Abstract
The ability to accurately monitor one's own memory is an important feature of normal memory function. Converging evidence from neuroimaging and lesion studies have implicated the dorsolateral prefrontal cortex (DLPFC) in memory monitoring. Here we used high definition transcranial direct stimulation (HD-tDCS), a non-invasive form of brain stimulation, to test whether the DLPFC has a causal role in memory monitoring, and the nature of that role. We used a metamemory monitoring task, in which participants first attempted to recall the answer to a general knowledge question, then gave a feeling-of-knowing (FOK) judgment, followed by a forced choice recognition task. When participants received DLPFC stimulation, their feeling-of-knowing judgments were better predictors of memory performance, i.e., they had better memory monitoring accuracy, compared to stimulation of a control site, the anterior temporal lobe (ATL). Effects of DLPFC stimulation were specific to monitoring accuracy, as there was no significant increase in memory performance, and if anything, there was poorer memory performance with DLPFC stimulation. Thus we have demonstrated a causal role for the DLPFC in memory monitoring, and showed that electrically stimulating the left DLPFC led people to more accurately monitor and judge their own memory.
Keywords: tDCS, HD-tDCS, prefrontal, memory, metamemory, feeling-of-knowing
1. Introduction
The ability to accurately monitor one's own memory, is one component of what is referred to as metamemory, and is an important feature of normal memory function (Nelson & Narens, 1990). For example, accurate memory monitoring can lead to better regulation of learning and memory, thereby improving overall performance (Thiede, Anderson, & Therriault, 2003). Furthermore, certain clinical populations have shown impairments in memory monitoring, independent of memory impairments (Stepanie Cosentino, Metcalfe, Butterfield, & Stern, 2007). Despite the critical role of monitoring in the effective use of memory, less is known about the neural mechanisms subserving memory monitoring accuracy, and whether it can be enhanced using non-invasive brain stimulation.
One common way to evaluate memory monitoring is by determining accuracy on metamemory tasks, such as the feeling-of-knowing (FOK) task (Nelson & Narens, 1990). Typical FOK tasks ask participants to recall a target piece of information, and when they fail to do so, they are asked to predict the likelihood that they will remember the answer later (i.e., to make a feeling-of-knowing judgment), followed by a recognition test for the target information (Hart, 1965; Kikyo & Miyashita, 2004; Maril, Simons, Mitchell, Schwartz, & Schacter, 2003; Metcalfe, Schwartz, & Joaquim, 1993; Schwartz & Metcalfe, 1992). Monitoring accuracy, also referred to as metamemory accuracy, is then assessed by determining how well the individual's subjective FOK ratings relate to their objective memory performance (Benjamin & Diaz, 2008; Nelson, 1984; Pannu & Kaszniak, 2005).
Monitoring accuracy depends on how well the information used to make these metamemory judgments relates to memory accuracy (Chua, Hannula, & Ranganath, 2012). FOK judgments have been shown to be based on cue familiarity (Metcalfe et al., 1993; Reder, 1987), target accessibility (Koriat, 1993), and the combination of the two (Chua & Solinger, 2015; Koriat & Levy-Sadot, 2001). FOKs can be based on a rapid evaluation of the cue, with more familiar cues leading to higher FOKs (Metcalfe et al., 1993; Reder, 1987). Sufficient cue familiarity then leads to a memory retrieval search, and higher FOKs are given when more partial information accessed, regardless of whether this partial information is correct (Koriat, 1993). By this view, FOK accuracy (i.e., monitoring accuracy in the FOK task) depends on the quality of the memory processes, in that judgments are based on the outcomes of the search attempts for the target (Koriat, 1993).
Indeed, there is some evidence that FOK and memory may not be completely independent processes. Memory impairments have been associated with reduced FOK accuracy (Bacon et al., 1998; Perrotin, Belleville, & Isingrini, 2007). However, other studies have shown that impaired memory does not have to be associated with reduced FOK accuracy (Schacter, 1983; Shimamura & Squire, 1986). For example, comparisons of Korsakoff's patients and other amnesics, both of whom had reduced memory performance, showed that only Korsakoff's patients showed impaired FOK accuracy (Shimamura & Squire, 1986). This difference was attributed to the frontal lobe pathology seen in Korsakoff's patients, but not in the other amnesics.
The role of the prefrontal cortex in FOKs may be related to its role in executive functioning (Fernandez-Duque, Baird, & Posner, 2000; Pannu & Kaszniak, 2005; Perrotin, Tournelle, & Isingrini, 2008; Shimamura, 2000; Souchay, Isingrini, & Espagnet, 2000). Recent work using neuropsychological testing showed that executive functioning contributed to FOK accuracy, and that this effect was much larger than the contribution of memory ability (Perrotin et al., 2008). Furthermore, several neuroimaging studies have shown that activity in the DLPFC increases with increasing demands on retrieval monitoring (Dobbins, Foley, Schacter, & Wagner, 2002; Gallo, McDonough, & Scimeca, 2010), with monitoring being one of the executive functions.
