Abstract
Background
The maintenance of harmful alcohol use can be considered a reiterated decision in favour of alcohol in concrete drinking occasions. These decisions are often made despite an intention to quit or reduce alcohol consumption. We tested if a hyperactive reward system and/or an impaired cognitive control system contribute to such unfavourable decision-making.
Methods
In this fMRI study, men with modest to harmful drinking behaviour, which was measured using the Alcohol Use Disorders Identification Test (AUDIT), repeatedly made decisions between alcoholic and nonalcoholic drinks. Based on prior individual ratings, decision pairs were created with an alcoholic decision option considered more desirable but less beneficial by the participant. By correlating AUDIT scores with brain activation during decision-making, we determined areas explicitly related to pro-alcohol decisions in men with greater drinking severity.
Results
Thirty-eight men participated in our study. Behaviourally, we found a positive correlation between AUDIT scores and the number of decisions for desired alcoholic drinks compared with beneficial nonalcoholic drinks. The fMRI results show that AUDIT scores were positively associated with activation in areas associated with reward and motivation processing (i.e., ventral striatum, amygdala, medial prefrontal cortex) during decisions favouring a desired, nonbeneficial alcoholic drink. Conversely, we did not find hypoactivation in areas associated with self-control (dorsolateral prefrontal cortex). These effects were not present when participants chose a desired, nonbenefical, nonalcoholic drink.
Limitations
The men participating in our study had to be abstinent and would potentially consume an alcoholic drink at the end of the experiment. Hence, we did not define manifest alcohol dependence as an inclusion criterion and instead focused on less severely affected individuals.
Conclusion
Our results indicate that with growing drinking severity, decisions for alcoholic drinks are associated with increasing activity in reward-associated neural systems, rather than decreasing activity in self-control–associated systems.
Introduction
The maintenance of harmful alcohol use implies reiterated decisions to consume alcohol in concrete drinking occasions. These decisions are often made despite an intention to quit or reduce alcohol consumption. Although there is quite a large body of evidence on neural responsivity to alcohol cues or neural mechanisms of general decision-making capacities in individuals with alcohol use disorders, the neural processes during real drinking decisions remain largely unclear.
Dual-process models of addiction1,2 state the importance of 2 distinct but interacting systems during decisions for and against alcohol consumption. On the one hand, a reward system (also referred to as an impulsive, motivational, or reflexive system) has been implicated in the immediate emotional assessment of stimuli and automatic (approach) behaviour. On the other hand, a cognitive control system (also referred to as a deliberative or reflective system) that modulates this primary assessment by integration of higher-order considerations, such as long-term effects of a possible decision, has been suggested. In theory, both a hyperactive reward system and an impaired control system may contribute to addictive behaviour. Indeed, behavioural and neuroimaging data suggest alterations in both systems in individuals with substance use disorders.
Alcohol-dependent or heavily drinking individuals show subjective craving3 and automatic approach tendencies4,5 when confronted with alcoholic drinks, and a substantial body of literature suggests that such addiction-related behaviour is associated with an overactive reward system. Specifically, fMRI studies have consistently linked alcohol cue reactivity (i.e., brain responses to the presentation of alcohol stimuli) with the amygdala, ventral striatum and ventromedial pre-frontal cortex (VMPFC) in both alcohol-dependent patients6–14 and heavy drinkers.15–17
Moreover, alcohol-dependent patients showed activation of the ventral striatum and VMPFC when approaching versus avoiding alcohol compared with fruit juice in a joystick task,18 and activation of the amygdala and ventral striatum has been reported to correlate with subjective craving in alcohol-dependent patients.14,18 Thus, hyperactivity in reward-associated neural systems appears to play a role in craving and approach behaviour. Conversely, this enhanced response to alcohol-related stimuli may be accompanied by an attenuated response to nonalcoholic rewarding stimuli.12,18 This neuroimaging finding is behaviourally paralleled by a loss of interest in activities that are not related to alcohol consumption.
On the other hand, previous findings suggest impaired self-control function in alcohol-dependent or heavily drinking individuals. At the behavioural level, these individuals show a preference for short-term rather than long-term rewards,19 as well as for riskier decision options.20 At the neural level, this may correspond to attenuated activity of the second system in dual system models of decision-making. This control system supposedly modifies automatic behaviour by integrating goals related to long-term benefits.
In healthy individuals, dorsolateral prefrontal cortex (DLPFC) activation has been associated with a preference for long-term over short-term rewards,21,22 whereas disruption of the DLPFC by repetitive transcranial magnetic stimulation (rTMS) has been shown to promote impulsive decision behaviour.23 In an fMRI study on healthy dieters choosing between a tastier and a healthier food product, decisions in favour of the healthier product were correlated with increased DLPFC activation.24 In line with this finding, lesions of the DLPFC led to the inability to change dysfunctional decision patterns.25 In indivdiuals with substance use disorders, neuro-imaging studies have shown attenuated DLPFC activity during inhibitory control tasks.26,27 Furthermore, the DLPFC was more active in smokers when using cognitive strategies to suppress craving.28 Taken together, these findings suggest that functional and structural alterations in self-control areas could lead to the inability to resist craving despite the intention to quit drinking.
Behavioural and neuroimaging data suggest that alterations in the reward as well as in the control system contribute to addictive behaviour. An overwhelming desire (associated with hyperactivation of reward-associated circuits) as well as impaired control processes (associated with hypoactivation in control-associated areas) may contribute to the maintenance of substance use despite awareness of its harmful consequences. The aforementioned fMRI studies either focused on passive exposure to alcohol-related stimuli (thus studying responsivity of the reward system to alcohol cues, independent of actual decision-making situations) or on general decision-making tasks, such as the Iowa Gambling Task29 or the Monetary Delayed Discounting Task30 (thus studying control processes independent of alcohol stimuli). Hence, these studies mainly focused either on reward or control processes in addiction. The present study addressed the question of how both systems interact during real-life drinking decisions and how this interplay is altered with increasing drinking severity.
For this purpose, we used an fMRI task where individuals with widely differing drinking severity decided between alcoholic and nonalcoholic drinks. The decision options were individually designed in a way that participants experienced a conflict between the desire and benefit associated with the respective drinks. We implemented a real-world decision by scheduling scanning sessions on Friday or Saturday evenings and by serving one of the chosen drinks directly after scanning. By this means, the paradigm established by Hare and colleagues24 was adopted to elucidate the neural mechanisms of decisions for desired, nonbeneficial alcoholic drinks. Specifically, we tested if increased activity of reward areas (hypothesis of overwhelming desire), decreased activity of self-control areas (hypothesis of impaired control processes) or a combination of both promotes harmful pro-alcohol decisions.
