Abstract
Background
Arterial stiffness may contribute to depression via cerebral microvascular damage, but evidence for this is scarce. We therefore investigated whether arterial stiffness is associated with depressive symptoms and whether cerebral small vessel disease contributes to this association.
Methods
This cross-sectional study included a subset of participants from the AGES-Reykjavik study second examination round, which was conducted from 2007 to 2011. Arterial stiffness (carotid–femoral pulse wave velocity [CFPWV]), depressive symptoms (15-item geriatric depression scale [GDS-15]) and cerebral small vessel disease (MRI) were determined. Manifestations of cerebral small vessel disease included higher white matter hyperintensity volume, subcortical infarcts, cerebral microbleeds, Virchow–Robin spaces and lower total brain parenchyma volume.
Results
We included 2058 participants (mean age 79.6 yr; 59.0% women) in our analyses. Higher CFPWV was associated with a higher GDS-15 score, after adjustment for potential confounders (β 0.096, 95% confidence interval [CI] 0.005–0.187). Additional adjustment for white matter hyperintensity volume or subcortical infarcts attenuated the association between CFPWV and the GDS-15 score, which became nonsignificant (p > 0.05). Formal mediation tests showed that the attenuating effects of white matter hyperintensity volume and subcortical infarcts were statistically significant. Virchow–Robin spaces, cerebral microbleeds and cerebral atrophy did not explain the association between CFPWV and depressive symptoms.
Limitations
Our study was limited by its cross-sectional design, which precludes any conclusions about causal mediation. Depressive symptoms were assessed by a self-report questionnaire.
Conclusion
Greater arterial stiffness is associated with more depressive symptoms; this association is partly accounted for by white matter hyperintensity volume and subcortical infarcts. This study supports the hypothesis that arterial stiffness leads to depression in part via cerebral small vessel disease.
Introduction
Depression and depressive symptoms are frequently encountered in older individuals.1 Depressive symptoms in older populations, even in the absence of a clinical diagnosis of depression, are associated with a 1.5- to 2-fold higher mortality risk.2,3 The pathobiology of late-life depression is incompletely understood, but it has been suggested that arterial stiffness is involved.4
Arterial stiffness impairs the cushioning function of large arteries, reduces wave reflection and increases pressure and flow pulsatility, which transmits distally and damages the microcirculation.5,6 Microvascular damage leads to impaired vasoreactivity and cerebral hypoperfusion7 and can manifest itself as cerebral small vessel disease, including white matter hyperintensities, subcortical infarcts, Virchow–Robin spaces, cerebral microbleeds and cerebral atrophy.8,9 Cerebral small vessel disease, in turn, may predispose to depression via disruption of frontal and subcortical structures involved in mood regulation.10,11 In accordance, previous studies6,12–17 have shown an association between arterial stiffness and cerebral small vessel disease. The studies were conducted in the general population6,12–14,17 (sample sizes ranged from 30313 to 1460 individuals14), or in selected clinical populations (e.g., individuals with diabetes [n = 178]15 or hypertension [n = 167]16). Most of these studies, but not all,13 were cross-sectional by design. Furthermore, recent longitudinal data of the Age, Gene/Environment Susceptibility (AGES)-Reykjavik study (n = 1949)18 and the Three City (3C) study (n = 1214)19 have shown that markers of cerebral small vessel disease are associated with incident depressive symptoms.
However, only 3 studies4,20,21 have evaluated the association between arterial stiffness and depression, and these studies yielded inconsistent results. The Rotterdam Study (n = 3704)4 and a small case–control study (n = 46),20 which both included older individuals (mean age of both study samples 72 yr), showed an association between greater arterial stiffness and presence of depression. In contrast, the Netherlands Study of Depression and Anxiety (NESDA; n = 618) did not find an association between arterial stiffness and depression.21 However, the NESDA included a relatively young sample (mean age 47 yr). The contribution of arterial stiffness and cerebral small vessel disease to depression may be more important in older people. In addition, the foregoing studies did not evaluate whether the presence of cerebral small vessel disease contributes to the association (if any) between arterial stiffness and depression.
In view of the above findings, we investigated in a large population-based cohort of older men and women whether arterial stiffness was associated with depressive symptoms and whether any such association was explained by manifestations of cerebral small vessel disease, including white matter hyperintensities, subcortical infarcts, Virchow–Robin spaces, cerebral microbleeds and cerebral atrophy.
