalloproteinase-9 expression and lipid peroxidation in these organs developed or increased. These findings demonstrate that compression SCI leads to a SIRS that imparts damage to key organs such as the lungs and kidneys. We proposed a model in which the SIRS is initiated by pro-inflammatory mediators released from the lesion into the circulation after the SCI (Campbell et al., 2005; Wang et al., 1997). This activates circulating neutrophils and monocytes as well as endothelial cells of systemic and spinal cord blood vessels, providing the opportunity for the leukocytes, particularly neutrophils, to engage in adhesion molecule-mediated diapedesis into uninjured organs such as the lungs and kidneys as well as the injured spinal cord. This influx of neutrophils leads to organ damage and dysfunction that would negatively impact on recovery from traumatic SCI (Campbell et al., 2003, 2005; Gris et al., 2008; Perry et al., 2003; Wilcockson et al., 2002).
The purpose of the present study was to ascertain whether a treatment that we have put forth to prevent the acute inflammatory response within the injured spinal cord, would also be effective in reducing SIRS after SCI. This treatment is intravenous delivery of a monoclonal antibody (mAb) against the CD11d subunit of the CD11d/CD18 integrin expressed by neutrophils and monocytes. This CD11dβ2 integrin family member binds to VCAM-1 in rats and to ICAM-3 and VCAM-1 in humans, facilitating firm adhesion to extracellular matrix molecules (Grayson et al., 1998; Van der Vieren et al., 1999, 1995). When administered within 2–6 h after SCI, the anti-CD11d mAb blocks migration of neutrophils and monocytes into the injured cord, leading to decreased oxidative damage to the cord, to intraspinal tissue sparing and to improved neurological outcomes (Ditor et al., 2006; Fleming et al., 2008; Gris et al., 2004; Oatway et al., 2005; Saville et al., 2004). In the current study, we delivered the anti-CD11d mAb at 2 h post moderate clip compression SCI at the 4th or 12th thoracic segments and assessed inflammation, oxidative activity and cellular damage within the lung at 12 h post-injury. In most of the lung analyses we compared high and low thoracic injuries to evaluate the importance of level of injury on the intensity of the SIRS and on the effectiveness of the mAb treatment. We studied the lung extensively as our previous study demonstrated a robust SIRS in the lung after SCI (Gris et al., 2008), but limited analyses also were performed on the kidney and liver.
Materials and methods
Spinal cord injury and antibody treatment
All protocols for these experiments were done in accordance with the policies established by the Canadian Council on Animal Care. Fifty female Wistar rats (Charles River, St. Constant, Quebec) weighing 200–220 g were used and the 4th (T4) or 12th (T12) thoracic spinal cord segment was moderately injured by clip compression as described previously (Bao et al., 2004; Weaver et al., 2001). After the T4 or T12 SCI, rats were given one treatment with the anti-CD11d mAb 217 L (1.0 mg/kg, via the tail vein) at 2 h after injury followed by a 12 h survival time post-injury. Control rats were given an isotype-matched mAb (1B7) instead of the anti-CD11d mAb. Ten rats per treatment were used to study effects at each site of injury. One set of five rats was used for biochemical assays and a second set of five was used for immunocytochemistry. Another group of rats without injury was used as an uninjured control group (n=10). Occasionally the uninjured groups contained 6 rats for the biochemical assays. The mAb 217L and 1B7 were generously provided by the former ICOS Corporation (mAb currently owned by Eli Lilly & Co., Indianapolis, IN).
Tissue preparation for Western blotting analysis and morphological examination
For morphological examination, uninjured animals and animals 12 h after injury were anesthetized and perfused transcardially with saline, followed by 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.2–7.4. Lungs were perfused separately via the pulmonary artery. Lung (left), liver (right lobe) and kidney (left) were removed, and post-fixed for 24 h at 4 °C, cryoprotected in increasing concentrations of sucrose and sectioned into 25 μm sections for immunohistochemical staining. For biochemical and Western blotting analyses, uninjured rats and the rats at 12 h post-injury were perfused with cold 0.9% NaCl first transcardially and then via the pulmonary artery. Lung (left), liver (right lobe) and kidney (left) were removed and stored at −80 °C until they were used.
Assessing infiltration of phagocytic leukocytes
Infiltration of phagocytic leukocytes was detected by an MPO assay, by immunohistochemical staining of tissue sections and by Western blotting. For the MPO assay, the tissue (lung, liver and kidney) homogenates were incubated in a 96-well plate in a developing buffer that consisted of 100 μl of K-PBS and 100 μl of o-dianosinisidine (12.5 mg per 10 ml distilled water and 9 μl of 0.05% H2O2). The reaction was stopped by addition of 100 μl of 1% NaH3 in each well. The plate was scanned using a 96-well plate reader (Multiskan Ascent, Thermo Fisher Scientific, Waltham, MA) at a wavelength of 450 nm. For every plate, one standard curve in triplicate was performed using MPO from human leukocytes (Sigma Chemical Company, St. Louis, MO) (Bao et al., 2004).
For immunohistochemical staining, randomly selected sections from lung liver and kidney were processed free-floating for staining as described previously (Weaver et al., 2001). A rabbit anti-rat neutrophil polyclonal antibody diluted 1:20,000 (Anthony et al., 1998) (gift of Dr. Daniel Anthony, Oxford University, Oxford, UK) was used to identify neutrophil infiltration and an anti-rat ED-1 antibody (1:500, Serotec, Raleigh, NC; analogous to CD68 in humans) was used to identify phagocytic macrophages in the tissues. Sections were next incubated overnight with biotinylated donkey anti-rabbit antibody (1:1000 dilution) (Jackson Laboratories, West Grove, PA). The immunoreactivity was visualized using diaminobenzidine [(DAB) Sigma Chemical Company, as before] as a chromogen. The slides were viewed using an Olympus microscope (BX50, Olympus America Inc., Center Valley, PA) and photomicrographs were acquired using a digital camera (Retiga, Quantitative Imaging Corporation, Burnaby, BC, Canada) and Image Pro software version 5.1 (Media Cybernetics, Silver Spring, MD).
Macrophage ED-1 protein expression in the lung, liver and kidney was quantified by Western blot analysis. We also used the anti-neutrophil antibody in the Western blots, searching for a means of quantifying the neutrophil presence in the organ homogenates. This experiment was successful, yielding a clear band at 56 kDa. Proteins derived from tissues homogenates were loaded onto 7% or 10% polyacrylamide gel separated by SDS/PAGE using a Bio-Rad Mini-Protean 3 apparatus (Bio-Rad, Hercules, CA), and transferred to polyvinylidene difluoride (PVDF) membranes (0.45 μm pore size, Millipore, Mississauga, ON). The membranes were first blocked with 5% non-fat powdered milk and then incubated with the ED-1 antibody or the anti-neutrophil antibody followed by incubation with horse-radish peroxidase (HP)-conjugated donkey anti-mouse secondary antibody. The signal was developed using an enhanced chemiluminescence (ECL plus) detection system (Amersham, Oakville, ON). Band intensity was measured using Lab Works software (UVP, Upland, CA). Densitometric values were normalized for protein loading using β-actin (antibody from Sigma, St. Louis, MO) as a loading control and for local background. Molecular weights of the proteins detected were determined using known molecular weight protein standards (BioRad Prestained Precision Protein Standards).
Assessing oxidative enzymes and Nuclear Factor-κB
Oxidative enzymes in lung were detected by immunohistochemical staining of the catalytic subunit (gp91phox) of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase with an mouse anti-rat gp91phox antibody (1:500, Upstate biotechnology, Lake Placid, NY) and quantified by Western blot analysis using methods described previously (Bao et al., 2004). Another two oxidative enzymes, inducible nitric oxide (iNOS) and COX-2 were also detected by Western blot analysis with polyclonal rabbit anti-iNOS (Oxford Biomedical Research, Oxford, MI) and polyclonal rabbit anti-COX-2 (Cayman Chemical, Ann Arbor, MI) antibodies as previously described (Bao et al., 2004). The inflammatory factor, transcription factor Nuclear Factor-κB (NF-κB) was identified by a mouse anti-rat NF-κB antibody, (Abcam Inc, Cambridge MA) using immunohistochemical staining of lung sections and identified by Western blot analysis.
Assessing free radical production (DCFH-DA assay)
To assess free radical production in the lung and kidney, we used 2′-7′-dichlorofluorescin diacetate (DCFH-DA) as a probe for free radical detection and the resulting DCF formation was monitored by the fluorescence intensity. DCFH-DA is hydrolyzed to DCFH by esterase cleavage of the diacetate group. This compound is oxidized by reactive oxygen species to form the fluorescent compound, 2′-7′-dichlorofluorescein (DCF). For ex vivo detection of free radicals, an aliquot of the lung or kidney homogenate sample (25 μl) from the same animals used for MPO assay was incubated with 0.1 mM DCFH-DA at 37 °C for 30 min. The formation of the oxidized fluorescent derivative DCF was monitored at an excitation wavelength of 488 nm and an emission wavelength of 525 nm using a fluorescence spectrophotometer as described previously (Bao et al., 2005). Background fluorescence was corrected by the inclusion of parallel blanks. The formation of reactive oxygen species was quantified using a DCF standard curve, and results were expressed as nmol DCF/mg protein.
