Abstract
Objective
To evaluate the impact of an episode of intermenstrual bleeding on the probability of conception in a menstrual cycle (fecundability).
Design
Prospective, time to pregnancy cohort study
Setting
Community-based cohort
Patient(s)
Women trying to conceive, ages 30-44 years, without known infertility
Intervention(s)
None
Main Outcome Measure(s)
Current cycle and subsequent cycle fecundability
Result(s)
A total of 549 women provided 1,552 complete cycles for analysis. Intermenstrual and luteal bleeding were reported in 36% and 34% of cycles, respectively. Ninety-three percent of all intermenstrual bleeding was luteal. Cycles in which women had intermenstrual bleeding or luteal bleeding were significantly less likely to result in conception (fecundability ratio (FR)=0.23, 95% Confidence Interval (CI): 0.16-0.34 and FR=0.22, 95% CI:0.14-0.33 ). Women with an episode of intermenstrual and luteal bleeding had a significant increase in probability of pregnancy in the subsequent cycle (FR=1.61, 95% CI:1.15-2.25 and FR=2.01, 95%CI:1.52-2.87, respectively).
Conclusion(s)
Intermenstrual bleeding significantly decreases the odds of conceiving in that cycle; however, it does not appear to negatively impact a woman’s immediate future reproductive potential.
Clinical trials registration number
Keywords: intermenstrual bleeding, luteal bleeding, bleeding, fecundability, natural fertility
Introduction
Menses occurs as a response to the late luteal phase drop in progesterone after failure of the corpus luteum (1-4). Bleeding during the cycle at a time separate from menses, known as intermenstrual bleeding, is relatively common (5-7). Intermenstrual bleeding is thought to be caused by structural abnormalities or hormonal imbalances. Possible structural abnormalities include uterine polyps or fibroids. Hormonal imbalances include luteal phase defects attributed to poor corpus luteal development and inadequate progesterone secretion.
These underlying pathologies, which manifest themselves in intermenstrual bleeding, may interfere with fertility. Structural abnormalities, such as uterine polyps and fibroids, can cause uterine cavity distortion and interfere with implantation (8-11). Genital tract infections and endometriosis may result in an endometrial inflammatory response and upper reproductive tract scarring, which also hinders conception (12, 13). Luteal phase defects, which commonly result in intermenstrual bleeding during the luteal phase, may result in dysfunctional endometrial development, which could interfere with implantation or maintenance of pregnancy. (14-18).
The association between intermenstrual bleeding and natural fertility has not been previously evaluated. As intermenstrual bleeding may represent underlying pathologies which could interfere with implantation, we hypothesized that intermenstrual bleeding would impair a woman’s fertility. We sought to determine the impact of intermenstrual bleeding and luteal bleeding on fecundability, the probability of conceiving in a given cycle, in the current cycle and in the next consecutive cycle.
Material and Methods
This is a sub-study within Time to Conceive (TTC), an ongoing time-to-pregnancy study approved by the Institutional Review Board of the University of North Carolina. English-speaking women between 30 and 44 years of age, who were attempting to conceive for 3 months or less, were eligible for participation in the study. This analysis includes women recruited between April 2008 and April 2015. Women were recruited by direct advertising, online and on-air marketing strategies. Women with a history of infertility, polycystic ovarian disease, pelvic inflammatory disease, endometriosis, pelvic radiation, or with a partner with a history of infertility were excluded from participation. After informed consent was obtained, women completed a baseline questionnaire, which included survey of demographics, height, weight, and medical history for both the participant and her partner and of behaviors such as tobacco, alcohol, and caffeine use. The baseline questionnaire also queried duration of pregnancy attempt by asking specific questions regarding prior birth control methods: type, duration of use in the past year, and date of cessation; date participant started having intercourse without preventing pregnancy; and number of menstrual cycles at risk for pregnancy.
While attempting to conceive, women recorded information in a daily diary and were subsequently followed without intervention until pregnancy was detected. The daily diary included information on vaginal bleeding or spotting (as reported by women without any specific definition or instruction), markers of ovulation (cervical mucus scores, temperature, and ovulation test results), acts of intercourse, and pregnancy test results. Women provided daily data for up to four months if no positive pregnancy test occurred. If women were not pregnant after the fourth month, a monthly diary was completed for the remainder of the study, up to 12 months, or until pregnancy was achieved. Women were provided free home pregnancy tests, with a sensitivity of 20 mIU human chorionic gonadotropin (hCG) per mL, and standardized pregnancy testing instructions. Specifically, women were instructed to test for pregnancy on days 28, 31, and 34 of their cycles if they did not have menstrual bleeding.