Many fMRI studies have shown greater activation with increasing levels of FOKs in many PFC subregions (Chua, Schacter, & Sperling, 2009; Elman, Klostermann, Marian, Verstaen, & Shimamura, 2012; Kikyo, Ohki, & Miyashita, 2002; Maril, Simons, Weaver, & Schacter, 2005), and this tends to be most consistent in left lateral prefrontal cortex (Chua et al., 2009; Kikyo & Miyashita, 2004; Kikyo et al., 2002; Maril et al., 2003). There are several possible explanations for why activity in the PFC modulates with FOK: 1) activity in the PFC may provide an index of the subjective feeling per se (Chua et al., 2009), 2) activity in the PFC increases with retrieval monitoring demands (Henson, Rugg, Shallice, & Dolan, 2000; Henson, Shallice, & Dolan, 1999), and trials with higher FOKs tend to require weighing and monitoring of more information, or 3) the PFC may modulate based on the amount of target information accessed and higher levels of FOKs may be associated with increased target access and partial retrieval (Maril et al., 2005).
The role of the PFC in FOKs has yet to be tested using non-invasive brain stimulation, which has become a useful tool in cognitive neuroscience research and has potential use for development as an intervention (Berryhill, Peterson, Jones, & Stephens, 2014; Sparing & Mottaghy, 2008). Transcranial direct current stimulation (tDCS) involves passing a low level current between stimulation and reference electrodes placed on the scalp, with the stimulating electrode typically being placed over the targeted brain region (for review, see Nitsche et al., 2008). Stimulation using a positively charged electrode, typically referred to as anodal stimulation, has been shown to increase neural firing rates, with after effects that last beyond the stimulation duration. Conventional tDCS typically places large electrodes that are spaced relatively far apart on the scalp, thereby stimulating both the targeted brain region and surrounding and connected structures (Bai, Dokos, Ho, & Loo, 2014). More focal methods of tDCS have been developed, termed High Definition tDCS (HD-tDCS), which use a 4 × 1 ring electrode, and which better restricts the stimulation to the region of interest (Datta et al., 2009; Kuo et al., 2013).
In this study, we use HD-tDCS to test the whether or not the DLPFC has a causal role in the feeling-of-knowing using a general knowledge task, and to examine the nature of that role. We targeted the left DLPFC because it has modulated by level of FOK across multiple kinds of tasks (Reggev, Zuckerman, & Maril, 2011). Paralleling lesion studies that have compared patients with frontal and temporal lesions to examine metamemory versus memory contributions to FOK accuracy (Shimamura & Squire, 1986), we compared left DLPFC stimulation to left anterior temporal lobe (ATL) stimulation because the left ATL has been shown to be critical for semantic memory (Hodges, Patterson, Oxbury, & Funnell, 1992; Mummery et al., 2000). If the role of the DLPFC in FOKs is to index the subjective feeling per se (Chua et al., 2009), excitation of the DLPFC should lead to higher FOKs compared to ATL and sham stimulation. In contrast, if the role of the DLPFC is in retrieval monitoring (Henson et al., 2000, 1999), then excitation of the DLPFC should lead to increased monitoring accuracy. Finally, if DLPFC activity relates to the amount of information accessed (Maril et al., 2005), then both DLPFC and ATL stimulation should lead to higher FOK ratings compared to sham, and potentially improved memory accuracy. In summary, the goals of this study were to use HD-tDCS to examine the role of the left DLPFC and left ATL in memory and metamemory for general knowledge questions.
2. Materials and Methods
2.1 Participants
Thirty healthy, English speaking, right-handed, Brooklyn College students participated in this 3-session, research study for course credit (1 credit per hour) or for pay ($15/hour). Three participants failed to return for all three visits, and the data from the 27 participants (13F/14M, ages 18-25, Mean ± SEM: 20.3 ± 0.31 years) who completed the study were analyzed. G*Power 3.1 (Faul, Erdfelder, Lang, & Buchner, 2007) was used to determine that data from 27 subjects were needed for 80% power to detect a medium-sized effect (η2=0.06) for a repeated measures ANOVA with 1 group and 3 measurements. Participants were screened before each experiment to ensure they were suitable to participate. Participants were free from neurologic and psychiatric illness, nor had any open wounds on the scalp and/or face. Each participant provided written consent in a manner approved by the Human Research Protection Program at the City University of New York (CUNY).
2.3 High Definition Transcranial Direct Current Stimulation (HD-tDCS)
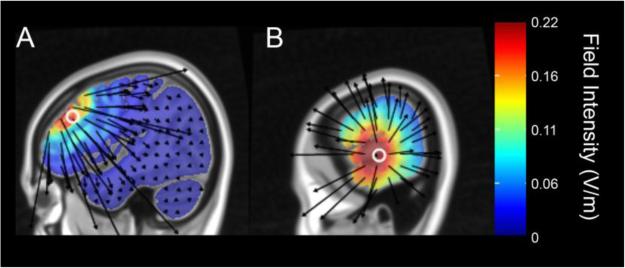
HD-tDCS was administered using the Soterix 4×1 adaptor for the Soterix 1×1 tDCS Low-Intensity Stimulator (Model 1224-B, Soterix Medical, New York, NY). For DLPFC and sham stimulation sessions, the anode was placed at the F3 position (using the 10-20 system) and 4 return electrodes were placed at positions AF3, F1, F5, and FC3. For ATL stimulation, the anode was placed at T7 and 4 return electrodes were placed at FT7, FT9, C5, and TP7. The stimulation electrodes were sintered Ag/AgCl ring electrodes (outer radius: 12mm; inner radius 6mm). The electrodes were fixed on an EEG cap with HD Electrode holders (Soterix Medical, New York, NY) and filled with Signa Gel to ensure electroconductivity to the scalp. In each montage, the anode was set to deliver a total current of 2 mA, and the return electrodes shared the same current intensity (0.5 mA each). These montages were chosen based on computational models that generated stimulated current maps using HDExplore (Soterix Medical, New York, NY) that showed good current flow in the left DLPFC and left ATL, respectively (Figure 1). During active stimulation, participants received 2mA of stimulation for 20 minutes, whereas during sham stimulation, the current ramped up to 2 mA and then back down during the first ~30 sec, remained at 0.1 mA for 19 minutes, and then ramped up to 2mA and down again at the end of the sham period for the last 30 seconds. Participants completed 3 sessions, approximately one week apart, and typically at the same time of day, with separate DLPFC, ATL, and sham HD-tDCS sessions; sessions were counterbalanced for stimulation and list order across subjects.
