Abstract
Objective
To determine if very early serum hCG, a marker of trophoblast differentiation, is associated with adverse perinatal outcomes in singleton pregnancies.
Design
Retrospective cohort study
Setting
University fertility program
Patients
360 singleton IVF live births
Interventions
Serial hCG measurements were used to determine the within-woman slope for hCG (hCG rise).
Main Outcomes Measures
Primary outcomes included birth weight and gestational age at delivery. Statistical comparisons utilized t-test, chi-square test, and linear and logistic regression as appropriate.
Results
hCG rise was positively associated with birth weight but not gestational age at delivery. Infant gender, gestational age, and type of embryo transfer ("fresh" vs. frozen/thawed) were significantly associated with birth weight and confounded the associations of interest. hCG rise was slower among subjects delivering an infant with low birth weight (slope: 0.386 ±0.05 vs. 0.407 ±0.06,) or small for gestational age (slope: 0.371 ±0.07 vs. 0.406 ±0.06,). Analysis of hCG rise by quartile showed that, when compared to the first quartile (slowest), subjects with a rate of hCG rise in the fourth quartile (fastest) had a significantly decreased risk of delivering an infant of low birth weight [OR = 0.32, 95% CI (0.11–0.92)]. No relationship was noted between hCG rise and hypertensive disorders of pregnancy.
Conclusions
Slower, very early first trimester hCG rise is associated with low birth weight but not gestational age at delivery among singleton IVF conceptions. The rate of increase in serum hCG may reflect early trophoblast differentiation and placentation and, possibly, predict subsequent development.
Keywords: human chorionic gonadotropin, hCG, low birth weight, adverse pregnancy outcomes, in vitro fertilization
Introduction
Adverse pregnancy outcomes may result, in part, from an underlying defect in early placentation (1). This period of pregnancy is difficult to study because most women do not routinely present for obstetric care until later in the first trimester. However, women who conceive following in vitro fertilization (IVF) are monitored frequently and early in pregnancy with serial hormone measurements and thus represent a unique population ideal for study of early placentation events.
Placental trophoblast invasion begins soon after embryo attachment and continues through the first trimester. It is hypothesized that assessment of serial hormone levels, such as of human chorionic gonadotropin (hCG) at this early stage of pregnancy may be useful in predicting early placental development and possibly perinatal outcome. Serum hCG, a hormone secreted by placental syncytiotrophoblasts and detectable in the serum as early as 6–12 days after ovulation (2), rises rapidly during early pregnancy, peaks around the 10th–11th week of gestation, and is routinely measured in women undergoing IVF following embryo transfer (ET).
While the expected rate of increase in hCG has been defined for an ongoing intrauterine gestation in both the assisted reproductive and medically unassisted pregnancy populations (3, 4), the association between rise in serial hCG and pregnancy outcome, in particular adverse outcome at the time of delivery, remains unclear. Previous studies have shown that low initial serum hCG levels following ET are negatively associated with pregnancy viability (5, 6) and that initial level and rise of hCG may predict live delivery rate (7). However, data demonstrating the association between the rate of early hCG increase and pregnancy outcomes at delivery is lacking.
Therefore, the goal of this study was to interrogate the association between the rate of increase in hCG in the early first trimester and adverse pregnancy outcomes related to birth weight and gestational age at the time of delivery in a cohort of women who conceived with IVF and subsequently delivered a singleton live born infant. We hypothesized that the rate of increase in serial hCG levels may serve as a surrogate marker for trophoblast differentiation, and hence placentation, and that extremes in the rates of increase may identify women at risk for adverse pregnancy outcomes.
Materials and Methods
This retrospective cohort study assessed singleton live births conceived with the assistance of IVF from 2005–2009 at the Hospital of the University of Pennsylvania (HUP). We chose these years for analysis because, during that time period, the same laboratory protocols were used for all cases, including culture media (LifeGlobal) and oxygen tension (95% air / 5% CO2). Included patients had only one gestational sac at the time of a 6-week ultrasound and two or more serial hCG serum concentrations. Conceptions after both "fresh" and frozen/thawed ETs were included. Baseline patient demographics and characteristics were obtained from medical records and serum hCG levels following ET were abstracted from the electronic database. Donor egg cycles and twin and triplet pregnancies were excluded from analysis. This study was approved by the University of Pennsylvania Institutional Review Board (protocol number 813313).
Primary outcomes included birth weight (grams) and gestational age (days) at delivery. Secondary pregnancy outcomes included specific adverse events including delivery of an infant with: low birth weight (LBW, <2,500g), preterm delivery (<37 weeks), small for gestational age (SGA, <10th percentile) (8), gestational hypertension, and preeclampsia as defined by internationally accepted diagnostic criteria (9). Infant NICU admission was collected as a surrogate marker of infant health, although infant follow-up after hospital discharge was unavailable.
