Abstract
With therapeutic successes and improved survival after a cancer diagnosis in childhood, increasing numbers of cancer survivors are at risk of subsequent treatment-related morbidities, including cataracts. While it is well known that the lens of the eye is one of the most radiosensitive tissues in the human body, the risks associated with radiation doses less than 2 Gy are less understood, as are the long- and short-term cataract risks from exposure to ionizing radiation at a young age. In this study, we followed 13,902 five-year survivors of childhood cancer in the Childhood Cancer Survivor Study cohort an average of 21.4 years from the date of first cancer diagnosis. For patients receiving radiotherapy, lens dose (mean: 2.2 Gy; range: 0–66 Gy) was estimated based on radiotherapy records. We used unconditional multivariable logistic regression models to evaluate prevalence of self-reported cataract in relationship to cumulative radiation dose both at five years after the initial cancer diagnosis and at the end of follow-up. We modeled the radiation effect in terms of the excess odds ratio (EOR) per Gy. We also analyzed cataract incidence starting from five years after initial cancer diagnosis to the end of follow-up using Cox regression. A total of 483 (3.5%) cataract cases were identified, including 200 (1.4%) diagnosed during the first five years of follow-up. In a multivariable logistic regression model, cataract prevalence at the end of follow-up was positively associated with lens dose in a manner consistent with a linear dose-response relationship (EOR per Gy = 0.92; 95% CI: 0.65–1.20). The odds ratio for doses between 0.5 and 1.5 Gy was elevated significantly relative to doses <0.5 Gy (OR = 2.2; 95% CI: 1.3–3.7). The results from this study indicate a strong association between ocular exposure to ionizing radiation and long-term risk of pre-senile cataract. The risk of cataract increased with increasing exposure, beginning at lens doses as low as 0.5 Gy. Our findings are in agreement with a growing body of evidence of an elevated risk for lens opacities in populations exposed to doses of ionizing radiation below the previously suggested threshold level of 2 Gy.
INTRODUCTION
With improved cancer treatment and increased life expectancy over recent decades, the number of long-term cancer survivors in the U.S. is growing, having increased from 1.5% in 1971 (1) to 3.1% in 2011, reaching over 13 million individuals (2). Although the majority of cancer cases in the U.S. occur in people ages 65 and older, the number of pediatric cancers is increasing. As of 2011, there were estimated to be over 388,501 childhood cancer survivors in the U.S. (2), and this number is expected to grow, along with the concomitant morbidities associated with treatment (3).
A major element in the treatment and improved survival of childhood cancers is radiotherapy (4), including cranial or craniospinal radiotherapy. While used with curative intent, radiation therapy is nevertheless associated with an increased risk of adverse outcomes such as second cancers and sensory or psychosocial problems (5). Specifically, it has been reported that childhood cancer survivors are at higher risk of neurosensory complications, including ocular diseases (6). One of the most investigated ocular sequelae of ionizing radiation exposure are cataracts (7). Since a child’s visual system is immature and develops during the initial years of life, the consequences of future visual deprivation caused by cataracts and aphakia in children can be important and include anisometropia, aniseikonia and lack of accommodation (8). Moreover, compared to adults, pediatric cataract surgery tends to be more complicated, requiring additional treatments to decrease the postoperative inflammation, secondary glaucoma, refractive instability of the developing eye and early and severe visual axis opacification. With respect to radiation-associated sequelae, most of the reported studies in pediatric cancer patients (9) were limited by relatively small sample sizes and a lack of quantitative assessment of lens dose.
Whelan et al. (10) evaluated specific cancer treatments and risk of developing several different types of late ocular complications five or more years after diagnosis in the Childhood Cancer Survivor Study (CCSS). They found an increased risk of cataract at doses greater than 2 Gy to the eye. Recently, several studies have suggested that cataracts are associated with doses below 2 Gy (11, 12), suggesting that cataracts should more correctly be classified as stochastic rather than deterministic outcomes, as had been thought in the past (13). The current study aimed to extend the previous CCSS cataract analysis (10) in terms of follow-up period and to further characterize the dose-response relationship between radiotherapy and occurrence of cataract, with a particular focus on risk at lens doses under 2 Gy.
METHODS
Study Population
The CCSS cohort study is comprised of five-year survivors of childhood cancer diagnosed before age 21 at any of 26 institutions in the U.S. and Canada between January 1, 1970 and December 31, 1986 and who participated in one or more surveys administered over a span of nearly two decades (n = 14,357) (14, 15). Eligible types of first cancer included leukemia, central nervous system (CNS) cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, kidney cancer (Wilms’ tumor), neuroblastoma, soft tissue sarcoma and bone sarcoma. The CCSS study protocol and summaries of cohort characteristics are available at: http://ccss.stjude.org. At study entry (1992–1994), all eligible participants were sent a 24-page self-administered baseline questionnaire that included questions on demographic variables, cataracts and other medical conditions, surgical procedures and multiple other health outcomes. For eligible participants who were known to have died after 5 years of follow-up, their next of kin were asked to complete the baseline questionnaire. These initial questionnaires were completed between 1992 and 2002. Of all initial participants, 8,013 also completed a follow-up questionnaire in 2007 with questions regarding cataract status. Copies of the baseline and follow-up questionnaires are available at: http://ccss.stjude.org. A comparison of the 7,748 patients with the 2007 questionnaire, which corresponds to nonresponders (excluding patients who died) indicates minor differences in mean age at cancer diagnosis (7.9 vs. 8.3 years), sex (49.7 vs. 58.1% males) and type of cancer. All of these differences were small (η2 < 0.01 or Cramer’s V < 0.1) and do not suggest an important response bias.
Excluded from the current analysis were children who were diagnosed with cataract prior to their first cancer diagnosis (n = 82), became legally blind before their first primary cancer diagnosis (n = 253), were of unknown cataract or blindness status at baseline (n = 11) or had missing baseline data on age at cataract diagnosis (n = 58). Because an unknown number of persons in the latter group may have had their cataracts diagnosed before their cancer, we elected to exclude the entire group rather than impute missing ages. Cancer survivors with missing documentation about radiotherapy (yes/no) were also excluded from the analysis (n = 51). The total eligible study population included 13,902 subjects. Those known to have had radiotherapy but with insufficient data to assess radiation dose (n = 230) were included in the overall analysis and classified as having unknown dose. Of the eligible study participants, 7,748 also completed the 2007 follow-up questionnaire.