Figure 1.
Model of brain current flow using HD-Explore™ during HD-tDCS for A) left DLPFC stimulation with the anode placed at the F3 position and 4 return electrodes at positions AF3, F1, F5, and FC3, and B) left ATL stimulation with the anode placed at T7 and 4 return electrodes at FT7, FT9, C5, and TP7.
2.4 Stimuli and Procedure
Stimuli consisted of 300 general knowledge questions that had previously been normed using a CUNY sample (Mangels, Abraham, Hoxha, Bakdash, & Adali, 2015), and were presented using Psychopy (Peirce, 2007). There were 4 additional practice questions used to familiarize participants with the task.
HD-tDCS began at the start of the general knowledge task. After the instructions, participants first viewed a general knowledge question on the screen and were asked to recall the answer and type it in. If they did not know the answer, they were instructed to type “idk” to indicate “I don't know.” After 45 seconds, or a subject response, the trial advanced. This was followed by a FOK judgment, in which they had a maximum of 30 seconds to indicate the likelihood that they would recognize the correct answer on a 1-10 scale. This was then followed by a recognition judgment in which they had a maximum of 15 seconds to choose which among 4 answers was the correct answer to the general knowledge question. One of the answers was the correct answer, and the three incorrect answers were the most frequently given incorrect answers given by other CUNY students (Mangels et al., 2015). There were 100 questions per session, and the three sets of questions were matched on difficulty based on previous norming with CUNY students. Stimulation condition and question set were counterbalanced for order across participants.
2.5 Data Analysis
FOK accuracy was assessed in a trial-by-trial manner by calculating da, a measure based in signal detection theory used to index relative metamemory accuracy and is thought to be superior to other measures of monitoring resolution (Benjamin & Diaz, 2008; Toth, Daniels, & Solinger, 2011). To compute da, we used the procedure described by Benjamin and Diaz (2008), using the formula √2y0/(1+m2), where y0 is the y intercept and m2 is the slope of a normal deviate isosensitivity function.
The relationships between memory, the level of FOK expressed, FOK accuracy, and HD-tDCS were analyzed using repeated measures ANOVAs and post-hoc t-tests in SPSS 22.0. Mauchly's test of sphericity was used to determine whether or not the assumption of sphericity was met. If the assumption was violated, the Greenhouse-Geisser correction was applied and the epsilon (ε) is reported. Results were considered significant at p<0.05. Post-hoc t-tests were Bonferroni corrected for multiple comparisons, with the adjusted criterion reported in the text.
3. Results
There were moderate levels of correct recall of answers to general knowledge across stimulation sessions (Table 1), and the effect of stimulation on the number of correctly recalled answers approached significance [F(1.52, 39.64)=3.23, p<0.065, ε=0.76, η2=0.11]. For the recognition test, subjects performed well above the chance rate of 0.25 (Table 1). There were no differences in the overall proportion of correctly recognized answers [F(2, 52)=0.19, p>0.80, η2=0.007], or in the proportion of non-recalled answers that were later recognized [F(1.62, 42.07)=0.49, p>0.6, ε=0.81, η2=0.015] between stimulation sessions.
Table 1.
Memory performance (Mean ± SEM) for recall (# of correct answers) and recognition (proportion correct) for each stimulation condition.
