Abstract
Aims
General obesity, measured by the body mass index (BMI), and abdominal adiposity, measured as waist circumference (WC) and waist-to-hip ratio (WHR), are associated with heart failure and cardiovascular events. However, the relationship of general and abdominal obesity with subclinical left ventricular (LV) dysfunction is unknown. We assessed the association of general and abdominal obesity with subclinical LV systolic dysfunction in a population-based elderly cohort.
Methods and Results
Participants from the Cardiovascular Abnormalities and Brain lesions study underwent measurement of BMI, WC, and WHR. LV systolic function was assessed by two-dimensional echocardiographic LV ejection fraction (LVEF) and speckle-tracking global longitudinal strain (GLS). The study population included 729 participants (mean age 71±9 years, 60% women). In multivariate analysis, higher BMI (but not WC and WHR) was associated with higher LVEF (β=0.11, p=0.003). Higher WC (β=0.08, p=0.038) and higher WHR (β=0.15, p<0.001) were associated with lower GLS, whereas BMI was not (p=0.720). Compared with normal WHR, high WHR was associated with lower GLS in all BMI categories (normal, overweight, and obese), and was associated with subclinical LV dysfunction by GLS both in participants without (adjusted OR=2.0, 95% CI=1.1-3.6, p=0.020) and with general obesity (adjusted OR=5.4, 95% CI=1.1-25.9, p=0.034). WHR was incremental to BMI and risk factors in predicting LV dysfunction.
Conclusion
Abdominal adiposity was independently associated with subclinical LV systolic dysfunction by GLS in all BMI categories. BMI was not associated with LV dysfunction. Increased abdominal adiposity may be a risk factor for LV dysfunction regardless of the presence of general obesity.
Keywords: Obesity, Abdominal adiposity, Systolic dysfunction, Global longitudinal strain, Echocardiography
Introduction
In the United States, more than one third of the adult population is obese.1 Obesity is associated with an excess of cardiovascular risk factors, and is a major predictor of cardiovascular disease and mortality.2-4 Obese and overweight individuals have significantly greater prevalence of left ventricular (LV) diastolic dysfunction and higher risk of heart failure compared to those with normal weight.5,6 The body mass index (BMI) has been often adopted as a measure of body size because easily determined from height and weight measurements. However, measures of abdominal adiposity, such as waist circumference (WC) and waist-to-hip ratio (WHR), are strongly associated with cardiovascular risk factors and metabolic abnormalities,7,8 and have been shown to be strong predictors of incident cardiovascular events, especially in the elderly.9-15
Although obesity is an established predictor of heart failure, it is not clear whether it is related to early abnormalities in LV systolic function independent of risk factors and cardiac adaptations associated with the excess body weight. Also, it is not known whether general and abdominal measures of body size share similar relationships with LV function. In previous studies, despite the documented obesity-related changes in LV structure (increased mass, concentric geometry) and diastolic function,5 LV systolic function measured by LV ejection fraction (LVEF) or midwall shortening was not affected in obese subjects, or was even increased.16-18 Recent studies showed that LV longitudinal strain, a measure of myocardial fiber deformation in the longitudinal direction, can detect subclinical LV systolic dysfunction in a significant proportion of individuals with normal LVEF, and that such dysfunction strongly predicts future cardiovascular events.19 Although some studies have documented an impaired LV longitudinal strain in obese subjects, this evidence was limited to small, selected samples often including young individuals.20-22 There is lack of data regarding the association of general and abdominal obesity metrics with subclinical LV dysfunction in population setting and especially in the elderly, in whom the relationship of anthropometric measures with body composition may substantially change. Accordingly, we assessed the relationship of different body size metrics with LV systolic dysfunction assessed by traditional and novel echocardiographic techniques in a middle-aged to elderly population-based cohort.
Methods
Study population
Participants from the Cardiovascular Abnormalities and Brain Lesion (CABL) study underwent two-dimensional echocardiographic assessment of LV function using traditional and speckle-tracking imaging. CABL based its recruitment on the Northern Manhattan Study (NOMAS), a population-based prospective study designed to investigate the epidemiology and risk factors for stroke and cardiovascular disease that enrolled 3,298 participants from the community living in northern Manhattan between 1993 and 2001. The study design and recruitment details of NOMAS have been described previously.23 Beginning in 2003, participants were invited to participate in a brain MRI substudy if they: 1) were at least 50 years of age; 2) had no contraindications to MRI; and 3) did not have a prior diagnosis of stroke. From September 2005 to July 2010, NOMAS MRI participants that voluntarily agreed to undergo an extensive cardiovascular evaluation were included in CABL. Of the 1,004 CABL participants, 854 had echocardiographic exams available in digital format, 125 of whom were excluded because of suboptimal image quality for speckle-tracking analysis, leading to a final study sample of 729. Written informed consent was obtained from all study participants. The study complies with the Declaration of Helsinki and was approved by the Institutional Review Boards of Columbia University Medical Center and of the University of Miami.
