Abstract
OBJECTIVE
We hypothesized that the genetic makeup impacts on functional behavior of the uterine cervix. Therefore, we compared the biomechanical properties of uterine cervix in postpartum 2 strains of mice that differ in their underlying regenerative collagen remodeling characteristics: MRL/MpJ+/+ (MRL: high regenerative repair) and C57BL/6 (C57: low regenerative high fibrotic repair).
METHODS
Cervical tensile proprieties were assessed on day (d) 3, 15, and 60 postpartum in MRL (n=14) and C57 (n=13) mice (4-5 animals at each time point). Stress-strain curves were generated using Shimadzu EZ-test instrumentation. Cervical tissue was stretched by 0.42 mm/min. until rupture. Parameters of viscoelasticity including slope (a measure of stiffness - force needed to produce similar extension), yield point (YP; moment when tissue changes its proprieties from elastic to plastic), and break point (BP; measure of tissue strength) were recorded and analyzed blindly between strains. Data were normalized to the weight of the tissue and analyzed by 2-way ANOVA. Histological evaluation and collagen birefringence of the uterine cervix (MRL: n=4; C57: n=4) was performed 5d post-delivery.
RESULTS
At 3 and 15 d postpartum cervices of MRL mice were significantly more compliant than those of C57 (P<0.001). MRL mice displayed a significant increase in stiffness from d3 to d 60 [slope, median ± SEM: d3: 3.1 ± 0.5 vs. d15: 20.3 ± 4.9 vs. d60: 33.1 ± 3.5 N/mm/gram, P<0.001]. In contrast, the stiffness of C57 cervices reached maximum on d15 [slope d3: 14.1 ± 4.3 vs. d15: 40.0 ± 6.5 N/mm/gram, p=0.02] and rested at a similar level on d60. [d60: 26.1 ± 7.0 N/mm/gram, d60 vs. d15: P=0.937]. More force was required to reach YP in C57 on d3 (C57: 72.5 ± 14.7 vs. MRL: 19.9 ± 1.6 N/gram, P<0.001) but not on either d15 (C57: 156.1 ± 27.5 vs. MRL: 109.2 ± 26.0 N/gram, P=0.120) or on d60 (C57: 143.4 ± 26.5 vs. MRL: 164.5 ± 18.7 N/gram, P=0.412). There was a significant decrease in BP in both strains on both d15 and d60 compared with d3 postpartum (P=0.856 for strain, P=0.008 for d). MRL mice displayed significantly less cervical collagen birefringence compared to C57 control (P<0.001) but increased proteoglycan staining and increased water content.
CONCLUSION
We provide evidence that genetic makeup may impact on cervical tissue remodeling and function. There are significant differences in postpartum cervical stiffness and compliance which vary with the regenerative collagen remodeling phenotype.
INTRODUCTION
The current working model of parturition indicates that this process is composed of several major steps: a long conditioning preparatory phase, followed by a relatively short active labor and postpartum recovery phase.1 During gestation, the conditioning phase leads to gradual ripening of the cervix, a process which commonly occurs at a different time frame from that of the uterine myometrium.2,3 Thus, even though part of the uterus, the cervix is viewed as a separate, complex, and heterogeneous organ.4
The process of cervical ripening and postpartum recovery are prerequisites for a normal pregnancy outcome but also for preservation of the future natural reproductive potential. It is believed that the biology of the cervical conditioning, active labor and recovery phase depends on the complex interaction between fibrous connective tissue, the extracellular matrix (collagen, elastin and proteoglycans), smooth muscle, fibroblasts, nitric oxide and hormonal factors such as progesterone, estrogens, androgens, prostaglandins and relaxin.2,5,6,7,8 In addition, the inflammatory cascade is actively involved and there is persuasive evidence to suggest that the apoptotic cascade plays an important role.9,10 For example, cell proliferation is highest in early pregnancy whereas apoptosis increases progressively in later pregnancy to reach maximal levels in the postpartum time period. 10
A clear understanding of the biologic mechanisms that regulate cervical remodeling during pregnancy and postpartum is greatly needed.11 But while there has been a progressive focus on preparatory and active labor phase of cervical ripening, less attention was paid to the reparative and transformational process of the cervix in postpartum despite the importance of this process for future pregnancies.10,12
The MRL/MpJ+/+ (MRL) mouse strain is unique in its capacity for regenerative remodeling with complete closure of ear holes post-injury.13, 14 The advantage of this strain of mice is that its regenerative ability can be directly compared to that of other mouse strains which do not hold similar remodeling properties such as that of the C57Bl/6 (C57) mice. This reparative process in MRL mice has been associated with the formation of a blastema-like structure (a mass of mesenchymal cells from which new tissue is differentiated) and increased vascularization.15 A number of studies have now shown that the process of wound healing in MRL mice is faster, more complete, with increased swelling, angiogenesis, fibroblast migration, extracellular matrix deposition and decreased collagen deposition, scarring and fibrosis.14,16,17 Using backcross mapping a number of genetic linkage studies demonstrated that the regenerative remodeling phenotype of MRL mice is genetically determined.18 Approximately 20 quantitative trait loci (QTLs) on at least 7 chromosomes were identified and proposed as being responsible for the different wound healing/regeneration phenotype of the MRL mice. Epistatic loci-to-loci interactions as well as interactions with the environment have been linked to the regeneration phenotype of the MRL genetic background. 19,20
We hypothesized that inborn genetic remodeling phenotypes impact on future morphology and functional behavior of the uterine cervix. Therefore, we compared the tensile strength properties of the postpartum cervices in 2 strains of mice that differ in their underlying extracellular matrix remodeling characteristics: MRL (high regenerative repair) and C57 (low regenerative and high fibrotic repair relative to the MRL background).