Methods
AGES-Reykjavik Study
For the present study, we used cross-sectional data from the AGES-Reykjavik Study second examination, which took place from 2007 to 2011. The AGES-Reykjavik Study is a population-based cohort study originating from the Reykjavik Study, as described elsewhere.22 It aimed to investigate genetic and environmental factors and biological mechanisms leading to major clinical and subclinical disorders in old age, including those prevalent in neurocognitive, vascular, musculoskeletal, body compositional and metabolic symptoms. The AGES-Reykjavik Study was approved by the National Bioethics Committee in Iceland (approval number: VSN-00–063), and by the National Institute on Aging Intramural Institutional Review Board. All participants gave written informed consent. Briefly, from 2002 to 2006, 5764 surviving participants in the Reykjavik Study were examined. From 2007 to 2011 there was a follow-up examination of all surviving participants who agreed to participate (n = 3316). Reasons for not attending the follow-up examination included death (n = 1039), refusal (n = 1198) and loss to follow-up (n = 211).
Arterial stiffness
We assessed arterial stiffness by determining carotid–femoral pulse wave velocity (CFPWV), as previously described.6 Briefly, after 15–20 min in a supine posture, we measured brachial blood pressure and arterial tonometry with an electrocardiogram obtained from the carotid and femoral arteries using a custom transducer (Cardiovascular Engineering, Inc.).6 Transit distance from the carotid to femoral arteries was assessed with body surface measurements from the suprasternal notch to the carotid and femoral pulse recording sites. We computed CFPWV as the pulse wave transit distance divided by the transit time of the pulse wave from the carotid to femoral arteries, with adjustment for parallel transmission of the arterial pulse wave in the brachiocephalic artery and aortic arch.6 Higher CFPWV indicates greater arterial stiffness.
Depressive symptoms
We assessed depressive symptoms using the 15-item Geriatric Depression Scale (GDS-15; score range 0–15).23 Higher GDS-15 scores indicate the presence of more depressive symptoms. The GDS-15 score can be used as a continuous as well as a dichotomous variable.23 In the present study, we used the continuous variable as the primary outcome, because this offers the highest power to detect associations. In secondary analyses we also evaluated associations with dichotomous measures of depressive symptoms, defined as a dichotomous GDS-15 score of 6 or higher23 and/or use of antidepressant medication (tricyclic anti-depressants, selective serotonin reuptake inhibitors, other non-tricyclics and monoamine oxidase inhibitors), as assessed based on medication bottles brought to the clinic. In addition, the association with apathy was evaluated. The following 3 items of the GDS-15 score are related to apathy: 1) “Have you dropped many of your activities and interests?”; 2) “Do you prefer to stay at home, rather than going out and doing new things?”; and 3) “Do you feel full of energy?”.24,25 As described previously,24,25 we distinguished an apathy subscale that included all apathy items (range 0–3; GDS-3A). Presence of apathy was defined as a GDS-3A score of 2 or 3 versus no apathy (score of 0 or 1).
Brain MRI measures
Image acquisition
All eligible participants were offered a high-resolution brain MRI acquired on the same study-dedicated 1.5 T Signa Twin-speed scanner (General Electric Medical Systems). The same imaging protocol was used as in the first examination of the AGES-Reykjavik Study, which is described elsewhere,22,26,27 and included the following sequences: 3-dimensional spoiled-gradient recalled T1-weighted, proton density/T2-weighted fast spin-echo, fluid-attenuated inversion recovery (FLAIR) and T2*-weighted gradient-echo type echo-planar image (GRE-EPI). All images were acquired to give full brain coverage, with slices angled parallel to the anterior commissure– posterior commissure line in order to give reproducible image views in the oblique–axial plane.
