Abstract
Background
We evaluated the diagnostic rate of diabetes using fasting plasma glucose (FPG), 2-hour plasma glucose (2h PG) after 75 g oral glucose tolerance test (OGTT), and glycosylated hemoglobin (HbA1c) levels, and we elucidated the pathophysiologic characteristics and risk factors that give rise to diabetes in patients with prediabetes.
Methods
The data of 236 patients who had the OGTT at Konkuk University Hospital were analyzed. Fasting, 30, and 120 minutes blood glucose levels and insulin levels were measured. The diagnostic rate of diabetes was assessed using FPG, 2h PG, and HbA1c levels. The clinical data and insulin resistance and secretion evaluations were compared using indexes according to the fasting glucose level.
Results
Among 236 subjects, 97 (41.1%) were diabetics and 102 (43.2%) were prediabetics. The rate of diabetes diagnosis by one of the individual criteria was 56.7%, 53.6%, and 84.5% for FPG, HbA1c, and 2h PG, respectively. When two criteria were used to diagnose diabetes, 72.2% of the diabetic patients were identified by FPG and HbA1c, while 100% were identified by FPG and 2h PG, and 91.7% were identified by 2h PG and HbA1c. The HbA1c cut-off value for 2h PG ≥200 mg/dL was 6.1%, and the FPG cut-off value was 115 mg/dL. In impaired fasting glucose subjects, the HbA1c level, Matsuda index, and insulinogenic index were associated with risk of occurrence of overt diabetes (P<0.01).
Conclusion
This study suggests that performing additional OGTT for patients with FPG ≥110 mg/dL or HbA1c ≥6.1% is helpful to reclassify their glucose tolerance status and evaluate their potential for progressing to overt diabetes.
Keywords: Diabetes mellitus, Diagnosis, Glucose tolerance test, Prediabetic state
INTRODUCTION
Diabetes mellitus (DM) and its related complications, such as cardiovascular diseases, acquired blindness, chronic kidney disease, and non-traumatic limb loss, are major causes of morbidity and mortality in Korea [1]. According to a recent report by the International Diabetes Federation, approximately 382 million people were diagnosed with diabetes in 2013, and it is predicted that this will increase to approximately 600 million by 2035 [2]. It was actually estimated that the number of people suffering from diabetes will reach 382 million by 2030 in 2004, but in fact, this number was already reached by 2013, indicating that the number of diabetic patients is increasing much faster than estimated [3]. Such a phenomenon has also been observed in Korea, where the prevalence of diabetes has risen from 8.9% to 12.4% from 2001 to 2010 [4]. Furthermore, the prevalence of prediabetes has also been reported to be 38.3%, implying that the increasing trend of diabetes will continue [4]. Additionally, more of the younger populations are now suffering from diabetes due to westernized lifestyles and eating habits, and there is an increasing rate of obesity, which suggests that diabetes will remain as a public health burden [5]. It has already been proven by multiple studies that strict blood glucose control is essential to prevent chronic complications of diabetes. Tight glycemic control through active intervention soon after diagnosis has been shown to prevent microvascular complications as well as macrovascular complications, denoting that early and active treatment is important [6,7]. In addition, identifying patients with impaired glucose tolerance (IGT) or impaired fasting glucose (IFG), which is a previous stage of diabetes, and inhibiting their further progression by taking preventive measures can also be another method to relieve the socioeconomic burden caused by diabetes [8].
The currently used methods to identify diabetes include the fasting plasma glucose (FPG) level test, oral glucose tolerance test (OGTT), and glycosylated hemoglobin (HbA1c) level test [9]. OGTT used to be the gold standard for diagnosing diabetes and prediabetes; however, it is now being used less because of its low reproducibility and time-consuming disadvantages [10]. However, the diagnostic value of OGTT now requires a re-evaluation considering the fact that diabetic patients with increasing postprandial glucose can be omitted due to the low concordance rate of the standards for diagnosing diabetes between FPG, 2-hour plasma glucose (2h PG) after 75 g OGTT, and HbA1c levels, while OGTT can reveal insulin resistance and β-cell dysfunction that are the fundamental pathophysiology of the state of diabetes [11]. Furthermore, it has been reported that the diagnosis of diabetes is difficult solely based on FPG and HbA1c levels because postprandial glucose increases before fasting glucose in Korean diabetic patients aged more than 60 years [12].
Here, we re-evaluated the diagnostic value of OGTT for diabetes and prediabetes with patients who underwent OGTT between September 2010 and September 2014. The necessity of OGTT for further classification of glucose dysregulation status was determined by investigating the differences in pathophysiologic characteristics of the occurrence of diabetes in patients with IFG according to their fasting glucose level.
METHODS
Study population
The data of patients who were reported to have a problem with FPG (≥100 mg/dL) during regular medical checkups and received an OGTT from September 2010 to September 2014 in the division of Endocrinology, Department of Internal Medicine, Konkuk University Hospital, were collected. Additionally, the data of 23 healthy volunteers who took the OGTT and 18 patients who received gestational diabetes mellitus treatment and subsequently took the OGTT at 6 to 8 weeks postpartum were included. Patients with a history of diabetes or currently undergoing medical therapy were excluded. Female patients who continued to use insulin syringes after gestational diabetes were also excluded. Informed consent was obtained from healthy volunteers, and the protocols were approved by the Konkuk University Hospital Institutional Review Board.