For each study participant, detailed information about cancer treatment was obtained from the medical records. Radiotherapy records were photocopied and sent to the CCSS Radiation Dosimetry Center at the University of Texas MD Anderson Cancer Center. Chemotherapy information was abstracted according to a standardized protocol and included beginning and ending dates for all chemotherapy agents and doses (mg/m2) and routes of administration for 22 specific agents. Abstract forms are available at: http://ccss.stjude.org.
Outcome
The primary outcome measure for these analyses was the self-reported diagnosis of cataract among study participants using the baseline questionnaire and the 2007 follow-up questionnaire. The question on cataracts was phrased: “Have you ever been told by a doctor or other health care professional that you have, or have had, cataract”. For a “yes” response, the respondent was asked to indicate the age at first diagnosis of a cataract. In the event that a survivor reported a cataract but did not report the age at first occurrence in either the first (n=58) or follow-up questionnaire (n=45), the date of cataract diagnosis was set to the last day of follow-up. In the 2007 follow-up questionnaire, study participants were also asked whether they had a previous cataract surgery and its date. Self-reported cataract surgery was also accepted as evidence of having had cataract(s).
Radiation Dosimetry
Cutoff dates for radiation treatment accrual were set at 5 years after the date of diagnosis of the first primary cancer. This cutoff date was chosen because some childhood survivors may have been treated later than 5 years at a different institution; such later treatments generally would have been unknown to study investigators. Therefore, to quantify radiation exposure, right and left lens doses were calculated for all cancer survivors who underwent radiotherapy in the five years after diagnosis of their first primary cancer. Dose-response analysis was based on the maximal dose to either the left or the right lens because questions regarding cataract were not specific to the left or right eye. Dose reconstruction was performed by the collaborating medical physicists using radiotherapy records. Captured data included dates of therapy and information on beam energy, field location (diagrams and/or photos if available) and size, presence of field blocks, age at treatment and total dose. As previously described (16), doses were determined using a calculation model based on measurements (in and out of beam) in a water phantom. When the eyes were entirely outside the treatment field, e.g., eye relative to a mantle field, the dose was based on the calculation model, which takes into account the distance of the organ from the field edge, field size and energy. For patients whose records noted that the eyes or lenses were in the beam and blocked, a 10% block transmission factor was applied. For whole-brain irradiated patients, both eyes were assumed to have been blocked based on diagrams in their individual records, including those from the early 1970s; the eye blocking in diagrams was consistent with whole-brain portal images in a standard reference textbook from that era (17). Despite the considerable effort given to dose reconstruction, some uncertainty persists with respect to possible dosimetric error in the lower dose range (e.g., <0.5 Gy). Evaluation of potential dose misclassification was included in sensitivity analysis, as described below.
Statistical Analysis
Both prevalence analyses, using unconditional logistic regression models, and incidence analyses, using Cox proportional hazards models, were conducted. For prevalence analyses, the time referents were: 1. the fifth anniversary of the date of childhood cancer diagnosis; and 2. the end of the follow-up period. Prevalence odds ratios (ORs) at five years after the initial cancer diagnosis were estimated because of the CCSS cohort eligibility requirement that childhood cancer patients survive a minimum of five years. Duration of survival and cataract occurrence among otherwise eligible childhood cancer patients who did not survive five years are unknown, as this information was not recorded. Incidence analysis was used to estimate hazard ratios (HRs) for follow-up times beginning five years after the initial cancer diagnosis. The end of follow-up was the earliest date of cataract diagnosis (or cataract surgery), date of loss of one or both eyes (or becoming legally blind), date of death or date of last questionnaire.
Cumulative incidence of cataract beginning five years after the date of initial cancer diagnosis was calculated according to a method described previously (18), with adjustment for competing risk of death or blindness [RStudio 0.97.551, Rogue Wave Software (bit.ly/1TYNEnW)]. To account for cataracts that occurred among this cohort of five-year survivors, we calculated the cumulative proportion affected at end of follow-up, namely, the prevalence at five years after diagnosis of the initial cancer plus the cumulative incidence thereafter.
Cataract risk factors other than exposure to ionizing radiation were evaluated as follows. Chemotherapy drugs were categorized into major groups (epipodophyllotoxins, cytosine arabinoside, anthracyclines, platinum-based compounds, methotrexate, alkylating agents, bleomycin) and administered dose tertiles of each agent in chemotherapy categories were calculated for each patient (19). Answers regarding alcohol intake were categorized as: (a) never (or none for >2 years); (b) some alcohol in the last two years, but <5 drinks per month; (c) 5–16 drinks per month and (d) 17 or more drinks per month. A single drink of alcohol was considered to be equivalent to a glass of wine, a can of beer or a shot of mixed spirits. Smoking status was categorized into never smokers (smoked <100 cigarettes during their lifetime), former smokers (smoked ≥100 cigarettes lifetime and currently not smoking or unsure about their smoking status) and current smokers. Other potential risk factors included self-reported history of diabetes and ever use of corticosteroids, a significant risk factor for the development of posterior subcapsular cataract (20).
We used the IBM SPSS for Windows (version 22.0) for unconditional logistic regression modeling to evaluate cataract prevalence and the PEANUTS program in Epicure (HiroSoft, Seattle, WA) (21) to fit Cox’s proportional hazards regression models for cataract incidence among five-year survivors. Attained age was used as the primary time scale. In these analyses, the effect of radiation dose to the lens was modeled using a linear prevalence excess odds ratio (EOR) and an excess relative risk (ERR) term. Indicator variables for the potential confounders that were associated with changes in the relative risk point estimates in a multivariable model (sex, age at diagnosis of first cancer, type of first cancer, diabetes and chemotherapy) were included in the models as conventional log-linear relative risk terms. Dose was included both as a continuous and categorical variable, with a dose of <0.5 Gy as the reference category. Potential modifiers of radiation-related risk were included as product terms with radiation dose. We assessed goodness-of-fit of the regression models using the likelihood ratio test.