Image analysis
We evaluated several manifestations of cerebral small vessel disease. White matter hyperintensity volume (WMHV) and total brain parenchyma volume (an indicator of cerebral atrophy) were computed automatically with a previously described image analysis pipeline28 and were calculated as the percentage of total intracranial volume (ICV). We considered lower total brain parenchyma volume to be a manifestation of cerebral small vessel disease, as cerebral small vessel disease may contribute to generalized loss of brain parenchyma via, among others, microinfarcts29 and loss of white matter integrity.30 Subcortical infarcts were evaluated as described previously26 and defined as a brain parenchyma defect with a minimum diameter of 4 mm, not extending into the cortex, with a signal intensity equal to cerebrospinal fluid on all pulse sequences (i.e., T2-weighted, proton density–weighted and FLAIR) and surrounded by an area of high signal intensity on FLAIR images. Parenchymal defects in the subcortical area with evidence of hemosiderin on the T2*-weighted GRE-EPI scan were labelled as resorbed hematomas and were excluded from the definition of subcortical infarcts. In addition, Virchow–Robin spaces were evaluated separately and defined as defects in the subcortical area without a rim or area of high signal intensity on FLAIR and without evidence of hemosiderin on the T2*-weighted GRE-EPI scan. We considered the presence of Virchow–Robin spaces to be a manifestation of cerebral small vessel disease, as they are associated with endothelial dysfunction, which may play a role in the pathogenesis of cerebral small vessel disease.8 Cerebral microbleeds were defined as a focal area of signal void within the brain parenchyma visible on T2*-weighted GRE-EPI scans and were identified as described previously.27
Confounding variables
Education (categorized into primary, secondary and college/university education) was measured at baseline. All other confounding variables were measured during the second examination of the AGES-Reykjavik Study. Smoking history (categorized into none, former and current smoker) was assessed using a questionnaire. Use of antihypertensive medication was assessed using a questionnaire and by assessing medication bottles brought to the clinic. We evaluated brachial artery mean arterial pressure and heart rate during the arterial stiffness measurements by using a computer-controlled manometer and an electrocardiogram, respectively.6 Gait speed, a measure of physical performance,31 was determined as the time in seconds needed to walk 6 metres at a usual pace. Diabetes mellitus was defined as a self-reported doctor’s diagnosis of diabetes, use of blood glucose–lowering drugs or fasting blood glucose level of 7.0 mmol/L or higher. The coronary calcium score (categorized into sex-specific quartiles), a measure of atherosclerosis, was based on computed tomography scan.32 The Digit Symbol Substitution Test (DSST), a measure of cognitive function, was also administered.22
Statistical analysis
We performed our statistical analyses using PASW statistics version 21. We inverse-transformed CFPWV to reduce heteroscedasticity and multiplied it by −1000 to restore directionality and to convert the units to milliseconds per metre. In addition, CFPWV was entered as a sex-specific Z score in all models. We logarithmically transformed WMHV to normalize the skewed distribution, and we entered WMHV and lower total brain parenchyma volume as Z scores in all models.
Linear and logistic regression analyses were used to estimate the association between CFPWV, the GDS-15 score and markers of cerebral small vessel disease. Models were adjusted for the following potential confounders (selected based on previous literature and knowledge): age, sex, education level, smoking, DSST, gait speed, BMI, diabetes, mean arterial pressure, heart rate, coronary calcium score and the use of antihypertensive medication. Associations are reported as unstandardized regression coefficients with corresponding 95% confidence intervals (CI).
We used mediation analysis33 to test the hypothesis that manifestations of cerebral small vessel disease (that is, higher WMHV, presence of subcortical infarcts, Virchow–Robin spaces, cerebral microbleeds and lower total brain parenchyma volume) are on the potential causal pathway of the association between arterial stiffness and depressive symptoms. The model quantifies the degree to which a variable statistically moderates the association of a dependent variable with an independent variable. We used bootstrapping (10 000 samples) to calculate bias-corrected 95% CIs of the explained effects using the PROCESS statistical package for PASW statistics.33 The magnitude of the explained effect was calculated as a percentage of the total direct effect.
We performed the following secondary analyses. Logistic analysis was performed to evaluate the association between CFPWV and a dichotomous measure of depressive symptoms. It has been suggested24 that cerebral small vessel disease may be associated with, in particular, apathy-related or motivation-related symptoms of depression, but not with mood-related symptoms. Therefore, we performed logistic regression analyses to evaluate the association between CFPWV and individual apathy items of the GDS-15 score as the outcome. To minimize the potential confounding effect of stroke, we repeated the analyses after excluding individuals with a clinical diagnosis of stroke. In addition, it has been suggested that deep or infratentorial cerebral microbleeds (i.e., microbleeds located in the basal ganglia, thalamus, brainstem and cerebellum) are more closely associated with vascular disease, whereas lobar microbleeds might be more closely associated with amyloid angiopathy.34 Therefore, analyses were repeated with presence of deep cerebral microbleeds only, instead of the presence of any microbleed. Finally, we investigated whether the association between arterial stiffness and depressive symptoms differed by sex by adding interaction terms between arterial stiffness and sex to the fully adjusted models. We found no such interaction (p = 0.57) and, therefore, all results are presented for men and women combined.
