Abstract
Background
GABAergic and glutamatergic neurotransmitter systems are central to the pathophysiology of depression and are potential targets of repetitive transcranial magnetic stimulation (rTMS). We assessed the effect of 10-Hz rTMS over the left dorsolateral prefrontal cortex (DLPFC) of patients with major depressive disorder on the levels of medial prefrontal cortex (MPFC) γ-aminobutyric acid (GABA) and the combined resonance of glutamate and glutamine (Glx) as assessed in vivo with proton magnetic resonance spectroscopy (1H MRS).
Methods
Currently depressed individuals between the ages of 23 and 68 years participated in a 5-week naturalistic, open-label treatment study of rTMS, with 1H MRS measurements of MPFC GABA and Glx levels at baseline and following 5 weeks of the rTMS intervention. We applied rTMS pulses over the left DLPFC at 10 Hz and 80%–120% of motor threshold for 25 daily sessions, with each session consisting of 3000 pulses. We assessed therapeutic response using the 24-item Hamilton Rating Scale for Depression (HAMD24). The GABA and Glx levels are expressed as ratios of peak areas relative to the area of the synchronously acquired and similarly fitted unsuppressed voxel water signal (W).
Results
Twenty-three currently depressed individuals (7 men) participated in the study. GABA/W in the MPFC increased 13.8% (p = 0.013) in all depressed individuals. There were no significant effects of rTMS on Glx/W. GABA/W and Glx/W were highly correlated in severely depressed patients at baseline but not after TMS.
Limitations
The primary study limitations are the open-label design and the inclusion of participants currently taking stable regimens of antidepressant medications.
Conclusion
These results implicate GABAergic and glutamatergic systems in the mechanism of action of rTMS for major depression, warranting further investigation in larger samples.
Introduction
Transcranial magnetic stimulation (TMS) is an effective treatment for major depressive disorder (MDD), particularly in individuals resistant to first-line antidepressants.1–3 Effect sizes of TMS from recent meta-analyses range from 0.39 to 0.55.4,5 Less is known about the neurobiological mechanisms of antidepressant response to TMS. Emerging evidence suggests that in addition to its effects on the dorsolateral prefrontal cortex (DLPFC), TMS also affects structures to which the DLPFC projects, including the medial prefrontal cortex (MPFC).6 The MPFC is overactive in individuals with MDD,7 and functional connectivity between the DLPFC and MPFC predicts response to TMS.8–10 Little is known about how connectivity between the DLPFC and MPFC gives rise to the antidepressant effect of TMS.
Other antidepressants appear to act in part by modulating the excitability of cortical circuits. Levels of γ-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the brain, which are generally decreased in depressed individuals,11 have been reported to increase after treatment with selective serotonin reuptake inhibitors (SSRIs)12 and electro-convulsive therapy (ECT).13 Although TMS affects the balance of excitation and inhibition in cortical circuits,14 it is unknown whether changes in excitation–inhibition balance mediate antidepressant response.
In this naturalistic study, we tested whether TMS alters the levels of GABA and those of the combined resonance of glutamate and glutamine (Glx) in patients with MDD. Specifically, the standard J-edited spin echo difference proton magnetic resonance spectroscopy (1H MRS) technique was implemented at 3 T to measure levels of MPFC GABA and Glx before and after a 25-day course of 10-Hz TMS treatment over the left DLPFC. Based on the majority of reports in patients with MDD on the neurochemical response to anti-depressant therapy,11,12,15 we hypothesized that TMS would selectively increase MPFC GABA levels while minimally affecting those of Glx.
Methods
Participants
Participants in this study were outpatients meeting DSM-IV-TR criteria for a nonpsychotic major depressive episode who provided written informed consent. Patients were referred by the outpatient clinic in the Department of Psychiatry at Weill Cornell Medical College, New York, USA. Also enrolled were self-referred patients who directly contacted our TMS program.
To be eligible, the patients were required to meet criteria for treatment-resistance, defined as a failure to respond to at least 2 previous antidepressant trials at adequate doses for at least 8 weeks during the current episode. Patients were also required to have a minimum score of 18 on the 24-item Hamilton Rating Scale for Depression (HAMD24). Patients were excluded if they were actively suicidal with plan or intent; were pregnant or lactating; had bipolar I disorder or a psychotic disorder; had been in their current episode for longer than 3 years; had a history of clinically significant personality disorder, as established in the diagnostic interview; or had a substance abuse disorder or substance dependence within the past 3 years, metal implants, a history of seizure disorder or other neurologic disorder, or a closed head injury with loss of consciousness.
Participants were allowed to continue prior medications, provided that the doses remained unchanged for 4 weeks before beginning the study and throughout the course of TMS. All aspects of our experimental protocol were approved by the Institutional Review Board of Weill Cornell Medical College and were conducted in accordance with federal and institutional human research guidelines.