RESULTS
The mean age of the study population at time of diagnosis of the first cancer was 8.3 years, and male survivors accounted for 53.7% of the cohort. Leukemia was the most common first cancer (n = 4,736) followed by Hodgkin lymphoma (n = 1,900) and tumors of the CNS (n = 1667) (Table 1). The median ionizing radiation dose to the lens of the eye among all irradiated patients (n = 7,792) was 2.8 Gy (range 0–66 Gy), with the highest median dose among irradiated survivors of soft tissue sarcoma (6.5 Gy), CNS cancer (4.5 Gy) and leukemia (3.2 Gy) (Table 2).
TABLE 1.
Baseline Characteristics of the Study Population and Numbers of Cataract Cases (Prevalent at Five Years and Incident Thereafter) among Five-Year Survivors of Childhood Cancer in the Childhood Cancer Survivor Study
| Number | Percentage (%) | Cataract cases |
||
|---|---|---|---|---|
| Total | 13,902 | 100.0 | 483 | |
| Type of first cancer | Leukemia | 4,736 | 34.1 | 275 |
| Soft tissue sarcoma | 1,187 | 8.5 | 60 | |
| Central nervous system | 1,667 | 12.0 | 48 | |
| Non-Hodgkin lymphoma | 1,059 | 7.6 | 32 | |
| Neuroblastoma | 940 | 6.8 | 20 | |
| Hodgkin lymphoma | 1,900 | 13.7 | 29 | |
| Bone sarcoma | 1,170 | 8.4 | 12 | |
| Kidney (Wilms’ tumor) | 1,243 | 8.9 | 7 | |
| Age (in years) at first cancer diagnosis | <1 | 971 | 7.0 | 27 |
| 1–<5 | 4,581 | 33.0 | 195 | |
| 5–<10 | 3,093 | 22.2 | 113 | |
| 10–<15 | 2,820 | 20.3 | 71 | |
| 15–21 | 2,437 | 17.5 | 77 | |
| Cumulative follow-up time (in years) since first cancer diagnosis |
<5 | 13,902 | 100.0 | 200 |
| 5–<15 | 13,480 | 97.0 | 162 | |
| 15–<25 | 9,988 | 74.1 | 64 | |
| 25–38 | 5,001 | 50.1 | 57 | |
| Age (in years) at end of follow-up | <15 | 1,350 | 9.7 | 237 |
| 15–<25 | 3,236 | 23.3 | 122 | |
| 25–<35 | 4,828 | 34.7 | 66 | |
| 35–58 | 4,488 | 32.3 | 58 | |
| Sex | Male | 7,462 | 53.7 | 259 |
| Female | 6,440 | 46.3 | 224 | |
| Education | 1–8 years | 2,478 | 17.8 | 107 |
| Some high school | 2,150 | 15.5 | 64 | |
| Completed high school | 5,835 | 42.0 | 189 | |
| Completed college | 2,044 | 14.7 | 79 | |
| Post-graduate | 669 | 4.8 | 15 | |
| Missing | 726 | 5.2 | 29 | |
| Body mass index | Underweight (<18.5 kg/m2) | 1,627 | 11.7 | 64 |
| Normal (18.5–<25 kg/m2) | 7,137 | 51.3 | 243 | |
| Overweight (25–<30 kg/m2) | 2,951 | 21.2 | 98 | |
| Obese class I (30–<35 kg/m2) | 960 | 6.9 | 34 | |
| Obese class II/III (≥35 kg/m2) | 432 | 3.1 | 15 | |
| Missing | 795 | 5.7 | 29 | |
| Smoking | Never | 10,063 | 72.4 | 377 |
| Current | 2,112 | 15.2 | 46 | |
| Former | 1,193 | 8.6 | 36 | |
| Missing | 534 | 3.8 | 24 | |
| Diabetes status | No | 13,355 | 96.1 | 440 |
| Yes | 249 | 1.8 | 24 | |
| Missing | 298 | 2.1 | 19 | |
| Radiotherapy | No | 5,735 | 41.3 | 84 |
| Yes | 8,167 | 58.7 | 399 | |
| Use of corticosteroids at therapy | Dexamethasone | 970 | 7.0 | 49 |
| Prednisone | 5,755 | 41.4 | 271 | |
| Hydrocortisone | 652 | 4.7 | 23 | |
| Chemotherapy drugs | Epipodophyllotoxins | 941 | 6.8 | 57 |
| Cytosine arabinoside | 3,039 | 21.9 | 205 | |
| Anthracyclines | 4,817 | 34.6 | 248 | |
| Platinum compounds | 640 | 4.6 | 25 | |
| Methotrexate | 5,545 | 39.9 | 277 | |
| Alkylating agents | 6,314 | 45.4 | 269 | |
| Bleomycin | 688 | 4.9 | 4 |
TABLE 2.
Dose to the Lens (Gy) According to Type of Primary Cancer among Patients Receiving Radiotherapy
| Dose (Gy) | |||||
|---|---|---|---|---|---|
| Cancer type | Numbera | Mean | Median | Minimum | Maximum |
| Leukemia | 2,727 | 0.05 | 3.18 | <0.001 | 30.6 |
| Soft tissue sarcoma | 593 | 0.58 | 6.53 | <0.001 | 66.0 |
| Central nervous system | 943 | 0.14 | 4.54 | 0.022 | 43.8 |
| Non-Hodgkin lymphoma | 602 | 0.20 | 2.33 | 0.002 | 58.0 |
| Neuroblastoma | 386 | 0.28 | 1.58 | 0.002 | 57.2 |
| Hodgkin lymphoma | 1519 | 0.02 | 1.46 | 0.006 | 12.7 |
| Bone cancer | 348 | 0.24 | 0.94 | <0.001 | 61.2 |
| Kidney (Wilms’) | 674 | 0.01 | 0.16 | 0.012 | 4.5 |
| Total | 7,792 | 0.06 | 2.76 | <0.001 | 66.0 |
Including 145 patients who first received radiation exposure after the cutoff date.
During the study period, there were 483 cataract cases reported among 13,902 study participants (3.5%); 200 were diagnosed during the first five years after first cancer diagnosis and 283 after five years. During the entire study period, the median time from date of first cancer diagnosis to the onset of cataracts was 9.6 years, with a maximum of 37 years. If the 58 subjects that were excluded from the study because of missing baseline data on age at cataract diagnosis were included, the proportion with cataracts would increase to 3.9% (=541/13,960). A total of 113 cataract surgeries were reported among participants who completed the 2007 follow-up questionnaire (31% of the patients in the group who reported cataracts).