Risk factors and body size assessment
Cardiovascular risk factors were ascertained through direct examination and interview by trained research assistants. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg, or self-reported history of hypertension or use of anti-hypertensive medication. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or self-reported history of diabetes or use of diabetes medications. Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL, self-report of hypercholesterolemia, or use of lipid-lowering treatment. Cigarette smoking, either at the time of the interview or in the past, was recorded. Coronary artery disease was defined as history of myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention. The race-ethnicity classification was based on self-identification, and modeled after the U.S. Census. Height and weight were measured using a standard scale. BMI was calculated as weight (kilograms) divided by height (meters) squared. Normal BMI was defined as < 25 kg/m2, overweight was defined as BMI between 25 and 29.9 kg/m2, and obesity was defined as BMI ≥ 30.0 kg /m2. Waist and hip circumferences were measured using flexible measuring tape with participants standing and relaxed without heavy outer garments. WC was measured at the level of the umbilicus, and hip circumference was measured at the level of the greater trochanters. WHR was defined as WC divided by hip circumference. Increased abdominal adiposity was defined as recommended by the World Health Organization (WC >88 cm in women and >102 cm in men; WHR ≥0.85 in women and ≥0.90 in men).24
Echocardiographic assessment
Two-dimensional echocardiography
Transthoracic echocardiography was performed using a commercially available system (iE 33, Philips, Andover, MA) by a trained, registered cardiac sonographer according to a standardized protocol. LV dimensions were measured from a parasternal long-axis view according to the recommendations of the American Society of Echocardiography,25 and LV mass was calculated with a validated method26 and indexed by height2.7. LV volumes (indexed by body surface area) and LVEF were calculated using the biplane modified Simpson's rule. LV mass/volume ratio (M/V), an indicator of LV geometry, was calculated as LV mass/end-diastolic volume. LV diastolic function was assessed from the apical 4-chamber view by means of the peak early velocity (E) of the trans-mitral flow and the early diastolic velocity (e′) of the mitral annulus (average of septal and lateral site) by pulsed-wave tissue-Doppler, as previously described.27,28 The ratio E/e′ was calculated as an indicator LV diastolic function and filling pressure.
Speckle-tracking strain imaging
Speckle-tracking analysis was performed off-line using commercially available software (Philips QLAB Advanced Quantification Software version 8.1). Analysis of LV myocardial deformation over the longitudinal axis was performed from two-dimensional gray-scale loops by automatic tracking of myocardial speckles after manual selection of landmark points as previously described.29 Global LV longitudinal systolic strain (GLS) was calculated from the apical 4-chamber and 2-chamber views. At least two cardiac cycles were recorded at a frame rate ≥ 45 fps, and were averaged for strain analysis. Abnormal GLS was previously defined as GLS > -14.7% (GLS is a negative number, therefore less negative values correspond to smaller systolic longitudinal shortening), representing the cut-off identifying the lower 5% of the GLS distribution in a healthy subgroup of participants.19 Inter-observer reproducibility of GLS measurement was assessed in a random sample of 20 study participants. Intra-class correlation coefficient for GLS was 0.85. Mean difference between measurements was 0.08±2.4%, and the coefficient of variation (standard deviation/mean) was 0.09.
Statistical analysis
Data are presented as mean ± standard deviation, and median and interquartile range for continuous variables and as percentage for categorical variables. The Student's t-test and chi-square test were used to assess differences between groups. Simple correlations were assessed by Pearson's correlation coefficients. Linear regressions were used to assess the association of parameters of LV function with body size measures, and unstandardized (B) and standardized (β) parameter estimates and standard errors (SE) were reported. Logistic regression analyses were performed to assess the risk of abnormal GLS in different quartiles of body size metrics, and adjusted odds ratios (OR) and 95% confidence intervals (CI) were calculated. Covariates were selected based on their univariate association with body size metrics. The likelihood ratio test was used in nested logistic models to evaluate the incremental value of body size metrics over risk factors in predicting LV dysfunction by GLS. For all statistical analyses, a two-tailed p<0.05 was considered significant. Statistical analyses were performed using IBM SPSS Statistics software version 22 (IBM Corp., Armonk, NY).
Results
Clinical characteristics of the study cohort
Demographics and clinical characteristics of study participants and associations with body size metrics are reported in Table 1. The study population consisted of 729 participants (mean age 71.5±9.4 years, 60.5% women). Mean BMI was 27.8±4.6 kg/m2, WC was 95.2±11.9 cm, and WHR was 0.91±0.08. BMI showed a good correlation with WC (β=0.71, p<0.001), whereas the correlation with WHR was weaker although statistically significant (β=0.11, p=0.004).
