Abstract
Background
Social anxiety disorder (SAD) and Williams–Beuren syndrome (WBS) are 2 conditions with major differences in terms of genetics, development and cognitive profiles. Both conditions are associated with compromised abilities in overlapping areas, including social approach, processing of social emotional cues and gaze behaviour, and to some extent they are associated with opposite behaviours in these domains. We examined common and distinct patterns of brain activation during a facial emotion processing paradigm in patients with SAD and WBS.
Methods
We examined patients with SAD and WBS and healthy controls matched by age and laterality using functional MRI during the processing of happy, fearful and angry faces.
Results
We included 20 patients with SAD and 20 with WBS as well as 20 matched controls in our study. Patients with SAD and WBS did not differ in the pattern of limbic activation. We observed differences in early visual areas of the face processing network in patients with WBS and differences in the cortical prefrontal regions involved in the top–down regulation of anxiety and in the fusiform gyrus for patients with SAD. Compared with those in the SAD and control groups, participants in the WBS group did not activate the right lateral inferior occipital cortex. In addition, compared with controls, patients with WBS hypoactivated the posterior primary visual cortex and showed significantly less deactivation in the right temporal operculum. Participants in the SAD group showed decreased prefrontal activation compared with those in the WBS and control groups. In addition, compared with controls, participants with SAD showed decreased fusiform activation. Participants with SAD and WBS also differed in the pattern of activation in the superior temporal gyrus, a region that has been linked to gaze processing.
Limitations
The results observed in the WBS group are limited by the IQ of the WBS sample; however, the specificity of findings suggests that the pattern of brain activation observed for WBS is more likely to reflect a neurobiological substrate rather than intellectual impairment per se.
Conclusion
Patients with SAD and WBS showed common and specific patterns of brain activation. Our results highlight the role of cortical regions during facial emotion processing in individuals with SAD and WBS.
Introduction
Social anxiety disorder (SAD) is a highly prevalent psychiatric condition.1 The core feature of the disorder is a marked and persistent fear of social situations in which the person is exposed to unfamiliar people or possible scrutiny by others.2
A condition of great interest in relation to the study of SAD is Williams–Beuren syndrome (WBS), a rare neurodevelopmental disorder caused by a heterozygous deletion of 25–28 genes on chromosome band 7q11.23.3 Williams–Beuren syndrome is associated with abnormal development across multiple cognitive, emotional and social domains, which leads to a unique phenotype characterized by an uneven profile in cognitive functions and a distinctive social profile.4 Patients with SAD and WBS show major differences in terms of genetics, development and cognitive profiles; both show compromised abilities in some overlapping areas and, to some extent, display opposite behaviours in these domains. For instance, the main characteristic of the WBS social phenotype is a markedly increased social drive, particularly toward strangers.4,5 Thus, whereas people with SAD are typically shy and withdrawn when faced with unfamiliar people and social settings,6 individuals with WBS display outgoing, hypersocial behaviour, and they exhibit an unusual attraction to unfamiliar people.4,5,7 Similarly, people with SAD typically fear and avoid eye contact,8 whereas individuals with WBS show an unusual degree of attention and fixation toward faces and eyes.9,10 It should be noted, however, that the pattern of social fearlessness observed in individuals with WBS coexists with high levels of nonsocial anxiety.11
Atypical emotion processing is another feature shared by individuals with WBS and SAD. The evidence indicates that people with WBS show reduced ability to detect social threat signals, such as perceiving negative emotions through facial expressions and voices,12 or to detect angry faces,13 but they show a positive bias toward processing happy faces.14 By contrast, people with SAD are typically hypervigilant to facial expressions, and they rapidly avoid facial stimuli perceived as threatening.15 Evidence from neuroimaging studies of SAD and WBS suggests that alterations in regions involved in the threat-detecting system may underlie the aberrant patterns of facial emotion processing in individuals with these disorders and may contribute to explaining part of the neural substrate of the exaggerated/diminished fear responses to social cues that characterize SAD and WBS, respectively.16 Research on WBS has reported diminished amygdala response to negative facial expressions17–19 and heightened amygdala response to happy faces.18 Regarding SAD, hyperactivation of limbic regions has been a widely replicated finding across studies.16 Abnormal functioning of cortical prefrontal regions involved in the top–down modulation of anxiety has also been proposed to be a core deficit in patients with SAD20–23 and may contribute to abnormal regulation of the amygdala in those with WBS.17
With the aim of identifying common and specific neural correlates during facial emotion processing in patients with SAD and WBS, to our knowledge, we compared for the first time fMRI neural responses during a facial emotion processing paradigm among adults with SAD and WBS and healthy controls. We predicted that major differences would emerge in the activation of brain regions involved in the fear response, including limbic regions, such as the amygdala, and in cortical prefrontal regions involved in the top–down regulation of this response.
Methods
Participants
We recruited patients with SAD, patients with WBS and healthy controls of both sexes aged 18–60 years to participate in the study. Participants were matched by chronological age and laterality.
Individuals with SAD were recruited using a poster advertisement. Interested individuals contacted the study centre and underwent a preliminary phone interview. They then completed a screening visit and a physical examination. To be included in the study, they had to be outpatients with a primary psychiatric diagnosis of generalized SAD based on DSM-IV-TR criteria2 and the Mini-International Neuropsychiatric Interview (MINI)24 and have a Liebowitz Social Anxiety Scale (LSAS)25 score greater than 50. Patients with relevant medical or neurologic disorders or other DSM-IV Axis-I disorders were not considered for inclusion. In addition, we excluded individuals receiving any current psychotherapy or pharmacological treatment. Participants with WBS were recruited from the Hospital del Mar (Barcelona), in collaboration with the Spanish National and Regional WBS Associations. We confirmed the diagnosis of WBS in all participants using molecular methods, documenting a 1.55Mb or 1.83Mb heterozygous deletion at 7q11.23. Participants with WBS were evaluated with the MINI interview to confirm absence of SAD. Healthy controls were recruited using a poster advertisement. Interested individuals contacted the study centre and underwent a preliminary telephone interview. We assessed healthy controls using the MINI psychiatric interview to ensure absence of psychiatric conditions, and they underwent a complete psychical examination. To be included, controls had to have an LSAS score lower than 30. All participants (SAD, WBS, control) were required to be free of any history of substance dependence or current substance abuse, confirmed with urine toxicity and breath alcohol screening. The presence of a prosthesis or metal pacemaker was an exclusion criterion common to all 3 groups.
Behavioural assessments included the LSAS25 for social anxiety, the State–Trait Anxiety Inventory (STAI)26 for general anxiety, the use of a 0–100 mm visual analogue scale (VAS) for rating state anxiety, and the Hamilton Rating Scale for Depression (HAM-D).27 Written informed consent was obtained from all participants. For the WBS group, written informed consent was also obtained from parents. The study was approved by the local ethics committee (CEIC-IMAS, CEIC-H.Clínic, Barcelona) and was in compliance with the Declaration of Helsinki.