Table 3 shows the prevalence of cataract at five years and end of follow-up period, as well as incidence density per 1,000 person-years (PY) from 5 years forward. Among patients with available dose assessment (n = 13,672), lens dose was associated with an increased prevalence of cataract at the end of follow-up. The unadjusted prevalence increased from 1.3% among patients exposed to less than 0.5 Gy, to 6.1% among survivors exposed to 2.50–3.49 Gy, and reached 40.6% for the highest dose group (20–60 Gy), with a prevalence OR of 57.0 (34.4–94.2) compared to patients exposed to less than 0.5 Gy.
TABLE 3.
Multivariable Models for Prevalence of Cataract at Five Years after Initial Cancer Diagnosis and End of Follow-Up and Risk of Incident Cataract among Those Surviving Five Years or More
| Baseline characteristics | Prevalence at 5 years | |||||
|---|---|---|---|---|---|---|
| Cases | Percentage (%) |
OR | 95% CI | |||
| Age | Per 1 year | 200 | 1.4 | 0.99 | 0.96 | 1.02 |
| Sex | Male | 107 | 1.4 | 1 (ref.) | ||
| Female | 93 | 1.4 | 1.08 | 0.80 | 1.46 | |
| Type of first cancer | Bone sarcoma | 3 | 0.3 | 1 (ref.) | ||
| CNS | 15 | 0.9 | 0.75 | 0.21 | 2.73 | |
| HL | 2 | 0.1 | 0.26 | 0.04 | 1.72 | |
| Kidney (Wilms’) | 3 | 0.2 | 1.34 | 0.26 | 6.96 | |
| Leukemia | 112 | 2.4 | 6.61 | 1.98 | 22.02 | |
| NHL | 8 | 0.8 | 1.55 | 0.40 | 6.08 | |
| Neuroblastoma | 12 | 1.3 | 3.40 | 0.89 | 12.94 | |
| Soft tissue sarcoma | 45 | 3.8 | 3.37 | 0.98 | 11.57 | |
| Radiation dose to lens, Gy (mean) | <0.5 (0.03) | 35 | 0.4 | 1 (ref.) | ||
| 0.5–<1.5 (1.05) | 6 | 0.5 | 4.21 | 1.64 | 10.82 | |
| 1.5–<2.5 (2.09) | 7 | 0.4 | 0.59 | 0.26 | 1.35 | |
| 2.5–<3.5 (2.94) | 30 | 2.1 | 2.57 | 1.53 | 4.33 | |
| 3.5–<5.0 (4.30) | 8 | 2.2 | 7.01 | 3.16 | 15.53 | |
| 5.0–<10.0 (6.29) | 39 | 6.1 | 20.66 | 12.56 | 33.97 | |
| 10.0–<20.0 (13.26) | 26 | 15.2 | 26.76 | 15.20 | 47.10 | |
| 20–60 (41.02) | 36 | 35.6 | 140.48 | 74.71 | 264.15 | |
| EOR/Gy | 1.53 | 0.79 | 2.27 | |||
| Incidence follow-up ≥5 years | Prevalence at end of follow-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | IDa | HRb | 95% CI | Cases | Percentage (%) |
OR | 95% CI | ||
| 283 | 1.26 | 0.99 | 0.97 | 1.02 | 483 | 3.5 | 0.99 | 0.97 | 1.01 |
| 152 | 1.31 | 1 (ref.) | 259 | 1.9 | |||||
| 131 | 1.21 | 1.03 | 0.81 | 1.30 | 224 | 1.6 | 1.10 | 0.91 | 1.33 |
| 9 | 0.46 | 1 (ref.) | 12 | 1.0% | 1 (ref.) | ||||
| 33 | 1.36 | 1.00 | 0.46 | 2.20 | 48 | 2.9% | 0.78 | 0.39 | 1.53 |
| 27 | 0.81 | 1.02 | 0.46 | 2.28 | 29 | 1.5% | 1.00 | 0.48 | 2.07 |
| 4 | 0.18 | 0.41 | 0.12 | 1.35 | 7 | 0.6% | 0.63 | 0.24 | 1.64 |
| 163 | 2.23 | 2.38 | 1.15 | 4.90 | 275 | 5.8% | 3.18 | 1.70 | 5.95 |
| 24 | 1.37 | 1.76 | 0.80 | 3.87 | 32 | 3.0% | 1.73 | 0.86 | 3.45 |
| 8 | 0.50 | 0.89 | 0.33 | 2.40 | 20 | 2.1% | 1.47 | 0.68 | 3.16 |
| 15 | 0.78 | 0.99 | 0.42 | 2.32 | 60 | 5.1% | 1.66 | 0.84 | 3.27 |
| 70 | 0.54 | 1 (ref.) | 105 | 1.3 | 1 (ref.) | ||||
| 17 | 0.76 | 1.73 | 0.95 | 3.14 | 23 | 1.8 | 2.17 | 1.29 | 3.67 |
| 55 | 1.90 | 2.46 | 1.67 | 3.61 | 62 | 3.5 | 1.84 | 1.31 | 2.59 |
| 57 | 2.20 | 2.26 | 1.54 | 3.33 | 87 | 6.1 | 2.81 | 2.05 | 3.84 |
| 21 | 3.79 | 5.88 | 3.54 | 9.79 | 29 | 8.1 | 7.68 | 4.92 | 11.99 |
| 24 | 2.78 | 4.91 | 2.99 | 8.08 | 63 | 9.8 | 9.97 | 7.01 | 14.17 |
| 29 | 22.50 | 30.02 | 18.95 | 47.57 | 55 | 32.2 | 25.95 | 17.47 | 38.56 |
| 5 | 8.30 | 18.58 | 7.24 | 47.68 | 41 | 40.6 | 56.95 | 34.44 | 94.17 |
| 0.80 | 0.47 | 1.14 | 0.92 | 0.65 | 1.20 | ||||
Notes. OR = odds ratio; HR = hazard ratio; CI = confidence interval; CNS = central nervous system; HL=Hodgkin lymphoma; NHL=non-Hodgkin lymphoma; EOR = excess odds ratio.