Methods
Participants
We recruited men between 20 and 60 years old through advertising for participation in the study. Exclusion criteria were withdrawal symptoms when abstinent, cannabis consumption 4 weeks before participation and substance dependence other than alcohol and/or nicotine. Participants were told before they enrolled in the study that there would be urine toxicology tests on a random basis. In practice, this random screening was not performed, and we relied on the participants’ self-disclosure instead. In addition, to be eligible for participation, individuals were required to have no other DSM-IV Axis-I disorders and no history of head trauma or neurologic disorders. To guarantee a general awareness of health issues, participants were asked about eating habits and health awareness in the screening interview.
Participants were screened for DSM-IV criteria for alcohol abuse and alcohol dependence using the Mini-International Neuropsychiatric Interview (M.I.N.I.).31 As participants received real drinks at the end of the experiment, we did not include abstinent or immediately treatment-seeking participants to avoid the risk of provoking relapses. After the experiment, all participants were given information on addiction counselling centres and treatment possibilities.
Participants completed the following questionnaires concerning drinking behaviour: the Alcohol Use Disorders Identification Test32 (AUDIT; assessing harmful drinking on a scale of 0 to 40), the Obsessive Compulsive Drinking Scale33 (OCDS) and the Alcohol Dependence Scale34 (ADS). The AUDIT was used as the main variable modelling severity of harmful drinking.
We collected the following additional information to allow strict control over confounding variables and potential psychiatric comorbidities. Handedness was assessed using the Edinburgh Handedness Inventory35 (EHI), and the Matrix Reasoning Test of the Wechsler Adult Intelligence Scale36 (WAIS) was used as a proxy of general intelligence. We assessed depressive symptoms using the Beck Depression Inventory (BDI), anxiety using the State-Trait Anxiety Inventory37 (STAI) and impulsiveness using the Barratt Impulsiveness Scale38 (BIS) and the Monetary Choice Questionnaire39 (MCQ). The Lifetime Drinking History (LDH40) was used to assess the participants’ drinking behaviour over the lifespan.
The study was approved by the Ethical Committee of the Charité, Universitätsmedizin Berlin. After complete description of the study, written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Experimental setting
Participants were instructed not to drink anything for 2 hours before the scanning session to ensure a basic level of thirst. Because participants arrived at the scanning site 90 min before the fMRI session to perform the ratings and fill out consent forms and questionnaires, we can say for certain that they did not drink within this timeframe. Moreover, every session was scheduled for evenings before either weekends or public holidays to guarantee drinking willingness. Before the experiment, a minibar with drinks was presented to the participant in a room near the scanning room, and the participants were told that 1 of the decisions made during the experiment would be implemented after the experiment.
Ratings
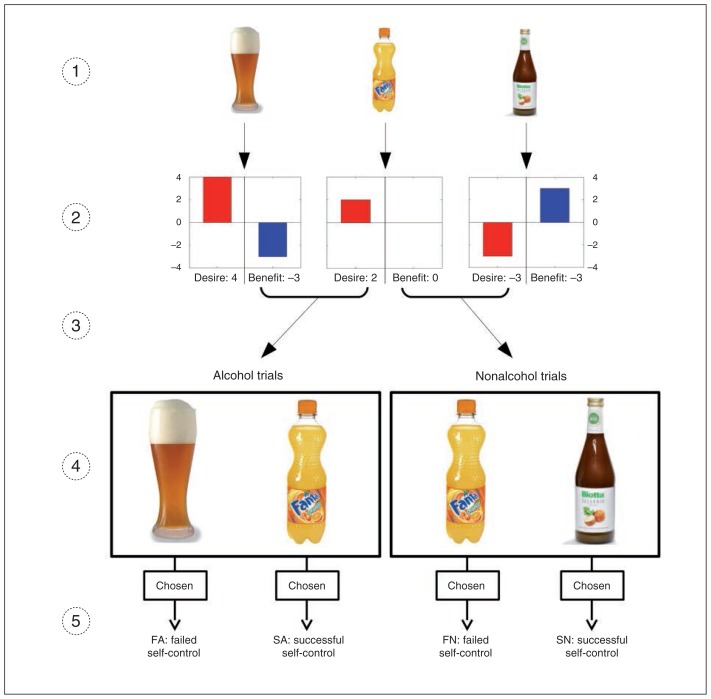
Prior to scanning, participants rated 120 photographs depicting alcoholic drinks (e.g., beer, wine, liquor) as well as a variety of nonalcoholic drinks (e.g., lemonade, milk, juice) with regard to desire and beneficence. The wording of the 2 questions was (translated from German) “In your honest opinion, how great is your desire to have this drink right now?” for the desire rating and “How beneficial/harmful would it be to have this drink?” for the benefit rating. In both cases, the scale reached from −4 to 4, with 0 as a neutral value (Fig. 1). The drinks were presented using high-resolution colour pictures matched for luminance and size between alcoholic and nonalcoholic items. We used the ratings to create conflicting pairs of a more beneficial and a more desired drink in the decision task. The image set’s suitability to create such conflicting pairs was investigated beforehand and optimized in a behavioural pilot study involving 8 participants.
Fig. 1.
Stimulus set, ratings and conditions of the decision task. (1) The stimulus set comprised images of 120 alcoholic and nonalcoholic drinks. (2) These drinks were rated by the participant in terms of desire to drink and beneficence of the drink. (3) Pairs of drinks inducing a conflict between desire and benefit were generated based on the individual ratings. (4) During the fMRI session, the participant chose between the 2 drinks. (5) All decisions made by participants during the decision task were assigned to 1 of 4 conditions: successful self-control in an alcohol–nonalcohol conflict (SA; e.g., choosing the nonalcoholic item), failed self-control in an alcohol–nonalcohol conflict (FA; e.g., choosing the alcoholic item), successful self-control in a nonalcohol–nonalcohol conflict (SN; e.g., choosing the less desired, more beneficial nonalcoholic item), and failed self-control in a nonalcohol–nonalcohol conflict (FN; e.g., choosing the less beneficial, more desired nonalcoholic item).
Decision task
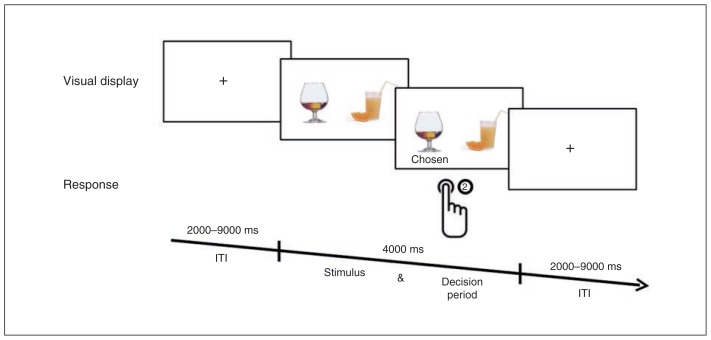
In the decision task, 2 images of drinks were presented simultaneously, followed by a fixation cross (Fig. 2). Within a 4-s interval, participants chose (by button press) between 2 nonalcoholic drinks or between an alcoholic and a nonalcoholic drink. The decisions involving an alcoholic drink are hereafter referred to as “alcohol trials,” and those with 2 non-alcoholic drinks are refferred to as “nonalcohol” trials. Decision options were presented in such a way that they induced a conflict within the participant between the desire and the benefit associated with the consumption of the respective drinks. That is, based on the prescan ratings, decision options were presented where 1 drink was considered more beneficial and the other more desirable by the participant. Moreover, “close” conflict pairs that differed by only 1 point on both scales were excluded from analysis.