Infant birth weight, date of delivery, and gender were reported by the patient as part of routine post-treatment follow-up for reporting to the Society for Assisted Reproductive Technologies (SART) and were confirmed with delivery records, when available. The outcomes of gestational hypertension, preeclampsia, and NICU admission were available for a subset of patients who received care and delivered at the primary institution (Hospital of the University of Pennsylvania, HUP).
Exposure was defined as the increase in hCG over time, or slope. Our focus was the rise of hCG within the first six weeks of gestation, when the natural log-transformed hCG is considered linear (4). Values of hCG greater than 10,000 mIU/mL or values obtained from women of a gestational age greater than six weeks, or more than 28 days from egg retrieval, were excluded.
hCG values were transformed using natural log to better meet the assumptions of regression modeling and reduce the influence of large values. For each woman, a slope, or rate of rise in log-transformed hCG levels was determined using simple linear regression for her data only and also using linear mixed-effects (LME) regression for the entire sample which allows for within- and between-subject variation in the rate of hCG rise (10). Both estimates of rise were evaluated as a predictor of adverse pregnancy outcome and ultimately, LME regression estimates were used. While the two estimates are highly correlated with one another (Pearson correlation coefficient = 0.910, p<0.001), slope estimate using LME utilizes within- and between-subject data to enhance precision in slope estimates.
For this analysis, hCG rise is presented as a one-day change on the natural log scale along with corresponding two-day percentage increases in hCG (normal scale).
Serum hCG concentrations were measured with either the Abbott AxSYM (Abbott Laboratories, Abbott Park, IL) or DPC Immulite (Diagnostic Products Corporation, Los Angeles, CA) total immunoassay systems. Both the interassay and intraassay coefficients of variation were below 10%. Results are expressed as milli- International Units per milliliter (mIU/mL), using the third international reference hCG standard (3rd IS).
The association between the rate of increase in hCG, or hCG rise, and pregnancy outcomes was assessed using t-test, chi-square test, and linear and logistic regression where appropriate. hCG rise was explored as a continuous variable and categorized into quartiles. Multivariable linear or logistic regression was used to explore the effect of potential confounders using backwards selection. For each hypothesized confounder, effect modification, or interaction with the primary exposure variable (hCG rise) was first examined and included if significant. Variables with ≥15% change in the regression coefficient were identified as confounders and included in final multivariable modeling.
The statistical software packages SAS 9.2 (SAS Institute Inc., Cary, NC) and Stata 11.2 (Statacorp LP, College Station, TX) were used for statistical analysis.
Results
Three-hundred-sixty IVF cycles resulting in a singleton live birth met inclusion criteria. From an original cohort of 687 live births, IVF cycles were excluded for the following reasons: twin (n=180) or triplet (n=7) gestations, donor egg cycles (n=68), >1 gestational sac seen at 6-week ultrasound (i.e. no "vanishing twins" were included; n=22], or incomplete or missing serial hCG data (n=50). Subjects had, on average, 2.44 ±0.9 (mean ±SD) embryos transferred and 3.1 ±0.98 serial hCG measurements beginning 15.9 ±1.7 days after egg retrieval. Sixty-nine of 360 (19.2%) of the study population conceived following frozen ET.
Baseline characteristics for the included study population are displayed in supplemental table 1. The majority of patients were Caucasian and non-Hispanic. The mean initial hCG level was 287±231 mIU/L. The median slope was 0.407, which corresponds to a two-day increase in hCG of 126% and the mean ± SD slope was 0.405 ±0.06. Among the entire study population, 90/360 (25%) contributed two hCG values, 202/360 (56.1%) contributed three hCG values, and 68/360 (18.9%) contributed four or more hCG values.
We first examined the association between hCG rise and the two primary outcomes, birth weight (grams) and gestational age (days) at delivery. Unadjusted linear regression demonstrated that hCG rise was significantly positively associated with birth weight (p=0.002). For a one quartile change in slope of hCG rise (or 0.039), birth weight increased by 62.0 grams.
