Abstract
Specific growth factors induce formation and differentiation of excitatory and inhibitory synapses, and are essential for brain development and function. Fibroblast growth factor 22 (FGF22) is important for specifying excitatory synapses during development, including in the hippocampus. Mice with a genetic deletion of FGF22 (FGF22KO) during development subsequently have fewer hippocampal excitatory synapses in adulthood. As a result, FGF22KO mice are resistant to epileptic seizure induction. In addition to playing a key role in learning, the hippocampus is known to mediate mood and anxiety. Here, we explored whether loss of FGF22 alters affective, anxiety or social cognitive behaviors in mice. We found that relative to control mice, FGF22KO mice display longer duration of floating and decreased latency to float in the forced swim test, increased immobility in the tail suspension test, and decreased preference for sucrose in the sucrose preference test, which are all suggestive of a depressive-like phenotype. No differences were observed between control and FGF22KO mice in other behavioral assays, including motor, anxiety, or social cognitive tests. These results suggest a novel role for FGF22 specifically in affective behaviors.
Keywords: depression, fibroblast growth factor 22, knockout mouse, forced swim test, tail suspension test, sucrose preference test
1. Introduction
Fibroblast growth factors (FGFs) are known to play important roles in synapse formation and maintenance (1-3). Altered expression of, or mutations in, FGFs and their receptors have been implicated in the pathogenesis of many neuropsychiatric diseases, including depression (4), altered social behavior (5), seizures (6) and intellectual disability (6).
FGF22 is a critical determinant of excitatory synapses in the developing hippocampus (1). When FGF22 is deleted in mice (FGF22KO mice), excitatory synapses in the hippocampus fail to form during development, and this defect persists into adulthood (1). The deletion of FGF22 renders mice resistant to seizure kindling in a pentylenetetrazol (PTZ) kindling model of epilepsy (1), and FGF22KO mice do not display seizure-induced neuropathology, such as increased hippocampal mossy fiber sprouting and hilar cell death, even when seizures are induced (7). FGF22 has been implicated in the organization of other excitatory synapses in the brain, including retinogeniculate synapses (8) and mossy fiber-dentate granule cell synapses in the cerebellum (3), as well as the peripheral neuromuscular junction (NMJ) synapse (9).
The hippocampus is implicated in mood, anxiety, and learning and memory (10-12). Since FGF22 is critical for excitatory synapse formation in the hippocampus, we hypothesized that FGF22KO mice may have abnormal affective, anxiety-like, and social cognitive behaviors. Here we performed a battery of behavioral studies and found that FGF22KO mice exhibit passive stress coping behaviors in affective reactivity tests, including the forced swim test and the tail suspension test, the tests that are often used to screen for antidepressants. The FGF22KO mice also prefer sucrose to a lesser degree than WT littermates, suggestive of anhedonia, the failure to engage in pleasurable activity. These alterations in affective tasks are independent of any changes in anxiety-like behaviors, social cognition, and motor phenotypes. Our results reveal a unique role for FGF22 in affective behaviors and suggest that FGF22KO mice may be an ethologically useful animal model for screening antidepressant compounds.
2. Materials and Methods
2.1. Animals
The generation of FGF22KO mice has been described previously (1). FGF22KO mice were backcrossed with C57BL/6J mice for at least 20 generations. All mice were bred within our colony, and all wild-type (WT) animals were FGF22KO littermates. Studies were conducted using adult mice (8-30 weeks old). All mice were housed by sex in groups of two to five. Mice were maintained in cages with a 14-h/10-h light/dark cycle for a minimum of one week prior to behavioral experiments. The average ambient temperature was 22°C and mice were provided with food and water ad libitum. All experiments were conducted during the animals' light cycle. Air purifiers (Honeywell, Southborough, MA) were used during all experiments to mask ambient noise. For all experiments, examiner was blinded to mouse genotype. We used several cohorts of mice and used them in multiple tasks. Being mindful of potential order of testing effects (13, 14), we tested the mice using the least stressful tasks first and the most stressful tasks last, in the following order: rotarod, open field, light-dark box, elevated zero maze, social recognition, forced swim test, and tail suspension test. Mixed cohorts of males and females were used in each genotype group in all tests except rotarod, open field, and tail suspension, where groups were male (precise numbers of animals for each task can be found in Supplementary Table 1). Although the rotarod and open field groups were entirely male, in the social recognition task females and males (both WT and FGF22KO) displayed similar exploration (Supplementary Figure 1), suggesting that motor exploratory behaviors are similar between males and females in our cohorts. Sucrose preference test was performed on experiment-naïve animals in their home cages without masking of ambient noise. All experiments were conducted according to the National Institute of Health guidelines for animal care and were approved by the University Committee on the Use and Care of Animals of the University of Michigan (PRO00003549 and PRO00004242) and the Institutional Animal Care and Use Committees at Boston Children's Hospital (13-11-2528).
2.2. Behavioral Procedures
2.2.1. Open Field (OF)
Animals were permitted to explore a transparent acrylic arena for 5 minutes under white room lights at 33 lux as previously described (15). Total distance traveled and percent time in periphery of arena were calculated by Limelight automated software via a video camera mounted above the arena (Actimetrics, Wilmette, IL).
2.2.2. Rotarod
Animals were trained to run on rubber-coated rod which accelerated from 4 rpm to 40 rpm (Ugo Basile, Comerio, Italy) over a 5-minute testing period as previously described (15). Animals underwent one training session per day for 5 consecutive days. Time to passively rotate on the rod or fall off the rod was recorded for each trial.