MATERIALS AND METHODS
Animals
Two strains of pregnant mice (MRL and C57) nulliparous female mice were acquired from Jackson Laboratory (Bar Harbor, Maine, USA), maintained on a 12:12 hours light cycle and allowed free access to food and water. Animals were bred in our facilities under a rigorous protocol (day (d) 1=day sperm plug observed). For the purpose of this study, we analyzed data from 35 animals (MRL: n=18 and C57: n=17). All the procedures were conducted in accordance with the standards outlined by the National Institute of Health guide for the Care and Use of laboratory animals, under protocols approved by the Institution of Animal Care and Research Advisory Committee.
To avoid possible variation in stretching of the uterine cervix due to differences in fetal mass and number of the pups, animals were anesthetized with ketamine 90-100 mg/kg (Ketalar, Parke-Davis, Morris Plains, NJ) and xylazine 10 mg/kg (Gemini, Rugby Lab., Rockville Center, NY) on d18 of pregnancy (24 hours before the expected day of delivery). Cesarean section was performed under sterile conditions. Once the abdomen was opened a longitudinal incision was made along the anti-mesometrial border in the mid-portion of each uterine horn. The fetuses and their placenta were gently extruded through the hysterotomy incision and fetal and maternal weight (before and after Cesarean) recorded. The uterine incision was closed with continuous lock stitches of 5-0 polyglycolic acid suture. Animals were observed until they recovered and become mobile. Immediate postoperative analgesia was provided to the animals (butorphanol 2 mg/kg s.c. prn. q 4 hours sc.). Animals were sacrificed by carbon dioxide inhalation at 3 days (d) (MRL: n=5, C57BL/6: n=4), 5 d (MRL: n=5, C57BL/6: n=5), 15 d (MRL: n=4, C57BL/6: n=4), and 60 d (MRL: n=5, C57BL/6: n=5) following surgery.
The cervix was defined as the less vascular tissue with parallel lumina between the uterine horns and the vagina. 21 Immediately after death, the cervix was isolated and transected at the utero-cervical junction with the aid of a dissecting microscope. Biomechanical testing was performed on cervices at d3, d15 and d60 postpartum while cervices from mice of d5 postpartum were used for histology.
Biomechanical properties of the cervix
The tensile properties of the isolated cervix were determined based on a previously described protocol. 22 Briefly, following collection the cervix was weighed (wet weight) and then anchored in a tissue bath filled with 10mM HEPES/PBS solution (pH=7.4), by means of a silk thread passed through the lumen of each of the cervical canal. The Shimadzu EZ-test instrumentation (Shimadzu North America, Columbia, MD) was used to stretch the tissue by 0.42 mm /minute (sampling rate: 20 Hz; duration of stretching: 0.7 sec.; duration or equilibration: 59.3 sec.). Representative stress-strain curves of the uterine cervix for both strain of mice are presented in Figure 1. Parameters such as slope (measure of stiffness), yield point (YP; moment when tissue changes its proprieties from elastic to plastic), break point (BP; measure of tissue strength), displacement at YP and BP were recorded and analyzed as previously described. 22,23 Once the intermittent stretching protocol was initiated, it was continued past the YP until the BP was reached. After stretching, the cervix was placed on an aluminum foil and dried in an oven overnight for weight measurement.
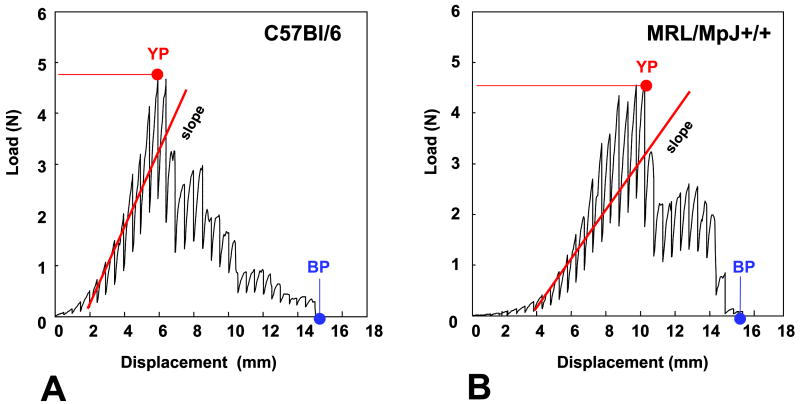
Figure 1. Load-displacement curves of the uterine cervix.