Results
Of the 3316 participants of the second examination of the AGES-Reykjavik Study, 648 had missing brain MRI data, 419 had missing data on arterial tonometry and 77 had missing data on depressive symptoms. Reasons for missing MRI data were contraindications (n = 272), refusal/nonattendance (n = 337) or technical reasons (no qualitatively acceptable MRI data available for all necessary sequences; n = 39). Missing data on tonometry was due to logistical or technical reasons. Of the remaining 2172 participants, we excluded those with a diagnosis of dementia (all subtypes; n = 114), which was diagnosed according to international guidelines35 by a panel that included a geriatrician, a neurologist, a neuropsychologist and a neuroradiologist, as described elsewhere.22 Excluded participants were more likely to be older (81.2 yr v. 76.6 yr), less educated (primary school or less: 23.6% v. 18.9%) and to have a higher body mass index (BMI; 27.3 v. 26.5) and to have higher prevalences of diabetes (17.6% v. 11.6%) and stroke (11.5% v. 8.2%; all p < 0.05).
The final study sample for the present analysis consisted of 2058 participants. Characteristics of the study population are described in Table 1. Briefly, participants had a mean age of 79.6 years, and 59.0% were women. Median CFPWV was 12.5 m/s (interquartile range [IQR] 10.4–15.7). The median GDS-15 score was 2 (IQR 1–3); 109 (5.3%) participants had a GDS-15 score of 6 or higher. A total of 294 (14.3%) participants used antidepressant medication.
Table 1.
Characteristics of the study population (n = 2058)
| Characteristic | Mean ± SD, median [IQR] or no. (%) |
|---|---|
| General characteristics | |
| Age, yr | 79.6 ± 4.6 |
| Female sex | 1214 (59.0) |
| Smoking status | |
| Nonsmoker | 897 (43.6) |
| Former | 983 (47.8) |
| Current | 177 (8.6) |
| Education | |
| Primary or less | 398 (18.9) |
| Secondary | 1084 (52.7) |
| College/university | 584 (28.4) |
| Digit Symbol Substitution Test score | 30 ± 10 |
| Body mass index | 26.5 ± 3.9 |
| Diabetes | 239 (11.6) |
| Stroke | 169 (8.2) |
| Systolic blood pressure, mm Hg | 145 ± 21 |
| Diastolic blood pressure, mm Hg | 70 ± 11 |
| Use of antihypertensive medication | 1473 (71.6) |
| Brain MRI measures | |
| White matter hyperintensity volume, mL | 15 [8–28] |
| Subcortical infarcts | 202 (9.8) |
| Virchow–Robin spaces | 364 (17.7) |
| Cerebral microbleeds | 603 (29.3) |
| Total brain parenchyma volume, mL | 1067 ± 99 |
IQR = interquartile range; SD = standard deviation.
Association between arterial stiffness and depressive symptoms
Higher CFPWV (per standard deviation [SD]) was significantly associated with a higher GDS-15 score (β 0.096, 95% CI 0.005–0.187, explained variance by CFPWV R2 = 0.003) after adjustment for confounders but without adjustment for any of the potential mediators.
Contribution of manifestations of cerebral small vessel disease to the association between arterial stiffness and depressive symptoms
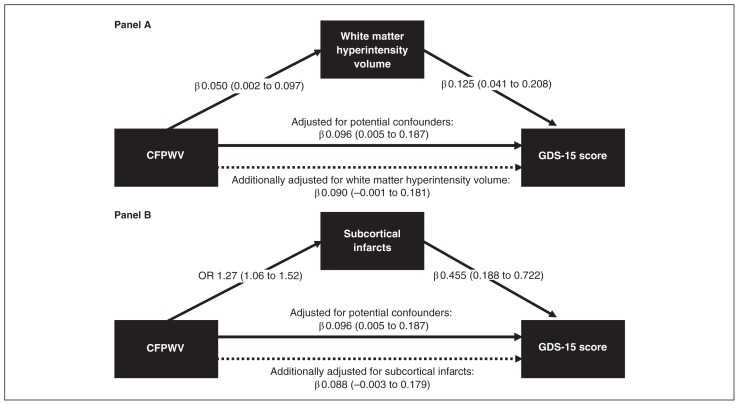
Higher CFPWV was significantly associated with a higher WMHV, and higher WMHV was significantly associated with a higher GDS-15 score (Fig. 1A). When we adjusted the association between CFPWV and the GDS-15 score for WMHV, the association was attenuated and was no longer statistically significant (Fig. 1A). The explained effect by WMHV was significant and was 6% of the total direct effect between CFPWV and the GDS-15 score (Table 2).
Fig. 1.