Incidence density (ID) per 1,000 person-years.
Mutually adjusted for all variables.
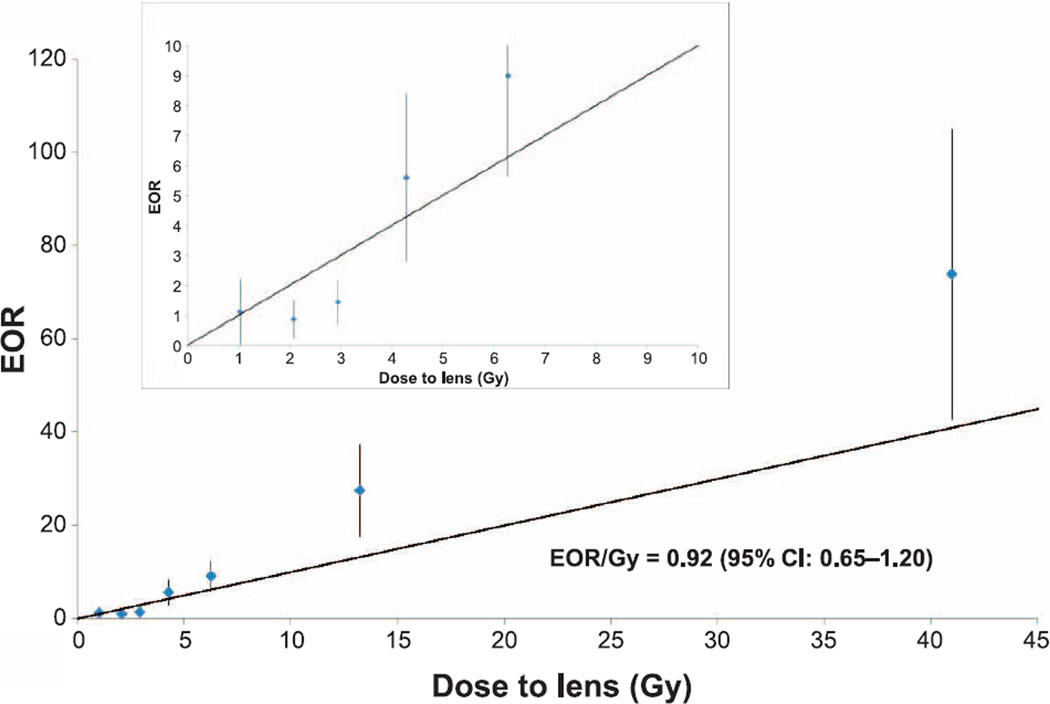
In multivariable logistic regressions, exposure to 0.5–1.5 Gy was associated with higher prevalence of cataract both after five years and at end of follow-up compared to patients exposed to <0.5 Gy to the lens of the eye with respective ORs of 4.2 (95% CI: 1.6–10.8) and 2.1 (95% CI: 1.2–3.5). Prevalence of cataract increased with increasing radiation dose (Table 3). Adjusted EORs per Gy at the end of the follow-up period are shown in Fig. 1. The slopes of the linear model were 1.53/Gy (95% CI, 0.79–2.27) at five years from initial cancer diagnosis and 0.92 (95% CI, 0.65–1.20) at the end of follow-up period. Similar linear slopes (1.51 and 0.98, respectively) were calculated when analyses were limited to patients with exposure of <3.5 Gy to the lens of the eye. There was no significant deviation from linearity when compared with quadratic and linear-quadratic models.
FIG. 1.
Adjusted excess prevalence odds ratio (EOR) and 95% confidence intervals for cataract prevalence at the end of the follow-up, according to lens dose. Inner panels show results for doses <10 Gy.
Other than lens dose, cataract at end of follow-up was independently associated with treatment with cytosine arabinoside (OR = 1.5; 95% CI: 1.2–2.0) and doxorubicin (1.5; 95% CI: 1.2–1.9). Methotrexate therapy was inversely associated with the prevalence of cataracts (OR = 0.6; 0.4–0.9). There was no indication for statistical interaction between lens dose and any of these agents (P > 0.2). Similarly, we did not find a positive interaction between use of corticosteroids with radiotherapy and risk of cataract compared to no use of corticosteroids. Other independent risk factors for cataract were leukemia as a first cancer (3.6; 1.8–7.1) and diabetes (3.2; 2.0–5.1).
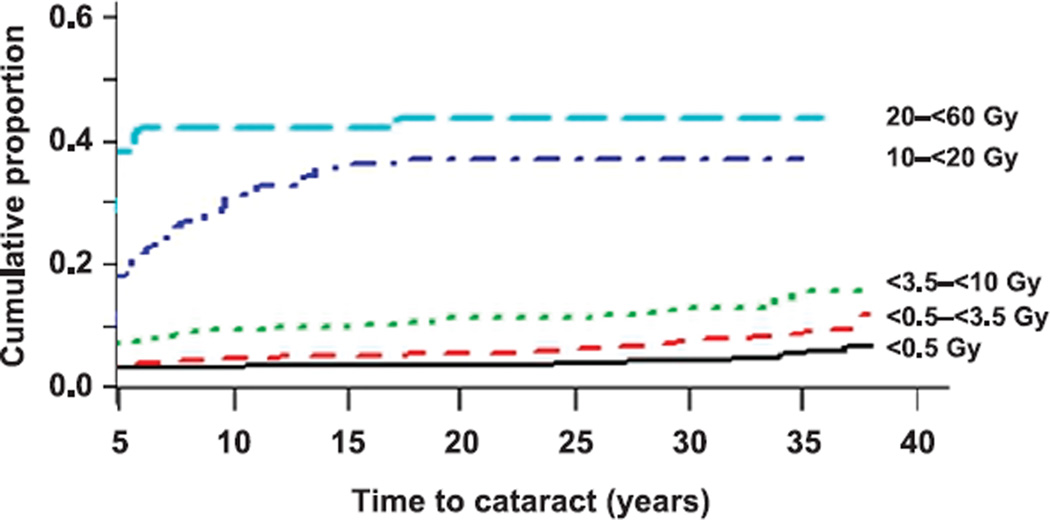
When follow-up time began at five years after the date of diagnosis of the first cancer, the incidence density of cataract per 1,000 PY among patients exposed to less than 0.5 Gy to the lens of the eye was 0.5, increasing to 1.9 and 2.8 among those exposed to 1.5–2.49 Gy and 5.0– 9.99 Gy, respectively. In patients exposed to 20–60 Gy, the incidence density was 8.3 per 1,000 PY. Among 101 patients in the 20–60 Gy dose category, there were 36 cases of cataract in the first five years and 5 cases thereafter. A total of 23 patients in this dose group with more than 20 years of follow-up had no indication of cataract. The cumulative proportion of cataract at five years of follow-up and onward, by lens dose, is shown in Fig. 2. In analyzing the incidence of cataract beginning five years after the initial cancer diagnosis date, the ERR/Gy was 0.80 (95% CI: 0.47–1.14). All linear slopes were highly significantly different from zero (P < 0.0001). We also modeled the EOR per Gy for 106 cataract surgeries reported by participants with available lens doses in the 2007 follow-up questionnaire. The slope of the linear model was 0.84/Gy (95% CI: 0.26–1.35). There was no evidence for interaction between lens dose and age at cancer diagnosis or patient’s sex (P > 0.5).