| Recall | Overall Recognition | Recognition of non-recalled | |
|---|---|---|---|
| Sham | 36.37 (± 2.47) | 0.57 (± 0.021) | 0.39 (± 0.022) |
| DLPFC | 33.04 (± 2.06) | 0.57 (± 0.023) | 0.40 (± 0.027) |
| ATL | 38.22 (± 2.47) | 0.58 (± 0.019) | 0.36 (± 0.029) |
DLPFC = Dorsolateral Prefrontal Cortex; ATL = Anterior Temporal Lobe
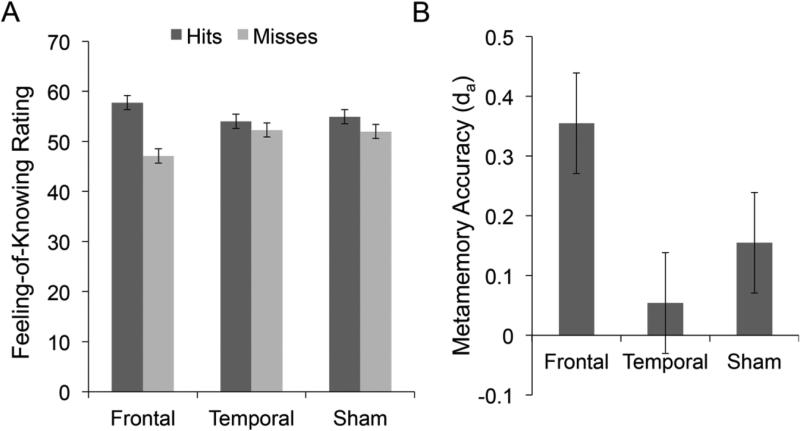
To examine whether stimulation had an effect on the level of subjective FOK given, we next conducted a recognition accuracy (using non-recalled items) × stimulation repeated measures ANOVA on FOK ratings. There was a significant main effect of recognition accuracy, with higher FOK ratings given for correctly recognized answers compared to incorrectly recognized answers [F(1,26)=12.88, p<0.001, ηp 2=0.35], which was qualified by a significant accuracy × stimulation interaction [F(1.52,39.61)=7.52, p<0.004, ε=0.76, ηp2=0.22]. Follow up ANOVAs showed no differences in FOK ratings for correct responses [F(1.59,41.39)=0.798, p>0.4, ε=0.80] or incorrect responses [F(2,52)=2.64, p<0.081] across stimulation sessions. To further probe the accuracy x stimulation interaction, we conducted two-way repeated measures ANOVAs to test for differences in FOK ratings for correct and incorrect responses by stimulation condition. There were significant accuracy by stimulation interactions when comparing DLPFC to sham [F(1,26)=7.66, p<0.01], and DLPFC to ATL [F(1,26)=9.72, p<0.004], but not ATL to sham [F(1,26)=0.56, p>0.46]. Post-hoc paired t-tests, considered significant at p<0.017 to Bonferroni correct for multiple comparisons, comparing FOK ratings for correct and incorrect recognition across stimulation sessions showed a significant difference between FOK ratings for correct and incorrect recognition for the frontal session [t(26)=3.94, p<0.001], whereas there was no difference in FOK ratings for correct and incorrect recognition during sham [t(26)=2.04, p<0.053] and temporal sessions [t(26)=1.10, p>0.28] (Figure 2a). Thus, the significant interaction was driven by a bigger difference in FOK ratings for correct and incorrect recognition with DLPFC stimulation compared to the other conditions.
Figure 2.
DLPFC stimulation led to improved metamemory accuracy, as shown by A) a bigger difference in FOK ratings for correct and incorrect recognition, and B) da, a signal detection-based, trial-by-trial measure of metamemory accuracy. Error bars represent within-subjects standard errors of the mean (Loftus & Masson, 1994).
We next examined relative FOK accuracy using da (Benjamin & Diaz, 2008) to index monitoring resolution in a trial-by-trial manner. As one might expect based on the significant recognition accuracy × stimulation interaction effect on FOK ratings, there was a significant difference in da based on stimulation [F(2,52)=3.98, p<0.03, η2=0.13]. Post-hoc paired t-tests were considered significant at p<0.017 to Bonferroni correct for multiple comparisons, and showed better FOK accuracy in the DLPFC session compared to the ATL session [t(26)=2.55,p<0.017], but the differences between DLPFC and sham [t(26)=1.64, p<0.11] and ATL and sham [t(26)=1.26, p>0.22] did not reach significance.
4. Discussion
Excitation of the left DLPFC using HD-tDCS showed enhanced monitoring accuracy relative to an active control, namely excitation of the left ATL. Moreover, HD-tDCS over the left DLPFC produced effects that were specific to metamemory accuracy, with no improvements in memory performance, indicating a specific and causal role for the DLPFC in metamemory accuracy that cannot be attributed to a global improvement in memory performance. These findings show that the DLPFC plays a causal role in memory monitoring, independent from memory, and that electrical stimulation to the DLPFC can improve monitoring accuracy.
Our results showed a single dissociation, with improved metamemory accuracy with HD-tDCS over the left DLPFC, and no significant effects on recall or recognition. Although the ANOVA testing for effects of stimulation on recall of general knowledge approached significance (p<0.065), there was no evidence that excitation of the left DLPFC improved recall; the subthreshold difference between sessions was driven by greater recall under left ATL compared to left DLPFC stimulation [t(26)=2.314, p<0.03]. This is consistent previous evidence for a role of the left ATL in semantic memory (Hodges et al., 1992; Mummery et al., 2000). More importantly, the improvement in metamemory accuracy seen with HD-tDCS over the left DLPFC was independent of memory performance.
It is worth noting that the improvement in metamemory accuracy, as measured by da, was seen only when comparing HD-tDCS over the DLPFC to HD-tDCS over the ATL, and not sham tDCS. We believe an active control is a superior control than sham because this was a within subjects design and it is more likely that participants may have detected differences in active vs. sham stimulation due to the physical sensations of HD-tDCS on the scalp. Nevertheless, although the trial-by-trial measure of metamemory accuracy, da, did not show differences between DLPFC and sham HD-tDCS and puts some limits on our conclusions, and it is possible that our effect comes from decreased metamemory accuracy with ATL stimulation in combination with increased metamemory accuracy with DLPFC stimulation. However, examination of the FOK ratings for correct and incorrect responses did show a bigger difference in FOK ratings for DLPFC compared to ATL stimulation and sham HD-tDCS, suggesting that the performance differences were indeed driven by improved metamemory accuracy in the DLPFC.