Measurements
Systolic and diastolic blood pressures were measured on the left upper arm after 5 minutes of rest in a sitting position using an automatic blood pressure monitor (HEM-907-E; OMROM, Tokyo, Japan). Abdominal circumference was measured in a standing position at the midway between the lower costal margin and the iliac crest. After fasting for 8 hours or more, blood was drawn from the antecubital vein of each participant. The samples were properly processed, refrigerated at 2℃ to 8℃, and analyzed within 24 hours of transportation. Fasting glucose was measured using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). High-performance liquid chromatography-723G7 (Tosoh, Tokyo, Japan) was used to check HbA1c. Insulin concentrations were estimated using an electrochemiluminescence method (COBAS E 411; Roche Diagnostics, Mannheim, Germany). The intra-assay and interassay coefficients of variation for the biochemical assays ranged between 3.1% and 7.6%. The blood levels of total cholesterol, triglycerides, high density lipoprotein, and low density lipoprotein cholesterol levels were measured using a Toshiba 200FR Autoanalyser (Toshiba Medical Systems Co. Ltd., Tokyo, Japan). High-sensitivity C-reactive protein was measured with an immunoturbidimetric method (CRP II Latex X2; Denka Seiken Co. Ltd., Tokyo, Japan) using an autoanalyzer (Toshiba).
Calculations
For evaluation of insulin resistance, the homeostasis model assessment of insulin resistance (HOMA-IR), the modified Matsuda index for whole body insulin sensitivity, and the Gutt insulin sensitivity index (ISI) were estimated as follows [13,14,15,16]:
β-Cell function was measured using the homeostasis model assessment of pancreatic β-cell function (HOMA-B), the insulinogenic index (IGI), and the oral disposition index (DI) by calculating as follows [13,17]:
| DI= Matsuda index×IGI |
Ins0, fasting plasma insulin (µIU/mL); Ins30, plasma insulin 30 minutes after glucose intake (µIU/mL); Ins120, plasma insulin 120 minutes after glucose intake (µIU/mL); Glc0, fasting plasma glucose (mg/dL); Glc30, plasma glucose 30 minutes after glucose intake (mg/dL); and Glc120, plasma glucose 120 minutes after glucose intake (mg/dL).
Total area under the curve (AUC) for insulin and AUC for glucose were calculated using the trapezoidal rule, and a ratio of the two was created (AUCins/glu). The glycemic status outcomes for this study were defined by the following American Diabetes Association criteria [9]: diabetes as FPG ≥126 mg/dL or 2h PG ≥200 mg/dL, or both; isolated IFG (iIFG) as FPG 100 to 125 mg/dL and 2h PG <140 mg/dL; isolated IGT (iIGT) as 2h PG after 75 g OGTT 140 to 199 mg/dL and FPG <100 mg/dL; NGT as FPG <100 mg/dL and 2h PG <140 mg/dL; and combined IFG and IGT (IFG+IGT) as FPG 100 to 125 mg/dL and 2h PG 140 to 199 mg/dL. Prediabetes was defined as iIFG, iIGT, or combined IFG and IGT.
Statistical analysis
All analyses were conducted using SPSS version 19.0 (IBM Co., Armonk, NY, USA). Data were presented as the mean±standard deviation for the continuous variables and the number of cases and as a percentage of the nominal variables. Statistical significance for comparisons between two groups was tested using the Mann-Whitney U test for continuous variables. Analysis of variance was used to compare the mean of three or more groups. Categorical variables were compared using Pearson chi-square test or Fisher exact test. The pointwise area under the receiver operating characteristic (ROC) curve was used to define the FPG, 2h PG, and A1c cut-off values for diagnosing diabetes. To identify the factors involved in overt diabetes mellitus in the subgroup whose FPG was between 100 and 125 mg/dL, logistic-regression models adjusted for body mass index (BMI), FPG, HbA1c, C-peptide, free fatty acid, triglycerides, Matsuda index, and IGI were used. Statistical significance was defined as a 2-tailed P<0.05.
RESULTS
Baseline characteristics
The baseline characteristics and metabolic parameters of the subjects are shown in Table 1. The study subjects included 122 men and 114 women. The subjects were divided into five groups according to the glucose tolerance state: NGT, iIFG, iIGT, IFG+IGT, and overt DM.
Table 1. Baseline characteristics and metabolic parameters of the subjects according to glucose tolerance status.
| Characteristic | NGT | iIFG | iIGT | IFG+IGT | Overt DM | P value |
|---|---|---|---|---|---|---|
| Number (total n=236) | 37 | 51 | 15 | 36 | 97 | |
| Male sex, % | 35.14 | 52.00 | 33.33 | 60.00 | 57.58 | 0.310 |
| FHx, % | 29.73 | 39.58 | 61.54 | 53.13 | 55.10a | 0.285 |
| Age, yr | 38.70±14.28 | 54.20±13.64a | 49.80±14.56a | 54.97±11.17a | 54.75±10.76a | 0.049 |