FIG. 2.
Cumulative proportion of five-year childhood cancer survivors affected with cataract according to lens dose. Results include prevalent cataracts at five years after initial cancer diagnosis and incident cataracts thereafter.
DISCUSSION
The results of the current study provide risk estimates for the occurrence of cataract among pediatric cancer survivors with follow-up spanning more than 35 years. They indicate a strong association between ocular exposure to ionizing radiation and long-term risk of pre-senile cataract. In survivors who had a lens dose of ≥20 Gy, 35.6% reported developing a cataract within 5 years after initial cancer diagnosis, accounting for 88% of all cataract cases in this dose group. However, among survivors exposed to less than 5 Gy, most of the cases occurred after five years from cancer diagnosis. This demonstrates the inverse relationship between latency and dose, and the need for long-term follow-up for assessing cataract risk after low-dose exposures (7).
Current radiation protection guidelines are predicated on the assumption that cataractogenesis is a deterministic event and requires a threshold radiation dose before cataracts will develop (13). A previous published study (10) of 12,480 survivors, also included in the current study cohort using baseline questionnaire data, only revealed a significantly increased risk of cataract at doses greater than 2 Gy to the eye. The current analysis shows evidence of a dose response for lens doses in the range of 0.5–1.49 Gy (and higher) based on well-estimated individual radiation doses. Our observation of elevated risk of cataract from a dose of ≥0.5 Gy supports the recent change in the position statement (13) of the International Commission on Radiological Protection (ICRP) on reducing the putative human threshold values for radiation cataractogenesis to 0.5 Gy from previous values of 2–8 Gy. In this analysis of cancer survivors, we did not investigate cataract risk in irradiated patients exposed to doses lower than 0.5 Gy separately from nonirradiated patients, due to the uncertainty in dose estimation at very low doses and the small number of cases in this group (n = 9).
The relationship between radiotherapy and cataract formation has been described in many studies since the 1950s (7). During childhood and adolescence, the number of both epithelial cells and fibers in the human lens increases by approximately 45–50% (22), raising the possibility of age-related sensitivity to radiation in cataract formation. This has been supported by studies among pediatric and adult cancer survivors, and particularly after single-dose total-body irradiation in bone marrow transplant recipients (23, 24). These studies have shown that cataract occurred in at least 20% of irradiated patients within several years after treatment (25). Among them, in at least three series of patients with unshielded eyes, the cumulative incidence reached 100% within 10 years of exposure (25). In a previous study of infants who were exposed to a lenticular dose of 1 Gy for skin hemangiomas, an increase of one month in age at exposure was associated with lower risk for developing a posterior subcapsular opacity but higher risk for developing cortical opacity (26). Lack of information on type of opacity may therefore explain why we did not observe evidence of an effect of age at radiation exposure on cataract risk among our cohort of children and adolescents.
The results of the current analysis agree with several studies of atomic bomb survivors that have shown significant radiation effects for cataract, particularly with posterior subcapsular (PSC) and cortical lens opacities (27). In one study of survivors exposed before age 13 that used the lens opacity classification system II (LOC-II), the odds ratios per 1 Sv for PSC and cortical cataract were 1.41 (95% CI: 1.21–1.64) and 1.29 (95% CI: 1.12–1.49), respectively. A similar association between lens dose and PSC or cortical cataract (OR = 1.65; 95% CI: 1.18–2.30) was calculated among Chernobyl cleanup workers (28).
In addition to the effects of radiotherapy, the chemotherapeutic agents doxorubicin and cytosine arabinoside, typically used for the management of childhood malignancies such as leukemia and Hodgkin lymphoma, also were associated with elevated risk of cataract independent of radiation exposure. Cataract formation can be a side effect of oxidative damage to cell membranes due to doxorubicin and cytosine arabinoside. Such damage has been demonstrated in rabbit eyes (29) as well as in small studies of pediatric cancer patient populations (25). Similar to our study, the magnitude of risk associated with exposure to chemotherapy generally was lower than that associated with radiotherapy (30). Cataractogenesis is also a documented feature of corticosteroids. As in several previous studies (31–33), the association between radiotherapy and risk of cataract was not affected by steroid use. Our data interpretation is limited since we lacked information on dose and duration of steroid use, which are important determinants for steroid-induced cataract (34, 35). The results from the current study also indicate that use of methotrexate was associated with a lower incidence of cataract independent of radiotherapy. Treating lens epithelial cells with methotrexate and actinomycin D proved to be an effective method for preventing posterior capsule opacification in rabbits (36). In a retrospective cohort study of children with juvenile idiopathic arthritis-associated uveitis, it was also reported that there was a significantly lower risk of cataract surgery over time in patients treated with methotrexate (37). These observations are of potential value for caregivers treating children at high risk of therapy-induced cataract.