Table 1. Demographics and clinical characteristics of the study population and univariate relationships with body size metrics.
| β values | ||||
|---|---|---|---|---|
| Variable | N=729 | BMI | WC | WHR |
| Age, years | 71.5±9.4 (71, 13) | -0.14† | -0.01 | 0.06 |
| Women, n (%) | 441 (60.5) | 0.15† | -0.18† | -0.36† |
| BMI, kg/m2 | 27.8±4.6 (27, 6) | - | 0.71† | 0.11† |
| WC, cm | 95.2±11.9 (95, 15) | 0.71† | - | 0.60† |
| WHR | 0.91±0.08 (0.91, 0.10) | 0.11† | 0.60† | - |
| Race-ethnicity | ||||
| Caucasians, n (%) | 104 (14.3) | Ref. | Ref. | Ref. |
| African-Americans, n (%) | 123 (16.9) | 0.12* | 0.03 | -0.04 |
| Hispanics, n (%) | 488 (66.9) | 0.20† | 0.06 | 0.002 |
| Other, n (%) | 14 (1.9) | 0.02 | -0.02 | -0.02 |
| Systolic BP, mmHg | 135.2±16.7 (134, 22) | 0.16† | 0.13† | 0.06 |
| Diastolic BP, mmHg | 78.2±9.4 (79, 14) | 0.18† | 0.17† | 0.09* |
| Hypertension, n (%) | 569 (78.1) | 0.18† | 0.18† | 0.11† |
| Anti-hypertensive medications, n (%) | 515 (70.6) | 0.18† | 0.18† | 0.10† |
| Diabetes mellitus, n (%) | 205 (28.1) | 0.14† | 0.15† | 0.08* |
| Hypercholesterolemia, n (%) | 483 (66.3) | 0.06 | 0.03 | 0.02 |
| Cholesterol-lowering medications, n (%) | 361 (49.5) | 0.07 | 0.06 | 0.02 |
| Coronary artery disease, n (%) | 48 (6.6) | 0.05 | 0.10† | 0.07* |
| Cigarette smoking, n (%) | 388 (53.2) | -0.09* | 0.05 | 0.10† |
| High school education, n (%) | 335 (46.0) | -0.11† | -0.06 | -0.09* |
Values in table are mean ± SD (median, interquartile range) for continuous variables, numerosity (percent) for proportions, and standardized coefficients (β) from linear regressions.
p<0.05.
p<0.01. BMI: Body mass index. WC: Waist circumference. WHR: Waist-to-hip ratio. BP: Blood pressure.
Higher BMI was associated with younger age, female sex, higher blood pressure, hypertension, diabetes, African-American or Hispanic race-ethnicity, no history of cigarette smoking, and lower education level (all p<0.05). WC and WHR were not associated with age, but were associated with male sex, blood pressure, hypertension, diabetes, coronary artery disease, and lower education level (all p<0.05).
Body size and LV structure and function
Echocardiographic characteristics of the study population and their association with body size parameters are shown in Table 2. BMI was significantly associated with lower LV volumes, greater LV mass, lower e′, and higher E/e′ (all p<0.05). WC and WHR were not associated with LV volumes, but were significantly associated with greater LV mass and LV M/V, and lower e′ and E/e′ (all p<0.05).
Table 2. Echocardiographic variables and univariate relationships with body size metrics.
| β values | ||||
|---|---|---|---|---|
| Variable | N=729 | BMI | WC | WHR |
| LV end-diastolic volume, ml/m2 | 53.7±15.5 (52, 17) | -0.09* | -0.05 | 0.03 |
| LV end-systolic volume, ml/m2 | 20.1±10.1 (18, 8) | -0.09* | -0.02 | 0.03 |
| LV mass, g | 181.6±48.5 (176, 61) | 0.18† | 0.33† | 0.27† |
| LV mass/height2.7, g/m2.7 | 50.0±13.3 (48, 16) | 0.27† | 0.19† | 0.11† |
| LV M/V | 2.0±0.6 (2, 0.7) | 0.06 | 0.14† | 0.12† |
| LVEF, % | 63.3±7.2 (64, 7) | 0.11† | -0.01 | -0.06 |
| GLS, % | -17.0±3.3 (-17, 4) | 0.05 | 0.17† | 0.22† |
| Left atrial volume, ml/m2 | 24.8±7.9 (24, 9) | -0.07 | -0.03 | -0.01 |
| e′, cm/s | 7.3±1.7 (7, 2) | -0.08* | -0.10† | -0.11† |
| E/e′ | 10.3±3.3 (10, 3) | 0.14† | -0.08* | -0.002 |
Values in table are mean ± SD (median, interquartile range) for continuous variables, numerosity (percent) for proportions, and standardized coefficients (β) from linear regressions.
p<0.05.
p<0.01. BMI: Body mass index. WC: Waist circumference. WHR: Waist-to-hip ratio. LV: Left ventricular. M/V: Mass/volume ratio. LVEF: LV ejection fraction. GLS: Global longitudinal strain.
Univariate correlations of BMI, WC, and WHR with LV systolic function are shown in Table 2 and Figure 1. Higher BMI correlated weakly but significantly with higher LVEF (r=0.11, p<0.01), whereas WC and WHR were not correlated with LVEF. Higher WC and WHR were correlated with less negative GLS (r=0.17 and r=0.22, respectively, both p<0.001), indicative of more impaired longitudinal LV systolic function; BMI did not show a significant correlation with GLS (r=0.05, p=0.22). In multivariate regression analyses (Table 3), after adjusting for demographics and risk factors including LV mass and geometry, higher BMI remained associated with higher LVEF (β=0.11, p=0.005), whereas it was not correlated with GLS (p=0.954). WC and WHR remained independently and significantly correlated with lower GLS (β=0.08, p=0.026 and β=0.15, p<0.001, respectively), but not with LVEF (p=0.104 and p=169, respectively).