Definitions
Menses was defined as 3 or more days of bleeding or spotting (with at least one day of bleeding), followed by 2 consecutive days without bleeding or spotting. The first day of a cycle was defined as the first day of bleeding occurring during menses. Bleeding episodes (including both “bleeding” and “spotting” observations reported in the daily diary), which did not meet the definition of a menses or began prior to cycle day 20, were classified as intermenstrual bleeding. Luteal bleeding, a subset of intermenstrual bleeding, was defined as any non-menstrual bleeding episode, which occurred during the luteal phase. Ovulation was estimated to have occurred on day after a positive ovulation predictor test result. If ovulation tests results were not available, then ovulation was defined as either the last day of type 4 cervical mucus or 14 days prior to the first day of menses or reported pregnancy. In coding the bleeding variables, we included only cycles 21-35 days in length in attempt to exclude anovulatory cycles. For modeling, cycles were only included if entries were made for all days in the cycle or if intermenstrual bleeding was noted in an incomplete cycle. Cycles with missing data and no intermenstrual bleeding were coded as missing.
Bivariate analyses were conducted to compare women based on their intermenstrual and luteal bleeding patterns in their first study cycle. Fisher’s exact test and the Kruskal-Wallis test were used to evaluate relationships between potential covariates and intermenstrual or luteal bleeding for categorical and continuous variables, respectively. Subsequently, discrete-time Cox proportional hazards models with time-varying (cycle-specific) exposure variables were created to determine the impact of either intermenstrual bleeding or luteal bleeding on probability of pregnancy in that cycle (current cycle fecundability) and in the next cycle (subsequent cycle fecundability). These models account for both right censoring and left truncation (due to women enrolling in cycles 1, 2, 3 or 4 or their pregnancy attempt), which were present in the data. In this model, a fecundability ratio (FR) of less than 1.0 suggests reduced fecundability. To adjust for potential confounders, covariates were added to the models. The full model was reduced to include only covariates strongly predictive of pregnancy in our cohort or in prior studies. Adjusted Kaplan-Meier curves were also created using the intermenstrual and luteal bleeding status in the first cycle as the exposure of interest.
Model 1 included the following covariates: age, race, body mass index (BMI), gravidity, mean cycle length, education level and smoking. Maternal age was modeled with 3 categories (<35 years, 35-37 years and >37 years), education level was categorized into 4 groups (less than a college degree, college graduate, some graduate level work, and graduate/professional degree), and BMI was categorized into 4 groups: underweight (<18.5 kg/m2), normal (≥18.5 and <25 kg/m2), overweight (≥25 and <30 kg/m2), and obese (≥30 kg/m2), In an attempt to more clearly delineate the impact of an isolated episode of intermenstrual or luteal bleeding on subsequent fecundability, model 2 included all variables in model 1 in addition to adjusting for intermenstrual bleeding in the outcome cycle. Thus, model 1 evaluates the impact of intermenstrual bleeding in a cycle on pregnancy outcome in the subsequent cycle, regardless of the presence or absence of intermenstrual bleeding in the outcome cycle. In comparison, model 2 evaluates the impact of intermenstrual bleeding in a cycle on pregnancy outcome in the subsequent cycle, adjusted for presence or absence of intermenstrual bleeding in the outcome cycle.
Results
A total of 1,552 cycles from 575 women were included in this analysis, with 196 cycles containing missing data on bleeding. Sixty-one percent of women enrolled in the study become pregnant, and 249 women (43%) conceived during the daily diary portion of the study. Fecundability in the first cycle of attempt was 18%. Although the study enrolled women between 30 and 45 years of age, 68% of the participants were less than 35 years of age, 19% between 35 and 37 years of age, and 13% 38 years or older. Participants tended to be Caucasian (77%) and highly educated (63% with a graduate degree). The majority of women had a normal BMI (60%), while 3% were underweight and 37% were overweight or obese.
In the first observed cycle, 38% and 34% of women had intermenstrual and luteal bleeding, respectively. Further, intermenstrual and luteal bleeding was reported in 36% and 34% of all observed cycles, respectively. Ninety-three percent of all intermenstrual bleeding was luteal bleeding. Figure 1 depicts the number of days of intermenstrual and luteal bleeding per woman in the first observed cycle. Of women who contributed at least 2 cycles, 51% of those who had intermenstrual bleeding in the first cycle also had intermenstrual bleeding in the subsequent cycle. No significant differences in baseline characteristics were observed between women who had intermenstrual or luteal bleeding in the first observed cycle and those who did not (Table 1).