2.2.3. Elevated Zero Maze (EZM)
Elevated zero maze was performed as previously described (16) with some modifications, detailed below. The maze is a 6-cm-wide ring (outer diameter 70cm), divided into 4 quadrants with alternating walled (closed) and unwalled (open) areas, elevated on 70cm legs. Testing was performed under low light conditions (3 lux). Mice were placed into an open area to start, and allowed to explore for 5 minutes. Mice were considered to be in an open area if their nose and more than 50% of their body was in the open area. Total time spent in open and closed compartments was calculated by Limelight automated software via a video camera mounted above the box (Actimetrics, Wilmette, IL).
2.2.4. Light-Dark Box (LDB)
Light-dark box test was performed as previously described (17). Mice were placed into the lit compartment of a 46-cm-long box divided into a lit opaque white acrylic area (2/3 of surface area) and a lidded dark compartment made of black acrylic (1/3 of surface area). Animals were allowed to freely explore both areas of the acrylic box for 5 minutes, and total time spent in each compartment was calculated by Limelight automated software via a video camera mounted above the box (Actimetrics, Wilmette, IL).
2.2.5. Social Recognition (SR)
Social recognition testing was performed as previously described (18) with some modifications. Testing was performed in a 3-chambered transparent acrylic arena with overhead light set at 6 lux. Mice were habituated for 10 minutes in the middle chamber of the arena, and then allowed to explore all 3 chambers for 10 minutes. During the sociability stage of the task (hereafter called the “object phase”), an empty wire cup was placed in one outer chamber, and a novel C57BL/6J mouse (same sex as test mouse) was placed under an identical wire cup in the opposite outer chamber; the test mouse was allowed to explore all chambers for 10 minutes and movement was recorded with Limelight software via a video camera mounted above the test area. After the object phase, social novelty preference (hereafter called the “social phase”) was assessed by placing a second novel C57BL/6J mouse under the first wire cup (which had previously been empty) and the test mouse was allowed to explore all chambers for 10 minutes. The time between each phase of the task was approximately 1 minute. Videos were scored manually for exploration of objects and mice by a trained observer blinded to genotype.
2.2.6. Forced Swim Test
Animals were placed into a 4L beaker of tepid water (∼25°C) for 5 minutes, and their behavior was recorded using a video camera mounted on the same level as the base of the beaker. Video recordings were reviewed and scored by a trained observer blinded to genotype. Animals were assessed for total duration of floating and latency to start floating in the water. Mice were judged to be floating when making only the movements necessary to keep their heads above water.
2.2.7. Tail Suspension Test
Animals were suspended by a piece of paper tape wrapped around the base of their tails 10cm above a piece of opaque white acrylic for 5 minutes. Behavior was recorded using a video camera mounted on the level of the acrylic base. Video recordings were reviewed and scored by a trained observer blinded to genotype. Animals were assessed for total duration of immobility. Mice were judged to be immobile when making only the movements necessary for breathing. Any time mice spent climbing their own tails was excluded. Duration of immobility is expressed as a percentage of time spent not tail-climbing.
2.2.8. Sucrose Preference Test
Mice were singly housed for the sucrose preference test. They were given two fresh water bottles in their home cage the morning of day 1, which were weighed before placement in the cage. Twenty-hour hours later, the water bottles were removed and weighed, then replaced with one water bottle and one bottle filled with 2% sucrose. Twenty-four hours later, the bottles were weighed and the bottle positions were switched, to control for any side position preferences in the mice. The bottle positions were switched daily for an additional 2 days, weighing the bottles each day before switching (2 days with each bottle in each position). Data are presented as the amount of sucrose consumed as a percentage of total liquid consumed over the testing period.
2.3. Statistical Analysis
Data were prepared and analyzed using GraphPad Prism 6.02 for Windows, and are graphically represented as mean ± standard error of the mean (SEM) for each group. Data were analyzed using the statistical test noted in results and figure legends. Results were considered significant when p<0.05 (denoted in all graphs as follows: *p<0.05; **p<0.01, ***p<0.001).
3. Results
3.1. Mice lacking FGF22 display normal exploratory and locomotive behavior
Because loss of FGF22 causes a decrease in number and size of excitatory synapses in the hippocampus, an essential brain structure for many affective and cognitive processes, our aim was to investigate whether FGF22KO mice have abnormalities in behaviors related to anxiety, mood, and learning and memory. Before performing such behavioral tests, we first confirmed that FGF22KO mice were not impaired in basic exploration or motor behaviors. In the open field test, FGF22KO mice traveled a similar distance overall compared to WT littermates (Student's unpaired two-tailed t-test, t(13)=0.44, p=0.67) (Fig. 1A), suggesting normal exploratory behavior. We also examined their ability to learn to walk on an accelerating rotarod to assess for motor strength and coordination. FGF22KO mice demonstrated normal ability to learn the accelerating rotarod task, including similar time to acquire the skill and normal ability to stay on the rod as it accelerated (main effect of genotype, repeated-measures ANOVA, F1,13 =0.1003, p=0.76; main effect of training for both groups, repeated-measures ANOVA, F4,52=23.61, p<0.0001; interaction effect, repeated-measures ANOVA, F4,52=0.1341, p=0.97) (Fig. 1B). These results confirm that FGF22KO mice have the basic motor and exploratory behaviors required to perform the behavioral assays we used.
Figure 1.