Facial emotion processing task
We assessed participants using a slightly modified version of the facial emotion processing task developed by Hariri and colleagues28; the task we used was identical to the paradigm described by Paulus and colleagues.29 During each 5-s trial, participants were presented with a target face (centre top) and 2 probe faces (bottom left and right) and were instructed to match the target to the probe expressing the same emotion; this was done by pressing a button in either their left or right hand in accordance with the position of the chosen probe. A block consisted of 6 consecutive trials in which the target face was happy, fearful or angry, and the probe faces included 2 of these 3 possible emotions (i.e., happy, fearful or angry). During the control (shape) condition, participants completed 5-s trials involving ovals or circles in an analogous configuration, and they were instructed to match the shape of the probe to the target. A total of 9 30-s blocks of faces (3 happy, 3 fearful and 3 angry) and 3 30-s blocks of the control condition were presented in a pseudorandomized order. A fixation cross was interspersed between each block (15-s duration). For each trial, response accuracy and response latency (reaction time) were obtained. The paradigm was presented visually on a laptop computer running presentation software (www.neurobehavioralsystems.com). The stimuli were displayed with MRI-compatible high-resolution goggles (VisuaStim Digital System, Resonance Technology Inc.). Participants’ responses were registered using a right- and a left-hand response device based on optical fibre transmission (Nordic NeuroLaboratories).
Functional MRI acquisition
Images were obtained using a 1.5 T Signa Excite system (General Electric) equipped with an 8-channel phased-array head coil and single-shot echoplanar imaging (EPI). Functional sequences consisted of gradient recalled acquisition in the steady state under the following parameters: repetition time (TR) 2000 ms, echo time (TE) 50 ms, pulse angle 90°, field of view (FOV) 24 cm, 64 × 64 pixel matrix, 4 mm slice thickness plus an interslice gap of 1 mm. Twenty-two interleaved slices parallel to the anterior–posterior commissure line were acquired to cover the whole brain. The functional time series consisted of 256 consecutive image sets obtained over 9 min. We also acquired an anatomic 3-dimensional (3D) fast spoiled gradient (SPGR) inversion-recuperation prepared sequence with 130 contiguous slices (TR 11.8 ms, TE 4.2 ms, flip angle 15°, FOV 30 cm, acquisition matrix 256 × 256 pixels, slice thickness 1.2 mm) for each participant.
Image processing and statistical analysis
We used a Microsoft Windows platform running MATLAB version 7 (The MathWorks Inc.) and Statistical Parametric Mapping software (SPM8; The Wellcome Department of Imaging Neuroscience). Image preprocessing involved motion correction, spatial normalization and smoothing using an 8 mm full-width at half-maximum Gaussian filter. Data were normalized to the standard SPM EPI template and resliced to 2 mm isotropic resolution in Montreal Neurological Institute (MNI) space. High-quality functional images were obtained in all cases. We performed a coverage check in the amygdala. No cases with relevant signal drop or anatomic distortion were identified. No cases with mean volume-to-volume translation above 0.25 mm were included. Mean volume-to-volume translation, however, significantly differed among the groups (WBS: 0.12 ± 0.08 mm; SAD: 0.045 ± 0.03 mm; control: 0.035 ± 0.01; WBS > SAD and WBS > HC, p < 0.001). Mean volume-to volume translation was used as a regressor in the group (second-level) analyses as a procedure to remove motion effects as previously described.30
First-level (single-subject) SPM contrast images were estimated for the following task effects of interest: all faces > shapes, fearful faces > shapes, angry faces > shapes and happy faces > shapes. For each participant, different task regressors were created for each task condition (cross fixation, shapes, angry, fear and happy) by specifying the onset and duration of each task block (multiple regressors option). A 4-s delay was applied to each separate regressor to account for the hemodynamic response delay, and a high-pass filter was used to remove low-frequency noise (1/128 Hz). The resulting first-level contrast images for each participant were then carried forward to second-level random effects (group) analyses. We used 1-sample and 2-sample t tests to estimate significant within- and between-group activation effects. In addition, SPM maps were generated, showing the correlation between brain activation and individual LSAS scores in participants with SAD and WBS. The ordinary least squares (OLS) model of SPM was used for this procedure. Results were considered to be significant with clusters above 1.032 mL (129 voxels) at a height threshold of p < 0.005, which satisfied the family-wise error (FWE) rate correction of pFWE < 0.05 according to recent Monte Carlo simulations.31 As an a priori key region of interest with a reduced anatomic volume, the amygdala region was additionally analyzed using small volume correction (SVC) and a threshold of pFWE < 0.05.
Additional statistical analyses included Student t tests to compare participants’ performance during fMRI assessment (accuracy rates and mean reaction time) and to test for differences in sample characteristics.
Results
Sample characteristics
We included 20 patients with SAD, 20 with WBS and 20 controls (age range 18–30 yr) in our study. The SAD group was notably homogeneous; participants all had the generalized type, with childhood onset of symptoms and significant distress and interference in the patient’s life, but with no current treatment that could confound the study results. Among patients in the WBS group, 19 had a 1.55Mb and 1 had a 1.83Mb heterozygous deletion at 7q11.23. Their mean IQ was 55 (WAIS-III scale; total IQ: 55, verbal IQ: 62, performance IQ: 57). Table 1 shows the sample characteristics. Participants in the SAD group scored significantly higher on social anxiety (LSAS) and trait anxiety (STAI-T) than those in the WBS and control groups. State anxiety scores before the fMRI session (both STAI-S and VAS), denoting anticipatory anxiety, were significantly higher in participants with SAD and WBS than in controls. After the experimental session, state anxiety scores were almost normalized in the WBS group but remained at higher levels in the SAD group.
Table 1.