Representative load-displacement curves are shown for C57Bl/6 (Panel A) and MRL/MpJ+/+ (Panel B) at 3 days postpartum. The mid-slope of the regression lines of the linear ascending part of the curve (elastic phase) (shown in red) is a measure of material stiffness. The yield point (YP: moment when tissue changes its proprieties from elastic to plastic) and break point (BP: moment when tissue is physically disrupted) are indicated with arrows.
Histology
Having verified the time point at which the 2 strains of mice displayed marked differences in biomechanical properties of their cervices, an additional 8 animals (MRL: n=4 and C57: n=4) were sacrificed 5d post delivery. We aimed to identify histological changes that may explain the disparity. Following collection, the cervix was fixed overnight in formalin (Fisher Chemical, Fairlawn, NJ) and embedded in paraffin. Sections were cut to a thickness of 5 μm and stained with either hematoxylin and eosin (H&E) or Alcian blue (8GX, Fluka, Buchs, Switzerland) or Sirius Red (Direct Red 80; Aldrich, Milwaukee, WI). After rehydration, serial sections were immersed for 15 min. in 1% Alcian blue (in 3% aqueous acetic acid, pH 2.5) to visualize anionic glycoconjugates such as proteoglycans and glycosaminoglycans. Alcian blue-stained sections were counterstained with 1% neutral red (Sigma, St Louis, MO). 0.5% Sirius Red dissolved in alkaline solution (NaOH, pH 10.5) was used for assessment of collagen birefringence through microscopic cross-polars.3,24 The polyazo dye, Sirius red F3B ionically binds with the positively charged collagen. Large collagen fibers appear brilliant yellow-orange on a black background. According to Junqueira et al., the birefringence is highly specific for collagen and represents an index of the organization of collagen fibers.24 For each animal, fields in 2-3 sections of the uterine cervix (200 x magnification) were analyzed at random and digital images acquired using an Olympus U-STP polarizing light microscope equipped with an Olympus OLY-200 digital camera (Olympus, Melville, NY). Attention was paid so that sections chosen for analysis to be are at similar bi-luminal level for both MRL and C57BL/6 mice. Fixed light settings were maintained throughout the digital acquisition of all images. Images were analyzed using Adobe Photoshop 7.0 (Adobe Systems) and the mean luminosity (light intensity of the pixels) was considered proportional to the amount of birefringent collagen per field, as previously reported. 3,23 Results were reported as units of luminosity. Luminosity was quantified from 32 (background luminosity) to 255 (bright white). By averaging the luminosity values from the acquired fields, for each animal, a semi-quantitative estimation of the amount of collagen was obtained for each tissue biopsy.
Statistical analysis
The normality of the data distribution was first tested using the Kolmogorov-Smirnov test. Analysis was performed with the aid of SigmaSTAT 2.03 statistical software (Jandel Corporation, San Rafael, CA). The data are presented as their mean ± standard error of the mean (SEM). Slopes of the upper, lower and mid-point regression lines were calculated to characterize the sample’s resistance to stretch. A larger slope indicates a higher resistance to stretch, and therefore increased stiffness. Comparisons between the 2 groups of animals were performed by Student t-tests. Comparisons between tensile visco-elestic properties of the cervix at different days post Cesarean delivery were performed by 2-way ANOVA with mouse strain and time points as the two independent variables. A P<0.05 was considered to indicate statistical significance.
RESULTS
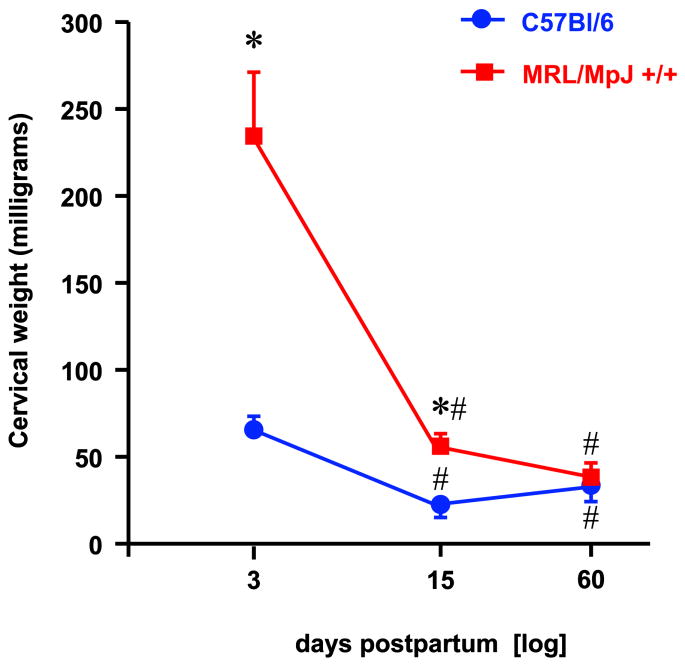
The total fetal mass of MRL mice at the time of Cesarean was significantly higher (MRL: 8.7±0.6 vs. 6.0±1.0 grams, P=0.027) impacted by their increased litter size (MRL: 10 [7-11] vs. C57: 7 [6-10] fetuses, P=0.008). There was no difference in average fetal weight between the two strains (P=0.349) but the maternal body weight (both before and after Cesarean) of the MRL mice was at least 50% larger (P<0.001) than that of C57 mice. There were significant differences in the weight of the uterine cervix between the 2 strains of mice on d3 and d15 but not on d60 postpartum (2-way ANOVA, P<0.001) (Figure 2). As the difference in weight of the cervices on d3 and d15 between MRL and C57 may relate both to the difference in organ size and to the amount of edema, we further analyzed the dry cervical weight and derived the cervical water content through the difference from the wet weight. Although the dry weights did not differ significantly either between strains (P=0.094) or with the interval postpartum (P=0.105), the calculated water content differed with both variables (P<0.001) with significant level of interaction between them (P<0.001). These findings suggest that the differences in cervical wet weight between strains could be largely attributed to differences in water content.