Figure 1. Body size and left ventricular function.
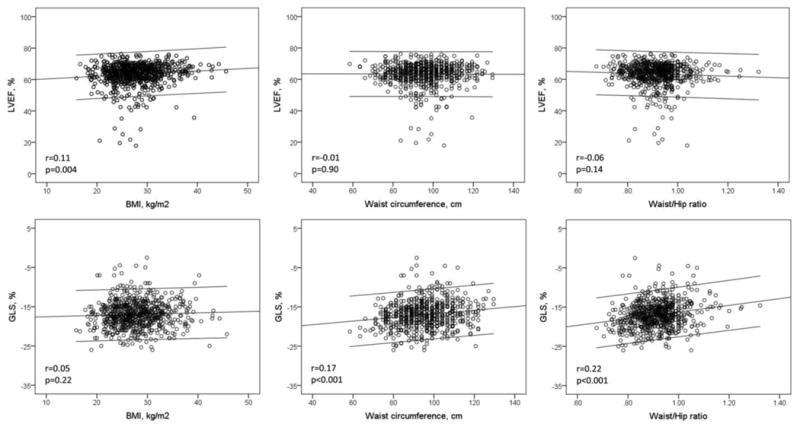
Scatterplots showing the association of body size metrics (BMI, WC, WHR) with LVEF and GLS.
Table 3. Multivariate linear regressions of LV systolic function with body size metrics.
| LVEF | GLS | |||||
|---|---|---|---|---|---|---|
| B (SE) | β | P value | B (SE) | β | P value | |
| BMI | 0.18 (0.06) | 0.11 | 0.003 | -0.010 (0.03) | -0.014 | 0.720 |
| WC | 0.04 (0.02) | 0.06 | 0.104 | 0.02 (0.01) | 0.08 | 0.038 |
| WHR | 4.87 (3.33) | 0.06 | 0.143 | 5.82 (1.52) | 0.15 | <0.001 |
Values in table represent change in systolic function parameters per unit increase of body size (B) with standard error (SE) and standardized correlation coefficients (β). LVEF: Left ventricular ejection fraction. GLS: Global longitudinal strain. BMI: Body mass index. WC: Waist circumference. WHR: Waist-to-hip ratio. Covariates: Age, sex, hypertension, anti-hypertensive medications, diabetes, coronary artery disease, cigarette smoking, education level, race-ethnicity, LV mass/height2.7, LV mass/volume, and E/e′.
In Figure 2 are shown GLS values in normal and high WHR across different BMI categories. In participants with normal BMI (<25 kg/m2), high WHR was significantly associate with lower GLS compared to the normal WHR group (-16.5±3.4% vs. -18.0±3.1%, p=0.002). In participants with BMI between 25 and 29.9 kg/m2, a high WHR was also associated with lower GLS compared to normal WHR (-16.7±3.2% vs. -17.8±3.3%, p=0.013). A similar finding was observed in the group with BMI ≥ 30 kg/m2, where GLS was significantly lower in those with high WHR compared to normal WHR (-16.6±3.4% vs. -18.0±3.1% respectively, p=0.024).
Figure 2. GLS by normal/high WHR in participants with BMI<25, BMI between 25 and 29.9), and BMI ≥30 kg/m2.
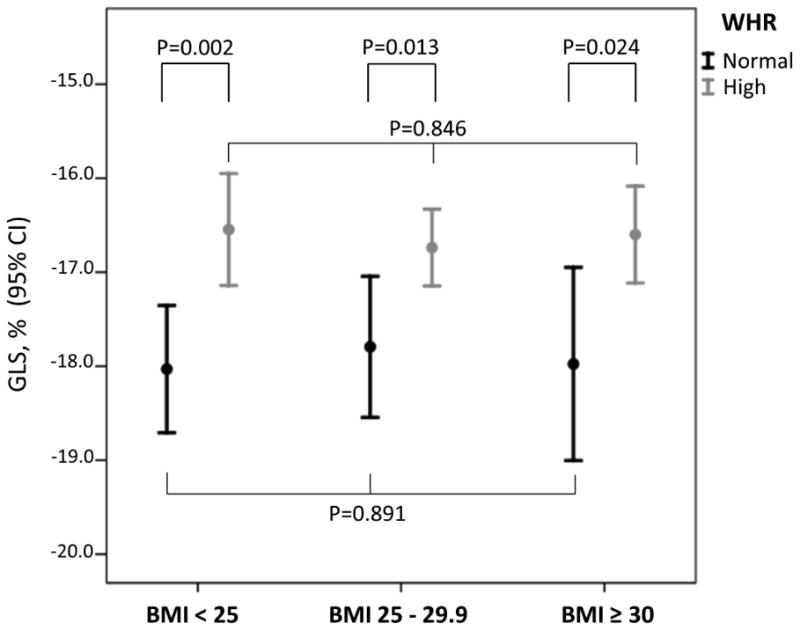
GLS was significantly reduced (less negative) in participants with high WHR in all BMI categories. The GLS values in participants with normal WHR were not significantly different across the BMI groups, and the same was observed for GLS values in high WHR participants.