Demographic and clinical characteristics of study participants
| Group; mean ± SD* | Statistical test (p value) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristic | SAD, n = 20 | WBS, n = 20 | Control, n = 20 | SAD v. WSB | SAD v. control | WSB v. control |
| Age, yr | 25.05 ± 5.25 | 25.42 ± 5.3 | 25.65 ± 6.31 | t36.527 = −0.662 (0.51) | t36.724 = −0.736 (0.75) | t33.353 = 0.205 (0.84) |
| Sex, male:female | 14:6 | 11:9 | 12:8 | χ2 = 3.801 (0.15) | χ2 = 3.801 (0.15) | χ2 = 3.801 (0.15) |
| LSAS total score | 75.8 ± 14.7 | 14.3 ± 9.2 | 13.1 ± 8.6 | t32.008 = 15.827 (< 0.001) | t30.679 = 16.432 (< 0.001) | t37.810 = −0.406 (0.69) |
| STAI-Trait total score | 33.1 ± 11.4 | 19.5 ± 12.3 | 11.7 ± 7.4 | t37.751 = 3.629 (0.001) | t32.825 = 7.031 (< 0.001) | t31.285 = 1.588 (0.022) |
| STAI-State Total Score | ||||||
| Before fMRI | 32.1 ± 7.2 | 21.9 ± 14.4 | 9.6 ± 6.4) | t28.129 = 2.840 (0.008) | t37.506 = 10.334 (< 0.001) | t31.285 = −2.412 (0.002) |
| After fMRI | 24.6 ± 6.4 | 9.3 ± 8.1 | 9.4 ± 8.3 | t36.116 = 6.579 (< 0.001) | t35.767 = 6.424 (< 0.001) | t37.980 = 0.058 (0.95) |
| VAS state anxiety | ||||||
| Before fMRI | 49.2 ± 21.4 | 33.6 ± 30 | 8.3 ± 22.2 | t32.964 = 2.160 (0.038) | t19.505 = 11.940 (0.001) | t33.213 = −2.424 (0.021) |
| After fMRI | 35.4 ± 27.4 | 5 ± 9 | 7.6 ± 12.5 | t30.670 = 4.078 (0.001) | t25.196 = 4.944 (0.001) | t36.185 = 0.586 (0.56) |
| HAM–D | 5.0 ± 4.4 | 2.6 ± 3.9 | 0.95 ± 1.4 | t34.639 = 1.720 (0.94) | t20.576 = 3.856 (0.001) | t22.358 = 1.734 (0.10) |
HAM-D = Hamilton Rating Scale for Depression; LSAS = Liebowitz Social Anxiety Scale; SAD = social anxiety disorder; STAI = State-Trait Anxiety Inventory; VAS = visual analogue scale; WBS = Wlliams–Beuren syndrome.
Unless indicated otherwise.
Behavioural performance during fMRI
Appendix 1, available at jpn.ca, shows mean reaction times and accuracy rates for each group. Overall, participants with WBS responded significantly slower (234 ms slower on average) across all conditions (fear, angry, happy, control-shape) than those with SAD or controls (p < 0.001). There were no significant differences in reaction times between the SAD and control groups in any of the 4 conditions. Accuracy rates were higher for participants with SAD and controls than for participants with WBS (SAD: 98.69%, mean no. of errors 0.95 ± 0.84; control: 99.62%, mean no. of errors 0.28 ± 0.66; WBS: 73.62%, mean no. of errors 19 ± 11.1; p < 0.001).
Functional MRI results during the Hariri task
Williams–Beuren syndrome
In the 1-sample analysis, the matching task for all faces (angry, fearful, happy, all-faces v. shapes) compared with the control shape condition was associated with significant activation in the visual cortex, the right fusiform gyrus, the lateral temporal cortex, the amygdala, the prefrontal cortex and the anterior cingulate cortex (ACC; Table 2). Overall, the specific analysis for the happy, angry and fearful conditions showed similar patterns of activation involving these regions (Appendix 1). However, matching happy faces produced significant activation in the right amygdala (MNI coordinates: x, y, z = 28, 4, −28; cluster size 60 (SVC); t = 3.65) and left amygdala (MNI coordinates: x, y, z = −12, −2, −12; cluster size 634; t = 4.73), whereas no amygdala activation was observed under the angry or fearful conditions (Appendix 1).
Table 2.
Regions showing significant activation during the processing of emotional faces (all faces v. shapes)
| SAD, n = 20 | WBS, n = 20 | Healthy control, n = 20 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Brain region | MNI space | Cluster size | t* | MNI space | Cluster size | t* | MNI space | Cluster size | t* | |
| Visual cortex | R | 18, −98, 2 | 14070 | 10.10 | 36, −96, −8 | 11972 | 6.75 | 30, −94, −6 | 25123 | 13.43 |
| L | −14, −98, 2 | 14070 | 7.38 | −36, −94, −8 | 11972 | 6.88 | −26, −96, −6 | 25123 | 12.26 | |
| Lateral occipital area | R | 46, −78, −2 | 14070 | 5.96 | — | — | — | 44, −82, −6 | 25123 | 9.31 |
| L | — | — | — | — | — | — | — | — | — | |
| Fusiform gyrus | R | 38, −54, −24 | 14070 | 7.21 | 40, −48, −20 | 11972 | 4.93 | 40, −50, −24 | 25123 | 9.01 |
| L | −40, −54, −18 | 14070 | 6.35 | — | — | — | −38, −54, −20 | 25123 | 9.00 | |
| Lateral temporal cortex | R | 66, −16, 10† | 1311† | 4.31† | 64, −18, −16 | 139 | 3.35 | — | — | — |
| L | — | — | — | — | — | — | ||||
| Superior temporal gyrus | R | 44, −24, 12† | 1311† | 2.98† | — | — | — | — | — | — |
| L | −58, −32, 12† | 1822† | 4.04† | — | — | — | −54, −28, 10† | 1101† | 3.43† | |
| Amygdala | R | 28, 4, −28‡ | 20‡ | 3.34‡ | 16, −6, −20 | 342 | 5.03 | |||
| L | — | — | — | −20, −8, −10 | 79‡ | 4.03 | −16, −4, −22 | 166 | 4.10 | |
| Prefrontal cortex | R | 44, 18, 26 | 1667 | 5.18 | 44, 22, 34 | 547 | 4.29 | 48, 18, 28 | 1651 | 5.97 |
| L | — | — | — | −40, 24, 24 | 2005 | 4.66 | −54, 20, 32 | 3762 | 6.08 | |
| Anterior cingulate cortex | R | 0, 30, 32† | 1304† | 4.89† | 12, 18, 42 | 197 | 4.51 | 4, −26, 54† | 215† | 3.05† |
| L | — | — | — | — | — | — | — | — | — | |
FWE = family-wise error; L = left; MNI = Montreal Neurological Institute; R = right; SAD = social anxiety disorder; WBS = Wlliams–Beuren syndrome.
Statistics correspond to a corrected threshold pFWE < 0.05 estimated using Monte Carlo simulations.
Shows deactivation.
Denotes small volume correction, pFWE < 0.05.
