INTRODUCTION
Liver metastasis of breast cancer (LMBC) develops in more than 50% of breast cancer patients [1], and one third of patients with metastatic breast cancer have metastases only in the liver [2]. Liver metastasis of breast cancer usually portends a poor prognosis. However, neoadjuvant therapy followed by partial liver resection to remove the residual metastatic tumor has been shown to provide survival benefit for these patients [2], and the tumor response to neoadjuvant therapy is a significant predictive indicator of recurrence-free survival [2, 3].
Tumor response to neoadjuvant therapy is measured by the pathologic response, defined by estimating the percentage of residual tumor cells in the resected tumor. This method of measuring tumor response is useful, but estimating the percentage of residual tumor cells is prone to inter- and intra-observer variations. A new method of defining tumor response to neoadjuvant therapy by measuring the residual tumor thickness at the tumor–normal tissue interface (TNI) was shown to be a significant predictive indicator of recurrence-free survival in patients with resected liver metastases of colorectal cancer [4]. We have observed that the patterns of residual tumor cells and fibro-collagenous proliferation in resected LMBC after neoadjuvant therapy are similar to those in resected liver metastases of colorectal cancer. We hypothesized that the residual tumor thickness at TNI can predict recurrence-free survival in patients with LMBC as well. We measured the tumor response to neoadjuvant therapy using the residual tumor thickness at TNI in resected liver metastases of breast cancer, and evaluated its association with recurrence-free survival.
MATERIALS AND METHODS
Patient Selection
This retrospective study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center. We identified 89 patients with LMBC who underwent partial liver resection at MD Anderson Cancer Center between 1997 and 2010 by searching the databases of the Departments of Surgical Oncology and Pathology. Twenty patients were excluded because they did not undergo neoadjuvant therapy for the metastatic tumor and 21 others were excluded because the pathology slides were not available for review. We included the remaining 48 patients in the study.
The electronic medical records of these patients were reviewed. The relevant clinical data were abstracted, including patient's age, size and histological grade of tumor, status of lymph node metastasis, status of hormonal and Her2/neu receptors of the tumor, whether liver metastasis was synchronous or metachronous, number of cycles of preoperative chemotherapy, whether liver resection was major or minor, status of surgical margins, and clinical follow-up. The duration of follow up was from January 1997 to December 31, 2010.
Assessment of Pathologic Response by Percentage of Residual Tumor Cells
Two pathologists (JZ and DR) who were blinded to the clinical information, treatment regimen, and outcome evaluated the hematoxylin-eosin (HE) stained slides of metastatic tumor nodules. The third pathologist (DM), who was also blinded to the clinical information, treatment regimen, and outcome resolved any discrepancy between the two pathologists. The percentage of residual tumor cells was assessed as described by Blazer et al [5]. All HE-stained sections of a tumor nodule were assessed, and the mean of percentages was determined. For patients with multiple tumor nodules, the mean of the percentages of residual tumor cells in all nodules was determined.
The pathologic response was categorized as described as complete response (0% residual tumor cells), major response (less than 50% residual tumor cells), and minor response (50% or more residual tumor cells).
Assessment of Tumor Response by Residual Tumor Thickness at Tumor-Normal Tissue Interface
The same pathologists performed this assessment and any discrepancy was resolved in the same manner.
The residual tumor thickness at the TNI was assessed as described by Maru et al. Briefly, the largest thickness of un-interrupted tumor cells perpendicular to the TNI in each metastatic tumor nodule was measured in millimeters under a microscope. Multiple measurements were taken for each tumor nodule, and the largest measurement was used for analysis. In cases with multiple tumor nodules, the largest measurement of all nodules was used for analysis.
Collection of Clinical Data
The patient's clinical data were collected through independent review of the electronic medical records (AA, AB and DA). The recurrence-free survival time was measured from the date of surgery to the date of recurrence or death.
Statistical Methods
The Spearman correlation coefficient was used to assess the inter-observer variation between the two pathologists, as well as the correlation of the residual tumor thickness at the TNI and the pathologic response. A receiver-operating curve for the tumor thickness at TNI was constructed and the strength of correlation was shown as the area under the curve. The Kaplan-Meier method was used to evaluate recurrence-free survival time, and the log-rank test was used to assess the differences between groups. The Cox proportional hazards model was used to identify predictors of recurrence-free survival time in both univariate and multivariate analyses of the clinicopathologic parameters. A p ≤ 0.05 was considered statistically significant. Statistics and graphs were generated using SPSS version 16.0.1 and Graph Pad Prism version 5.02 software (GraphPad Software, Inc.).