Association between carotid–femoral pulse wave velocity (CFPWV) and the 15-item Geriatric Depression Scale (GDS-15) score and the explained effects of this association by (A) white matter hyperintensity volume and (B) subcortical infarcts. Solid lines indicate significant associations; dashed lines indicate nonsignificant associations. Associations are given as regression coefficients (β) or odds ratios (ORs), and corresponding 95% confidence intervals (CIs). The CFPWV and white matter hyperintensity volume are indicated per higher standard deviation (SD). All associations are adjusted for age, sex, education level, smoking, digit symbol substitution test score, gait speed, body mass index, diabetes, mean arterial pressure, heart rate, coronary calcium score and use of antihypertensive medication.
Table 2.
Explained effects of white matter hyperintensity volume, subcortical infarcts, Virchow–Robin spaces, cerebral microbleeds and lower total brain parenchyma volume of the association between carotid-femoral pulse wave velocity and GDS-15 score*
| Manifestations of cerebral small vessel disease | β | 95% CI | %† |
|---|---|---|---|
| White matter hyperintensity volume (per +1 SD) | 0.006 | 0.001 to 0.017 | 6% |
| Subcortical infarcts | 0.008 | 0.002 to 0.020 | 9% |
| Virchow–Robin spaces | 0.001 | −0.003 to 0.007 | 1% |
| Cerebral microbleeds | 0.001 | −0.00 to 0.007 | 1% |
| Lower total brain parenchyma volume (per −1 SD) | −0.002 | −0.009 to 0.003 | −2% |
CI = confidence interval; GDS-15: 15-item geriatric depression scale; SD = standard deviation.
All associations adjusted for age, sex, education level, smoking, Digit Symbol Substitution Test score, gait speed, body mass index, diabetes, mean arterial pressure, heart rate, coronary calcium score and use of antihypertensive medication.
Magnitude of the explained effect relative to the total direct effect between carotid–femoral pulse wave velocity and GDS-15 score.
Similarly, higher CFPWV was significantly associated with the presence of subcortical infarcts (Fig. 1B), and the presence of subcortical infarcts was significantly associated with a higher GDS-15 score (Fig. 1B). When we adjusted the association between CFPWV and the GDS-15 score for subcortical infarcts, the association was attenuated and was no longer significant (Fig. 1B). The effect of subcortical infarcts was significant and was 9% of the total direct effect between CFPWV and the GDS-15 score (Table 2).
When WMHV and subcortical infarcts were entered simultaneously into the mediation model, they explained 12% of the total direct effect between CFPWV and the GDS-15 score.
In contrast, Virchow–Robin spaces, cerebral microbleeds and total brain parenchyma volume did not attenuate the association between CFPWV and the GDS-15 score (Table 2 and Appendix 1, Fig. S1, available at jpn.ca). Higher CFPWV was associated with the presence of Virchow–Robin spaces and cerebral microbleeds, but these lesions were not associated with depressive symptoms. In addition, lower total brain parenchyma volume was significantly associated with more depressive symptoms, but CFPWV was not associated with lower total brain parenchyma volume.
Secondary analyses
We found that CFPWV was not significantly associated with a dichotomous measure of depressive symptoms (i.e., GDS-15 score ≥ 6) or use of antidepressant medication (n = 336) (odds ratio [OR] 1.10, 95% CI 0.96–1.26). In addition, CFPWV was not significantly associated with a presence of depressive symptoms, defined as a GDS-15 score of 6 or higher, irrespective of the use of antidepressant medication (n = 109) (OR 1.02, 95% CI 0.81–1.29). We found that CFPWV was not associated with apathy (i.e., GDS-3A score of 2 or 3) after adjustment for all potential confounders (OR 1.07, 95% CI 0.96–1.19). After the exclusion of individuals with stroke (n = 223), the explained effects by WMHV and subcortical infarcts did not materially change (Appendix 1, Table S1). Finally, when we repeated the analyses with the presence of deep or cerebral microbleeds instead of the presence of any microbleed, results did not materially change (data not shown).
Discussion
We evaluated whether arterial stiffness is associated with depressive symptoms and whether any such association can be explained by cerebral small vessel disease. We had 2 main findings. First, arterial stiffness, as determined by CFPWV, was independently associated with a higher level of depressive symptoms. Second, this association was explained in part (12%) by WMHV and subcortical infarcts, but not by Virchow–Robin spaces, cerebral microbleeds and cerebral atrophy.