| BMI, kg/m2 | 22.57±3.58 | 24.49±3.79 | 24.08±2.34 | 25.33±3.13a | 25.83±3.05a | 0.053 |
| AC, cm | 76.97±9.91 | 87.56±9.00a | 85.94±6.25 | 88.48±8.23a | 89.85±7.35a | 0.383 |
| SBP, mm Hg | 118.27±15.29 | 125.67±15.05 | 121.73±12.67 | 122.64±15.46 | 128.18±15.33a | 0.175 |
| DBP, mm Hg | 73.51±11.11 | 73.71±11.10 | 72.47±6.55 | 72.91±10.68 | 77.36±10.03a | 0.043 |
| Glc0, mg/dL | 91.68±5.78 | 109.14±6.48a,c,e | 92.93±6.06b,d,e | 111.78±6.77a,c,d | 128.30±17.82a,b,c,d | <0.001 |
| Glc30, mg/dL | 147.79±25.03 | 178.30±42.00a,e | 168.71±23.52e | 191.77±25.43a,e | 219.41±36.57a,b,c,d | <0.001 |
| Glc120, mg/dL | 105.14±19.93 | 113.78±18.92c,d,e | 164.33±11.04a,b,e | 173.56±15.82a,b,e | 235.20±47.64a,b,c,d | <0.001 |
| Ins0, µIU/mL | 8.32±2.63 | 9.04±3.57e | 7.61±2.68 | 10.84±4.82 | 11.99±7.88a,b | 0.011 |
| Ins30, µIU/mL | 51.04±29.29 | 46.65±29.21 | 43.27±22.92 | 58.07±49.92e | 37.63±31.97d | 0.048 |
| Ins120, µIU/mL | 36.37±25.64 | 42.26±32.63d | 76.74±40.80 | 99.37±70.36a,b | 68.22±44.04a | 0.001 |
| HOMA-IR | 1.89±0.63 | 2.44±1.03e | 1.72±0.52e | 2.99±1.32 | 3.87±2.87a,b,c,d | <0.001 |
| Matsuda index | 7.03±2.70 | 5.92±2.36d,e | 4.81±1.60a | 4.02±1.97a,b | 3.84±1.54a,b | <0.001 |
| GUTT ISI | 50.89±9.97 | 44.74±12.51a,c,d,e | 31.10±4.21a,b | 27.74±7.17a,b | 23.45±5.77a,b | <0.001 |
| HOMA-B | 108.87±39.81 | 71.49±27.89a | 101.42±58.43e | 81.69±38.50a | 69.44±42.47a,c | 0.023 |
| IGI | 1.00±0.99 | 0.61±0.56a,e | 0.47±0.26a | 0.60±0.50a | 0.31±0.31a,b | 0.001 |
| DI | 6.26±6.08 | 2.94±2.92a,e | 2.54±0.80a | 1.49±0.63a | 0.96±0.65a,b | <0.001 |
| AUC Glc0–30 | 197.30±23.05 | 237.32±37.00a,e | 215.62±21.28d,e | 250.80±21.91a,b,e | 288.46±41.14a,b,c,d | <0.001 |
| AUC Ins0–30 | 890.26±461.85 | 842.63±472.99 | 766.37±381.13 | 1,031.22±755.56 | 736.81±544.48 | 0.107 |
| AUCRIns/Glc30 | 4.58±2.45 | 3.62±2.00 | 3.59±1.84 | 4.04±2.75 | 2.57±1.72a,d | 0.002 |
| AUC Glc0–120 | 823.11±109.32 | 960.91±146.96a,d,e | 1,039.09±93.79a,b,e | 1,150.66±93.16a,b,e | 1,422.50±197.59a,b,c,d | <0.001 |
| AUC Ins0–120 | 4,917.71±2,572.32 | 4,738.65±2,623.26d | 7,032.13±2,979.20 | 7,724.77±4,770.00a,b | 5,412.98±3,504.01 | 0.018 |
| AUCRIns/Glc120 | 6.10±3.23 | 5.10±2.73 | 6.79±2.92 | 6.50±3.77e | 3.92±2.55a,d | 0.001 |
| C-peptide, ng/mL | 1.65±0.56 | 1.98±0.59e | 1.82±0.42e | 2.37±0.92a | 2.50±0.88a,b,c | 0.001 |
| HbA1c, % | 5.53±0.35 | 5.80±0.41a,e | 6.00±0.38a,e | 6.05±0.43a,e | 6.59±0.58a,b,c,d | <0.001 |
| TC, mg/dL | 188.43±37.80 | 204.98±44.97 | 201.07±47.55 | 199.63±38.23 | 202.19±42.25 | 0.951 |
| TG, mg/dL | 82.33±41.30 | 128.98±88.94 | 154.71±103.63 | 172.03±164.49a | 153.63±88.13a | 0.317 |
| HDL-C, mg/dL | 60.13±15.28 | 56.46±15.82e | 50.79±11.85 | 51.91±12.45 | 49.92±12.00a,b | 0.045 |
| LDL-C, mg/dL | 109.75±31.08 | 126.80±42.89 | 116.77±33.70 | 119.78±31.61 | 128.33±36.35 | 0.561 |
| hs-CRP, mg/L | 0.11±0.15 | 0.15±0.22 | 0.10±0.12 | 0.09±0.09 | 0.13±0.17 | 0.445 |
Values are presented as mean±standard deviation.
NGT, normal glucose tolerance; iIFG, isolated impaired fasting glucose; iIGT, isolated impaired glucose tolerance (FPG <100 mg/dL, 140 mg/dL≤2h PG<200 mg/dL); DM, diabetes mellitus; FHx, family history of diabetes; BMI, body mass index; AC, abdominal circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; Glc0, fasting plasma glucose; Glc30, plasma glucose 30 minutes after glucose intake; Glc120, plasma glucose 120 minutes after glucose intake; Ins0, fasting plasma insulin; Ins30, plasma insulin 30 minutes after glucose intake; Ins120, plasma insulin 120 minutes after glucose intake; HOMA-IR, homeostasis model assessment of insulin resistance; Gutt ISI, Gutt insulin sensitivity index; HOMA-B, homeostasis model assessment of pancreatic β-cell function; IGI, insulinogenic index; DI, oral disposition index; AUC Glc, area under the curve for glucose; AUC Ins, area under the curve for insulin; AUCRIns/Glc, a ratio of the AUC Ins/AUC Glc; HbA1c, glycosylated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hs-CRP, high sensitivity C-reactive protein.
aP<0.05 vs. NGT, bP<0.05 vs. iIFG, cP<0.05 vs. iIGT, dP<0.05 vs. IFG+IGT, eP<0.05 vs. overt DM.
Among 236 subjects, 97 (41.1%) were diabetics, 102 (43.2%) were prediabetics (iIFG, iIGT, and IFG+IGT groups), and 37 (15.7%) had normal glucose tolerance. The mean age was higher in the prediabetes and overt DM group compared with the NGT group (P<0.05). BMI was high in the IFG plus IGT and overt DM groups compared with the NGT group (22.57±3.58, 25.33±3.13, and 25.83±3.05, respectively). The systolic and diastolic blood pressures were high in the overt DM group compared to the NGT group (P<0.05). The mean HbA1c levels in NGT, iIFG, iIGT, IFG+IGT, and overt DM subjects were 5.53±0.35, 5.80±0.41, 6.00±0.38, 6.05±0.43, and 6.59±0.58, respectively (P<0.001).