Figure 2. Cervical weight post-delivery in MRL/MpJ+/+ and C57Bl/6 mice.
Statistical analysis: 2-way ANOVA followed by post-hoc Student-Newman-Keuls tests. * P<0.05 vs C57Bl/6; # P<0.05 vs. day 3 post-delivery of the same strain.
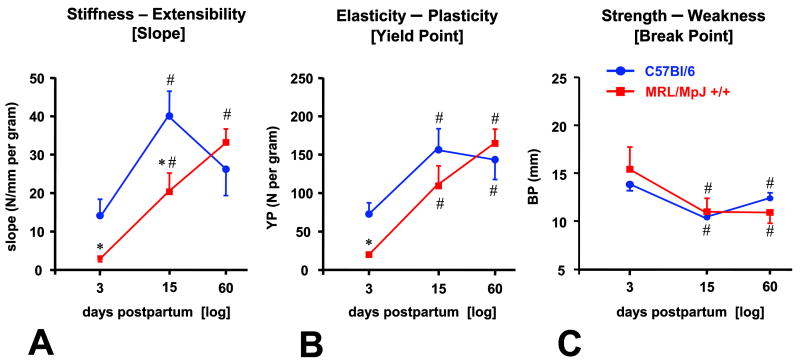
MRL cervices displayed a significant increase in stiffness over time [slope, d3 (n=5): 3.1 ± 0.5 vs. d15 (n=4): 20.3 ± 4.9 vs. d60 (n=5): 33.1 ± 3.5 N/mm/gram, P< 0.001] (Figure 3A). C57 cervices reached maximal stiffness on d15 [slope d3 (n=4): 14.1 ± 4.3 vs. d15 (n=4): 40.0 ± 6.5 N/mm/gram, p=0.02] and remained at similar level on d60. [d60 (n=5): 26.1 ± 7.0 N/mm/gram, d60 vs. d15: P=0.937]. There were significant differences in visco-elasticity of the cervix between the 2 strains on d3 and d15 with the cervix of the MRL being more compliant (MRL vs. C57, P<0.001).
Figure 3.
Analysis of the load-displacement relationships of cervices from day 3, 15 and 60 post-delivery demonstrating differences in the slope (Panel A: measure of stiffness), yield point (Panel B: measure of plasticity) and break point (Panel C: measure of strength). Statistical analysis: 2-way ANOVA followed by post-hoc Student-Newman-Keuls tests. * P<0.05 vs C57Bl/6; # P<0.05 vs. day 3 post-delivery of the same strain.
YP was affected similarly in both strains (Figure 3B). More force was required to reach YP in C57 on d3 (C57: 72.5 ± 14.7 vs. MRL: 19.9 ± 1.6 N/gram, P<0.001) but not on either d15 (C57: 156.1 ± 27.5 vs. MRL: 109.2 ± 26.0 N/gram, P=0.120) or on d60 (C57: 143.4 ± 26.5 vs. MRL: 164.5 ± 18.7 N/gram, P=0.412).
BP evolved similarly between the 2 strains (Figure 3C). There was a significantly decrease in BP in both strains on both d15 and d60 compared with d3 postpartum indicating a significant loss in strength which occurred independently of the genetic phenotype. Less tissue displacement was required to reach BP on d15 and d60, in both MRL and C57 compared with d3 (2-way ANOVA, P=0.856 for strain, P=0.008 for d).
Representative histological images of the uterine cervices are displayed Figure 4. Examination of Sirius red stained tissue sections through cross-polars showed that the C57 displayed significantly more abundant large collagen fibers in brilliant yellow-orange compared to MRL mice consistent with increased collagen birefringence (C57: 66.9 ± 1.1 vs. MRL: 38.7 ± 2.5 luminosity units, t-test, P< 0.001). No discernable differences in cellular populations or inflammatory infiltrate between the 2 mouse strains could be identified on H&E staining. However, the extracellular matrix of MRL mice cervices stained more intensely with Alcian blue indicative of more abundant acid mucins (proteglycans and glycosaminoglycans).
