Abstract
Background
Alterations in the innate immune/inflammatory system may underlie the pathophysiology of schizophrenia, but we do not understand the mechanisms involved. The main agents of innate immunity are the Toll-like receptors (TLRs), which detect molecular patterns associated with damage and pathogens. The TLR first reported was TLR4, and it is still the most studied one.
Methods
We aimed to describe putative modifications to the TLR4 proinflammatory pathway using 2 different strategies in 2 cohorts of patients with schizophrenia and matched controls: 1) quantification of protein and mRNA expression in postmortem prefrontal cortex samples from 30 patients with schizophrenia and 30 controls, and 2) identification of single nucleotide polymorphisms associated with the risk of schizophrenia using whole blood samples from 214 patients with schizophrenia and 216 controls.
Results
We found evidence of alterations in the expression of the initial elements of the TLR4 signalling pathway (TLR4, Myeloid differentiation primary response gene 88 [MyD88] and nuclear factor-κ B [NF-κB]) in the PFC of patients with schizophrenia. These alterations seem to depend on the presence/absence of antipsychotic treatment at death. Moreover, a polymorphism within the MyD88 gene was significantly associated with schizophrenia risk.
Limitations
The use of 2 different approaches in 2 different cohorts, the lack of a complementary neuropsychiatric group, the possible confounding effects of antipsychotic treatment and suicide are the main limitations of our study.
Conclusion
The evidence from this dual approach suggests there is an altered innate immune response in patients with chronic schizophrenia in which the TLR4 proinflammatory pathway could be affected. Improved understanding of the stimuli and mechanisms responsible for this response could lead to improved schizophrenia treatment and better control of the side effects of current antipsychotics.
Introduction
There is increasing evidence of activation of the innate immune system in psychosis, although the precise role this activation plays in the etiology, degree and evolution of the structural/functional brain alterations remains unclear.1,2
Innate immunity is a nonspecific homeostatic response; however, if uncontrolled, it becomes harmful.3 The response is initiated through the family of Toll-like receptors (TLRs), which are pattern recognition receptors that detect circulating pathogen-associated molecular patterns (PAMPs) that are present in pathogens but not in mammalian cells. These patterns trigger a complex proinflammatory cascade that can regulate central nervous system (CNS) homeostasis and even promote pathology.4 Toll-like receptors are highly expressed in immune cells5 and in different types of CNS cells.6 This ubiquitous distribution suggests that TLRs play other roles in non–pathogen-associated CNS diseases/injuries, presumably through recognizing a number of endogenous molecules released from damaged tissues (damage-associated molecular patterns [DAMPs]).7
The most studied member of the TLR family is TLR4, which mostly responds to lipopolysaccharide (LPS) from Gram-negative bacteria8 through its coreceptor: myeloid differentiation protein-2.9 Through recruiting some other adaptor proteins, such as the myeloid differentiation factor 88 (MyD88), TLR4 acquires specificity to intracellular signalling. After various consecutive steps in the transduction pathway (i.e., activation of specific kinases, such as interleukin [IL]1 receptor-associated kinase 1 [IRAK1]), the prototypic inflammatory nuclear factor-κ B (NF-κB) is activated. This in turn triggers expression of the enzymes inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) and the activation of cytokines, such as IL-1β and IL-6. The activation of these proinflammatory mediators in the brain can produce an accumulation of oxidative and nitrosative molecules, which can attack membrane phospholipids thereby causing cell damage via lipid peroxidation.10 There are endogenous counterbalancing mechanisms, such as activation of the anti-inflammatory nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ)11,12 (Appendix 1, Fig. S1, available at jpn.ca).
Recently, a peripheral imbalance of these pro-/anti-inflammatory pathways was reported in patients with first-episode psychosis (FEP) and in patients with chronic schizophrenia with an acutely exacerbated condition.13–15 However, only a few studies report altered TLR expression or response to immune stimuli in patients with schizophrenia.16,17
A recent update reviews the randomized controlled trials of the efficacy of anti-inflammatory compounds in patients with schizophrenia.18 However, the effects of long-term anti-psychotic treatment on the TLR4 signalling pathway and the putative therapeutic use of compounds capable of modulating this pathway remains largely unexplored.
Interestingly, results from several genome-wide association studies (GWAS) in patients with schizophrenia have reported significant associations in chromosome regions that contain different genes related to the immune system and inflammation.19–22 Although no genetic association studies include TLR4 in patietns with schizophrenia, an increased frequency of the TLR4 rs11536891 polymorphism was recently reported in patients with early-onset bipolar disorder compared with controls.23
Several circumstances provide good reasons to study TLR4 in schizophrenia populations specifically. First, some studies indicate that TLR4 may play a significant role in neurodevelopment and plasticity.24 Second, TLR4 could participate decisively in the priming alterations of the fetomaternal immune system after infection/stress.25 Third, an altered microbiome and signs of intestinal inflammation, increased intestinal barrier permeability and bacterial translocation are present in patients with schizophrenia.26 Finally, paliperidone regulates stress-induced activation of TLR4 in animal models.27
Taking into consideration all this background, in the present study we aimed to determine and report putative modifications of the TLR4 signalling pathway by using 2 different approaches: 1) quantification of protein and mRNA expression in postmortem prefrontal cortex (PFC) samples and 2) genetic association analysis to identify single nucleotide polymorphisms (SNPs) associated with schizophrenia.
Methods
Postmortem human brain studies
Sample
Human brain samples were obtained from autopsies (Basque Institute of Legal Medicine, Bilbao, Spain) in compliance with research policies and ethical committees for postmortem brain studies. After a retrospective search for antemortem medical information, we obtained postmortem PFC brain samples from 30 individuals who had a diagnosis of schizophrenia according to DSM-IV and from 30 controls matched for sex and age (± 4 yr) in a paired design. The criteria for selecting controls were absence of neuropsychiatric disorders and absence of drug abuse. We preferentially selected samples from controls with sudden and unexpected deaths in order to avoid possible confounding agonal influences. We screened blood from all sample donors to determine the presence of antipsychotics, other drugs and ethanol. According to the absence or presence of antipsychotic drugs in the toxicological screening, the schizophrenia samples (and their matched contols) were divided into 2 subgroups: antipsychotic-free (n = 17) and antipsychotic-treated (n = 13). Characteristics of the definitive schizophrenia–control pairs are shown in Appendix 1, Table S1. After completion of the assays, because the results of the 2 matched control subgroups did not differ for confounding factors (Appendix 1, Table S1), they were pooled for statistical analysis.