Repetitive TMS protocol
All patients completed 25 sessions of rTMS over the left DLPFC over a 5-week period using the NeuroStar TMS Therapy System (Neuronetics, Inc.). We assessed treatment response using the HAMD24 at baseline and 1–3 days after completion of the 5-week course of treatment. Individual sessions consisted of 37.5 min (3000 pulses; 30-s duty cycle, 4 s on, 26 s off) of 10-Hz excitatory TMS daily for 25 days (Monday–Friday for a 5-week period). The standard “Figure 8” NeuroStar TMS coil was centred over the scalp via the Beam F3 method16 using surface distances between the nasion, inion, tragus and vertex as landmarks. Resting motor threshold (MT) was defined as the stimulus strength over the thumb area of the motor cortex that produced visually detectable thumb movement on 50% of trials. We measured MT before the first treatment and on every fifth treatment thereafter. We aimed to apply repetitive stimulation at 120% of the resting MT,1 and applied intensities of 80%–120% (mean 86.4% ± 14.3%) to all participants except for 2, who reported scalp pain above an intensity of 80% of resting MT. Resting MT and stimulation intensity for each participant are described in Appendix 1, Table S2, available at jpn.ca.
Magnetic resonance neuroimaging procedures
All neuroimaging studies, which included limited structural brain MRI examination and single-voxel 1H MRS of the MPFC, were conducted on a research-dedicated 3.0 T GE MR system with an 8-channel phased-array head coil at the Citi-group Biomedical Imaging Center of Weill Cornell Medical College. All patients underwent 2 neuroimaging sessions that occurred before (mean 2.5 ± 2.3 d) and shortly after (mean 1.0 ± 1.1 d) completing the 5-week course of TMS.
Structural MRI
A 3-plane, low-resolution, high-speed scout imaging series was obtained, followed by a series of high-resolution scans, consisting of standard axial, coronal and sagittal T1-, T2- and spin density–weighted scans that were appropriately obliqued for prescribing the 1H MRS voxel in the MPFC. In addition, we obtained a T1-weighted spoiled gradient-recalled echo (SPGR) volumetric scan and an axial Fast Fluid-Attenuated Inversion Recovery (FLAIR) scan for brain tissue segmentation and detection of exclusionary focal brain lesions, respectively.
1H MRS
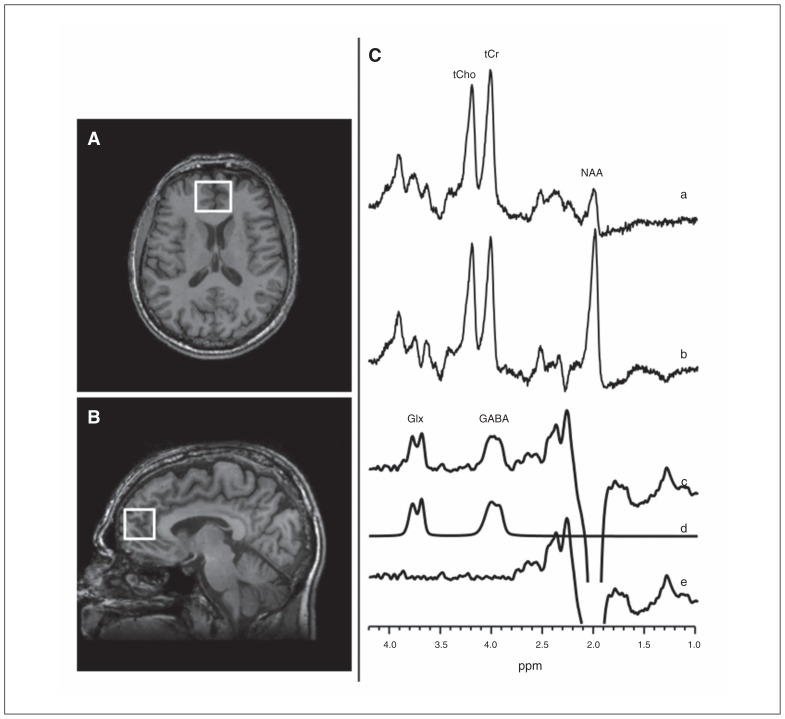
The GABA-edited 1H MRS data were acquired using the standard J-edited spin echo difference method17 and then processed as described recently16,18 (Fig. 1, Appendix 1). Each spectrum was recorded from a single 2.5 × 2.5 × 3.0 cm voxel prescribed in the MPFC, which includes a rostral component of the anterior cingulate cortex (Fig. 1A and B).
Fig. 1.
(A, B) Magnetic resonance images showing medial prefrontal cortex (MPFC) voxel size and location. (C) J-editing spectra obtained using volume-selective point-resolved spectroscopy (PRESS) with the editing radiofrequency pulse (a) on and (b) off. (c) The difference of the spectra in (a) and (b), showing (c) the edited γ-aminobutyric acid (GABA) and combined resonance of glutamate and glutamine (Glx) peaks, with (d) best-fit model spectrum of (c), and (e) the residuals of the difference between the edited (c) and best-fit (d) spectra. The data were acquired in 13 min from a 2.5 × 2.5 × 3.0 cm3 voxel using an echo time (TE) of 68 ms and a repetition time (TR) of 1500 ms, and 256 interleaved excitations (512 total) with editing pulse on or off.