Figure 4. Representative histological sections of the uterine cervix on day 5 post-delivery in MRL/MpJ+/+ and C57Bl/6 mice.
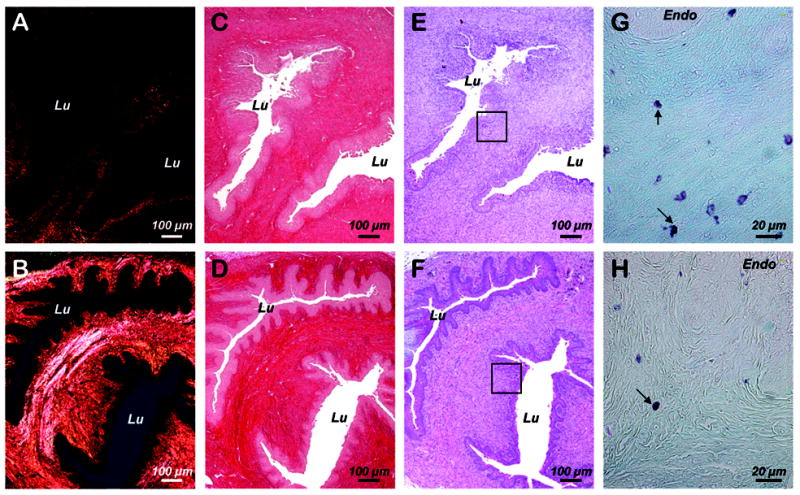
Sections were stained with Sirius red and visualized in either polarized light (Panel A: MRL/MpJ+/+, Panel B: C57Bl/6) or white light. (Panel C: MRL/MpJ+/+, Panel D: C57Bl/6). Adjacent hematoxylin & eosin stained sections are shown in Panel E: (MRL/MpJ+/+) and Panel F (C57Bl/6). The squared areas are displayed at higher magnification in Panel G (MRL/MpJ+/+) and Panel H (C57Bl/6) after Alcian blue/neutral red staining to illustrate acid mucins stained in light blue. Arrows point to metachromatically stained mast cells. Lu: lumen of the endocervical canal. Endo: Endocervical epithelium.
DISCUSSION
The results outlined in this study suggest that the tensile visco-elastic behavior of the postpartum uterine cervix varies with the genetic background and is phenotype dependent. By using 2 strains of mice that differ in their underlying regenerative collagen remodeling characteristics, MRL/MpJ+/+ (high regenerative repair) and C57BL/6 (low regenerative and high fibrotic repair) we have demonstrated significant differences in the stiffness and elasticity of the uterine cervix in the immediate postpartum period. Yet, the end result for these 2 indicators was similar 60 days post-delivery. Interestingly, the cervical strength was more advanced in the immediate compared to the late postpartum, but this phenomenon did not appear to be phenotype dependent. From a histological perspective, the decreased stiffness of the MRL cervices was associated with decreased collagen birefringence, increased proteoglycan staining and increased water content.
Persuasive data seems to suggest that the mechanical behavior of connective tissues in general, and that of the uterine cervix in particular is complex and is governed by interactions between the solid and a liquid component.25 Collagen remains perhaps the most critical element responsible for maintenance of tissue structural integrity.26 Other elements of the extracellular matrix such as proteoglycans, elastin, decorin and tissue hydration reliant on the amount of negatively charged glycosaminoglycans and levels of vascular endothelial growth factor (VEGF) have also been described as important determinants of cervical biomechanics.27,28
Attention of most investigators has concentrated on the mechanisms responsible for the gradual cervical softening and dilatation during pregnancy and/or labor. However, although the cervix in postpartum must undergo a similar but reversed remodeling process to protect the reproductive tract from exposure to a potentially infectious environment, much less attention was paid to this process. 32,29,30,31 Currently, it is advocated that the phenomenon of cervical involution is characterized by an extensive process of connective tissue remodeling. For example, in humans, 48 hours postpartum there is a 2 to 3-fold increase in the cervical mRNA for collagen I and III, compared to non-pregnant state.31 An increase in the transcriptional levels for proteoglycans (biglycan, decorin) was also reported.31 Although the initiating signaling events are still unclear, most recent data seem to emphasize the role of individuals’ genetic makeup.32,33,34,35 Microarray technology has been used to thoroughly map the genes differentially expressed in the mouse cervical tissue during pregnancy and hours into postpartum.32 Based on expression patterns it has been concluded that cervical ripening requires a downregulation of collagen assembly genes, increased synthesis of glycosaminoglycans that disrupt the matrix, such as hyaluronan, increased metabolism of progesterone and changes in epithelial barrier properties. 36 In contrast, the latter phases of dilation and immediate postpartum recovery are associated with increased assembly of mature collagen, synthesis of matrix proteins that promote a dense connective tissue, activation of inflammatory responses, prostaglandin synthesis, changes in epithelial barrier properties and differentiation.36 Though the authors did not specifically search for genes up-or down-regulated in the later postpartum periods, based on their results, it seems reasonable to assume that proteins involved in enzymatic activity, intra-cellular signaling, DNA transcription and repair, apoptosis, cellular growth and differentiation may play an important role as the cervix transitions to the non-pregnant state.