The finding that excitation of the DLPFC improved metamemory accuracy, rather than increasing memory accuracy or the level of FOK expressed, is consistent with a more general role for the DLPFC in retrieval monitoring aspects of executive functioning (Dobbins et al., 2002; Gallo et al., 2010). Indeed, previous neuropsychological work has shown that executive functioning accounts for a relatively large amount of the variance in FOK accuracy (Perrotin et al., 2008). When examining the contributions of specific executive functions, namely shifting, updating, and inhibition, shifting ability was shown to be the main executive function that predicted FOK accuracy (Perrotin et al., 2008). One possibility is that shifting relates to FOK accuracy because shifting ability may allow switching back and forth among multiple sources of information used to make the FOK judgment (i.e., cue familiarity, target access, general ability) and this shifting leads to more accurate FOK judgments because multiple inputs are considered. Further research will need to further unpack the mechanism by which HD-tDCS over the DLPFC improved metamemory accuracy, but is seems likely that it may be through improving more general, executive processes, possibly related to shifting.
One question is whether our finding that excitation of the left DLPFC improves metamemory accuracy would extend to other metamemory judgments. FOK judgments in semantic and episodic memory tasks have been shown to have, at least in part, different neural bases (Reggev et al., 2011). However, the left DLPFC showed a similar role in episodic and semantic FOKS (Reggev et al., 2011), suggesting that our results would generalize across FOKs tasks. Whether or not excitation of the left DLPFC would show similar improvements in other, non-FOK, memory monitoring tasks is an open question. Different metamemory tasks are known to have different cognitive (Chua & Solinger, 2015) and neural bases (Chua et al., 2009), and the excitation of the DLPFC may show different effects in these tasks (Ryals, Rogers, Gross, Polnaszek, & Voss, 2015).
Another issue worth noting is that HD-tDCS over other prefrontal regions could also lead to improvements in FOK accuracy. We chose the DLPFC because of its well known role in retrieval monitoring (Dobbins et al., 2002; Gallo et al., 2010; Henson et al., 1999), correlation with FOK rating (Chua et al., 2009; Maril et al., 2003), and because many conventional tDCS studies have targeted the DLPFC (e.g., Gray, Brookshire, Casasanto, & Gallo, 2015; Javadi & Walsh, 2012; Keeser et al., 2011). However, given the rostrocaudal hierarchical organization of the prefrontal cortex (Badre, 2008), stimulation over multiple prefrontal regions could show similar effects. Indeed, lesion and fMRI evidence have shown that the vmPFC is associated with FOK accuracy (Modirrousta & Fellows, 2008; Schnyer et al., 2004; Schnyer, Nicholls, & Verfaellie, 2005), making it a candidate target for future work.
This is a single HD-tDCS study, and as with any single study, should be interpreted with some caution. This is especially relevant given recent meta-analyses of tDCS research that have not found any consistent effects of tDCS on cognition in healthy, young adults (Horvath, Forte, & Carter, 2014, 2015; Tremblay et al., 2014). First, it is worth noting, as the authors acknowledge, that the meta-analyses are limited by the lack of comparable data, and there were often 2-3 studies in each domain examined (Horvath et al., 2015). Second, these analyses were based on conventional tDCS, not HD-tDCS, and it is unknown whether the same findings would hold for HD-tDCS, and there are not enough HD-tDCS papers to undertake such meta-analyses. Nevertheless, the results of the meta-analyses raise the question of whether tDCS has any real effects on brain function. Even if the effects on cognition are seemingly variable, effects on motor evoked potentials are more reliable (Horvath et al., 2014), suggesting that tDCS can have consistent effects, and that there is a need to identify additional variables that may determine whether or not tDCS has effects on cognition. One strong possibility is that tDCS effects emerge only when a task is sufficiently challenging (Berryhill et al., 2014) or the behavior is sub-optimal (Berryhill & Jones, 2012; Kalu, Sexton, Loo, & Ebmeier, 2012). Indeed, we have shown that the effect of tDCS over the parietal cortex on false recognition in the Deese-Roediger-McDermott paradigm, which is a challenging task, is reproducible (Pergolizzi & Chua, 2015). The current study used moderately difficult general knowledge questions with a challenging recognition task with difficult lures, thus setting up a situation in which participants may not be performing optimally and modulation of brain activity could enhance performance. Indeed, the authors of the meta-analyses acknowledge that differences in a subjects’ states could explain the inconsistent effects of tDCS shown in their meta-analyses (Horvath et al., 2015). Although we present a single, within subjects, HD-tDCS study, the results are consistent with previous lesion and neuroimaging studies implicating the prefrontal cortex in metamemory accuracy (Schnyer et al., 2004, 2005). Future work will examine whether task difficulty and state effects have a role in the effects of tDCS on memory and metamemory.