Assessing lipid peroxidation, protein nitration and cell death
Malondialdehyde (MDA) is used as a marker for lipid peroxidation, and was quantified in the homogenates of the lung, liver and kidney using a thiobarbituric acid reactive substances (TBARS) assay as described previously (Bao et al., 2004). A standard curve was established using MDA bis(dimethyl acetal) (Sigma-Aldrich), and lipid peroxidation was expressed as nmol of TBARS/g tissue. Lipid peroxidation in lung was also detected by the presence of 4-hydroxynonenal (HNE)-bound proteins by Western blots, using a mouse anti-HNE monoclonal antibody (1:5000, Alpha Diagnostic International, San Antonio, TX) and 10% polyacylamide gels. Protein nitration in the lung was detected by immunohistochemical staining and quantified by Western blot analysis with an antibody to nitrotyrosine (anti-Ntyr, Upstate, Lake Placid, NY) and cell death in the lung was quantified by Western blotting for caspase-3 (anti-caspase-3, Upstate, Lake Placid, NY).
Statistical analyses
Mean values are expressed±standard error (S.E.). Results were subjected to parametric statistical analysis using one-way analysis of variance [ANOVA (Snedecor and Cochran 1989)]. This analysis included data from the uninjured rats, from rats with injury at T4 and from those with injury at T12. Although some of the data appear to be suitable for a two-way ANOVA, the uninjured group did not have two levels of treatment and comparisons between the uninjured group and the two different SCI groups were essential, making it necessary to use a one-way ANOVA. Differences between means were determined by the post hoc Student Neuman Keuls test. Significance was accepted at P<0.05. The power of the performed tests always exceeded 0.80.
Results
Anti-CD11d treatment reduces lung neutrophils and macrophages after SCI
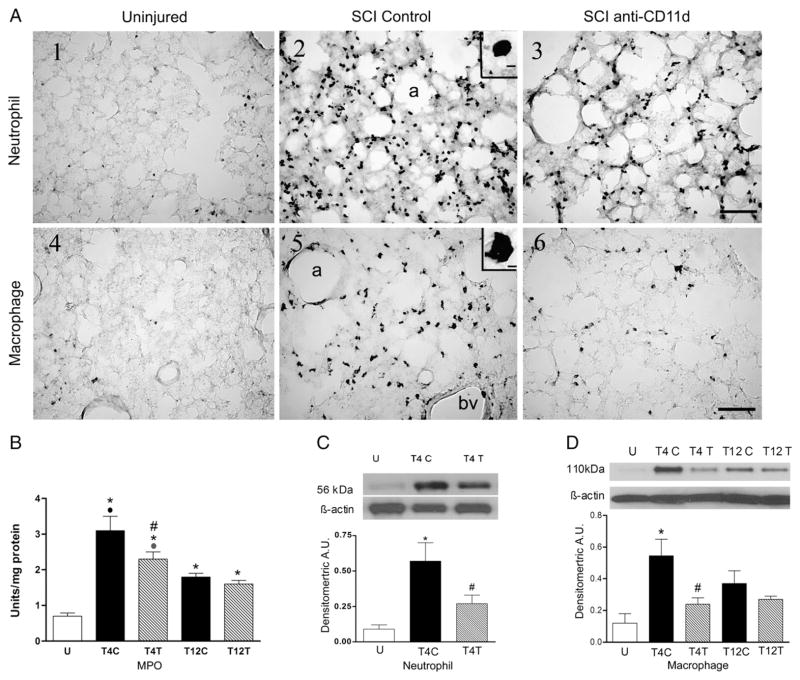
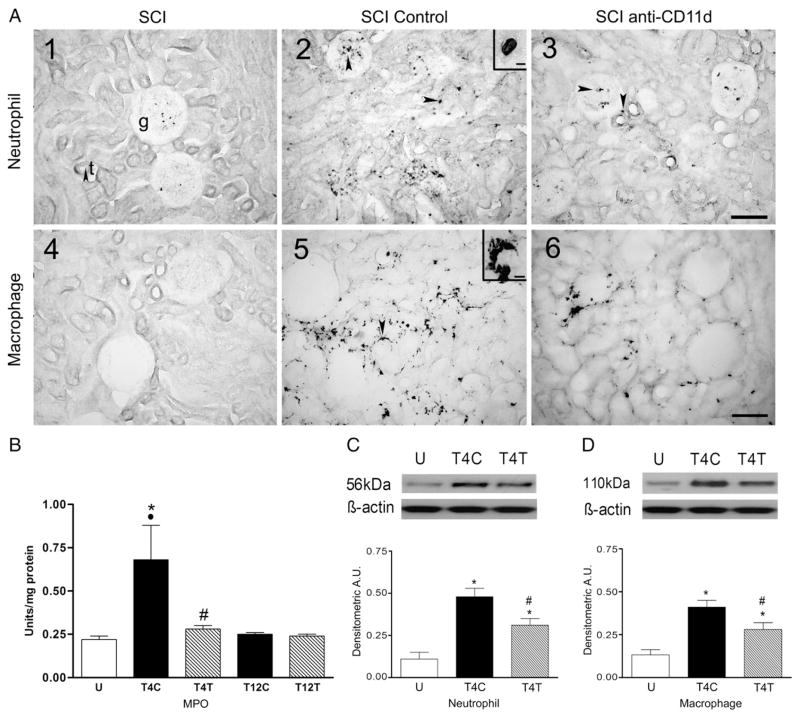
SCI at T4 caused an influx of neutrophils into the lung at 12 h after the injury (Fig. 1A1–3). The inset in A2 shows the morphology of the cells with lobed nuclei that is typical of neutrophils. In the uninjured rat, very few neutrophils stained with the anti-neutrophil antibody were present in the lung (Fig. 1A1), found almost exclusively within blood vessels, whereas after T4 injury in SCI control rats, an infiltrate of neutrophils appeared within the alveoli and extravascular tissue (Fig. 1A2). The rats treated with the anti-CD11d mAb had visibly fewer neutrophils within the lung (Fig. 1A3). The neutrophil influx into lungs of rats after T12 SCI was less apparent when examined after anti-neutrophil staining (data not shown). MPO activity in lung homogenates, assessed as an estimate of neutrophil activity, changed significantly with SCI (Fig. 1B; ANOVA, F4,21 =17.46, P<0.001), increasing by about 4-fold in the lungs of T4 control SCI rats (P<0.001). This increase was significantly attenuated by anti-CD11d treatment (P=0.017). After T12 SCI, the MPO activity increased significantly but by less than 3-fold (P=0.005), and this change was not different in rats treated with the anti-CD11d mAb. The increase in MPO after the T4 injury was significantly greater than after the T12 injury in control SCI rats (P<0.001). In the anti-CD11d-treated rats the MPO increase after T4 injury only tended to be greater than after the T12 injury (P=0.09).
Fig. 1.
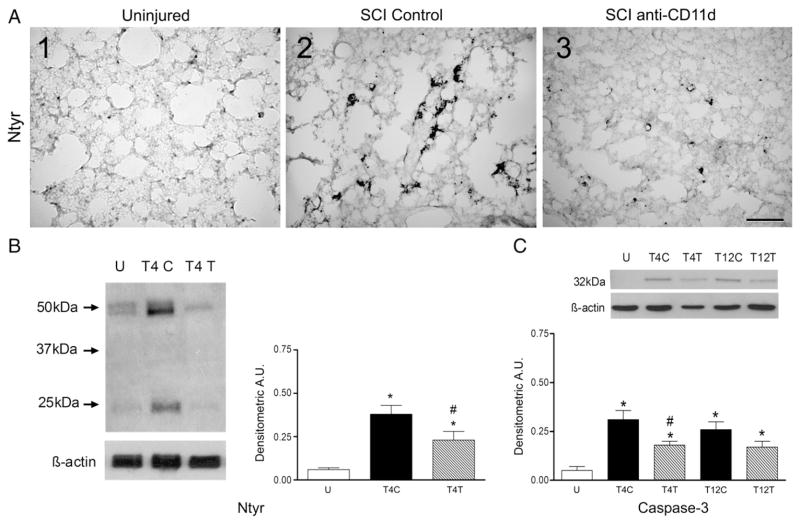
The anti-CD11d treatment decreases neutrophils and macrophages in the lung at 12 h after SCI. A) Photomicrograph of lung sections immunostained by an anti-neutrophil antibody (panels 1–3) and by an ED-1 antibody to detect macrophages (panels 4–6) from an uninjured rat, a T4 SCI control rat and a T4 SCI rat treated with the anti-CD11d mAb (SCI anti-CD11d). Insets in A2 and A5 show high power detail of stained cells. a, alveolus; bv, blood vessel. Scale bar=100 μm in A3 (applies to A1–3) and A6 (applies to A4–6) and 10 μm in insets. B) MPO activity in lung homogenates from T4 and T12 SCI rats (n=5 per group) and from uninjured rats (n=6). C) Neutrophil protein, identified by Western blotting in lung homogenates from uninjured and SCI rats (n=5, all groups) expressed in arbitrary units (A.U.). A representative autoradiogram of a Western blot showing relative protein expression, compared to loading controls (β-actin), is shown above the bar graph. D) Macrophage protein (ED-1) expression (Western blotting) in lung homogenates from uninjured and SCI rats (n=4, SCI controls; n=5, uninjured and anti-CD11d treated). In this and all figures: values are means±S.E.; symbols indicating significance refer to the ‘a posteriori’ Student Neuman Keul’s test for all comparisons (P≤0.05) *, significantly different from uninjured; #, significantly different from SCI control; ●, significantly different from T12 SCI control.