Figure 1.
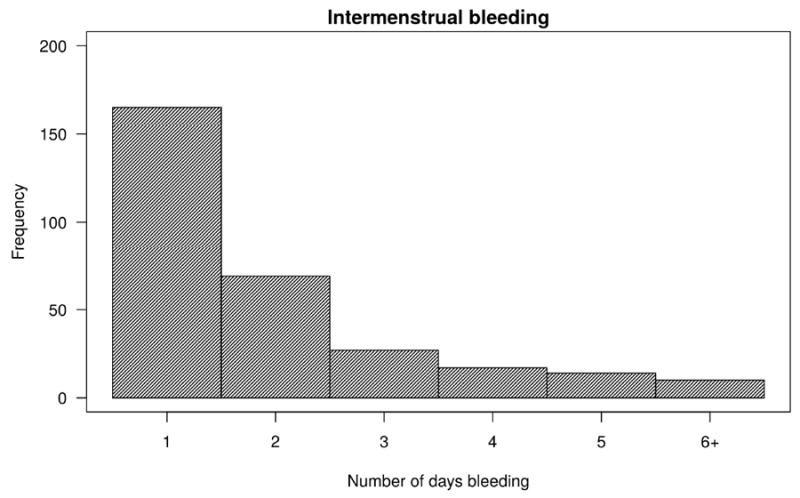
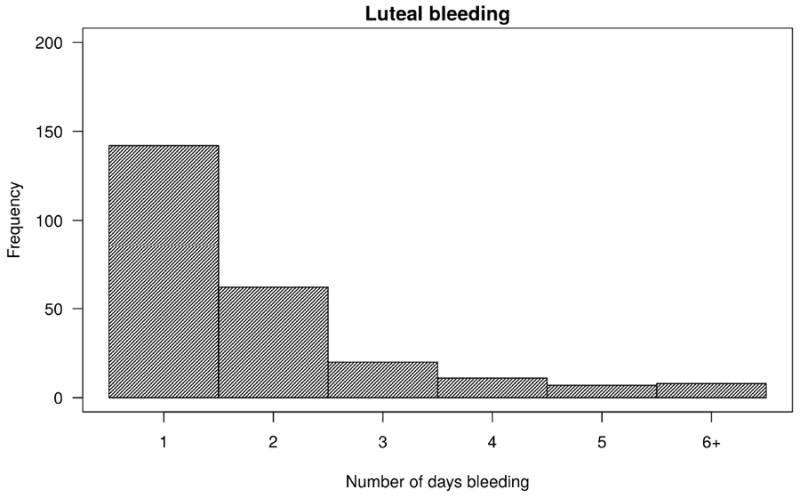
Number of days of intermenstrual bleeding (A) and luteal bleeding (B) in the first observed cycle (a total of 549 first observed cycles)
Table 1.