Insulin resistance (HOMA-IR, Matsuda index, Gutt ISI) and β-cell function (HOMA-B, IGI, DI) parameters showed significant differences in the prediabetes and overt diabetes groups compared with controls (NGT). The Matsuda index and Gutt ISI were lower in the combined IFG+IGT group and overt DM group compared with the iIFG group (Table 1).
Concordance rate among FPG, 2h PG, and HbA1c criteria for the diagnosis of diabetes
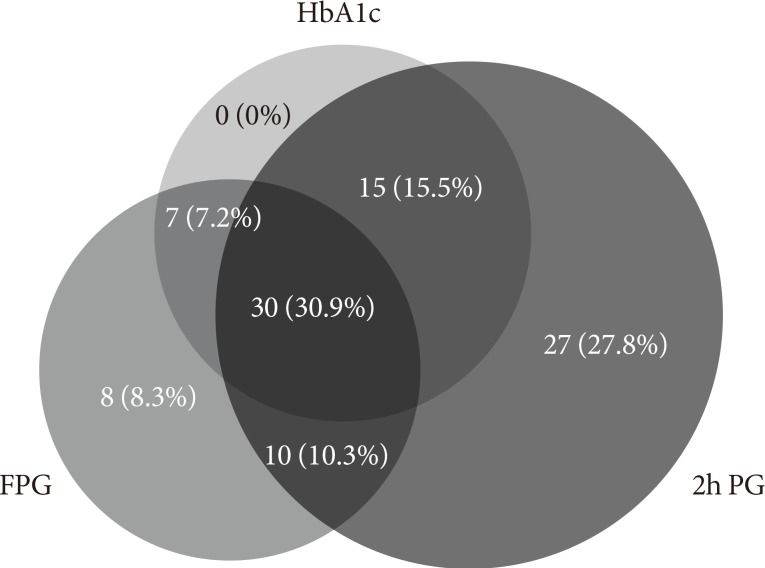
Among 97 diabetic patients, 30 patients (30.9%) satisfied all of the FPG, HbA1c, and 2h PG standards; 41.2% satisfied the FPG and 2h PG standards; 38.1% satisfied the FPG and HbA1c standards; and 46.4% satisfied the 2h PG and HbA1c standards. When only one of the individual criteria was used to diagnose diabetes, the rate of diabetes diagnosis was 56.7%, 53.6%, and 84.5% by FPG, HbA1c, and 2h PG, respectively (Fig. 1). When two of these criteria were used to diagnosis diabetes, 72.2% of diabetic patients were identified by FPG and HbA1c, while 100% were identified by FPG and 2h PG, and 91.7% were identified by 2h PG and HbA1c.
Fig. 1. The concordance rate between the three diagnostic criteria of diabetes: fasting plasma glucose (FPG) ≥126 mg/dL; 2-hour plasma glucose (2h PG) after 75 g oral glucose tolerance test ≥200 mg/dL; and glycosylated hemoglobin (HbA1c) ≥6.5%.
Optimal cut-off values of FPG, 2h PG, and HbA1C for detecting diabetes
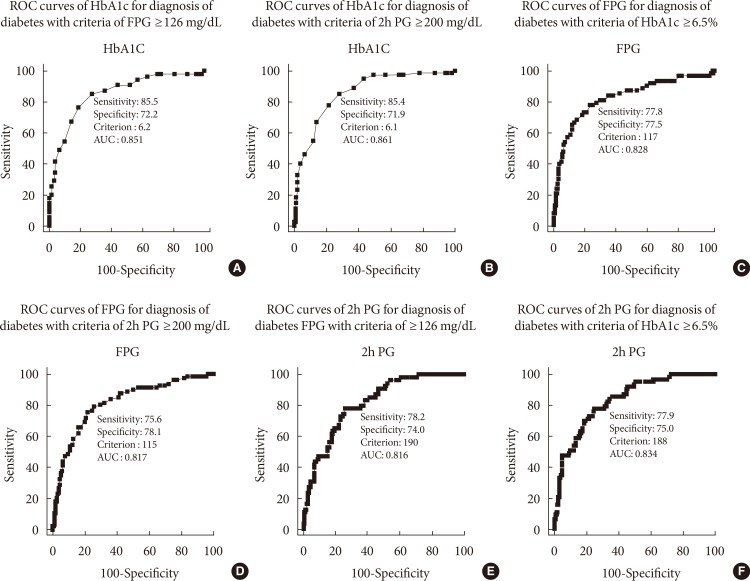
For the diagnosis of diabetes with criteria of FPG ≥126 mg/dL, an HbA1c cut-off value of 6.2% and 2h PG value of 190 mg/dL were required. For the diagnosis of diabetes with criteria of 2h PG ≥200 mg/dL, an HbA1c cut-off value of 6.1% and FPG value of 115 mg/dL were required. For the diagnosis of diabetes with criteria of HbA1c ≥6.5%, an FPG cut-off value of 117 mg/dL and 2h PG value of 188 mg/dL were required (Fig. 2).
Fig. 2. Receiver operating characteristic (ROC) curve of (A, B) glycosylated hemoglobin (HbA1c) and (C,D) fasting plasma glucose (FPG) levels for the diagnosis of diabetes with criteria of FPG ≥126 mg/dL or (E, F) 2-hour plasma glucose (2h PG) after 75 g oral glucose tolerance test ≥200 mg/dL or HbA1c ≥6.5%. AUC, area under the curve.