 , tended to differ from T12 SCI anti-CD11d treated. U: uninjured; T4C: T4 SCI control; T4T: T4 SCI anti-CD11d-treated; T12C: T12 SCI control; T12T: T12 SCI anti-CD11d-treated.
, tended to differ from T12 SCI anti-CD11d treated. U: uninjured; T4C: T4 SCI control; T4T: T4 SCI anti-CD11d-treated; T12C: T12 SCI control; T12T: T12 SCI anti-CD11d-treated.
Additional quantification of the effects of the anti-CD11d treatment on the neutrophil infiltrate at T4 was done using Western blotting. The anti-neutrophil antibody detected the neutrophil protein at a molecular weight of 56 kDa. After the T4 SCI, the 56 kDa neutrophil protein expression changed significantly (Fig. 1C; ANOVA, F2,12 =7.80, P=0.007) increasing by more than 6-fold, when compared to values in the uninjured rats (P=0.006). The anti-CD11d treatment reduced this increase significantly, resulting in an increase about half as great as in the SCI controls (P=0.029).
The normal uninjured lung contains a population of resident ED-1-immunoreactive macrophages within the tissue parenchyma surrounding the alveoli (Fig. 1A4). An example of a large irregular macrophage is shown in the A5 inset. At 12 h after T4 SCI in control SCI rats, the density of this macrophage population appeared increased (Fig. 1A5). These cells were similar in morphology and location to those in the uninjured lungs. After anti-CD11d treatment, the macrophage presence in the lungs appeared less than in the control SCI rats (Fig. 1A6). After T12 SCI, the increase in lung macrophages in tissue sections was less apparent (data not shown). Expression of ED-1 in lung homogenates changed significantly after T4 SCI (Fig. 1D, ANOVA, F4,19 =4.89, P=0.007), increasing by 5-fold in control SCI rats when compared to uninjured rats (P=0.004). The anti-CD11d treatment reduced this change significantly (by more than half, P=0.029); the values in these rats were not different from those in uninjured rats. The injury in control T12 SCI rats did not significantly increase the macrophage ED-1 within the lungs despite a ~3-fold change (P=0.102). At T12, the values in anti-CD11d-treated rats were not different from those of the control SCI rats or the uninjured rats.
Anti-CD11d treatment reduces expression of NF-κB and oxidative enzymes, and the concentration of free radicals in lung after SCI
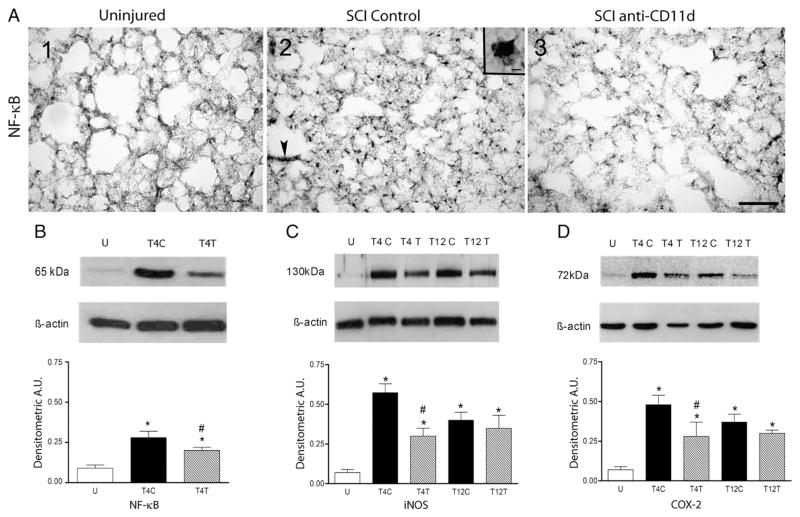
The inflammatory process within the lung was also evident by the expression of the inflammatory transcription factor NF-κB as shown in Fig. 2A. This nuclear transcription factor was expressed at low levels in the uninjured lung (Fig. 2A1) but was highly evident in the lungs of T4 SCI control and anti-CD11d-treated rats (Fig. 2A2,3). The NF-κB appeared to be in the nuclei of the neutrophils and macrophages (inset Fig. 2A2). NF-κB-stained cells were within the lung parenchyma, often adjacent to alveoli (arrow in A2). The distribution of these cells resembled that of the inflammatory cells shown in Fig. 1. In Western blots of lung homogenates, NF-κB expression changed significantly after T4 SCI (Fig. 2B; ANOVA, F2,12 =11.74, P =0.001), increasing by 3-fold in the lungs of the control SCI rats (P=0.001). The anti-CD11d treatment diminished this increased significantly, by ~50% (P=0.026).
Fig. 2.
The anti-CD11d treatment decreases expression of NF-κB, iNOS and COX-2 in the lung at 12 h after SCI. A) Photomicrograph of lung sections immunostained for NF-κB from uninjured and T4 SCI rats (panels A1–3). Inset in A2 shows detail of a cell resembling a macrophage with a stained nucleus. Many cells also resembled neutrophils. Arrow indicates clusters of cells next to alveolus. Scale bar=100 μm in A3 (applies to A1–3) and 10 μm in inset of A2. B) NF-κB was identified by Western blotting in uninjured and T4 SCI rat lungs (n=5, all groups). C) iNOS expression was examined in uninjured and T4 or T12 SCI rat lungs (n=4 SCI controls; n=5, uninjured and anti-CD11d treated). D) COX-2 expression was also evaluated in these rats (n=5, all groups). *, significantly different from uninjured; #, significantly different from SCI controls.
Enzymes associated with oxidative injury to the spinal cord were evaluated in the lung homogenates using Western blotting. Expression of iNOS changed significantly in lung homogenates after SCI (Fig. 2C; ANOVA, F4,19 =9.08, P < 0.001), increasing by 8-fold after T4 SCI (P<0.001). The anti-CD11d treatment reduced this change to a 4-fold increase (P=0.017). After T12 SCI, iNOS expression increased by 6-fold (P=0.004) and the treatment had no significant effect on this increase. No significant differences were detected between the responses at T4 vs. T12. COX-2 expression in the lung homogenates also changed after SCI (Fig. 2D; ANOVA, F4,20 =7.94, P<0.001), increasing by 7-fold after T4 SCI (P<0.001). This increase was significantly less (4-fold greater than uninjured) after anti-CD11d treatment (P=0.014). After T12 SCI, COX-2 expression increased by 5.5-fold (P=0.004) and mAb treatment did not significantly alter this response. Again, no significant differences were detected between the T4 and T12 injuries.