Our finding that HD-tDCS over the left DLPFC improves monitoring accuracy demonstrates a causal role for the left DLPFC in retrieval monitoring. Accurate memory monitoring can serve to improve learning and memory (Thiede et al., 2003) and can be impaired in certain clinical populations (Stepanie Cosentino et al., 2007). Impaired memory monitoring can delay seeking help and, therefore, early detection of memory impairments (Stephanie Cosentino, 2014). Our findings indicate that the left DLPFC has a causal role in monitoring accuracy, and that HD-tDCS over the left DLPFC is a potential target for developing interventions for impairments in metamemory. Further research is needed to determine whether improvement occurs in clinical populations, and the extent and duration of these effects.
Highlights.
We stimulated the prefrontal cortex during a feeling-of-knowing memory task
Prefrontal excitation led to improved memory monitoring accuracy
Prefrontal excitation did not improve semantic memory retrieval
Consistent with a role of the prefrontal cortex in memory monitoring
Acknowledgements
This work was supported by the National Institute of General Medical Sciences and the National Institute on Aging under award number SC2AG046910. The content is solely responsible of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bacon E, Danion JM, Kauffmann-Muller F, Schelstraete MA, Bruant A, Sellal F, Grange D. Confidence level and feeling of knowing for episodic and semantic memory: an investigation of lorazepam effects on metamemory. Psychopharmacology. 1998;138(3-4):318–25. doi: 10.1007/s002130050677. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9725754. [DOI] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends in Cognitive Sciences. 2008;12(5):193–200. doi: 10.1016/j.tics.2008.02.004. doi:10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Bai S, Dokos S, Ho K-A, Loo C. A computational modelling study of transcranial direct current stimulation montages used in depression. NeuroImage. 2014;87:332–44. doi: 10.1016/j.neuroimage.2013.11.015. doi:10.1016/j.neuroimage.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Benjamin AS, Diaz M. Measurement of relative metamnemoic accuracy. In: Dunlosky J, Bjork RA, editors. Handbook of memory and metamemory. Psychology Press; New York: 2008. pp. 73–94. [Google Scholar]
- Berryhill ME, Jones KT. tDCS selectively improves working memory in older adults with more education. Neuroscience Letters. 2012;521(2):148–51. doi: 10.1016/j.neulet.2012.05.074. doi:10.1016/j.neulet.2012.05.074. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Peterson DJ, Jones KT, Stephens JA. Hits and misses: leveraging tDCS to advance cognitive research. Frontiers in Psychology. 2014;5(July):800. doi: 10.3389/fpsyg.2014.00800. doi:10.3389/fpsyg.2014.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Hannula DE, Ranganath C. Distinguishing highly confident accurate and inaccurate memory: Insights about relevant and irrelevant influences on memory confidence. Memory. 2012;20(1):48–62. doi: 10.1080/09658211.2011.633919. doi:10.1080/09658211.2011.633919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Sperling RA. Neural correlates of metamemory: a comparison of feeling-of-knowing and retrospective confidence judgments. Journal of Cognitive Neuroscience. 2009;21(9):1751–1765. doi: 10.1162/jocn.2009.21123. doi:10.1162/jocn.2009.21123.Neural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Solinger LA. Building metamemorial knowledge over time: insights from eye tracking about the bases of feeling-of-knowing and confidence judgments. Frontiers in Psychology. 2015;6 doi: 10.3389/fpsyg.2015.01206. doi:10.3389/fpsyg.2015.01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S. Metacognition in Alzheimer's Disease. In: Fleming SM, Frith CD, editors. The Cognitive Neuroscience of Metacognition. Springer-Verlag; Berlin, Heidelberg: 2014. pp. 389–407. [Google Scholar]
- Cosentino S, Metcalfe J, Butterfield B, Stern Y. Objective metamemory testing captures awareness of deficit in Alzheimer's disease. Cortex. 2007;43(7):1004–1019. doi: 10.1016/s0010-9452(08)70697-x. doi:10.1016/S0010-9452(08)70697-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulation. 2009;2(4):201–7. 207, e1. doi: 10.1016/j.brs.2009.03.005. doi:10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35(5):989–96. doi: 10.1016/s0896-6273(02)00858-9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12372291. [DOI] [PubMed] [Google Scholar]
- Elman JA, Klostermann EC, Marian DE, Verstaen A, Shimamura AP. Neural correlates of metacognitive monitoring during episodic and semantic retrieval. Cognitive, Affective & Behavioral Neuroscience. 2012;12(3):599–609. doi: 10.3758/s13415-012-0096-8. doi:10.3758/s13415-012-0096-8. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Baird J. a, Posner MI. Executive attention and metacognitive regulation. Consciousness and Cognition. 2000;9(2 Pt 1):288–307. doi: 10.1006/ccog.2000.0447. doi:10.1006/ccog.2000.0447. [DOI] [PubMed] [Google Scholar]
- Gallo DA, McDonough IM, Scimeca J. Dissociating source memory decisions in the prefrontal cortex: fMRI of diagnostic and disqualifying monitoring. Journal of Cognitive Neuroscience. 2010;22(5):955–69. doi: 10.1162/jocn.2009.21263. doi:10.1162/jocn.2009.21263. [DOI] [PubMed] [Google Scholar]