RESULTS
Patient and Tumor Characteristics
The patients included 48 women, with a median age of 43 years (range 26 to 64 years). The median number of metastatic tumor nodules was one (range 1 to 7). Twenty patients (42%) had multiple liver metastases. The median tumor size was 1.6 cm (range 0 to 7 cm). Table 1 summarizes the characteristics of the patients and the tumors. The median duration of follow-up was 52.1 months (range 14.2 to 89.9 months).
Table 1.
Predictors of Recurrence Free Survival
| Recurrence Free Survival | |||||||
|---|---|---|---|---|---|---|---|
| Variable | No. of Patients | Univariate Analysis | Multivariate Analysis | ||||
| P | HR | 95% CI | P | HR | 95% CI | ||
| Age | |||||||
| <50 years | 36 | ||||||
| ≥50 years | 12 | 0.188 | 1.75 | 0.76-4.00 | NS | ||
| Pathologic Response | |||||||
| Major | 19 | 0.01 | 0.32 | 0.133-0.758 | NS | ||
| Minor | 29 | ||||||
| Tumor Thickness at TNI | |||||||
| ≤3 mm | 23 | ||||||
| >3 mm | 25 | 0.004 | 3.02 | 1.37-6.65 | 0.001 | 4.11 | 1.76-9.61 |
| # Tumor Nodules | |||||||
| <2 | 28 | 0.467 | 1.32 | 0.62-2.79 | |||
| ≥2 | 20 | ||||||
| Tumor Size | |||||||
| ≤5 cm | 43 | ||||||
| >5 cm | 5 | 0.006 | 4.24 | 1.51-11.94 | NS | ||
| Liver Resection | |||||||
| major | 30 | 0.851 | 1.07 | 0.51-2.26 | |||
| minor | 18 | ||||||
| Surgical Margin | |||||||
| positive | 4 | 0.009 | 5.20 | 1.50-17.97 | 0.002 | 8.3 | 2.20-31.28 |
| negative | 44 | ||||||
| Tumor Type | |||||||
| ductal type | 44 | 0.920 | 1.06 | 0.32-3.57 | |||
| lobular type | 3 | ||||||
| mixed type | 1 | ||||||
| Estrogen Receptor Status | |||||||
| positive | 31 | 0.340 | 0.66 | 0.28-1.54 | |||
| negative | 14 | ||||||
| no data | 10 | ||||||
| Progesterone Receptor Status | |||||||
| positive | 23 | 0.882 | 0.94 | 0.43-2.06 | |||
| negative | 21 | ||||||
| no data | 4 | ||||||
| ER and PR Status | |||||||
| positive | 14 | 0.340 | 1.51 | 0.647-3.53 | |||
| negative | 31 | ||||||
| no data | 3 | ||||||
| Her2/neu Status | |||||||
| positive | 20 | 0.583 | 0.795 | 0.35-1.81 | |||
| negative | 21 | ||||||
| no data | 7 | ||||||
| Synchronous liver Disease | |||||||
| yes | 16 | 0.443 | 0.71 | 0.30-1.67 | |||
| no | 32 | ||||||
| Primary Neoadjuvant Chemo | |||||||
| yes | 17 | 0.244 | 1.63 | 0.74-3.56 | |||
| no | 31 | ||||||
| Neoadjuvant Chemotherapy for Liver Metastasis>6 cycle | |||||||
| yes | 32 | 0.349 | 0.67 | 0.29-1.55 | |||
| no | 16 | ||||||
P, p value; HR, Hazard Ratio; NS, not significant; ER, estrogen receptor; PR, progesterone receptor; Chemo, chemotherapy.
The neoadjuvant therapy for the liver metastasis included chemotherapy alone (16), hormonal therapy alone (4), chemotherapy with trastuzumab/lapatinib (19), chemotherapy with hormonal therapy (5), and chemotherapy with bevacizumab (4). Seventeen patients also had neoadjuvant therapy for their primary breast cancer prior to the mastectomy.