These data are consistent with the hypothesis that arterial stiffness increases the risk of depressive symptoms in part via cerebral microvascular damage. Arterial stiffness may cause microvascular damage via an increased pulsatile load on the microcirculation.36,37 This increased load causes direct microvascular damage and induces a microvascular remodelling response. Microvascular remodelling initially serves to limit the penetration of the pulsatile pressure load into the microcirculatory system by raising vascular resistance. Yet, this protective response may ultimately become unfavourable, leading to impaired vasoreactivity, cerebral hypoperfusion and microvascular ischemia.7,36 Ischemia may damage frontal and subcortical structures or their connecting pathways involved in mood regulation and, hence, may lead to depression.7,10,11
Previous studies have shown associations of CFPWV or local carotid stiffness with various manifestations of cerebral small vessel disease, including WMHV6,12,17 and subcortical or lacunar infarcts.6,12,17 In addition, previous studies have shown an association between depression and WMHV19,38 and lacunar infarcts.38,39 Furthermore, a population-based study4 has shown that higher CFPWV and local carotid stiffness were associated with depression. The present study shows that the association between arterial stiffness and depressive symptoms is in part explained by cerebral small vessel lesions and thereby provides additional evidence consistent with the role of arterial stiffness in modulating the emergence of late-life depressive symptoms.
However, a relatively large part of the association between arterial stiffness and depressive symptoms remained unexplained after taking into account the effects of WMHV and subcortical infarcts. This remaining association may be due to vascular brain lesions not (directly) captured in the MRI scans (e.g., microinfarcts and loss of white matter integrity) that we did not incorporate into the analyses. In addition, it is possible that only a subset of the participants who scored poorly on the GDS-15 have vascular-related disease. Finally, although we adjusted for a large series of potential confounders, we cannot exclude the possibility of residual confounding.
Cerebral small vessel disease encompasses different lesions found on neuroimaging.6,8 Specific clinical consequences of each lesion type and their location are, however, not completely understood. In the present study, WMHV and subcortical infarcts explained a part of the association between arterial stiffness and depressive symptoms, whereas Virchow–Robin spaces, cerebral microbleeds and cerebral atrophy did not. Although higher CFPWV was associated with presence of Virchow–Robin spaces and cerebral microbleeds, these lesions were not associated with depressive symptoms. It is possible that Virchow–Robin spaces and cerebral microbleeds play a role early in the pathogenesis of cerebral small vessel disease,40 whereas WMHs and subcortical infarcts represent more advanced disease states. In addition, although lower brain parenchyma volume was associated with a higher level of depressive symptoms, brain parenchyma volume was not associated with CFPWV. Cerebral atrophy is strongly determined by factors other than vascular disease, particularly the process of neurodegeneration.8 This may have resulted in an underestimation of the association between CFPWV and brain parenchyma volume in the older population included in this study.
Strengths of the present study are the large population-based sample of older individuals, the comprehensive brain MRI assessment of various manifestations of cerebral small vessel disease and the extensive characterization of participants, which enabled us to adjust for a series of potential confounders.
In view of global aging and the increased prevalence of arterial stiffness with age, efforts at favourably influencing arterial stiffness may have significant public health implications for preventing vascular-related depression. Arterial stiffness may be favourably influenced by lifestyle modifications, such as weight loss, increased (habitual) physical activity and dietary modifications (e.g., low consumption of sodium). In addition, drugs, such as angiotensin receptor-2 agonists (e.g., compound 21), may lower arterial stiffness, possibly beyond any blood-pressure lowering effects.41
Limitations
Our study has some limitations. First, the cross-sectional design precludes any conclusions about a temporal association between arterial stiffness, cerebral small vessel disease and depressive symptoms. The evidence provided in the present study is, therefore, necessarily circumstantial. Nevertheless, it provides important support for more definitive investigations. It has been suggested that the association between depression and arterial stiffness may be bidirectional.21 Depressive symptoms may lead to arterial stiffness via multiple mechanisms, including inflammation, endothelial dysfunction and unfavourable lifestyle habits.42–44 Indeed, a recent small clinical trial showed that treatment of depression may decrease arterial stiffness.45 Further longitudinal studies are needed to clarify this issue. Furthermore, the associations between arterial stiffness, cerebral small vessel disease and depressive symptoms may reflect more direct measures of common underlying mechanisms (e.g., inflammation and oxidative stress). Second, depressive symptoms were assessed using a self-report questionnaire rather than a structured interview. Therefore, no information was available on the presence of a major depressive disorder. Third, it is possible that death or attrition between the AGES-1 and -2 studies resulted in a disproportional loss of people who were likely at high risk for depression. The associations between arterial stiffness, cerebral small vessel diseaes and depression found in the present study sample may be biased if associations substantively differed in people who were lost to follow-up. We consider this biased attrition unlikely, in which case the loss of the individuals reduces our power to detect existing associations. Fourth, the level and severity of depressive symptoms in this study was relatively low compared with depression rates in other European countries,46 but our rates are consistent with those found in a previous study conducted in Iceland.46 This low symptomatology may, however, have reduced the effect sizes and sensitivity to detect associations. This may explain why an association was found between arterial stiffness and a continuous depressive symptom score, but not with a dichotomous depression score. Analyses with a continuous outcome, in general, have higher statistical power than analyses with a dichotomous outcome. Finally, a relatively high number of individuals used antidepressant medication (14.3%) compared with the number of individuals with a GDS-15 score of 6 or higher (5.3%). Misclassification of the dichotomous depression score may have occurred because antidepressant medication is also prescribed for other reasons, and this may have led to an underestimation of the association between arterial stiffness and the dichotomous depression score.