Specimens of PFC (Brodmann area [BA] 9) were dissected at autopsy (0.5–1 g tissue) following standard procedures.28 They were immediately stored at −80°C until assayed. Brain pH values were obtained at autopsy, and the RNA integrity number (RIN) was also assayed, as previously reported, as quality indexes.29 The group averages for these parameters are shown in Appendix 1, Table S1.
Preparation of nuclear and cytosolic extracts from tissue samples
We used a modified procedure based on the method of Schreiber and colleagues30 (see Appendix 1 for details).
Western blot analysis
To determine the expression levels of TLR4, MyD88, iNOS and COX-2, PFC samples were homogenized by sonication in phosphate-buffered saline (PBS) mixed with a protease inhibitor cocktail (Complete®, Roche Farma) (pH = 7), followed by centrifugation at 12 000g for 10 min at 4° C. In the case of NF-κB and PPARγ, nuclear extracts were analyzed, whereas for the inhibitory subunit of NF-κB, IκBα, cytosolic extracts were used. Detailed information on primary and secondary antibodies can be found in Appendix 1.
Real-time polymerase chain reaction analysis
We prepared total cytoplasmic RNA from the PFC samples using TRIzol (Invitrogen). Aliquots were converted to complementary DNA (cDNA) using random hexamer primers. Quantitative changes in messenger RNA (mRNA) levels were estimated using real-time polymerase chain reaction. We calculated relative mRNA concentrations from the take-off point of the reactions using the software provided; glyceraldehyde 3-phosphate dehydrogenase (GADPH) and tubulin levels were used to normalize the data. The sequences of the primers can be found in Appendix 1.
Lipid peroxidation
Lipid peroxidation was measured using the thiobarbituric acid test for malondialdehyde (MDA) following the method described by Das and Ratty31 (Appendix 1).
Nitrite levels
As the stable metabolites of nitric oxide, NO2-levels were measured using the Griess method (see Appendix 1 for details).
Statistical analysis
Data were analyzed using the GraphPad and InvivoStat programs. We processed each pair of samples for a case and matched control in parallel to control for experimental variance. Semiquantitative values for mRNA and protein were tested for a Gaussian distribution and compared using a Student unpaired t test and 1-way analysis of variance (ANOVA), followed by the Fisher least significant difference (LSD) post hoc test. Correlations were analyzed to evaluate the influence of the postmortem delay, storage time and body mass index (BMI). When positive, we tested the results using analysis of covariance (ANCOVA) with the corresponding factor as a covariate. The results are expressed as means ± standard errors of the mean. We considered results to be significant at p < 0.05.
Genetic association study
Participants
A total of 216 patients with schizophrenia diagnosed using the Structured Clinical Interview for DSM-IV (SCID-DSM-IV)32 were recruited at the Psychiatric Service of the Hospital Clínic, Barcelona, Spain. We excluded 2 patients from the study owing to technical genotyping problems, leaving 214 patients (139 men, 75 women, mean age 30.6 ± 11.3 yr) for our analysis. We selected 216 healthy controls matched for age and sex (140 men, 76 women, mean age 30.4 ± 11.2 yr) for the purpose of this study. A questionnaire was administered to each participant in an interview to elicit demographic information, smoking habits and personal medical history. Moreover, the clinical information was checked against clinical records. Controls were excluded if they reported a history of cancer or mental disorder. All the participants were white and had previously participated in other genetic studies of schizophrenia risk.33 We obtained written informed consent and blood samples from each participant, and the Ethics Committee of the Hospital Clínic approved the study protocol.
Sample preparation
We collected blood samples from participants in ethylene-diaminetetraacetic acid (EDTA; BD Vacutainer EDTA tubes, Becton Dickinson), and genomic DNA was extracted with the MagNA Pure LC DNA Isolation Kit III and an LC MagNA Pure system (Roche Diagnostics GmbH). We measured DNA concentration and quality spectrophotometrically using a NanoDrop 2000 (Thermo Fisher Scientific).
SNP selection and genotyping
We selected SNPs located in 9 genes known to be involved in the regulation of the TRL4 pathway, in accordance with data published in PubMed, Ensembl and the Genetic Association Database. Our aim was to find SNPs with functionality based on the consequence of the nucleotide change (e.g., missense), the location within the gene (e.g., regulatory region) or previously described associations (with protein levels, inflammatory processes or schizophrenia risk and other mental disorders; Table 1). We excluded SNPs with frequencies lower than 5%. Some SNPs were rejected before genotyping owing to the assay rules. A total of 22 SNPs (Table 1) were genotyped using the MassArray assay with the Sequenom genotyping system at the Santiago de Compostela node of the Spanish National Genotyping Centre (CeGen). For quality control, 10 samples were genotyped in duplicate for all the SNPs analyzed, with 100% concordance.
Table 1.
Twenty-two single nucleotide polymorphisms included in the genetic association study
| Gene | SNP | Chr. position | Gene position | Major/minor alleles | Minor allele frequency | References* |
|---|---|---|---|---|---|---|
| LBP | rs2232571 | 20:36974084 | 5′ upstream | T/C | 0.134 | 62 |
| CD14 | rs5744455 | 5:140013307 | 5′ upstream | C/T | 0.208 | 63 |
| rs5744441 | 5:140016847 | 5′ upstream | C/T | 0.213 | 64 | |
| TLR4 | rs4986790 | 9:120475302 | Nonsynonymous | A/G | 0.056 | 61 |
| rs4986791 | 9:120475602 | Nonsynonymous | C/T | 0.053 | 61 | |
| rs11536889 | 9:120478131 | 3′UTR | G/C | 0.144 | 65 | |
| MyD88 | rs4988453 | 3:38179254 | 5′ upstream | C/A | 0.042 | 66 |
| rs4988457 | 3:38182136 | Noncodifying exon | C/G | 0.043 | 66 | |
| rs7744 | 3:38184021 | 3′UTR | A/G | 0.153 | 50 | |
| IRAK4 | rs4251545 | 12:44180295 | Nonsynonymous | G/A | 0.110 | 67 |
| IRAK1 | rs3027898 | X:153275890 | 3′ downstream | A/C | 0.199 | 68 |
| NFKB1 | rs28362491 | 4:103422155 | 5′ upstream | ATTG/- | 0.332 | 69 |
| rs72696119 | 4:103422504 | 5′UTR | C/G | 0.329 | 70 | |
| rs2272676 | 4:103423326 | Splicing region | G/T | 0.274 | NR | |
| rs230529 | 4:103457418 | Intron region | G/A | 0.329 | 71 | |
| rs4699030 | 4:103503824 | Intron region | G/C | 0.338 | 71 | |
| rs148268461 | 4:103534740 | Splicing region | -/A | 0.053 | NR | |
| IL6 | rs1800796 | 7:22766246 | 5′ upstream | G/C | 0.069 | 72 |
| rs1800795 | 7:22766645 | 5′ upstream | G/C | 0.350 | 58 | |
| IL1B | rs1143634 | 2:113590390 | Synonymous | C/T | 0.227 | 58 |
| rs1143627 | 2:113594387 | 5′UTR | T/C | 0.378 | 73 | |
| rs16944 | 2:113594867 | 5′ upstream | G/A | 0.375 | 58 |
NR = no reference; SNP = single nucleotide polymorphism; UTR = untranslated region.