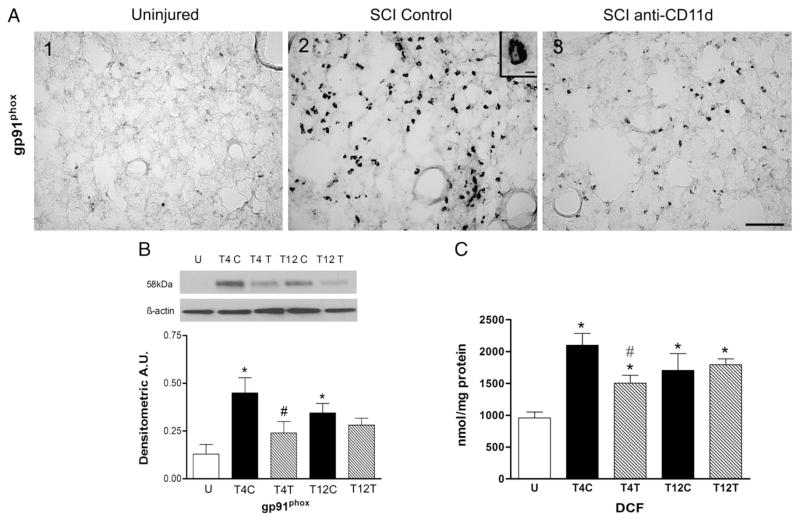
The pattern of changes in expression of the enzyme gp91phox followed that of iNOS and COX-2. Little staining for gp91phox was present in lung sections from the uninjured rats (Fig. 3A1) but, after T4 SCI, gp91phox expression was abundant throughout the lungs, in a pattern of distribution that resembled that of the inflammatory infiltrate (Fig. 3A2). The cells stained for gp91phox had morphology similar to that of neutrophils and macrophages and the inset in Fig. 3A2 shows a cell resembling a neutrophil. The gp91phox expression was much less evident in lungs of rats that had been treated with anti-CD11d (Fig. 3A3). When assayed by Western blotting, expression of the oxidative enzyme gp91phox was significantly changed after SCI (Fig. 3B; ANOVA, F4,20 =5.75, P=0.003), increasing by 5-fold in the lung homogenates after T4 SCI (P=0.002). Treatment with the anti-CD11d mAb reduced this increase by half (P=0.05) to levels no longer different from the uninjured values. After T12 SCI, gp91phox expression increased by 3.5-fold (P=0.02) and the mAb treatment had no significant effect on this increase. The changes in expression of this enzyme after T4 injury were not significantly different from those after T12 injury.
Fig. 3.
The anti-CD11d treatment decreases expression of gp91phox and production of free radicals in the lung at 12 h after SCI. A) Photomicrograph of lung sections immunostained for gp91phox from uninjured and T4 SCI rats (panels A1–3). Inset in A2 shows detail of stained cell resembling a neutrophil (irregular pale nucleus). Scale bar=100 μm in A3 (applies to A1–3) and 10 μm in inset of A2. B) gp91phox was identified by Western blotting in uninjured rats and in T4 and T12 SCI rat lungs (n=5, all groups). C) The concentration of DCF was assayed as a free radical marker in lung homogenates from uninjured (n=6) and T4 and T12 SCI rats (n=5 per group). *, significantly different from uninjured; #, significantly different from T4 SCI control; #, tendency for significant difference from T4 SCI control.
A quantitative DCF assay for free radicals in lung homogenates revealed significant changes in DCF after SCI (Fig. 3C; ANOVA, F4,21 =7.54, P < 0.001). In the T4 control SCI rats, a 2-fold increase in DCF occurred (P<0.001) and anti-CD11d treatment tended to reduce this response (P=0.075). DCF concentrations increased by 1.8-fold in the lung after T12 SCI (P=0.008) and the anti-CD11d treatment did not alter this response. The DCF concentrations in lung after T4 SCI did not differ from those after T12 SCI.
Anti-CD11d treatment reduces lipid peroxidation, protein nitration and cell death in lung after SCI
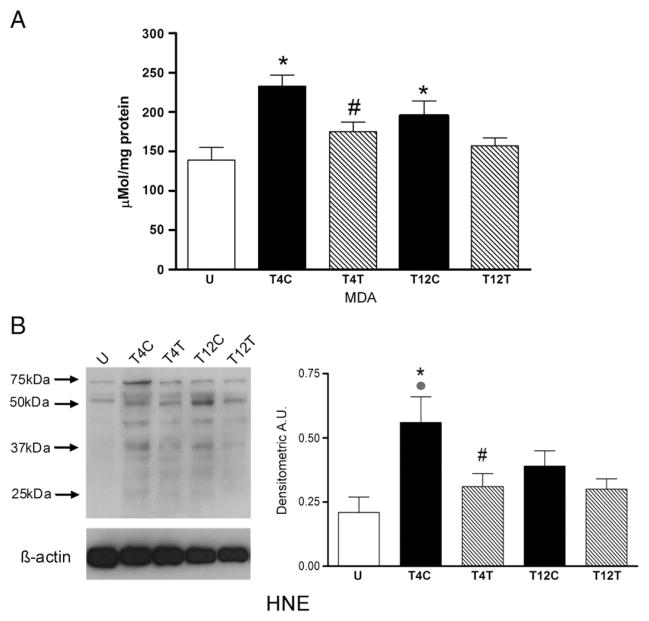
Lung damage resulting from oxidative activity and the presence of reactive oxygen species was evaluated by examining lipid peroxidation of cell membranes, tyrosine nitration of proteins and expression of the pro-apoptotic enzyme caspase-3. Lipid peroxidation was first estimated by assaying relative levels of malondialdehyde (MDA). This product of lipid peroxidation was significantly altered by SCI (Fig. 4A; ANOVA, F4,21 =5.90, P=0.002), increasing by ~70% after T4 SCI (P=0.002). Anti-CD11d treatment reduced this increase significantly (P=0.038) to values not different from those in uninjured rats. SCI at T12 caused a ~40% increase in lung lipid peroxidation (P=0.05) and this response was not significantly altered by treatment with the anti-CD11d mAb. However, the value in the treated group was not different from that in uninjured rats. Values after T4 SCI did not differ from those after T12 injury. Western blotting for the presence of hydroxynonenol-bound proteins (HNE) also revealed changes in lipid peroxidation after SCI (Fig. 4B; ANOVA, F4,20 =4.04, P =0.015). After T4 SCI, lung HNE increased significantly by ~2.7-fold (P=0.009). This increase was reduced by anti-CD11d treatment (P=0.035), to values not different from those in uninjured rats. After T12 SCI this measure of lipid peroxidation was not significantly increased. Lung HNE values in T12 control and anti-CD11d treated SCI rats were not different from each other. In control SCI rats, HNE values after T4 injury tended to be greater than after T12 injury (P=0.081).
Fig. 4.
The anti-CD11d treatment decreases lipid peroxidation in the lung at 12 h after SCI. A) Lipid peroxidation was assessed by the TBARS assay for malondialdehyde (MDA) in lung homogenates from uninjured (n=6) and T4 or T12 SCI rats (n=5 per group). B) Lipid peroxidation was also assayed by Western blotting for 4-hydroxynonenol (HNE)-bound proteins in these groups of rat lungs (n=5 per group). Western blot illustrates an example of HNE-bound protein expression at several molecular weights. *, significantly different from uninjured; #, significantly different from T4 SCI control;
 , T4 SCI control tended to differ from T12 SCI control.
, T4 SCI control tended to differ from T12 SCI control.
Oxidative injury to proteins was evaluated by examining nitrotyrosine expression. Immunocytochemical staining for nitrotyrosine in lung sections of uninjured rats revealed very small punctate areas of protein nitration (Fig. 5A1). The amounts of nitrotyrosine increased substantially in sections from T4 control SCI rats, appearing as large irregular deposits in cells within the lung parenchyma (Fig. 5A2). These cells may have been alveolar epithelial cells. After anti-CD11d treatment, the nitrotyrosine in the lung sections was markedly reduced (Fig. 5A3). Quantification of the protein nitration in the lung by Western blotting revealed significant changes after SCI (Fig. 5B; ANOVA, F2,12 =14.03, P < 0.001). Nitrotyrosine increased by 6.7-fold after T4 SC (P<0.001) and treatment with the anti-CD11d mAb significantly reduced this response by 60% (P=0.030).
Fig. 5.
The anti-CD11d treatment decreases protein nitration and caspase-3 expression in the lung at 12 h after SCI. A) Photomicrograph of lung sections immunostained for nitrotyrosine (Ntyr) from uninjured and T4 SCI rats (panels 1–3). Scale=100 μm in A3. B) Nitrotyrosine was assayed in lung homogenates in uninjured and T4 SCI rats (n=5, all groups). C) Caspase-3 expression was evaluated by Western blotting in uninjured and T4 or T12 SCI rat lungs (n=4 SCI controls; n=5, uninjured and anti-CD11d treated). *, significantly different from uninjured; #, significantly different from T4 SCI control.
Caspase-3 was examined as a marker of apoptotic cell death in the lungs. Quantification of caspase-3 expression by Western blotting revealed very limited expression of this enzyme in lungs of uninjured rats but significant changes in expression after SCI (Fig. 5C; ANOVA, F4,19 =7.49, P < 0.001). After T4 SCI, caspase-3 expression increased significantly by 5.7 fold (P<0.001) and anti-CD11d treatment reduced this increase by 38% (P=0.04). Likewise after T12 SCI, caspase-3 increased significantly by 5-fold (P=0.003) but anti-CD11d treatment did not significantly alter this response. Caspase-3 expression did not differ between rats with injury at T4 vs. T12 in control or anti-CD11d-treated groups.