Conclusion
The present study shows an association between greater arterial stiffness and more depressive symptoms in a general older population, and this association is in part explained by WMHV and subcortical infarcts.
Acknowledgements
The AGES-Reykjavik Study was funded by National Institutes of Health (NIH) (contract N01-AG-12100) and the Intramural Research Program of the National Institute on Aging, USA; the Icelandic Heart Association and the Icelandic Parliament, Iceland; and by grants from the National Institutes of Health, National Heart, Lung and Blood Institute (HL094898) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK082447).
Footnotes
Competing interests: G. Mitchell is owner of Cardiovascular Engineering, Inc., a company that designs and manufactures devices that measure vascular stiffness. The company uses these devices in studies that evaluate the effects of diseases and interventions on vascular stiffness. He has served as a consultant for Novartis, Servier and Merck, and has a patent (6331162) for a pulse wave velocity measurement device. A. Levey has a patent application pending for precise estimation of GFR using a panel of filtration markers. No other competing interests declared.
Contributors: T. van Sloten, G. Mitchell, M. van Buchem, P. Jonsson, T. Harris, V. Gudnason and L. Launer designed the study. G. Mitchell, S. Sigurdsson, M. Garcia, A. Levey and V. Gudnason acquired the data, which T. van Sloten, G. Mitchell, R. Henry and C. Stehouwer analyzed. T. van Sloten and L. Launer wrote the article, which all authors reviewed and approved for publication.
References
- 1.Byers AL, Yaffe K, Covinsky KE, et al. High occurrence of mood and anxiety disorders among older adults: The National Comorbidity Survey Replication. Arch Gen Psychiatry. 2010;67:489–96. doi: 10.1001/archgenpsychiatry.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penninx BW, Geerlings SW, Deeg DJ, et al. Minor and major depression and the risk of death in older persons. Arch Gen Psychiatry. 1999;56:889–95. doi: 10.1001/archpsyc.56.10.889. [DOI] [PubMed] [Google Scholar]
- 3.Pulska T, Pahkala K, Laippala P, et al. Follow up study of longstanding depression as predictor of mortality in elderly people living in the community. BMJ. 1999;318:432–3. doi: 10.1136/bmj.318.7181.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiemeier H, Breteler MM, van Popele NM, et al. Late-life depression is associated with arterial stiffness: a population-based study. J Am Geriatr Soc. 2003;51:1105–10. doi: 10.1046/j.1532-5415.2003.51359.x. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–45. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain. 2011;134:3398–407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–74. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–97. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 12.Tsao CW, Seshadri S, Beiser AS, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–91. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosano C, Watson N, Chang Y, et al. Aortic pulse wave velocity predicts focal white matter hyperintensities in a biracial cohort of older adults. Hypertension. 2013;61:160–5. doi: 10.1161/HYPERTENSIONAHA.112.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poels MM, Zaccai K, Verwoert GC, et al. Arterial stiffness and cerebral small vessel disease: the Rotterdam Scan Study. Stroke. 2012;43:2637–42. doi: 10.1161/STROKEAHA.111.642264. [DOI] [PubMed] [Google Scholar]
- 15.Laugesen E, Hoyem P, Stausbol-Gron B, et al. Carotid-femoral pulse wave velocity is associated with cerebral white matter lesions in type 2 diabetes. Diabetes Care. 2013;36:722–8. doi: 10.2337/dc12-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henskens LH, Kroon AA, van Oostenbrugge RJ, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52:1120–6. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- 17.Brisset M, Boutouyrie P, Pico F, et al. Large-vessel correlates of cerebral small-vessel disease. Neurology. 2013;80:662–9. doi: 10.1212/WNL.0b013e318281ccc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Sloten TT, Sigurdsson S, van Buchem MA, et al. Cerebral small vessel disease and association with higher incidence of depressive symptoms in a general elderly population: the AGES-Reykjavik Study. Am J Psychiatry. 2015;172:570–8. doi: 10.1176/appi.ajp.2014.14050578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godin O, Dufouil C, Maillard P, et al. White matter lesions as a predictor of depression in the elderly: the 3C-Dijon study. Biol Psychiatry. 