1H MRS data processing and quantitation
Details of the MRS data quality assessment criteria and procedures used in this study to retain or reject spectra for inclusion in group analyses are provided in Appendix 1. For all the cases that fulfilled our quality assessment criteria, spectral peak areas, which are proportional to the concentrations of the associated metabolites, were obtained as illustrated in Fig. 1C (traces [a–f]). Briefly, the GABA and Glx resonances in the J-edited difference spectra were modelled as a linear combination of pseudo-Voigt lineshape functions and then fitted in the frequency domain using a robust and highly optimized public-domain Levenberg–Marquardt nonlinear least squares minimization routine.19 For normalization across participants, the GABA and Glx levels were expressed as ratios of peak areas relative to the area of the synchronously acquired and similarly fitted unsuppressed voxel water signal (W).
Assessment of voxel tissue heterogeneity
To estimate the proportions of grey matter, white matter and cerebrospinal fluid (CSF) contained in each voxel of interest, we used MEDx software (Medical Numerics) to segment the brain tissue based on the signal-intensity histogram of each participant’s volumetric (SPGR) MRI. In-house software developed in MATLAB (MathWorks) was then implemented to generate a segmentation mask for each voxel, from which the proportions of grey matter, white matter and CSF were determined. These were then compared between baseline and post-TMS for the whole group and, in case of significant differences, included in the statistical model as covariates.
Statistical analysis
A TMS responder was defined as having at least a 50% reduction in HAMD24 scores post-TMS compared with baseline. To test our 2 primary hypotheses, we used a paired t test or Wilcoxon signed rank test wherever appropriate to compare GABA/W and Glx/W before and after TMS in all participants. In an exploratory analysis, we used linear regression to assess the effect of independent variables, including TMS-responder status, age, sex, baseline HAMD score, TMS intensity (% of resting MT), history of antipsychotic or mood stabilizer use, lifetime number of antidepressant trials, current benzodiazepine use (lorazepam equivalents in milligrams), and current SSRI use on 2 dependent variables: percent change in GABA/W and in Glx/W from before to after TMS. Stepwise variable selection with the Akaike Information Criterion was used to select the best-fitting set of independent variables. We performed linear regression to test for potential linear associations between neurotransmitter levels (GABA/W and Glx/W) and the severity of depressive symptoms. All reported p values are 2-tailed at α = 0.05 except the primary hypotheses, where α = 0.025 after adjusting for multiple comparisons using the Bonferroni method. We used IBM SPSS Statistics version 22 and R software version 3.1.1 to perform all the statistical analyses.20
Results
Demographics and sample characteristics
In the aggregate, 25 patients with treatment-resistant depression were enrolled in this study, and all completed the 5-week course of TMS. Data for 2 patients were excluded from all the analyses owing to significant differences in head tilt in the magnet between the baseline and post-TMS neuroimaging scans, leaving 23 outpatients for subsequent analyses: 21 with MDD, 2 with bipolar II disorder (mean age 41.7 ± 15.9 yr; 7 [30%] men). The clinical and demographic characteristics of the final sample before and after the 5-week course of TMS are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of study participants
| Group; no. (%) or mean ± SD [range] | |||
|---|---|---|---|
|
|
|||
| Characteristic | All MDD, n = 23 | TMS responders, n = 8 | TMS nonresponders, n = 15 |
| Age, yr | 41.7 ± 15.9 [21–68] | 37.0 ± 16.1 [23–66] | 44.2 ± 15.8 [21–68] |
| Sex | |||
| Male | 7 (30.4) | 2 (25) | 5 (33) |
| Female | 16 (69.6) | 6 (75) | 10 (67) |
| Illness history | |||
| Episode duration, mo | 14.5 ± 10.1 [2–36] | 17.4 ± 11.6 [3–36] | 12.9 ± 9.2 [2–36] |
| No. of episodes | 4.9 ± 2.4 [2–10] | 4.4 ± 2.3 [2–8] | 5.1 ± 2.4 [2–10] |
| Suicide attempts | 0.7 ± 1.0 [0–4] | 0.9 ± 0.8 [0–2] | 0.6 ± 1.1 [0–4] |
| Psychiatric hospitalizations | 1.4 ± 1.6 [0–5] | 0.6 ± 0.7 [0–2] | 1.8 ± 1.7 [0–5] |
| Medication-naive | 0 (0) | 0 (0) | 0 (0) |
| Medication-free | 4 (17) | 3 (38) | 1 (7) |
| HAMD24 baseline | 28.7 ± 5.6 [18–39] | 28.1 ± 6.1 [22–37] | 29.1 ± 5.5 [18–39] |
| HAMD24 final | 18.8 ± 8.2 [3–37] | 10.5 ± 5.0 [3–17] | 23.2 ± 5.7 [15–37] |
| Comorbidity | |||
| ADHD | 1 (4) | 1 (12.5) | 0 (0) |
| Any anxiety disorder | 3 (13) | 1 (12.5) | 2 (13) |
ADHD = attention-deficit/hyperactivity disorder; HAMD24 = 24-item Hamilton Rating Scale for Depression; MDD = major depressive disorder; SD = standard deviation; TMS = transcranial magnetic stimulation.