Anti-CD11d treatment reduces the influx of inflammatory cells into the kidney after SCI as well as intrarenal oxidative activity and damage
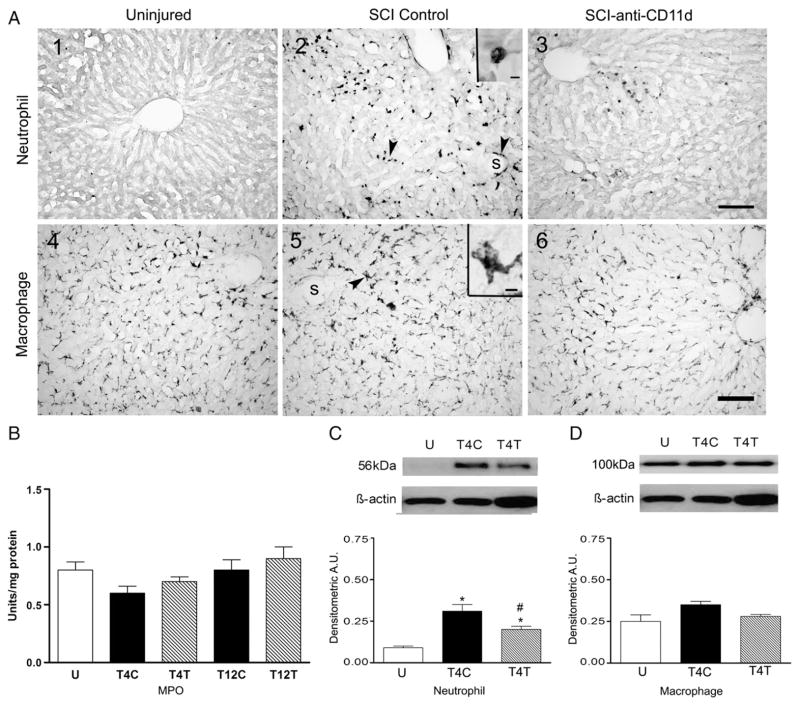
In kidney sections from uninjured rats, neutrophils were found predominantly within blood vessels of the glomeruli and not within the kidney parenchyma (Fig. 6A1). After T4 SCI, the density of neutrophils within the glomeruli appeared markedly increased and neutrophils were also found within the parenchyma, often adjacent to tubules (Fig. 6A2, see arrows). In rats after anti-CD11d treatment, the density of neutrophils within the glomeruli and adjacent to tubules was reduced but remained more prevalent than in kidneys of uninjured rats (Fig. 6A3, see arrows). After T12 SCI, the density of neutrophils in the kidney sections did not appear different from that in uninjured rats (data not shown).
Fig. 6.
The anti-CD11d treatment decreases neutrophils and macrophages in the kidney at 12 h after SCI. A) Photomicrograph of kidney sections from uninjured and T4 SCI rats, immunostained by the anti-neutrophil antibody (A1–3) and by the ED-1 antibody (A4–6). Insets in A2 and A5 show high power detail of cells with morphology typical of neutrophils and macrophages, respectively. g, glomerulus; t, tubule. Arrow in A1 points to a tubule in cross section. Arrows on remaining photo micrographs explained in text. Scale bar=100 μm in A3 (applies to A1–3) and A6 (applies to A4–6) and 10 μm in insets of A2 and A5. B) MPO activity was assayed in kidney homogenates from uninjured (n=6) and T4 and T12 SCI rats (n=5 both groups). C) Neutrophil protein expression was examined by Western blotting in uninjured and T4 SCI rats (n=5 both groups). D) Macrophage protein (ED-1) expression was also detected by Western blotting in these rat kidneys (n=5 per group). *, significantly different from uninjured; #, significantly different from T4 SCI controls; ●, significant difference between T4 and T12 control SCI rats.
MPO activity in kidney homogenates changed significantly with T4 SCI (Fig. 6B; ANOVA, F4,21 =4.91, P=0.006), increasing by about 3-fold in the kidneys of T4 control SCI rats (P=0.008). This increase was significantly attenuated in anti-CD11d-treated rats (P=0.005). After T12 SCI, the MPO activity was not different from that in uninjured rats, and values in control SCI and anti-CD11d-treated rats were very similar. The increase in kidney MPO after the T4 injury was significantly greater than after the T12 injury (P=0.007) in control SCI rats but not in anti-CD11d-treated rats. Quantification of the effects of the anti-CD11d treatment on the neutrophil infiltrate into kidneys after T4 SCI was done using Western blotting. After the SCI, expression of the 56 kDa neutrophil protein in the kidney changed significantly (Fig. 6C; ANOVA, F2,12 =19.00, P < 0.001), increasing by 4.5-fold when compared to values in the uninjured rats (P<0.001). The anti-CD11d treatment significantly reduced this increase by 34% (P=0.019).
Tissue sections of the normal uninjured kidney rarely contained ED-1-immunoreactive macrophages (Fig. 6A4). After T4 injury in control SCI rats, the density of this macrophage population appeared increased, particularly adjacent to the tubules (Fig. 6A5). The arrow in A5 points to a macrophage next to a tubule. After anti-CD11d treatment, the macrophage presence in the kidneys appeared to be reduced compared to that in the control SCI rats (Fig. 6A6). After T12 SCI, the increase in kidney macrophages was less apparent and was similar in tissue sections from control and anti-CD11d treated rats (data not shown). Kidney homogenates were examined by Western blotting for ED-1 and expression of the macrophage marker changed significantly after T4 SCI (Fig. 6D, ANOVA, F2,12 =15.01, P < 0.001). ED-1 expression increased by 3-fold within the kidneys of control SCI rats (P<0.001). The anti-CD11d treatment reduced this change significantly by 33% (P=0.020).
Oxidative activity within the kidney was estimated by the DCF assay for free radicals. Low levels of DCF were present in the kidneys of uninjured rats and SCI significantly altered the concentrations of this free radical detector (ANOVA, F4,21 =7.32, P<0.001). DCF concentrations increased by 2-fold in the kidney of T4 control SCI rats (P<0.001) and anti-CD11d treatment significantly reduced this response by 27% (P = 0.024). Injury at T12 did not increase concentrations of DCF within the kidney in either SCI control or anti-CD11d-treated rats and values in these two groups were not different from each other. DCF concentrations in kidneys of T4 control SCI rats were significantly greater than those in T12 SCI control rats (P=0.002) but values in anti-CD11d-treated rats of the T4 and T12 injury groups did not differ from each other.
Lipid peroxidation within the kidney was estimated by assaying relative levels of MDA. This product of lipid peroxidation was significantly altered by SCI (ANOVA, F4,21 =6.13, P =0.002), increasing by ~34% after T4 SCI (P=0.006) compared to that in uninjured rats. Anti-CD11d treatment reduced this increase significantly (P=0.05) to values not different from those in uninjured rats. SCI at T12 caused a ~37% increase in kidney lipid peroxidation (P=0.004) and this response was not altered by treatment with the anti-CD11d mAb. Values after T4 SCI did not differ from those after T12 injury.
Anti-CD11d treatment reduces neutrophils in the liver after SCI
The liver is also infiltrated by neutrophils after SCI. In uninjured rats no neutrophils were found within the tissue parenchyma of liver sections and a few neutrophils were within the vascular sinusoids (Fig. 7A1). After T4 injury, abundant neutrophils were present throughout the liver, within the parenchyma among the hepatocytes and surrounding the sinusoids (Fig. 7A2, see arrows). The morphology of the cell in the inset of Fig. 7A2 is typical of a neutrophil. The neutrophils adjacent to the sinusoids may have just undergone diapedesis. Liver sections from anti-CD11d-treated rats after T4 SCI had visibly fewer neutrophils than in the control SCI rats but appeared to have more of these cells than uninjured rats (Fig. 7A3). Sections of liver after T12 SCI also contained neutrophils and the relative numbers did not appear changed after anti-CD11d treatment (data not shown). In the liver homogenates, MPO activity was not significantly changed after T4 or T12 SCI (Fig. 7B). In contrast, quantification of the 56 kDa neutrophil protein by Western blotting with the anti-neutrophil antibody revealed significant changes after T4 SCI (Fig. 7C; ANOVA, F2,12 =15.7, P < 0.001), with 3.6-fold increases in the livers of control SCI rats (P<0.001) and a 36% reduction of this increase in rats with anti-CD11d treatment (P=0.016).
Fig. 7.