Fig. 2.
Test sequence in the decision task. Two drinks were presented simultaneously. Participants had to choose 1 of the drinks within 4000 ms by pressing a button. After pressing the button, a fixation cross was presented for a variable intertrial interval (ITI) lasting 2000–9000 ms.
Depending on the participants’ prior ratings and the real decision, each trial was subsumed under 1 of 4 conditions that were defined as follows (Fig. 1):
SA: successful self-control in an alcohol–nonalcohol conflict (e.g., choosing the less desired, more beneficial nonalcoholic item),
FA: failed self-control in an alcohol–nonalcohol conflict (e.g., choosing the more desired, less beneficial alcoholic item),
SN: successful self-control in a nonalcohol–nonalcohol conflict (e.g., choosing the less desired, more beneficial nonalcoholic item), and
FN: failed self-control in a nonalcohol–nonalcohol conflict (e.g., choosing the more desired, less beneficial nonalcoholic item).
The decision task was split into 4 runs of 50 trials each. For 12 of the participants, the ratings did not allow us to create the 200 conflicting stimulus pairs, so fewer trials were tested (range 50–192 decisions per participant). The reason for this was a correlation between desire and benefit ratings in these participants, which led to a reduced number of pairs with conflict between benefit and desire of the drinks and — because only pairs with this conflict were shown to the participant — to a reduced number of decisions. However, a confounding effect of this imbalance is unlikely since the number of trials per participant was not correlated with our variable of interest, the AUDIT scores (p = 0.77).
The general functionality of the task and the stimulus set was tested with a proof-of-concept analysis comparing blood-oxygen level–dependent (BOLD) responses between alcohol and nonalcohol trials (FA + SA) > (FN + SN). As expected, this analysis yielded strong effects in the posterior and anterior cingulate cortex and the medial prefrontal cortex (inter alia, family-wise error [FWE]–corrected whole brain analysis). Because of their replicative character, these results are not reported in the Results section.
FMRI data acquisition and preprocessing
We used a Siemens Trio 3 T scanner equipped with a 12-channel head coil to acquire MRI volumes. T2*-weighted gradient-echo echo-planar images (EPI) containing 36 axial slices (3.5 mm thick, interleaved) without interslice gap were acquired with the following imaging parameters: repetition time (TR) 2250 ms, echo time (TE) 30 ms, flip angle 80°, matrix size 64 × 64 and field of view (FOV) 134 mm, resulting in a voxel size of 3.5 × 3.5 × 3.5 mm. Images were acquired in an oblique orientation of 30° to the anterior commissure–posterior commissure line. High resolution T1-weighted structural data were collected for anatomic localization, with TR 900 ms, TE 2.52 ms, matrix size 256 × 256, FOV 256 mm, 192 slices (1 mm thick) and flip angle 9°.
We preprocessed functional scans using SPM8 software.41 Functional images were corrected for slice-acquisition time (using sinc interpolation), realigned and unwarped. The high-resolution T1 image was coregistered with the mean EPI image and subsequently segmented. Images were normalized using DARTEL and the segmented grey and white matter maps. Finally, images were spatially smoothed with an 8 mm full-width at half-maximum Gaussian kernel.
First-level analyses
After preprocessing, individual data analysis was performed using SPM8. For each participant, we used the onsets of presentation of the decision options to generate regressors for the 4 conditions (SA, FA, SN, FN) in an event-related design (see the Decision task section and Fig. 1). We used the realignment parameters of the motion correction as covariates of no interest. Subsequently, specific t contrast images (see the Contrast testing section) were created and entered into the second-level group analyses.
Second-level analyses
For every contrast image created in the first-level analyses, we performed a group-level correlation analysis between AUDIT scores and contrast-specific brain activation using the Multiple Regression Design of SPM (see the “Contrast testing” section). Because there was an association between AUDIT scores and age (r = 0.27, p = 0.10), we included age as a covariate of no interest to preclude a confounding influence of age differences. For this analysis, we used a priori regions of interest (ROIs) for small-volume α error adjustment. Based on prior studies on neural correlates of alcohol-related cue reactivity, craving and approach behaviour, we included the amygdala, striatum and MPFC as ROIs to test our hypothesis of overwhelming desire. These ROIs are hereafter referred to as “reward-associated areas,” although this wording certainly does not cover all cognitive processes previously proposed for these areas. Conversely, we used the DLPFC as an ROI to test our hypothesis of impaired control processes (“control-associated area”). The striatum, amygdala and MPFC were defined as described by Beck and colleagues7 using a combination of anatomic hypotheses and previous fMRI findings regarding alcohol cue reactivity. As the DLPFC is anatomically not clearly defined and has not been reported in cue reactivity studies, a functionally defined ROI was downloaded from an online atlas.42 All imaging results are presented with a significance threshold of p < 0.05, small volume–corrected for the amygdala, striatum, MPFC and DLPFC ROIs and using FWE correction to account for multiple testing.
Contrast testing
To study how brain activation during pro-alcohol decisions varies with drinking severity, we correlated AUDIT scores with specific BOLD contrasts obtained during the decision task. We aimed to identify 2 types of brain regions: areas whose activation was positively correlated with drinking severity during pro-alcohol decisions (reward-associated areas according to the hypothesis of overwhelming desire) and areas whose activation was negatively correlated with drinking severity (control-associated areas according to the hypothesis of impaired control processes).
To ensure the specificity of our findings for alcohol trials, we used decisions for more desired drinks in nonalcohol trials (FN trials) as a control condition (resulting in the contrast AUDIT × [FA − FN]). To further ensure the specificity for trials with a failure in self-control (i.e., to preclude a sole alcohol effect causing activations in AUDIT × [FA − FN]), we then subtracted the analogous contrast for successful self-control trials. This calculation yielded the interaction contrast AUDIT × [(FA − FN) − (SA − SN)], which represents the impact of growing drinking severity on activation during decisions for the more desired alcoholic drink compared with both decisions for the more desired nonalcoholic drink and decisions against the alcoholic drink. Thus, the contrasts AUDIT × (FA − FN) and AUDIT × [(FA − FN) − (SA − SN)] can be used to test the hypothesis of overwhelming desire (enhanced activation of reward areas during pro-alcohol decisions with growing drinking severity). Analogically, the inverse correlations −AUDIT × (FA − FN) and −AUDIT × [(FA − FN) − (SA − SN)] were computed indicating which areas show decreasing activations during pro-alcohol decisions with growing drinking severity (test for hypothesis of impaired control processes).