The differences in demographic and metabolic characteristics between each group after subdivision of IFG subjects according to FPG level
The IFG groups were subdivided into group 1 (100≤FPG<110) and group 2 (110≤FPG<126) according to previous Korean epidemiologic data [18] and were compared with the control group. Glucose dysregulation status, degree of insulin resistance, and β-cell dysfunction were worse in groups 1 and 2 compared with the normal controls. In the case of group 2, whose FPG level was higher than group 1, the postload 30 and 120 minutes glucose and HbA1c levels were higher; the portion of overt DM was also higher in group 2, with 38.5% compared with the 13.3% of group 1 (P=0.004) (Table 2). When comparing groups 1 and 2 from the point of view of the two basic pathophysiologies of the state of diabetes, insulin resistance represented by the HOMA-IR, Matsuda index, and Gutt ISI, did not show any difference. However, HOMA-B, IGI, and DI, which represent β-cell function, were statistically significantly lower in group 2 compared with group 1 (Table 2).
Table 2. Demographic and metabolic characteristics of the subjects with fasting plasma glucose between 100 and 125 mg/dL subdivided into two groups according to glucose level.
| Characteristic | NGT | Group 1 (100≤FPG<110) | Group 2 (110≤FPG<126) | P value |
|---|---|---|---|---|
| Number | 37 | 45 | 79 | |
| Male sex, n (%) | 13 (35.1) | 23 (51.1) | 45 (57.0) | 0.090 |
| FHx, % | 29.73 | 41.5a | 53.8b | 0.046 |
| Age, yr | 38.70±14.28 | 56.09±14.11a | 54.51±10.85b | <0.001 |
| BMI, kg/m2 | 22.57±3.58 | 24.03±3.44a | 25.84±3.09b,c | <0.001 |
| AC, cm | 76.97±9.91 | 86.23±9.18a | 89.02±7.27b | <0.001 |
| SBP, mm Hg | 118.27±15.29 | 125.71±17.20a | 124.94±14.09b | 0.054 |
| DBP, mm Hg | 73.51±11.11 | 73.11±12.48 | 75.16±10.18 | 0.556 |
| FPG Glc0, mg/dL | 91.68±5.78 | 104.38±5.78a | 116.3±4.70b,c | <0.001 |
| Glc30, mg/dL | 147.79±25.03 | 174.51±42.26a | 195.33±28.73b,c | <0.001 |
| Glc120, mg/dL | 105.14±19.93 | 151.73±53.02a | 179.28±37.29b,c | <0.001 |
| Ins0, µIU/mL | 8.32±2.63 | 10.16±2.63a | 10.29±4.50b | 0.046 |
| Ins30, µIU/mL | 51.04±29.29 | 51.41±29.70 | 46.47±37.80 | 0.708 |
| Ins120, µIU/mL | 36.37±25.64 | 67.67±44.76a | 69.48±57.39b | 0.007 |
| HOMA-IR | 1.89±0.63 | 2.62±1.15a | 2.95±1.30b | <0.001 |
| Matsuda index | 7.03±2.70 | 5.02±2.46a | 4.74±2.21b | <0.001 |
| GUTT ISI | 50.89±9.98 | 36.55±14.23a | 32.43±13.50b | <0.001 |
| HOMA-B | 108.87±39.81 | 88.35±36.48a | 69.95±31.11b,c | <0.001 |
| IGI | 1.00±0.99 | 0.69±0.49a | 0.46±0.48b,c | 0.001 |
| DI | 6.26±6.08 | 3.40±2.51a | 1.33±1.65b,c | <0.001 |
| AUC Glc0–30 | 197.30±23.05 | 229.86±35.41a | 256.93±25.03b,c | <0.001 |
| AUC Ins0–30 | 890.26±461.85 | 929.90±483.49 | 848.69±602.73 | 0.761 |
| AUCR30 | 4.58±2.45 | 4.05±1.92 | 3.31±2.29 | 0.021 |
| AUC Glc0–120 | 823.11±109.32 | 1021.42±198.68a | 1,170.95±199.03b,c | <0.001 |
| AUC Ins0–120 | 4,917.71±2,572.32 | 6,280.68±3,300.33 | 5,651.65±3,713.21 | 0.325 |
| AUCRIns/Glc | 6.10±3.23 | 6.10±2.67 | 4.86±3.09 | 0.117 |
| C-peptide, ng/mL | 1.65±0.56 | 2.11±0.71a | 2.35±0.90b | <0.001 |
| HbA1c, % | 5.53±0.35 | 5.88±0.47a | 6.14±0.43b,c | <0.001 |
| TC, mg/dL | 188.43±37.80 | 206.55±41.35 | 203.67±44.86b | 0.119 |
| TG, mg/dL | 82.33±41.30 | 159.66±151.30a | 147.03±86.42b | 0.002 |
| HDL-C, mg/dL | 60.13±15.28 | 54.96±13.51 | 52.21±14.22b | 0.025 |
| LDL-C, mg/dL | 109.75±31.08 | 126.53±39.05a | 128.77±39.52b | 0.075 |
| hs-CRP, mg/L | 0.11±0.15 | 0.10±0.10 | 0.13±0.16 | 0.671 |
| AST, IU/L | 20.38±5.61 | 26.86±10.13a | 27.64±9.34b | <0.001 |
| ALT, IU/L | 20.12±13.72 | 30.59±20.34a | 29.84±20.16b | 0.029 |
| Overt DM, % | - | 13.3 | 38.5c | 0.004 |
Values are presented as mean±standard deviation.