In multivariate analyses (Table 4), an increased WHR significantly predicted LV dysfunction by GLS in both non-obese (adjusted OR=2.0, 95% CI=1.1-3.6, p=0.020) and obese participants (adjusted OR=5.4, 95% CI=1.1-25.9, p=0.034).
Table 4. Multivariate logistic regressions of LV systolic dysfunction by GLS with abnormal WHR in obese and non-obese participants.
| Adjusted OR (95% CI) | P value | |
|---|---|---|
| BMI < 30 (n=522) | ||
| Normal WHR | Reference | - |
| High WHR | 2.0 (1.1-3.6) | 0.020 |
|
| ||
| BMI ≥ 30 (n=207) | ||
| Normal WHR | Reference | - |
| High WHR | 5.4 (1.1-25.9) | 0.034 |
Values in table are adjusted ORs and 95% CI for abnormal GLS. OR: Odds ratio. CI: Confidence interval. WHR: Waist-to-hip ratio. Covariates: Age, sex, hypertension, anti-hypertensive medications, diabetes, coronary artery disease, cigarette smoking, education level, race-ethnicity, LV mass/height2.7, LV mass/volume, and E/e′.
The incremental value of abdominal adiposity over risk factors and BMI in predicting LV dysfunction by GLS is shown in Figure 3. When added to a model including risk factors (-2 log likelihood=631.7, chi-square=85.2), BMI did not increase the model prediction (-2 log likelihood=631.0, chi-square change=0.6, p=0.423). The further addition of WC (-2 log likelihood=620.4, chi-square change=10.7, p=0.001) and WHR (-2 log likelihood=616.0, chi-square change=15.0, p<0.001) resulted in a significant and incremental prediction of abnormal GLS.
Figure 3. Incremental value of abdominal obesity metrics toward LV dysfunction.
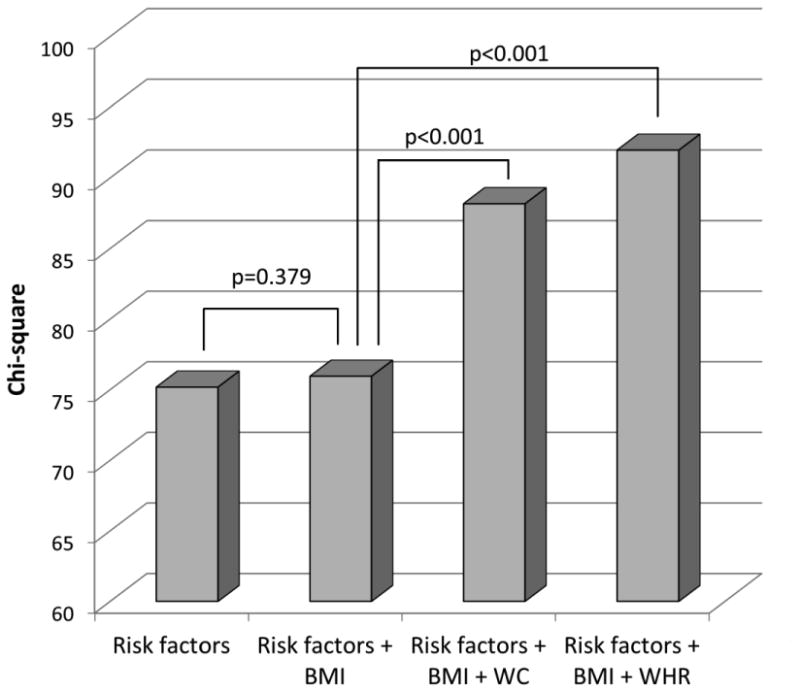
WC and WHR showed significant incremental value over risk factors and BMI in predicting LV dysfunction by GLS.
Discussion
In this study, we found that WC and WHR were independently associated with subclinical LV systolic dysfunction measured by LV longitudinal strain. Abdominal adiposity was associated with subclinical LV dysfunction in normal weight, overweight, and obese individuals, as defined by BMI. Since our study was carried out in a community-based cohort with mostly normal LVEF, abdominal adiposity can be considered therefore a risk factor for subclinical LV dysfunction at a stage when LVEF is still normal. We also found that in the absence of an increased abdominal adiposity (i.e. when WHR is normal), increasing BMI was not associated with worsening GLS. Similarly, an increase in BMI was not associated with a GLS reduction in subjects with increased abdominal adiposity.