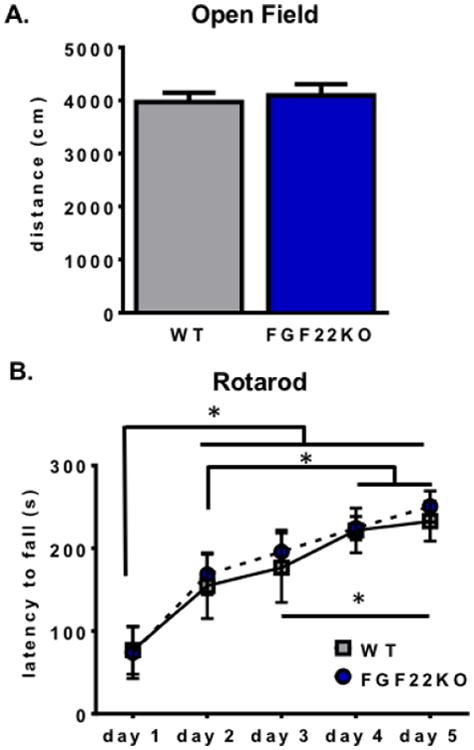
Mice lacking FGF22 display normal exploratory and locomotive behavior. A. FGF22KO mice traverse the same overall distance in the open field test as WT animals (Student's unpaired two-tailed t-test, t(13)=0.44, p=0.67). B. FGF22KO mice demonstrate normal motor learning and endurance on the rotarod test. Significant effect of day of training (repeated-measures ANOVA, F4,52=23.61, p<0.0001); *=p<0.05, Tukey's HSD. There was no effect of genotype (repeated-measures ANOVA, F1,13 =0.1003, p=0.76) and no interaction effect (repeated-measures ANOVA, F4,52=0.1341, p=0.97).
3.2. Mice lacking FGF22 display normal anxiety-like behaviors
We then examined FGF22KO mice for changes in anxiety-related behaviors using several measures. In the elevated zero maze, increases or reductions in anxiety-like behaviors manifest as more or less time in the enclosed parts of the track, respectively (19). When mice were placed in the elevated zero maze under low-light conditions (3 lux), both genotypes exhibited a preference for the closed parts of the maze (two-way ANOVA, main effect of maze part, F1,96=107.9, p<0.0001; *** p<0.001, Tukey's HSD), but there was no main effect of genotype (two-way ANOVA, main effect of genotype, F1,96=0, p>0.99; interaction effect, F1,96=0.55, p=0.46). We also examined anxiety-like behavior using another test, the light-dark box, in which the motivation to explore novel areas conflicts with the desire to avoid exposure in open areas (20, 21). To exaggerate any potential differences present, this test was performed under higher-light conditions (21 lux). As with the elevated zero maze, both WT and FGF22KO mice preferred dark parts of the maze over light parts (two-way ANOVA, main effect of box part, F1,92=336.9, p<0.0001, *** p<0.001, Tukey's HSD) but there was no main effect of genotype (main effect of genotype, F1,92=0, p>0.99; interaction effect, F1,92=2.24, p=0.14). Finally, we assessed the animals' behavior in an open arena, under higher-light conditions. In the open field test, time spent in the center of the field is associated with reduced anxiety, whereas time spent in the periphery, also known as thigmotaxis, is associated with higher anxiety (22, 23). Both WT and FGF22KO mice preferred the periphery of the open arena to the center (two-way ANOVA, main effect of open field part, F1,26=842.9, p<0.0001, *** p<0.001, Tukey's HSD) (Fig. 2C). Again, there was no main effect of genotype, (main effect of genotype, F1,26=0, p>0.99; interaction effect, F1,26=0.64, p=0.43). Taken together, these results demonstrate that FGF22KO animals exhibit normal anxiety-like behaviors.
Figure 2.
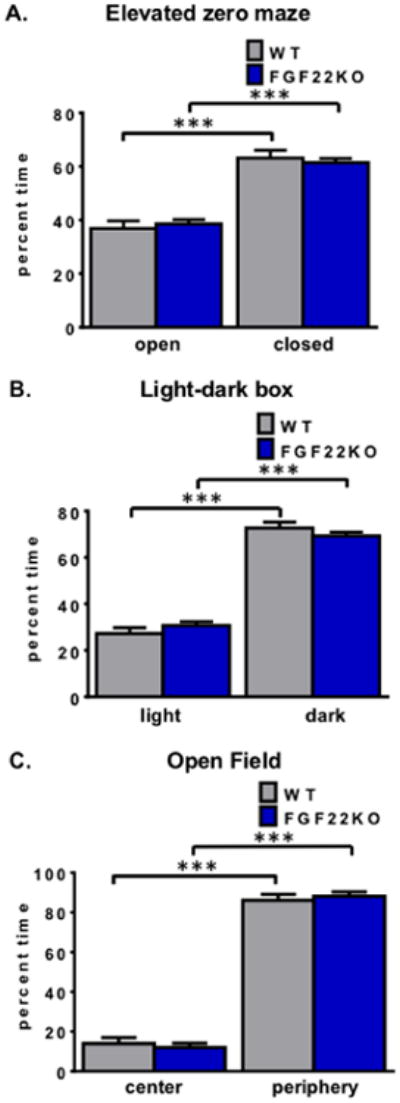
Mice lacking FGF22 display normal anxiety-like behaviors. A. FGF22KO mice spend the same amount of time in closed and open parts of the elevated zero maze as WT littermates (two-way ANOVA, main effect of maze part, F1,96=107.9, p<0.0001; *** p<0.001, Tukey's HSD; main effect of genotype, F1,96=0, p>0.99; interaction effect, F1,96=0.55, p=0.46). B. FGF22KO and WT mice spend the same amount of time in the dark compartment of the light-dark box (two-way ANOVA, main effect of box part, F1,92=336.9, p<0.0001, *** p<0.001, Tukey's HSD; main effect of genotype, F1,92=0, p>0.99; interaction effect, F1,92=2.24, p=0.14). C. FGF22KO and WT mice spend the same amount of time in the periphery of the open field arena (two-way ANOVA, main effect of open field part, F1,26=842.9, p<0.0001, *** p<0.001, Tukey's HSD; main effect of genotype, F1,26=0, p>0.99; interaction effect, F1,26=0.64, p=0.43).