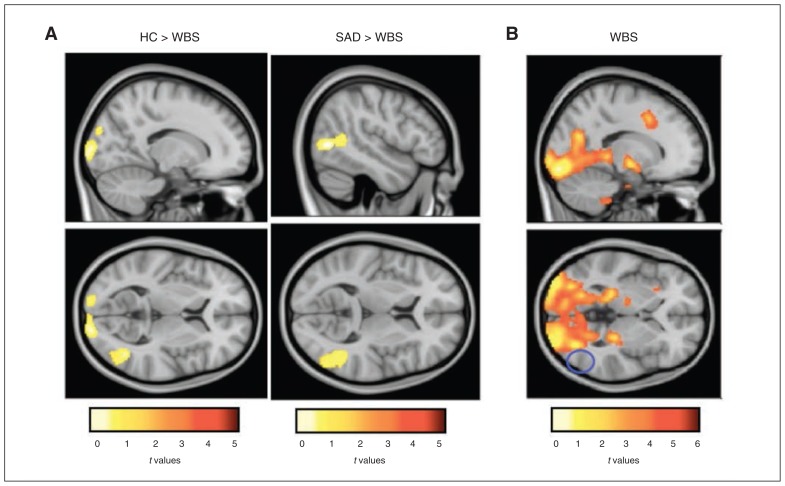
Between-group analyses showed that compared with the SAD and control groups, the WBS group showed an absence of activation in the right inferior occipital area (Fig. 1; Table 3). Moreover, we found that relative to controls, participants with WBS showed significantly less activation in the posterior region of the primary visual cortex (occipital pole) and significantly less deactivation in the right middle temporal gyrus/temporal operculum and the right supplementary motor area (Table 3). Overall, the specific analysis for the happy, angry and fearful conditions showed similar patterns of activation for these regions (Appendix 1). However, we found that whereas no between-group differences in the deactivation in the temporal operculum under the happy or angry conditions emerged, matching fearful faces (fearful v. shapes) yielded to less deactivation in the bilateral temporal operculum and the insular region in the WBS group than in the control group (Appendix 1).
Fig. 1.
Differential activation between groups in response to matching emotional faces and the control shape condition (all faces v. shapes). (A) Between-group differences for matching emotional faces (all faces) compared with the control shape condition. (B) Within-group map showing significant activation for the Williams–Beuren syndrome (WBS) group for the same contrast. The blue circle reflects the lateral occipital area, with no activation in the WBS group. HC = healthy controls; SAD = social anxiety disorder.
Table 3.
Differential activation between groups in response to matching emotional faces and the control shape condition (all faces v. shapes)
| All faces v. shapes | Cluster size | MNI space | t* |
|---|---|---|---|
| SAD > HC | — | — | — |
| SAD < HC† | — | — | — |
| Left medial prefrontal cortex | 361 | −8, 18, 56 | 3.96 |
| Left lateral prefrontal cortex | 989 | −42, 24, 24 | 4.23 |
| Right fusiform gyrus | 649 | 30, −74, −16 | 3.54 |
| Left fusiform gyrus | 245 | −28, −74, −10 | 3.59 |
| Left precuneus | 1611 | −4, −68, 42 | 3.81 |
| Right lateral temporal cortex | 172 | 68, −28, −10 | 4.28 |
| Left lateral temporal cortex | 362 | −62, −44, −14 | 3.79 |
| Cerebellum | 764 | 12, −56, −46 | 4.20 |
| HC > WBS | |||
| Right primary visual cortex | 449 | 18, −98, 6 | 3.70 |
| Left primary visual cortex | 159 | −16, −98, −2 | 3.16 |
| Right lateral occipital area | 375 | 42, −62, 8 | 3.85 |
| HC < WBS‡ | |||
| Right medial temporal gyrus temporal operculum | 130 | 64, −18, −16 | 3.45 |
| Right supplementary motor area | 425 | 12, −18, 68 | 3.16 |
| SAD > WBS | |||
| Right lateral occipital area | 351 | 48, −68, 2 | 4.04 |
| SAD < WBS† | |||
| Medial prefrontal cortex/ACC | 430 | −4, 50, 16 | 3.29 |
| Left dorsal prefrontal cortex | 602 | −24, 30, 40 | 4.07 |
| Left superior temporal gyrus | 185 | −52, −42, −14 | 3.95 |
| Right lateral temporal cortex | 381 | 64, −20, −18 | 4.34 |
| Left lateral temporal cortex | 177 | −58, −46, −12 | 3.84 |
ACC = anterior cingulate cortex; HC = healthy controls; MNI = Montreal Neurological Institute; SAD = social anxiety disorder; WBS = Wlliams–Beuren syndrome.
Statistics correspond to a corrected threshold pFWE < 0.05 estimated using Monte Carlo simulations.
More activation in controls, more deactivation in SAD.
More deactivation in controls.
Social anxiety disorder
In the 1-sample analysis the matching task for all faces (angry, fearful, happy, all faces v. shapes) compared with the control shape condition was associated with significant activation in the visual cortex, right lateral occipital area, fusiform gyrus, amygdala and prefrontal cortex and with significant deactivation in the lateral temporal cortex, superior temporal gyrus and right ACC (Table 2). The specific analysis for the happy, angry and fearful conditions showed similar patterns of activation involving these regions (Appendix 1).
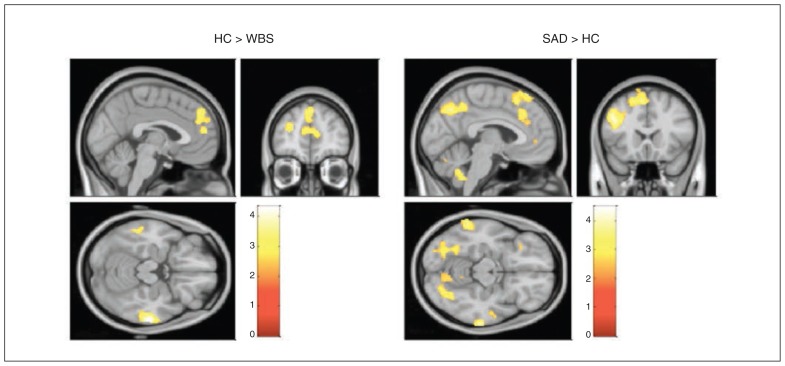
Between-group analyses for matching faces (all faces v. shapes) showed that relative to the WBS and control groups, the SAD group showed significantly less activation in the prefrontal cortex and lateral temporal cortex (more activation in the WBS and control groups v. more deactivation in the SAD group). Additionally, we found that compared with controls, patients with SAD showed less activation in the bilateral fusiform gyrus, left precuneus and cerebellum (more activation in controls, more deactivation in patients with SAD). Compared with the WBS group, the SAD group showed decreased activation in the left superior temporal gyrus (more activation in the WBS group, more deactivation in the SAD group; Fig. 2 and Table 3). Overall, the emotion-specific analysis showed similar patterns of activation for most of these regions (Appendix 1). However, the specific analysis for the angry condition (angry v. shapes) revealed additional decreased activation in a cluster extending to the left orbitofrontal cortex, left amygdala, left stratium and left caudate in patients with SAD relative to controls (MNI coordinates: x, y, z = −10, −4, 20; cluster size 7068; t = 4.07; Appendix 1).
Fig. 2.
Differential activation between groups in response to matching emotional faces and the control shape condition (all faces v. shapes). Between-group analysis maps show differences in brain activation in response to matching emotional faces (all faces) and the control shape condition. HC = healthy control; SAD = social anxiety disorder; WBS = Williams–Beuren syndrome.