Patient characteristics for the overall sample and stratified by intermenstrual bleeding1
| Characteristic | Overall (n=549) | No IM Bleeding (n=336) | IM Bleeding (n=204) | P value | No luteal bleeding (n=357) | Luteal bleeding (n=187) | P value |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age (years) | 0.99 | 0.90 | |||||
| <35 | 68% | 68% | 69% | 68% | 69% | ||
| 35-37 | 19% | 19% | 18% | 19% | 18% | ||
| >37 | 13% | 13% | 13% | 13% | 13% | ||
|
| |||||||
| Race | 0.47 | 0.17 | |||||
| Non-Hispanic Caucasian | 77% | 77% | 75% | 78% | 73% | ||
| Other | 23% | 23% | 25% | 22% | 27% | ||
|
| |||||||
| Education level | 0.83 | 0.64 | |||||
| Less than college degree | 7% | 7% | 8% | 7% | 8% | ||
| College degree | 20% | 20% | 19% | 21% | 17% | ||
| Some graduate work | 10% | 10% | 11% | 10% | 11% | ||
| Completed postgraduate | 63% | 63% | 62% | 62% | 64% | ||
|
| |||||||
| Gravid | 0.59 | 0.24 | |||||
| No | 49% | 48% | 50% | 47% | 52% | ||
| Yes | 51% | 52% | 50% | 53% | 48% | ||
|
| |||||||
| BMI (kg/m2) | 0.09 | 0.17 | |||||
| <18.5 | 3% | 2% | 4% | 2% | 4% | ||
| 18.5-24.9 | 60% | 58% | 64% | 57% | 65% | ||
| 25-29.9 | 21% | 24% | 16% | 24% | 17% | ||
| ≥30 | 16% | 16% | 16% | 17% | 14% | ||
|
| |||||||
| Current smoking | 0.09 | 0.09 | |||||
| No | 99% | 98% | 100% | 98% | 100% | ||
| Yes | 1% | 2% | 0% | 2% | 0% | ||
|
| |||||||
| Attempt cycle at study entry | 0.76 | 0.81 | |||||
| 1 | 17% | 17% | 16% | 17% | 17% | ||
| 2 | 43% | 44% | 41% | 44% | 40% | ||
| 3 | 22% | 21% | 25% | 21% | 25% | ||
| 4 | 12% | 11% | 12% | 11% | 12% | ||
| 5 | 6% | 7% | 5% | 6% | 6% | ||
|
| |||||||
| Recent hormone contraception2 | 0.59 | 0.93 | |||||
| No | 55% | 54% | 57% | 55% | 56% | ||
| Yes | 45% | 46% | 43% | 45% | 44% | ||
|
| |||||||
| Mean cycle length (days ± SD) | 29 (2) | 29 (2) | 29(2) | 0.67 | 29 (2) | 29 (2) | 0.47 |
|
| |||||||
| Mean menses length (days ± SD) | 5 (1) | 5 (1) | 5 (1) | 0.77 | 5 (1) | 5 (1) | 0.58 |
|
| |||||||
| Mean intercourse days (days ± SD) | 7 (4) | 7 (4) | 6 (4) | 0.26 | 7 (4) | 6 (4) | 0.34 |
|
| |||||||
| Tested for pregnancy (at least once) | 0.86 | 0.18 | |||||
| No | 42% | 42% | 43% | 40% | 47% | ||
| Yes | 58% | 58% | 57% | 60% | 53% | ||
|
| |||||||
| Partner’s age (years) | 0.72 | 0.72 | |||||
| <50 | 99% | 98% | 99% | 98% | 99% | ||
| ≥50 | 1% | 2% | 1% | 2% | 1% | ||
|
| |||||||
| Partner race | 0.54 | 0.92 | |||||
| Non-Hispanic Caucasian | 76% | 74% | 77% | 75% | 76% | ||
| Other | 24% | 26% | 23% | 25% | 24% | ||
|
| |||||||
| Partner education level | 0.77 | 0.42 | |||||
| Less than college degree | 17% | 18% | 15% | 18% | 14% | ||
| College degree | 28% | 29% | 27% | 30% | 26% | ||
| Some graduate/masters | 9% | 9% | 9% | 8% | 10% | ||
| Completed postgraduate | 46% | 44% | 49% | 44% | 50% | ||
IM, intermenstrual
Patient characteristics in the first complete study cycle
Oral contraceptive pills, contraceptive patch, or contraceptive vaginal ring use in the past year
Both unadjusted and adjusted cycle-specific fecundability ratios revealed lower current cycle fecundability in cycles with intermenstrual bleeding but higher subsequent cycle fecundability in cycles with intermenstrual bleeding (Table 2). Cycles in which women had either intermenstrual or luteal bleeding were significantly less likely to result in conception, with an adjusted FR of 0.23 (95% CI: 0.16-0.34) and 0.22 (95% CI: 0.14-0.33), respectfully (model 1). Cycles in which women had intermenstrual or luteal bleeding were associated with an increase in probability of conception in the subsequent cycle, with an adjusted FR of 1.61 (95% CI: 1.15-2.25) and FR=2.01 (95%CI:1.52-2.87), respectively, in the fully adjusted model (model 2).
Table 2.