2008;63:663–9. doi: 10.1016/j.biopsych.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Paranthaman R, Greenstein AS, Burns AS, et al. Vascular function in older adults with depressive disorder. Biol Psychiatry. 2010;68:133–9. doi: 10.1016/j.biopsych.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Seldenrijk A, van Hout HP, van Marwijk HW, et al. Depression, anxiety, and arterial stiffness. Biol Psychiatry. 2011;69:795–803. doi: 10.1016/j.biopsych.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 17:1982–1983. 37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 24.Ligthart SA, Richard E, Fransen NL, et al. Association of vascular factors with apathy in community-dwelling elderly individuals. Arch Gen Psychiatry. 2012;69:636–42. doi: 10.1001/archgenpsychiatry.2011.1858. [DOI] [PubMed] [Google Scholar]
- 25.Grool AM, Geerlings MI, Sigurdsson S, et al. Structural MRI correlates of apathy symptoms in older persons without dementia: AGES- Reykjavik Study. Neurology. 2014;82:1628–35. doi: 10.1212/WNL.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scher AI, Gudmundsson LS, Sigurdsson S, et al. Migraine headache in middle age and late-life brain infarcts. JAMA. 2009;301:2563–70. doi: 10.1001/jama.2009.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. Cerebral micro-bleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79:1002–6. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigurdsson S, Aspelund T, Forsberg L, et al. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik study. Neuroimage. 2012;59:3862–70. doi: 10.1016/j.neuroimage.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol. 2011;70:774–80. doi: 10.1002/ana.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kloppenborg RP, Nederkoorn PJ, Grool AM, et al. Cerebral small-vessel disease and progression of brain atrophy: the SMART-MR study. Neurology. 2012;79:2029–36. doi: 10.1212/WNL.0b013e3182749f02. [DOI] [PubMed] [Google Scholar]
- 31.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidal JS, Sigurdsson S, Jonsdottir MK, et al. Coronary artery calcium, brain function and structure: the AGES-Reykjavik Study. Stroke. 2010;41:891–7. doi: 10.1161/STROKEAHA.110.579581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–74. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Press; 1994. [Google Scholar]
- 36.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–60. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–4. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 38.Grool AM, Gerritsen L, Zuithoff NP, et al. Lacunar infarcts in deep white matter are associated with higher and more fluctuating depressive symptoms during three years follow-up. Biol Psychiatry. 2013;73:169–76. doi: 10.1016/j.biopsych.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Saavedra Perez HC, Direk N, Hofman A, et al. Silent brain infarcts: a cause of depression in the elderly? Psychiatry Res. 2013;211:180–2. doi: 10.1016/j.pscychresns.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Schreiber S, Bueche CZ, Garz C, et al. The pathologic cascade of cerebrovascular lesions in SHRSP: Is erythrocyte accumulation an early phase? J Cereb Blood Flow Metab. 2012;32:278–90. doi: 10.1038/jcbfm.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell GF. Arterial stiffness and hypertension. Hypertension. 2014;64:13–8. doi: 10.1161/HYPERTENSIONAHA.114.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry. 2003;54:248–61. doi: 10.1016/s0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- 43.van Sloten TT, Schram MT, Adriaanse MC, et al. Endothelial dysfunction is associated with a greater depressive symptom score in a general elderly population: the Hoorn Study. Psychol Med. 2014;44:1403–16. doi: 10.1017/S0033291713002043. [DOI] [PubMed] [Google Scholar]
- 44.Broadley AJ, Korszun A, Abdelaal E, et al. Metyrapone improves endothelial dysfunction in patients with treated depression. J Am Coll Cardiol. 2006;48:170–5. doi: 10.1016/j.jacc.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 45.Oulis P, Kouzoupis A, Kyrkou K, et al. Reversal of increased arterial stiffness in severely depressed women after 6-week antidepressant treatment. J Affect Disord. 2010;122:164–6. doi: 10.1016/j.jad.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Copeland JR, Beekman AT, Braam AW, et al. Depression among older people in Europe: the EURODEP studies. World Psychiatry. 2004;3:45–9. [PMC free article] [PubMed] [Google Scholar]