Prior studies using microarray technology have revealed that the differential gene expression profile between MRL and C57 mice is characterized by enhanced collagen remodeling signals of the “high regenerative” phenotype mediated by a metabolic shift toward a low inflammatory response and an enhanced rapid tissue repair.15,16,17,37 In our study we demonstrated the MRL and C57 mice also differ significantly in cervical stiffness and elasticity immediately post-delivery. We have also shown that approximately 2 weeks post-delivery the uterine cervix breaks easier in both phenotypes. Hence, the information gained from this investigation provides functional ground that these 2 strains of mice might differ in gene action at the level of the uterine cervix. Further studies should be carried out to identify genes that are transcriptionally regulated during the phase of cervical involution and thus provide insight into molecular mechanisms controlling the biomechanics and the rate of cervical remodeling.
The triple helix arrangement allows collagen to cross-link into complex 3-D networks of fibrils, fibers and bundles that dictate both firmness and mechanical strength during non-pregnant and pregnant states.38 A growing body of evidence suggests that the orientation and thickness of collagen fibers show a relationship with mechanical strength.39 Collagen has a natural birefringence due its 3-D arrangement of fibers. The fibers’ thickness and orientation determine polarization colors, a property enhanced by Sirius red dye binding. Furthermore, in the cervix as well as in other tissues with complex mechanical behavior, such as cartilage and blood vessels, the relative ratio of collagen to proteoglycans is an important determinant of visco-elasticity, compliance and ultimate of function.22 The differences in biomechanical properties between MRL and C57 cervices paralleled by differences in collagen birefringence and proteoglycan abundance provide the proof of concept that the genetic background may also impact upon tissue biomechanics by concurrently modifying the 3-D arrangement of the collagen fibers and the relative ratio of collagen to proteoglycans in the uterine cervix.
Although there is evidence that inflammatory cells infiltrate the cervix prior to labor, the role of inflammation in modulating the process of cervical remodeling remains controversial.1,40 Interestingly, one prior study showed that neutrophil migration and activation was not initiated within the mouse cervix until shortly after delivery.30 In our study, the histological examination of the cervices could not recognize marked differences in cellular populations or inflammatory infiltrate between the MRL and C57 mice, 5d post delivery. Based solely on our histological data we cannot minimize the role of inflammation in the process of cervical remodeling. We argue that genes implicated in the expression of inflammatory mediators such as cytokines (interleukins [IL] 1, -6, -8) or matrix metallo-proteases (MMPs) may be involved in cervical remodeling in the absence of histological evidence of inflammation.30
Prior studies on differences in regenerative ear healing capacity identified that both the MRL and C57 mice generated an inflammatory response after wounding with cells that are MMP (MMP-2 and MMP-9) positive.41 C57 mice had, however, higher number of cells positive for tissue inhibitors of MMPs (TIMPs). Why MMPs express at a higher level and the TIMPs at a lower level in the MRL mice is not clear. Prior studies attributed this to the inflammatory cytokines TNFα and IL-1. 42 It has been reported that MRL macrophages are dysregulated in these two cytokine responses. The ear hole closure phenotype is a heritable quantitative trait known to interact with the environmental factors such as levels in steroid hormones. 17, 43 Rather than investigating particular expression of individual effectors or signaling regulators, we took a more global approach to identify differences in cervical recovery post-pregnancy between the two genetic backgrounds. Since this is the first study to propose a connection between wound healing phenotype and cervical function, our results highlight the importance of future research into the concerted role of inflammation, extracellular matrix remodeling and responsible molecular factors in the process of cervical involution post-delivery. Taking into account the differences in the genetic background of the MRL and C57 mice, our experimental model may be useful in stimulating future research to provide insight into the interaction between genetic and environmental factors as relates to cervical incompetence and preterm birth.