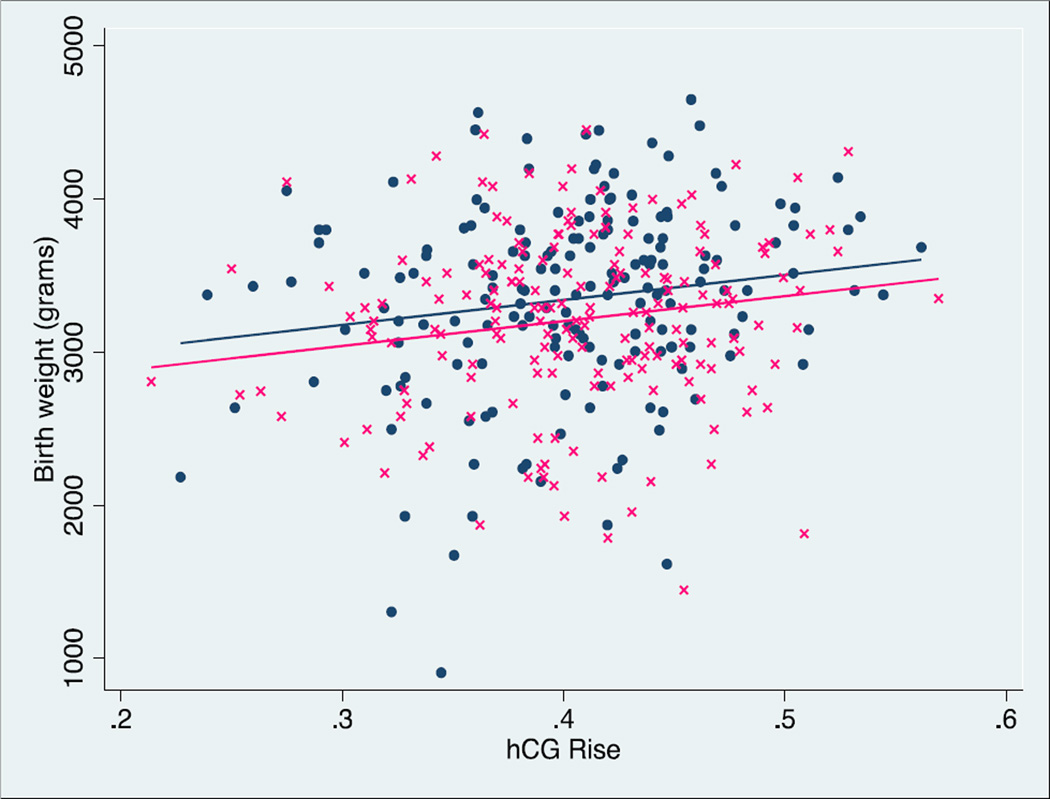
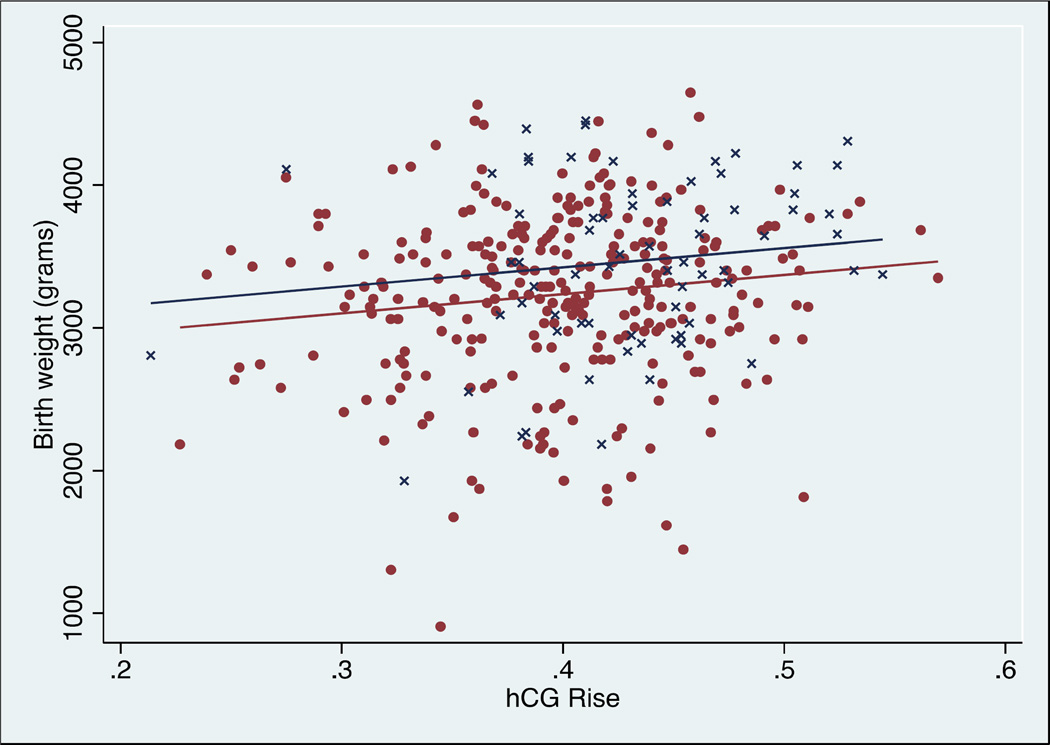
The effect of age, race, ethnicity, parity, type of ET ("fresh" vs. frozen/thawed), number of embryos transferred, infant gender, number of serial hCGs, initial hCG level, and gestational age on this association was assessed using multivariable linear regression. Infant gender, type of ET, gestational age, and race confounded the association between hCG rise and birth weight. Independently, and as expected, gestational age had a significant positive effect on birth weight. For each one-day increase in gestational age, birth weight increased by 27.5 grams (p<0.001). Female infants had a significantly lower predicted birth weight (−193.3 grams compared to males, p<0.001) and infants born following a frozen/thawed ET had a significantly higher predicted birth weight (+150.9 grams compared to fresh, p=0.012). The association between hCG rise and birth weight, stratified by infant gender (Figure 1) and by "fresh" versus frozen/thawed ET (Figure 2) are shown. We examined whether the association was different in the fresh versus frozen transfers. Details of this evaluation are found in Supplemental Figure 2. A statistical test for effect modification due to type of transfer (fresh vs frozen) did not achieve statistically significance, p=0.263.