The mean lifetime number of major depressive episodes was 4.9 ± 2.4. Two participants had a history of hypomania and thus met a diagnosis of bipolar II disorder. Four patients had psychiatric comorbidities: 1 had attention-deficit/hyperactivity disorder, 2 had obsessive–compulsive disorder and 1 had generalized anxiety disorder. Of the 23 patients included in the final sample, 19 (83%) were taking antidepressants, and a number were taking mood stabilizers, anti-psychotics, or other medications (Appendix 1, Table S1).
Effects of TMS treatment on depressive symptoms
On average, patients’ depressive symptoms, as assessed using the HAMD24, improved by 9.9 points (from 28.7 to 18.8) from the first to the final TMS treatment sessions (95% confidence interval [CI] −9.0 to −4.3). Depressive symptoms in the 8 responders improved on average by 17.6 points (from 28.1 to 10.5) from the first to the final TMS treatment sessions (95% CI −12.1 to −7.5). In contrast (and by definition), depressive symptoms did not improve in the 15 nonresponders, changing on average by 5.9 points (from 29.1 to 23.2) from the first to the final TMS treatment sessions (95% CI −4.2 to 0.8).
1H MRS voxel tissue heterogeneity
Table 2 provides the proportions of grey matter, white matter and CSF in the MPFC voxel for baseline and post-TMS, as determined by tissue segmentation, as well as the mean unsuppressed voxel tissue water signal (W) for baseline and post-TMS. Using paired t tests, we detected no differences in percentage of grey matter (t22 = 0.69, p = 0.49), percentage of white matter (t22 = 1.10, p = 0.28), or percentage of CSF (t22 = 0.80, p = 0.44) between baseline and post-TMS for the whole group. Levels of the reference voxel water signal did not differ from baseline to post-TMS for the whole group (t22 = 0.35, p = 0.73). Therefore, we henceforth use simply GABA or Glx to mean GABA/W or Glx/W, respectively.
Table 2.
GABA/W concentration, grey matter, white matter, and CSF percentages before and after TMS
| Timing; mean ± SD or no. (%) | ||
|---|---|---|
|
|
||
| Measure; group | Pre-TMS | Post-TMS |
| All participants, n = 23 | ||
| ACC GABA/W | 2.66 × 10−3 ± 0.53 × 10−3 | 2.98 × 10−3 ± 0.60 × 10−3* |
| ACC Glx/W | 2.14 × 10−3 ± 0.41 × 10−3 | 2.15 × 10−3 ± 0.43 × 10−3 |
| ACC water | 1.75 × 1012 ± 0.52 × 1012 | 1.79 × 1012 ± 0.48 × 1012 |
| Grey matter | 53.1 (2.8) | 52.8 (2.9) |
| White matter | 31.5 (2.9) | 32.0 (3.2) |
| CSF | 15.4 (2.1) | 15.1 (2.0) |
| TMS responders, n = 8 | ||
| ACC GABA/W | 2.68 × 10−3 ± 0.66 × 10−3 | 3.08 × 10−3 ± 0.60 × 10−3 |
| ACC Glx/W | 1.98 × 10−3 ± 0.44 × 10−3 | 2.16 × 10−3 ± 0.32 × 10−3 |
| ACC water | 1.71 × 1012 ± 0.47 × 1012 | 1.90 × 1012 ± 0.48 × 1012 |
| Grey matter | 51.4 (2.3) | 50.7 (2.0) |
| White matter | 32.3 (1.9) | 33.9 (2.2) |
| CSF | 16.3 (1.0) | 15.4 (1.9) |
| TMS nonresponders, n = 15 | ||
| ACC GABA/W | 2.65 × 10−3 ± 0.48 × 10−3 | 2.92 × 10−3 ± 0.61 × 10−3 |
| ACC Glx/W | 2.23 × 10−3 ± 0.38 × 10−3 | 2.14 × 10−3 ± 0.49 × 10−3 |
| ACC water | 1.77 × 1012 ± 0.56 × 1012 | 1.73 × 1012 ± 0.49 × 1012 |
| Grey matter | 53.9 (2.7) | 54.0 (2.6) |
| White matter | 31.1 (3.3) | 31.0 (3.2) |
| CSF | 15.0 (2.5) | 15.0 (2.1) |
ACC = anterior cingulate cortex; CSF = cerebrospinal fluid; GABA/W = γ-aminobutyric acid level relative to unsuppressed voxel tissue water; Glx = combined resonance of glutamate and glutamine; SD = standard deviation; TMS = transcranial magnetic stimulation.
Significantly increased ACC GABA in the whole group before compared with after TMS (p = 0.01).