Body size metrics and cardiovascular risk
Although BMI is more frequently used as a measure of obesity in clinical studies due to the ease of acquisition and well documented association with cardiovascular events and mortality,4,11,30-32 measures of abdominal adiposity have been shown to be associated with a range of metabolic abnormalities, including decreased glucose tolerance, reduced insulin sensitivity, and dyslipidemia.8 The observation from our and previous studies that abdominal adiposity metrics, especially WHR, are not well correlated with BMI, suggest that these measures have the potential to show a different or additional impact on cardiovascular risk. In a large study conducted in 29 countries, WC and BMI, although well-correlated, often did not agree in the classification of obese subjects, and WC showed to carry significant information regarding the amount of visceral adipose tissue regardless of the BMI category.33 In the INTERHEART study, the Cardiovascular Health Study, and the Health, Aging and Body Composition Study, abdominal obesity measures predicted cardiovascular events and heart failure independent of BMI, whereas the latter did not after adjusting for abdominal obesity metrics.10,34,35 In the Emerging Risk Factors Collaboration, WHR showed higher hazard ratios for cardiovascular disease than BMI, and was associated with incident coronary artery disease in all BMI categories.36 In other studies, abdominal obesity was also found to be a strong predictor of cardiovascular events, but it did not show clear superiority over BMI.11 Our findings are consistent with those from a recent report from the Third National Health and Nutrition Examination Survey (NHANES III) that investigated the impact of different types of obesity defined by BMI and WHR on total and cardiovascular mortality in 15,184 individuals from the community.15 In that study, Sahakyan and coll. showed that an increased abdominal adiposity significantly predicted total and cardiovascular mortality regardless of BMI-defined obesity, and that having an increased WHR was a stronger predictor of mortality than having an increased BMI alone.
Body size metrics and LV systolic dysfunction
Obesity is associated with LV structural and functional changes resulting in increased cardiac output to support the expansion of both fat and fat-free mass associated with the increased body size.37-40 These adaptive mechanisms often result in LV mass increase, concentric geometry, and preserved or increased LVEF, as shown in our and previous studies.16-18,21 While the relationship between obesity and LV diastolic dysfunction is well documented and is likely multifactorial,5 involving chronic increase in pre- and after-load and LV mass and geometry changes, insulin resistance, and increased oxidative stress resulting in impaired myocardial relaxation, the relationship of obesity with systolic function is more elusive. Despite the consistent finding of a higher LVEF in obese subjects, some studies found associations between BMI and LV strain;21,22,41 however, these studies were mostly conducted in small, selected samples and in cohorts of significantly younger age than ours, in which the increase in BMI is paralleled by an increase of both fat and fat-free mass.39 In the elderly, a progressive loss of fat-free mass and an increase in fat mass is common, suggesting that abdominal adiposity parameters might be better indicators of obesity and cardiovascular risk in the elderly than BMI. This observation might also help reconcile conflicting results from previous studies. In fact, studies that reported a better prognostic value of WC and WHR over BMI were those conducted in older populations.10,33-35,42,43 In a recent study, Wang et al. found that obesity was related lower longitudinal strain, however no difference in strain were found between metabolic healthy and unhealthy obese subjects.44 In this study, the mean age of the population was significantly younger than ours, and therefore the prevalence of subclinical LV dysfunction is likely low, a factor that may have contributed to the results.
The mechanism of the association of GLS with abdominal adiposity is not known. Abdominal adiposity is associated with cardiovascular and metabolic risk factors which may in part mediate its association with GLS. In fact, GLS has been shown to be associated with cardiovascular risk factors, arterial stiffness, coronary artery calcium, and silent cerebrovascular disease.45-47 GLS, however, remained associated with abdominal adiposity even after accounting for traditional cardiovascular risk factor. Abdominal adiposity may exert negative effects on myocardial function through several pathways involving inflammatory cytokines, renin-angiotensin-aldosterone system activation, insulin resistance and hyperinsulinemia, and lipotoxicity from lipid accumulation in the cardiac tissue;48,49 all these stimuli may result in impaired myocardial energetics, myocytes apoptosis, and increased fibrosis. Since the LV contraction in the longitudinal direction is often the first sign of an initial impairment in systolic function, GLS may be able to detect LV dysfunction when LVEF is still in the normal range. Given the demonstrated negative prognostic value of an impaired GLS towards cardiovascular events, early treatment strategies in subjects with abdominal obesity might improve the outcome in this group, a hypothesis that needs further investigation.
Our study adds to the evidence documenting the relationship between obesity and subclinical LV dysfunction. Since an abnormal GLS has been demonstrated to be a powerful and independent predictor of cardiovascular outcome even in the general population,19 its impairment may represent an early stage in the progression toward symptomatic heart failure, and may be considered as another feature of the stage B of the heart failure classification. However, the relationship of obesity with heart failure is complex. While obesity is a risk factor for the development of heart failure, its presence has been associated with better prognosis once the disease has developed, a phenomenon known as “obesity paradox”. The relationship between body size, body composition and LV function in patients with overt heart failure is not well characterized, and more studies are needed to investigate whether it plays a role in the obesity paradox.
Strengths and limitations
Our study was the first to assess the relationship between general and abdominal obesity metrics with LV function using advanced imaging techniques in a large, tri-ethnic, community-based cohort of middle-aged to elderly subjects. Our population had the ideal risk profile to assess such relationship, a context that may provide useful clinical information in elderly patients at high cardiovascular risk. We performed the study analyses using multivariate models controlling for known risk factors and confounders affecting LV function, including strong determinants such as LV mass and geometry. Our study also has limitations that need to be considered. The cross-sectional design of the analysis allowed us to describe associations between abdominal adiposity metrics and systolic dysfunction, and although we controlled for possible confounders, it is not possible to draw definitive conclusions regarding cause-effect relationships. Finally, although we used clinically accepted metrics of body size (BMI, WC, WHR), the assessment of fat mass and fat-free mass, helpful to better understand the relationships between body size and body composition, was not available in our study.