Figure 1.
Linear regression of hCG rise versus birth weight in male infants (navy filled circles) and females (pink crosses). There is a significant positive association between hCG rise and birth weight (p=0.002), n=360.
Figure 2.
Linear regression of hCG rise versus birth weight of singleton pregnancies following "fresh" embryo transfer cycles (maroon filled circles) or following frozen/thawed embryo transfer cycles (navy crosses). There is a significant positive association between hCG rise and birth weight (p=0.002), n=360.
In a multivariable linear model the effect of race on this association was no longer significant and removed from the model. The final model of the association between hCG rise and birth weight included gestational age, ET type ("fresh" vs. frozen/thawed), and infant gender. After adjusting for infant gender and type of transfer, it was demonstrated that a one SD increase in hCG rise resulted in a 50.7 gram increase in birth weight (p=0.033).
Similarly, the association between the rate of hCG rise and gestational age at delivery was determined. No association was noted between hCG rise and gestational age (p= 0.178). Analysis of potential confounders did not change this finding.
The mean rate of hCG increase was then compared between subjects with and without specific adverse clinical outcomes (Table 1) using two-sample t-tests. Subjects who delivered a LBW infant had a significantly slower hCG rise (p=0.035) compared to those who did not. Similarly, subjects delivering a SGA infant also showed a significantly slower hCG rise (p=0.038). There was no statistically significant difference in mean hCG rise among subjects with preterm delivery compared to subjects without, although a trend was noted (p=0.096). Among those with delivery records available for review (n=170), there was no association between mean hCG rise and gestational hypertension (p=0.908), preeclampsia (p=0.849), or infant NICU admission (p=0.222).
Table 1.
Association of hCG rise and adverse perinatal outcomes.
| Outcome | n (%) | Rate of hCG Rise |
Two-Day Increase (%) |
*P-value | |
|---|---|---|---|---|---|
| Low birth weight | Yes | 40 (11.1) | 0.386 ±0.05 | 116.4 | 0.035 |
| No | 319 (88.6) | 0.407 ±0.06 | 125.7 | ||
| Preterm delivery | Yes | 47 (13.1) | 0.391 ±0.06 | 118.6 | 0.096 |
| No | 313 (86.9) | 0.407 ±0.06 | 125.7 | ||
| Small for gestational age | Yes | 13 (3.6) | 0.371 ±0.07 | 110.0 | 0.038 |
| No | 346 (96.4) | 0.406 ±0.06 | 125.2 | ||
| Gestational hypertension | Yes | 11 (6.5) | 0.409 ±0.09 | 126.6 | 0.908 |
| No | 156 (91.8) | 0.407 ±0.06 | 125.7 | ||
| Preeclampsia | Yes | 7 (4.1) | 0.411 ±0.07 | 127.5 | 0.849 |
| No | 160 (94.1) | 0.407 ±0.06 | 125.7 | ||
| Neonatal intensive care unit admission | Yes | 21 (12.4) | 0.391 ±0.07 | 118.6 | 0.222 |
| No | 149 (87.7) | 0.409 ±0.06 | 126.6 |
P-value for two-tail t-test
For ease of interpretation, hCG rise was stratified into quartiles. The association with adverse pregnancy outcome is presented in Table 2. The first (slowest) quartile served as the reference quartile. Unadjusted odds ratios (OR) for adverse outcomes showed that the risk of delivering an infant of low birth weight, when compared to the first quartile, decreased as rate of hCG rise increased (p=0.018 for overall trend). When individual quartiles of hCG rise were assessed, this association between hCG rise and risk of adverse outcome was significantly decreased among individuals in the fourth quartile (OR = 0.32, 95%CI (0.11–0.92)). After adjustment for previously identified covariates (gestational age, infant gender, and embryo transfer type) the association between the fastest rate of hCG rise (fourth quartile) and a lower risk for low birth weight persisted (OR = 0.13, 95%CI (0.02–0.70)).
Table 2.