Two important strengths of the current study are that it is based on one of the largest pediatric cancer survivor cohorts and has the longest follow-up. Access to medical records, including treatment records, provided valuable information on therapeutic exposures and other risk factors. Additional study strengths were the detailed information about radiotherapy and chemotherapy for nearly all members of the cohort, as well as individual radiation dose reconstruction. However, this study also has some limitations worthy of consideration. We relied on self-reported information about cataracts and had no independent confirmation of these reports nor on the type of lens opacity; thus, we cannot exclude the possibility that some misclassification of outcome occurred. However, previous studies suggested that the positive predictive values of self-reported cataracts and cataract-extractions were relatively high (76% and 95%, respectively) (38). When we limited the analyses to cataract extraction (a more clinically valid outcome) by analyzing data from 55% of the study population that completed the 2007 follow-up questionnaire, the dose-response results were similar to those for the primary analysis.
Other limitations include an unclear dose-response below 0.5 Gy, potential residual confounding by other risk factors and possible surveillance bias. The low doses to the lens of the eyes calculated in our study were outside the therapeutic field. These doses are the result of leakage from the medical treatment head, scatter from collimation devices and scatter within the patient’s body and thus are subject to considerable uncertainty (39). In addition, data on several factors that have been linked to cataract risk were missing in our study, including nutrition, sunlight exposure and blunt eye trauma (40), however, there is no reason to believe that any of these were related to level of exposure to ionizing radiation. Cancer survivors who received radiotherapy to the head or neck may undergo more screening tests and thus may have a higher chance of being diagnosed early with cataract compared to patients who did not have radiotherapy to the head or neck. However, restricting the analysis to five-year survivors and analyzing prevalence of cataract at the end of follow-up lessen potential surveillance/detection bias by reducing the effect of early diagnosis. For a small proportion of patients, age at cataract diagnosis was missing and was set to the last day of the latest available follow-up. This may have resulted in a minor underestimation of the true incidence rate of cataract in the cohort. Additional bias could result from radiotherapy treatments in other settings after the first five years of follow-up. This nondifferential classification error is not likely to be related to the study end point.
In summary, results of the current investigation indicate that survivors of childhood cancer who were exposed to 0.5 Gy or above to the lens of the eye are at increased risk for cataract. Our findings are consistent with a growing body of evidence suggesting an elevated risk for lens opacities in populations exposed to doses well below previously suggested threshold levels for radiation cataract (7) with, apparently, a linear dose response (41). For many cancer patients, radiation dose to the target tissue cannot be reduced because it would adversely affect curative efficacy. However, accurate radiation dose estimates and shielding design for the lens of the eye provide an opportunity to maintain the effectiveness of radiation treatments for young patients while reducing the risk of cataract. Future efforts should focus on methods to reduce cataracts in high-risk patients and to educate healthcare providers about the importance of screening this population for the development of cataracts.
Acknowledgments
This project would not have been possible without the generous participation of the survivors who have contributed to the Childhood Cancer Survivor Study (CCSS), as well as the efforts of the CCSS data abstractors and survey interviewers. We thank the investigators of the institutions that participated in the CCSS. This study was supported, in part, by the Intramural Research Program of the National Cancer Institute (NCI) Division of Cancer Epidemiology and Genetics (Bethesda, MD) and the Maccabi Institute for Health Services Research (Tel Aviv, Israel). The Childhood Cancer Survivor Study is supported by the National Cancer Institute [grant no. U24 CA55727 (GT Armstrong, Principal Investigator)] and funding from the American Lebanese Syrian Associated Charities.
REFERENCES
- 1.Frieden TR, Jaffe HW, Stephens JW, Thacker SB, Zasa S. Cancer survivors–United States, 2007. MMWR Morb Mortal Wkly Rep. 2011;60:269–272. [PubMed] [Google Scholar]
- 2.Bethesda: National Cancer Institute; 2014. SEER cancer statistics review, 1975–2011. (1.usa.gov/1Lxaou3) [Google Scholar]
- 3.Mariotto AB, Rowland JH, Yabroff KR, Scoppa S, Hachey M, Ries L, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 4.Inskip P, Curtis R. New malignancies following childhood cancer in the United States, 1973–2002. Int J Cancer. 2007;121:2233–2240. doi: 10.1002/ijc.22827. [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 6.Lackner H, Benesch M, Schagerl S, Kerbl R, Schwinger W, Urban C. Prospective evaluation of late effects after childhood cancer therapy with a follow-up over 9 years. Eur J Pediatr. 2000;159:750–758. doi: 10.1007/pl00008340. [DOI] [PubMed] [Google Scholar]
- 7.Kleiman N. Radiation cataract. Ann ICRP. 2012;41:80–97. doi: 10.1016/j.icrp.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Baradaran-Rafii A, Shirzadeh E, Eslani M, Akbari M. Optical correction of aphakia in children. J Ophthalmic Vis Res. 2014;9:71–82. [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman D, Constine L. Late effects of cancer treatment. In: Halperin E, Constine L, Trabell N, editors. Pediatric radiation oncology. Philadelphia: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 10.Whelan K, Stratton K, Kawashima T, Waterbor J, Castleberry R, Stovall M, et al. Ocular late effects in childhood and adolescent cancer survivors: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2010;54:103–109. doi: 10.1002/pbc.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chodick G, Bekiroglu N, Hauptmann M, Alexander BH, Freedman DM, Doody MM, et al. Risk of cataract after exposure to low doses of ionizing radiation: a 20-year prospective cohort study among US radiologic technologists. Am J Epidemiol. 2008;168:620–631. doi: 10.1093/aje/kwn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neriishi K, Nakashima E, Akahoshi M, Hida A, Grant EJ, Masunari N, et al. Radiation dose and cataract surgery incidence in atomic bomb survivors, 1986–2005. Radiology. 2012;265:167–174. doi: 10.1148/radiol.12111947. [DOI] [PubMed] [Google Scholar]