Conclusions
In this community cohort of prevalently elderly subjects, abdominal adiposity was associated with subclinical LV systolic dysfunction, independent of cardiovascular risk factors and other confounders. The presence of abdominal adiposity significantly increased the risk of subclinical LV systolic dysfunction both in participants with and without general obesity. Cardiovascular risk stratification in the elderly might be improved by the assessment of simple abdominal adiposity measures.
Acknowledgments
The authors wish to thank Janet De Rosa, MPH (project manager), Rafi Cabral, MD, Michele Alegre, RDCS, and Palma Gervasi-Franklin (collection and management of the data).
Funding: This work was supported by the National Institute of Neurological Disorders and Stroke [grant number R01 NS36286 to M.D.T. and R37 NS29993 to R.L.S. and M.S.E.].
Footnotes
Conflict Of Interest: None declared.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 3.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 5.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 7.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 8.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61:646–653. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 11.Loehr LR, Rosamond WD, Poole C, McNeill AM, Chang PP, Folsom AR, Chambless LE, Heiss G. Association of multiple anthropometrics of overweight and obesity with incident heart failure: the Atherosclerosis Risk in Communities study. Circ Heart Fail. 2009;2:18–24. doi: 10.1161/CIRCHEARTFAILURE.108.813782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28:850–856. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 13.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjonneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, May AM, Bueno-de-Mesquita HB, van Duijnhoven FJ, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quiros JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 14.Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. 2010;121:237–244. doi: 10.1161/CIRCULATIONAHA.109.887893. [DOI] [PubMed] [Google Scholar]
- 15.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, Coutinho T, Jensen MD, Roger VL, Singh P, Lopez-Jimenez F. Normal-Weight Central Obesity: Implications for Total and Cardiovascular Mortality. Ann Intern Med. 2015;163:827–835. doi: 10.7326/M14-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JA, Bluemke DA. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3:266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pascual M, Pascual DA, Soria F, Vicente T, Hernandez AM, Tebar FJ, Valdes M. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–1156. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Simone G, Devereux RB, Mureddu GF, Roman MJ, Ganau A, Alderman MH, Contaldo F, Laragh JH. Influence of obesity on left ventricular midwall mechanics in arterial hypertension. Hypertension. 1996;28:276–283. doi: 10.1161/01.hyp.28.2.276. [DOI] [PubMed] [Google Scholar]
- 19.Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL, Di Tullio MR. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16:1301–1309. doi: 10.1002/ejhf.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, vila-Roman VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 21.Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 22.Kishi S, Armstrong AC, Gidding SS, Colangelo LA, Venkatesh BA, Jacobs DR, Jr, Carr JJ, Terry JG, Liu K, Goff DC, Jr, Lima JA. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the CARDIA study (Coronary Artery Risk Development in Young Adults) JACC Heart Fail. 2014;2:500–508. doi: 10.1016/j.jchf.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden-Albala B, Di Tullio MR, Homma S, Elkind MS, Paik MC. Improving Global Vascular Risk Prediction With Behavioral and Anthropometric Factors The Multiethnic NOMAS (Northern Manhattan Cohort Study) J Am Coll Cardiol. 2009;54:2303–2311. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. report of a WHO expert consultation. Geneva: Geneva: Dec 8-11, 2008. 2011. Waist circumference and waist-hip ratio. [Google Scholar]
- 25.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 27.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of diabetes and hypertension on left ventricular diastolic function in a high-risk population without evidence of heart disease. Eur J Heart Fail. 2010;12:454–461. doi: 10.1093/eurjhf/hfq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo C, Jin Z, Sera F, Lee ES, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Left ventricular systolic dysfunction by longitudinal strain is an independent predictor of incident atrial fibrillation: a community-based cohort study. Circ Cardiovasc Imaging. 2015;8:e003520. doi: 10.1161/CIRCIMAGING.115.003520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levitan EB, Yang AZ, Wolk A, Mittleman MA. Adiposity and incidence of heart failure hospitalization and mortality: a population-based prospective study. Circ Heart Fail. 2009;2:202–208. doi: 10.1161/CIRCHEARTFAILURE.108.794099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 32.