Unadjusted and adjusted odds ratios for adverse outcomes (95% confidence interval) by quartile of hCG rise.
| hCG Rise Quartile | Low birth weight | Preterm delivery | Small for gestational age | |||
| OR | AOR | OR | AOR | OR | AOR | |
| 1st quartile | Ref | Ref | Ref | Ref | Ref | Ref |
| 2nd quartile | 0.92 (0.40, 2.08) | 0.87 (0.28, 2.64) | 1.00 (0.46, 2.19) | 1.06 (0.48, 2.36) | 0.13 (0.02, 1.11) | 0.14 (0.02, 1.16) |
| 3rd quartile | 0.54 (0.22, 1.37) | 0.79 (0.23, 2.71) | 0.49 (0.20, 1.23) | 0.50 (0.20, 1.26) | 0.56 (0.16, 2.00) | 0.62 (0.17, 2.26) |
| 4th quartile | 0.32 (0.11, 0.92) | 0.13 (0.02, 0.70) | 0.55 (0.23, 1.33) | 0.59 (0.24, 1.47) | 0.13 (0.02, 1.09) | 0.15 (0.02, 1.30) |
| Trend (p-value) | 0.018 | 0.028 | 0.08 | 0.111 | 0.067 | 0.107 |
| hCG Rise Quartile | Gestational hypertension | Preeclampsia | Neonatal intensive care unit admission | |||
| OR | AOR | OR | AOR | OR | AOR | |
| 1st quartile | Ref | Ref | Ref | Ref | Ref | Ref |
| 2nd quartile | 0.50 (0.04, 5.74) | 0.62 (0.05, 7.22) | 1.03 (0.14, 7.65) | 1.14 (0.15, 8.74) | 0.56 (0.17, 1.87) | 0.52 (0.10, 2.77) |
| 3rd quartile | 2.16 (0.37, 12.5) | 2.54 (0.43, 15.0) | # | # | 0.33 (0.08, 1.33) | 0.54 (0.09, 3.30) |
| 4th quartile | 2.05 (0.36, 11.8) | 2.65 (0.44, 15.8) | 1.50 (0.24, 9.46) | 1.97 (0.30, 12.7) | 0.56 (0.17, 1.87) | 0.81 (0.16, 4.13) |
| Trend (p-value) | 0.230 | 0.153 | 0.881 | 0.667 | 0.244 | 0.774 |
All outcomes adjusted for infant gender and embryo transfer type (fresh versus frozen). Low birth weight and NICU admission also adjusted for gestational age at delivery. P-value presented for trend. OR = odds ratio; AOR = adjusted odds ratio, # = no event
A similar, although non-significant, trend was noted for delivery of a SGA infant and preterm delivery for women in the upper quartiles of hCG rise. We found no association between the quartile of hCG rise and hypertensive disorders nor for NICU admission.
Given reported differences in perinatal outcomes among "fresh" versus frozen/thawed ETs (11–15), we further investigated the difference in hCG rise among these two groups in our population. We compared the rate of increase in hCG levels among "fresh" and frozen/thawed embryo transfer cycles assessing curves stratified by absolute hCG value, and days from gestation, allowing to isolate the earliest portion of the curve generated by serial hCG values (Supplemental Figure 1). Rise in hCG following frozen/thawed ET was significantly higher than "fresh" ET regardless of absolute hCG value or gestational age.
Discussion
The primary goal of the present study was to assess the association between the rate of hCG rise in the early first trimester and adverse perinatal outcomes, hypothesizing that hCG rise may be a marker of trophoblast differentiation and, thus, invasion and placentation. We assessed the association of slope of hCG rise and perinatal outcomes in three ways: a) the correlation of the slope based on the rise of serial hCG serum concentrations with birth weight and gestational age, b) a comparison of the slope of hCG in those with and without a specific adverse perinatal outcome, and c) the odds of adverse perinatal outcome depending on the quartile of the hCG slope. These data demonstrated that early first trimester maternal serum hCG is associated with pregnancy outcomes beyond the first trimester. These findings suggest that events occurring in the periimplantation period may have long-term impact on fetal heath, possibly related to trophoblast differentiation and early placentation.
Using linear regression of log-transformed hCG values, we were able to approximate the overall rate of hCG increase for each subject starting approximately 15–16 days from egg retrieval (or about 4 weeks gestation). The median rate of two-day increase in hCG was 126%, consistent with our prior work reporting a two-day increase of 124% (4). The association between hCG rise and birth weight was confounded by three important factors, infant gender, type of embryo transfer ("fresh" vs. frozen/thawed), and gestational age at delivery. For a given rate of hCG rise, male infants, infants conceived following a frozen embryo transfer, and infants who were delivered at a later gestational age had, on average, a greater birth weight. However, even after controlling for these factors we demonstrate a positive association of early hCG rise and birth weight, while no association was noted between hCG rise and gestational age (Figure 1). When comparing the slope of early hCG rise in those who did or did not have an adverse perinatal outcome, we observed similar findings: the average slope was slower for infants delivered of low birth weight or small for gestational age (Table 1). There was a non-significant trend towards a slower slope for infants delivered preterm. Of note, there was no apparent difference in hCG slope for women who developed gestational hypertension or preeclampsia. Although we had limited power to detect differences amongst these relatively low prevalence outcomes, this observation may suggest that the second wave of invasion occurring in the late first and early second trimester of pregnancy, a time of different hCG dynamics and not studied in the present investigation, may be responsible for the pathologic placentation associated with gestational hypertensive disorders.