Acknowledgments
Supported from RO3 HD50249 (CSB)
References
- 1.Garfield RE, Saade G, Buhimschi C, Buhimschi I, Shi L, Shi SQ, Chwalisz K. Control and assessment of the uterus and cervix during pregnancy and labour. Hum Reprod Update. 1998;4:673–95. doi: 10.1093/humupd/4.5.673. [DOI] [PubMed] [Google Scholar]
- 2.Buhimschi I, Ali M, Jain V, Chwalisz K, Garfield RE. Differential regulation of nitric oxide in the rat uterus and cervix during pregnancy and labour. Hum Reprod. 1996;11:1755–66. doi: 10.1093/oxfordjournals.humrep.a019481. [DOI] [PubMed] [Google Scholar]
- 3.Buhimschi CS, Buhimschi IA, Zhao G, Funai E, Peltecu G, Saade GR, Weiner CP. Biomechanical properties of the lower uterine segment above and below the reflection of the urinary bladder flap. Obstet Gynecol. 2007;109:691–700. doi: 10.1097/01.AOG.0000236448.61465.95. [DOI] [PubMed] [Google Scholar]
- 4.Buhimschi CS, Buhimschi IA, Malinow A, Garfield RE, Weiner CP. The forces of labor. Fetal and Maternal Medicine Reviews. 2003;14(4):273–307. [Google Scholar]
- 5.Ludmir J, Sehdev HM. Anatomy and physiology of the uterine cervix. Clinic Obstet Gynecol. 2000;43:433–9. doi: 10.1097/00003081-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Marx SG, Wentz MJ, Mackay LB, Schlembach D, Maul H, Fittkow C, et al. Effects of progesterone on iNOS, COX-2, and collagen expression in the cervix. J Histochem Cytochem. 2006;54:623–39. doi: 10.1369/jhc.5A6759.2006. [DOI] [PubMed] [Google Scholar]
- 7.Ji H, Dailey TL, Long V, Chien EK. Androgen-regulated cervical ripening: a structural, biomechanical, and molecular analysis. Am J Obstet Gynecol. 2008;198:543.e1–9. doi: 10.1016/j.ajog.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Steer PJ. The endocrinology of parturition in the human. Baillieres Clin Endocrinol Metab. 1990;4:333–49. doi: 10.1016/s0950-351x(05)80054-0. [DOI] [PubMed] [Google Scholar]
- 9.Lee HY, Sherwood OD. The effects of blocking the actions of estrogen and progesterone on the rates of proliferation and apoptosis of cervical epithelial and stromal cells during the second half of pregnancy in rats. Biol Reprod. 2005;73:790–7. doi: 10.1095/biolreprod.105.043984. [DOI] [PubMed] [Google Scholar]
- 10.Leppert PC. Proliferation and apoptosis of fibroblasts and smooth muscle cells in rat uterine cervix throughout gestation and the effect of the antiprogesterone onapristone. Am J Obstet Gynecol. 1998;178:713–25. doi: 10.1016/s0002-9378(98)70481-8. [DOI] [PubMed] [Google Scholar]
- 11.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 12.Ramos JG, Varayoud J, Bosquiazzo VL, Luque EH, Muñoz-de-Toro M. Cellular turnover in the rat uterine cervix and its relationship to estrogen and progesterone receptor dynamics. Biol Reprod. 2002;67:735–42. doi: 10.1095/biolreprod.101.002402. [DOI] [PubMed] [Google Scholar]
- 13.Leferovich JM, Bedelbaeva K, Samulewicz S, Zhang XM, Zwas D, Lankford EB, Heber-Katz E. Heart regeneration in adult MRL mice. Proc Natl Acad Sci USA. 2001;98:9830–5. doi: 10.1073/pnas.181329398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kench JA, Russell DM, Fadok VA, Young SK, Worthen GS, Jones-Carson J, et al. Aberrant wound healing and TGF-beta production in the autoimmune-prone MRL/+ mouse. Clin Immunol. 1999;92:300–10. doi: 10.1006/clim.1999.4754. [DOI] [PubMed] [Google Scholar]
- 15.Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- 16.Naseem RH, Meeson AP, Michael Dimaio J, White MD, Kallhoff J, Humphries C, Goetsch SC, De Windt LJ, Williams MA, Garry MG, Garry DJ. Reparative myocardial mechanisms in adult C57BL/6 and MRL mice following injury. Physiol Genomics. 2007;30:44–52. doi: 10.1152/physiolgenomics.00070.2006. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald J, Rich C, Burkhardt D, Allen J, Herzka AS, Little CB. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 18.McBrearty BA, Clark LD, Zhang XM, Blankenhorn EP, Heber-Katz E. Genetic analysis of a mammalian wound-healing trait. Proc Natl Acad Sci USA. 1998;95:11792–7. doi: 10.1073/pnas.95.20.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masinde GL, Li X, Gu W, Davidson H, Mohan S, Baylink DJ. Identification of wound healing/regeneration quantitative trait loci (QTL) at multiple time points that explain seventy percent of variance in (MRL/MpJ and SJL/J) mice F2 population. Genome Res. 2001;11:2027–33. doi: 10.1101/gr.203701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heber-Katz E, Chen P, Clark L, Zhang XM, Troutman S, Blankenhorn EP. Regeneration in MRL mice: further genetic loci controlling the ear hole closure trait using MRL and M.m. Castaneus mice. Wound Repair Regen. 2004;12:384–92. doi: 10.1111/j.1067-1927.2004.012308.x. [DOI] [PubMed] [Google Scholar]
- 21.Harkness MRL, Harkness RD. Changes in the physiological properties of the uterine cervix of the rat during pregnancy. J Physiol. 1959;148:524–47. doi: 10.1113/jphysiol.1959.sp006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buhimschi IA, Dussably L, Buhimschi CS, Ahmed A, Weiner CP. Physical and biomechanical characteristics of rat cervical ripening are not consistent with increased collagenase activity. Am J Obstet Gynecol. 2004;191:1695–704. doi: 10.1016/j.ajog.2004.03.080. [DOI] [PubMed] [Google Scholar]