Includes 1 relevant reference on which the polymorphism selection was based. In cases of NR, the polymorphism was selected based just on its gene position. These references can be found in Appendix 1, available at jpn.ca.
Statistical analysis
Sample size and statistical power calculations were performed using Quanto 1.2 software. These calculations were based on allele frequencies in population genetics data from 1000 genomes (Ensembl). Given the sample size and assuming a 5% level of significance, we were able to detect odds ratios of 1.9–2.3 with more than 80% statistical power when we analyzed SNPs with allele frequencies of 0.05–0.4.
We calculated means and standard deviations for continuous variables. Univariate analysis (χ2 test for categorical variables and Student t test for continuous variables) was used to identify variables associated with the risk of schizophrenia using SPSS statistics software version 20 (IBM). No differences in age or sex were detected. However, we observed significant differences in smoking habits (50.0% of patients v. 37.9% of controls, p = 0.024). To account for this, the statistical analysis was adjusted accordingly.
After quality control of the genotyping, all the SNPs were included in the statistical analysis, as all of them showed consistent clustering and high genotyping rates (> 90% of samples), and none of them was out of Hardy–Weinberg equilibrium. To estimate the independent contribution of each SNP to schizophrenia risk, we assessed genotype frequencies by means of multivariate methods based on logistic regression analysis, with the analysis adjusted for variables significantly more common in patients with schizophrenia (smoking habits). For this, we used the SNPassoc R package.34 The haplotype analysis was also performed using the SNPassoc R package. We performed SNP interaction analysis using the multifactor dimensionality reduction (MDR), as described elsewhere,35 and the MDR 2.0 software, available from the open-source MDR project (www.epistasis.org/software.html). Further information on the methods used in the SNP interaction analysis can be found in Appendix 1.
To account for the multiple testing issue, we applied a Bonferroni correction (0.05/number of SNPs analyzed) for the single SNP analysis (after Bonferroni correction, results were considered to be significant at p < 0.002) and permutation for the interaction analysis (the p value of the best model was corrected for multiple testing by 1000 permutation cycles using the MDR Permutation Testing Module 1.0).
Results
TLR4 pathway in postmortem human brain samples of patients with schizophrenic and matched controls
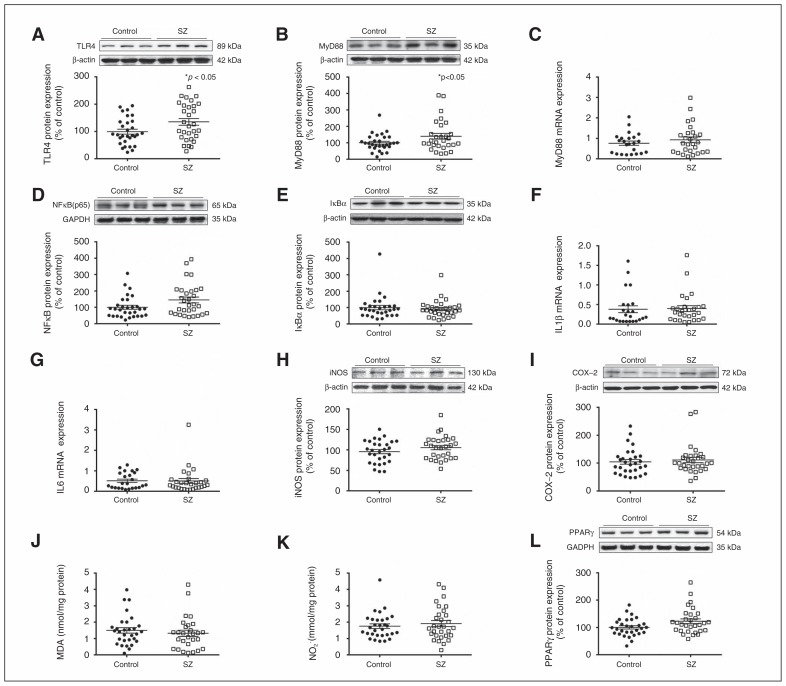
TLR4 and MyD88 protein expression were higher in the postmortem PFC samples of patients with schizophrenia than matched controls (Fig. 1A and B). In addition, TLR4 displayed a positive correlation with storage time. The subsequent ANCOVA, controlling for storage time, confirmed the elevated TRL4 expression in patients with schizophrenia versus controls (p = 0.010). MyD88 mRNA levels did not differ between the groups (Fig. 1C). NF-κB expression in nuclear extracts was also higher in patients with schizophrenia than controls (Fig. 1D), but there were no changes in the protein levels of IκBα in cytosolic extracts (Fig. 1E); IL-1β and IL6 mRNA levels were also the same for the control and schizophrenia groups (Fig. 1F and G).
Fig. 1.
Toll-like receptor-4 (TLR4) pathway in postmortem human brain samples from patients with schizophrenia (SZ) and matched controls. (A) TLR4 and (B) myeloid differentiation factor 88 (MyD88) protein levels. (C) Relative messenger RNA (mRNA) levels of MyD88. Protein levels of (D) nuclear factor κB (p65) and (E) inhibitory protein κBα. Messenger RNA levels of (F) interleukin (IL)1β and (G) IL6. Protein levels of (H) inducible nitric oxide synthase (iNOS), (I) cyclooxygenase-2 (COX-2), (J) malondialdehyde (MDA) and (K) nitrite levels (NO2). (L) Protein levels of peroxi-some proliferator-activated receptor γ (PPARγ) in the prefrontal cortex of patients with schizophrenia and matched controls. The densitometric data of the respective bands of interest are normalized with respect to β-actin/glyceraldehyde 3-phosphate dehydrogenase (lower band). *p < 0.05 compared with controls; unpaired Student t test analysis. Data represent the means ± standard errors of the mean.