- 13.ICRP Publication 118. Ottawa, Canada: International Commission on Radiological Protection; 2012. ICRP statement on tissue reactions / early and late effects of radiation in normal tissues and organs – threshold doses for tissue reactions in a radiation protection context. (bit.ly/1LYN7Mr) [DOI] [PubMed] [Google Scholar]
- 14.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 15.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stovall M, Weathers R, Kasper C, Smith SA, Travis L, Ron E, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher G. Textbook of radiotherapy. 3rd. Philadelphia: Lee & Febiger; 1980. [Google Scholar]
- 18.Pintilie M. Competing risks: a practical perspective. Chichester, UK: John Wiley & Sons; 2006. [Google Scholar]
- 19.Neglia J, Friedman D, Yasui Y, Mertens A, Hammond S, Stovall M, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 20.Jobling A, Augusteyn R. What causes steroid cataracts? A review of steroid-induced posterior subcapsular cataracts. Clin Exp Optom. 2002;85:61–75. doi: 10.1111/j.1444-0938.2002.tb03011.x. [DOI] [PubMed] [Google Scholar]
- 21.Preston D, Lubin J, Pierce D. EPICURE: risk regression and data analysis software. Seattle, WA: HiroSoft International Corporation; 1991. [Google Scholar]
- 22.Rosen E. The lens. In: Yanoff M, Duker J, editors. Ophthalmology. St. Louis: Mosby; 2004. [Google Scholar]
- 23.Deeg HJ, Flournoy N, Sullivan KM, Sheehan K, Buckner CD, Sanders JE, et al. Cataracts after total body irradiation and marrow transplantation: a sparing effect of dose fractionation. Int J Radiat Oncol Biol Phys. 1984;10:957–964. doi: 10.1016/0360-3016(84)90163-9. [DOI] [PubMed] [Google Scholar]
- 24.Stava C, Beck M, Vassilopoulou-Sellin R. Cataracts among cancer survivors. Am J Clin Oncol. 2005;28:603–608. doi: 10.1097/01.coc.0000175291.51232.48. [DOI] [PubMed] [Google Scholar]
- 25.Leiper AD. Non-endocrine late complications of bone marrow transplantation in childhood: part II. Br J Haematol. 2002;118:23–43. doi: 10.1046/j.1365-2141.2002.03471.x. [DOI] [PubMed] [Google Scholar]
- 26.Hall P, Granath F, Lundell M, Olsson K, Holm L. Lenticular opacities in individuals exposed to ionizing radiation in infancy. Radiat Res. 1999;152:190–195. [PubMed] [Google Scholar]
- 27.Hammer GP, Scheidemann-Wesp U, Samkange-Zeeb F, Wicke H, Neriishi K, Blettner M. Occupational exposure to low doses of ionizing radiation and cataract development: a systematic literature review and perspectives on future studies. Radiat Environ Biophys. 2013;52:303–319. doi: 10.1007/s00411-013-0477-6. [DOI] [PubMed] [Google Scholar]
- 28.Worgul BV, Kundiev Y, Sergiyenko N, Chumak V, Vitte P, Medvedovsky C, et al. Cataract among Chernobyl cleanup workers: Implications regarding permissible eye exposure. Radiat Res. 2007;167:233–243. doi: 10.1667/rr0298.1. [DOI] [PubMed] [Google Scholar]
- 29.Bayer A, Evereklioglu C, Demirkaya E, Altun S, Karslioglu Y, Sobaci G. Doxorubicin-induced cataract formation in rats and the inhibitory effects of hazelnut, a natural antioxidant: a histopathological study. Med Sci Monit. 2005;11:BR300–BR304. [PubMed] [Google Scholar]
- 30.Leahey A, Teunissen H, Friedman D, Moshang T, Lange B, Meadows A. Late effects of chemotherapy compared to bone marrow transplantation in the treatment of pediatric acute myeloid leukemia and myelodysplasia. Med Pediatr Oncol. 1999;32:163–169. doi: 10.1002/(sici)1096-911x(199903)32:3<163::aid-mpo1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Bray L, Carey P, Proctor S, Evans R, Hamilton P. Ocular complications of bone marrow transplantation. Br J Ophthalmol. 1991;75:611–614. doi: 10.1136/bjo.75.10.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Marco R, Dassio D, Vittone P. A retrospective study of ocular side effects in children undergoing bone marrow transplantation. Eur J Ophthalmol. 1996;6:436–439. doi: 10.1177/112067219600600416. [DOI] [PubMed] [Google Scholar]
- 33.Lappi M, Rajantie J, Uusitalo R. Irradiation cataract in children after bone marrow transplantation. Graefes Arch Clin Exp Ophthalmol. 1990;228:218–221. doi: 10.1007/BF00920023. [DOI] [PubMed] [Google Scholar]
- 34.Ng JS, Wong W, Law RW, Hui J, Wong EN, Lam DS. Ocular complications of paediatric patients with nephrotic syndrome. Clin Experiment Ophthalmol. 2001;29:239–243. doi: 10.1046/j.1442-9071.2001.00426.x. [DOI] [PubMed] [Google Scholar]
- 35.Tichelli A, Gratwohl A, Egger T, Roth J, Prunte A, Nissen C, et al. Cataract formation after bone marrow transplantation. Ann Int Med. 1993;119:1175–1180. doi: 10.7326/0003-4819-119-12-199312150-00004. [DOI] [PubMed] [Google Scholar]
- 36.Sternberg K, Terwee T, Stachs O, Guthoff R, Löbler M, Schmitz K. Drug-induced secondary cataract prevention: experimental ex vivo and in vivo results with disulfiram, methotrexate and actinomycin D. Ophthalmic Res. 2010;44:225–236. doi: 10.1159/000316696. [DOI] [PubMed] [Google Scholar]
- 37.Sijssens K, Rothova A, Van De Vijver D, Stilma J, De Boer J. Risk factors for the development of cataract requiring surgery in uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol. 2007;144:574–579. doi: 10.1016/j.ajo.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 38.Bowie H, Congdon N, Lai H, West S. Validity of a personal and family history of cataract and cataract surgery in genetic studies. Invest Ophthalmol Vis Sci. 2003;44:2905–2908. doi: 10.1167/iovs.02-1055. [DOI] [PubMed] [Google Scholar]
- 39.Taylor M, Kron T. Consideration of the radiation dose delivered away from the treatment field to patients in radiotherapy. J Med Phys. 2011;36:59–71. doi: 10.4103/0971-6203.79686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodge WG, Whitcher JP, Satariano W. Risk factors for age-related cataracts. Epidemiol Rev. 1995;17:336–346. doi: 10.1093/oxfordjournals.epirev.a036197. [DOI] [PubMed] [Google Scholar]
- 41.Little MP. A review of non-cancer effects, especially circulatory and ocular diseases. Radiat Environ Biophys. 2013;52:435–449. doi: 10.1007/s00411-013-0484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]