Cycle-specific fecundability ratios (FR)* for patients with intermenstrual bleeding
| Unadjusted FR (95% CI) | Adjusted FR1 (95% CI) | Adjusted FR2 (95% CI) | |
|---|---|---|---|
|
| |||
| Current cycle fecundability | |||
| Intermenstrual bleeding | 0.22 (0.15-0.32) | 0.23 (0.16-0.34) | |
| Luteal bleeding | 0.21 (0.14-0.31) | 0.22 (0.14-0.33) | |
|
| |||
| Subsequent cycle fecundability | |||
| Intermenstrual bleeding | 1.20 (0.91-1.58) | 1.25 (0.94-1.66) | 1.61 (1.15-2.25) |
| Luteal bleeding | 1.36 (1.04-1.77) | 1.46 (1.11-1.91) | 2.01 (1.52-2.87) |
Based on a Cox proportional hazards model
Model 1: adjusted for age, race, education level, gravidity, smoking, BMI, and mean cycle length with absence of bleeding as the reference group.
Model 2: adjusted for bleeding in the outcome cycle in addition to all variables adjusted for in model 1, with absence of bleeding as the reference group.
Adjusted Kaplan-Meier curves with 95% CI demonstrated that the cumulative probability of pregnancy over 12 months was not different for women who had intermenstrual bleeding and those without intermenstrual bleeding in the first observed cycle (Figure 2). Although women with intermenstrual bleeding in the first observed cycle had slightly lower fertility for approximately the first 6 months of conception attempt, by 12 months there was no significant difference in cumulative probability of pregnancy nor were the curves statistically significantly different (p=0.29). Further, for luteal bleeding, the curves are very similar throughout the 12 months, also without significant statistical difference (p=0.64).
Figure 2.
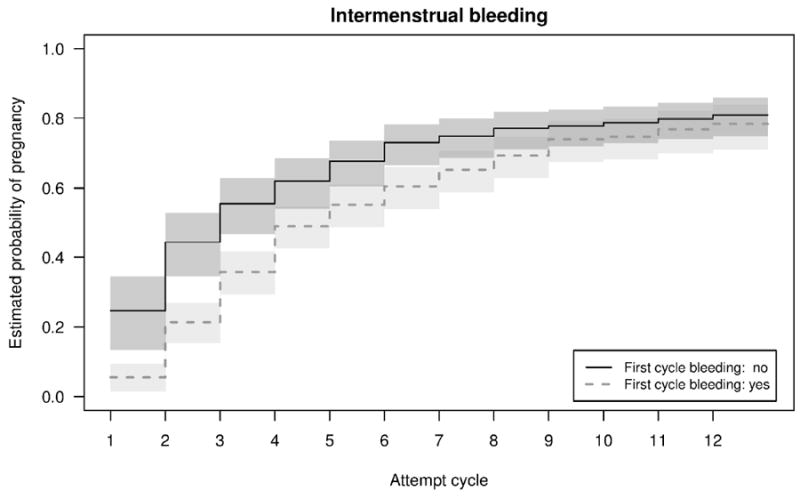
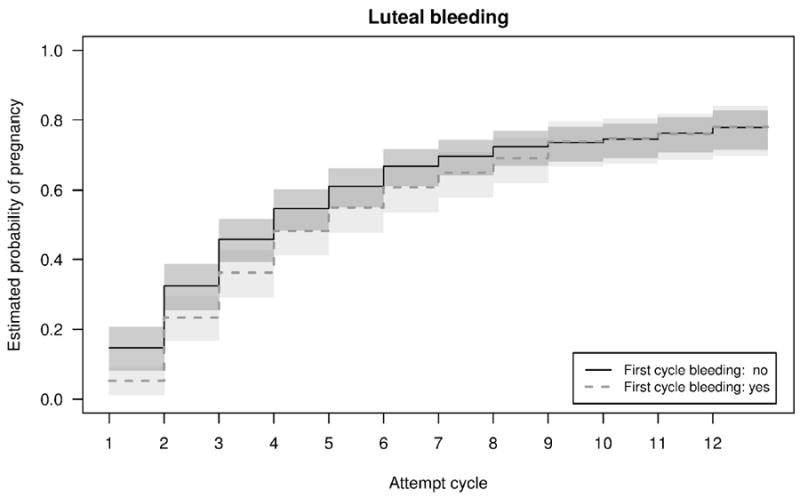
Adjusted Kaplan Meier curves by intermenstrual bleeding (A) and luteal bleeding (B) in the first observed cycle.
Discussion
Intermenstrual bleeding was relatively common in our cohort, and the vast majority of intermenstrual bleeding occurred in the luteal phase. Intermenstrual bleeding was not associated with any specific patient characteristics. Women who had an episode of intermenstrual bleeding were significantly less likely to conceive in that same cycle. However, an episode of intermenstrual bleeding did not decrease the probability of pregnancy in the subsequent cycle. In fact, intermenstrual bleeding was associated with an increase in probability of conception in the next consecutive cycle. Further, one episode of intermenstrual bleeding did not result in any difference in probability of conception after 12 months.