3.3. Mice lacking FGF22 display depressive-like behaviors
We next asked whether FGF22KO mice displayed behavioral differences that might suggest depressive-like behaviors. The forced swim test is commonly used as a preclinical behavioral screen for antidepressant medications, where increased escape-targeted swimming behavior correlates with a drug's antidepressant effect (24). By corollary, animals that float in this test are thought to exhibit hopelessness, amotivation, and a passive style of coping with stress, which are characteristics present in depression (24, 25). Interestingly, FGF22KO spent significantly more time floating in the forced swim test than WT littermates (Student's unpaired two-tailed t-test, t(33)=2.62, p=0.01) (Fig. 3A). Additionally, they stopped swimming sooner than WT mice, shown as a significant decrease in the latency to begin floating (Student's unpaired two-tailed t-test, t(33)=2.48, p=0.02) (Fig. 3B). To confirm these results, we tested the animals with another test that assesses stress coping style, the tail suspension test. As in the forced swim test, increased immobility during tail suspension is correlated with increased passive stress-coping phenotypes (26, 27). Again, we observed a significant increase in the amount of time FGF22KO animals spent immobile compared to WT littermates (Student's unpaired two-tailed t-test, t(16)=2.17, p=0.045) (Fig. 3C). Finally, we tested whether animals exhibit behaviors suggestive with anhedonia, the reduction of engagement in pleasurable activity, in the sucrose preference test. Anhedonia is one of the core features of depression in humans. In this test, animals are provided with both water and 2% sucrose solution for several days to measure the degree of their preference for sucrose over water. Typically, mice prefer sucrose at a very high ratio (28). Using a separate cohort of experiment-naïve mice, we observed that WT mice demonstrate a significant preference for sucrose over water, whereas FGF22KO animals prefer sucrose to a lower extent, consistent with lack of pleasure-motivated behavior or anhedonia (Student's unpaired two-tailed t-test, t(29)=4.66, p<0.0001) (Fig. 3D). These data suggest that FGF22KO animals exhibit alterations in behavior consistent with a depression-like phenotype.
Figure 3.
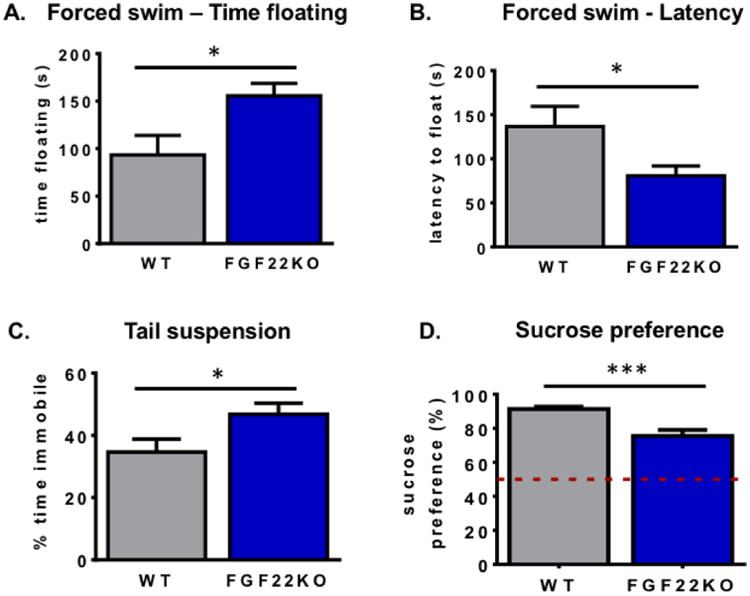
Mice lacking FGF22 display depressive-like behaviors. FGF22KO mice spend more time floating (A.) and start floating more quickly (B.) than their WT littermates (Student's unpaired two-tailed t-test, t(33)=2.62, *p<0.05 and t(33)=2.48, *p<0.05, respectively). C. FGF22KO mice spend more time immobile than WT littermates (Student's unpaired two-tailed t-test, t(16)=2.17, *p<0.05). D. FGF22KO mice drink less sucrose than WT littermates in a sucrose preference test (Student's unpaired two-tailed t-test, t(29)=4.66, ***p<0.001). Dotted line indicates chance level of discrimination (50%).