Behavioural analyses
For the 4 conditions SA, FA, SN and FN, we calculated the number of trials per condition and subject-wise mean response times. To check the validity of the AUDIT scores, we computed Pearson correlations between AUDIT scores and other alcohol-related measures (OCDS, ADS).
As a proxy of general impulsiveness, we correlated AUDIT scores with the general proportion of failed self-control trials (all failed self-control trials ÷ by all trials). As a measure of tendency to more likely fail in alcohol trials, we computed the ratio of failed self-control rates between alcohol and non-alcohol trials, referred to as “alcohol-specific failed self-control.” It was correlated with AUDIT scores to check if this alcohol-specific failed self-control was more likely to occur in participants with more severe drinking.
We compared mean response times (RTs) between the different conditions as another measure of impulsive decision making. Analogous to contrast testing of imaging data, the interaction contrast of response times ([FAReact − FNReact] − [SAReact − SNReact]) was used to ensure the highest possible specificity for failed self-control decisions in favour of alcohol.
Results
Participants
We recruited 44 men to participate in the study. Five of them had to be excluded from the analysis for technical reasons, and 1 was excluded because of an incidental finding, leaving 38 men for data analysis. All participants were right-handed. Seventeen participants fulfilled DSM-IV criteria for alcohol abuse and 2 further fulfilled the criteria for alcohol dependence. Table 1 summarizes the final sample’s demographic and behavioural features.
Table 1.
Sample characteristics and behavioural results
| Characteristic* | No. participants | Range | Mean ± SD | Pearson R |
|---|---|---|---|---|
| Age, yr | 38 | 23 to 49 | 32.53 ± 7.13 | 0.27 |
| Age at first drunken stupor, yr | 38 | 12 to 18 | 15.16 ± 1.59 | 0.19 |
| Alcohol Dependence Scale Score | 36 | 25 to 54 | 32 ± 7.04 | 0.83† |
| Alcohol-specific failed self-control (ratio of failed self-control rates between alcohol and nonalcohol trials) | 37 | 0.07 to 3.25 | 1.18 ± 0.56 | 0.41‡ |
| AUDIT score | 38 | 2 to 30 | 11.08 ± 7.05 | — |
| No. drinking d/wk in the last mo | 38 | 0.25 to 6 | 2.98 ± 2.04 | 0.48† |
| No. of drinks per drinking d in the last mo | 38 | 3 to 12 | 8.16 ± 2.95 | 0.54† |
| Barratt Impulsiveness Scale score | 38 | 42 to 171 | 69.43 ± 25.37 | 0.13 |
| Beck Depression Inventory score | 35 | 21 to 119 | 27.94 ± 16.27 | 0.11 |
| Edinburgh Handedness Inventory quotient | 38 | 10 to 100 | 81.75 ± 19.53 | 0.05 |
| Interaction effec in response times [(FA − FN) − (SA −SN)] | 37 | −848.53 to 876.82 | −5.7 ± 401.14 | 0.37‡ |
| IQ | 34 | 70.00 to 115.00 | 96.91 ± 10.87 | 0.14 |
| Lifetime Drinking History alcohol intake per mo, g | 38 | 82 to 9465 | 1762 ± 1774 | 0.56† |
| Lifetime Drinking History total alcohol intake, g | 38 | 4861 to 2 754 299 | 390 481 ± 524 030 | 0.51† |
| Response time for FA trials, ms | 38 | 972.57 to 2354.06 | 1499.08 ± 287.90 | 0.36‡ |
| Response time for FN trials, ms | 38 | 934.76 to 2303.95 | 1536.92 ± 316.19 | 0.19 |
| Response time for SA trials, ms | 37 | 996.96 to 3063.50 | 1811.08 ± 479.95 | −0.22 |
| Response time for SN trials, ms | 38 | 994.33 to 3031.50 | 1864.11 ± 438.21 | 0.20 |
| Monetary Choice Questionnaire — Discounting Index score | 38 | 0.0003 to 69 | 0.019 ± 0.019 | 0.20 |
| Obsessive Compulsive Drinking Scale score | 35 | 2 to 28 | 11.09 ± 6.16 | 0.77† |
| Proportion of failed self-control trials in all trials | 38 | 0.11 to 0.98 | 0.72 ± 0.22 | 0.03 |
| State-Trait Anxiety Inventory score | 38 | 45 to 52 | 48.92 ± 1.81 | 0.01 |
| Total abstinence, mo | 36 | 0 to 7 | 1.45 ± 1.95 | 0.16 |
| Total drinking, yr | 38 | 5 to 34 | 16 ± 7 | 0.30 |
| Education, yr | 38 | 10 to 22 | 16.36 ± 2.79 | −0.15 |
AUDIT = Alcohol Use Disorders Identification Test; FA = failed self-control in an alcohol–nonalcohol conflict; FN = failed self-control in a nonalcohol–nonalcohol conflict; SA = successful self-control in an alcohol–non-alcohol conflict; SD = standard deviation; SN = successful self-control in a nonalcohol–nonalcohol conflict.
Eighteen participants were smokers and 20 were not (p = 0.21, 2-sample t test).
Significant at a threshold of p < 0.01.
Significant at a threshold of p < 0.05.
Behavioural results
To check the validity of AUDIT measures, we computed correlations between AUDIT, OCDS, ADS and LDH scores. These analyses revealed a significant correlation between AUDIT and OCDS (r = 0.768, t36 = 7.19, p < 0.001), AUDIT and ADS (r = 0.828, t36 = 8.86, p < 0.001) and AUDIT and alcohol consumption per month (r = 0.561, t36 = 4.06, p < 0.001) as well as for the entire life (r = 0.513, t36 = 3.59, p = 0.001) as measured with LDH. There was a positive correlation between drinking severity, as reflected in the AUDIT scores, and our behavioural measure of alcohol-specific failed self-control (see the Behavioural analyses section; r = 0.41, t36 = 2.70, p = 0.012). That is, with increasing AUDIT scores, participants failed more often in alcohol than in nonalcohol trials. Moreover, with increasing AUDIT scores, participants made significantly faster decisions in alcohol trials than in nonalcohol trials in failed compared with successful self-control (interaction effect for response times AUDIT × [(FAResp − FNResp) − (SAResp − SNResp)] (r = −0.371, t36 = −2.40, p = 0.024).
There was no significant correlation between AUDIT scores and EHI scores, intelligence (matrices subtest of WAIS), BDI scores, years of education, STAI scores and impulsiveness (general proportion of failed self-control, BIS, MCQ), excluding these variables as possible confounders (Table 1).