Correlation of brain activation with LSAS scores
Correlational analyses showed that there were no significant positive or negative correlations between LSAS scores and brain activation in any of the 4 conditions in either the SAD or the WBS groups.
Discussion
Using fMRI the present study examined, to our knowledge, for the first time common and specific patterns of brain activation during a facial emotion matching task among patients with SAD, patients with WBS and healthy controls. Contrary to our expectations, limbic activity did not differ among the groups. Instead, we observed major differences in early visual areas of the face processing network32 in patients with WBS and in cortical prefrontal regions involved in the top–down regulation of anxiety21 and the fusiform gyrus in patients with SAD. Interestingly, we found that the SAD and WBS groups differed in the activation in the superior temporal gyrus (STG), a region that has been linked to gaze processing.33
Specific findings in the WBS group
Previous studies of WBS have reported a diminished amygdala response to negative facial expressions17–19 and a heightened amygdala response to happy faces,18 a pattern that might explain part of the hypersocial fearless behaviour typical of this population. We did not observe significant differences in amygdala activation between the WBS group and either the SAD or control groups. It should be noted, however, that although no significant between-group differences emerged, the between-groups analysis revealed that whereas matching angry or fearful faces did not produce amygdala activation in the WBS group, there was significant bilateral amygdala activation under the happy condition, a pattern that resembles findings reported in previous studies.18–20
Another finding in our WBS group was that relative to controls, matching faces (v. matching shapes) produced significantly less activation in the posterior region of the primary visual cortex corresponding to the foveal vision,34 which is responsible for visual acuity and processing of features and fine details.35 Furthermore, in comparison with both the SAD and control groups, the WBS group did not activate the right lateral inferior occipital cortex, a region that might correspond to the so-called occipital face area (OFA; for a review see the study by Pitcher and colleagues36). Human lesion studies37 and transcranial magnetic stimulation (TMS) studies have provided strong evidence that the right OFA plays a major role in face processing,36,38–40 particularly the processing of face parts.41,42
Traditional hierarchical feed-forward models of visual processing32,36 maintain that the involvement of the OFA in face processing is restricted to the initial feature-based analysis that is then fed forward to other higher-order face-selective regions, such as the fusiform face area (FFA) in the fusiform gyrus in order to process complex aspects. However, available data from brain-damaged patients43 and fMRI studies44 has challenged the notion that face processing occurs in a strictly hierarchical feed-forward manner. For instance, it has been observed that face-preferential activation in higher-order visual regions of the right hemisphere, such as the FFA, as observed in the present study, can emerge in the absence of OFA activation45 and even in the presence of structural damage to this region.43 Furthermore, evidence from TMS studies suggests that the OFA might be involved in other higher-level perception abilities,38 such as the discrimination of facial expressions,46 identity47 or judgment of trustworthiness from faces.48 On this basis, Atkinson and Adolphs38 proposed a more interactive model in which higher-level face perception abilities rely on an intact face-processing network, including the OFA, whereas lower-level categorization abilities, such as discriminating faces from objects, can be achieved without OFA. Other authors even propose a nonhierarchical model in which the FFA is responsible for holistically early face detection, whereas the OFA contributes to subsequent refinement through fine-grained analysis of facial features.43,49 The potential involvement of the OFA in higher-perceptual abilities, such as the discrimination of facial expressions46 and judgment of trustworthiness from faces,48 is interesting in light of existing evidence showing that individuals with WBS have difficulty identifying facial emotional expressions12–14 and that they tend to perceive unfamiliar faces abnormally positively.7
In our study, individuals with WBS showed hypoactivation in the posterior region of the primary visual cortex, corresponding to foveal vision, as well as absence of activation in the right OFA. Similar to the SAD and control participants, however, participants with WBS showed significant activation in the right fusiform gyrus. Taken together our results suggest that individuals with WBS might fail to accurately process facial features and fine-grained details, with a more coarse/holistic impression being produced instead. This could be related, at least in part, to the low-level visual phenotype described in patients with WBS, characterized by decreased retinal thickness, abnormal optic disk concavity and impaired magnocellular pathway, involved in the detection of temporal changes in the visual scene.50 The documented dorsal stream vulnerability and impaired 3D visual integration in patients with WBS is associated with distinct neural correlates and cognitive strategies to reach visual coherence.51,52 The information from facial features and fine-grained details might be critical not only for accurate processing of facial expressions,46 but also for the rapid detection of threat-related signals, such as fearful faces.53,54
Deficient input from visual areas may also have contributed to the absence of an amygdala response to threat-related faces observed in our study. Interestingly, Sarpal and colleagues55 reported significant reductions in functional connectivity between the FFA and the amygdala during the presentation of threatening faces. Since a previous study in the same sample found diminished amygdala activation to threatening faces,17 Sarpal and colleagues proposed that input from facial stimuli from ventral stream areas might gain less access to amygdala and regulatory prefrontal regions and contribute to the reduced amygdala activation and associated lack of social fear in patients with WBS.55 Overall, our results extend previous findings by suggesting that deficient input from face-processing regions conveying fine-grained information that is important for accurately processing facial expressions and detecting threat signals might contribute to the fearless social phenotype and difficulties in detecting threat-related signals that is typical this population.
Finally, we observed that relative to controls, participants with WBS showed less deactivation in the right middle temporal gyrus and the temporal operculum. Deactivation in these regions has been shown to be modulated by task demand, with more demanding tasks producing greater deactivation, particularly in the insular cortex.56 As matching faces is a more complex task than matching shapes, our results may indicate that the greater deactivation expected for the more difficult condition (face matching) did not emerge in the WBS group, reflecting the relative strength and salience to faces typical of this population. Moreover, emotion-specific analysis showed that whereas differences in deactivation for these regions did not emerge in the WBS group under the happy or angry conditions, matching fearful faces (a more difficult emotion to process for a typical control) yielded to less deactivation in the bilateral temporal operculum and insular region in the WBS group than in the control group, a pattern that might further reflect the impaired processing of fear typical of this population. Task-invariant patterns associated with task difficulty have also been reported in previous studies of WBS.17 Furthermore, some of these regions, such as the insula, are thought to mediate awareness by integrating cognitive, interoceptive and affective information,57 and previous evidence has shown that functional and structural alterations in this region predict specific measures of the hypersocial personality in patients with WBS.58 Thus, another possible explanation is that the pattern of deactivations observed in the WSB group may reflect an alteration in the awareness processes mediated by this region.
Specific findings in the SAD group
With regards to SAD, our major finding was that relative to the WBS and control group, participants with SAD showed no enhanced limbic response, with decreased activation in medial/dorsal prefrontal regions. However, under the angry condition, participants with SAD showed less activation in the left amygdala and surrounding areas than controls.