The anti-CD11d treatment decreases neutrophils but not macrophages in the liver at 12 h after SCI. A) Photomicrograph of liver sections from uninjured and T4 SCI rats, immunostained by the anti-neutrophil antibody (A1–3) and by the ED-1 antibody (A4–6). Insets in A2 and A4 show high power detail of stained cells. s, liver sinusoid. Arrows explained in text. Scale bar=100 μm in A3 (applies to A1–3) and A6 (applies to A4–6), and 10 μm in insets of A2 and 5. B) MPO activity was assayed in liver homogenates of uninjured (n=6) and SCI rats with injury at T4 or T12 (n=5 all groups). C) Neutrophil protein expression was revealed by Western blotting in uninjured and T4 SCI rat livers (n=5 both groups). D) Macrophage protein (ED-1) expression was also examined by Western blotting in these rat livers (n=5 both groups). *, significantly different from uninjured; #, significantly different from T4 SCI control.
Staining for macrophages by ED-1 after T4 SCI revealed these cells within the liver of uninjured, control SCI and anti-CD11d-treated rats (Fig. 7A4–6, arrow in A5). The large irregular cell in the inset of Fig. 7A5 resembles a tissue macrophage. These macrophages likely were Kupffer cells. The relative abundance of these cells was similar among the groups of rats. Indeed, Western blotting for the ED-1 in liver homogenates showed no significant difference between macrophages in uninjured rats and in the two SCI groups (Fig. 7D).
Free radicals and lipid peroxidation within the liver were estimated with the DCF and MDA assays, respectively, in tissue homogenates from T4 and T12 SCI rats. The variability in the measurements of free radicals and lipid peroxidation in the liver led to an insufficient power (<0.80) in the statistical analyses of these data. Despite this limitation, increases in liver free radical (DCF) concentrations were detected after SCI (ANOVA, F4,21 =3.48, P =0.025). The value in the T12 control SCI rats (3337± 1233 nmol/mg protein) was significantly increased by 80% in comparison to that in uninjured rats (1855±202 nmol/mg protein). No changes after T4 injury or changes due to anti-CD11d treatment could be detected. The DCF values after T12 SCI did not differ from those after T4 SCI. Changes in lipid peroxidation were found in liver homogenates after SCI (ANOVA, F4,21 =3.08, P =0.038) with significant 25% increases in MDA in T4 SCI rats (P=0.024). Concentrations of MDA in uninjured, T12 control, and anti-CD11d-treated T4 and T12 SCI rats were very similar. The liver MDA concentrations in all of the groups (average 480±49 μM/mg protein) were more than twice the concentrations found in the lung (180±33 μM/mg protein) and kidney (184±21μM/mg protein).
Discussion
SCI is a severe trauma that initiates an early innate immune response to the injured tissue. In a review of systemic inflammation after general trauma, Lenz et al. (2007) state that ‘an overly vigorous pro-inflammatory host defense response may lead to non-specific organ injury as part of this uncontrolled immune activation. Modulation of this response is a major therapeutic goal, to direct it toward injured tissue and infection, while sparing other vital organs’. Such modulation was our goal in the treatment of SIRS after SCI with the anti-CD11d mAb. This treatment was successful, decreasing the influx of neutrophils and activation of macrophages in the lung and kidney, and reducing oxidative activity and the resulting tissue damage in these organs. Although the liver was also invaded by neutrophils after SCI, no changes in macrophages were present at 12 h post-injury, the time of our analysis, and little liver damage was detected. Accordingly, although the anti-CD11d treatment decreased the influx of neutrophils, it had no other effects on the liver. Our secondary hypothesis that the segmental level of SCI would impact the magnitude of the SIRS was partially substantiated. MPO activity in the lung and kidney and DCF detection in the kidney were increased significantly more by a T4 than by a T12 injury, and the same tendencies for such differences in some of the other measures did not attain significance. In contrast to the inconsistent segmental differences in induction of the SIRS, the anti-CD11d treatment was always effective after T4 but not after T12 SCI.
Anti-CD11d treatment of SIRS in the lung, kidney and liver
After SCI the SIRS was readily apparent in the lung, as we described previously (Gris et al., 2008). The current study extended those findings, revealing upregulation of the pro-inflammatory transcription factor NF-κB and of the oxidative enzymes MPO, iNOS, COX-2 and gp91phox, production of free radicals, induction of tissue damage due to lipid peroxidation and protein nitration, and upregulation of the apoptosis-related protease caspase-3. All of these contributors to the SIRS after T4 SCI were significantly reduced by a single treatment with the anti-CD11d mAb. In this first investigation of the anti-CD11d mAb in the context of SIRS, we chose a single time point, 12 h after injury, to facilitate examining two sites of injury and effects in three organs. At this time, our previous study had demonstrated a significant neutrophil influx into the lung and the onset of tissue damage (Gris et al., 2008). As the anti-CD11d mAb effectively limits neutrophil diapedesis (Saville et al., 2004), our first inquiry was to determine treatment effects on neutrophil infiltration. The increased macrophage presence in the lung at 12 h after SCI may entail monocyte influx as well as activation of resident macrophages. Likewise, within 4 h after SCI, expression of the chemokines CINC-1 and CCL-2 is induced in the liver, leading to intrahepatic neutrophil and monocyte recruitment and activation of resident macrophages (Kupffer cells) (Campbell et al., 2005). The chemokine production in the liver increased in parallel with the density of recruited leukocytes and activated Kupffer cells; CCL-2 protein was identified in these cells. Accordingly, the reduction of visible macrophages in tissue sections and of ED-1 on Western blots is likely due to the limitation of neutrophil and monocyte migration with the subsequent reduction of their impact to activate the Kupffer cells. Further studies are needed to examine treatment effects on the later time course of SIRS and to examine, in detail, effects on macrophages within organs.
The kidney was infiltrated by neutrophils after the T4 SCI and intrarenal macrophages were more apparent as well. Again, the macrophages may have been of monocytic origin or may have been resident, newly activated macrophages within the organ. The presence of free radicals and lipid peroxidation demonstrated that the kidney was subject to the adverse effects of SIRS, particularly after T4 injury. The reduced kidney inflammation and damage after T12 injury may occur because a T12 injury is below the level of renal innervation and would not cause any loss of neural control of the renal circulation, a factor that may be relevant to the induction of inflammation in an organ. Furthermore, a T12 injury would not cause serious systemic hemodynamic disruption (see later discussion), another factor that could be deleterious to the kidney. The anti-CD11d treatment blocked the inflammation and increased oxidative activity within the kidney.
Neutrophil and monocyte migration into the liver after SCI and other forms of trauma has been well documented (Campbell et al., 2005; Wilcockson et al., 2002) and is known to promote production of chemokines and cytokines, as described earlier, that are key to the SIRS after CNS trauma. We too observed a significant influx of neutrophils into the liver at 12 h after the T4 SCI. But this response was not accompanied by increased MPO activity or apparent changes in the large population of resident macrophages, the Kupffer cells. A small increase in lipid peroxidation occurred after the T4 injury but the TBARS measure of tissue aldehydes showed that basal levels were three times greater in the liver than in the lung or kidney. The highly metabolic functions of the liver, and its role in detoxification, entail ongoing oxidative activity and production of aldehydes (Comporti et al., 2009) that appear minimally impacted by SCI. Failure of the anti-CD11d treatment to affect the liver macrophages, free radicals or lipid peroxidation may have occurred because they are inherent to liver function and respond to stimuli different from those provided by infiltrating neutrophils or monocytes. Furthermore, the relatively small numbers of neutrophils in the dense liver parenchyma may not have been adequate to enhance oxidative activity within this organ. A recent study of hepatic inflammation after SCI used intravital microscopy and revealed a significant influx of leukocytes into the organ within 2 h after compression or transection injury of the spinal cord (Hundt et al., 2011). Hepatocyte death detected by propidium iodide was noted only after the more severe transection injury, in keeping with the minimal impact on the liver that we noted with our milder compression injury. The circumscribed effect of the anti-CD11d treatment in the liver may still have had benefits outside this organ. By limiting the influx of neutrophils and monocytes into the liver, the anti-CD11d treatment likely limited the hepatic production of chemokines and cytokines that promotes the SIRS throughout other organs.
Differences in SIRS due to segmental site of SCI
Activity of the oxidative enzyme MPO increased substantially in the lungs and kidneys at 12 h after T4 SCI and this response was cord segment-specific as the increases after T12 SCI were significantly smaller. In the kidney, increases in free radical production (that typically correlate well with the upregulation or activation of oxidative enzymes) also occurred to a greater degree after the T4 injury. This difference between injuries at different segmental levels may relate to their unequal influences on the sympathetic nervous system and the production of adrenal and sympathetic nerve catecholamines. Injury at T4 isolates the segments containing preganglionic neurons controlling the visceral blood vessels and the adrenal gland from the tonic excitatory drive provided by the brainstem. Stimulation of the spinal neurons in the T4 segment by physical trauma would initially stimulate release of catecholamines from sympathetic nerve terminals and from the adrenal gland for a few hours. Then, because the central tonic drive to these neurons is lost after SCI, circulating levels of catecholamines become significantly reduced (Maiorov et al., 1997; Mathias et al., 1975). The injury at T12 would have a much lesser impact, if any, on circulating catecholamines because the sympathetic preganglionic innervation of the adrenal glands and of visceral resistance blood vessels are not directly injured and remain under control by the brainstem. Catecholamines may be relevant to the segmental differences we observed as they have significant anti-inflammatory influences on neutrophils. Catecholamines can inhibit neutrophil oxidative burst and generation of free radicals (Trabold et al., 2007; Yasui et al., 2006), effects relevant to the MPO activity and free radical production during SIRS. Catecholamines also have inhibitory actions on tissue macrophages (Johnson 2002). Accordingly, the loss of circulating catecholamines after SCI would permit greater oxidative activity of neutrophils and activation of macrophages.