Expression of the iNOS and COX-2 enzymes was unaltered in patients with schizophrenia compared with controls (Fig. 1H and I). Similarly, PFC samples from patients with schizophrenia did not present a significant change in the lipid peroxidation index MDA (Fig. 1J) or NO2 levels (Fig. 1K) when compared with controls.
In order to study counterbalancing mechanisms, we evaluated PPARγ expression. Western blot studies showed an increase in nuclear PPARγ protein levels in the PFC of patients with schizophrenia compared with controls (Fig. 1L). TLR4 pathway results were controlled for suicide, which was the cause of death of a large number of patients with schizophrenia. No influence was observed except for PPARγ protein levels, where the difference between patients with schizophrenia and controls was attenuated (F1,57 = 3.09, p = 0.08).
Effect of antipsychotic treatment on TLR4 pathway
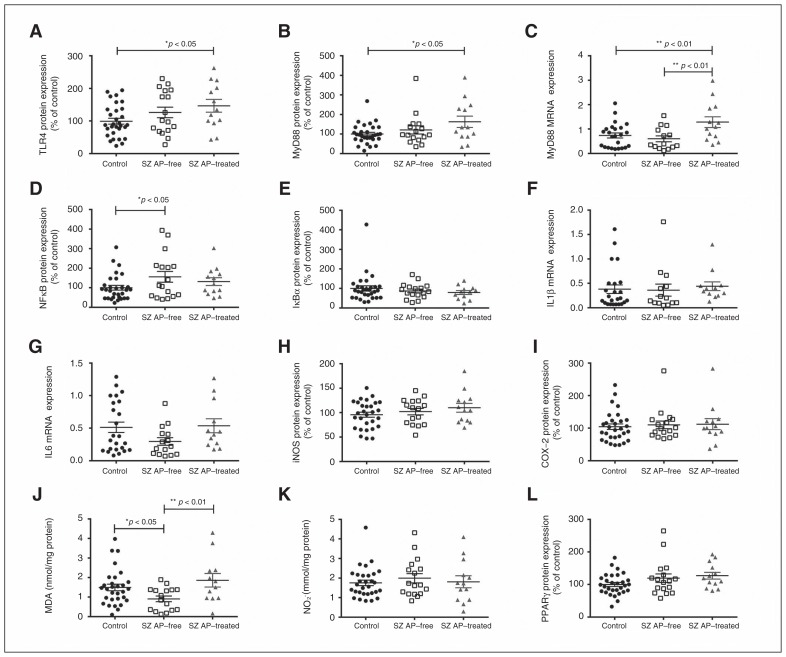
In order to determine the possible effect of antipsychotic treatment on the TLR4 proinflammatory pathway, patients with schizophrenia were divided into 2 groups: antipsychotic-free and antipsychotic-treated at time of death. The treated patients presented higher levels of TLR4 and MyD88 protein expression than controls (Fig. 2A and B). No significant differences were found between antipsychotic-free patients and controls or antipsychotic-treated patients in either parameter, although, especially for TLR4, the confidence interval for antipsychotic-free patients extended to the median of the treated patients. At the MyD88 mRNA expression level, treated patients presented higher levels than both control and antipsychotic-free patients, with no major differences between the latter 2 groups (Fig. 2C). This suggests that this effect could be not only medication-associated, but also genetically mediated.
Fig. 2.
Effect of antipsychotic treatment on Toll-like receptor-4 (TLR4) pathway in postmortem human brain samples of patients with schizophrenia (SZ) and matched controls. (A) TLR4 and (B) myeloid differentiation factor 88 (MyD88) protein levels. (C) Relative messenger RNA (mRNA) levels of MyD88. Protein levels of (D) nuclear factor κB (p65) and (E) inhibitory protein κBα. Messenger RNA levels of (F) interleukin (IL)1β and (G) IL6. Protein levels of (H) inducible nitric oxide synthase (iNOS) and (I) cyclooxygenase-2 (COX-2), (J) malondialdehyde (MDA) and (K) nitrite levels (NO2). Protein levels of (L) peroxisome proliferator-activated receptor γ (PPARγ) in the prefrontal cortex of patients with schizophrenia free from or treated with antipsychotics and matched controls. *p < 0.05, **p < 0.01; 1-way analysis of variance followed by the Fisher least significant difference post hoc test. Data represent the means ± standard errors of the mean.
Moreover, antipsychotic-free patients showed higher NF-κB expression than the control group (Fig. 2D). The remaining parameters studied in blood did not differ between the antipsychotic-free and treated patients at the time of death, except for MDA levels, which appeared lower in antipsychotic-free patients than controls and higher in antipsychotic-free patients than in treated patients (Fig. 2J). Post hoc differences in MDA levels between controls and antipsychotic-free patients (p = 0.020) were attenuated when we controlled for suicide (p = 0.06).
Genetic association study
The genotype frequencies of 3 SNPs differed between controls and patients with schizophrenia (Table 2). While 4.2% of controls were homozygous for the minor frequency allele of MyD88 rs7744, no cases were homozygous for the same allele (p = 0.001). Regarding IL6 rs1800795, about 20% more controls than patients with schizophrenia were carriers of the minor frequency allele (p = 0.007). Moreover, differences in genotype frequencies of IL1B rs1143634 were also found: while only 3.7% of controls were homozygous for the minor frequency allele, 7.5% of patients with schizophrenia were homozygous for the same allele (p = 0.037). MyD88 rs7744 was the only SNP that remained significantly associated with schizophrenia after Bonferroni correction. Haplotypes in MyD88 (rs4988453–rs4988457–rs7744) and IL6 (rs1800796–rs1800795) were nominally associated. The frequency of the MyD88 CCG haplotype, containing the minor frequency allele rs7744, was higher in controls than in patients with schizophrenia (18.5% v. 11.3%, p = 0.014). In the same way, the IL6 GC haplotype, containing the minor frequency allele rs1800795, was more common in controls than in patients with schizophrenia (18.5% v. 11.3%, p = 0.045). In both cases, the haplotype associations were no stronger than those found with the single polymorphisms alone. In fact, rs7744 and rs1800795 were responsible for the MyD88 and IL6 haplotype associations, respectively.
Table 2.