3.4. Mice lacking FGF22 display normal social behaviors and social recognition in a social cognitive task
Finally, we examined whether FGF22KO mice have deficits in social recognition and social preference. To address this question, we first asked whether FGF22KO mice would display normal preferences for novel animals in a social recognition test. We found that FGF22KO mice exhibit similar preferences as their WT littermates for animals compared to objects in the social recognition test (two-way ANOVA, main effect of test object, F1,66=71.21, p<0.0001; *** p<0.001, Tukey's HSD) (Fig. 4A), with no main effect of genotype (main effect of genotype, F1,66=0.013, p=0.91; interaction effect, F1,66=0.096, p=0.76). FGF22KO mice, similarly to their WT littermates, prefer novel animals over animals they have previously investigated (two-way ANOVA, main effect of test animal, F1,66=21.63, p<0.0001; *** p<0.001, Tukey's HSD) (Fig. 4B). Again, there was no main effect of genotype (main effect of genotype, F1,66=1.45, p=0.23; interaction effect, F1,66=0.79, p=0.38). There were no differences in the amount of time FGF22KO and WT mice spent exploring stimuli during any phase of the task (Student's unpaired two-tailed t-test, t(33)=0.19, p=0.85 for object phase; Student's unpaired two-tailed t-test, t(33)=1.31, p=0.20 for social phase) (Fig. 4C), supporting the open field data showing no differences in motor exploratory behavior (Fig. 1A and Supplementary Fig. 1). Since there is a 60-second delay between the social preference (object phase) and social recognition (social phase) stages of the test, this also confirms that FGF22KO mice have functional short-term memory, at least for conspecifics.
Figure 4.
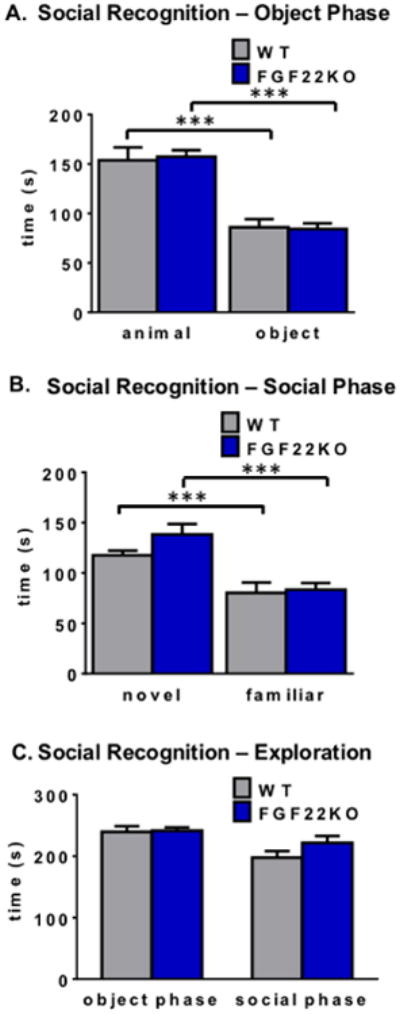
FGF22KO mice display normal learning and memory in a social cognitive task. A. In a standard social preference task, WT and FGF22KO animals prefer a novel animal over an object (two-way ANOVA, main effect of test object, F1,66=71.21, p<0.0001; ***Tukey's HSD p<0.001; main effect of genotype, F1,66=0.013, p=0.91; interaction effect, F1,66=0.096, p=0.76). B. In the social recognition stage of the task, WT and FGF22KO animals prefer a novel animal over a familiar one (two-way ANOVA, main effect of test animal, F1,66=21.63, p<0.0001; ***Tukey's HSD p<0.001; main effect of genotype, F1,66=1.45, p=0.23; interaction effect, F1,66=0.79, p=0.38). C. FGF22KO mice spend a similar amount of time exploring stimuli during the object phase as WT littermates (Student's unpaired two-tailed t-test, t(33)=0.19, p=0.85). Both FGF22KO and WT mice also explore stimuli during the social phase to a similar extent (Student's unpaired two-tailed t-test, t(33)=1.31, p=0.20).
4. Discussion
FGF22 is critical for the presynaptic organization of excitatory synapses in the hippocampus (1). Here we investigated the behavioral manifestations of the loss of FGF22 in adult mice. We found that FGF22KO mice display behaviors consistent with a depressive-like phenotype in three behavioral paradigms: the forced swim test, the tail suspension test, and the sucrose preference test. The fact that anhedonia-like behavior (lack of sucrose preference) was observed in an independent cohort of FGF22KO animals supports our findings of depression-like behaviors in the initial cohort of FGF22KO mice. Interestingly, our results show that loss of FGF22 during development does not affect general neurological functioning, as evidenced by normal motor and exploratory behaviors. There is also no obvious effect of FGF22 loss on anxiety-like behaviors. Additionally, loss of FGF22 does not negatively affect social behaviors or social memory. These results suggest a specific role for FGF22 in affective behaviors.
Since FGF22 is required for normal excitatory synapse development in the hippocampus, and the hippocampus has been implicated in affective behaviors modeled by the forced swim and tail suspension tests (28, 29), our results are consistent with the hypothesis that loss of excitatory synapses in the hippocampus is responsible for the altered behaviors observed in FGF22KO mice, including a passive style of coping with stress and anhedonia. Of course, it is also possible that there are other brain regions involved in the behaviors we observed, such as the prefrontal cortex (30-32), and we are currently investigating the neural circuits that may be involved in the behavioral manifestations of FGF22 deletion. Nevertheless, our results raise the intriguing question: why does loss of FGF22, which has been shown to be important in both the central and peripheral nervous system, cause such a specific behavioral phenotype? One potential answer is that there may be mechanisms that compensate for the lack of appropriate excitatory synapses, but the compensation might be limited spatially. For example, knockout mice for FGFR2, a receptor for FGF22, show transient defects at the neuromuscular junction (9), but more prolonged synaptic defects in the cerebellum (3). The question of how specific synapses compensate for the loss of FGF22 remains to be answered.