Residual Tumor Thickness at the TNI Correlated with the Pathologic Response
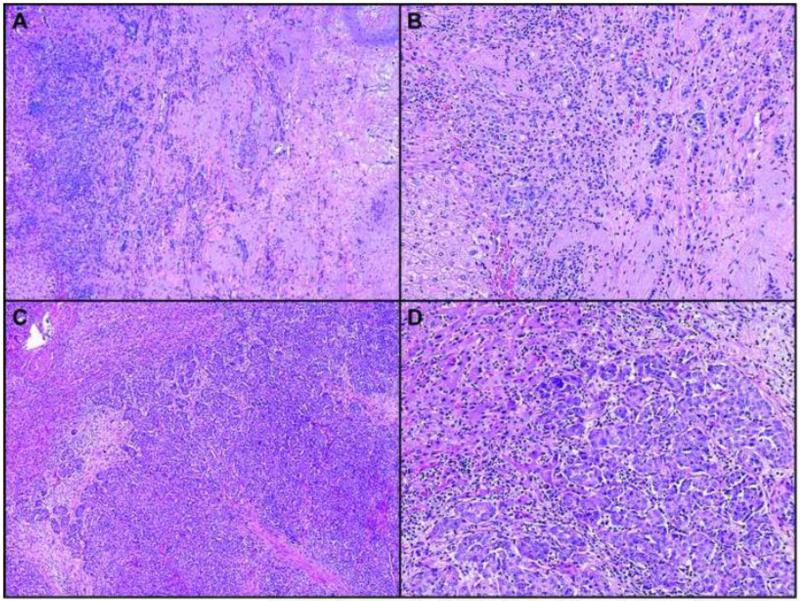
Nineteen (40%) patients had complete or major pathologic response, and 29 (60%) had a minor response (Figure 1). The correlation coefficient between the two pathologists for percentage of residual tumor cells was 0.95.
Figure 1.
Hematoxylin-eosin–stained sections (4X and 10X) show major (A and B) and minor (C and D) pathologic response to neoadjuvant chemotherapy for liver metastasis from breast cancer.
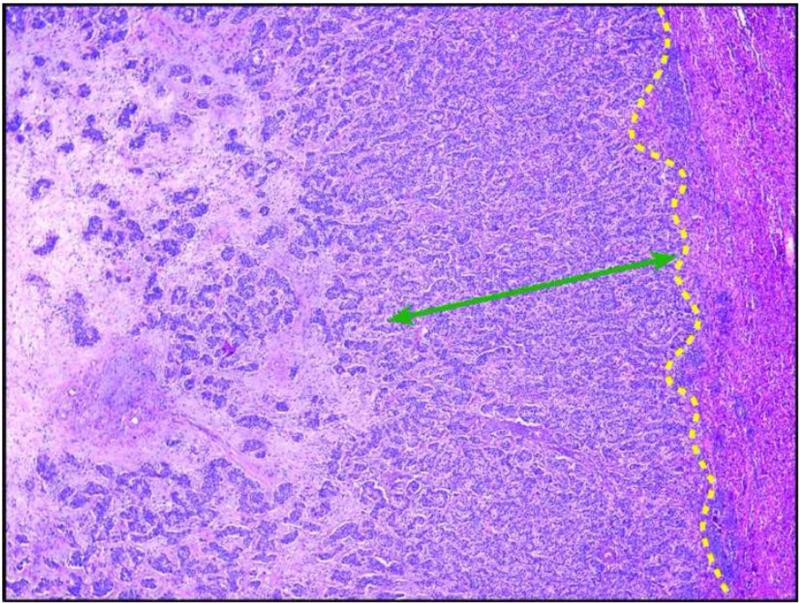
The median residual tumor thickness at the TNI was 2.75 mm (range 0 to 13 mm). Twenty-five (52%) patients had tumor thickness > 3 mm (Figure 2). The correlation coefficient between the two pathologists for tumor thickness at the TNI was 0.89.
Figure 2.
Hematoxylin-eosin–stained section (10X) demonstrates the method of measuring tumor thickness at the tumor-normal interface. The yellow dotted line indicates the tumor-normal interface.
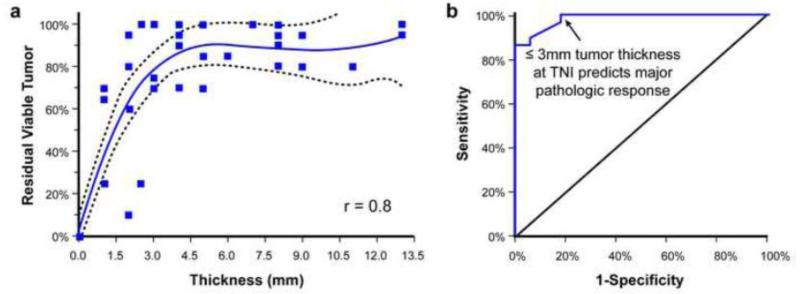
The residual tumor thickness ≤ 3 mm at the TNI correlated with major pathologic response and the tumor thickness > 3 mm at the TNI correlated with minor pathologic response (Figure 3).
Figure 3.
A. Scatter plot correlating the residual tumor cells with tumor thickness at the tumor-normal interface. The solid line indicates the regression line, and the dotted line indicates the 95% confidence interval. B. Receiver operating curve demonstrating the cutoff value to differentiate a major from a minor pathologic response.