Primary hypothesis: GABA and Glx levels before and after TMS treatment
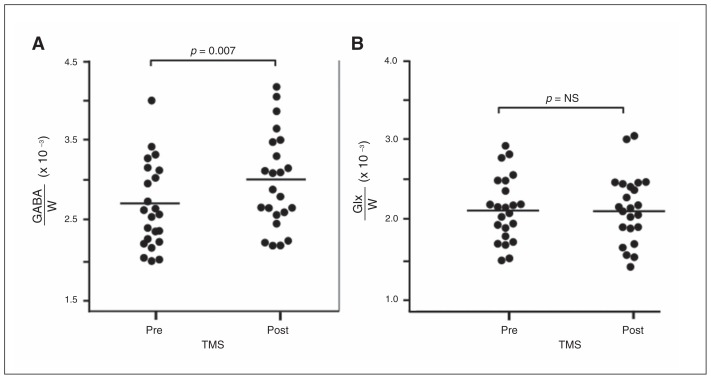
In all participants (Fig. 2A), the mean MPFC GABA level was higher post-TMS than at baseline (2.98 × 10−3 ± 0.60 × 10−3 v. 2.66 × 10−3 ± 0.53 × 10−3, mean change in GABA 13.8%, paired t22 = 2.69, p = 0.013). Among responders, there was a significantly higher mean MPFC GABA level post-TMS than at baseline (3.08 × 10−3 ± 0.60 × 10−3 v. 2.68 × 10−3 ± 0.66 × 10−3, mean change in GABA 17.4%, Wilcoxon V = 2, p = 0.023). Among nonresponders, there was a trend toward higher mean MPFC GABA levels post-TMS than at baseline (2.92 × 10−3 ± 0.61 × 10−3 v. 2.65 × 10−3 ± 0.48 × 10−3, mean change in GABA 11.9%, Wilcoxon V = 26, p = 0.06).
Fig. 2.
γ-Aminobutyric acid/unsuppressed voxel water signal (GABA/W) levels in the medial prefrontal cortex (MPFC) in depressed patients before and after a 25-session treatment with transcranial magnetic stimulation (TMS) over the left dorsolateral prefrontal cortex (DLPFC). Each graph shows GABA levels for the sample both at baseline (pre-) and post-TMS. (A) Full sample of 23 participants. Average GABA/W increases by 13.8% (p = 0.013). (B) Subgroup of TMS responders. GABA/W increases by 17.4% post-TMS compared with baseline (p = 0.07). In the subgroup of TMS nonresponders there was an 11.9% change in GABA/W post-TMS compared with baseline (p = 0.10). Glx = combined resonance of glutamate and glutamine; NS = nonsignificant.
If response was defined more liberally as a greater than 30% reduction in HAMD24 scores,21 there were 12 responders and 11 nonresponders. Among responders, there was a significantly higher mean MPFC GABA level post-TMS than at baseline (3.03 × 10−3 ± 0.61 × 10−3 v. 2.63 × 10−3 ± 0.62 × 10−3, mean change in GABA 17.4%, Wilcoxon V = 2, p = 0.001). Among nonresponders, MPFC GABA levels post-TMS were greater than at baseline (2.92 × 10−3 ± 0.61 × 10−3 v. 2.70 × 10−3 ± 0.44 × 10−3); this difference was smaller than in responders and was nonsignificant (mean change in GABA 9.9%, Wilcoxon V = 18, p = 0.21).
The presence of a psychiatric comorbidity influenced the magnitude of change of post-TMS GABA from baseline. One participant in the responder group (defined by 50% reduction in HAMD24 score) had a comorbid disorder (ADHD), and 5 participants in the nonresponder group had comorbidities (2 had bipolar II Disorder, 2 had obsessive–compulsive disorder and 1 had generalized anxiety disorder). The 1 responder with ADHD had a large (55%) increase in post-TMS GABA from baseline, which significantly impacted the average GABA increase in the responder group. Among the 7 responders without comorbidities, there was a significantly higher mean MPFC GABA level post-TMS (mean change in GABA 12.0%, Wilcoxon V = 2, p = 0.047). Among nonresponders, MPFC GABA levels post-TMS were also larger than at baseline, although this difference did not reach significance (mean change in GABA 10.9%, Wilcoxon V = 11, p = 0.11).
In all participants (Fig. 2B), mean MPFC Glx levels were unchanged post-TMS relative to baseline (2.15 × 10−3 ± 0.43 × 10−3 v. 2.14 × 10−3 ± 0.41 × 10−3, paired t22 = 0.05 p = 0.96). Among responders, mean MPFC Glx levels were unchanged post-TMS relative to baseline (2.16 × 10−3 ± 0.32 × 10−3 v. 1.98 × 10−3 ± 0.44 × 10−3, Wilcoxon V = 11, p = 0.38). Similarly, among nonresponders, mean MPFC Glx levels were unchanged post-TMS relative to baseline (2.14 × 10−3 ± 0.49 × 10−3 v. 2.23 × 10−3 ± 0.38 × 10−3, Wilcoxon V = 75, p = 0.42). Mean MPFC Glx levels remained unchanged when we used the definition of a response of greater than 30% reduction in HAMD24 score and when we removed participants with psychiatric comorbidities from our analyses.
Demographic variables versus GABA and Glx levels before and after TMS
Regression analysis of percent change in GABA with stepwise selection retained age, sex, current SSRI use and daily lorazepam use as predictors, and the model had an adjusted R2 value of 0.31. Percent GABA change was significantly and positively associated with age (t = 2.15, p = 0.046) and male sex (t = 2.59, p = 0.018) and showed a trend toward significance for a negative association with current use of SSRI (t = −2.09, p = 0.05) and current daily use of lorazepam (t = −1.81, p = 0.09)
Percent change in GABA was not associated with baseline HAMD24 score, TMS responder status, TMS intensity, lifetime number of antidepressant trials, or history of anti-psychotic or mood stabilizer use.