Imaging results
To study the effect of increasing drinking severity on brain activation during failed self-control in favour of alcohol (pro-alcohol decisions), we correlated AUDIT scores with activation during failed self-control in alcohol compared with failed self-control in nonalcohol trials.
Hyperactivated areas during pro-alcohol decisions
According to the hypothesis of overwhelming desire, reward-associated areas should show enhanced activation during pro-alcohol decisions, and this hyperactivation should increase with growing drinking severity.
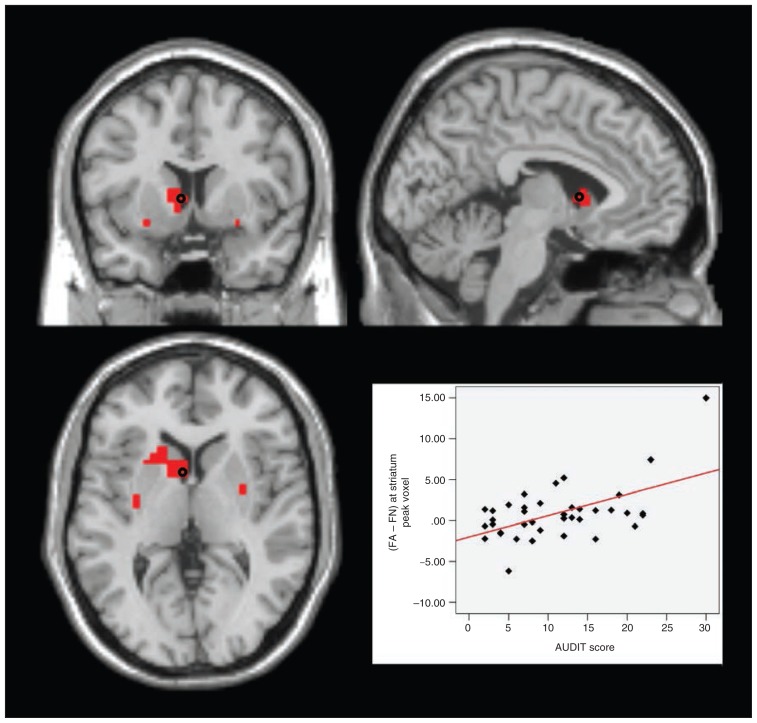
The corresponding analysis testing positive correlations between drinking severity and brain activation during pro-alcohol decisions (i.e., AUDIT × [FA − FN]) revealed significant results in the bilateral striatum (peak left in Montreal Neurological Institute [MNI] space: x, y, z = −4, 7, 4, t35 = 4.34, pFWE = 0.013, extent = 9; peak right: x, y, z = 35, −18, −7, t35 = 3.81, pFWE = 0.046, extent = 9; clusters were localized in the ventral striatal parts), in the bilateral MPFC (peak left: x, y, z = 0, 60, 18, t35 = 4.29, pFWE = 0.018, extent = 82; peak right: x, y, z = 4, 56, 18, t35 = 4.71, pFWE = 0.005, extent = 105), and in the left DLPFC (peak: x, y, z = −18, 18, 60, t35 = 4.87, pFWE = 0.002, extent = 50). Notably, these correlations were driven by both a positive AUDIT × FA correlation and a negative AUDIT × FN correlation (Appendix 1, Figs. S1–S3, available at jpn.ca), indicating enhanced activation of reward-associated areas during decisions in favour of alcohol as well as attenuated activation during decisions in favour of desirable nonalcoholic drinks.
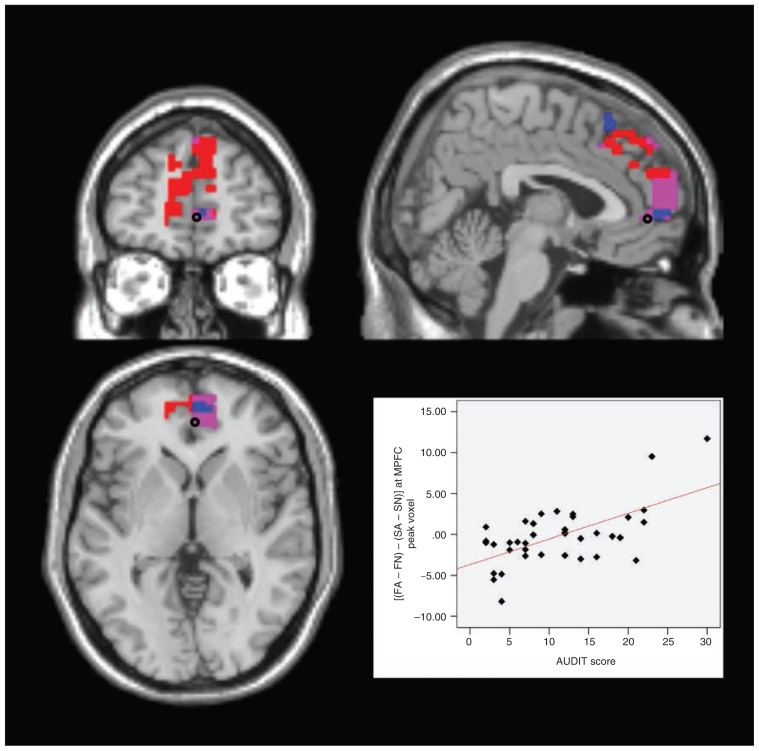
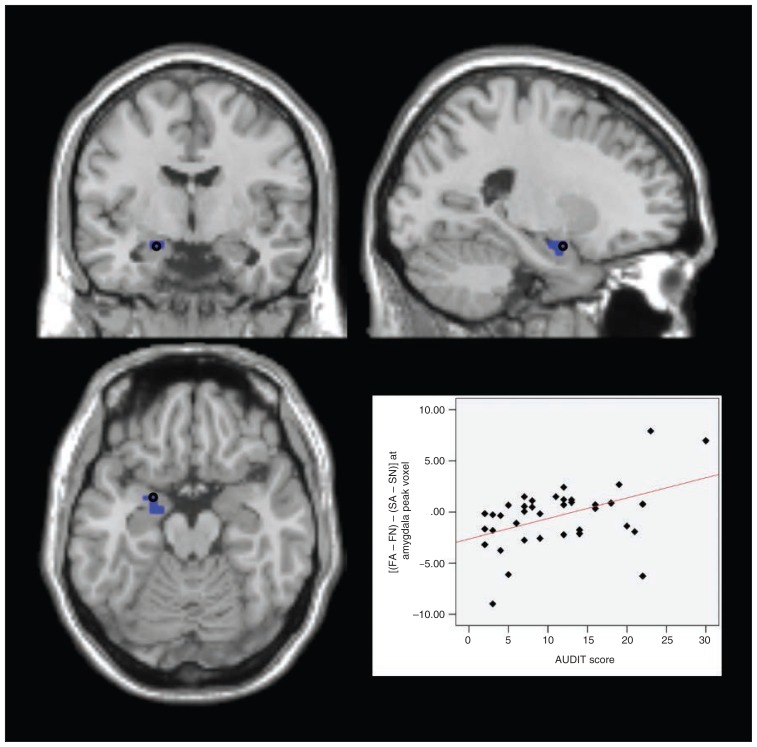
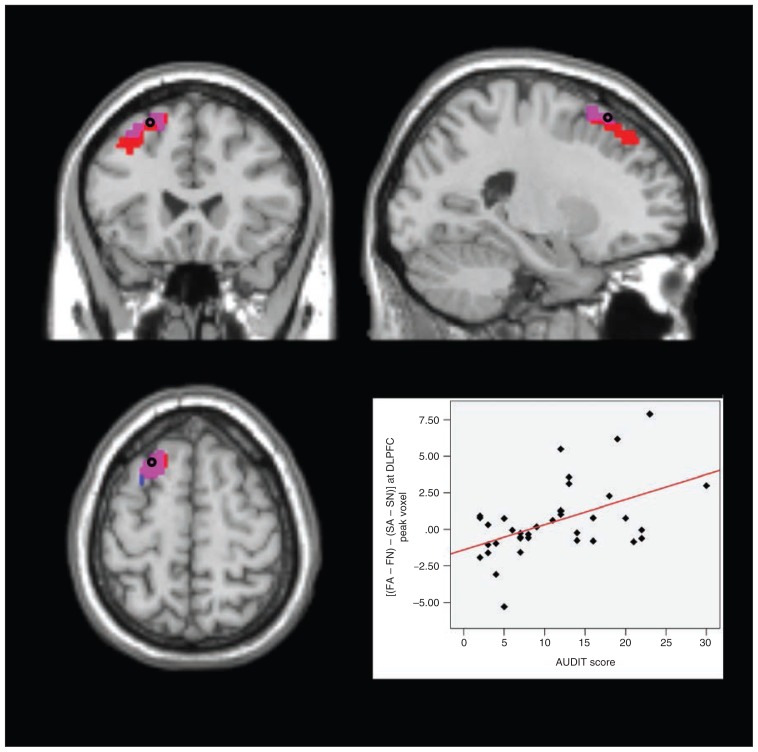
To preclude a sole alcohol effect causing the activations in AUDIT × (FA − FN), we then subtracted the analogous activation for successful self-control trials from the above contrast. For the resulting analysis, AUDIT × [(FA − FN) − (SA − SN)], we found significant results in the left amygdala (peak: x, y, z = −21, 0, −18, t35 = 3.64, pFWE = 0.011, extent = 3) and in the left DLPFC (peak: x, y, z = −28, 11, 63, t35 = 4.14, pFWE = 0.014, extent = 9) as well as the bilateral MPFC (peak left: x, y, z = 0, 56, 4, t35 = 4.45, pFWE = 0.012, extent = 56; peak right: x, y, z = 4, 49, 0, t35 = 4.52, pFWE = 0.008, extent = 60). That is, with growing drinking severity, these areas showed increasing activations in failed compared with successful self-control in alcohol compared with nonalcohol trials (Fig. 3, Fig. 4, Fig. 5, Fig. 6).
Fig. 3.
Failed self-control in alcohol trials — medial prefrontal cortex (MPFC). Section views showing significant clusters for AUDIT × (FA − FN) and AUDIT × [(FA − FN) − (SA − SN)] within the MPFC at a threshold of p < 0.05, family-wise error–corrected. Custers are presented at a threshold of p < 0.005, uncorrected. Red: MPFC cluster with higher activity in participants with higher drinking severity in failed self-control in alcohol compared with nonalcohol trials (AUDIT × [FA − FN]) Blue: MPFC cluster with higher activity in participants with higher drinking severity in failed compared with successful self-control in alcohol compared with nonalcohol trials (AUDIT × [(FA − FN) − (SA − SN)]) Violet: overlap between these 2 clusters. Plot: effect of interaction contrast (FA − FN) − (SA − SN) at the marked peak voxel (Montreal Neurological Institute space: x, y, z = 4, 49, 0) plotted subject-wise against AUDIT score. AUDIT = Alcohol Use Disorders Identification Test; FA = failed self-control in an alcohol–nonalcohol conflict; FN = failed self-control in a nonalcohol–nonalcohol conflict; SA = successful self-control in an alcohol–non-alcohol conflict; SN = successful self-control in a nonalcohol–nonalcohol conflict.