Residual Tumor Thickness at the TNI Predicts the Recurrence-Free Survival Time
Residual tumor thickness > 3 mm at the TNI, minor pathologic response, tumor size > 5 cm, and positive surgical margin were associated with shorter recurrence-free survival by univariate analysis. Furthermore, residual tumor thickness > 3 mm at the TNI and positive surgical margin were associated with shorter recurrence-free survival also by multivariate analysis. Table 1 shows the correlation of clinical and pathological variables with recurrence-free survival time by univariate and multivariate analysis.
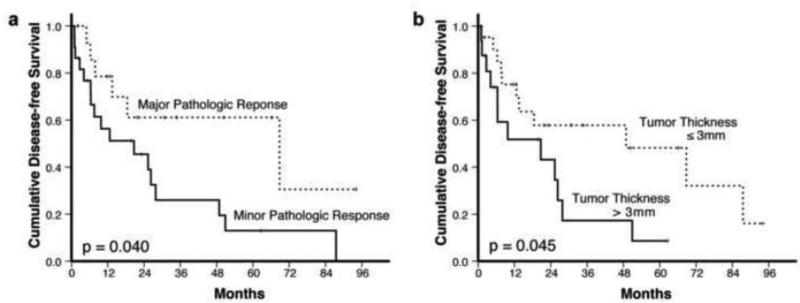
Figure 4 shows that both the pathologic response (p = 0.040) and the residual tumor thickness at the TNI (p = 0.045) correlated with the recurrence-free survival time as determined by the Kaplan-Meier method.
Figure 4.
Kaplan-Meier curves demonstrate the recurrence-free survival in association with A. pathologic response, and B. the residual tumor thickness at tumor-normal interface.
DISCUSSION
Our study shows that the residual tumor thickness at the TNI can predict recurrence-free survival time in patients with LMBC. The residual tumor thickness at the TNI provides an objective measure of tumor response to neoadjuvant therapy by measuring the thickness of the residual tumor in millimeters under a microscope. While we cannot eliminate variations of measuring with a ruler, the variations are expected to be smaller than those by estimating. In our study, the inter-observer agreement in measuring the residual tumor thickness at the TNI was very good (r = 0.89). Although the correlation coefficient of inter-observer agreement in the estimation of the residual tumor cells was slightly higher in our study, we believe that is due to the pre-assessment standardization of estimating the percentage of residual tumor cells. However, such standardization is not routinely performed in everyday practice. Moreover, no significant difference exists between the two correlation coefficients. The residual tumor thickness at the TNI can serve as another outcome endpoint in patients with LMBC.
The residual tumor thickness at the TNI and the pathologic response can be synergistic. The pathologic response by estimating the percentage of residual tumor cells is the only pathologic marker currently used to measure tumor response to neoadjuvant therapy. Because tumor thickness at the TNI correlates with pathologic response, the two methods can be used together to check for possible errors in the measurement or the estimation. For example, if a tumor has a major pathologic response by the percentage of residual tumor cells but the residual tumor thickness is > 3 mm at the TNI, the histologic slides and the specimen should be re-examined to detect a possible error that may have occurred in the evaluation. Using both methods together can improve the accuracy of the measurements.
We realize that the study was retrospective, and the neoadjuvant therapy included five different therapeutic regimens. Because the number of patients on each regimen was small, we could not compare the effectiveness of the different regimens. Our study suggests that survival benefit is associated with the tumor response to neoadjuvant therapy regardless of the therapeutic regimen. However, this finding should be confirmed in a prospective study comparing the effectiveness of different therapeutic regimens. We do not know if the method of residual tumor thickness at the TNI would apply to other metastatic tumors to the liver or to other body sites such as lung. Further study is needed to confirm its validity in those situations.
In summary, the residual tumor thickness at the TNI predicts recurrence-free survival time in patients with LMBC. The residual tumor thickness at the TNI provides another outcome endpoint in patients who underwent liver resection of metastatic breast cancer after neoadjuvant therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None
References
- 1.Vlastos G, Smith DL, Singletary SE, et al. Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol. 2004;11:869–874. doi: 10.1245/ASO.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Rene adam M, PhD, Jinane Krissat MD, Marie-Pierre Bralet MD, et al. Is Liver Resection Justified for Patients With Hepatic Metastases From Breast Cancer? Annals of Surgery. 2006;244:897–908. doi: 10.1097/01.sla.0000246847.02058.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyld L, Gutteridge E, Pinder SE, et al. Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer. 2003;89:284–290. doi: 10.1038/sj.bjc.6601038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maru DM, Kopetz S, Boonsirikamchai P, et al. Tumor thickness at the tumor-normal interface: a novel pathologic indicator of chemotherapy response in hepatic colorectal metastases. Am J Surg Pathol. 2010;34:1287–1294. doi: 10.1097/PAS.0b013e3181eb2f7b. [DOI] [PubMed] [Google Scholar]
- 5.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]