Exploratory analysis: MPFC GABA versus Glx correlations before and after TMS
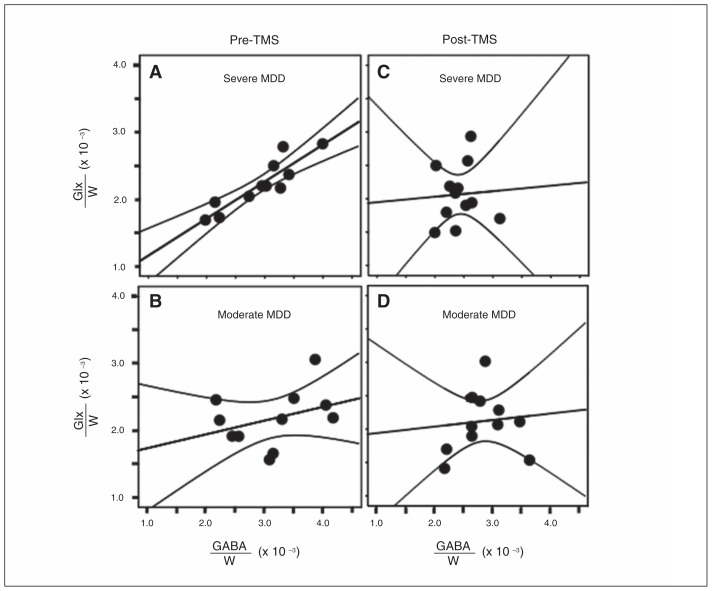
Given that metabolic GABA is synthesized from glutamate by the enzyme L-glutamic acid decarboxylase (GAD) and that the GABA shunt converts to glutamate, it is generally of interest to explore associations between the 2 neurotransmitters, especially since they both are postulated to play an important role in many neuropsychiatric disorders, including MDD.22,23 Our exploratory investigation of such associations revealed GABA to correlate strongly with Glx in participants with severe depression (i.e., HAMD24 > 2724, n = 11) at baseline (r = 0.90, p < 0.001; Fig. 3A), but not in patients with moderate depression (HDRD24 < = 27, n = 12, r = 0.06, p = 0.86; Fig. 3B). Post-TMS, GABA and Glx were uncorrelated (r = 0.35, p = 0.29) in the severe depression group (Fig. 3C), with still no GABA and Glx correlation (r = 0.10, p = 0.77) in the moderate depression group (Fig. 3D).
Fig. 3.
γ-Aminobutyric acid/unsuppressed voxel water signal (GABA/W) in the medial prefrontal cortex (MPFC) plotted by depression severity (first and second rows) and baseline and post–transcranial magnetic stimulation (TMS; first and second columns). (A) GABA/W and combined resonance of glutamate and glutamine (Glx)/W are tightly correlated in severely depressed patients at baseline (r = 0.90, p < 0.001) and (B) uncorrelated in moderately depressed patients (r = 0.06, p = 0.86). (C) In patients who were severely depressed before treatment, this correlation was reduced post-TMS (r = 0.35, p = 0.29). (D) GABA/W and Glx/W remained uncorrelated in moderately depressed patients post-TMS (r = 0.10, p = 0.77). MDD = major depressive disorder.
Discussion
The novelty of our study is its focus on examining changes in brain GABA and Glx levels in patients with depression before and after a course of therapeutic TMS. We found GABA elevations in the MPFC post-TMS relative to levels at baseline in a cohort of adults with MDD. The GABA elevations were greater in responders than in nonresponders (17.4% v. 11.9%). Differences between responders and nonresponders were more pronounced (17.4% v. 9.9%) if response was defined more liberally as a greater than 30% reduction in HAMD24 scores. In contrast, we found no differences or changes in Glx between baseline and post-TMS.
The main result of the present study, that the antidepressant effect of rTMS was associated with elevation of GABA levels relative to baseline, is consistent with and extends prior observations about GABA in depression and the effects of antidepressant treatments. The observed increase in GABA was also seen in nonresponders, suggesting that GABA increase may contribute to the antidepressant mechanism of TMS, but this association was not causal. Levels of GABA are low in the depressed brain, supported by convergent evidence from CSF samples,25 GABAergic neuronal density26 and GAD67 expression27 in postmortem brains. Prior MRS studies revealed GABA reductions in depressed individuals compared with healthy controls,11,28 with more pronounced MPFC reductions in a treatment-refractory subgroup.15 Further, and also in support of the GABA- deficit hypothesis of depression, a variety of different anti-depressants seem to function, in part, by elevating GABA. These include SSRIs,12 the monoamine-oxidase inhibitor phenelzine,29 the GABA-reuptake inhibitor tiagabine30 and ECT.13
We did not observe changes in Glx levels, either in the whole group or in TMS-responder or nonresponder subgroups. Glx levels have been found to be reduced in the MPFC at baseline in patients with depression,22 and these levels have normalized in the lateral prefrontal cortex,31 MPFC32 and amygdala after ECT;31 in the DLPFC after TMS for pediatric depression;33 and in the anterior cingulate cortex in healthy individuals after TMS.34 The difference between our findings and those previously reported in healthy individuals after TMS may reflect a difference in Glx homeostasis in the MPFC in patients with depression.