- Gray SJ, Brookshire G, Casasanto D, Gallo DA. Electrically stimulating prefrontal cortex at retrieval improves recollection accuracy. Cortex. 2015;73:188–194. doi: 10.1016/j.cortex.2015.09.003. doi:10.1016/j.cortex.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Hart JT. Memory and the feeling-of-knowing. J. of Educational Psychology. 1965;56(4):208–216. doi: 10.1037/h0022263. doi:10.1037/h0022263. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. Journal of Cognitive Neuroscience. 2000;12(6):913–23. doi: 10.1162/08989290051137468. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11177413. [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain : A Journal of Neurology. Pt. 1999;1227:1367–81. doi: 10.1093/brain/122.7.1367. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10388802. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. SEMANTIC DEMENTIA. Brain. 1992;115(6):1783–1806. doi: 10.1093/brain/115.6.1783. doi:10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O. Evidence that transcranial direct current stimulation (tDCS) Generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy Human subjects: A systematic review. Neuropsychologia. 2014;66:213–236. doi: 10.1016/j.neuropsychologia.2014.11.021. doi:10.1016/j.neuropsychologia.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-session Transcranial Direct Current Stimulation (tDCS). Brain Stimulation. 2015;8(3):535–50. doi: 10.1016/j.brs.2015.01.400. doi:10.1016/j.brs.2015.01.400. [DOI] [PubMed] [Google Scholar]
- Javadi AH, Walsh V. Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimulation. 2012;5(3):231–41. doi: 10.1016/j.brs.2011.06.007. doi:10.1016/j.brs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Kalu UG, Sexton CE, Loo CK, Ebmeier KP. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychological Medicine. 2012;42(9):1791–800. doi: 10.1017/S0033291711003059. doi:10.1017/S0033291711003059. [DOI] [PubMed] [Google Scholar]
- Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, Padberg F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2011;31(43):15284–93. doi: 10.1523/JNEUROSCI.0542-11.2011. doi:10.1523/JNEUROSCI.0542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikyo H, Miyashita Y. Temporal lobe activations of “feeling-of-knowing” induced by face-name associations. NeuroImage. 2004;23:1348–57. doi: 10.1016/j.neuroimage.2004.08.013. doi:10.1016/j.neuroimage.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Kikyo H, Ohki K, Miyashita Y. Neural correlates for feeling-of-knowing: an fMRI parametric analysis. Neuron. 2002;36:177–86. doi: 10.1016/s0896-6273(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Koriat A. How do we know that we know ? The accessibility model of the feeling of knowing. Psychological Review. 1993;100(4):609–639. doi: 10.1037/0033-295x.100.4.609. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8255951. [DOI] [PubMed] [Google Scholar]
- Koriat A, Levy-Sadot R. The combined contributions of the cue-familiarity and accessibility heuristics to feelings of knowing. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27(1):34–53. doi:10.1037//0278-7393.27.1.34. [PubMed] [Google Scholar]
- Kuo H-I, Bikson M, Datta A, Minhas P, Paulus W, Kuo M-F, Nitsche MA. Comparing cortical plasticity induced by conventional and high- definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimulation. 2013;6(4):644–8. doi: 10.1016/j.brs.2012.09.010. doi:10.1016/j.brs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using Confidence Intervals in Within-Subjects Designs. Psychonomic Bulletin & Review. 1994;1:476–490. doi: 10.3758/BF03210951. Retrieved from papers3://publication/uuid/0DFB63AE-D78E-44E2-B870-1341DCA37BBC. [DOI] [PubMed] [Google Scholar]
- Mangels JA, Abraham D, Hoxha O, Bakdash J, Adali S. Trusting information in social networks: How do we determine the credibility of the information we receive?. Annual Meeting of the Association for Psychological Science; New York, NY.. 2015. [Google Scholar]
- Maril A, Simons JS, Mitchell JP, Schwartz BL, Schacter DL. Feeling- of-knowing in episodic memory: An event-related fMRI study. Neuroimage. 2003;18:827–836. doi: 10.1016/s1053-8119(03)00014-4. doi:10.1016/S1063-8119(03)00014-4. [DOI] [PubMed] [Google Scholar]
- Maril A, Simons JS, Weaver JJ, Schacter DL. Graded recall success: an event-related fMRI comparison of tip of the tongue and feeling of knowing. NeuroImage. 2005;24(4):1130–8. doi: 10.1016/j.neuroimage.2004.10.024. doi:10.1016/j.neuroimage.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Schwartz B, Joaquim S. The cue-familiarity heuristic in metacognition. Journal of Experimental Psychology. Learning, Memory, and Cognition. 1993;19(4):851–861. doi: 10.1037//0278-7393.19.4.851. Retrieved from http://psycnet.apa.org/journals/xlm/19/4/851/ [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Fellows LK. Medial prefrontal cortex plays a critical and selective role in “feeling of knowing” meta-memory judgments. Neuropsychologia. 2008;46(12):2958–65. doi: 10.1016/j.neuropsychologia.2008.06.011. doi:10.1016/j.neuropsychologia.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47(1):36–45. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10632099. [PubMed] [Google Scholar]
- Nelson TO. A comparison of current measures of the accuracy of feeling-of- knowing predictions. Psychological Bulletin. 1984;95(1):109. [PubMed] [Google Scholar]