Although several studies have reported an exaggerated limbic response in patients with SAD (see the study by Binelli and colleagues16) others have failed to find limbic hyperactivity20–22 or have even found a decreased amygdala response.59 For example, Pujol and colleagues20 used a paradigm in which participants viewed prerecorded videos of themselves performing a task in front of an audience, a highly distressing situation for patients with SAD. The study found normal subcortical and limbic response with reduced activation in cortical prefrontal areas in patients with SAD compared with controls. In this line, Ziv and colleagues22 examined behavioural and brain responses using 3 distinct socioemotional tasks in a sample of participants with SAD. They found that negative emotion ratings were greater in the SAD group across all tasks, although there were no differences in brain responses in the amygdala and insula between the SAD and control groups on any of the tasks. However, differential brain responses between groups were observed in frontal, occipital and temporal regions. The authors explained these results by suggesting that ratings of negative emotion might be less tightly coupled with increased limbic activity than typically thought, and they further proposed that deficits in higher cognitive processes involved in emotion regulation may be a core deficit of SAD. Using the same tasks and the same sample, the authors then examined the neural response while participants were instructed to downregulate negative emotion reactions.23 The study found that relative to controls, patients with SAD showed reduced late brain responses in prefrontal regions, particularly when reappraising harsh faces. In addition, the study found that reduced late responses in the prefrontal cortex in patients with SAD were related to less reduction in negative emotion ratings when reappraising negative self-beliefs. In line with these studies, we found that patients with SAD showed normal limbic activity (except in the angry condition), with decreased activation in cortical prefrontal regions involved in the top–down mechanisms of emotion regulation.21 Our results therefore extend previous findings by demonstrating abnormal prefrontal activity in the SAD group compared with both the WBS and control groups, supporting the notion that deficient cognitive regulation may be a core feature of SAD.20–23
We also found that relative to controls, patients with SAD showed decreased activation in the bilateral fusiform gyrus. This result is consistent with those of an earlier study of SAD that found fusiform hypoactivation in response to faces.60 However, other studies in this context have reported fusiform hyperactivity61–63 or no fusiform activation at all.64,65 Recently, Frick and colleagues63 found increased reactivity in the bilateral fusiform gyrus in response to fearful as opposed to neutral faces as well as greater fusiform connectivity with the right amygdala in patients with SAD compared with controls. At first sight, it may seem that our results contradict those reported by Frick and colleagues63; however, in our study, people with SAD showed decreased fusiform activation and decreased amygdala reactivity under the angry condition (i.e., precisely in response to an expression that is particularly threatening to individuals with SAD).
Interestingly, a previous study in a nonclinical sample of patients with social anxiety found a modulation effect from the fusiform gyrus to the amygdala in response to facial emotion.66 In that study, social anxiety ratings were associated with amygdala response only after controlling for the participants’ level of activation in the fusiform gyrus. Furthermore, fusiform response to fearful faces showed a negative correlation only with those behavioural assessments related to avoidance. Hypoactivation of the fusiform gyrus has also been reported by studies of autism-spectrum disorder (ASD)67 and in Fragile X syndrome,68 2 conditions that show a relevant clinical overlap with SAD, including the characteristic gaze avoidance. In the context of ASD and Fragile X syndrome, hypoactivation of the fusiform gyrus is thought to arise from diminished gaze fixation,67,68 while variations in eye fixation modulate amygdala activation in those with ASD.67
Taken together our findings suggest that fusiform hypoactivation may reflect the use of avoidance strategies and/or diminished gaze fixation to facial stimuli during the task by patients with SAD. This would further explain inconsistencies in the results regarding fusiform reactivity, both among previous studies60–63 and within individual studies,61 since this reactivity may vary depending on the use of avoidant strategies and the type of paradigm adopted.61 Further studies using eye-tracking methodology are necessary to confirm this hypothesis. The pattern of fusiform hypoactivity and decreased amygdala activation observed in participants with SAD relative to controls in response to angry faces fits with previous findings that suggest a modulator effect from the fusiform gyrus to amygdala activation in response to emotional faces, particularly those expressing threat.66
Common finding: the role of eye gaze
We found that relative to participants with SAD, those with WBS showed greater activation in the left STG. Note that differences in brain activation for the STG emerged only in the SAD versus WBS comparison. This is an interesting finding, as the STG region has been strongly implicated in eye-gaze processing,33 and lesions in this region produce important difficulties in gaze contact.69 This result seems to fit nicely with the pattern of gaze avoidance typical of patients with SAD8 as well as with the increased attention and fixation to eyes and gaze that is characteristic of those with WBS.9 However, these conclusions remain speculative because no eye-tracking system was used in our study and because the paradigm we used may not be adequate for such a purpose.
Limitations
A number of further limitations should also be mentioned. The face paradigm adopted in our study was not designed to discriminate between the effect of different emotional expressions, as different emotions (target and probes) appear in each picture. The brain activation results observed in the WBS group are limited, and may be partly mediated by the cognitive profile (IQ) of our WBS group. Relative to participants in the SAD and control groups, those in the WBS group showed significant differences in accuracy rates and reaction times. However, the specificity of findings suggests that the pattern of brain activation observed in the WBS group is not entirely a result of immature cognitive profile. Previous studies reported abnormal patterns of brain activation in response to emotional faces in samples with WBS with a similar IQ to our participants compared with both typically developing and developmentally delayed controls.17–19 Therefore, our results are more likely to be proximal to reflect a neurobiological substrate rather than intellectual impairment per se.
Conclusion
To our knowledge, this study is the first to compare the neural responses to emotional faces in patients with SAD and WBS, 2 conditions that are, to some extent, opposed to one another when it comes to certain clinical features. Contrary to our expectations, the SAD and WBS groups did not differ in the pattern of activation in limbic regions. Instead, we observed major differences between the groups in visual areas of the face processing network and in distal cognitive areas involved in the top–down control of anxiety. Relative to participants in the SAD group, those in the WBS group showed greater activation in the STG, a region that has been related to gaze processing. Taken together our results highlight the role of cortical regions during facial emotion processing in patients with SAD and WBS and suggest that alterations within and between the regions involved in the face processing network, the fear response and the top–down mechanisms of anxiety regulation may underlie the altered processing of facial emotional cues and partially explain the social phenotype of exaggerated/diminished responses to social threat typical of patients with SAD and WBS.
Acknowledgements
This study was performed in part with grants: Instituto de Carlos III (ISCIII) – FIS: PI14/01411 (R. Martín-Santos), ISCIII-G03/184 (R. Martín-Santos), and the support of Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement: SGR2009/1435 and SGR2014/1411 (R. Martín-Santos).
Footnotes
Competing interests: None declared.