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 33.Nazare JA, Smith J, Borel AL, Aschner P, Barter P, Van GL, Tan CE, Wittchen HU, Matsuzawa Y, Kadowaki T, Ross R, Brulle-Wohlhueter C, Almeras N, Haffner SM, Balkau B, Despres JP. Usefulness of Measuring Both Body Mass Index and Waist Circumference for the Estimation of Visceral Adiposity and Related Cardiometabolic Risk Profile (from the INSPIRE ME IAA Study) Am J Cardiol. 2015;115:307–315. doi: 10.1016/j.amjcard.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 34.Nicklas BJ, Cesari M, Penninx BW, Kritchevsky SB, Ding J, Newman A, Kitzman DW, Kanaya AM, Pahor M, Harris TB. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54:413–420. doi: 10.1111/j.1532-5415.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 35.Djousse L, Bartz TM, Ix JH, Zieman SJ, Delaney JA, Mukamal KJ, Gottdiener JS, Siscovick DS, Kizer JR. Adiposity and incident heart failure in older adults: the cardiovascular health study. Obesity (Silver Spring) 2012;20:1936–1941. doi: 10.1038/oby.2011.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wormser D, Kaptoge S, Di AE, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bella JN, Devereux RB, Roman MJ, O'Grady MJ, Welty TK, Lee ET, Fabsitz RR, Howard BV. Relations of left ventricular mass to fat-free and adipose body mass. The Strong Heart Study Investigators Circulation. 1998;98:2538–2544. doi: 10.1161/01.cir.98.23.2538. [DOI] [PubMed] [Google Scholar]
- 38.Collis T, Devereux RB, Roman MJ, de Simone G, Yeh JL, Howard BV, Fabsitz RR, Welty TK. Relations of stroke volume and cardiac output to body composition: the Strong Heart Study. Circulation. 2001;103:820–825. doi: 10.1161/01.cir.103.6.820. [DOI] [PubMed] [Google Scholar]
- 39.Palmieri V, de Simone G, Arnett DK, Bella JN, Kitzman DW, Oberman A, Hopkins PN, Province MA, Devereux RB. Relation of various degrees of body mass index in patients with systemic hypertension to left ventricular mass, cardiac output, and peripheral resistance (The Hypertension Genetic Epidemiology Network Study) Am J Cardiol. 2001;88:1163–1168. doi: 10.1016/s0002-9149(01)02054-9. [DOI] [PubMed] [Google Scholar]
- 40.Rider OJ, Lewandowski A, Nethononda R, Petersen SE, Francis JM, Pitcher A, Holloway CJ, Dass S, Banerjee R, Byrne JP, Leeson P, Neubauer S. Gender-specific differences in left ventricular remodelling in obesity: insights from cardiovascular magnetic resonance imaging. Eur Heart J. 2013;34:292–299. doi: 10.1093/eurheartj/ehs341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosmala W, Jedrzejuk D, Derzhko R, Przewlocka-Kosmala M, Mysiak A, Bednarek-Tupikowska G. Left ventricular function impairment in patients with normal-weight obesity: contribution of abdominal fat deposition, profibrotic state, reduced insulin sensitivity, and proinflammatory activation. Circ Cardiovasc Imaging. 2012;5:349–356. doi: 10.1161/CIRCIMAGING.111.969956. [DOI] [PubMed] [Google Scholar]
- 42.Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord. 2001;25:1730–1735. doi: 10.1038/sj.ijo.0801787. [DOI] [PubMed] [Google Scholar]
- 43.de Hollander EL, Bemelmans WJ, Boshuizen HC, Friedrich N, Wallaschofski H, Guallar-Castillon P, Walter S, Zillikens MC, Rosengren A, Lissner L, Bassett JK, Giles GG, Orsini N, Heim N, Visser M, de Groot LC. The association between waist circumference and risk of mortality considering body mass index in 65- to 74-year-olds: a meta-analysis of 29 cohorts involving more than 58 000 elderly persons. Int J Epidemiol. 2012;41:805–817. doi: 10.1093/ije/dys008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang YC, Liang CS, Gopal DM, Ayalon N, Donohue C, Santhanakrishnan R, Sandhu H, Perez AJ, Downing J, Gokce N, Colucci WS, Ho JE. Preclinical Systolic and Diastolic Dysfunctions in Metabolically Healthy and Unhealthy Obese Individuals. Circ Heart Fail. 2015;8:897–904. doi: 10.1161/CIRCHEARTFAILURE.114.002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russo C, Jin Z, Takei Y, Hasegawa T, Koshaka S, Palmieri V, Elkind MS, Homma S, Sacco RL, Di Tullio MR. Arterial wave reflection and subclinical left ventricular systolic dysfunction. J Hypertens. 2011;29:574–582. doi: 10.1097/HJH.0b013e328342ca56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandes VR, Polak JF, Edvardsen T, Carvalho B, Gomes A, Bluemke DA, Nasir K, O'Leary DH, Lima JA. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47:2420–2428. doi: 10.1016/j.jacc.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 47.Russo C, Jin Z, Homma S, Elkind MS, Rundek T, Yoshita M, DeCarli C, Wright CB, Sacco RL, Di Tullio MR. Subclinical left ventricular dysfunction and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Circulation. 2013;128:1105–1111. doi: 10.1161/CIRCULATIONAHA.113.001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 49.Kosmala W, Plaksej R, Przewlocka-Kosmala M, Kuliczkowska-Plaksej J, Bednarek-Tupikowska G, Mazurek W. Matrix metalloproteinases 2 and 9 and their tissue inhibitors 1 and 2 in premenopausal obese women: relationship to cardiac function. Int J Obes (Lond) 2008;32:763–771. doi: 10.1038/sj.ijo.0803794. [DOI] [PubMed] [Google Scholar]