NGT, normal glucose tolerance; FPG, fasting plasma glucose; FHx, family history of diabetes; BMI, body mass index; AC, abdominal circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; Glc30, plasma glucose 30 minutes after glucose intake; Glc120, plasma glucose 120 minutes after glucose intake; Ins0, fasting plasma insulin; Ins30, plasma insulin 30 minutes after glucose intake; Ins120, plasma insulin 120 minutes after glucose intake; HOMA-IR, homeostasis model assessment of insulin resistance; Gutt ISI, Gutt insulin sensitivity index; HOMA-B, homeostasis model assessment of pancreatic β-cell function; IGI, insulinogenic index; DI, oral disposition index; AUC Glc, area under the curve for glucose; AUC Ins, area under the curve for insulin; AUCRIns/Glc, a ratio of the AUC Ins/AUC Glc; HbA1c, glycosylated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hs-CRP, high sensitivity C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; DM, diabetes mellitus.
aP<0.05 NGT vs. group 1, bP<0.05 NGT vs. group 2, cP<0.05 group 1 vs. group 2.
Risk factors associated with overt DM in a subgroup with FPG 100 to 125 mg/dL.
Patients who were diagnosed with prediabetes with a fasting glucose of 100 to 125 mg/dL and patients who were diagnosed with diabetes were compared for risk factors of diabetes (Table 3). We found that high HbA1c, high HOMA-IR, low IGI, and low DI were associated with the occurrence of diabetes even if the patients were within the same fasting glucose range (Table 3).
Table 3. Comparison of risk factors between prediabetes and overt diabetes in a subgroup whose fasting glucose was 100 to 125 mg/dL.
| Characteristic | Group 1 (prediabetes) |
Group 2 (overt diabetes) |
P value |
|---|---|---|---|
| Number | 87 | 36 | |
| Male sex, % | 54.0 | 55.6 | 1.00 |
| FHx, % | 45.1 | 58.3 | 0.231 |
| Age, yr | 54.52±12.56 | 57.111±10.27 | 0.375 |
| BMI, kg/m2 | 24.85±3.53 | 25.77±2.31 | 0.092 |
| AC, cm | 87.92±8.66 | 88.65±6.21 | 0.495 |
| SBP, mm Hg | 124.41±15.23 | 1,216.97±15.47 | 0.546 |
| DBP, mm Hg | 73.38±10.82 | 76.61±11.44 | 0.236 |
| Glc0, mg/dL | 110.23±6.70 | 116.17±6.23 | 0.000 |
| Glc30, mg/dL | 183.65±36.74 | 200.15±28.51 | 0.026 |
| Glc120, mg/dL | 138.51±34.45 | 243.36±31.65 | 0.000 |
| Ins0, µIU/mL | 9.79±4.20 | 11.05±4.67 | 0.121 |
| Ins30, µIU/mL | 51.19±37.90 | 37.57±21.81 | 0.120 |
| Ins120, µIU/mL | 63.37±55.92 | 81.59±45.17 | 0.028 |
| HOMA-IR | 2.67±1.18 | 3.16±1.31 | 0.022 |
| Matsuda index | 5.24±2.39 | 3.89±1.70 | 0.012 |
| GUTT ISI | 38.64±13.60 | 22.59±4.79 | 0.007 |
| HOMA-B | 75.76±32.93 | 76.60±36.22 | 0.987 |
| IGI | 0.61±0.53 | 0.34±0.30 | 0.007 |
| DI | 2.42±2.46 | 1.00±0.57 | 0.000 |
| AUC Glc0–30 | 242.68±32.38 | 260.79±26.18 | 0.010 |
| AUC Ins0–30 | 918.56±605.37 | 721.88±368.18 | 0.176 |
| AUCR30 | 4.05±1.92 | 3.31±2.29 | 0.047 |
| AUC Glc0–120 | 1,036.33±158.17 | 1,363.18±138.63 | 0.000 |
| AUC Ins0–120 | 5,772.31±3,754.30 | 6,035.24±3,208.23 | 0.476 |
| AUCRIns/Glc | 5.58±3.16 | 4.51±2.52 | 0.194 |
| C-peptide, ng/mL | 2.14±0.76 | 2.53±0.95 | 0.028 |
| HbA1c, % | 5.90±0.43 | 6.40±0.40 | 0.000 |
| TC, mg/dL | 202.78±42.17 | 208.75±47.23 | 0.555 |
| TG, mg/dL | 146.71±126.05 | 162.77±79.45 | 0.048 |
| HDL-C, mg/dL | 54.59±14.63 | 49.94±12.02 | 0.124 |
| LDL-C, mg/dL | 123.93±38.59 | 136.91±40.15 | 0.144 |
| hs-CRP, mg/L | 0.12±0.18 | 0.12±0.12 | 0.199 |
Values are presented as mean±standard deviation.
FHx, family history of diabetes; BMI, body mass index; AC, abdominal circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG Glc0, fasting plasma glucose; Glc30, plasma glucose 30 minutes after glucose intake; Glc120, plasma glucose 120 minutes after glucose intake; Ins0, fasting plasma insulin; Ins30, plasma insulin 30 minutes after glucose intake; Ins120, plasma insulin 120 minutes after glucose intake; HOMA-IR, homeostasis model assessment of insulin resistance; Gutt ISI, Gutt insulin sensitivity index; HOMA-B, homeostasis model assessment of pancreatic β-cell function; IGI, insulinogenic index; DI, oral disposition index; AUC Glc, area under the curve for glucose; AUC Ins, area under the curve for insulin; AUCRIns/Glc, a ratio of the AUC Ins/AUC Glc; HbA1c, glycosylated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hs-CRP, high sensitivity C-reactive protein.
To uncover the variables related to overt diabetes in the IFG group, we performed a multiple logistic regression analysis and discovered that the levels of HbA1c, Matsuda Index, and IGI were statistically significantly correlated with overt diabetes (Table 4).
Table 4. Logistic regression of risk factors associated with overt diabetes mellitus in the subgroup whose fasting pasma glucose was 100 to 125 mg/dL.