SCI can also interrupt a postulated cholinergic anti-inflammatory pathway, resulting in heightened systemic inflammatory responses after cervical or high-to-mid thoracic SCI (Rosas-Ballina and Tracey 2009). This pathway is thought to regulate the release of pro-inflammatory cytokines; accordingly, the absence of this restraint could promote cytokine-induced activation of neutrophils and monocyte/macrophages. As this pathway involves sympathetic neurons emanating from the mid-thoracic spinal cord, an injury in this location (such as T4) would certainly disrupt this modulatory influence whereas the system would remain functional after a T12 injury.
Although MPO activity and free radical concentrations were differentially affected by the location of the injury, the other measures that we examined were not. Larger changes in several variables such as the activity of oxidative enzymes, macrophage activation and lipid peroxidation occurred after injury at T4, but these differences from injury at T12 were not usually statistically significant. Perhaps MPO activity and DCF production were more greatly affected by the rostral injury because they are among the most sensitive responses of the activated neutrophil. The other variable may be less easily changed. Furthermore, our study at 12 h after injury emphasized the role of the neutrophils. At later times, such as 2–3 days after injury, when macrophages make a greater contribution, differences between the segments of injury may have a greater impact on the SIRS response elicited.
Segmental differences in anti-CD11d treatment effects
The absence of a clear benefit from the anti-CD11d treatment after T12 SCI deserves comment. As the actions of the mAb were on leukocytes in the circulation and perhaps in the organs, the distinction between effects of injury at one level vs. another is perplexing. In some cases, the lack of response to the anti-CD11d mAb may be due to a relatively small inflammatory response. The responses to T12 injury may have fallen below a threshold degree of inflammation that is optimal for activation of the CD11d integrin, thereby failing to provide a target for the action of the mAb. Alternatively, a lesser upregulation of the tissue binding partner, VCAM-1, after T12 injury could limit CD11d/CD18 binding and leukocyte extravasation. This could be argued for many of the lung and kidney responses to T12 injury. Another reason for lack of treatment effects after T12 SCI might relate to distribution of the anti-CD11d mAb. The T4 SCI generates a large increase in arterial pressure, followed by a large vasodilation and hypotension that gradually abates over a period of one or two days (Maiorov et al., 1997). The vasodilation may provide optimal delivery of the mAb to the circulation of the target organs. Furthermore the changes in circulating catecholamines may alter integrin expression or the state of integrin activation by the leukocytes (Wahle et al., 2005), perhaps maximizing binding of the mAb. SCI at T12 does not evoke large changes in arterial pressure (Weaver, L.C., unpublished observations) and is unlikely to change circulating catecholamines.
Significance of the findings
Trauma is one of the main reasons for death worldwide and the SIRS, with subsequent multi-organ failure, is a key contributor to that death (Lenz et al., 2007). After cord injury, clinical studies show that SIRS, with its associated organ failure, contributes significantly to the morbidity and mortality of SCI patients (Acosta et al., 1998). The most vulnerable organs appear to be the lungs and kidneys (Catz et al., 2002; DeVivo et al., 1999; O’Connor 2005). Moreover, progression of secondary damage after SCI may be exacerbated by a background of systemic inflammation. In a study of patients during the first two weeks after SCI, we found that all had increased oxidative activity in circulating neutrophils and monocytes and increased expression of integrins by these leukocytes, consistent with an ongoing SIRS, particularly within the first 72 h after their injury (Bao et al., 2009, 2011). Therefore, developing a treatment that can block the onset of SIRS is an important goal. The anti-CD11d treatment appears to fulfill that goal. This treatment for SIRS best targets injuries in the upper spinal segments, a finding of significance as more than half of clinical spinal cord injuries occur in the cervical and high thoracic regions (National Spinal Cord Injury Statistics Center, https://www.nscisc.uab.edu).
The duration of the anti-CD11d treatment is important and future studies will examine a 72 h course of treatment to include the interval when the macrophage contribution is more fully developed. Treatment probably can be discontinued after 72 h as many aspects of the SIRS wane between 3 and 7 days after SCI (Gris et al., 2008). In addition, a short time course of anti-CD11d treatment avoids the risk of immunosuppression, a phenomenon that develops later after SCI. Immune impairment likely contributes to the septicemia, pneumonia, renal and gastrointestinal tract infections and renal failure that are significant issues after SCI (Cruse et al., 1996; McKinley et al., 1999). With higher levels of injury the function of natural killer cells, T-cells, B cells and macrophages may be suppressed in humans and animals within weeks of the injury (Cruse et al., 1992, 2000; Lucin et al., 2007). Adhesion molecule expression by lymphocyte and neutrophils also can be decreased by 2 weeks after injury (Cruse et al., 2000). These are important considerations but they occur later in the injury time course than the early hours/days during which the overactive SIRS predominates. The evidence for SIRS in the first days after SCI demonstrates that the innate immune system is fully functional at this time and limiting the SIRS by an anti-inflammatory treatment such as the anti-CD11d mAb is much needed.
Acknowledgments
This research was supported by a grant from the Canadian Institutes of Health Research. The authors thank Eli Lilly and Company for donating the anti-CD11d mAb (271L) and isotype matched control mAb (1B7). We are indebted to Dr. Kristine Kikly of Eli Lilly for her constructive comments regarding this work. We also appreciate the critical review by Dr. Canio Polosa.
References
- Acosta JA, Yang JC, Winchell RJ, Simons RK, Fortlage DA, Hollingsworth-Fridlund P, Hoyt DB. Lethal injuries and time to death in a level I trauma center. J Am Coll Surg. 1998;186:528–533. doi: 10.1016/s1072-7515(98)00082-9. [DOI] [PubMed] [Google Scholar]
- Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, Walker K. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr Biol. 1998;8:923–926. doi: 10.1016/s0960-9822(07)00373-9. [DOI] [PubMed] [Google Scholar]
- Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 2004;88:1335–1344. doi: 10.1046/j.1471-4159.2003.02240.x. [DOI] [PubMed] [Google Scholar]
- Bao F, Dekaban GA, Weaver LC. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. J Neurochem. 2005;94:1361–1373. doi: 10.1111/j.1471-4159.2005.03280.x. [DOI] [PubMed] [Google Scholar]
- Bao F, Bailey CS, Gurr KR, Bailey SI, Rosas-Arellano MP, Dekaban GA, Weaver LC. Increased oxidative activity in human blood neutrophils and monocytes after spinal cord injury. Exp Neurol. 2009;215 (2):308–316. doi: 10.1016/j.expneurol.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Bao F, Bailey CS, Gurr KR, Bailey SI, Rosas-Arellano MP, Brown A, Dekaban GA, Weaver LC. Human spinal cord injury causes specific increases in surface expression of Beta integrins on leukocytes. J Neurotrauma. 2011;28 (2):269–280. doi: 10.1089/neu.2010.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran H, Yarmush ML, Berthiaume F. Dynamics of tissue neutrophil sequestration after cutaneous burns in rats. J Surg Res. 2000;93 (1):88–96. doi: 10.1006/jsre.2000.5955. [DOI] [PubMed] [Google Scholar]
- Bhatia RK, Pallister I, Dent C, Jones SA, Topley N. Enhanced neutrophil migratory activity following major blunt trauma. Injury. 2005;36 (8):956–962. doi: 10.1016/j.injury.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Blight AR. Macrophages and inflammatory damage in spinal cord injury. J Neurotrauma. 1992;9 (Supp 1):S83–S91. [PubMed] [Google Scholar]
- Campbell SJ, Hughes PM, Iredale JP, Wilcockson DC, Waters S, Docagne F, Perry VH, Anthony DC. CINC-1 is an acute-phase protein induced by focal brain injury causing leukocyte mobilization and liver injury. FASEB J. 2003;17 (9):1168–1170. doi: 10.1096/fj.02-0757fje. [DOI] [PubMed] [Google Scholar]