- 23.Buhimschi CS, Buhimschi IA, Yu C, Wang H, Sharer DJ, Diamond MP, Petkova AP, Garfield RE, Saade GR, Weiner CP. The effect of dystocia and previous cesarean uterine scar on the tensile properties of the lower uterine segment. Am J Obstet Gynecol. 2006;194:873–83. doi: 10.1016/j.ajog.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–55. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 25.Myers KM, Paskaleva AP, House M, Socrate S. Mechanical and biochemical properties of human cervical tissue. Acta Biomater. 2008;4:104–16. doi: 10.1016/j.actbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol. 1995;38:267–79. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Sfakianaki AK, Buhimschi IA, Ravishankar V, Bahtiyar MO, Dulay AT, Buhimschi CS. Relationships of maternal serum levels of vascular endothelial growth factor and tensile strength properties of the cervix in a rat model of chronic hypoxia. Am J Obstet Gynecol. 2008;198:223.e1–7. doi: 10.1016/j.ajog.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 28.Kokenyesi R, Woessner JF., Jr Relationship between dilatation of the rat uterine cervix and a small dermatan sulfate proteoglycan. Biol Reprod. 1990;42:87–97. doi: 10.1095/biolreprod42.1.87. [DOI] [PubMed] [Google Scholar]
- 29.Ruscheinsky M, De la Motte C, Mahendroo M. Hyaluronan and its binding proteins during cervical ripening and parturition: Dynamic changes in size, distribution and temporal sequence. Matrix Biol. 2008;27:487–97. doi: 10.1016/j.matbio.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod. 2006;74:236–45. doi: 10.1095/biolreprod.105.044891. [DOI] [PubMed] [Google Scholar]
- 31.Westergren-Thorsson G, Norman M, Björnsson S, Endrésen U, Stjernholm Y, Ekman G, Malmström A. Differential expressions of mRNA for proteoglycans, collagens and transforming growth factor-beta in the human cervix during pregnancy and involution. Biochim Biophys Acta. 1998;1406:203–13. doi: 10.1016/s0925-4439(98)00005-2. [DOI] [PubMed] [Google Scholar]
- 32.Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction. 2007;134:327–40. doi: 10.1530/REP-07-0032. [DOI] [PubMed] [Google Scholar]
- 33.Xu H, Gonzalez JM, Ofori E, Elovitz MA. Preventing cervical ripening: the primary mechanism by which progestational agents prevent preterm birth? Am J Obstet Gynecol. 2008;198:314.e1–8. doi: 10.1016/j.ajog.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Bukowski R, Hankins GD, Saade GR, Anderson GD, Thornton S. Labor-associated gene expression in the human uterine fundus, lower segment, and cervix. PLoS Med. 2006;3:e169. doi: 10.1371/journal.pmed.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H, Gonzalez JM, Ofori E, Elovitz MA. Preventing cervical ripening: the primary mechanism by which progestational agents prevent preterm birth? Am J Obstet Gynecol. 2008;198:314.e1–8. doi: 10.1016/j.ajog.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Timmons BC, Mahendroo M. Processes regulating cervical ripening differ from cervical dilation and postpartum repair: insights from gene expression studies. Reprod Sci. 2007;14:53–62. doi: 10.1177/1933719107309587. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Mohan S, Gu W, Baylink DJ. Analysis of gene expression in the wound repair/regeneration process. Mamm Genome. 2001;12:52–9. doi: 10.1007/s003350010230. [DOI] [PubMed] [Google Scholar]
- 38.Bank RA, Tekoppele JM, Janus GJ, Wassen MH, Pruijs HE, Van der Sluijs HA, Sakkers RJ. Pyridinium cross-links in bone of patients with osteogenesis imperfecta: evidence of a normal intrafibrillar collagen packing. J Bone Miner Res. 2000;15:1330–6. doi: 10.1359/jbmr.2000.15.7.1330. [DOI] [PubMed] [Google Scholar]
- 39.Leppert PC, Kokenyesi R, Klemenich CA, Fisher J. Further evidence of a decorin-collagen interaction in the disruption of cervical collagen fibers during rat gestation. Am J Obstet Gynecol. 2000;182:805–11. doi: 10.1016/s0002-9378(00)70329-2. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Invest. 2005;12:145–55. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Gourevitch D, Clark L, Chen P, Seitz A, Samulewicz SJ, Heber-Katz E. Matrix metalloproteinase activity correlates with blastema formation in the regenerating MRL mouse ear hole model. Dev Dyn. 2003;226:377–87. doi: 10.1002/dvdy.10243. [DOI] [PubMed] [Google Scholar]
- 42.Alleva DG, Kaser SB, Beller DI. Aberrant cytokine expression and autocrine regulation characterize macrophages from young MRL+/+ and NZB/W F1 lupus-prone mice. J Immunol. 1997;159:5610–9. [PubMed] [Google Scholar]
- 43.Heber-Katz E, Leferovich JM, Bedelbaeva K, Gourevitch D. Spallanzani’s mouse: a model of restoration and regeneration. Curr Top Microbiol Immunol. 2004;280:165–89. doi: 10.1007/978-3-642-18846-6_5. [DOI] [PubMed] [Google Scholar]