Our prospective observation demonstrates that both intermenstrual bleeding and luteal bleeding are relatively common in women trying to conceive. More than ninety percent of bleeding episodes occur in the luteal phase. Previous studies have reported that between 5-13% of women have intermenstrual bleeding (5-7). In a population of women trying to conceive, Mikolajczyk et al. reported that 8% of their cohort (total n=74) had non-menstrual bleeding (6). However, the authors disclose that many more bleeding episodes were defined which were likely non-menstrual but were not recognized as such by the participants, likely underestimating the true prevalence of intermenstrual bleeding. Dasharathy et al. reported that 4.8% of women (total n=259) reported having non-menstrual bleeding (7). However, this may under represent the general population as an inclusion criteria for the study was regular menstrual cycles and patients with intermenstrual bleeding may interpret their cycles as irregular. Another evaluation of menstrual cycles characteristics reported that 13.3% of reproductive aged women had intermenstrual bleeding based on a single administration of a questionnaire (total n=3,941) (5). The prevalence of intermenstrual bleeding in our cohort is higher than previously reported. Our study may differ from others based on our definition of intermenstrual bleeding. In our study, premenstrual spotting, even as little as one day of spotting prior to menses, was considered intermenstrual bleeding. In addition, women were queried daily with regard to bleeding or spotting, which may have resulted in a higher response rate when compared to other methods of ascertainment. Despite this difference, intermenstrual bleeding appears to be relatively common in women trying to conceive.
Intermenstrual bleeding was associated with a lower probability of pregnancy in the current cycle. In IVF cycles, luteal bleeding has been shown to be a poor prognostic sign for conception in the current cycle. A secondary analysis of a randomized trial (n=365) revealed that women with luteal bleeding after IVF were less likely to achieve pregnancy than women without bleeding (45% versus 74%, respectively) (19). In this study, women who did get pregnant but had luteal bleeding were also more likely to have a failing pregnancy than those who did not have bleeding (50% versus 27%, respectively) (19). A cross-sectional, survey study found that women with intermenstrual bleeding were more likely to have a history of infertility (5). Our study differs in 1) the exposure: an episode of intermenstrual bleeding versus history of intermenstrual bleeding, 2) the outcome: fecundability versus history of infertility and 3) study design: prospective cohort versus cross-sectional.
An episode of intermenstrual bleeding did not reduce the probability of pregnancy in the next consecutive cycle. In fact, an isolated episode of intermenstrual bleeding was associated with an increase in the probability of conception in the subsequent cycle. When controlling for intermenstrual bleeding in the outcome cycle, this association was even more pronounced. Intermenstrual bleeding may represent undetected early pregnancy loss. Wilcox et al. evaluated a cohort of women attempting pregnancy (n=221) and reported an incidence of unrecognized early pregnancy loss of 22%, the majority of which had hCG levels which would escape detection on standard urinary pregnancy tests (20, 21). The mean cycle length in cycles with unrecognized pregnancy loss was 32 days (as compared to 29 days in cycles without a loss), thus menses was not markedly disturbed by the failed pregnancy (21). Although our data did not show a difference in mean cycle length between women who had intermenstrual bleeding and those who did not, we did restrict our cohort to cycles less than 35 days in length in an attempt to exclude anovulatory cycles from the analysis. This may have limited our ability to detect a difference in cycle length between cycles with intermenstrual bleeding and those without. In addition, a secondary analysis of the cohort from Wilcox et al. revealed that the overall pattern of bleeding in patients with an early pregnancy loss (<6 weeks) was very similar to normal menses with only a slight increase in number of light spotting days in women with pregnancy loss (22). Therefore, very early pregnancy loss may be indistinguishable from luteal phase bleeding and menses on the basis of bleeding patterns alone.