Significant genotype associations
| Group; no. (%) | ||||
|---|---|---|---|---|
|
|
||||
| SNP | Schizophrenia | Control | OR (95% CI) | p value* |
| rs7744 (MyD88) | ||||
| AA+AG | 214 (100.0) | 207 (95.8) | 1.00 | |
| GG | 0 (0.0%) | 9 (4.2) | 0.00 (0.0–0.0) | 0.001 |
| rs1800795 (IL6) | ||||
| GG | 105 (49.1) | 85 (39.4) | 1.00 | |
| CG+CC | 109 (50.9) | 131 (60.6) | 0.56 (0.5–0.9) | 0.007 |
| rs1143634 (IL1B) | ||||
| CC+CT | 198 (92.5) | 208 (96.3) | 1.00 | |
| TT | 16 (7.5) | 8 (3.7) | 2.59 (1.0–6.5) | 0.037 |
CI = confidence interval; OR = odds ratio; SNP = single nucleotide polymorphism.
Logistic regression analysis adjusted for smoking habit. Results were considered to be significant at p < 0.002 after Bonferroni correction.
The results of the exhaustive MDR analysis are given in Table 3. A 5-way model including the rs1800795 (IL6), rs7744 (MyD88), rs2272676 (NFKB1), rs1143627 (IL1B) and rs3027898 (IRAK1) variants performed best overall (training accuracy: 0.721; testing accuracy: 0.602) with a cross-validation consistency of 100/100 (permutation testing p = 0.010). The stepwise addition of the 5 variants increased the accuracy from 0.561 to 0.602. We constructed a multilocus attribute with the 5 SNPs. Carriers of the predisposing attribute (69.9% of cases v. 30.1% of controls) were almost 7 times more likely to develop schizophrenia than those without the attribute (odds ratio 6.9, 95% confidence interval 4.4–10.6, p < 0.001). The predisposing genetic attribute we constructed correctly predicted 76.9% of cases (sensitivity), 67.3% of controls (specificity) and 72.1% of cases and controls (accuracy). Moreover, 69.9% of predicted cases were actual cases (precision).
Table 3.
Results of the multifactor dimension reduction analysis showing the best model of all possible 1- to 5-loci interactions and their outcome parameters
| Model | Training accuracy* | Testing accuracy† | CVC‡ | p value§ |
|---|---|---|---|---|
| rs1800795 | 0.561 | 0.561 | 100/100 | 0.46 |
| rs2272676–rs3027898 | 0.586 | 0.420 | 63/100 | > 0.99 |
| rs1800795–rs2272676–rs3027898 | 0.616 | 0.421 | 61/100 | 0.39 |
| rs1800795–rs7744–rs2272676–rs3027898 | 0.661 | 0.406 | 44/100 | 0.99 |
| rs1800795–rs7744–rs2272676–rs1143627–rs3027898¶ | 0.721 | 0.602 | 100/100 | 0.020 |
CVC = cross-validation consistency.
Accuracy in the 100-fold training data set.
Accuracy in the 100-fold testing data set.
100-fold CVC.
p value of the best model corrected by 1000 permutations.
Odds ratio 6.9 (95% confidence interval 4.4–10.6), p < 0.001, sensitivity 76.9%, specificity 67.3%, accuracy 72.1%, precision 69.9%.
Discussion
In the present study, evidence of the involvement of an altered TLR4 pathway in patients with schizophrenia was found in both of the research approaches adopted. We considered it interesting to evaluate the TLR4 pathway at different stages, from genes to protein level, to strengthen the evidence of its role in the pathophysiology of schizophrenia.
In our search for polymorphisms, we tried to find possible etiological mechanisms, and through the postmortem study, we could check possible lifetime effects (including confounding factors, such as age, BMI or medication) on the state and response of the innate immune system in a chronic clinical manifestation of the disease.
First, we found evidence of higher expression of TLR4 and MyD88 in the postmortem PFC of patients with schizophrenia. TLR4 upregulation in this brain area has also been demonstrated in the PFC of alcoholic adolescents.36 In accordance with our results, NF-κB upregulation had previously been found in the postmortem PFC of patients with schizophrenia and in patients with other psychiatric diseases, such as depression or autism.37–39 Recently, meta-analysis of gene coexpression networks in the postmortem PFC of patients with schizophrenia and controls found quantitative and clustering alterations in the module including immunity-related genes, such as TLR2, CD14, MYD88 and NFKBIA.40
Previous studies demonstrated a peripheral imbalance in the TLR4 signalling pathway in patients with schizophrenia. Enhanced TLR-dependent responses were observed, producing a massive release of IL6 or TNF-α.16 In addition, other authors found increased TLR4 expression in the monocytes of patients with schizophrenia.17 Interestingly, these monocytes are less reactive to viral infection.
However, the origin of TLR4 activation in individuals with psychotic diseases remains unknown. Some nonexcluding mechanisms have been proposed. First, given the key role played by LPS in TLR4 stimulation, increased intestinal barrier permeability and bacterial translocation have been reported in patients with schizophrenia, as in those with major depression. Patients present an altered microbiome and, in some cases, intestinal inflammation.26 In addition, DAMPs are believed to be involved in the pathophysiology of psychotic disease,41 but their precise role remains unclear. Second, there is growing preclinical evidence that preperinatal infections can induce maternal immune activation and inflammatory and oxidonitrosative stress that can lead to neurodevelopmental damage and behavioural abnormalities in the progeny.25,42–45 In fact, activation of TLRs by infection has been reported to affect the fetomaternal immune response and cause behavioural abnormalities in descendants.44,45
Other possible sources of activation of the innate immune system have been recently suggested for major depressive disorder and could also be applicable to schizophrenia. These include psychosocial stressors, physical inactivity, obesity, smoking, poor dental care or overall hygiene and sleep deprivation.46 All these putative mechanisms of activation could activate other TLRs; this is especially relevant considering that the degree and type of inflammatory response could vary depending on the specific combinations of TLRs activated at the microglial level.47 This dynamic and heterogenic activation further complicates the study and elucidation of the role of TLR4 in physiologic/pathophysiological CNS conditions.
Efforts should be made to determine whether symptomatic onset of the disease occurs in a vulnerable brain (immunologically primed months or years before symptoms manifest) or whether symptoms that will increase the deleterious effects of inflammation or infection develop in genetically prone individuals.