Fig. 4.
Failed self-control in alcohol trials — amygdala. Section views showing significant clusters for AUDIT × (FA − FN) and AUDIT × [(FA − FN) − (SA − SN)] within the amygdala at a threshold of p < 0.05, family-wise error–corrected. Clusters are presented at a threshold of p < 0.005, uncorrected. Blue: amygdala cluster with higher activity in participants with higher drinking severity in failed compared with successful self-control in alcohol compared with nonalcohol trials (AUDIT × [(FA − FN) − (SA − SN)]). Plot: effect of interaction contrast (FA − FN) − (SA − SN) at the marked peak voxel (Montreal Neurological Institute space: x, y, z = −21, 0, −18) plotted subject-wise against AUDIT score. AUDIT = Alcohol Use Disorders Identification Test; FA = failed self-control in an alcohol–nonalcohol conflict; FN = failed self-control in a nonalcohol–nonalcohol conflict; SA = successful self-control in an alcohol–non-alcohol conflict; SN = successful self-control in a nonalcohol–nonalcohol conflict.
Fig. 5.
Failed self-control in alcohol trials — striatum. Section views showing significant clusters for AUDIT × (FA − FN) within the striatum at a threshold of p < 0.05, family-wise error–corrected. Clusters are presented at a threshold of p < 0.005, uncorrected. Red: striatal clusters with higher activity in participants with higher drinking severity in failed self-control in alcohol compared with nonalcohol trials (AUDIT × [FA − FN]). Plot: effect of contrast (FA − FN) at the marked peak voxel (Montreal Neurological Institute space: x, y, z = −4, 7, 4) plotted subject-wise against AUDIT score. AUDIT = Alcohol Use Disorders Identification Test; FA = failed self-control in an alcohol–nonalcohol conflict; FN = failed self-control in a nonalcohol–nonalcohol conflict.
Fig. 6.
Failed self-control in alcohol trials — dorsolateral prefrontal cortex (DLPFC). Section views showing significant clusters for AUDIT × (FA − FN) and AUDIT × [(FA − FN) − (SA − SN)] within the DLPFC at threshold of p < 0.05, family-wise error–corrected. Clusters are presented at a threshold of p < 0.005, uncorrected. Red: DLPFC cluster with higher activity in participants with higher drinking severity in failed self-control in alcohol compared with nonalcohol trials (AUDIT × [FA − FN]). Blue: DLPFC cluster with higher activity in participants with higher drinking severity in failed compared with successful self-control in alcohol compared with nonalcohol trials (AUDIT × [(FA − FN) − (SA − SN)]). Violet: overlap between these 2 clusters Plot: effect of interaction contrast (FA − FN) − (SA − SN) at the marked peak voxel (Montreal Neurological Institute space: x, y, z = −28, 11, 63) plotted subject-wise against AUDIT score. AUDIT = Alcohol Use Disorders Identification Test; FA = failed self-control in an alcohol–nonalcohol conflict; FN = failed self-control in a nonalcohol–nonalcohol conflict; SA = successful self-control in an alcohol–nonalcohol conflict; SN = successful self-control in a nonalcohol–nonalcohol conflict.