We hypothesize that the luteal bleeding observed in our cohort may represent unrecognized early pregnancy loss. Women with an isolated episode of luteal bleeding may represent a more fertile subset of the population. In a prospective cohort of women attempting pregnancy (n=221), Wilcox et al. reported that women with an unrecognized pregnancy loss were more likely to get pregnant in the next consecutive cycle (35% versus 25% of women without a pregnancy) (21). Similar findings have been reported in infertile patients as well. Bates et al. reported that in women undergoing IVF (n=1,679), those with a an early loss in their first IVF cycle had higher rates a conception in the subsequent cycle over those without a pregnancy (37% versus 27%) (23). Croucher et al. evaluated women undergoing a second IVF cycle (n=2,396) and found those women with a biochemical pregnancy loss in their first cycle were more likely to conceive in their second IVF cycle as compared to women without a pregnancy (36% versus 24%) (24). An evaluation of consecutive IVF cycles (n=1,141) also revealed that women with a prior pregnancy loss had an increased probability of pregnancy in the subsequent IVF cycle (48% versus 34.5%) (25). Thus, patients with an isolated prior early pregnancy loss likely have greater reproductive potential than women who have never achieved pregnancy.
When evaluating the impact of one episode of intermenstrual or luteal bleeding on the probability of pregnancy we did not observe a significant difference in the cumulative pregnancy rate at 1 year (or conversely probability of infertility) for those women who bled and those who did not bleed. This study was not designed to examine the impact of repetitive episodes of intermenstrual bleeding on fertility. However, considering that intermenstrual bleeding was associated with lower fecundability in the current cycle, one might deduce that repetitive episodes of intermenstrual bleeding over multiple cycles would result in overall lower fertility.
To our knowledge, ours is the first study to examine the impact of intermenstrual and luteal phase bleeding on subsequent fertility in a population of unproven reproductive potential. Our study does have limitations. The cohort was composed of mostly Caucasian, well-educated, and older women. These findings may not be generalizable to other groups. Also, detection bias may be present due to our pregnancy testing protocol. Women in our study were instructed to test for pregnancy on days 28, 31, and 34 of their cycles if they did not have menstrual bleeding. Thus, as women with intermenstrual bleeding, and specifically luteal bleeding, may have perceived this bleeding as the onset of a new cycle and not tested for pregnancy. Strengths of this study include the size of the cohort, modeling with adjustment for potential confounders, and the prospective nature of this study in a non-infertile population trying to conceive. Furthermore, recall bias was reduced by use of the daily diary to record bleeding.
In summary, our study reveals that an episode of intermenstrual bleeding significantly decreases the odds of conception in the current cycle; however, it does not appear to impact a woman’s future reproductive potential. The increase in subsequent cycle fecundability seen with luteal bleeding could be due to an early, unrecognized pregnancy loss and represent a more fertile segment of the population. Therefore, in ovulatory patients with a single episode of luteal bleeding, expectant management is likely the best course of action as many will conceive without intervention in subsequent attempt cycles.
Acknowledgments
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, grant nos. R21 HD060229 and R01 HD067683.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Natalie M. Crawford, Email: natalie_crawford@med.unc.edu.
David A. Pritchard, Email: dpritch@live.unc.edu.
Amy H. Herring, Email: amy_herring@unc.edu.
Anne Z. Steiner, Email: anne_steiner@med.unc.edu.
References
- 1.Wise LA, Mikkelsen EM, Rothman KJ, Riis AH, Sorensen HT, Huybrechts KF, et al. A prospective cohort study of menstrual characteristics and time to pregnancy. American journal of epidemiology. 2011;174:701–9. doi: 10.1093/aje/kwr130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harlow SD, Ephross SA. Epidemiology of menstruation and its relevance to women’s health. Epidemiologic reviews. 1995;17:265–86. doi: 10.1093/oxfordjournals.epirev.a036193. [DOI] [PubMed] [Google Scholar]
- 3.Direito A, Bailly S, Mariani A, Ecochard R. Relationships between the luteinizing hormone surge and other characteristics of the menstrual cycle in normally ovulating women. Fertility and sterility. 2013;99:279–85. doi: 10.1016/j.fertnstert.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Chiazze L, Jr, Brayer FT, Macisco JJ, Jr, Parker MP, Duffy BJ. The length and variability of the human menstrual cycle. Jama. 1968;203:377–80. [PubMed] [Google Scholar]