- Nelson TO, Narens L. Metamemory: a theoretical framework and new findings. In: Bower GH, editor. The psychology of learning and motivation. 26th ed. Vol. 26. Academic Press; San Diego: 1990. pp. 125–141. [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Pascual-Leone A. Transcranial direct current stimulation: state of the art 2008. Brain Stimulation. 2008;1(3):206–23. doi: 10.1016/j.brs.2008.06.004. doi:10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Pannu JK, Kaszniak AW. Metamemory experiments in neurological populations: a review. Neuropsychology Review. 2005;15(3):105–30. doi: 10.1007/s11065-005-7091-6. doi:10.1007/s11065-005-7091-6. [DOI] [PubMed] [Google Scholar]
- Peirce JW. PsychoPy--Psychophysics software in Python. Journal of Neuroscience Methods. 2007;162(1-2):8–13. doi: 10.1016/j.jneumeth.2006.11.017. doi:10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi D, Chua EF. Transcranial direct current stimulation (tDCS) of the parietal cortex leads to increased false recognition. Neuropsychologia. 2015;66:88–98. doi: 10.1016/j.neuropsychologia.2014.11.012. doi:10.1016/j.neuropsychologia.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Perrotin A, Belleville S, Isingrini M. Metamemory monitoring in mild cognitive impairment: Evidence of a less accurate episodic feeling-of-knowing. Neuropsychologia. 2007;45(12):2811–26. doi: 10.1016/j.neuropsychologia.2007.05.003. doi:10.1016/j.neuropsychologia.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Perrotin A, Tournelle L, Isingrini M. Executive functioning and memory as potential mediators of the episodic feeling-of-knowing accuracy. Brain and Cognition. 2008;67(1):76–87. doi: 10.1016/j.bandc.2007.11.006. doi:10.1016/j.bandc.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Reder LM. Strategy selection in question answering. Cognitive Psychology. 1987;19(1):90–138. doi:10.1016/0010-0285(87)90005-3. [Google Scholar]
- Reggev N, Zuckerman M, Maril A. Are all judgments created equal? An fMRI study of semantic and episodic metamemory predictions. Neuropsychologia. 2011;49(5):1332–42. doi: 10.1016/j.neuropsychologia.2011.01.013. doi:10.1016/j.neuropsychologia.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Ryals AJ, Rogers LM, Gross EZ, Polnaszek KL, Voss JL. Cerebral Cortex. New York, N.Y.: 2015. Associative Recognition Memory Awareness Improved by Theta-Burst Stimulation of Frontopolar Cortex. p. bhu311. 1991 doi:10.1093/cercor/bhu311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL. Feeling of knowing in episodic memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1983;9(1):39–54. [Google Scholar]
- Schnyer DM, Nicholls L, Verfaellie M. The role of VMPC in metamemorial judgments of content retrievability. Journal of Cognitive Neuroscience. 2005;17(5):832–46. doi: 10.1162/0898929053747694. doi:10.1162/0898929053747694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyer DM, Verfaellie M, Alexander MP, LaFleche G, Nicholls L, Kaszniak AW. A role for right medial prefontal cortex in accurate feeling-of- knowing judgements: evidence from patients with lesions to frontal cortex. Neuropsychologia. 2004;42(7):957–66. doi: 10.1016/j.neuropsychologia.2003.11.020. doi:10.1016/j.neuropsychologia.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Metcalfe J. Cue familiarity but not target retrievability enhances feeling-of-knowing judgments. Journal of Experimental Psychology. Learning, Memory, and Cognition. 1992;18(5):1074–83. doi: 10.1037//0278-7393.18.5.1074. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. Toward a cognitive neuroscience of metacognition. Consciousness and Cognition. 2000;9(2 Pt 1):313–23. doi: 10.1006/ccog.2000.0450. discussion 324–6. doi:10.1006/ccog.2000.0450. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. Memory and metamemory: a study of the feeling-of-knowing phenomenon in amnesic patients. Journal of Experimental Psychology. Learning, Memory, and Cognition. 1986;12(3):452–60. doi: 10.1037//0278-7393.12.3.452. doi:10.1037/0278-7393.12.3.452. [DOI] [PubMed] [Google Scholar]
- Souchay C, Isingrini M, Espagnet L. Aging, episodic memory feeling-of- knowing, and frontal functioning. Neuropsychology. 2000;14(2):299–309. doi: 10.1037//0894-4105.14.2.299. [DOI] [PubMed] [Google Scholar]
- Sparing R, Mottaghy FM. Noninvasive brain stimulation with transcranial magnetic or direct current stimulation (TMS/tDCS)-From insights into human memory to therapy of its dysfunction. Methods (San Diego, Calif.) 2008;44(4):329–37. doi: 10.1016/j.ymeth.2007.02.001. doi:10.1016/j.ymeth.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Thiede KW, Anderson MCM, Therriault D. Accuracy of metacognitive monitoring affects learning of texts. Journal of Educational Psychology. 2003;95(1):66–73. doi:10.1037/0022-0663.95.1.66. [Google Scholar]
- Toth JP, Daniels KA, Solinger LA. What you know can hurt you: Effects of age and prior knowledge on the accuracy of judgments of learning. Psychology and Aging. 2011;26(4):919–931. doi: 10.1037/a0023379. doi:10.1037/a0023379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Lepage J-F, Latulipe-Loiselle A, Fregni F, Pascual-Leone A, Théoret H. The uncertain outcome of prefrontal tDCS. Brain Stimulation. 2014;7(6):773–83. doi: 10.1016/j.brs.2014.10.003. doi:10.1016/j.brs.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]