Dividing hCG rise into quartiles allowed us to assess the ‘dose-response’ relationship between exposure and outcome. The odds ratio for having a LBW infant decreased as the quartile of hCG rise increased, ultimately becoming significantly reduced for subjects in the fourth (fastest) quartile. A test for trend showed that each quartile increase in slope was significantly negatively associated with LBW in unadjusted and adjusted analyses (Table 2). There were non-significant trends for greater odds of preterm delivery and small for gestational age infant, but no association with hCG slope and gestational hypertension or preeclampsia. There was no association with the odds of neonatal intensive care unit admission and hCG rise.
Several authors have noted elevated second trimester hCG levels following frozen ET when compared to fresh ET (16–18), however these studies did not specifically assess rates of first trimester increase. We found that the association of hCG level and type of conception was constant even when we stratified our analysis based on gestational age and absolute hCG concentration, suggesting the difference in hCG rise is already apparent as early as 15 days post-IVF retrieval (and remains unaltered) (Supplemental Figure 1).
It has been hypothesized that the non-physiologic maternal environment during a “fresh ET” may affect early trophoblast differentiation and placentation and help explain the association of lower perinatal morbidity following frozen ET when compared to fresh ET in both single-center and population based studies (11–15). However, this mechanism may be independent of early hCG rise over time as we noted an association with low birth weight and slow hCG rise after both fresh and frozen transfers. The association with obstetrical outcome and hCG rise was in the same direction, with a similar strength of association, when data are stratified by fresh vs frozen conception (data not shown). Moreover, the slope of hCG rise in conceptions after fresh or frozen embryo transfer was not different in this cohort (Supplemental figure 2).
Currently, there is no definitive explanation for the cellular mechanisms underlying our observations and to explain how early hCG rise correlates with placental health and fetal growth. Nevertheless, it can be hypothesized that variations in trophoblast differentiation towards the invasive/extravillous phenotype versus the hCG-producing/villous phenotype are at the center of these findings (19). This remains to be elucidated.
Our study differs from previous studies that have assessed maternal serum markers and adverse pregnancy outcomes. We capture an earlier period of gestation and assess a maternal serum marker of placentation as it changes over time. Previous authors have assessed maternal serum markers collected as part of aneuploidy screening and correlation with preeclampsia, low birth weight, still birth, and preterm delivery (19–23). However, the use of hCG alone has not been shown to have meaningful clinical utility to predict adverse pregnancy outcomes and is best when used in combination with other screening measures (24, 25). Findings have been inconsistent including that late first trimester hCG levels in women who develop preeclampsia or growth restriction, are only slightly reduced, if at all, and that in the second trimester, hCG levels may in fact be elevated in those with adverse outcomes (26). This finding may be attributable to a ‘hypoperfusion-leakage’ phenomenon occurring after the failure of an adequate second wave of trophoblast invasion in the late first and early second trimester (27). Although a low hCG level as early as 12 days after cleavage-stage ET has been recently associated with a higher risk of pre-eclampsia (28), we did not observe such an association. This may be due to the limited pre-eclampsia events in our cohort to allow us to adequately assess the relationship between hCG and preeclampsia. It is plausible that low hCG levels with compensatory faster rise may reflect dysfunctional placentation and be a marker of adverse outcomes in this population later in pregnancy. The assessment of serial hCG in the early first trimester has primarily been used to assess viability, with slower rise associated with miscarriage and faster rise with live birth (6, 7).
Limitations of our study include its retrospective nature. While we ascertained hCG curves and demographic information on the majority of subjects that met our inclusion criteria, missing data, such as smoking status, BMI and past history of preterm delivery/low birth weight were not available. Nevertheless, smoking is low in this patient population and is only a theoretical possibility that a history of a poor obstetrical outcome would affect the hCG rise in a subsequent pregnancy. We also did not analyze the impact of the pre conception hormonal milieu (i.e. estrogen or progesterone levels). The effect of these potential confounding factors would be an important future direction of this line of research. We attempted to reduce any effect that ‘vanishing twins’ may have on hCG curves, and on perinatal outcomes, by limiting our study to only pregnancies with one gestational sac at six-week ultrasound. However, it was not feasible to limit our analysis to only cycles with a single ET due to limited number of single ETs (29/360, 8.1%) in our cohort during the period of this study. However we are confident that any confounding based on a potential biochemical vanishing twin would be minimal.