In addition to synapse formation, FGF22 may have additional roles during development and in adulthood, which may account for the depression-like phenotype in FGF22KO mice. It is known that FGF22 contributes to reactive neurogenesis in the adult dentate gyrus under certain conditions, such as seizure induction by pentylenetetrazol (7). Abnormal adult neurogenesis in the dentate gyrus has been hypothesized to contribute to depression (33). Thus, it is possible that FGF22KO mice have abnormal adult neurogenesis that induces abnormal affective behaviors, although at least some mouse models lacking adult neurogenesis do not exhibit abnormal stress-coping behaviors either on the forced swim test (34) or the tail suspension test (35) at baseline.
Alterations in the levels of FGFs and their receptors, FGFRs, have been observed in postmortem brain tissue from depressed patients (4, 36). Mice lacking FGFR1 signaling in telencephalic neurons have fewer cortical inhibitory interneurons, resulting in hyperactivity without other signs of learning problems (37, 38). Mice lacking FGF2 also display hyperactivity, correlated with decreased corticostriatal glutamatergic fibers (39). Interestingly, mice overexpressing FGF2 have an increase in hippocampal neuronal excitability and susceptibility to kainate-induced seizures (40), which suggests that FGF2 has variable functions depending upon the area of the brain studied. There may also be a critical developmental time period that determines how FGF2 alters behavior, including anxiety and depression, during adulthood (41, 42). Mice with inducible loss of FGFR2 have mild deficits in spatial learning, which depend on the developmental period in which FGFR2 expression was lost (43). Thus, multiple FGFs may play a role in the development and clinical manifestations of depression-related behaviors and other mental disorders in a temporally and spatially regulated manner.
In summary, our studies demonstrate the importance of FGF22 in affective behaviors in mice. This suggests the possibility that, conversely, upregulation or activation of FGF22 in certain brain areas could treat or reduce the risk of illnesses that feature deficits in stress coping and emotional reactivity as core manifestations of disease, such as depression. However, it should be noted that since global loss of FGF22 prevents seizure induction under certain experimental conditions (1), any intervention that increases FGF22 levels should be evaluated for seizure risk. Nonetheless, our results suggest that FGF22KO mice could be used as an animal model to screen for antidepressant compounds and that the appropriate control of FGF22 or FGF22 regulators, such as fibroblast growth factor binding proteins (FGFBPs) and fibroblast growth factor receptor like 1 (FGFRL1) (44), may lead to treatment of certain aspects of affective disorders.
Supplementary Material
Supplementary Figure 1. Male and female mice display similar motor exploratory behaviors. In a standard social preference task, male and female WT and FGF22KO animals spend similar amounts of time exploring stimuli during the object phase (two-way ANOVA, F(1,31) genotype=6.7×10-5, p=0.99, F(1,31) sex=0.13, p=0.72, F(1,31) interaction=2.02, p=0.17) and the social phase (two-way ANOVA, F(1,31) genotype=2.01, p=0.17, F(1,31) sex=1.35, p=0.25, F(1,31) interaction=1.69, p=0.20).
Supplementary Table 1. Animals used for behavioral tests. Total number of animals tested in each task is shown in each column, with numbers of male and female animals in each group is shown in parentheses.
Highlights.
Mice lacking fibroblast growth factor 22 (FGF22KO) have depressive-like behaviors.
FGF22KO mice have normal motor, exploratory, and social behaviors.
Fibroblast growth factor 22 plays specific roles in affective behaviors.
Acknowledgments
We thank Shannon Moore for technical assistance. We also thank Georgia Gunner and Nick Andrews of the Neurodevelopmental Behavioral Core at Boston Children's Hospital (IDDRC, P30 HD18655) for technical help. We thank members of the Murphy and Umemori labs for helpful comments on the paper.
Abbreviations
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- FGF22
fibroblast growth factor 22
- FGF22KO
fibroblast growth factor 22 knockout
- NMJ
neuromuscular junction
- PTZ
pentylenetetrazol
- WT
wild-type
Footnotes
The authors report no biomedical financial interests or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Aislinn J. Williams, Email: aiwillia@umich.edu.
Patricia Yee, Email: Patricia.Yee@childrens.harvard.edu.
Mitchell C. Smith, Email: mitchl@umich.edu.
Geoffrey G. Murphy, Email: murphyg@umich.edu.
Hisashi Umemori, Email: Hisashi.Umemori@childrens.harvard.edu.