It is worth discussing the differential results concerning the balance of pro-/anti-inflammatory pathways between the postmortem brain samples evaluated here and those previously reported at the systemic level in patients with FEPS and chronic schizophrenia.13–15 A significant increase in intracellular components of the NF-κB-dependent main proinflammatory pathway (iNOS, COX-2 expression, and NO2 and MDA levels), along with a significant decrease in the anti-inflammatory receptor PPARγ, have been reported in patients with FEPS and chronic schizophrenia. However, in our PFC samples, these proinflammatory parameters remained unaltered, and the expression of PPARγ increased. This switch in the pro-/anti-inflammatory balance suggests that the degree and progression of the inflammatory process together with the nature of its autoregulatory mechanisms vary in the different stages of the illness or the different organic compartments studied. Although mRNA expression of IL-1β and IL-6 was not altered in the postmortem PFC samples from patients with schizophrenia, a recent meta-analysis reported increased peripheral IL-1β and IL6 levels in acute psychosis that normalizes with antipsychotic treatment.48
In this particular clinical sample, the increased expression of TLR4/MyD88 seems to be predominantly influenced by the pharmacological treatment at the moment of death, which thus constitutes an important confounding factor. Although the anti-inflammatory/antioxidant role of anti-psychotics has previously been reported,49 their direct upstream effects on TLR4/MyD88 expression levels remained unexplored in human samples. Recent data in stress-based animal models show a potential regulatory role of paliperidone on TLR4 activation in the PFC.28
One of our most striking results is the higher content of MDA found in the PFC of patients with schizophrenia treated with antipsychotics compared with both antipsychotic-free patients and controls. Our results agree with previous findings that report a neurotoxic profile for high doses or chronic use of antipsychotics.50,51 Further studies are needed to extend these findings to other brain areas or degrees of psychotic disease.
The results we obtained in the genetic association approach also suggest that the TLR4 signalling pathway is involved in the etiology of schizophrenia. Our results could indicate that individuals homozygous for the MyD88 rs7744 minor allele (GG) may have protection from schizophrenia. There are only a few studies of the presence or functional role of this polymorphism in human disorders. MyD88 rs7744 has been associated with autoimmune or inflammatory processes,52–54 but its biological function has not yet been identified. As it is located in the 3′ untranslated region (UTR) region, MyD88 rs7744 could influence the stability of mRNA, thereby compromising normal protein levels. Moreover, it has been reported that an alternatively spliced form of MyD88 acts as a negative regulator of TLR4, which leads to a reduced inflammatory response.55 It may be that rs7744 was in linkage disequilibrium with another polymorphism that could influence this alternative splicing of the MyD88 gene. Either possibility would explain the reduced schizophrenia risk observed in individuals homozygous for the MyD88 rs7744 minor allele. Interestingly, MYD88 is located on chromosome 3p21.3-p22, a region previously linked with schizophrenia.56 More recently, several GWAS of schizophrenia have consistently reported positive signals in the 3p21 region, particularly in the ITIH family of genes,22,57,58 which codify for molecules that also seem to play an important role in inflammation.59
Our genetic results show that other genes related to the TLR4 signalling pathway could also be involved in schizophrenia. Nominal associations were found for IL6 rs1800795 and IL1B rs1143634. Interestingly, both polymorphisms have previously been associated with the disease.60–62 Moreover, IL6 rs1800795, located in the promoter region, has been associated with increased IL-6 blood levels61 and with reduced hippocampal volume in antipsychotic-naive patients with schizophrenia.63
We also propose a predictive genetic model for schizophrenia risk that includes the MyD88, IL6 and IL1B genes, as well as another 2 key genes in the intracellular signalling of the TLR4 pathway: NFKB1 and IRAK. The model identifies different genotype combinations of these 5 genes that allow the classification of patients with schizophrenia and healthy controls with high sensitivity and specificity.
Our genetic results suggest that polymorphisms in genes related to the TLR4 pathway play a role in the pathophysiology of schizophrenia, which would be in line with results from GWAS that report significant signals in regions that include different genes also related to the immune system and inflammation.19–22
Limitations
An important limitation of the present study was that the 2 approaches were applied in 2 different cohorts. No immune marker other than genetic ones could be examined in the genetic cohort because no suitable samples were collected for such a purpose. Then, in order to detect a possible genetic effect, we did not consider it appropriate to increase the heterogeneity by considering the 2 cohorts together, particularly taking into account the small increase of sample size that would have been achieved. In the future, when more postmortem samples have been collected, it will be interesting to assess the effect of the SNPs in that independent cohort.
At the moment, the significance of the higher expression of TLR4 and MyD88 in patients treated with antipsychotics compared with higher NF-κB in antipsychotic-free patients is difficult to interpret in a meaningful way. However, we must consider the fact that NF-κB is a master inflammatory molecule that could also be activated in mechanisms that are not directly related to the TLR4/MyD88 pathway.
In this vein, it is worth noting that the great majority of patients have experienced chronic treatment, even in the case of the antipsychotic-free group. The presence of a statistically non-significant trend toward elevated TLR4/MyD88 expression in antipsychotic-free patients could be interpreted in the context of a previous treatment period. With this type of postmortem brain samples, it is almost impossible to find medication-naive patients, but the toxicological screening that we performed guarantees no antipsychotic medication use in the month preceding death in antipsychotic-free patients.
Unfortunately, cytokine levels are studied only at the mRNA level, and there is a lack of concordance between the mRNA findings concerning cytokines and the genetic polymorphisms and haplotypes found. Further studies are required to check the functional consequences of these polymorphisms.
The predictive capacity of our genetic model for schizophrenia, which includes SNPs in 5 TLR4 pathway genes, would need to be validated in independent populations. It is important to highlight that MyD88 rs7744 and IL6 rs1800795 were the genetic variants that explained the highest percentage of the total entropy of the predictive model.
Finally, another limitation was the presence of several potentially confounding factors in some of the individuals included in the postmortem study, such as suicide as the cause of death.
Conclusion
The evidence from this dual approach suggests the existence of an altered innate immune response in patients with chronic schizophrenia, in which the TLR4 pathway could be one of those affected. Further research is needed to determine the state of the innate immune system in the different grades of psychotic disease (paying special attention to the potential confounding factor of antipsychotic treatment), the etiologic relevance of the polymorphisms found, and whether the pharmacological modulation of TLR4-dependent inflammatory and oxidonitrosative stress is useful for the management of the symptomatology and chronic treatment of psychotic pathologies.