As already suggested, GABA elevation in the MPFC may contribute to the antidepressant mechanism of TMS. Optogenetic studies in rodents indicate that homeostasis in the ratio of excitation to inhibition in MPFC microcircuits plays a critical role in supporting normal circuit function and PFC-dependent behaviours.35 In patients, GABA deficits may contribute to findings of MPFC hyperactivity and elevated functional connectivity that have been reported in patients with depression,36 all of which have been shown to normalize after antidepressant treatment with SSRIs,37 TMS,9 ECT38 and deep brain stimulation.39 The lack of increase of Glx after TMS may highlight a seizure-dependent antidepressant mechanism specific to ECT suggested by postictal elevations in Glutamate after spontaneous seizures.40
Medial PFC GABA and Glx correlated significantly at baseline but not after TMS owing to elevations in GABA that were unaccompanied by Glx elevations. In keeping with our pre-TMS findings, GABA and Glx in the MPFC have been found to correlate highly in a pooled sample of patients with schizophrenia and healthy controls.41 Transcranial magnetic stimulation may unlink the GABA and Glx metabolism, change proportions of the pools of protein-bound neurotransmitter, or change compartmental localization.
Elevation of GABA in the MPFC may exert its antidepressant effect in part through its impact on the functional connectivity of resting-state networks. Functional connectivity of the subgenual anterior cingulate decreases during TMS for depression, targeting the left DLPFC9 and dorsomedial prefrontal cortex10 while baseline connectivity of the subgenual anterior cingulate predicts response to TMS.8,9 The effect of MPFC GABA elevations on functional connectivity of the subgenual anterior cingulate awaits further study.
Limitations
Our study has several limitations. First, it is a naturalistic study, and the lack of a group of healthy controls makes it difficult to determine if the change in GABA reflected normalization of a GABA deficit or an elevation above the normal level. Second, the heterogeneity of our patient population and their concurrent medication treatment raises a potential confound between different causes of GABA elevation. While our inclusion of currently depressed patients with bipolar II disorder may be considered another limitation of this study since it increases sample heterogeneity, this is mitigated by the fact that studying individuals with symptom clusters that may cross diagnostic boundaries is in line with the new US National Institute of Mental Health Research Domain Criteria (RDoC) framework for studying mental disorders.42,43 Current SSRI and benzodiazepine use were negatively associated with increase in GABA, suggesting that treatment with these medications may have partially prevented GABA elevations in a subgroup of participants. Third, owing to discomfort, some participants could not be treated at the standard stimulus strength of 120% of MT. Finally, a general limitation related to 1H MRS is the inability of the standard J-editing method to separate glutamate from glutamine, yielding the composite resonance that is commonly referred to as “Glx.” However, we recently obtained preliminary evidence that strongly suggests that the J-edited Glx peak consists primarily of glutamate, with little or no glutamine contribution.44,45
Conclusion
The major inhibitory neurotransmitter system represented by GABA is at the intersection of the pathophysiology of depression as well as mechanisms of its treatment. Transcranial magnetic stimulation over the left DLPFC improves depressive symptoms, and this is correlated with selective elevations of GABA in the MPFC, which has dense reciprocal connections with the DLPFC. This may indicate both restoration of GABA-ergic synapses as well as improved homeostatic balance with the potentially excitotoxic glutamatergic system. Future studies should elucidate whether these neurochemical changes mediate plasticity of the resting-state networks that show elevated functional connectivity in patients with depression.
Acknowledgements
The authors thank Rebecca Gordon and Jude Allen for technical assistance in administering transcranial magnetic stimulation and Rebecca Gordon for administering the Hamilton Rating Scales for Depression.
Footnotes
Funding: This work was supported by grants from the Brain and Behavior Research Foundation (National Alliance for Research on Schizophrenia and Depression Young Investigator Award) to M. Dubin and by funds from the Department of Psychiatry at Weill Cornell Medical College. C. Liston was supported by grants from the National Institutes of Health (K99 MH097822) and the DeWitt Wallace Reader’s Digest Foundation at Weill Cornell. These sources of funding had no further role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Competing interests: M. Dubin reports receiving a materials transfer from Neuronetics, Inc to complete this study, but received no compensation from Neuronetics. K. Lapidus serves on the advisory board for Halo Neuro, Inc., has received devices from Medtronic and Brainsway, and consults for LCN Consulting, Inc. No other competing interests declared.
Contributors: M. Dubin and D. Shungu designed the study. M. Dubin, X. Mao, Z. Goodman and D. Shungu acquired the data, which M. Dubin, X. Mao, S. Banerjee, K. Lapidus, G. Kang, C. Liston and D. Shungu analyzed. M. Dubin, S. Banerjee, K. Lapidus, C. Liston and D. Shungu wrote the article, which all authors reviewed and approved for publication.