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| Odds ratio (CI) | P value | Odds ratio (CI) | P value | |
| BMI, kg/m2 | 1.093 (0.97–1.24) | 0.157 | - | - |
| FPG, mg/dL | 1.14 (1.07–1.22) | <0.001 | - | - |
| HbA1c, % | 22.75 (6.12–84.59) | <0.001 | 19.57 (2.40–159.41) | 0.005 |
| C-peptide, ng/mL | 1.70 (1.06–2.72) | 0.027 | - | - |
| TG, mg/dL | 1.00 (0.99–1.01) | 0.490 | - | - |
| Matsuda index | 0.73 (0.55–0.96) | 0.022 | 0.60 (0.39–0.93) | 0.022 |
| IGI | 0.254 (0.008–0.82) | 0.022 | 0.054 (0.003–0.912) | 0.043 |
BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; TG, triglyceride; IGI, insulinogenic index.
DISCUSSION
The results showed that it is significantly more accurate to use OGTT in addition to FPG and HbA1c tests when diagnosing diabetes. In addition, we also showed that even if the blood glucose level is within the IFG range, β-cell dysfunction progresses with increasing FPG levels, which in turn raises the possibility of having overt diabetes. Furthermore, we found that the level of HbA1c, Matsuda index, and IGI are associated factors that increase the risk of having overt diabetes.
In this study, we confirmed the results of previous studies that claimed the low concordance rate between the standards of the three methods used to diagnose diabetes [18,19,20]. This low concordance rate may be partly due to low mean HbA1c levels (6.5%±0.58%). In diabetic patients with more than a 9% HbA1c level, the diagnostic concordance rate between FPG, 2h PG, and HbA1c level has been reported to be relatively high [21]. However, we could not assess the concordance rate of the three methods according to HbA1c levels because of the low number of patients with an HbA1c level >7%.
A total of 97 diabetic patients who were diagnosed by either FPG or 2h PG with OGTT were analyzed, and within these subjects, only 41.2% were diagnosed with diabetes by both FPG and 2h PG, which is similar to the results from a pooled analysis in a previous Korean epidemiological study that reported 46%. Additionally, only 30.9% (n=30) of the patients matched the criteria for all three methods, which was similar to the 31.9% reported in previous research based on a Korean population [12]. These results directly indicated the low concordance rate of the criteria of the three methods; therefore, diagnosing patients with diabetes based on only one of these methods will lead to omitting a large portion of other diabetic patients. In another point of view, when diabetes was classified based on FPG and HbA1c without 2h PG, 27.8% of the patients were misclassified as non-diabetic patients, which was much higher than the 0% or 8.3% misclassification by 2h PG and FPG or 2h PG and HbA1c, respectively, denoting the importance of OGTT for a more accurate diagnosis. It was previously reported that an increase in 2h PG after 75 g OGTT was observed before an increase in fasting glucose levels in old-aged diabetic patients in Korea [12]. Considering this, although the convenience for diabetic patients is important, OGTT will likely be required in certain cases.
To identify differences in diagnostic values, we used a ROC curve to calculate the optimal cut-off values for each diabetes diagnosis method. The appropriate HbA1c thresholds for FPG ≥126 mg/dL and 2h PG ≥200 mg/dL were 6.2% and 6.1%, respectively, which are both lower than the currently used threshold of 6.5%. These data are in agreement with previous Korean or American data [12,19,22]. Thus, the current HbA1c criteria have high specificity but low sensitivity, leading to approximately one-third of the patients being misclassified. Consequently, individuals with HbA1c levels higher than 6.1% should additionally have the OGTT to more precisely determine their glucose regulation status and receive more appropriate treatments. In the case of FPG, the appropriate thresholds for HbA1c ≥6.5% and 2h PG ≥200 mg/dL were 117 and 115 mg/dL, respectively, which are both ~10 mg/dL lower than the currently used 126 mg/dL. This implies that even if the FPG level is within the IFG range, a further HbA1c check or OGTT is required if the FPG level is on the edge.
Previously, we showed that the appropriate FPG threshold for 2h PG of 200 mg/dL was 110 mg/dL by a previous pooled analysis of Korean epidemiology data [23] and that when the IFG group was further subdivided into stage 1 IFG (FPG 100 to 109 mg/dL) and stage 2 IFG (FPG 110 to 125 mg/dL), stage 2 IFG had a poorer metabolic profile and a higher percentage of people with diabetes by OGTT [18]. Based on this method, we also subdivided the IFG in this study into subgroup 1 (FPG 100 to 109 mg/dL) and subgroup 2 (FPG 110 to 125 mg/dL) and compared them with each other and the normal control group. We found that in subgroup 2, which had a higher FPG level, the prevalence of diabetes was higher (38.5% vs. 13.3%, P=0.004). In addition, although there were no significant differences in insulin resistance parameters, HOMA-B, IGI, and DI, which represent β-cell function, were statistically significantly worse in subgroup 2. This indicated that β-cell function, which is the fundamental basis for the progression of diabetes and plays an important pathophysiologic role in Asians, including Koreans, can be different between individuals depending on their level of FPG despite being in the IFG range [24,25]. Consequently, different diagnostic and treatment approaches could be required for subgroups 1 and 2.
By performing a multiple logistic regression analysis in the IFG group for the potential risk factors included in overt DM criteria after having an OGTT, we showed that HbA1c, the Matsuda index, and IGI showed significant correlations. Thus, OGTT can be utilized to measure a patient's insulin resistance status and β-cell function to predict the possibility of further diabetes progression and plan a suitable follow-up schedule and education for each patient.
Several limitations exist for this study. First, the number of study participants was low and data were collected from only one hospital, so the applicability of this study to the general population can be narrow. Therefore, further studies should be undertaken to confirm that it is more accurate to use OGTT in addition to FPG or HbA1c tests when diagnosing diabetes.