- 5.Rowland AS, Baird DD, Long S, Wegienka G, Harlow SD, Alavanja M, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology (Cambridge, Mass) 2002;13:668–74. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Mikolajczyk RT, Louis GM, Cooney MA, Lynch CD, Sundaram R. Characteristics of prospectively measured vaginal bleeding among women trying to conceive. Paediatric and perinatal epidemiology. 2010;24:24–30. doi: 10.1111/j.1365-3016.2009.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasharathy SS, Mumford SL, Pollack AZ, Perkins NJ, Mattison DR, Wactawski-Wende J, et al. Menstrual bleeding patterns among regularly menstruating women. American journal of epidemiology. 2012;175:536–45. doi: 10.1093/aje/kwr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livingstone M, Fraser IS. Mechanisms of abnormal uterine bleeding. Human reproduction update. 2002;8:60–7. doi: 10.1093/humupd/8.1.60. [DOI] [PubMed] [Google Scholar]
- 9.Practice Committee of American Society for Reproductive Medicine. Myomas and reproductive function. Fertility and sterility. 2008;90:S125–30. doi: 10.1016/j.fertnstert.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Taylor E, Gomel V. The uterus and fertility. Fertility and sterility. 2008;89:1–16. doi: 10.1016/j.fertnstert.2007.09.069. [DOI] [PubMed] [Google Scholar]
- 11.Ferenczy A. Pathophysiology of endometrial bleeding. Maturitas. 2003;45:1–14. doi: 10.1016/s0378-5122(03)00068-9. [DOI] [PubMed] [Google Scholar]
- 12.Heitmann RJ, Langan KL, Huang RR, Chow GE, Burney RO. Premenstrual spotting of >/=2 days is strongly associated with histologically confirmed endometriosis in women with infertility. American journal of obstetrics and gynecology. 2014;211:358.e1–6. doi: 10.1016/j.ajog.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 13.Soper DE. Pelvic inflammatory disease. Obstetrics and gynecology. 2010;116:419–28. doi: 10.1097/AOG.0b013e3181e92c54. [DOI] [PubMed] [Google Scholar]
- 14.Practice Committee of the American Society for Reproductive Medicine. The clinical relevance of luteal phase deficiency: a committee opinion. Fertility and sterility. 2012;98:1112–7. doi: 10.1016/j.fertnstert.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 15.Schliep KC, Mumford SL, Hammoud AO, Stanford JB, Kissell KA, Sjaarda LA, et al. Luteal phase deficiency in regularly menstruating women: prevalence and overlap in identification based on clinical and biochemical diagnostic criteria. The Journal of clinical endocrinology and metabolism. 2014;99:E1007–14. doi: 10.1210/jc.2013-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones GE. Some newer aspects of the management of infertility. Journal of the American Medical Association. 1949;141:1123–9. doi: 10.1001/jama.1949.02910160013004. illust. [DOI] [PubMed] [Google Scholar]
- 17.Muechler EK, Huang KE, Zongrone J. Superovulation of habitual aborters with subtle luteal phase deficiency. International journal of fertility. 1987;32:359–65. [PubMed] [Google Scholar]
- 18.Sonntag B, Ludwig M. An integrated view on the luteal phase: diagnosis and treatment in subfertility. Clinical endocrinology. 2012;77:500–7. doi: 10.1111/j.1365-2265.2012.04464.x. [DOI] [PubMed] [Google Scholar]
- 19.Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Patterns of luteal phase bleeding in in vitro fertilization cycles supplemented with Crinone vaginal gel and with intramuscular progesterone--impact of luteal estrogen: prospective, randomized study and post hoc analysis. Fertility and sterility. 2011;95:617–20. doi: 10.1016/j.fertnstert.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. The New England journal of medicine. 1999;340:1796–9. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. The New England journal of medicine. 1988;319:189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 22.Promislow JH, Baird DD, Wilcox AJ, Weinberg CR. Bleeding following pregnancy loss before 6 weeks’ gestation. Human reproduction (Oxford, England) 2007;22:853–7. doi: 10.1093/humrep/del417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bates GW, Jr, Ginsburg ES. Early pregnancy loss in in vitro fertilization (IVF) is a positive predictor of subsequent IVF success. Fertility and sterility. 2002;77:337–41. doi: 10.1016/s0015-0282(01)02988-0. [DOI] [PubMed] [Google Scholar]
- 24.Croucher CA, Lass A, Margara R, Winston RM. Predictive value of the results of a first in-vitro fertilization cycle on the outcome of subsequent cycles. Human reproduction (Oxford, England) 1998;13:403–8. doi: 10.1093/humrep/13.2.403. [DOI] [PubMed] [Google Scholar]
- 25.Kalu E, Thum MY, Abdalla H. Prognostic value of first IVF cycle on success of a subsequent cycle. Journal of assisted reproduction and genetics. 2011;28:379–82. doi: 10.1007/s10815-010-9534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