References
- 1.O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–16. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34:522–34. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- 3.George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–16. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 4.Schutter DJLG. Antidepressant efficacy of high-frequency trans-cranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychol Med. 2009;39:65–75. doi: 10.1017/S0033291708003462. [DOI] [PubMed] [Google Scholar]
- 5.Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873–84. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- 6.Paus T, Castro-Alamancos MA, Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci. 2001;14:1405–11. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- 7.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 8.Fox MD, Buckner RL, White MP, et al. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liston C, Chen AC, Zebley BD, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–26. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salomons TV, Dunlop K, Kennedy SH, et al. Resting-state corticothalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology. 2014;39:488–98. doi: 10.1038/npp.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanacora G, Mason GF, Rothman DL, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–7. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 12.Sanacora G, Mason GF, Rothman DL, et al. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–5. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 13.Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160:577–9. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald PB, Fountain S, Daskalakis Z. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–96. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- 15.Price RB, Shungu DC, Mao X, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beam W, Borckardt JJ, Reeves ST, et al. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2009;2:50–4. doi: 10.1016/j.brs.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothman DL, Petroff OA, Behar KL, et al. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–6. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geramita M, van der Veen JW, Barnett AS, et al. Reproducibility of prefrontal g-aminobutyric acid measurements with J-edited spectroscopy. NMR Biomed. 2011;24:1089–98. doi: 10.1002/nbm.1662. [DOI] [PubMed] [Google Scholar]
- 19.Markwardt C. Non-linear least squares fitting in IDL with MPFIT 2008. Proc Astron Data Anal Softw Syst XVIII Astron Soc Pac. 2009;411:251–4. [Google Scholar]
- 20.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: Available: www.R-project.org/ [Google Scholar]
- 21.Rush AJ, Kraemer HC, Sackeim HA, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31:1841–53. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 22.Hasler G, van der Veen JW, Tumonis T, et al. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 23.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rush AJ, First MB, Blacker D. Handbook of psychiatric measures, second edition. Washington, DC: American Psychiatric Publishing, Inc; 2007. [Google Scholar]
- 25.Mann JJ, Oquendo MA, Watson KT, et al. Anxiety in major depression and cerebrospinal fluid free gamma-aminobutyric acid. Depress Anxiety. 2014;31:814–21. doi: 10.1002/da.22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maciag D, Hughes J, O’Dwyer G, et al. Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies. Biol Psychiatry. 2010;67:465–70. doi: 10.1016/j.biopsych.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson M, Weickert CS, Wyatt E, et al. Decreased glutamic acid decarboxylase67 mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43:970–7. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg DR, Macmaster FP, Mirza Y, et al. Reduced anterior cingulate glutamate in pediatric major depression: a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:700–4. doi: 10.1016/j.biopsych.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Todd KG, Baker GB. Neurochemical effects of the monoamine oxidase inhibitor phenelzine on brain GABA and alanine: a comparison with vigabatrin. J Pharm Pharm Sci. 2008;11:14s–21s. doi: 10.18433/j34s38. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter LL, Schecter JM, Tyrka AR, et al. Open-label tiagabine monotherapy for major depressive disorder with anxiety. J Clin Psychiatry. 2006;67:66–71. doi: 10.4088/jcp.v67n0110. [DOI] [PubMed] [Google Scholar]
- 31.Michael N, Erfurth A, Ohrmann P, et al. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol Med. 2003;33:1277–84. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Narr KL, Woods RP, et al. Glutamate normalization with ECT treatment response in major depression. Mol Psychiatry. 2013;18:268–70. doi: 10.1038/mp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X-R, Kirton A, Wilkes TC, et al. Glutamate alterations associated with transcranial magnetic stimulation in youth depression: a case series. J ECT. 2014;30:242–7. doi: 10.1097/YCT.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 34.Michael N, Gosling M, Reutemann M, et al. Metabolic changes after repetitive transcranial magnetic stimulation (rTMS) of the left prefrontal cortex: a sham-controlled proton magnetic resonance spectroscopy (1H MRS) study of healthy brain. Eur J Neurosci. 2003;17:2462–8. doi: 10.1046/j.1460-9568.2003.02683.x. [DOI] [PubMed] [Google Scholar]
- 35.Yizhar O, Fenno LE, Prigge M, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–8. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy SH, Evans KR, Krüger S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 38.Nobler MS, Oquendo MA, Kegeles LS, et al. Decreased regional brain metabolism after ECT. Am J Psychiatry. 2001;158:305–8. doi: 10.1176/appi.ajp.158.2.305. [DOI] [PubMed] [Google Scholar]
- 39.Hamani C, Mayberg H, Stone S, et al. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–8. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 40.Simister RJ, McLean MA, Salmenpera TM, et al. The effect of epileptic seizures on proton MRS visible neurochemical concentrations. Epilepsy Res. 2008;81:36–43. doi: 10.1016/j.eplepsyres.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex g-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–59. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- 42.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 43.Insel TR. The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–7. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 44.Shungu DC. Demystifying the “Glx” peak that co-edits with GABA by J-editing. Cardiff; Wales, UK: 2013. [Google Scholar]
- 45.Milak MS, Proper CJ, Mulhern ST, et al. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.83.. [DOI] [PMC free article] [PubMed] [Google Scholar]