Hypoactivated areas during pro-alcohol decisions
According to the hypothesis of impaired control, the control-associated areas should show attenuated activation during pro-alcohol decisions, and the activation of these areas should further decrease with growing drinking severity.
The analysis testing negative correlations between drinking severity and brain activation during pro-alcohol decisions (i.e., −AUDIT × [FA − FN]) revealed no significant results, even after lowering the significance threshold to p < 0.001, uncorrected. Likewise, the more specific contrast −AUDIT × [(FA − FN) − (SA − SN)] revealed no significant results, even after lowering the threshold to p < 0.001, uncorrected. That is, there were no areas showing decreasing activations with growing drinking severity in failed compared with successful self-control in alcohol compared with nonalcohol trials.
Discussion
We used fMRI to study the so-called reward and control networks during real-life decisions for and against alcohol. For this purpose, participants with widely differing drinking severity made decisions between more beneficial and more desired alcoholic and nonalcoholic drinks. We found that with increasing drinking severity, participants showed enhanced activations in the bilateral ventral striatum and MPFC as well as in the left amygdala and DLPFC during pro-alcohol decisions (failed self-control in alcohol trials). The specificity of our findings for failed self-control in alcohol trials is documented by the interaction contrast AUDIT × [(FA − FN) − (SA − SN)] that precludes a sole alcohol effect as well as a sole effect of failed self-control. Behaviourally, our fMRI finding was paralleled by an alcohol-related decision bias: with increasing drinking severity, participants failed more frequently and responded significantly faster in alcohol compared with nonalcohol trials.
Earlier studies in individuals with alcohol use disorders have implicated the striatum, amygdala and MPFC in reward processing and have linked activation in these areas to craving and approach behaviour.7–12,15–17,43 However, to our knowledge, this is the first study to demonstrate that a hyperactivation of these reward-associated areas is associated not only with the development of craving, but also with real decisions in favour of alcoholic drinks.
Besides enhanced responses of the reward system, we hypothesized that areas of the control system would be hypoactive during failed self-control, resulting in pro-alcohol decisions. However, contrary to our hypotheses, we found that these decisions were associated with hyperactivation in the DLPFC, a brain area related to self-control processes.22–24,26,27,44 This unexpected finding may represent compensatory processes (i.e., enhanced though insufficient self-control efforts in harmful drinkers when confronted with alcohol). This would be in accordance with the clinical observation that individuals with alcohol use disorders tend to choose alcoholic drinks despite their awareness of the risks involved and the intention to quit or reduce drinking. Moreover, similar ineffective hyper-activations of control-associated areas have previously been reported in abstinent alcohol-dependent patients.45
In addiction research, there is an ongoing debate on whether harmful decisions for alcohol are due to enhanced responses in reward/motivation areas (overwhelming desire) or to a hypoactive self-control system (impaired control processes).1,2,46 Our results suggest that decisions for alcohol consumption are linked to a hyperactivation of the reward system (reflected in activations in the striatum, amygdala and MPFC) rather than a hypoactivation of the control system. Notably, we found not only increasing activation of reward areas in pro-alcohol trials with growing drinking severity, but also decreasing activation in nonalcohol trials (Appendix 1). These findings are in line with the “hijacking” hypothesis of the reward system, stating that individuals with addiction show both enhanced responses to addiction-related stimuli and attenuated responses to non–addiction-related rewards.18 Our findings suggest that both effects may play a role when individuals with harmful drinking behaviour choose between alcoholic and nonalcoholic drinks.
While we refer to the striatum, amygdala and MPFC as reward-associated areas in this article, we acknowledge that for each of these brain areas a variety of distinct psychological functions has been proposed. Although these proposed functions are mostly related to reward-processing, particular functional roles may be considered for each brain region. Specifically, the activation of the ventral striatum has been shown to be related to the occurrence of prediction errors and, therefore, to the guidance of learning processes. Altered activity in the ventral striatum and connectivity with the DLPFC (resulting in altered teaching signals) has been linked to the maintenance of harmful alcohol consumption.47 Thus, the reported association between drinking severity and activation in the ventral striatum during pro-alcohol decisions may be related to malfunction of prediction error signalling and consequently to altered learning processes.
Our study aimed to transfer the paradigm of Hare and colleagues24 from decisions between healthier and more desired food items in dieters to the context of (desired but unhealthy) alcohol consumption. Analogous to the study by Hare and colleagues, we distinguished between failed and successful self-control trials. A critical assumption in this type of paradigm is that study participants face a conflict between the desire to consume an attractive but nonbeneficial item and the awareness of the negative consequences of consumption. Because the decision options always consisted of a more desirable and a more beneficial item (as indicated by the participants’ individual ratings of the drinks), we believe that participants indeed experienced such conflict in our study; the awareness for nonbeneficial effects of the drinks was reinforced by the prescan ratings that required the participants to reflect on the drinks’ harmfulness. Because participants were screened for health considerations during the recruitment for the study and because all participants chose the less desired, more beneficial item in the self-control trials, we assume a general willingness to exert self-control among our study participants. Furthermore, the hyperactivation of the self-control–associated DLPFC indicates enhanced though unsuccessful self-control efforts during decisions for alcohol. In summary, there are good reasons to believe that pro-alcohol decisions in our fMRI study implied reduced self-control. That is, participants chose the desired alcoholic drink, although they were aware of the nonbeneficial effects that the consumption of this particular drink would have on their own health.
Limitations
Participants in our study had to be abstinent at the beginning and would potentially consume an alcoholic drink at the end of the experiment. Because we wanted to avoid inducing withdrawal symptoms or relapse in alcohol-dependent individuals, we did not recruit patients from our department for the study and did not define manifest alcohol dependence as an inclusion criterion. Instead, we focused on less severely affected individuals, assessing drinking severity as a continuous variable (AUDIT scores). Accordingly, we do not provide categorical comparisons between clinically defined groups (e.g., alcohol-dependent patients v. healthy controls), but rather regression analyses on individuals with a wide range of AUDIT scores. This means our study included indivdiuals showing different severities of alcohol-drinking behaviour, ranging from normal to riskful to abusive to even dependent alcohol consumption. In doing so, we followed current concepts of dependence and abuse that tend to abandon dichotomous classifications (e.g., “addicted” v. “healthy”) in favour of a more gradual concept of alcohol use disorders (DSM-5). However, with only 2 participants fulfilling the DSM-IV criteria for alcohol dependence, further research is required to confirm the validity of our results in a larger sample of more severely affected individuals.