- Campbell SJ, Perry VH, Pitossi FJ, Butchart AG, Chertoff M, Waters S, Dempster R, Anthony DC. Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am J Pathol. 2005;166:1487–1497. doi: 10.1016/S0002-9440(10)62365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz A, Thaleisnik M, Fishel B, Ronen J, Spasser R, Fredman B, Shabtay E, Gepstein R. Survival following spinal cord injury in Israel. Spinal Cord. 2002;40 (11):595–598. doi: 10.1038/sj.sc.3101391. [DOI] [PubMed] [Google Scholar]
- Comporti M, Arezzini B, Signorini C, Vecchio D, Gardi C. Oxidative stress, isoprostanes and hepatic fibrosis. Histol Histopathol: Cell Mol Biol. 2009;24:893–900. doi: 10.14670/HH-24.893. [DOI] [PubMed] [Google Scholar]
- Cruse JM, Lewis RE, Bishop GR, Kliesch WF, Gaitan E. Neuroendocrine-immune interactions associated with loss and restoration of immune system function in spinal cord injury and stroke patients. Immunol Res. 1992;11 (2):104–116. doi: 10.1007/BF02918615. [DOI] [PubMed] [Google Scholar]
- Cruse JM, Keith JC, Bryant ML, Jr, Lewis RE., Jr Immune system-neuroendocrine dysregulation in spinal cord injury. Immunol Res. 1996;15 (4):306–314. doi: 10.1007/BF02935314. [DOI] [PubMed] [Google Scholar]
- Cruse JM, Lewis RE, Dilioglou S, Roe DL, Wallace WF, Chen RS. Review of immune function, healing of pressure ulcers, and nutritional status in patients with spinal cord injury. J Spinal Cord Med. 2000;23 (2):129–135. doi: 10.1080/10790268.2000.11753520. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80 (11):1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Ditor DS, Bao F, Chen Y, Dekaban GA, Weaver LC. A therapeutic time window for anti-CD11d monoclonal antibody treatment yielding reduced secondary tissue damage and enhanced behavioral recovery following severe spinal cord injury. J Neurosurg Spine. 2006;5:343–352. doi: 10.3171/spi.2006.5.4.343. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquales-Style M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129 (Pt 12):3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Bao F, Chen Y, Hamilton EF, Relton JK, Weaver LC. α4β1 integrin blockade after spinal cord injury decreases damage and improves neurological function. Exp Neurol. 2008;214 (2):147–159. doi: 10.1016/j.expneurol.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340 (6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Grayson MH, Van der Vieren M, Sterbinsky SA, Gallatin WM, Hoffman PA, Staunton DE, Bochner BS. αdβ2 integrin is expressed on human eosinophils and functions as an alternative ligand for vascular cell adhesion molecule 1 (VCAM-1) J Exp Med. 1998;188:2187–2191. doi: 10.1084/jem.188.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gris D, Hamilton EF, Weaver LC. The systemic inflammatory response after spinal cord injury damages lungs and kidneys. Exp Neurol. 2008;211:259–270. doi: 10.1016/j.expneurol.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Hundt H, Fleming JC, Phillips JT, Lawendy A, Gurr KR, Bailey SI, Sanders D, Bihari R, Gray D, Parry N, Bailey CS, Badhwar A. Assessment of hepatic inflammation after spinal cord injury using intravital microscopy. Injury. 2011 doi: 10.1016/j.injury.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Johnson M. Effects of beta2-agonists on resident and infiltrating inflammatory cells. J Allergy Clin Immunol. 2002;110 (6 Suppl):S282–S290. doi: 10.1067/mai.2002.129430. [DOI] [PubMed] [Google Scholar]
- Kyono W, Coates TD. A practical approach to neutrophil disorders. Pediatr Clin North Am. 2002;49 (5):929–971. viii. doi: 10.1016/s0031-3955(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38 (12):1336–1345. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Lucin KM, Sanders VM, Jones TB, Malarkey WB, Popovich PG. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp Neurol. 2007;207 (1):75–84. doi: 10.1016/j.expneurol.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorov DN, Weaver LC, Krassioukov AV. Relationship between sympathetic activity and arterial pressure in conscious spinal rats. Am J Physiol. 1997;272:H625–H631. doi: 10.1152/ajpheart.1997.272.2.H625. [DOI] [PubMed] [Google Scholar]
- Mathias CJ, Christensen NJ, Corbett JL, Frankel HL, Goodwin TJ, Peart WS. Plasma catecholamines, plasma renin activity and plasma aldosterone in tetraplegic man, horizontal and tilted. Clin Sci Mol Med. 1975;49:291–299. doi: 10.1042/cs0490291. [DOI] [PubMed] [Google Scholar]
- McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402–1410. doi: 10.1016/s0003-9993(99)90251-4. [DOI] [PubMed] [Google Scholar]
- Oatway MA, Chen Y, Bruce JC, Dekaban GA, Weaver LC. An anti-CD11d integrin antibody treatment restores normal serotonergic projections to the dorsal, intermediate and ventral horns of the injured spinal cord. J Neurosci. 2005;25:637–647. doi: 10.1523/JNEUROSCI.3960-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor PJ. Survival after spinal cord injury in Australia. Arch Phys Med Rehabil. 2005;86 (1):37–47. [PubMed] [Google Scholar]
- Ott L, McClain CJ, Gillespie M, Young B. Cytokines and metabolic dysfunction after severe head injury. J Neurotrauma. 1994;11 (5):447–472. doi: 10.1089/neu.1994.11.447. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4 (2):103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Pickett GE, Campos-Benitez M, Keller JL, Duggal N. Epidemiology of traumatic spinal cord injury in Canada. Spine. 2006;31 (7):799–805. doi: 10.1097/01.brs.0000207258.80129.03. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague–Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265 (6):663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo M, Sawa Y, Takano H, Matsumiya G, Iwai S, Ono M, Hata H, Yamauchi T, Nishimura M, Fujino Y, Matsuda H. Effect of a polymorphonuclear elastase inhibitor (sivelestat sodium) on acute lung injury after cardiopulmo-nary bypass: findings of a double-blind randomized study. Surg Today. 2006;36 (4):321–326. doi: 10.1007/s00595-005-3160-y. [DOI] [PubMed] [Google Scholar]
- Saville LR, Pospisil CH, Mawhinney LA, Bao F, Simedria FC, Peters AA, O’Connell PJ, Weaver LC, Dekaban GA. A monoclonal antibody to CD11d reduces the inflammatory infiltrate into the injured spinal cord: a potential neuroprotective treatment. J Neuroimmunol. 2004;156:42–57. doi: 10.1016/j.jneuroim.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Snedecor G, Cochran WG. Statistical Methods. 8. Iowa State University Press; Iowa: 1989. [Google Scholar]
- Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, Naruo M, Okabe H, Takatsuki K. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79 (4):1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- Trabold B, Gruber M, Frohlich D. Functional and phenotypic changes in polymorphonuclear neutrophils induced by catecholamines. Scand Cardiovasc J. 2007;41 (1):59–64. doi: 10.1080/14017430601085948. [DOI] [PubMed] [Google Scholar]
- Van der Vieren M, Trong HL, Wood CL, Moore PF, St John T, Staunton DE, Gallatin WM. A novel leukointegrin, αdβ2, binds preferentially to ICAM-3. Immunity. 1995;3:683–690. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Van der Vieren M, Crowe DT, Hoekstra D, Vazeux R, Hoffman PA, Grayson MH, Bochner BS, Gallatin WM, Staunton DE. The leukocyte integrin αDβ2 binds VCAM-1: evidence for a binding interface between I domain and VCAM-1. J Immunol. 1999;163:1984–1990. [PubMed] [Google Scholar]
- Wahle M, Greulich T, Baerwald CG, Hantzschel H, Kaufmann A. Influence of catecholamines on cytokine production and expression of adhesion molecules of human neutrophils in vitro. Immunobiology. 2005;210 (1):43–52. doi: 10.1016/j.imbio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wang CX, Olschowka J, Wrathall JR. Increase of interleukin-1β mRNA and protein in the spinal cord following experimental traumatic injury in the rat. Brain Res. 1997;759:190–196. doi: 10.1016/s0006-8993(97)00254-0. [DOI] [PubMed] [Google Scholar]
- Weaver LC, Verghese P, Bruce JC, Fehlings MG, Krenz NR, Marsh DR. Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J Neurotrauma. 2001;18:1107–1119. doi: 10.1089/08977150152693782. [DOI] [PubMed] [Google Scholar]
- Wilcockson DC, Campbell SJ, Anthony DC, Perry VH. The systemic and local acute phase response following acute brain injury. J Cereb Blood Flow Metab. 2002;22 (3):318–326. doi: 10.1097/00004647-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Yasui K, Kobayashi N, Yamazaki T, Agematsu K, Matsuzaki S, Nakata S, Baba A. Differential effects of short-acting beta2-agonists on human granulocyte functions. Int Arch Allergy Immunol. 2006;139 (1):1–8. doi: 10.1159/000089516. [DOI] [PubMed] [Google Scholar]