Acknowledgements
This work was supported by the Instituto de Salud Carlos III (FIS 10/00123 and 13/1102); MINECO-FEDER Funds (SAF2013–48586-R); and regional authorities of Catalonia via the Secretaria d’Universitats i Recerca, Departament d’Economia i Coneixement (2014SGR441). The work was partly developed at the Centro Esther Koplowitz (Barcelona), CIBERSAM, and the Foundation Santander-UCM (GR 58/08). BGB is a Ramón y Cajal fellow (MINECO). The authors provide full disclosure of any and all biomedical financial interests and declare that there are no conflicts of interest. The authors thank staff of the Basque Institute of Legal Medicine, Bilbao for their cooperation in this work.
Footnotes
Competing interests: None declared.
Contributors: B. García-Bueno, P. Gassó and J. Leza designed the study. P. Gassó, A. Lafuente, K. MacDowell, S. Mas and J. Meana acquired the data, which B. García-Bueno, P. Gassó, L. Callado, M. Bernardo, A. Lafuente, J. Meana and J. Leza analyzed. B. García-Bueno, P. Gassó and J. Leza wrote the article, which all authors reviewed and approved for publication.
References
- 1.Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39:1174–9. doi: 10.1093/schbul/sbt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khandaker GM, Cousins L, Deakin J, et al. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2:258–70. doi: 10.1016/S2215-0366(14)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lampron A, Elali A, Rivest S. Innate immunity in the CNS: redefining the relationship between the CNS and Its environment. Neuron. 2013;78:214–32. doi: 10.1016/j.neuron.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Hanke ML, Kielian T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci (Lond) 2011;121:367–87. doi: 10.1042/CS20110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010 doi: 10.1155/2010/672395. pii:672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi O, Akira S. Toll-like receptors: their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625–35. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 9.Shimazu R, Akashi S, Ogata H, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madrigal JL, Garcia-Bueno B, Caso JR, et al. Stress-induced oxidative changes in brain. CNS Neurol Disord Drug Targets. 2006;5:561–8. doi: 10.2174/187152706778559327. [DOI] [PubMed] [Google Scholar]
- 11.García-Bueno B, Caso JR, Leza JC. Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev. 2008;32:1136–51. doi: 10.1016/j.neubiorev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Gárate I, Garcia-Bueno B, Madrigal JL, et al. Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biol Psychiatry. 2013;73:32–43. doi: 10.1016/j.biopsych.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 13.García-Bueno B, Bioque M, Mac-Dowell KS, et al. Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull. 2014a;40:376–87. doi: 10.1093/schbul/sbt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Bueno B, Bioque M, MacDowell KS, et al. Pro-/antiinflammatory dysregulation in early psychosis: results from a 1-year follow-up study. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu037. pii: pyu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez-Gras I, Pérez-Nievas BG, García-Bueno B, et al. The anti-inflammatory prostaglandin 15d-PGJ2 and its nuclear receptor PPARgamma are decreased in schizophrenia. Schizophr Res. 2011;128:15–22. doi: 10.1016/j.schres.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 16.McKernan DP, Dennison U, Gaszner G, et al. Enhanced peripheral toll-like receptor responses in psychosis: further evidence of a pro-inflammatory phenotype. Transl Psychiatry. 2011;1:e36. doi: 10.1038/tp.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller N, Wagner JK, Krause D, et al. Impaired monocyte activation in schizophrenia. Psychiatry Res. 2012;198:341–6. doi: 10.1016/j.psychres.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 18.Sommer IE, van Westrhenen R, Begemann MJ, et al. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40:181–91. doi: 10.1093/schbul/sbt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avramopoulos D, Pearce BD, McGrath J, et al. Infection and inflammation in schizophrenia and bipolar disorder: a genome wide study for interactions with genetic variation. PLoS One. 2015;10:e0116696. doi: 10.1371/journal.pone.0116696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia P, Wang L, Meltzer HY, et al. Common variants conferring risk of schizophrenia: a pathway analysis of GWAS data. Schizophr Res. 2010;122:38–42. doi: 10.1016/j.schres.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamshere ML, Walters JT, Smith R, et al. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDC-CAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol Psychiatry. 2013;18:708–12. doi: 10.1038/mp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliveira J, Busson M, Etain B, et al. Polymorphism of Toll-like receptor 4 gene in bipolar disorder. J Affect Disord. 2014;152–154:395–402. doi: 10.1016/j.jad.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 24.Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–81. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkatasubramanian G, Debnath M. The TRIPS (Toll-like receptors in immuno-inflammatory pathogenesis) hypothesis: a novel postulate to understand schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:301–11. doi: 10.1016/j.pnpbp.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Severance EG, Gressitt KL, Stallings CR, et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. 2013;148:130–7. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDowell KS, Caso J, Martin-Hernandez D, et al. Paliperidone prevents brain Toll-like receptor 4 pathway activation and neuroinflammation in rat models of acute and chronic restraint stress. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu070. pii:pyu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–22. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- 29.García-Sevilla JA, Alvaro-Bartolome M, Diez-Alarcia R, et al. Reduced platelet G protein-coupled receptor kinase 2 in major depressive disorder: antidepressant treatment-induced upregulation of GRK2 protein discriminates between responder and non-responder patients. Eur Neuropsychopharmacol. 2010;20:721–30. doi: 10.1016/j.euroneuro.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber E, Matthias P, Müller MM, et al. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das NP, Ratty AK. Studies on the effects of the narcotic alkaloids, cocaine, morphine, and codeine on nonenzymatic lipid peroxidation in rat brain mitochondria. Biochem Med Metab Biol. 1987;37:258–64. doi: 10.1016/0885-4505(87)90035-1. [DOI] [PubMed] [Google Scholar]
- 32.First MB, Frances AJ, Pincus HA, et al. DSM-IV in progress. Changes in substance-related, schizophrenic, and other primarily adult disorders. Hosp Community Psychiatry. 1994;45:18–20. doi: 10.1176/ps.45.1.18. [DOI] [PubMed] [Google Scholar]