Another limitation might be that, especially in small-sized regions of interest like the amygdala and the ventral striatum, we obtained significant results only in a small number of contiguous voxels. Further studies including a larger number of participants might help to also tackle the challenge of achieving larger effect sizes.
Finally, participants were told before they enrolled in the study that there would be urine toxicology tests on a random basis. In practice, this random screening was not performed, and we relied on the participants’ self-disclosure instead. Thus, drug consumption among participants cannot completely be excluded.
Conclusion
Taken together, our data suggest that failed self-control in decisions for alcohol in harmful drinkers is associated with a hyper-active reward system rather than a hypoactive control system. This result is in accordance with clinical findings suggesting that cognitive approaches in psychotherapy attempting to strengthen self-control processes show only moderate effects on relapse rates.45 The question arises how psychotherapeutic interventions could specifically address the strong automatic, implicit response of the reward system to alcohol-related cues. Cognitive bias modification therapy (CBMT) may represent such a treatment strategy. Recent studies investigated the therapeutic effects of this retraining of automatic approach tendencies and the associated hyperactivation of reward systems. In these studies, CBMT successfully reduced relapse rates 1 year later5,48 as well as craving-related alcohol cue reactivity in the amygdala.13 Targeting automatic tendencies rather than control processes may therefore be a promising direction for future therapies in individuals with alcohol use disorders.
Acknowledgements
The current project was supported by the German Research Foundation (DFG FOR 16/17 HE2597/14-1 to A. Heinz).
Footnotes
Competing interests: None declared.
Contributors: H. Stuke, S. Gutwinski, C. Wiers, T. Schmidt, H. Walter and F. Bermpohl designed the study. H. Stuke, S. Gröpper, J. Parnack and C. Gawron acquired the data, which H. Stuke, S. Gutwinski, C. Wiers, C. Attar, S. Spengler, A. Heinz and F. Bermpohl analyzed. H. Stuke and F. Bermpohl wrote the article, which all authors reviewed and approved for publication.
References
- 1.McClure SM, Bickel WK. A dual-systems perspective on addiction: contributions from neuroimaging and cognitive training. Ann N Y Acad Sci. 2014;1327:62–78. doi: 10.1111/nyas.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noël X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol. 2013;23:632–8. doi: 10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinz A, Beck A, Grusser SM, et al. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14:108–18. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoenmakers T, Wiers R, Field M. Effects of a low dose of alcohol on cognitive biases and craving in heavy drinkers. Psychopharmacology (Berl) 2008;197:169–78. doi: 10.1007/s00213-007-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiers RW, Eberl C, Rinck M, et al. Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol Sci. 2011;22:490–7. doi: 10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- 6.Lu L, Hope BT, Dempsey J, et al. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–9. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 7.Beck A, Wustenberg T, Genauck A, et al. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry. 2012;69:842–52. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- 8.Grüsser SM, Wrase J, Klein S, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- 9.Schneider F, Habel U, Wagner M, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–83. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- 10.Vollstädt-Klein S, Loeber S, Kirsch M, et al. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol Psychiatry. 2011;69:1060–6. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Myrick H, Anton RF, Li X, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 12.Wrase J, Schlagenhauf F, Kienast T, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–94. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 13.Wiers CE, Stelzel C, Gladwin TE, et al. Effects of cognitive bias modification training on neural alcohol cue reactivity in alcohol dependence. Am J Psychiatry. 2015;172:335–43. doi: 10.1176/appi.ajp.2014.13111495. [DOI] [PubMed] [Google Scholar]
- 14.Myrick H, Anton RF, Li X, et al. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–75. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ihssen N, Cox WM, Wiggett A, et al. Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cereb Cortex. 2011;21:1408–15. doi: 10.1093/cercor/bhq220. [DOI] [PubMed] [Google Scholar]
- 16.Claus ED, Ewing SW, Filbey FM, et al. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–96. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filbey FM, Claus E, Audette AR, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow ND, Wang GJ, Fowler JS, et al. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. BioEssays. 2010;32:748–55. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–89. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 20.Lane SD, Cherek DR. Analysis of risk taking in adults with a history of high risk behavior. Drug Alcohol Depend. 2000;60:179–87. doi: 10.1016/s0376-8716(99)00155-6. [DOI] [PubMed] [Google Scholar]
- 21.McClure SM, Ericson KM, Laibson DI, et al. Time discounting for primary rewards. J Neurosci. 2007;27:5796–804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crockett MJ, Braams BR, Clark L, et al. Restricting temptations: neural mechanisms of precommitment. Neuron. 2013;79:391–401. doi: 10.1016/j.neuron.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figner B, Knoch D, Johnson EJ, et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13:538–9. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- 24.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 25.Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- 26.Salo R, Ursu S, Buonocore MH, et al. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphet-amine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:706–9. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolla K, Ernst M, Kiehl K, et al. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–64. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kober H, Mende-Siedlecki P, Kross EF, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–6. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bechara A, Damasio AR, Damasio H, et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 30.Richards JB, Zhang L, Mitchell SH, et al. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–43. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview. (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 32.Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 33.Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–9. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 34.Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- 35.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 36.Climie EA, Rostad K. Test review: Wechsler Adult Intelligence Scale. J Psychoed Assess. 2011;29:581–6. [Google Scholar]
- 37.Spielberger CD. State-trait anxiety inventory: a comprehensive bibliography. Consulting Psychologists Press; Palo Alto, USA: 1989. [Google Scholar]
- 38.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 39.Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- 40.Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43:1157–70. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- 41.Penny WD, Friston KJ, Ashburner JT, et al. Statistical parametric mapping: the analysis of functional brain images: the analysis of functional brain images. Academic press; 2011. [Google Scholar]
- 42.Shirer WR, Ryali S, Rykhlevskaia E, et al. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–65. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiers CE, Stelzel C, Park SQ, et al. Neural correlates of alcohol-approach bias in alcohol addiction: The spirit is willing but the flesh is weak for spirits. Neuropsychopharmacology. 2014;39:688–97. doi: 10.1038/npp.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chanraud S, Martelli C, Delain F, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–38. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- 45.Charlet K, Beck A, Jorde A, et al. Increased neural activity during high working memory load predicts low relapse risk in alcohol dependence. Addict Biol. 2014;19:402–14. doi: 10.1111/adb.12103. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SQ, Kahnt T, Beck A, et al. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci. 2010;30:7749–53. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eberl C, Wiers RW, Pawelczack S, et al. Approach bias modification in alcohol dependence: do clinical effects replicate and for whom does it work best? Dev Cogn Neurosci. 2013;4:38–51. doi: 10.1016/j.dcn.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]