Second, it is very likely that an IFG patient will additionally have an OGTT due to the customary scheme of a regular medical checkup. This leads to the possibility of a selection bias where patients in an isolated IGT or overt diabetes group with normal FPG and high 2h PG might not be included. In our study, when diabetes was classified based on OGTT, no patient or only 8.3% of the patients, were misclassified as non-diabetic patients by 2h PG and FPG or 2h PG and HbA1c, respectively. The consequence of this is that it underestimates the percentage of diabetic patients diagnosed with OGTT, so we speculated that it would have little impact on the overall conclusion of this study.
Third, we did not check hemoglobin (Hb) and hematocrit levels in all subjects. While iron deficiency anemia can increase HbA1c levels in subjects with NGT or prediabetes, hemolytic anemia may decrease the HbA1c value [26,27]. Thus, the Hb level may affect the diagnosis of diabetes based on the HbA1c level.
In conclusion, the concordance rate of the criteria between each method that is used to diagnose diabetes is low. Therefore, considering the risk of chronic diabetic complications due to delayed detection, performing an additional OGTT during regular check-ups for patients with high FPG or HbA1c (FPG ≥110 mg/dL, HbA1c ≥6.1%) to reclassify their glucose regulation status and further evaluate their potential of progressing to overt diabetes in order to provide a more suitable individualized management is required.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Task Force Team for Basic Statistical Study of Korean Diabetes Mellitus of Korean Diabetes Association. Park Ie B, Kim J, Kim DJ, Chung CH, Oh JY, Park SW, Lee J, Choi KM, Min KW, Park JH, Son HS, Ahn CW, Kim H, Lee S, Lee IB, Choi I, Baik SH. Diabetes epidemics in Korea: reappraise nationwide survey of diabetes "diabetes in Korea 2007". Diabetes Metab J. 2013;37:233–239. doi: 10.4093/dmj.2013.37.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Jeon JY, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, Song KH, Won JC, Lim S, Choi SH, Jang MJ, Kim Y, Oh K, Kim DJ, Cha BY Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Prevalence of diabetes and prediabetes according to fasting plasma glucose and HbA1c. Diabetes Metab J. 2013;37:349–357. doi: 10.4093/dmj.2013.37.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus: present and future perspectives. Nat Rev Endocrinol. 2012;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 7.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 8.Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, Hamman RF, Ackermann RT, Engelgau MM, Ratner RE Diabetes Prevention Program Research Group. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005;142:323–332. doi: 10.7326/0003-4819-142-5-200503010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl 1):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 10.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 11.Bartoli E, Fra GP, Carnevale Schianca GP. The oral glucose tolerance test (OGTT) revisited. Eur J Intern Med. 2011;22:8–12. doi: 10.1016/j.ejim.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Lee H, Oh JY, Sung YA, Kim DJ, Kim SH, Kim SG, Moon S, Park Ie B, Rhee EJ, Chung CH, Kim BJ, Ku BJ. Optimal hemoglobin A1C cutoff value for diagnosing type 2 diabetes mellitus in Korean adults. Diabetes Res Clin Pract. 2013;99:231–236. doi: 10.1016/j.diabres.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Matsuda M. Reduced time points to calculate the composite index. Diabetes Care. 2010;33:e93. doi: 10.2337/dc10-0646. [DOI] [PubMed] [Google Scholar]
- 16.Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, Schneiderman N, Skyler JS, Marks JB. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000;47:177–184. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 17.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, Leonetti DL, McNeely MJ, Fujimoto WY, Kahn SE. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32:335–341. doi: 10.2337/dc08-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh JY, Lim S, Kim DJ, Kim NH, Kim DJ, Moon SD, Jang HC, Cho YM, Song KH, Ahn CW, Sung YA, Park JY, Shin C, Lee HK, Park KS Committee of the Korean Diabetes Association on the Diagnosis and Classification of Diabetes Mellitus. A report on the diagnosis of intermediate hyperglycemia in Korea: a pooled analysis of four community-based cohort studies. Diabetes Res Clin Pract. 2008;80:463–468. doi: 10.1016/j.diabres.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Kramer CK, Araneta MR, Barrett-Connor E. A1C and diabetes diagnosis: the Rancho Bernardo Study. Diabetes Care. 2010;33:101–103. doi: 10.2337/dc09-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu ZX, Walker KZ, O'Dea K, Sikaris KA, Shaw JE. A1C for screening and diagnosis of type 2 diabetes in routine clinical practice. Diabetes Care. 2010;33:817–819. doi: 10.2337/dc09-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 22.Kim JM, Hong JW, Won JC, Noh JH, Ko KS, Rhee BD, Kim DJ. Glycated hemoglobin value for fasting plasma glucose of 126 mg/dL in Korean: the 2011 Korea National Health and Nutrition Examination Survey. Diabetes Metab J. 2014;38:480–483. doi: 10.4093/dmj.2014.38.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh JY, Lim S, Kim DJ, Kim NH, Kim DJ, Moon SD, Jang HC, Cho YM, Song KH, Park KS Committee of the Korean Diabetes Association on the Diagnosis and Classification of Diabetes Mellitus. The diagnosis of diabetes mellitus in Korea: a pooled analysis of four community-based cohort studies. Diabet Med. 2007;24:217–218. doi: 10.1111/j.1464-5491.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 24.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 25.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- 26.English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58:1409–1421. doi: 10.1007/s00125-015-3599-3. [DOI] [PubMed] [Google Scholar]
- 27.Hong JW, Ku CR, Noh JH, Ko KS, Rhee BD, Kim DJ. Association between the presence of iron deficiency anemia and hemoglobin A1c in Korean adults: the 2011-2012 Korea National Health and Nutrition Examination Survey. Medicine (Baltimore) 2015;94:e825. doi: 10.1097/MD.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]