Contributors: M. Farré, L. Pérez-Jurado, J. Pujol and R. Martin-Santos designed the study. C. Binelli, A. Muñiz, S. Subira, R. Navines, L. Blanco-Hinojo, D. Perez-Garcia, M. Farré, L. Pérez-Jurado and J. Pujol acquired the data, which C. Binelli, L. Blanco-Hinojo, J. Crippa, J. Pujol and R. Martin-Santos analyzed. C. Binelli and R. Martin-Santos wrote the article, which all authors reviewed and approved for publication.
References
- 1.Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–84. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.APA. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 3.Pérez Jurado AL. Williams-Beuren syndrome: a model of recurrent genomic mutation. Horm Res. 2003;59:106–13. doi: 10.1159/000067836. [DOI] [PubMed] [Google Scholar]
- 4.Järvinen A, Korenberg JR, Bellugi U. The social phenotype of Williams syndrome. Curr Opin Neurobiol. 2013;23:414–22. doi: 10.1016/j.conb.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle TF, Bellugi U, Korenberg JR, et al. “Everybody in the world is my friend” hypersociability in young children with Williams syndrome. Am J Med Genet A. 2004;124A:263–73. doi: 10.1002/ajmg.a.20416. [DOI] [PubMed] [Google Scholar]
- 6.Stein MB, Stein D. Social anxiety disorder. Lancet. 2008;371:1115–25. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 7.Bellugi U, Adolphs R, Cassady C, et al. Towards the neural basis for hypersociability in a genetic syndrome. Neuroreport. 1999;10:1653–7. doi: 10.1097/00001756-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 8.Weeks JW, Howell A, Goldin P. Gaze avoidance in social anxiety disorder. Depress Anxiety. 2013;30:749–56. doi: 10.1002/da.22146. [DOI] [PubMed] [Google Scholar]
- 9.Riby DM, Hancock P. Do faces capture the attention of individuals with Williams syndrome or autism? Evidence from tracking eye movements. J Autism Dev Disord. 2009;39:421–31. doi: 10.1007/s10803-008-0641-z. [DOI] [PubMed] [Google Scholar]
- 10.Porter MA, Shaw TA, Marsh PJ. An unusual attraction to the eyes in Williams-Beuren syndrome: a manipulation of facial affect while measuring face scanpaths. Cogn Neuropsychiatry. 2010;15:505–30. doi: 10.1080/13546801003644486. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-García D, Granero R, Gallastegui F, et al. Behavioral features of Williams Beuren syndrome compared to Fragile X syndrome and subjects with intellectual disability without defined etiology. Res Dev Disabil. 2011;32:643–52. doi: 10.1016/j.ridd.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Plesa-Skwerer D, Faja S, Schofield C, et al. Perceiving facial and vocal expressions of emotion in individuals with Williams syndrome. Am J Ment Retard. 2006;111:15–26. doi: 10.1352/0895-8017(2006)111[15:PFAVEO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Santos A, Silva C, Rosset D, et al. Just another face in the crowd: evidence for decreased detection of angry faces in children with Williams syndrome. Neuropsychologia. 2010;48:1071–8. doi: 10.1016/j.neuropsychologia.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Dodd HF, Porter MA. I see happy people: attention bias towards happy but not angry facial expressions in Williams syndrome. Cogn Neuropsychiatry. 2010;15:549–67. doi: 10.1080/13546801003737157. [DOI] [PubMed] [Google Scholar]
- 15.Machado-de-Sousa JP, Arrais KC, Alves NT, et al. Facial affect processing in social anxiety: tasks and stimuli. J Neurosci Methods. 2010;193:1–6. doi: 10.1016/j.jneumeth.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Binelli C, Subirà S, Batalla A, et al. Common and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: a systematic review and voxel-based meta-analysis of functional resonance imaging studies. Neuropsychologia. 2014;64C:205–17. doi: 10.1016/j.neuropsychologia.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Meyer-Lindenberg A, Hariri AR, Munoz KE, et al. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–3. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- 18.Haas BW, Mills D, Yam A, et al. Genetic influences on sociability: heightened amygdala reactivity and event-related responses to positive social stimuli in Williams syndrome. J Neurosci. 2009;29:1132–9. doi: 10.1523/JNEUROSCI.5324-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mimura M, Hoeft F, Kato M, et al. A preliminary study of orbitofrontal activation and hypersociability in Williams syndrome. J Neurodev Disord. 2010;2:93–8. doi: 10.1007/s11689-009-9041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pujol J, Giménez M, Ortiz H, et al. Neural response to the observable self in social anxiety disorder. Psychol Med. 2013;43:721–31. doi: 10.1017/S0033291712001857. [DOI] [PubMed] [Google Scholar]
- 21.Goldin PR, Manber T, Hakimi S, et al. Neural bases of social anxiety disorder. Arch Gen Psychiatry. 2009;66:170–80. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziv M, Goldin P, Jazaieri H, et al. Is there less to social anxiety than meets the eye? Behavioral and neural responses to three socio-emotional tasks. Biol Mood Anxiety Disord. 2013;3:5. doi: 10.1186/2045-5380-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziv M, Goldin P, Jazaieri H, et al. Emotion regulation in social anxiety disorder: behavioral and neural responses to three socio-emotional tasks. Biol Mood Anxiety Disord. 2013;3:20. doi: 10.1186/2045-5380-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrendo L, Bobes M, Gilbert J, editors. Spanish version 5..0.0. Madrid: Instituto IAP; 1999. M.I.N.I. Mini International Neuropsychiatric Interview. [Google Scholar]
- 25.Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry. 1987;22:141–73. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- 26.Spielberger C. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists; 1983. [Google Scholar]
- 27.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 29.Paulus MP, Feinstein J, Castillo G, et al. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62:282–8. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- 30.Pujol J, Macià D, Blanco-Hinojo L, et al. Does motion-related brain functional connectivity reflect both artifacts and genuine neural activity? Neuroimage. 2014;101:87–95. doi: 10.1016/j.neuroimage.2014.06.065. [DOI] [PubMed] [Google Scholar]
- 31.Pujol J, Macià D, Garcia-Fontanals A, et al. The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. Pain. 2014;155:1492–503. doi: 10.1016/j.pain.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 32.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 33.Itier RJ, Batty M. Neural bases of eye and gaze processing: the core of social cognition. Neurosci Biobehav Rev. 2009;33:843–63. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavidor M, Walsh V. The nature of foveal representation. Nat Rev Neurosci. 2004;5:729–35. doi: 10.1038/nrn1498. [DOI] [PubMed] [Google Scholar]
- 35.Wright MJ, Johnston A. Spatiotemporal contrast sensivity and the visual field. Vision Res. 1983;23:983–9. doi: 10.1016/0042-6989(83)90008-1. [DOI] [PubMed] [Google Scholar]
- 36.Pitcher D, Walsh V, Duchaine B. The role of the occipital face area in the cortical face perception network. Exp Brain Res. 2011;209:481–93. doi: 10.1007/s00221-011-2579-1. [DOI] [PubMed] [Google Scholar]