References
- 1.Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature. 2010 Jun 10;465(7299):783–7. doi: 10.1038/nature09041. Epub 2010/05/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umemori H. Weaving the neuronal net with target-derived fibroblast growth factors. Dev Growth Differ. 2009 Apr;51(3):263–70. doi: 10.1111/j.1440-169X.2008.01079.x. Epub 2009/02/13. eng. [DOI] [PubMed] [Google Scholar]
- 3.Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004 Jul 23;118(2):257–70. doi: 10.1016/j.cell.2004.06.025. Epub 2004/07/21. eng. [DOI] [PubMed] [Google Scholar]
- 4.Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, et al. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A. 2004 Oct 26;101(43):15506–11. doi: 10.1073/pnas.0406788101. Epub 2004/10/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, et al. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav. 2008 Apr;7(3):344–54. doi: 10.1111/j.1601-183X.2007.00357.x. Epub 2007/10/03. eng. [DOI] [PubMed] [Google Scholar]
- 6.Williams AJ, Umemori H. The best-laid plans go oft awry: synaptogenic growth factor signaling in neuropsychiatric disease. Frontiers in synaptic neuroscience. 2014;6:4. doi: 10.3389/fnsyn.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CH, Umemori H. Suppression of epileptogenesis-associated changes in response to seizures in FGF22-deficient mice. Front Cell Neurosci. 2013;7:43. doi: 10.3389/fncel.2013.00043. Epub 2013/04/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Singh R, Su J, Brooks J, Terauchi A, Umemori H, Fox MA. Fibroblast growth factor 22 contributes to the development of retinal nerve terminals in the dorsal lateral geniculate nucleus. Front Mol Neurosci. 2012;4:61. doi: 10.3389/fnmol.2011.00061. Epub 2012/03/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox MA, Sanes JR, Borza DB, Eswarakumar VP, Fassler R, Hudson BG, et al. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell. 2007 Apr 6;129(1):179–93. doi: 10.1016/j.cell.2007.02.035. Epub 2007/04/10. eng. [DOI] [PubMed] [Google Scholar]
- 10.Dahlhaus R, Hines RM, Eadie BD, Kannangara TS, Hines DJ, Brown CE, et al. Overexpression of the cell adhesion protein neuroligin-1 induces learning deficits and impairs synaptic plasticity by altering the ratio of excitation to inhibition in the hippocampus. Hippocampus. 2010 Feb;20(2):305–22. doi: 10.1002/hipo.20630. [DOI] [PubMed] [Google Scholar]
- 11.Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10(1):47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Morellini F, Sivukhina E, Stoenica L, Oulianova E, Bukalo O, Jakovcevski I, et al. Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cerebral cortex. 2010 Nov;20(11):2712–27. doi: 10.1093/cercor/bhq017. [DOI] [PubMed] [Google Scholar]
- 13.McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiology & behavior. 2001 Aug;73(5):705–17. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- 14.Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiology & behavior. 2001 Jan;72(1-2):271–81. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- 15.McKinney BC, Murphy GG. The L-Type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learning & memory. 2006 Sep-Oct;13(5):584–9. doi: 10.1101/lm.279006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKinney BC, Schneider JS, Schafer GL, Lowing JL, Mohan S, Zhao MX, et al. Decreased locomotor activity in mice expressing tTA under control of the CaMKII alpha promoter. Genes Brain Behav. 2008 Mar;7(2):203–13. doi: 10.1111/j.1601-183X.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 17.McKinney BC, Chow CY, Meisler MH, Murphy GG. Exaggerated emotional behavior in mice heterozygous null for the sodium channel Scn8a (Nav1.6) Genes Brain Behav. 2008 Aug;7(6):629–38. doi: 10.1111/j.1601-183X.2008.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagnon JL, Korn MJ, Parent R, Tarpey TA, Jones JM, Hammer MF, et al. Convulsive seizures and SUDEP in a mouse model of SCN8A epileptic encephalopathy. Human molecular genetics. 2015 Jan 15;24(2):506–15. doi: 10.1093/hmg/ddu470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology. 1994 Sep;116(1):56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- 20.Bourin M, Hascoet M. The mouse light/dark box test. European journal of pharmacology. 2003 Feb 28;463(1-3):55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 21.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacology, biochemistry, and behavior. 1980 Aug;13(2):167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 22.Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacology, biochemistry, and behavior. 1988 Dec;31(4):959–62. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- 23.Gershenfeld HK, Paul SM. Mapping quantitative trait loci for fear-like behaviors in mice. Genomics. 1997 Nov 15;46(1):1–8. doi: 10.1006/geno.1997.5002. [DOI] [PubMed] [Google Scholar]
- 24.Kitada Y, Miyauchi T, Satoh A, Satoh S. Effects of antidepressants in the rat forced swimming test. European journal of pharmacology. 1981 Jun 19;72(2-3):145–52. doi: 10.1016/0014-2999(81)90269-7. [DOI] [PubMed] [Google Scholar]
- 25.Alcaro A, Cabib S, Ventura R, Puglisi-Allegra S. Genotype- and experience-dependent susceptibility to depressive-like responses in the forced-swimming test. Psychopharmacology. 2002 Nov;164(2):138–43. doi: 10.1007/s00213-002-1161-8. [DOI] [PubMed] [Google Scholar]
- 26.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85(3):367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 27.Vaugeois JM, Odievre C, Loisel L, Costentin J. A genetic mouse model of helplessness sensitive to imipramine. European journal of pharmacology. 1996 Dec 5;316(2-3):R1–2. doi: 10.1016/s0014-2999(96)00800-x. [DOI] [PubMed] [Google Scholar]