- 33.Lafuente A, Bernardo M, Mas S, et al. -141C Ins/Del polymorphism of the dopamine D2 receptor gene is associated with schizophrenia in a Spanish population. Psychiatr Genet. 2008;18:122–7. doi: 10.1097/YPG.0b013e3282fb0019. [DOI] [PubMed] [Google Scholar]
- 34.González JR, Armengol L, Sole X, et al. SNPassoc: an R package to perform whole genome association studies. Bioinformatics. 2007;23:644–5. doi: 10.1093/bioinformatics/btm025. [DOI] [PubMed] [Google Scholar]
- 35.Moore JH, Gilbert JC, Tsai CT, et al. A flexible computational framework for detecting, characterizing, and interpreting statistical patterns of epistasis in genetic studies of human disease susceptibility. J Theor Biol. 2006;241:252–61. doi: 10.1016/j.jtbi.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 36.Young AM, Vetreno RP, Qin L, et al. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Front Psychiatry. 2011;2:27. doi: 10.1016/j.nbd.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao JS, Kim HW, Harry GJ, et al. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in the postmortem frontal cortex from schizophrenia patients. Schizophr Res. 2013;147:24–31. doi: 10.1016/j.schres.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malki K, Pain O, Tosto MG, et al. Identification of genes and gene pathways associated with major depressive disorder by integrative brain analysis of rat and human prefrontal cortex transcriptomes. Transl Psychiatry. 2015;5:e519. doi: 10.1038/tp.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young AM, Campbell E, Lynch S, et al. Aberrant NF-kappaB expression in autism spectrum condition: a mechanism for neuroinflammation. Front Psychiatry. 2011;2:27. doi: 10.3389/fpsyt.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mistry M, Gillis J, Pavlidis P. Meta-analysis of gene coexpression networks in the post-mortem prefrontal cortex of patients with schizophrenia and unaffected controls. BMC Neurosci. 2013;14:105. doi: 10.1186/1471-2202-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pae CU, Drago A, Kim JJ, et al. The impact of heat shock protein 70 gene variations on clinical presentation and outcome in schizophrenic inpatients. Neuropsychobiology. 2009;59:135–41. doi: 10.1159/000218075. [DOI] [PubMed] [Google Scholar]
- 42.Dhandapani A, Narayanaswamy JC, Venkatasubramanian G. Adjuvant raloxifene treatment for negative symptoms of schizophrenia. Asian J Psychiatr. 2013;6:254–5. doi: 10.1016/j.ajp.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Reisinger S, Khan D, Kong E, et al. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther. 2015;149:213–26. doi: 10.1016/j.pharmthera.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Abrahams VM, Bole-Aldo P, Kim YM, et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–96. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 45.De MJ, Yaddanapudi K, Hornig M, et al. Induction of Toll-like receptor 3-mediated immunity during gestation inhibits cortical neurogenesis and causes behavioral disturbances. MBio. 2010;1 doi: 10.1128/mBio.00176-10. pii: e00176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberger K, Derkow K, Dembny P, et al. The impact of single and pairwise Toll-like receptor activation on neuroinflammation and neurodegeneration. J Neuroinflammation. 2014;11:166. doi: 10.1186/s12974-014-0166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacDowell KS, Garcia-Bueno B, Madrigal JL, et al. Risperidone normalizes increased inflammatory parameters and restores anti-inflammatory pathways in a model of neuroinflammation. Int J Neuropsychopharmacol. 2013;16:121–35. doi: 10.1017/S1461145711001775. [DOI] [PubMed] [Google Scholar]
- 50.Elmorsy E, Elzalabany LM, Elsheikha HM, et al. Adverse effects of antipsychotics on micro-vascular endothelial cells of the human blood-brain barrier. Brain Res. 2014;1583:255–68. doi: 10.1016/j.brainres.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Pillai A, Parikh V, Terry AV, Jr, et al. Long-term antipsychotic treatments and crossover studies in rats: differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2007;41:372–86. doi: 10.1016/j.jpsychires.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Chen Z, Nakajima T, Inoue Y, et al. A single nucleotide polymorphism in the 3′-untranslated region of MyD88 gene is associated with Buerger disease but not with Takayasu arteritis in Japanese. J Hum Genet. 2011;56:545–7. doi: 10.1038/jhg.2011.44. [DOI] [PubMed] [Google Scholar]
- 53.Matsunaga K, Tahara T, Shiroeda H, et al. The *1244 A>G polymorphism of MyD88 (rs7744) is closely associated with susceptibility to ulcerative colitis. Mol Med Rep. 2014;9:28–32. doi: 10.3892/mmr.2013.1769. [DOI] [PubMed] [Google Scholar]
- 54.Potter C, Cordell HJ, Barton A, et al. Association between anti-tumour necrosis factor treatment response and genetic variants within the TLR and NF{kappa}B signalling pathways. Ann Rheum Dis. 2010;69:1315–20. doi: 10.1136/ard.2009.117309. [DOI] [PubMed] [Google Scholar]
- 55.Burns K, Janssens S, Brissoni B, et al. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med. 2003;197:263–8. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis CM, Levinson DF, Wise LH, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He K, Wang Q, Chen J, et al. ITIH family genes confer risk to schizophrenia and major depressive disorder in the Han Chinese population. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:34–8. doi: 10.1016/j.pnpbp.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Ripke S, O’Dushlaine C, Chambert K, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–9. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Opal SM, Lim YP, Siryaporn E, et al. Longitudinal studies of inter-alpha inhibitor proteins in severely septic patients: a potential clinical marker and mediator of severe sepsis. Crit Care Med. 2007;35:387–92. doi: 10.1097/01.CCM.0000253810.08230.83. [DOI] [PubMed] [Google Scholar]
- 60.Paul-Samojedny M, Kowalczyk M, Suchanek R, et al. Functional polymorphism in the interleukin-6 and interleukin-10 genes in patients with paranoid schizophrenia–a case-control study. J Mol Neurosci. 2010;42:112–9. doi: 10.1007/s12031-010-9365-6. [DOI] [PubMed] [Google Scholar]
- 61.Xu M, He L. Convergent evidence shows a positive association of interleukin-1 gene complex locus with susceptibility to schizophrenia in the Caucasian population. Schizophr Res. 2010;120:131–42. doi: 10.1016/j.schres.2010.02.1031. [DOI] [PubMed] [Google Scholar]
- 62.Zakharyan R, Petrek M, Arakelyan A, et al. Interleukin-6 promoter polymorphism and plasma levels in patients with schizophrenia. Tissue Antigens. 2012;80:136–42. doi: 10.1111/j.1399-0039.2012.01886.x. [DOI] [PubMed] [Google Scholar]
- 63.Kalmady SV, Venkatasubramanian G, Shivakumar V, et al. Relationship between interleukin-6 gene polymorphism and hippocampal volume in antipsychotic-naive schizophrenia: Evidence for differential susceptibility? PLoS One. 2014;9:e96021. doi: 10.1371/journal.pone.0096021. [DOI] [PMC free article] [PubMed] [Google Scholar]