- 37.Bouvier SE, Engel SA. Behavioral deficits and cortical damage loci in cerebral achromatopsia. Cereb Cortex. 2006;16:183–91. doi: 10.1093/cercor/bhi096. [DOI] [PubMed] [Google Scholar]
- 38.Atkinson AP, Adolphs R. The neuropsychology of face perception: beyond simple dissociations and functional selectivity. Philos Trans R Soc Lond B Biol Sci. 2011;366:1726–38. doi: 10.1098/rstb.2010.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steeves J, Dricot L, Goltz H, et al. Abnormal face identity coding in the middle fusiform gyrus of two brain-damaged prosopagnosic patients. Neuropsychologia. 2009;47:2584–92. doi: 10.1016/j.neuropsychologia.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Rossion B, Hanseeuw B, Dricot L. Defining face perception areas in the human brain: a large-scale factorial fMRI face localizer analysis. Brain Cogn. 2012;79:138–57. doi: 10.1016/j.bandc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Pitcher D, Walsh V, Yovel G, et al. TMS evidence for the involvement of the right occipital face area in early face processing. Curr Biol. 2007;17:1568–73. doi: 10.1016/j.cub.2007.07.063. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Harris A, Kanwisher N. Perception of face parts and face configurations: an fMRI study. J Cogn Neurosci. 2010;22:203–11. doi: 10.1162/jocn.2009.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossion B, Caldara R, Seghier M, et al. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–95. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- 44.Jiang F, Dricot L, Weber J, et al. Face categorization in visual scenes may start in a higher order area of the right fusiform gyrus: evidence from dynamic visual stimulation in neuroimaging. J Neurophysiol. 2011;106:2720–36. doi: 10.1152/jn.00672.2010. [DOI] [PubMed] [Google Scholar]
- 45.Rossion B, Dricot L, Goebel R, et al. Holistic face categorization in higher order visual areas of the normal and prosopagnosic brain: toward a non-hierarchical view of face perception. Front Hum Neurosci. 2011;4:225. doi: 10.3389/fnhum.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitcher D, Garrido L, Walsh V, et al. Transcranial magnetic stimulation disrupts the perception and embodiment of facial expressions. J Neurosci. 2008;28:8929–33. doi: 10.1523/JNEUROSCI.1450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon-Harris LM, Mullin CR, Steeves JKE. TMS to the “occipital face area” affects recognition but not categorization of faces. Brain Cogn. 2013;83:245–51. doi: 10.1016/j.bandc.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Dzhelyova MP, Ellison A, Atkinson AP. Event-related repetitive TMS reveals distinct, critical roles for right OFA and bilateral posterior STS in judging the sex and trustworthiness of faces. J Cogn Neurosci. 2011;23:2782–96. doi: 10.1162/jocn.2011.21604. [DOI] [PubMed] [Google Scholar]
- 49.Rossion B. Constraining the cortical face network by neuroimaging studies of acquired prosopagnosia. Neuroimage. 2008;40:423–6. doi: 10.1016/j.neuroimage.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 50.Castelo-Branco M, Mendes M, Sebastião AR, et al. Visual phenotype in Williams-Beuren syndrome challenges magnocellular theories explaining human neurodevelopmental visual cortical disorders. J Clin Invest. 2007;117:3720–9. doi: 10.1172/JCI32556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernardino I, Castelhano J, Farivar R, et al. Neural correlates of visual integration in Williams syndrome: gamma oscillation patterns in a model of impaired coherence. Neuropsychologia. 2013;51:1287–95. doi: 10.1016/j.neuropsychologia.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Bernardino I, Rebola J, Farivar R, et al. Functional reorganization of the visual dorsal stream as probed by 3-D visual coherence in Williams syndrome. J Cogn Neurosci. 2014;26:2624–36. doi: 10.1162/jocn_a_00662. [DOI] [PubMed] [Google Scholar]
- 53.Pessoa L, Adolphs R. Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–83. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein T, Seymour K, Hebart M, et al. Rapid fear detection relies on high spatial frequencies. Psychol Sci. 2014;25:566–74. doi: 10.1177/0956797613512509. [DOI] [PubMed] [Google Scholar]
- 55.Sarpal D, Buchsbaum BR, Kohn PD, et al. A genetic model for understanding higher order visual processing: functional interactions of the ventral visual stream in Williams syndrome. Cereb Cortex. 2008;18:2402–9. doi: 10.1093/cercor/bhn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrison BJ, Pujol J, Contreras-Rodríguez O, et al. Task-induced deactivation from rest extends beyond the default mode brain network. PLoS ONE. 2011;6:e22964. doi: 10.1371/journal.pone.0022964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Craig AD. How do you feel – now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 58.Jabbi M, Kippenhan JS, Kohn P, et al. The Williams syndrome chromosome 7q11.23 hemideletion confers hypersocial, anxious personality coupled with altered insula structure and function. Proc Natl Acad Sci U S A. 2012;109:E860–6. doi: 10.1073/pnas.1114774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kilts CD, Kelsey JE, Knight B, et al. The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006;31:2243–53. doi: 10.1038/sj.npp.1301053. [DOI] [PubMed] [Google Scholar]
- 60.Gentili C, Gobbini MI, Ricciardi E, et al. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with Social Phobia and healthy subjects. Brain Res Bull. 2008;77:286–92. doi: 10.1016/j.brainresbull.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Straube T, Kolassa IT, Glauer M, et al. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry. 2004;56:921–30. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 62.Straube T, Mentzel HJ, Miltner WHR. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–8. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- 63.Frick A, Howner K, Fischer H, et al. Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Transl Psychiatry. 2013;3:e312. doi: 10.1038/tp.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stein MB, Goldin P, Sareen J, et al. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–34. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 65.Phan KL, Fitzgerald DA, Nathan PJ, et al. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–9. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Pujol J, Harrison BJ, Ortiz H, et al. Influence of the fusiform gyrus on amygdala response to emotional faces in the non-clinical range of social anxiety. Psychol Med. 2009;39:1177–87. doi: 10.1017/S003329170800500X. [DOI] [PubMed] [Google Scholar]
- 67.Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garrett AS, Menon V, MacKenzie K, et al. Here’s looking at you, kid: neural systems underlying face and gaze processing in Fragile X syndrome. Arch Gen Psychiatry. 2004;61:281–8. doi: 10.1001/archpsyc.61.3.281. [DOI] [PubMed] [Google Scholar]
- 69.Akiyama T, Kato M, Muramatsu T, et al. A deficit in discriminating gaze direction in a case with right superior temporal gyrus lesion. Neuropsychologia. 2006;44:161–70. doi: 10.1016/j.neuropsychologia.2005.05.018. [DOI] [PubMed] [Google Scholar]