- 28.Tang M, He T, Sun X, Meng QY, Diao Y, Lei JY, et al. Subregion-specific decreases in hippocampal serotonin transporter protein expression and function associated with endophenotypes of depression. Hippocampus. 2014 Apr;24(4):493–501. doi: 10.1002/hipo.22242. [DOI] [PubMed] [Google Scholar]
- 29.Tang M, He T, Meng QY, Broussard JI, Yao L, Diao Y, et al. Immobility responses between mouse strains correlate with distinct hippocampal serotonin transporter protein expression and function. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014 May;15:1–14. doi: 10.1017/S146114571400073X. [DOI] [PubMed] [Google Scholar]
- 30.Browne CA, Clarke G, Hanke J, Dinan TG, Schwegler H, Yilmazer-Hanke DM, et al. Alterations in prefrontal cortical serotonin and antidepressant-like behavior in a novel C3H/HeJxDBA/2J recombinant inbred mouse strain. Behavioural brain research. 2013 Jan 1;236(1):283–8. doi: 10.1016/j.bbr.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003 Jan 1;23(1):349–57. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Espejo EF, Minano FJ. Prefrontocortical dopamine depletion induces antidepressant-like effects in rats and alters the profile of desipramine during Porsolt's test. Neuroscience. 1999 Jan;88(2):609–15. doi: 10.1016/s0306-4522(98)00258-9. [DOI] [PubMed] [Google Scholar]
- 33.Lee MM, Reif A, Schmitt AG. Major depression: a role for hippocampal neurogenesis? Current topics in behavioral neurosciences. 2013;14:153–79. doi: 10.1007/7854_2012_226. [DOI] [PubMed] [Google Scholar]
- 34.Jedynak P, Kos T, Sandi C, Kaczmarek L, Filipkowski RK. Mice with ablated adult brain neurogenesis are not impaired in antidepressant response to chronic fluoxetine. Journal of psychiatric research. 2014 May 29; doi: 10.1016/j.jpsychires.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Iascone DM, Padidam S, Pyfer MS, Zhang X, Zhao L, Chin J. Impairments in neurogenesis are not tightly linked to depressive behavior in a transgenic mouse model of Alzheimer's disease. PloS one. 2013;8(11):e79651. doi: 10.1371/journal.pone.0079651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goswami DB, Jernigan CS, Chandran A, Iyo AH, May WL, Austin MC, et al. Gene expression analysis of novel genes in the prefrontal cortex of major depressive disorder subjects. Progress in neuro-psychopharmacology & biological psychiatry. 2013 Jun 3;43:126–33. doi: 10.1016/j.pnpbp.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller Smith K, Fagel DM, Stevens HE, Rabenstein RL, Maragnoli ME, Ohkubo Y, et al. Deficiency in inhibitory cortical interneurons associates with hyperactivity in fibroblast growth factor receptor 1 mutant mice. Biological psychiatry. 2008 May 15;63(10):953–62. doi: 10.1016/j.biopsych.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 38.Shin DM, Korada S, Raballo R, Shashikant CS, Simeone A, Taylor JR, et al. Loss of glutamatergic pyramidal neurons in frontal and temporal cortex resulting from attenuation of FGFR1 signaling is associated with spontaneous hyperactivity in mice. J Neurosci. 2004 Mar 3;24(9):2247–58. doi: 10.1523/JNEUROSCI.5285-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fadda P, Bedogni F, Fresu A, Collu M, Racagni G, Riva MA. Reduction of corticostriatal glutamatergic fibers in basic fibroblast growth factor deficient mice is associated with hyperactivity and enhanced dopaminergic transmission. Biological psychiatry. 2007 Aug 1;62(3):235–42. doi: 10.1016/j.biopsych.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Zucchini S, Buzzi A, Barbieri M, Rodi D, Paradiso B, Binaschi A, et al. Fgf-2 overexpression increases excitability and seizure susceptibility but decreases seizure-induced cell loss. J Neurosci. 2008 Dec 3;28(49):13112–24. doi: 10.1523/JNEUROSCI.1472-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhury S, Aurbach EL, Sharma V, Blandino P, Jr, Turner CA, Watson SJ, et al. FGF2 is a target and a trigger of epigenetic mechanisms associated with differences in emotionality: Partnership with H3K9me3. Proc Natl Acad Sci U S A. 2014 Jul 28; doi: 10.1073/pnas.1411618111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu S, Peng H, Wang L, Vasish S, Zhang Y, Gao W, et al. Elevated specific peripheral cytokines found in major depressive disorder patients with childhood trauma exposure: a cytokine antibody array analysis. Comprehensive psychiatry. 2013 Oct;54(7):953–61. doi: 10.1016/j.comppsych.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 43.Stevens HE, Jiang GY, Schwartz ML, Vaccarino FM. Learning and memory depend on fibroblast growth factor receptor 2 functioning in hippocampus. Biological psychiatry. 2012 Jun 15;71(12):1090–8. doi: 10.1016/j.biopsych.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinberg F, Zhuang L, Beyeler M, Kalin RE, Mullis PE, Brandli AW, et al. The FGFRL1 receptor is shed from cell membranes, binds fibroblast growth factors (FGFs), and antagonizes FGF signaling in Xenopus embryos. J Biol Chem. 2010 Jan 15;285(3):2193–202. doi: 10.1074/jbc.M109.058248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Male and female mice display similar motor exploratory behaviors. In a standard social preference task, male and female WT and FGF22KO animals spend similar amounts of time exploring stimuli during the object phase (two-way ANOVA, F(1,31) genotype=6.7×10-5, p=0.99, F(1,31) sex=0.13, p=0.72, F(1,31) interaction=2.02, p=0.17) and the social phase (two-way ANOVA, F(1,31) genotype=2.01, p=0.17, F(1,31) sex=1.35, p=0.25, F(1,31) interaction=1.69, p=0.20).
Supplementary Table 1. Animals used for behavioral tests. Total number of animals tested in each task is shown in each column, with numbers of male and female animals in each group is shown in parentheses.