Our study was conducted on a convenience sample specifically chosen to minimize potential confounding based on laboratory techniques and media. A post hoc power calculation determined that given our sample size, we had >90% power to detect a one-half SD difference in the outcome of LBW in this purposeful homogenous population. Estimating hCG rise for each individual with regression models introduced random error and may have reduced our precision, biasing results towards the null. To address this, we reduced potential modeling error using LME regression to more accurately account for within- and between-subject variation in hCG rise. Future study will be needed to address the effects of different media, oxygen tension or culture conditions.
We believe that our study has several strengths. First, we proposed a novel hypothesis, that events very early in pregnancy have an association with ultimate pregnancy outcome. We studied this association with respect to clinically meaningful endpoints that occur at the time of delivery and did not limit our analysis to first trimester endpoints such as the diagnosis of a viable pregnancy. Furthermore, we restricted our study population to single gestational sac early in the first trimester resulting in singleton live births, in an attempt to reduce the effect that multiple pregnancies (even “vanishing twins”) have on hCG curves and pregnancy outcomes.
Conclusion
In conclusion, we found that among a cohort of women who conceived after IVF and delivered a singleton live born infant, a faster rate of hCG rise in the first few weeks of early gestation was associated with a reduced risk of delivering a LBW infant, but not preterm delivery nor hypertensive disorders of pregnancy. This novel finding suggests that events of early pregnancy, such as trophoblast differentiation, implantation and early placental development, may play a crucial role in the health of the developing embryo and impact infant health. While our findings are intriguing, much work remains before the cellular mechanism of this clinical observation is elucidated and before this information can be applied clinically.
Supplementary Material
Acknowledgments
Funding and support: P50-HD068157 (CC); R01-HD036455, K24-HD060687 (KTB, MDS); Doris Duke Clinical Research Fellowship, (CBM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have nothing to disclose.
References
- 1.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "Great Obstetrical Syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011 Mar;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. The New England Journal of Medicine. 1999 Jun 10;340(23):1796–1769. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 3.Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W. Symptomatic patients with an early viable intrauterine pregnancy: HCG curves redefined. Obstet Gynecol. 2004 Jul;104(1):50–55. doi: 10.1097/01.AOG.0000128174.48843.12. [DOI] [PubMed] [Google Scholar]
- 4.Chung K, Sammel MD, Coutifaris C, Chalian R, Lin K, Castelbaum AJ, et al. Defining the rise of serum HCG in viable pregnancies achieved through use of IVF. Human Reproduction. 2006 Mar;21(3):823–828. doi: 10.1093/humrep/dei389. [DOI] [PubMed] [Google Scholar]
- 5.Porat S, Savchev S, Bdolah Y, Hurwitz A, Haimov-Kochman R. Early serum beta-human chorionic gonadotropin in pregnancies after in vitro fertilization: contribution of treatment variables and prediction of long-term pregnancy outcome. Fertil Steril. 2007 Jul;88(1):82–89. doi: 10.1016/j.fertnstert.2006.11.116. [DOI] [PubMed] [Google Scholar]
- 6.Stone BA, Vargyas JM, Ringler GE, March CM, Marrs RP. The rate at which serum total beta-subunit human chorionic gonadotropin increases after embryo transfer is a predictor of the viability of pregnancy and an identifier of determinants of pregnancy. Fertil Steril. 2006 Dec;86(6):1626–1633. doi: 10.1016/j.fertnstert.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 7.Shamonki MI, Frattarelli JL, Bergh PA, Scott RT. Logarithmic curves depicting initial level and rise of serum beta human chorionic gonadotropin and live delivery outcomes with in vitro fertilization: an analysis of 6021 pregnancies. Fertil Steril. 2009 May;91(5):1760–1764. doi: 10.1016/j.fertnstert.2008.02.171. [DOI] [PubMed] [Google Scholar]
- 8.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010 Feb;125(2):e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 9.Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstetrics and Gynecology. 2003 Jul;102(1):181–192. doi: 10.1016/s0029-7844(03)00475-7. [DOI] [PubMed] [Google Scholar]
- 10.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982 Dec;38(4):963–974. [PubMed] [Google Scholar]
- 11.Henningsen AK, Pinborg A, Lidegaard O, Vestergaard C, Forman JL, Andersen AN. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: Danish national sibling-cohort study. Fertility and Sterility. 2011 Mar 1;95(3):959–963. doi: 10.1016/j.fertnstert.2010.07.1075. [DOI] [PubMed] [Google Scholar]
- 12.Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstetrics and Gynecology. 2011 Oct;118(4):863–871. doi: 10.1097/AOG.0b013e31822be65f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kansal Kalra S, Ratcliffe SJ, Milman L, Gracia CR, Coutifaris C, Barnhart KT. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertility and Sterility. 2011 Feb;95(2):548–553. doi: 10.1016/j.fertnstert.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelkonen S, Koivunen R, Gissler M, Nuojua-Huttunen S, Suikkari AM, Hyden-Granskog C, et al. Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995–2006. Human Reproduction. 2010 Apr;25(4):914–923. doi: 10.1093/humrep/dep477. [DOI] [PubMed] [Google Scholar]
- 15.Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995–2006. Fertility and Sterility. 2010 Sep;94(4):1320–1327. doi: 10.1016/j.fertnstert.2009.05.091. [DOI] [PubMed] [Google Scholar]
- 16.Raty R, Virtanen A, Koskinen P, Anttila L, Forsstrom J, Laitinen P, et al. Serum free beta-HCG and alpha-fetoprotein levels in IVF, ICSI and frozen embryo transfer pregnancies in maternal mid-trimester serum screening for Down's syndrome. Human Reproduction. 2002 Feb;17(2):481–484. doi: 10.1093/humrep/17.2.481. [DOI] [PubMed] [Google Scholar]
- 17.Hui PW, Tang MH, Lam YH, Ng EH, Yeung WS, Ho PC. Maternal serum hCG and alpha-fetoprotein levels in pregnancies conceived after IVF or ICSI with fresh and frozen-thawed embryos. Human Reproduction. 2003 Mar;18(3):572–575. doi: 10.1093/humrep/deg153. [DOI] [PubMed] [Google Scholar]
- 18.Hui PW, Lam YH, Tang MH, Ng EH, Yeung WS, Ho PC. Maternal serum pregnancy-associated plasma protein-A and free beta-human chorionic gonadotrophin in pregnancies conceived with fresh and frozen-thawed embryos from in vitro fertilization and intracytoplasmic sperm injection. Prenat Diagn. 2005 May;25(5):390–393. doi: 10.1002/pd.1169. [DOI] [PubMed] [Google Scholar]
- 19.Smith GC, Crossley JA, Aitken DA, Pell JP, Cameron AD, Connor JM, et al. First-trimester placentation and the risk of antepartum stillbirth. JAMA. 2004 Nov 10;292(18):2249–2254. doi: 10.1001/jama.292.18.2249. [DOI] [PubMed] [Google Scholar]
- 20.Krantz D, Goetzl L, Simpson JL, Thom E, Zachary J, Hallahan TW, et al. Association of extreme first-trimester free human chorionic gonadotropin-beta, pregnancy-associated plasma protein A, and nuchal translucency with intrauterine growth restriction and other adverse pregnancy outcomes. Am J Obstet Gynecol. 2004 Oct;191(4):1452–1458. doi: 10.1016/j.ajog.2004.05.068. [DOI] [PubMed] [Google Scholar]
- 21.Kirkegaard I, Henriksen TB, Torring N, Uldbjerg N. PAPP-A and free beta-hCG measured prior to 10 weeks is associated with preterm delivery and small-for-gestational-age infants. Prenat Diagn. 2011 Feb;31(2):171–175. doi: 10.1002/pd.2671. [DOI] [PubMed] [Google Scholar]
- 22.Dugoff L, Hobbins JC, Malone FD, Porter TF, Luthy D, Comstock CH, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial) Am J Obstet Gynecol. 2004 Oct;191(4):1446–1451. doi: 10.1016/j.ajog.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 23.Canini S, Prefumo F, Pastorino D, Crocetti L, Afflitto CG, Venturini PL, et al. Association between birth weight and first-trimester free beta-human chorionic gonadotropin and pregnancyassociated plasma protein A. Fertility and Sterility. 2008 Jan;89(1):174–178. doi: 10.1016/j.fertnstert.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Dane B, Dane C, Kiray M, Cetin A, Koldas M, Erginbas M. Correlation between first-trimester maternal serum markers, second-trimester uterine artery doppler indices and pregnancy outcome. Gynecol Obstet Invest. 2010;70(2):126–131. doi: 10.1159/000303260. [DOI] [PubMed] [Google Scholar]
- 25.Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009 May;53(5):812–818. doi: 10.1161/HYPERTENSIONAHA.108.127977. [DOI] [PubMed] [Google Scholar]
- 26.Zhong Y, Tuuli M, Odibo AO. First-trimester assessment of placenta function and the prediction of preeclampsia and intrauterine growth restriction. Prenat Diagn. 2010 Apr;30(4):293–308. doi: 10.1002/pd.2475. [DOI] [PubMed] [Google Scholar]
- 27.Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991 Jul-Aug;12(4):301–308. doi: 10.1016/0143-4004(91)90339-h. [DOI] [PubMed] [Google Scholar]
- 28.Asvold BO, Vatten LJ, Tanbo TG, Eskild A. Concentrations of human chorionic gonadotropin in very early pregnancy and subsequent pre-eclampsia: a cohort study. Human Reprod. 2014 Jun;29(6):1153–1160. doi: 10.1093/humrep/deu068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.